Abstract
Healthy vascular function is primarily regulated by several factors including EDRF (endothelium-dependent relaxing factor), EDCF (endothelium-dependent contracting factor) and EDHF (endothelium-dependent hyperpolarizing factor). Vascular dysfunction or injury induced by aging, smoking, inflammation, trauma, hyperlipidaemia and hyperglycaemia are among a myriad of risk factors that may contribute to the pathogenesis of many cardiovascular diseases, such as hypertension, diabetes and atherosclerosis. However, the exact mechanisms underlying the impaired vascular activity remain unresolved and there is no current scientific consensus. Accumulating evidence suggests that the inflammatory cytokine TNF (tumour necrosis factor)-α plays a pivotal role in the disruption of macrovascular and microvascular circulation both in vivo and in vitro. AGEs (advanced glycation end-products)/RAGE (receptor for AGEs), LOX-1 [lectin-like oxidized low-density lipoprotein receptor-1) and NF-κB (nuclear factor κB) signalling play key roles in TNF-α expression through an increase in circulating and/or local vascular TNF-α production. The increase in TNF-α expression induces the production of ROS (reactive oxygen species), resulting in endothelial dysfunction in many pathophysiological conditions. Lipid metabolism, dietary supplements and physical activity affect TNF-α expression. The interaction between TNF-α and stem cells is also important in terms of vascular repair or regeneration. Careful scrutiny of these factors may help elucidate the mechanisms that induce vascular dysfunction. The focus of the present review is to summarize recent evidence showing the role of TNF-α in vascular dysfunction in cardiovascular disease. We believe these findings may prompt new directions for targeting inflammation in future therapies.
Keywords: inflammation, macrovascular circulation, microvascular circulation, nitric oxide, reactive oxygen species (ROS), tumour necrosis factor-α (TNF-α)
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; AGE, advanced glycation end-product; AMI, acute myocardial infarction; ASS, argininosuccinate synthase; CRP, C-reactive protein; DC, dendritic cell; EC, endothelial cell; EDHF, endothelium-dependent hyperpolarizing factor; EET, epoxyeicosatrienoic acid; EPC, endothelial progenitor cell; HDL, high-density lipoprotein; HMG-CoA, 3-hydroxy-3-methylglutaryl-CoA; HUVEC, human umbilical vein EC; ICAM-1, intercellular adhesion molecule-1; IHD, ischaemic heart disease; IL, interleukin; I/R, ischaemia/reperfusion; NF-κB, nuclear factor κB; IκB, inhibitor of NF-κB; IKK, IκB kinase; NOS, NO synthase; cNOS, constitutive NOS; eNOS, endothelial NOS; iNOS, inducible NOS; nNOS, neuronal NOS; O2•−, superoxide radical; ONOO−, peroxynitrite; PGI2, prostacyclin; RA, rheumatoid arthritis; RAGE, receptor for AGEs; ROS, reactive oxygen species; SCF, stem cell factor; SK1, sphingosine kinase 1; Sph1P, sphingosine-1-phosphate; TNF, tumour necrosis factor; TNFR, TNF receptor; t-PA, tissue plasminogen activator; VCAM-1, vascular cell adhesion molecule-1; XO, xanthine oxidase; ZOF rat, Zucker Obese Fatty rat
INTRODUCTION
IHD (ischaemic heart disease) accounts for over 500000 deaths annually in the United States. AMI (acute myocardial infarction), also known as a heart attack [1], is a common complication of IHD. AMI usually results from plaque rupture with thrombus formation in a coronary vessel, resulting in an acute reduction in blood supply to the downstream myocardium. Paradoxically, re-establishment of the blood supply can exacerbate vascular injury. Treatment of AMI, such as thrombolysis and other means of revascularization, often induce further vascular injury, which contributes to morbidity and mortality before normal cardiac function restores.
The endothelium is a functional barrier between the blood vessel and the blood stream, and was once considered to be relatively inert [2]. However, various functions of ECs (endothelial cells) have been elucidated, such as the control of fibrinolysis, coagulation, vascular tone, growth and immune response. The endothelium modulates vascular tone through several factors, including NO, PGI2 (prostacyclin) and EDHF (endothelium-dependent hyperpolarizing factor). A hallmark of IHD is the development of coronary vascular lesions, which are linked to well-known risk factors, such as diabetes and obesity conditions associated with increased levels of inflammatory markers (Figure 1). IHD accelerates the atherosclerotic process, the earliest event of which is endothelial dysfunction.
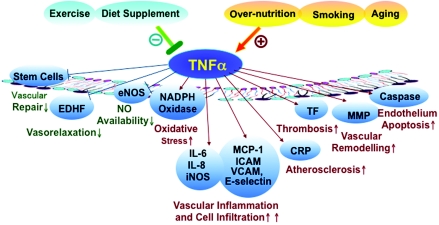
Figure 1. Pivotal role of TNF-α in vascular dysfunction.
Even though numerous risk factors, such as physical inactivity, smoking and over-nutrition, appear to contribute to the development of vascular dysfunction, normal aging is also an independent factor in the aetiology of cardiovascular diseases. There is evidence, however, that those seemingly diverse processes converge on modulating TNF-α signalling to lead to the generation of dysfunctional endothelium and the onset of vascular diseases. TNF-α induces the gene expression of various inflammatory cytokines and chemokines, either dependently or independently of the activation of transcriptional factors, such as NF-κB and AP-1 (activator protein 1). This TNF-α-mediated signalling initiates and accelerates atherogenesis, thrombosis, vascular remodelling, vascular inflammation, endothelium apoptosis, vascular oxidative stress and impaired NO bioavailability, which contribute to the blunted vascular function. Dietary supplements and exercise favourably reduce the risk of vascular dysfunction by inhibiting TNF-α production and (or) TNF-α-mediated signalling. Risk factors in orange demonstrate those factors that converge on TNF-α to induce vascular dysfunction. Factors in green denote those that protect against vascular damage mediated by TNF-α expression and signalling. TNF-α-induced pathophysiological conditions related to vascular function are shown in blue. Both vascular risk factors and protective factors affect the regulation of vascular functions by modulating TNF-α production and downstream signalling. MCP-1, monocyte chemoattractant protein-1; MMP, matrix metalloproteinase; TF, tissue factor.
ROLE OF TNF-α (TUMOUR NECROSIS FACTOR-α) IN ENDOTHELIAL DYSFUNCTION
NO is a free radical generated by NOS (NO synthase) in a two-step five-electron oxidation of the terminal guanidino nitrogen of L-arginine. Three isoforms of NOS have been characterized: eNOS (endothelial NOS), nNOS (neuronal NOS) and iNOS (inducible NOS). eNOS and nNOS are also called cNOS (constitutive NOS) [3]. TNF-α regulates NOS expression and/or activity, which exerts direct effects on NO production; for example, human aortic ECs treated with TNF-α for 8 h had induced iNOS mRNA expression, but down-regulated eNOS expression [4]. Other studies have also shown that TNF-α significantly decreased eNOS expression in ECs [5–8]. Unlike eNOS, iNOS is transcriptionally regulated and not normally produced in most cells. iNOS-derived RNS (reactive nitrogen species) initiate an ONOO− (peroxynitrite)-mediated mechanism and therefore contribute to nitrative stress and impair endothelial function.
Several mechanisms have been suggested for the induction/activation of NOS by TNF-α. Yoshizumi et al. [9] demonstrated that TNF-α markedly reduced mRNA levels of cNOS in HUVECs (human umbilical vein ECs) in a dose- and time-dependent manner without changing the rate of cNOS gene transcription. TNF-α appears to decrease cNOS mRNA levels by increasing the rate of mRNA degradation Another study, however, suggested that TNF-α increases eNOS activity in HUVECs [10]. Activation of eNOS by TNF-α requires activation of Akt (protein kinase B), a known eNOS activator, via Sph1P (sphingosine-1-phosphate) receptor activation. Sph1P receptor is activated by Sph1P, a sphingolipid involved in proliferation, survival, migration and differentiation of these cells, generated through N-SMase2 (neutral sphingomyelinase 2) and SK1 (sphingosine kinase 1) activation [10]. TNF-α-mediated activation of eNOS is accompanied by increased NO generation, which exerts protective effects on DC (dendritic cell) adhesion to endothelium induced by TNF-α itself. It has also been suggested that TNF-α may increase iNOS expression by activating NF-κB (nuclear factor κB) [11]. TNF-α-induced iNOS mRNA expression in microvascular ECs could be decreased by rooperol (a dicatechol from the South African plant Hypoxis rooperi) administration, which is an anti-inflammatory agent in the treatment of several inflammatory disorders [12]. In HUVECs, the effect of TNF-α on iNOS expression was not affected by statin treatment, whereas reduced eNOS expression was reversed by rosuvastatin and ceruvastatin by inhibiting HMG-CoA (3-hydroxy-3-methylglutaryl-CoA) reductase and subsequent blocking of isoprenoid synthesis [13].
Evidence suggests that TNF-α impairs endothelium-dependent and NO-mediated vasodilation in various vascular beds, e.g. mouse coronary arterioles [14], rat coronary arterioles [15], cat carotid arteries [16] and bovine small coronary arteries [17]. Picchi et al. [15] demonstrated that endothelial dysfunction in pre-diabetic metabolic syndrome is a result of the effects of TNF-α and the subsequent production of O2•− (superoxide radical). We have assessed the role of TNF-α in I/R (ischaemia/reperfusion) injury in TNF 1.6 mice, which overexpress TNF-α in cardiac tissue. Myocardial I/R initiated the increase in the expression of TNF-α, which induced activation of XO (xanthine oxidase) and the production of O2•−, leading to coronary endothelial dysfunction [18]. Gao et al. [14] showed that AGE (advanced glycation end-product)/RAGE (receptor for AGEs) and NF-κB signalling play a pivotal role in elevating circulating and/or local vascular TNF-α production. The increased TNF-α expression induces the production of ROS (reactive oxygen species), leading to endothelial dysfunction in Type 2 diabetes. Endothelial dysfunction associated with TNF-α in pathophysiological conditions is linked to excess production of ROS and a decrease in NO bioavailability.
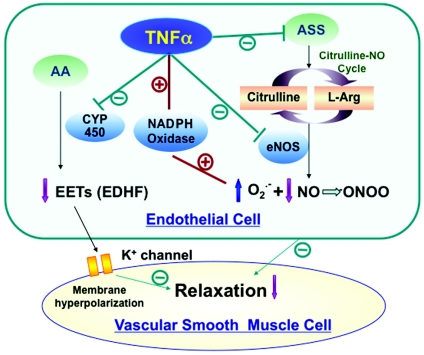
TNF-α appears to decrease the bioavailability of NO by (i) diminishing the production of NO [6,15,17,19], and (ii) enhancing the removal of NO [14]. Picchi et al. [15] reported that the real-time production of NO in isolated coronary arteries from ZOF rats (Zucker Obese Fatty rats; a model of pre-diabetic metabolic syndrome) and ACh (acetylcholine)-induced NO production were significantly lower in ZOF rats compared with the lean control rats. This result suggested that higher concentrations of circulating and protein expression of TNF-α diminished NO bioavailability in ZOF rat coronary arteries via the decreased expression of eNOS (Figure 2). Many studies have shown that the direct effects of TNF-α on eNOS are via down-regulating eNOS expression and diminishing NO production in diverse vasculatures [6,10,14,15]. In addition to eNOS, other factors are also involved in regulating NO production, and one of those factors is a functional citrulline/NO cycle [20–23]. The citrulline/NO cycle is regulated by ASS (argininosuccinate synthase). NO is synthesized from the conversion of L-arginine into L-citrulline mediated by eNOS, and ASS catalyses the rate-limiting step in the arginine regeneration through the citrulline/NO cycle and appears to be co-ordinately regulated with eNOS activity [24] (Figure 2). Goodwin et al. [6] have shown that TNF-α diminished the protein and mRNA expression of ASS in aortic ECs and directly resulted in the reduced production of NO. Gao and co-workers [14,15] reported that TNF-α impaired NO-mediated vasodilation in Type 2 diabetic coronary arterioles. A neutralizing antibody to TNF-α decreased the formation of ROS (O2•−, ONOO− and H2O2) and improved NO-mediated vasodilation. TNF-α stimulates the endothelial generation of ROS by activation of NADPH oxidase, perhaps via the subunits gp91phox, NOX-1, p47phox and p22phox (Figure 2).
Figure 2. Role of TNF-α in endothelial dysfunction.
TNF-α reduces the production of NO through the inhibition of the enzyme activities of ASS and eNOS, and enhances the removal of NO through the increase in NADPH-dependent O2•− production to react with NO to form ONOO−. As a consequence, TNF-α decreases the bioavailability of NO to induce relaxation of smooth muscle in the vasculature. TNF-α also diminishes EETs, one of the candidate EDHFs, via the inhibition of cytochrome P450 (CYP 450) enzyme activity. AA, arachidonic acid.
NO has been implicated as the major mediator of endothelium-dependent relaxation, but EDHF also plays an important role in regulating vascular tone and vasoreactivity, particularly in resistance blood vessels, where a small change in membrane potential causes a significant change in diameter [25]. A number of different factors have been considered as candidates for EDHFs, such as K+ ions, EET (epoxyeicosatrienoic acid) and H2O2 [26]. Current evidence suggests that EDHF-induced responses may be mediated by one or a combination of several factors in different vasculatures [25]. Type 2 diabetes impairs EDHF-mediated vasodilation [27]; however, the mechanisms have not been clearly elucidated. For example, the role of TNF-α in EDHF-mediated vascular dysfunction is controversial. Wimalasundera et al. [28] reported that TNF-α did not inhibit EDHF-dependent vasodilation, whereas Gillham et al. [29] measured a direct effect of TNF-α on EDHF-mediated vasodilation by incubation of 1 nmol/l TNF-α for 1 or 2 h with blood vessels from human omental arteries and showed that TNF-α impaired EDHF-mediated dilation. In addition, Kessler et al. [30] found that TNF-α reduced EDHF synthesis with direct measurement of hyperpolarization from porcine coronary arteries, and Park et al. [30a] have shown that EDHF-mediated dilation in coronary arterioles from Type 2 diabetic mice null for TNF-α (dbTNF−/dbTNF−) was enhanced compared with Type 2 diabetic (db/db) mice. The possible mechanism of impaired EDHF-mediated vasodilation by TNF-α may be via EETs, one of the candidates for EDHF. EETs are synthesized in the ECs from AA (arachidonic acid) through cytochrome P450 oxygenase. TNF-α down-regulated the protein expression of cytochrome P450 2C, which is the major family of cytochrome P450 mono-oxygenases in porcine aortic ECs [30] (Figure 2).
EFFECT OF TNF-α ON ROS PRODUCTION
The production of ROS can stimulate a cytokine cascade through NF-κB-induced transcriptional events, which then induce the expression of TNF-α [31,32]. TNF-α stimulates O2•− production in neutrophils and ECs, reportedly via CAPK (ceramide-activated protein kinase), NADPH oxidase [33], XO [34], NOS [35,36] etc. Many experimental studies suggest that increased O2•− production accounts for a significant proportion of the NO deficit in diabetic vessels. Potential sources of vascular O2•− production include NADPH-dependent oxidases [37,38], XO [39], lipoxygenase, mitochondrial oxidase and uncoupled NOS [40]. NADPH oxidase appears to be the principal source of O2•− production in several animal models of vascular disease, including diabetes [41]. Furthermore, NADPH oxidase proteins and activity are present in human blood vessels, including atherosclerotic coronary arteries [42], and in saphenous veins and mammary arteries from patients with coronary artery disease [43], which suggests that this oxidase system plays an important role in cardiovascular diseases [44]. Guzik et al. [45] have described the mechanisms of increased O2•− production in human diabetes mellitus. They found that basal O2•− release was significantly elevated in vessels from patients with diabetes. Western immunoblot analysis showed increased levels of the p22phox membrane-bound subunit and the p67phox and p47phox cytosolic subunits in both veins and arteries from patients with diabetes. Moreover, engagement of RAGE triggers signalling cascades in which activation of NADPH oxidase recruits multiple downstream pathways, including p21ras, the MAPKs (mitogen-activated protein kinases), the JAK (Janus kinase)/STAT (signal transducer and activator of transcription) pathway, PI3K (phosphoinositide 3-kinase), cdc42/rac and nuclear translocation of NF-κB [46]. As mentioned previously, NF-κB can be considered as a link between TNF-α and AGE/RAGE signalling because TNF-α enhanced RAGE expression by NF-κB activation [32,47–50].
TNF-α activates the transcription of NF-κB, which regulates the expression of genes involved in inflammation, oxidative stress and endothelial dysfunction [51–53]. TNF-α initiates the signalling cascades via the IKK [IκB (inhibitor of NF-κB) kinase] complex, which contains IKKα and IKKβ. TNF-α predominantly initiates signalling cascades acting through IKKβ [54,55]. The inhibitory protein IκBα is phosphorylated, ubiquitinated and degraded by the proteasome, releasing NF-κB to translocate into the nucleus. Under normal physiological conditions, the inflammatory response is terminated by binding NF-κB with the inhibitory protein IκB [56,57]. In ECs, NF-κB regulates the inducible expression of genes encoding TNF-α, IL (interleukin)-6, MCP-1 (monocyte chemoattractant protein-1) and adhesion molecules in diabetic mice [58]. Shoelson et al. [59] have detailed the role of IKKβ in inflammation-induced insulin resistance in obesity and Type 2 diabetes, with the genetic disruption of the IKKβ signalling pathways shown to improve insulin resistance. We have shown that blockade of IKKβ activity by sodium salicylate not only prevented insulin resistance, but also preserved coronary arteriolar vasodilation in Type 2 diabetic mice (J. Yang, Y. Park, H. Zhang, X. Xu, G.A. Laine, K.C. Dellsperger and C. Zhang, unpublished work). Furthermore, obese insulin-resistant subjects have endothelial dysfunction and resistance to endothelium-dependent insulin-mediated vasodilation [60,61]. Augmentation of insulin signalling may contribute to endothelium-dependent NO-mediated vasodilation in diabetic mice treated with sodium salicylate (J. Yang, Y. Park, H. Zhang, X. Xu, G.A. Laine, K.C. Dellsperger and C. Zhang, unpublished work). NF-κB induces TNF-α signalling to accentuate oxidative stress and endothelial dysfunction induced via an IKKβ-dependent mechanism, which may be associated with inflammatory and insulin signalling pathways seen in Type 2 diabetes. AGE/RAGE signalling, as noted above, stimulates the production of O2•−, which could exacerbate both oxidative stress and impaired bioavailability of NO. Thus it appears that impaired NO function may be the direct result of the overproduction of O2•− induced by TNF-α and AGE/RAGE. O2•− is the chemical precursor to many ROS, such as H2O2 and ONOO−.
ROLE OF TNF-α IN LIPID METABOLISM
Both clinical observations and basic research have indicated a potential link between inflammation and lipid metabolism. TNF-α acts as a key cytokine that affects and mediates intermediary metabolism, and a close relationship between TNF-α and lipid metabolism is supported by several studies. In patients with hyperlipidaemia, TNF-α levels correlated significantly with the concentrations of VLDL (very-low-density lipoprotein) -triacylglycerol (triglyceride) and -cholesterol, and negatively with HDL (high-density lipoprotein)-cholesterol [62]. Simvastatin and atorvastatin decrease TNF-α levels in subjects with hyperlipidaemia and hypercholesterolaemia [63–65]. Furthermore, patients with type IIa and IIb dyslipidaemia have an abnormal pattern of TNF-α. HMG-CoA reductase inhibitors (statins) and PPAR-α (peroxisome-proliferator-activated receptor-α) activators (fibrates) normalize TNF-α levels [66]. A high-cholesterol diet induces high levels of serum TNF-α concentration, whereas the mRNA expression of TNF-α is significantly reduced by atorvastatin treatment in hypercholesterolaemic rabbits [67]. TNF-α blockade could significantly affect lipid metabolism. Short-term administration of adaluminab, a fully human anti-TNF-α monoclonal antibody, to patients with active RA (rheumatoid arthritis), significantly increased HDL-cholesterol concentrations; in addition, the atherogenic index decreased [68]. Infliximab, a chimaeric anti-TNF-α monoclonal antibody, had similar results [69–71]. Administration of TNF-α has been demonstrated to directly interfere with the plasma lipid level and metabolic pathways. In mice, administration of TNF-α results in an acute increase in plasma triacylgycerol concentrations of 85%, and inhibition of TNF-α activity blocked the increase in serum triacylglycerols that is characteristically observed after LPS (lipopolysaccharide) treatment [72,73]. The effect of TNF-α on lipid metabolism is complicated, and the mechanisms are complex and take place at different levels and through different steps, from affecting protein expression to inhibiting enzyme activity. Collectively, these studies prompt the question: why does TNF-α produce different responses in different situations? With this controversial background and in conjunction with previous studies, there is ample rationale to study the role of TNF-α in lipid metabolism.
AGING AND TNF-α
Epidemiological studies have shown that even normal aging is an independent risk factor for cardiovascular diseases [74]. An aging-induced pro-inflammatory shift plays an important role in vascular regulatory mechanisms. Previous studies have suggested that circulating levels of TNF-α are elevated in the elderly [75]. Increased TNF-α production has been demonstrated in carotid arteries, aortic wall [76] and coronary arteries [77] of aged rodents. Age-related up-regulation of TNF-α in rat coronary arteries induced endothelial apoptotic cell death, which may lead to impaired endothelial function in the elderly [77]. At the cellular level, TNF-α reduced the growth rate and in vitro life span of ECs in both dose- and treatment-length-dependent manners, suggesting that the aging of ECs is modified by TNF-α exposure [78]. In contrast, inhibition of TNF-α exerts beneficial effects in aging-related pathophysiological changes. In vivo, chronic TNF-α inhibition by etanercept improves flow-mediated arterial dilation in resistance arteries of aged female rats [79], as well as down-regulates the expression of inflammatory markers, including iNOS and ICAM-1 (intercellular adhesion molecule-1), which are abundantly expressed in aged vessels. In carotid arteries of young animals, recombinant TNF-α induced endothelial dysfunction, oxidative stress and increased apoptosis and pro-inflammatory gene expression, mimicking many of the symptoms of vascular aging [74]. Thus dysregulation of TNF-α expression is associated with vascular aging, and anti-TNF-α treatment exerts anti-aging vasculoprotective effects.
To sum up, aging is an independent factor in vascular dysfunction. In the presence of other risk factors, such as smoking and over-nutrition, the development of endothelial dysfunction might be accelerated. Those risk factors converge on TNF-α to cause vascular oxidative stress, vascular remodelling, thrombosis, cell infiltration, apoptosis, vascular inflammation etc., and therefore lead to vascular damage (Figure 1).
TNF-α AND HIGH-FAT AND HIGH-CARBOHYDRATE DIETS
The effects of over-nutrition on endothelial dysfunction in healthy subjects and subjects with dyslipidaemia, the metabolic syndrome and diabetes have been examined in many studies. Endothelial function was markedly impaired by a high-fat meal that caused an acute hypertriacylglycerolaemia. This impairment was evident in patients with dyslipidaemia with baseline hypertriacylglycerolaemia, but not in controls with normotriacylglycerolaemia [80]. Compared with the control group, subjects with metabolic syndrome had reduced endothelial function, as assessed using the L-arginine test, and higher circulating levels of TNF-α. Following the high-fat meal, both triacylglycerol and TNF-α levels increased more in subjects with the metabolic syndrome than in normal subjects, whereas endothelial function decreased more in subjects with the metabolic syndrome [81]. Moreover, in healthy subjects, the high-fat meal increased plasma levels of TNF-α, IL-6, ICAM-1 and VCAM-1 (vascular cell adhesion molecule-1), while the high-carbohydrate meal had no effects in these subjects. In patients with diabetes, both meals significantly increased cytokine and adhesion molecule levels, but the increase lasted longer following the high-fat meal [82]. On the basis of the significant relationship between increases in TNF-α levels and decreases in endothelial function in subjects with the metabolic syndrome and diabetes [81], the mechanisms of TNF-α-induced endothelial dysfunction following high-energy diets has been extensively studied at the molecular and cellular levels. Intraluminal butter administration significantly increased TNF-α expression in lamina proprial macrophage and lymphocyte adherence to intestinal microvessels, accompanied by increases in the expression levels of ICAM-1, MAdCAM-1 (mucosal adhesion cell adhesion molecule-1) and VCAM-1. Furthermore, anti-TNF-α treatment attenuated the enhanced expression of adhesion molecules induced by butter administration [83]. Therefore high-energy diets may cause endothelial dysfunction, as well as potentiate TNF-α-mediated EC injury [84]. Reducing saturated fat and dietary cholesterol intake and avoiding excess calories remains the cornerstone of the dietary approaches to decrease the risk of vascular diseases.
ROLE OF EXERCISE IN CARDIOVASCULAR DISEASE
Pro-inflammation events, such as the increases in TNF-α, CRP (C-reactive protein), IL-6 and resistin, appear to produce their harmful effects, at least in part, by inducing endothelial dysfunction and also by decreasing endothelial NO generation. NO inhibits platelet adherence and aggregation, suppresses vasoconstriction, reduces the adherence of leucocytes to the endothelium and suppresses the proliferation of VSMCs (vascular smooth muscle cells) [85]. Of these pro-inflammatory cytokines, TNF-α is a key player in systemic low-level inflammation through stimulating the expression of adhesion molecules on ECs and thereby inducing endothelial dysfunction [85,86]. TNF-α is a strong biological driver of the metabolic syndrome, which is characterized by abdominal obesity, hypertension, a reduced level of HDL, elevated triacylglycerols and high-fasting glucose, and constitutes an important risk factor in atherosclerosis and Type 2 diabetes [86]. Keller et al. [87] have reported that TNF-α overexpression returned to normal levels after 1 h of acute swimming exercise in TNFR (TNF receptor)-knockout mice. In addition, chronic exercise appears to suppress pro-inflammatory factors, such as TNF-α, CRP and IL-6, and augment anti-inflammatory factors, including IL-4, IL-10, TGF-β (transforming growth factor-β) and adiponectin, even though these results showed discrepancies according to the modes, intensity and time duration of exercise [86,88–90]. Therefore the anti-inflammatory effects of exercise may offer protection against TNF-α-induced insulin resistance and the secondary development of cardiovascular dysfunction. In summary, regular exercise contributes to the prevention of cardiovascular dysfunction by controlling traditional cardiovascular risk factors, including HDL- and LDL (low-density lipoprotein)-cholesterol, improving antioxidant factors, such as SOD (superoxide dismutase) and glutathione peroxidase, elevating the anti-inflammatory effect and suppressing TNF-α, which is the main pro-inflammatory cytokine (Figure 1).
TNF-α AND STEM CELLS/PROGENITOR CELLS
Stem and progenitor cells possess the ability to self-regenerate and differentiate into many cell types, including cardiovascular-specific forms. The role of stem cells and progenitor cells in cardiovascular diseases has been widely investigated. As inflammation is involved in most cardiovascular diseases, understanding the communication and interaction between TNF-α and stem cells is important. Clinical evidence has shown that the serum TNF-α level was negatively correlated with peripheral blood CD34+ stem cells and circulating EPCs (endothelial progenitor cells) in the early and late stages of congestive heart failure, which might be related to the myelosuppressive effect of TNF-α [91]. A similar observation was also reported in a mouse model of congestive heart failure, with increased serum TNF-α levels and decreased bone marrow progenitor cells [92]. In vitro studies have indicated a causal relationship between TNF-α and suppression of haemopoietic stem cell growth. Rusten et al. [93] reported that TNF-α directly inhibited SCF (stem cell factor)-stimulated proliferation of CD34+ haemopoietic progenitor cells. Similar results were also demonstrated in human CD34+ myeloid leukaemia cells and primitive human bone marrow progenitor cells (CD34+CD38−) [94,95]. Interestingly, the inhibitory effects of TNF-α in these studies were consistently mediated by TNFR-I, but not TNFR-II. On the contrary, the TNFR-II signalling pathway has a protective profile on stem cell function. Chen et al. [96] have demonstrated that TNF-α-overexpressing cardiomyocytes attracted increased numbers of embryonic stem cells, mediated by TNFR-II in the embryonic stem cells. Treatment with TNFR-II-overexpressing mesenchymal stem cells attenuated cardiac dysfunction after myocardial infarction [97]. The expression of TNFR-II on bone-marrow-derived progenitor cells was required for ischaemia-induced neovascularization [98]. Thus distinct effects of TNF-α are mediated by different subtypes of TNFRs in stem cells, whereas the overall effect might be dependent on the expression level and ratio of these two receptors.
Apart from the direct effects, TNF-α is able to indirectly influence the fate of stem cells. TNF-α markedly stimulates production of GM-CSF (granulocyte/macrophage colony-stimulating factor), a strong mobilizer of stem cells from bone marrow [99]. Activation of the TNF-α/Fas pathway in lymphocytes in the bone marrow may play a pathogenic role in suppressing haemopoiesis [92]. In the peripheral circulation, EPC adhesion to HUVECs was significantly increased by TNF-α pre-treatment with HUVECs; the adhesion was mediated by the up-regulation of E-selectin on the cells. Interestingly, when EPCs rather than HUVECs were stimulated with TNF-α, EPC adhesion to HUVECs was not induced [100]. TNF-α also has effects on stem cell differentiation: administration of TNF-α switched the differentiation of these cells from granulocytes to almost complete production of macrophages when mouse Lin−Sac+ haemopoietic progenitor cells were cultured with SCF and IL-7 [101].
In summary, TNF-α plays an important role in regulating stem-cell-mediated vascular reparation and remodelling; however, the overall effect of TNF-α on stem cell mobilization, proliferation and function is complicated, depending on the TNFR subtypes and the presence of other cytokines as well as other cells (Figure 1).
ANTI-TNF-α TREATMENT IN CLINICAL STUDIES
As a potent pro-inflammatory trigger, the central role of TNF-α in vascular dysfunction has been demonstrated by the ability of agents that block the action of TNF-α to treat a range of cardiovascular disorders and inflammatory conditions, including AMI, heart failure, RA, diabetes, hyperlipidaemia and COPD (chronic obstructive pulmonary disease).
Intra-arterial TNF-α infusion in healthy volunteers and patients provides direct evidence about TNF-α-stimulated vascular dysfunction. In healthy volunteers, intra-arterial TNF-α at a dose of 80 or 240 ng/min for 30 min resulted in an acute local vascular inflammation that was associated with impaired endothelium-dependent vasomotion, as well as a sustained and substantial increase in endothelial t-PA (tissue plasminogen activator) release [102]. A dose of 17 ng/min for 60 min increased the basal bioavailability of the vasoconstrictor prostaglandin and reduced the basal bioavailability of NO, although it had no effects on endothelium-dependent vasomotion in healthy subjects [103]. In patients with coronary heart disease, intra-arterial TNF-α at a dose of 80 ng/min for 60 min caused an increase in t-PA concentrations without affecting blood flow [104]. In patients with Type 2 diabetes, intra-arterial infusion with TNF-α (10 ng·100 ml−1 of forearm volume·min−1 for 2 h) induced the impairment of endothelial function in resistance vessels [105].
On the basis of the important role of TNF-α in inducing endothelial dysfunction, clinical trials are under way to investigate the use of the three currently available TNF-α inhibitors (infliximab, etanercept or adalimumab), as well as others, in vascular disorders accompanied by several diseases. Chronic anti-inflammatory treatment with the anti-TNF-α antibody infliximab improved endothelial function of the brachial artery in patients with RA [106,107], systemic vasculitis [108] and Crohn's disease [109]. Anti-TNF-α therapy with etanercept, a recombinant TNFR that binds to and functionally inactivates TNF-α, and adalimumab, a fully human monoclonal antibody directed against TNF-α, were also able to improve endothelial function in patients with RA [110,111]. In patients with advanced heart failure, etanercept improves systemic endothelial vasodilator capacity [112], although studies using anti-TNF-α antibodies in chronic heart failure have been terminated prematurely owing to a lack of benefit on the rate of death or hospitalization [113]. However, although short-term etanercept treatment had a significant beneficial effect on systemic inflammatory markers, no improvement in vascular or metabolic insulin sensitivity was observed in obese patients with Type 2 diabetes [114]. In addition to TNF-α inhibitors, various statins, such as atorvastatin and simvastatin, and the ACEI (angiotensin-converting enzyme inhibitor) quinapril were reported to decrease circulating TNF-α levels, as well as improve endothelial function in patients with Type 2 diabetes, congestive heart failure, RA or hyperlipidaemia [65,115–121]. Therefore, although the efficacy and safety of anti-TNF-α biologicals have been extensively studied in treating vascular complications of inflammatory diseases, such as RA and Crohn's disease, the use of TNF-α inhibitors and antibodies in cardiovascular disease and Type 2 diabetes may need to be evaluated further by randomized controlled clinical trials or long-term observational studies. Furthermore, direct evidence may be needed to address the causal relationship between the effects of statins and ACEIs in attenuating TNF-α production and improving endothelial function.
To sum up, the evidence above suggests an important role of TNF-α in vascular dysfunction. These studies may support the long-term use of drugs that block TNF-α function to reduce the high incidence of cardiovascular disorders and vascular complications in various diseases.
CONCLUDING REMARKS
Recent research in animal models and humans provides compelling evidence identifying TNF-α as one of several regulators of vascular homoeostasis. Major progress has been made in unveiling the molecular mechanisms that underlie the multiple vasculoprotective actions of anti-TNF-α. However, many of the mechanisms proposed in the present review are based on in vitro studies and, thus, the physiological relevance of these findings remains to be confirmed in vivo. Our understanding of TNF-α and TNFR, especially with respect to structure–function relationships and their pathophysiological role in vascular dysfunction, is still in its infancy. There have been trials using TNF-α antagonists in heart failure, but there are very few trials using TNF-α antagonists (soluble receptors) in vascular disease. Although TNF-α antagonists (soluble receptors) have been shown to lack benefits on the rate of death or hospitalization in chronic heart failure, there have been trials using TNF-α antagonists in vascular complications of inflammatory diseases, such as RA and Crohn's diseases. These studies suggested beneficial effects of anti-TNF-α treatment in improving vascular function; however, there are very few trials applying anti-TNF-α treatment in vascular diseases and cardiovascular disorders related to Type 2 diabetes and the metabolic syndrome. Is it time to revisit antagonism of TNF-α in cardiovascular diseases? We believe that further investigations in this exciting field could facilitate the development of selective TNF-α antagonists with therapeutic potential in the management of diabetes and other vascular diseases.
FUNDING
This study was supported by a Pfizer Atorvastatin Research Award [grant number 2004-37)]; an American Heart Association Scientist Development Grant [grant number 110350047A]; and the National Institutes of Health [grant number RO1-HL077566] (to C. Z.).
References
- 1.Guyton A. C., Hall J. E. 6th edn. Philadelphia: Saunders; 1997. Human Physiology and Mechanism of Disease. [Google Scholar]
- 2.Stenvinkel P. Endothelial dysfunction and inflammation: is there a link? Nephrol. Dial. Transplant. 2001;16:1968–1971. doi: 10.1093/ndt/16.10.1968. [DOI] [PubMed] [Google Scholar]
- 3.Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 4.MacNaul K. L., Hutchinson N. I. Differential expression of iNOS and cNOS mRNA in human vascular smooth muscle cells and endothelial cells under normal and inflammatory conditions. Biochem. Biophys. Res. Commun. 1993;196:1330–1334. doi: 10.1006/bbrc.1993.2398. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J., Patel J. M., Li Y. D., Block E. R. Proinflammatory cytokines downregulate gene expression and activity of constitutive nitric oxide synthase in porcine pulmonary artery endothelial cells. Res. Commun. Mol. Pathol. Pharmacol. 1997;96:71–87. [PubMed] [Google Scholar]
- 6.Goodwin B. L., Pendleton L. C., Levy M. M., Solomonson L. P., Eichler D. C. Tumor necrosis factor-α reduces argininosuccinate synthase expression and nitric oxide production in aortic endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H1115–H1121. doi: 10.1152/ajpheart.01100.2006. [DOI] [PubMed] [Google Scholar]
- 7.Xia Z., Liu M., Wu Y., et al. N-acetylcysteine attenuates TNF-α-induced human vascular endothelial cell apoptosis and restores eNOS expression. Eur. J. Pharmacol. 2006;550:134–142. doi: 10.1016/j.ejphar.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 8.Seidel M., Billert H., Kurpisz M. Regulation of eNOS expression in HCAEC cell line treated with opioids and proinflammatory cytokines. Kardiol. Pol. 2006;64:153–158. [PubMed] [Google Scholar]
- 9.Yoshizumi M., Perrella M. A., Burnett J. C., Jr, Lee M. E. Tumor necrosis factor downregulates an endothelial nitric oxide synthase mRNA by shortening its half-life. Circ. Res. 1993;73:205–209. doi: 10.1161/01.res.73.1.205. [DOI] [PubMed] [Google Scholar]
- 10.De Palma C., Meacci E., Perrotta C., Bruni P., Clementi E. Endothelial nitric oxide synthase activation by tumor necrosis factor α through neutral sphingomyelinase 2, sphingosine kinase 1, and sphingosine 1 phosphate receptors: a novel pathway relevant to the pathophysiology of endothelium. Arterioscler. Thromb. Vasc. Biol. 2006;26:99–105. doi: 10.1161/01.ATV.0000194074.59584.42. [DOI] [PubMed] [Google Scholar]
- 11.Zhong L., You J., Sun Q. The role of NF-κB in the TNF-α-induced endothelial cell apoptosis. Zhonghua Yixue Zazhi. 1999;79:863–866. [PubMed] [Google Scholar]
- 12.Bereta J., Bereta M., Allison A. C., Kruger P. B., Koj A. Inhibitory effect of di-catechol rooperol on VCAM-1 and iNOS expression in cytokine-stimulated endothelium. Life Sci. 1997;60:325–334. doi: 10.1016/s0024-3205(96)00633-9. [DOI] [PubMed] [Google Scholar]
- 13.Jantzen F., Koneman S., Wolff B., et al. Isoprenoid depletion by statins antagonizes cytokine-induced down-regulation of endothelial nitric oxide expression and increases NO synthase activity in human umbilical vein endothelial cells. J. Physiol. Pharmacol. 2007;58:503–514. [PubMed] [Google Scholar]
- 14.Gao X., Belmadani S., Picchi A., et al. Tumor necrosis factor-α induces endothelial dysfunction in Leprdb mice. Circulation. 2007;115:245–254. doi: 10.1161/CIRCULATIONAHA.106.650671. [DOI] [PubMed] [Google Scholar]
- 15.Picchi A., Gao X., Belmadani S., et al. Tumor necrosis factor-α induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ. Res. 2006;99:69–77. doi: 10.1161/01.RES.0000229685.37402.80. [DOI] [PubMed] [Google Scholar]
- 16.Aoki N., Siegfried M., Lefer A. M. Anti-EDRF effect of tumor necrosis factor in isolated, perfused cat carotid arteries. Am. J. Physiol. 1989;256:H1509–H1512. doi: 10.1152/ajpheart.1989.256.5.H1509. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad M., Zhang Y., Papharalambus C., Alexander R. W. Role of isoprenylcysteine carboxyl methyltransferase in tumor necrosis factor-α stimulation of expression of vascular cell adhesion molecule-1 in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2002;22:759–764. doi: 10.1161/01.atv.0000015884.61894.dc. [DOI] [PubMed] [Google Scholar]
- 18.Zhang C., Xu X., Potter B. J., et al. TNF-α contributes to endothelial dysfunction in ischemia/reperfusion injury. Arterioscler. Thromb. Vasc. Biol. 2006;26:475–480. doi: 10.1161/01.ATV.0000201932.32678.7e. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg S., Xie J., Wang Y., et al. Tumor necrosis factor-α inhibits endothelium-dependent relaxation. J. Appl. Physiol. 1993;74:2394–2403. doi: 10.1152/jappl.1993.74.5.2394. [DOI] [PubMed] [Google Scholar]
- 20.Xie L., Hattori Y., Tume N., Gross S. S. The preferred source of arginine for high-output nitric oxide synthesis in blood vessels. Semin. Perinatol. 2000;24:42–45. doi: 10.1016/s0146-0005(00)80054-3. [DOI] [PubMed] [Google Scholar]
- 21.Goodwin B. L., Solomonson L. P., Eichler D. C. Argininosuccinate synthase expression is required to maintain nitric oxide production and cell viability in aortic endothelial cells. J. Biol. Chem. 2004;279:18353–18360. doi: 10.1074/jbc.M308160200. [DOI] [PubMed] [Google Scholar]
- 22.Hattori Y., Campbell E. B., Gross S. S. Argininosuccinate synthetase mRNA and activity are induced by immunostimulants in vascular smooth muscle. Role in the regeneration or arginine for nitric oxide synthesis. J. Biol. Chem. 1994;269:9405–9408. [PubMed] [Google Scholar]
- 23.Husson A., Brasse-Lagnel C., Fairand A., Renouf S., Lavoinne A. Argininosuccinate synthetase from the urea cycle to the citrulline-NO cycle. Eur. J. Biochem. 2003;270:1887–1899. doi: 10.1046/j.1432-1033.2003.03559.x. [DOI] [PubMed] [Google Scholar]
- 24.Oyadomari S., Gotoh T., Aoyagi K., Araki E., Shichiri M., Mori M. Coinduction of endothelial nitric oxide synthase and arginine recycling enzymes in aorta of diabetic rats. Nitric Oxide. 2001;5:252–260. doi: 10.1006/niox.2001.0344. [DOI] [PubMed] [Google Scholar]
- 25.Feletou M., Vanhoutte P. M. Endothelial dysfunction: a multifaceted disorder. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H985–H1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- 26.Fitzgerald S. M., Kemp-Harper B. K., Tare M., Parkington H. C. Role of endothelium-derived hyperpolarizing factor in endothelial dysfunction during diabetes. Clin. Exp. Pharmacol. Physiol. 2005;32:482–487. doi: 10.1111/j.1440-1681.2005.04216.x. [DOI] [PubMed] [Google Scholar]
- 27.De Vriese A. S., Verbeuren T. J., Van de Voorde J., Lameire N. H., Vanhoutte P. M. Endothelial dysfunction in diabetes. Br. J. Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wimalasundera R., Fexby S., Regan L., Thom S. A., Hughes A. D. Effect of tumour necrosis factor-α and interleukin 1β on endothelium-dependent relaxation in rat mesenteric resistance arteries in vitro. Br. J. Pharmacol. 2003;138:1285–1294. doi: 10.1038/sj.bjp.0705168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillham J. C., Myers J. E., Baker P. N., Taggart M. J. TNF-α alters nitric oxide- and endothelium-derived hyperpolarizing factor-mediated vasodilatation in human omental arteries. Hypertens. Pregnancy. 2008;27:29–38. doi: 10.1080/10641950701825796. [DOI] [PubMed] [Google Scholar]
- 30.Kessler P., Popp R., Busse R., Schini-Kerth V. B. Proinflammatory mediators chronically downregulate the formation of the endothelium-derived hyperpolarizing factor in arteries via a nitric oxide/cyclic GMP-dependent mechanism. Circulation. 1999;99:1878–1884. doi: 10.1161/01.cir.99.14.1878. [DOI] [PubMed] [Google Scholar]
- 30a.Park Y., Capobianco S., Gao X., Falck J. R., Dellsperger K. C., Zhang C. Role of EDHF in Type 2 diabetes-induced endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2008. doi:10.1152/ajpheart.01261.2007. [DOI] [PMC free article] [PubMed]
- 31.De Martin R., Hoeth M., Hofer-Warbinek R., Schmid J. A. The transcription factor NF-κB and the regulation of vascular cell function. Arterioscler. Thromb. Vasc. Biol. 2000;20:E83–E88. doi: 10.1161/01.atv.20.11.e83. [DOI] [PubMed] [Google Scholar]
- 32.Ye J., Wang L., Zhang X., Tantishaiyakul V., Rojanasakul Y. Inhibition of TNF-α gene expression and bioactivity by site-specific transcription factor-binding oligonucleotides. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;284:L386–L394. doi: 10.1152/ajplung.00134.2002. [DOI] [PubMed] [Google Scholar]
- 33.Sorescu D., Griendling K. K. Reactive oxygen species, mitochondria, and NAD(P)H oxidases in the development and progression of heart failure. Congest. Heart Failure. 2002;8:132–140. doi: 10.1111/j.1527-5299.2002.00717.x. [DOI] [PubMed] [Google Scholar]
- 34.Downey J. M., Omar B., Ooiwa H., McCord J. Superoxide dismutase therapy for myocardial ischemia. Free Radical Res. Commun. 1991;12–13:703–720. doi: 10.3109/10715769109145850. [DOI] [PubMed] [Google Scholar]
- 35.Pritchard K. A., Jr, Groszek L., Smalley D. M., et al. Native low-density lipoprotein increases endothelial cell nitric oxide synthase generation of superoxide anion. Circ. Res. 1995;77:510–518. doi: 10.1161/01.res.77.3.510. [DOI] [PubMed] [Google Scholar]
- 36.Cai H., Harrison D. G. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ. Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 37.Rajagopalan S., Kurz S., Munzel T., et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ushio-Fukai M., Zafari A. M., Fukui T., Ishizaka N., Griendling K. K. p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J. Biol. Chem. 1996;271:23317–23321. doi: 10.1074/jbc.271.38.23317. [DOI] [PubMed] [Google Scholar]
- 39.White C. R., Darley-Usmar V., Berrington W. R., et al. Circulating plasma xanthine oxidase contributes to vascular dysfunction in hypercholesterolemic rabbits. Proc. Natl. Acad. Sci. U.S.A. 1996;93:8745–8749. doi: 10.1073/pnas.93.16.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vasquez-Vivar J., Kalyanaraman B., Martasek P., et al. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc. Natl. Acad. Sci. U.S.A. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warnholtz A., Nickenig G., Schulz E., et al. Increased NADH-oxidase-mediated superoxide production in the early stages of atherosclerosis: evidence for involvement of the renin-angiotensin system. Circulation. 1999;99:2027–2033. doi: 10.1161/01.cir.99.15.2027. [DOI] [PubMed] [Google Scholar]
- 42.Azumi H., Inoue N., Takeshita S., et al. Expression of NADH/NADPH oxidase p22phox in human coronary arteries. Circulation. 1999;100:1494–1498. doi: 10.1161/01.cir.100.14.1494. [DOI] [PubMed] [Google Scholar]
- 43.Guzik T. J., West N. E., Black E., et al. Vascular superoxide production by NAD(P)H oxidase: association with endothelial dysfunction and clinical risk factors. Circ. Res. 2000;86:E85–E90. doi: 10.1161/01.res.86.9.e85. [DOI] [PubMed] [Google Scholar]
- 44.Griendling K. K., Sorescu D., Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ. Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 45.Guzik T. J., Mussa S., Gastaldi D., et al. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105:1656–1662. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- 46.Feng L., Matsumoto C., Schwartz A., Schmidt A. M., Stern D. M., Pile-Spellman J. Chronic vascular inflammation in patients with type 2 diabetes: endothelial biopsy and RT-PCR analysis. Diabetes Care. 2005;28:379–384. doi: 10.2337/diacare.28.2.379. [DOI] [PubMed] [Google Scholar]
- 47.Zou W., Amcheslavsky A., Takeshita S., Drissi H., Bar-Shavit Z. TNF-α expression is transcriptionally regulated by RANK ligand. J. Cell. Physiol. 2005;202:371–378. doi: 10.1002/jcp.20127. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka N., Yonekura H., Yamagishi S., Fujimori H., Yamamoto Y., Yamamoto H. The receptor for advanced glycation end products is induced by the glycation products themselves and tumor necrosis factor-α through nuclear factor-κB, and by 17β-estradiol through Sp-1 in human vascular endothelial cells. J. Biol. Chem. 2000;275:25781–25790. doi: 10.1074/jbc.M001235200. [DOI] [PubMed] [Google Scholar]
- 49.Yao J., Mackman N., Edgington T. S., Fan S. T. Lipopolysaccharide induction of the tumor necrosis factor-α promoter in human monocytic cells. Regulation by Egr-1, c-Jun, and NF-κB transcription factors. J. Biol. Chem. 1997;272:17795–17801. doi: 10.1074/jbc.272.28.17795. [DOI] [PubMed] [Google Scholar]
- 50.Santee S. M., Owen-Schaub L. B. Human tumor necrosis factor receptor p75/80 (CD120b) gene structure and promoter characterization. J. Biol. Chem. 1996;271:21151–21159. doi: 10.1074/jbc.271.35.21151. [DOI] [PubMed] [Google Scholar]
- 51.dela Paz N. G., Simeonidis S., Leo C., Rose D. W., Collins T. Regulation of NF-κB-dependent gene expression by the POU domain transcription factor Oct-1. J. Biol. Chem. 2007;282:8424–8434. doi: 10.1074/jbc.M606923200. [DOI] [PubMed] [Google Scholar]
- 52.Rimbach G., Valacchi G., Canali R., Virgili F. Macrophages stimulated with IFN-γ activate NF-κB and induce MCP-1 gene expression in primary human endothelial cells. Mol. Cell. Biol. Res. Commun. 2000;3:238–242. doi: 10.1006/mcbr.2000.0219. [DOI] [PubMed] [Google Scholar]
- 53.Kumar A., Takada Y., Boriek A. M., Aggarwal B. B. Nuclear factor-κB: its role in health and disease. J. Mol. Med. 2004;82:434–448. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- 54.Bonizzi G., Bebien M., Otero D. C., et al. Activation of IKKα target genes depends on recognition of specific κB binding sites by RelB:p52 dimers. EMBO J. 2004;23:4202–4210. doi: 10.1038/sj.emboj.7600391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lawrence T., Bebien M., Liu G. Y., Nizet V., Karin M. IKKα limits macrophage NF-κB activation and contributes to the resolution of inflammation. Nature. 2005;434:1138–1143. doi: 10.1038/nature03491. [DOI] [PubMed] [Google Scholar]
- 56.Tran K., Merika M., Thanos D. Distinct functional properties of IκBα and IκBβ. Mol. Cell. Biol. 1997;17:5386–5399. doi: 10.1128/mcb.17.9.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee E. G., Boone D. L., Chai S., et al. Failure to regulate TNF-induced NF-κB and cell death responses in A20-deficient mice. Science. 2000;289:2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Russo G., Leopold J. A., Loscalzo J. Vasoactive substances: nitric oxide and endothelial dysfunction in atherosclerosis. Vasc. Pharmacol. 2002;38:259–269. doi: 10.1016/s1537-1891(02)00250-1. [DOI] [PubMed] [Google Scholar]
- 59.Shoelson S. E., Herrero L., Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 60.Steinberg H. O., Chaker H., Leaming R., Johnson A., Brechtel G., Baron A. D. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J. Clin. Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cleland S. J., Petrie J. R., Ueda S., Elliott H. L., Connell J. M. Insulin-mediated vasodilation and glucose uptake are functionally linked in humans. Hypertension. 1999;33:554–558. doi: 10.1161/01.hyp.33.1.554. [DOI] [PubMed] [Google Scholar]
- 62.Jovinge S., Hamsten A., Tornvall P., et al. Evidence for a role of tumor necrosis factor α in disturbances of triglyceride and glucose metabolism predisposing to coronary heart disease. Metab. Clin. Exp. 1998;47:113–118. doi: 10.1016/s0026-0495(98)90203-7. [DOI] [PubMed] [Google Scholar]
- 63.Zubelewicz-Szkodzinska B., Szkodzinski J., Romanowski W., et al. Simvastatin decreases concentration of interleukin-2 in hypercholesterolemic patients after treatment for 12 weeks. J. Biol. Regul. Homeost. Agents. 2004;18:295–301. [PubMed] [Google Scholar]
- 64.Ascer E., Bertolami M. C., Venturinelli M. L., et al. Atorvastatin reduces proinflammatory markers in hypercholesterolemic patients. Atherosclerosis. 2004;177:161–166. doi: 10.1016/j.atherosclerosis.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 65.Marketou M. E., Zacharis E. A., Nikitovic D., et al. Early effects of simvastatin versus atorvastatin on oxidative stress and proinflammatory cytokines in hyperlipidemic subjects. Angiology. 2006;57:211–218. doi: 10.1177/000331970605700212. [DOI] [PubMed] [Google Scholar]
- 66.Okopien B., Krysiak R., Kowalski J., et al. Monocyte release of tumor necrosis factor-α and interleukin-1β in primary type IIa and IIb dyslipidemic patients treated with statins or fibrates. J. Cardiovasc. Pharmacol. 2005;46:377–386. doi: 10.1097/01.fjc.0000175455.46245.c8. [DOI] [PubMed] [Google Scholar]
- 67.Zhao S. P., Wu Z. H., Wu J., Hong S. C., Deng P. Effect of atorvastatin on tumor necrosis factor α serum concentration and mRNA expression of adipose in hypercholesterolemic rabbits. J. Cardiovasc. Pharmacol. 2005;46:185–189. doi: 10.1097/01.fjc.0000167017.69468.61. [DOI] [PubMed] [Google Scholar]
- 68.Popa C., Netea M. G., Radstake T., et al. Influence of anti-tumour necrosis factor therapy on cardiovascular risk factors in patients with active rheumatoid arthritis. Ann. Rheum. Dis. 2005;64:303–305. doi: 10.1136/ard.2004.023119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cauza E., Cauza K., Hanusch-Enserer U., Etemad M., Dunky A., Kostner K. Intravenous anti TNF-α antibody therapy leads to elevated triglyceride and reduced HDL-cholesterol levels in patients with rheumatoid and psoriatic arthritis. Wien. Klin. Wochenschr. 2002;114:1004–1007. [PubMed] [Google Scholar]
- 70.Spanakis E., Sidiropoulos P., Papadakis J., et al. Modest but sustained increase of serum high density lipoprotein cholesterol levels in patients with inflammatory arthritides treated with infliximab. J. Rheumatol. 2006;33:2440–2446. [PubMed] [Google Scholar]
- 71.Vis M., Nurmohamed M. T., Wolbink G., et al. Short term effects of infliximab on the lipid profile in patients with rheumatoid arthritis. J. Rheumatol. 2005;32:252–255. [PubMed] [Google Scholar]
- 72.Memon R. A., Grunfeld C., Moser A. H., Feingold K. R. Tumor necrosis factor mediates the effects of endotoxin on cholesterol and triglyceride metabolism in mice. Endocrinology. 1993;132:2246–2253. doi: 10.1210/endo.132.5.8477669. [DOI] [PubMed] [Google Scholar]
- 73.Feingold K. R., Marshall M., Gulli R., Moser A. H., Grunfeld C. Effect of endotoxin and cytokines on lipoprotein lipase activity in mice. Arterioscler. Thromb. 1994;14:1866–1872. doi: 10.1161/01.atv.14.11.1866. [DOI] [PubMed] [Google Scholar]
- 74.Csiszar A., Labinskyy N., Smith K., Rivera A., Orosz Z., Ungvari Z. Vasculoprotective effects of anti-tumor necrosis factor-α treatment in aging. Am. J. Pathol. 2007;170:388–398. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bruunsgaard H., Skinhoj P., Pedersen A. N., Schroll M., Pedersen B. K. Ageing, tumour necrosis factor-α (TNF-α) and atherosclerosis. Clin. Exp. Immunol. 2000;121:255–260. doi: 10.1046/j.1365-2249.2000.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Belmin J., Bernard C., Corman B., Merval R., Esposito B., Tedgui A. Increased production of tumor necrosis factor and interleukin-6 by arterial wall of aged rats. Am. J. Physiol. 1995;268:H2288–H2293. doi: 10.1152/ajpheart.1995.268.6.H2288. [DOI] [PubMed] [Google Scholar]
- 77.Csiszar A., Ungvari Z., Koller A., Edwards J. G., Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol. Genomics. 2004;17:21–30. doi: 10.1152/physiolgenomics.00136.2003. [DOI] [PubMed] [Google Scholar]
- 78.Shimada Y., Ito H., Kaji K., Fukuda M. Tumor necrosis factor reduces lifespan of human endothelial cells in vitro. Mech. Ageing Dev. 1990;55:245–254. doi: 10.1016/0047-6374(90)90152-6. [DOI] [PubMed] [Google Scholar]
- 79.Arenas I. A., Xu Y., Davidge S. T. Age-associated impairment in vasorelaxation to fluid shear stress in the female vasculature is improved by TNF-α antagonism. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H1259–H1263. doi: 10.1152/ajpheart.00990.2005. [DOI] [PubMed] [Google Scholar]
- 80.Giannattasio C., Zoppo A., Gentile G., et al. Acute effect of high-fat meal on endothelial function in moderately dyslipidemic subjects. Arterioscler. Thromb. Vasc. Biol. 2005;25:406–410. doi: 10.1161/01.ATV.0000152231.93590.17. [DOI] [PubMed] [Google Scholar]
- 81.Esposito K., Ciotola M., Sasso F. C., et al. Effect of a single high-fat meal on endothelial function in patients with the metabolic syndrome: role of tumor necrosis factor-α. Nutr. Metab. Cardiovasc. Dis. 2007;17:274–279. doi: 10.1016/j.numecd.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 82.Nappo F., Esposito K., Cioffi M., et al. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: role of fat and carbohydrate meals. J. Am. Coll. Cardiol. 2002;39:1145–1150. doi: 10.1016/s0735-1097(02)01741-2. [DOI] [PubMed] [Google Scholar]
- 83.Fujiyama Y., Hokari R., Miura S., et al. Butter feeding enhances TNF-α production from macrophages and lymphocyte adherence in murine small intestinal microvessels. J. Gastroenterol. Hepatol. 2007;22:1838–1845. doi: 10.1111/j.1440-1746.2007.04905.x. [DOI] [PubMed] [Google Scholar]
- 84.Hennig B., Toborek M., McClain C. J. High-energy diets, fatty acids and endothelial cell function: implications for atherosclerosis. J. Am. Coll. Nutr. 2001;20:97–105. doi: 10.1080/07315724.2001.10719021. [DOI] [PubMed] [Google Scholar]
- 85.Willerson J. T., Ridker P. M. Inflammation as a cardiovascular risk factor. Circulation. 2004;109:II2–II10. doi: 10.1161/01.CIR.0000129535.04194.38. [DOI] [PubMed] [Google Scholar]
- 86.Bruunsgaard H. Physical activity and modulation of systemic low-level inflammation. J. Leukocyte Biol. 2005;78:819–835. doi: 10.1189/jlb.0505247. [DOI] [PubMed] [Google Scholar]
- 87.Keller C., Keller P., Giralt M., Hidalgo J., Pedersen B. K. Exercise normalises overexpression of TNF-α in knockout mice. Biochem. Biophys. Res. Commun. 2004;321:179–182. doi: 10.1016/j.bbrc.2004.06.129. [DOI] [PubMed] [Google Scholar]
- 88.Flynn M., McFarlin B. K., Markofski M. A. The anti-inflammatory actions of exercise training. Am. J. Lifestyle Med. 2007;1:220–235. doi: 10.1177/1559827607300283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Lemos E. T., Reis F., Baptista S., et al. Exercise training is associated with improved levels of C-reactive protein and adiponectin in ZDF (type 2) diabetic rats. Med. Sci. Monit. 2007;13:BR168–BR174. [PubMed] [Google Scholar]
- 90.Das U. N. Anti-inflammatory nature of exercise. Nutrition. 2004;20:323–326. doi: 10.1016/j.nut.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 91.Valgimigli M., Rigolin G. M., Fucili A., et al. CD34+ and endothelial progenitor cells in patients with various degrees of congestive heart failure. Circulation. 2004;110:1209–1212. doi: 10.1161/01.CIR.0000136813.89036.21. [DOI] [PubMed] [Google Scholar]
- 92.Iversen P. O., Woldbaek P. R., Tonnessen T., Christensen G. Decreased hematopoiesis in bone marrow of mice with congestive heart failure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282:R166–R172. doi: 10.1152/ajpregu.2002.282.1.R166. [DOI] [PubMed] [Google Scholar]
- 93.Rusten L. S., Smeland E. B., Jacobsen F. W., et al. Tumor necrosis factor-α inhibits stem cell factor-induced proliferation of human bone marrow progenitor cells in vitro. Role of p55 and p75 tumor necrosis factor receptors. J. Clin. Invest. 1994;94:165–172. doi: 10.1172/JCI117303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hu X., Tang M., Fisher A. B., Olashaw N., Zuckerman K. S. TNF-α-induced growth suppression of CD34+ myeloid leukemic cell lines signals through TNF receptor type I and is associated with NF-κB activation. J. Immunol. 1999;163:3106–3115. [PubMed] [Google Scholar]
- 95.Ramsfjell V., Borge O. J., Cui L., Jacobsen S. E. Thrombopoietin directly and potently stimulates multilineage growth and progenitor cell expansion from primitive (CD34+ CD38−) human bone marrow progenitor cells: distinct and key interactions with the ligands for c-kit and flt3, and inhibitory effects of TGF-β and TNF-α. J. Immunol. 1997;158:5169–5177. [PubMed] [Google Scholar]
- 96.Chen Y., Ke Q., Yang Y., et al. Cardiomyocytes overexpressing TNF-α attract migration of embryonic stem cells via activation of p38 and c-Jun amino-terminal kinase. FASEB J. 2003;17:2231–2239. doi: 10.1096/fj.03-0030com. [DOI] [PubMed] [Google Scholar]
- 97.Bao C., Guo J., Lin G., Hu M., Hu Z. TNFR gene-modified mesenchymal stem cells attenuate inflammation and cardiac dysfunction following MI. Scand. Cardiovasc. J. 2007;42:56–62. doi: 10.1080/14017430701543556. [DOI] [PubMed] [Google Scholar]
- 98.Goukassian D. A., Qin G., Dolan C., et al. Tumor necrosis factor-α receptor p75 is required in ischemia-induced neovascularization. Circulation. 2007;115:752–762. doi: 10.1161/CIRCULATIONAHA.106.647255. [DOI] [PubMed] [Google Scholar]
- 99.Munker R., Gasson J., Ogawa M., Koeffler H. P. Recombinant human TNF induces production of granulocyte-monocyte colony-stimulating factor. Nature. 1986;323:79–82. doi: 10.1038/323079a0. [DOI] [PubMed] [Google Scholar]
- 100.Nishiwaki Y., Yoshida M., Iwaguro H., et al. Endothelial E-selectin potentiates neovascularization via endothelial progenitor cell-dependent and -independent mechanisms. Arterioscler. Thromb. Vasc. Biol. 2007;27:512–518. doi: 10.1161/01.ATV.0000254812.23238.2b. [DOI] [PubMed] [Google Scholar]
- 101.Fahlman C., Jacobsen F. W., Veiby O. P., McNiece I. K., Blomhoff H. K., Jacobsen S. E. Tumor necrosis factor-α (TNF-α) potently enhances in vitro macrophage production from primitive murine hematopoietic progenitor cells in combination with stem cell factor and interleukin-7: novel stimulatory role of p55 TNF receptors. Blood. 1994;84:1528–1533. [PubMed] [Google Scholar]
- 102.Chia S., Qadan M., Newton R., Ludlam C. A., Fox K. A., Newby D. E. Intra-arterial tumor necrosis factor-α impairs endothelium-dependent vasodilatation and stimulates local tissue plasminogen activator release in humans. Arterioscler. Thromb. Vasc. Biol. 2003;23:695–701. doi: 10.1161/01.ATV.0000065195.22904.FA. [DOI] [PubMed] [Google Scholar]
- 103.Nakamura M., Yoshida H., Arakawa N., Saitoh S., Satoh M., Hiramori K. Effects of tumor necrosis factor-α on basal and stimulated endothelium-dependent vasomotion in human resistance vessel. J. Cardiovasc. Pharmacol. 2000;36:487–492. doi: 10.1097/00005344-200010000-00011. [DOI] [PubMed] [Google Scholar]
- 104.Robinson S. D., Dawson P., Ludlam C. A., Boon N. A., Newby D. E. Vascular and fibrinolytic effects of intra-arterial tumour necrosis factor-α in patients with coronary heart disease. Clin. Sci. 2006;110:353–360. doi: 10.1042/CS20050268. [DOI] [PubMed] [Google Scholar]
- 105.Martens F. M., Rabelink T. J., op 't Roodt J., de Koning E. J., Visseren F. L. TNF-α induces endothelial dysfunction in diabetic adults, an effect reversible by the PPAR-γ agonist pioglitazone. Eur. Heart J. 2006;27:1605–1609. doi: 10.1093/eurheartj/ehl079. [DOI] [PubMed] [Google Scholar]
- 106.Hurlimann D., Forster A., Noll G., et al. Anti-tumor necrosis factor-α treatment improves endothelial function in patients with rheumatoid arthritis. Circulation. 2002;106:2184–2187. doi: 10.1161/01.cir.0000037521.71373.44. [DOI] [PubMed] [Google Scholar]
- 107.Cardillo C., Schinzari F., Mores N., et al. Intravascular tumor necrosis factor α blockade reverses endothelial dysfunction in rheumatoid arthritis. Clin. Pharmacol. Ther. 2006;80:275–281. doi: 10.1016/j.clpt.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 108.Booth A. D., Jayne D. R., Kharbanda R. K., et al. Infliximab improves endothelial dysfunction in systemic vasculitis: a model of vascular inflammation. Circulation. 2004;109:1718–1723. doi: 10.1161/01.CIR.0000124720.18538.DD. [DOI] [PubMed] [Google Scholar]
- 109.Schinzari F., Armuzzi A., De Pascalis B., et al. Tumor necrosis factor-α antagonism improves endothelial dysfunction in patients with Crohn's disease. Clin. Pharmacol. Ther. 2008;83:70–76. doi: 10.1038/sj.clpt.6100229. [DOI] [PubMed] [Google Scholar]
- 110.Bilsborough W., Keen H., Taylor A., O'Driscoll G. J., Arnolda L., Green D. J. Anti-tumour necrosis factor-α therapy over conventional therapy improves endothelial function in adults with rheumatoid arthritis. Rheumatol. Int. 2006;26:1125–1131. doi: 10.1007/s00296-006-0147-y. [DOI] [PubMed] [Google Scholar]
- 111.Gonzalez-Juanatey C., Llorca J., Sanchez-Andrade A., Garcia-Porrua C., Martin J., Gonzalez-Gay M. A. Short-term adalimumab therapy improves endothelial function in patients with rheumatoid arthritis refractory to infliximab. Clin. Exp. Rheumatol. 2006;24:309–312. [PubMed] [Google Scholar]
- 112.Fichtlscherer S., Rossig L., Breuer S., Vasa M., Dimmeler S., Zeiher A. M. Tumor necrosis factor antagonism with etanercept improves systemic endothelial vasoreactivity in patients with advanced heart failure. Circulation. 2001;104:3023–3025. doi: 10.1161/hc5001.101749. [DOI] [PubMed] [Google Scholar]
- 113.Mann D. L., McMurray J. J., Packer M., et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL) Circulation. 2004;109:1594–1602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 114.Dominguez H., Storgaard H., Rask-Madsen C., et al. Metabolic and vascular effects of tumor necrosis factor-α blockade with etanercept in obese patients with type 2 diabetes. J. Vasc. Res. 2005;42:517–525. doi: 10.1159/000088261. [DOI] [PubMed] [Google Scholar]
- 115.Radaelli A., Loardi C., Cazzaniga M., et al. Inflammatory activation during coronary artery surgery and its dose-dependent modulation by statin/ACE-inhibitor combination. Arterioscler. Thromb. Vasc. Biol. 2007;27:2750–2755. doi: 10.1161/ATVBAHA.107.149039. [DOI] [PubMed] [Google Scholar]
- 116.Sola S., Mir M. Q., Lerakis S., Tandon N., Khan B. V. Atorvastatin improves left ventricular systolic function and serum markers of inflammation in nonischemic heart failure. J. Am. Coll. Cardiol. 2006;47:332–337. doi: 10.1016/j.jacc.2005.06.088. [DOI] [PubMed] [Google Scholar]
- 117.Kovacs I., Toth J., Tarjan J., Koller A. Correlation of flow mediated dilation with inflammatory markers in patients with impaired cardiac function. Beneficial effects of inhibition of ACE. Eur. J. Heart Failure. 2006;8:451–459. doi: 10.1016/j.ejheart.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 118.Tikiz C., Utuk O., Pirildar T., et al. Effects of angiotensin-converting enzyme inhibition and statin treatment on inflammatory markers and endothelial functions in patients with longterm rheumatoid arthritis. J. Rheumatol. 2005;32:2095–2101. [PubMed] [Google Scholar]
- 119.Tousoulis D., Antoniades C., Vassiliadou C., et al. Effects of combined administration of low dose atorvastatin and vitamin E on inflammatory markers and endothelial function in patients with heart failure. Eur. J Heart Failure. 2005;7:1126–1132. doi: 10.1016/j.ejheart.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 120.Koh K. K., Son J. W., Ahn J. Y., et al. Vascular effects of diet and statin in hypercholesterolemic patients. Int. J. Cardiol. 2004;95:185–191. doi: 10.1016/j.ijcard.2003.05.018. [DOI] [PubMed] [Google Scholar]
- 121.Economides P. A., Caselli A., Tiani E., Khaodhiar L., Horton E. S., Veves A. The effects of atorvastatin on endothelial function in diabetic patients and subjects at risk for type 2 diabetes. J. Clin. Endocrinol. Metab. 2004;89:740–747. doi: 10.1210/jc.2003-031116. [DOI] [PubMed] [Google Scholar]