Abstract
Naturally acquired immunity to falciparum malaria protects millions of people routinely exposed to Plasmodium falciparum infection from severe disease and death. There is no clear concept about how this protection works. There is no general agreement about the rate of onset of acquired immunity or what constitutes the key determinants of protection; much less is there a consensus regarding the mechanism(s) of protection. This review summarizes what is understood about naturally acquired and experimentally induced immunity against malaria with the help of evolving insights provided by biotechnology and places these insights in the context of historical, clinical, and epidemiological observations. We advocate that naturally acquired immunity should be appreciated as being virtually 100% effective against severe disease and death among heavily exposed adults. Even the immunity that occurs in exposed infants may exceed 90% effectiveness. The induction of an adult-like immune status among high-risk infants in sub-Saharan Africa would greatly diminish disease and death caused by P. falciparum. The mechanism of naturally acquired immunity that occurs among adults living in areas of hyper- to holoendemicity should be understood with a view toward duplicating such protection in infants and young children in areas of endemicity.
INTRODUCTION
Each year malaria infects about one-half billion people, killing 1 million to 2 million and severely dampening economic development (44, 123, 133, 289, 321a, 321b). The parasitic Plasmodium species causing malaria persist and even flourish despite the availability of tools for prevention, control, and treatment. Those tools consist of an array of drugs, diagnostics, and insecticides and a detailed understanding of the breeding site preferences of the many anopheline mosquito vectors. Despite the tremendous strides in biotechnology during the past 5 decades and the application to malaria of the many breakthroughs in molecular biology, genetics, immunology, and vaccinology by talented researchers, useful vaccines of any type evade us. This review examines one factor that may contribute substantially to this failure: inadequate understanding of naturally acquired immunity (NAI).
The dawn of scientific understanding of malaria occurred on 6 November 1880, when Alphonse Laveran observed a male gametocyte exflagellating in a blood smear from an Algerian patient with malaria. This event marked the identification of plasmodia as the cause of malaria (181). Working in India in 1897, Ronald Ross identified plasmodial oocysts in the guts of mosquitoes fed on parasitemic birds, thereby implicating mosquitoes as the vector of malaria (261). William George McCallum confirmed plasmodial exflagellation as a process of sexual reproduction in 1897 (200, 201), and Batistta Grassi et al. confirmed anopheline mosquitoes as the vector of human malaria in 1900 (130).
Human malaria has persisted through the development of miracle drugs and insecticides, a global eradication effort, and 30 years of intensive efforts to develop a practical vaccine. Not only does malaria persist; it thrives. Today the global malaria situation is “serious and becoming worse” according to the WHO. The incidence and range of malaria, which were pushed to lows in about 1965 (the zenith of dichlorodiphenyltrichloroethane spraying campaigns), now increase sharply in areas of endemicity and spread into areas where control or eradication had been achieved. Worse still, this resurgence has been in progress for 40 years. Even as early as 1978 the historian Gordon Harrison wrote of the persistence of malaria in the face of such vigorous efforts to attack it, “Failure so universal, so apparently ineluctable, must be trying to tell us something. The lesson could be of course that we have proved incompetent warriors. It could also be that we have misconstrued the problem”(140). Three dominant factors account for the failure to maintain control: (i) parasite resistance to safe and affordable antimalarials, (ii) the almost complete demise of vector control programs in developing tropical and subtropical countries, and (iii) the failure to develop a practical vaccine that prevents malaria. Inadequate understanding of the mechanisms of naturally acquired clinical immunity against plasmodia may be an important factor contributing to the failure to develop a practical vaccine. We explore this possibility by examining the genesis and character of the current state of understanding of NAI.
In 1980, Bruce-Chwatt (50) wrote, “Malaria immunity may be defined as the state of resistance to the infection brought about by all those processes which are involved in destroying the plasmodia or by limiting their multiplication. Natural (innate) immunity to malaria is an inherent property of the host, a refractory state or an immediate inhibitory response to the introduction of the parasite, not dependent on any previous infection with it. Acquired immunity may be either active or passive. Active (acquired) immunity is an enhancement of the defense mechanism of the host as a result of a previous encounter with the pathogen (or parts thereof). Passive (acquired) immunity is conferred by the prenatal or postnatal transfer of protective substances from mother to child or by the injection of such substances.”
In humans, various types of acquired or adaptive immunity against plasmodia have been defined: (i) antidisease immunity, conferring protection against clinical disease, which affects the risk and extent of morbidity associated with a given parasite density; (ii) antiparasite immunity, conferring protection against parasitemia, which affects the density of parasites; and (iii) premunition, providing protection against new infections by maintaining a low-grade and generally asymptomatic parasitemia (171-174, 276). Here, protection is defined as objective evidence of a lower risk of clinical disease, as indicated by both the absence of fever (axillary temperature of >37.5°C) with parasitemia and lower densities of parasitemia.
Across sub-Saharan Africa where the disease is holoendemic, most people are almost continuously infected by P. falciparum, and the majority of infected adults rarely experience overt disease. They go about their daily routines of school, work, and household chores feeling essentially healthy despite a population of parasites in their blood that would almost universally prove lethal to a malaria-naive visitor. This vigor in the face of infection is NAI to falciparum malaria. Adults have NAI, but infants and young children, at least occasionally, do not. NAI is compromised in pregnant women, especially primigravidae, and adults removed from their routine infections apparently lose NAI, at least temporarily. Interventions that reduce exposure below a level capable of maintaining NAI risk the possibility of catastrophic rebound, as occurred in the highlands of Madagascar in the 1980s, with epidemic malaria killing more than 40,000 people (259). Routine exposure to hyper- to holoendemic malaria protects a majority of individuals while killing a minority. Aggressive interventions that consider only that vulnerable minority risk compromising or eliminating the solid protection against severe malaria in the majority.
This review summarizes what is understood about naturally acquired and experimentally induced immunity against malaria, with the help of evolving insights provided by biotechnology, and places these insights in the context of historical, clinical, and epidemiological observations. Apart from the practical importance of understanding NAI with respect to attacking holoendemic malaria, we also undertake this task to emphasize that NAI may be a good model for vaccine development. Consider a vaccine that allows infants and young children the same immunity enjoyed by their older siblings and parents: no disease with natural boosting, lifelong. Even if that concept were to be rendered superfluous by a safe eradication strategy, a more thorough understanding of NAI would almost certainly arm vaccinologists with other concepts to explore and adapt to specific populations. The exploration of NAI is key to the rational development and deployment of vaccines and other malaria control tools for almost any population at risk and, ultimately, a necessary foundation upon which to develop strategies of eradication by any means.
LIFE CYCLE AND GEOGRAPHIC DISTRIBUTION
Natural transmission of malaria occurs through exposure of a human host to the bite of an infective female anopheline mosquito. It is estimated that mosquitoes generally transmit fewer than 100 sporozoites per bite (241, 260, 306, 307). The traditional view has been that a feeding mosquito inoculates sporozoites from its salivary glands into the peripheral circulation of the host, which results, ultimately, in the invasion of hepatocytes. However, recent studies using intravital imaging have shown that sporozoites are injected by mosquitoes into the skin, where they can remain for up to 6 h (323), and that approximately one-third of those leaving the injection site may enter lymphatics and drain to the regional lymph nodes (7); other sporozoites trickle into the bloodstream and traffic to the liver, resulting in multiple potential sites for sporozoite-host interaction. In the P. berghei model, up to 80% of sporozoites injected by mosquito bite were estimated to infect the liver (122). Despite these relatively high numbers of sporozoites that leave the skin and move to the liver, the capacity of each of these sporozoites to result in asexual erythrocytic-stage infections is low. In humans, it takes the bites of five P. falciparum-infected mosquitoes to ensure that 100% of volunteers become infected (249, 310). In the P. yoelii rodent malaria model system, in which one can frequently infect 100% of inbred mice by intravenous administration of 20 sporozoites (the 50% infectious dose for BALB/c, C57BL/6, A/J, and B10.BR mice has been determined to be 4.9 to 10.6 sporozoites [32]), it takes the bites of six to eight infected mosquitoes to achieve 100% infection. This indicates that the majority of sporozoites inoculated by mosquitoes do not lead to productive infections.
It is now well established that Plasmodium sporozoites migrate through Kupffer cells and several hepatocytes before finally infecting a hepatocyte (157, 222). In hepatocytes, the uninucleate sporozoites undergo a cycle of asexual amplification called schizogony that lasts 2 to 10 days, depending on the species (a minimum of 5.5 days among plasmodia infecting humans), with each exoerythrocytic schizont containing as many as 30,000 uninucleate merozoites. Clinical symptoms of malaria are not manifest during liver-stage maturation. In P. vivax infection, sporozoites can remain within hepatocytes in dormant stages known as hypnozoites that can cause clinical relapses (177). Exoerythrocytic schizonts rupture, and the merozoites are then released into the bloodstream where they quickly invade red blood cells (90), commencing the erythrocytic stage of the disease that is responsible for the clinical symptoms. A recent study with the P. yoelii model shows that liver merozoites are released from hepatocytes as merosomes, clusters of 100 to 200 parasites surrounded by a host cell membrane (10).
The invading merozoite carries with it to the interior of the red blood cell an enveloping membrane derived from the host cell, and within an associated parasitophorous vacuole the parasite commences further asexual development. After maturation from the ring stage (immature trophozoite) to a trophozoite and then to a schizont, the parasite undergoes three to six mitotic divisions to yield 6 to 36 merozoites within each erythrocytic schizont (ranges depend on the species). After 48 h, the schizonts rupture, releasing merozoites into the bloodstream. Some of these invade uninfected red blood cells and repeat the cycle of blood schizongony.
Other parasites differentiate into male or female gametocytes that circulate independently in the peripheral blood. P. falciparum gametocytes appear in peripheral circulation about 7 to 15 days after the initial invasion of erythrocytes, but it remains unclear what factors stimulate gamecytogenesis (105). Without treatment, most patients with falciparum malaria will develop gametocytemia within 10 to 40 days after the onset of parasitemia (80, 82). P. vivax gametocytes, in contrast, appear in peripheral blood before clinical symptoms. If ingested by a feeding anopheline mosquito, these forms differentiate to gametes capable of combining to form a diploid zygote where meiosis occurs. The zygote differentiates to an invasive ookinete that penetrates the gut wall and attaches to the outer aspect of the mosquito gut. Bathed in hemolymph, the ookinete differentiates to an oocyst which balloons in size as schizogony produces many thousands of haploid sporozoites. The mature oocyst ruptures, and the sporozoites migrate actively to the mosquito salivary glands. The sporozoites penetrate the glands and rest in the channels bearing saliva, awaiting access to a vertebrate host.
Four species of plasmodia routinely infect humans: P. falciparum, P. vivax, P. malariae, and P. ovale. The greatest impact on human health in terms of mortality is from P. falciparum. The pan-tropical distribution of the parasite, its potentially lethal course of infection, and a profile of increasing resistance to chemoprophylactic and chemotherapeutic agents justifiably make this species a primary focus of concern. Nonetheless, P. vivax also constitutes an important burden on public health throughout most of the tropical and many subtropical or temperate latitudes. Although less often fatal, this infection causes a severely debilitating disease with frequent and sometime multiple episodes of relapse. Moreover, recent reports reveal a significant risk of severe disease and death caused by the same spectrum of syndromes typically linked to falciparum malaria (23, 126, 300). Resistance of P. vivax to standard antimalarials apparently emerged relatively recently compared to that of P. falciparum (252), but prophylactic and therapeutic failure of chloroquine against P. vivax now dominates over chloroquine-sensitive infections on the island of New Guinea (17, 22). At one study site in Indonesian New Guinea (Papua, formerly known as Irian Jaya), 95% of patients with slide-proven vivax malaria had ordinarily curative levels of drug when reporting ill to clinics (22). Primaquine, the only available drug capable of preventing relapses of vivax malaria, may routinely fail as a consequence of parasite resistance, tolerance, or very poor effectiveness driven by poor adherence to the 14-day treatment regimen (21). P. malariae also occurs throughout the tropics, but it tends to appear in isolated pockets and at relatively low frequency compared to P. falciparum or P. vivax. Chloroquine-resistant P. malariae has been reported from southern Sumatra, Indonesia (188). Microscopically confirmed P. ovale is exceedingly rare in eastern Indonesia, New Guinea, and the Philippines but is relatively common in West Africa. Little is known about the clinical susceptibility of this parasite to standard antimalarials. The geographical distribution, prevalence, lethality, and drug resistance risk of the human malaria parasites are summarized in Table 1.
TABLE 1.
Geographical distribution, prevalence, lethality, and drug resistance risk of the human malaria parasites
| Species | Range | Prevalence | Lethality risk | Drug resistance risk | Relapse | Animal reservoir |
|---|---|---|---|---|---|---|
| P. falciparum | Pan-tropical | High | High | High | No | No |
| P. vivax | Pan-tropical, temperate | High | High (?) | High | Yes | No |
| P. malariae | Tropical | Low, focal | Low | Low | No | No (?) |
| P. ovale | West Africa, Southeast Asia | Rare | Low | Low | Yes | No |
| P. knowlesi | Southeast Asia | Rare | High (?) | Low | No | Yes |
Recent work by Singh, Cox-Singh, and their colleagues in Malaysian Borneo provides compelling evidence that a fifth species may in fact routinely infect humans. They uncovered evidence of P. knowlesi, which naturally infects macaques on that island (and many other areas of southeast Asia), routinely infecting humans living in proximity to the monkeys, causing acute illness and some deaths (92, 93, 283).
Malaria is not an exclusively tropical disease. Numerous small outbreaks in North America over the past 20 years emphasize the fact that malaria reached well into temperate climates as recently as 100 years ago. Even today malaria in the Koreas and much of temperate Middle East illustrates the ability of these parasites (in particular P. vivax, with its ability to lay dormant in the liver for weeks, months, or years) to thrive under seasonally favorable conditions.
HISTORICAL OBSERVATIONS OF NAI
Knowledge of an acquired protection against malaria predates any information on the specific cause of the disease. European colonists in the tropics long understood their dangerous susceptibility to malaria compared to indigenous people. It was obvious to them that those exposed to malaria since birth enjoyed a very high degree of protection, although many attributed such distinctions to genetic constitution (not entirely erroneously) rather than to acquired immunity. In 1900, Robert Koch first reported a scientific basis for naturally acquired protection against malaria (171-174). Using cross-sectional studies of stained blood films, an innovation at that time, Koch examined the frequency and density of parasitemia in two distinct populations: (i) those in an area of low endemicity at Sukabumi, West Java, and (ii) those in an area of high endemicity at Ambarawa, Central Java. Koch deduced that protection against malaria was acquired only after heavy and uninterrupted exposure to the parasite. The relatively uniform distribution (frequency and density) of parasites across age groups at Sukabumi contrasted with the distinct age-dependent patterns seen at Ambarawa. Koch's report was followed by a number of cross-sectional microscopic studies of malaria in communities in Asia and Africa that largely corroborated the findings on Java. By 1920, the essential features of NAI had been described. It was accepted that natural immunity was (i) effective in adults after uninterrupted lifelong heavy exposure, (ii) lost upon cessation of exposure, (iii) species specific, (iv) somewhat stage specific, and (v) acquired at a rate which was dependent upon the degree of exposure (274, 277). These findings put into context the classic studies of epidemic malaria in India reported by Christophers (64, 65), Gill (127), Byam and Archibald (55), and Covell and Baily (88). Epidemic malaria killed tens of thousands of people of all ages because the level of exposure before the epidemic was not sufficient to induce protective immunity. Studies of the strain specificity of NAI awaited therapeutic challenge with malaria for advanced syphilis in the 2 decades remaining before penicillin appeared to supplant that therapy.
HISTORICAL OBSERVATIONS OF ACTIVELY ACQUIRED OR INDUCED IMMUNITY
The advent of “malariatherapy” of patients suffering from general paresis (neurosyphilis) in 1917 by von Wagner-Jauregg set the stage for conclusively demonstrating that effective immunity against malaria could be induced in humans. Malariatherapy effectively cured about one of three individuals treated in crowded syphilis sanitariums across Europe and the United States, a feat which earned von Wagner-Jauregg the Nobel Prize in 1927. A series of published clinical studies involving thousands of patients largely confirmed conclusions from field work (summarized in references 89, 161, and 208). Although achievable after a single infection, induction of adequate protective immunity usually required repeated infections, and protection against P. falciparum appeared to be acquired more slowly than that against P. vivax or P. malariae. Protective immunity to P. vivax did not persist for a long period of time in the absence of reexposure, as evidenced by the eradication of acute or chronic vivax malaria in previously malaria-naive prisoners and subsequent rechallenge studies with P. vivax sporozoites or trophozoites (326). Immunity was species specific, since immunity to a particular species conferred no protection against challenge with a heterologous species (67, 159, 161, 325). However, it was not necessarily strain specific, since protection could be obtained against challenge with heterologous strains as assessed by shortened clinical episodes and reduced levels of parasitemia, although the protection was not quite as effective as that against the homologous strain (38, 40, 161). Furthermore, it appeared that repeated infections brought about a broadening of specificity, i.e., often transcending the strain (267). A series of retrospective examinations of the malariatherapy studies with P. vivax and P. falciparum were published in 1999 and 2004 (80, 82). The apparently strict species specificity of acquired immunity described in these treatments figures later in the interpretation of observations from Indonesian New Guinea where P. falciparum and P. vivax occur together.
After the historic paper by Brown and Brown (47) demonstrating antigenic variation by P. knowlesi in rhesus macaques, a theory explaining the slow onset of immunity in the field emerged. It was reasoned that repeated infections achieved a sufficiently diverse repertoire of antigenic memory to eventually defeat most variants encountered in the wild. In other words, heavy exposure allowed an accumulation of antigenic memory such that, after 8 to 15 years, there resulted protective albeit nonsterilizing immunity. The relatively rapid onset of protective immunity to malaria in neurosyphilis patients receiving malaria therapy, which was seemingly incompatible with this interpretation, was turned into supporting evidence by pointing to the use of homologous strains in many of those experiments. The often superior protection provided by homologous versus heterologous strains (which was only obvious after the first rechallenge) has been the narrow focus of advocates of cumulative acquisition of NAI. Each episode of infection presumably induced immunity to only that strain. The broader view shows dramatic strain-transcending protection that rapidly eradicates the homologous strain advantage with repeated exposure. Indeed, Brown and Brown (47) themselves pointed to this phenomenon in an obscure and rarely cited section of their paper.
An important pitfall in most of those studies, including the challenge studies using nonhuman primates, was the exclusive use of adult subjects. The extrapolation of findings for adult subjects to a human population that includes susceptible infants and children may well have been a critical error. This issue will be detailed later in this paper.
CHARACTERISTICS OF NAI
The complexity of NAI among age groups increases when the level of exposure falls below those seen in the Sahel of Africa, an area of holoendemicity. Across even just Africa, transmission may be perennial and much less intense, sharply seasonal and as intense, or limited to sporadic epidemics at elevations challenging anopheline tolerances and capacity to serve as a vector. An exploration of the characteristics and determinants of NAI in these far more complex settings is beyond the scope of this review. Our purpose here is to explore the basis of NAI as it occurs in settings of perennial intense transmission.
The principal features of NAI have been defined, but little is known about the underlying mechanisms. The development of clinical and parasitological immunity to malaria is marked by the ability to control disease and parasite density. Parasite density is linked to disease, and diminished parasite counts almost certainly contribute to diminished risk of disease. The dominant factor driving protection from disease may be specific to effectors that diminish parasite numbers, but other effectors, e.g., responses that diminish proinflammatory cytokines, may also play a role. In areas of heavy transmission, the prevalence of parasitemia and the risk of morbidity and mortality caused by malaria decrease markedly with age beyond early childhood. Young children exhibit an “antidisease immunity” which affects the risk and extent of morbidity associated with a given parasite density. The protection seems to be rapidly acquired and results in reduced mortality or severe clinical disease, at least acutely. In contrast, the seemingly slowly acquired “antiparasite immunity” confers protection against high-density parasitemia and the attendant risk of severe disease (192). Sterilizing immunity against infection is never fully achieved, and an asymptomatic carrier status is the rule among adults. This phenomenon of a high degree of immune responsiveness together with the nearly permanent presence of relatively low densities of parasites was originally described by Koch in 1900 and is often termed “premunition” (276). In the absence of continual exposure, the solid immunity against severe disease is apparently relatively short lived. In its usual context, premunition suggests an immunity mediated directly by the presence of the parasites themselves and not as much the result of previous infections. Premunition in helminth infections is a good example; i.e., there is apparent protection from reinfection with resident worms that is immediately lost when the worms are eliminated. In the milieu of holoendemic transmission, it is difficult to sort out which may be the dominant factor of protection seen in older children and adults, i.e., circulating parasites or the immunity to them that persists even in their absence. Passive transfer experiments, described below, demonstrated an acquired and persistent immunity at work, rather than premunition in its usual context.
In naive individuals of any age, P. falciparum infection is almost always symptomatic, and clinical symptoms can be observed even at very low parasitemia levels. The immunity to asexual blood stages where transmission is low or seasonal has not been adequately explored, and this review does not fully describe the complexity of those settings. The persistence of low-grade, asymptomatic parasitemias, as in the Peruvian Amazon, for example (42), may involve mechanisms of immune protection distinct from those occurring in hyper- to holoendemic transmission. This review largely excludes immunity in settings intermediate between exposure of the genuinely malaria-naive and that of the chronically and heavily exposed. While intermediate exposures and states of immunity clearly represent important topics, the focus here aims at a grasp of NAI mechanisms in the perhaps less complex setting of exposure saturation and maximal levels of protection afforded. We consider this a logical starting point and basis for comparison for the exploration of immunity in intermediate-transmission settings.
In areas of heavy endemicity, disease is sometimes distinct from parasitemia, and both are age dependent. Among very heavily exposed children, high-density parasitemia may occur in the absence of overt clinical symptoms. The greatest disease risk for these children is severe anemia rather than cerebral malaria or failure of respiratory, renal, or hepatic systems. Adults in such areas rarely have high-density parasitemia, but when they do, the symptoms appear to be more severe than those in children with equal parasitemia density and where there is higher risk of disease (236). These rare symptomatic adults may represent the small fraction of the adult population who defy the odds of reinfection long enough for their immunity to wane.
The prevalence of parasitemia increases sharply beginning at about 20 weeks of age. Nonetheless, children remain remarkably resistant to high parasitemia, fever, and severe disease until about 6 months of age. This protection has been thought to be associated with the presence of maternal immunoglobulin G (IgG) antibodies, since IgG is acquired by the fetus in utero, mainly during the third trimester of pregnancy, and IgG levels decrease from birth over the first year of life. However, at least one study has ruled out maternal antibodies against malaria antigens as the basis of this protection (254). Alternatively, the protection of infants may be associated with parasite growth-inhibitory factors such as lactoferrin and secretory IgA found in breast milk and in maternal and infant sera (165). At between 4 and 10 months of age many infants are seronegative to specific malaria antigens (1, 254), but the kinetics of decay of maternal antibodies show interindividual variations which depend on the antigen and the epidemiological setting (119).
The risk of clinical disease increases from birth to about 6 months of age, depending on the transmission rate, and beginning at around 3 to 4 months of age, infants become susceptible to severe disease and death. The risk of cerebral malaria increases with age in children 2 to 4 years old. At about 2 to 5 years of age, the frequency of clinical disease begins to diminish and the risk of mortality sharply decreases. The age of onset of this protection is somewhat earlier with heavier transmission, but protection rarely occurs before the age of 2 years. The presence and density of P. falciparum parasitemia at any given time do not correlate well with clinical disease in areas of holoendemicity. Children may have high parasite loads but no symptoms, or they may have disease with low-density parasitemia. Parasite density in the peripheral circulation as measured by microscopic evaluation of blood smears does not always reflect the full parasite load, as mature parasites may be sequestered in deep organs. Longitudinal studies measuring parasitemias in blood daily and autopsy studies quantifying parasites in tissues are valuable to provide a more complete picture of the true parasite load. In addition, low-level submicroscopic parasitemias may be important in the onset and maintenance of immunity/premunition.
After the age of peak parasite prevalence, the number of clinical attacks of malaria per year dramatically declines, as does the risk of mortality. From adolescence onwards, severe disease very rarely occurs. Mild clinical episodes may still be quite common, and the cumulative incidence of parasitemia often approaches 100% within just a few months (19, 236). People having chronic, heavy, and largely uninterrupted exposure to infection develop and maintain a highly efficacious protection from severe disease at an age corresponding roughly with the onset of puberty. Studies by Kurtis and colleagues (178) suggest that the onset of puberty itself, rather than cumulative exposure linked to calendar age, may be a dominant factor in the onset of protective immunity. Intrinsic factors linked to a maturing immune system may be one key to understanding the molecular and cellular bases of NAI.
The characteristics of NAI against malaria substantially change during pregnancy. Despite the effective immunity against severe disease that comes with reaching adulthood in areas of heavy endemicity, pregnant women demonstrate a markedly increased susceptibility to malaria (indicated by increased parasite densities and risk of severe disease and death), particularly during first and second pregnancies (reviewed in reference 212). Significant correlations between host maternal parity and risk of pathogenic infection have been reported, and parasite density decreases as the number of gestations increases (87, 99). Maternal susceptibility to malaria infection during pregnancy is thought to be related to the physiological immunosupression that occurs during gestation (213) and the accumulation of erythrocytes infected with P. falciparum in the placenta through cytoadherence mechanisms (28). The specifics of malaria immunity during pregnancy and the associated pathology have being reviewed recently (29, 124, 256, 257) and will not be considered in detail here; however, host factors that may in part explain the susceptibility of pregnant women to malaria include (i) impairment of cellular immunity, as the concentrations of some cytokines (e.g., tumor necrosis factor alpha) in the placenta have been shown to be significantly higher among primigravidae with severe anemia than among other primigravidae and have been correlated with densities of P. falciparum-infected erythrocytes and of intervillous monocyte infiltrates in the placenta (258), and (ii) hormonal immunosuppression, where a sustained increase in the levels of hormones associated with pregnancy may underlie the increased susceptibility of pregnant women, particularly primigravidae, to malaria. Levels of corticosteroids, which suppress cell-mediated immunity, are substantially increased during the third trimester of pregnancy, in primiparous women, and in P. falciparum-infected pregnant women compared with other pregnant women (311). Cortisol concentrations have been found to be significantly higher in primigravidae than in multigravidae; conversely, plasma prolactin levels have been highest in multigravidae (37). Placental P. falciparum infection is also likely to have an effect on the development of immunity in the offspring due to in utero sensitization to parasite antigens (reviewed in references 45 and 107), and this research area has recently gained increasing interest.
EFFICACY OF NAI
NAI provides solid protection against severe morbidity and mortality. Older children and adults in areas of hyper- to holoendemicity rarely experience life-threatening complications caused by malaria. Even mild disease is relatively uncommon. Quantitative demonstrations of the relative immunity of adults living under conditions of hyper- to holoenemic transmission have corroborated this view. In a study in northern Ghana, an area of holoendemicity, 2% of adults in a wet-season cohort of 192 individuals had a first parasitemia after radical cure that was >20,000 parasites/μl. In that cohort, 97% had a parasitemia during the 16 weeks of follow-up (236). In contrast, 32% of infants and young children (age 6 to 24 months) in a wet-season cohort of 254 had parasite densities of >20,000/μl. Most of the subjects with these high-grade parasitemias were ill, children and adults virtually alike (78 of 81 children with fever, versus 3 of 4 adults). Prevention of high-density parasitemia appears to be the basis of adult protection against disease. The relative risk of wet-season parasitemia exceeding 20,000/μl in children was 21 (95% confidence interval [CI] = 8 to 78; P < 0.00001) relative to adults. In other words the protective efficacy of NAI against high-density parasitemia was 94%. The efficacy of adult protection against death has not been similarly measured, but we believe that it must approach 100%.
The risk of death among young children should also be put into the perspective that includes risk of the same among those who lack continuous chronic exposure to infection. There is no precise estimate of the risk of death with P. falciparum in the absence of immunity or chemotherapy in any age group. However, fatality rates among nonimmune Europeans traveling in areas of holoendemicity in Africa during the 19th century provide some clue to the magnitude of risk in malaria-naive adults. McGregor (207) recounted a European expedition on the Niger River during the middle of the 19th century in which 28% of Caucasians died of fevers presumed to be malaria, despite the likely availability of quinine. Mortality due to malaria among French troops posted in Senegal between 1819 and 1831 was reported to range from 9% to 57% (95). One may thus conservatively estimate 30% as the risk of death without acquired immunity or adequate chemotherapeutic management of malaria. In contrast, an estimated 2% of African children succumb to death caused by malaria before the age of 5 years (131). Thus, it seems that the African children, even those within the ages of highest vulnerability, enjoy quite a large degree of protection from severe disease and death. A study by Gupta et al. suggested that this is the case in areas of intense transmission, showing by mathematical modeling that immunity to noncerebral severe malaria may be acquired after only one or two infections (133a). The basis of that protection and how it fails in 1 million or so African children each year are very poorly understood. One study pointed to gamma interferon responses as the key difference between age-matched African children suffering mild or severe malaria (183). Likewise, it is not understood how changes in exposure to infection would affect that protection in either children or adults.
The early susceptibility of children, the seemingly long period of exposure for onset of protective immunity arising from its strain specificity, the requirement for nearly continuous exposure, and its nonsterilizing activity among the most protected have all discouraged vaccine strategists. We offer a different perspective in this review, i.e., that strain-transcending NAI may be acquired relatively quickly, after as few as three or four exposures, providing highly efficacious protection from hyperparasitemia and the attendant risk of severe disease. The key to understanding this perspective lies in careful consideration of the epidemiology of NAI in distinct settings of exposure. Such understanding reveals key insights of immediate relevance to vaccine development strategies and technical objectives.
EPIDEMIOLOGICAL ASPECTS OF NAI
Effect of Exposure
The distribution of malaria morbidity and mortality within communities depends directly upon transmission intensity. Traditionally, the level of malaria endemicity has been classified by reference to the spleen rate (the proportion of children with an enlarged spleen in a sample of the population) in malarious areas (50). More recently, however, it has been recognized that the entomological inoculation rate (EIR), which is the number of infectious mosquito bites received per person per unit of time (185), is a more direct measure of transmission intensity (30). In situations with annual EIRs of below about 10, the malaria prevalence rate is almost directly proportional to the EIR, and malaria transmission tends to be unstable and is considered to be of low to moderate intensity. At annual EIRs of above 10, individuals receive multiple infectious bites, and malaria transmission intensity is considered high and tends to be stable (321a). Where the risk of infection is low, almost all exposed people are at a substantial risk of debilitating or severe disease. Where the risk of infection is high, the risk of severe disease is limited to visitors, infants, young children, and pregnant women. In general, the more intense the transmission, the earlier and more confined the age range of susceptibility to disease. Significant associations have been shown between the intensity of exposure to biting infectious mosquitoes (EIR) and the incidence and density, but not prevalence, of P. falciparum parasitemia in children 6 months to 6 years old who reside in areas of endemicity (27, 31, 202, 203, 301, 304). The incidence of primary clinical episodes among the susceptible subpopulations usually peaks during the high-transmission season and decreases considerably during the low-transmission season.
Among people from areas where the disease is not endemic, a clear correlation exists between the severity of clinical disease and the density of P. falciparum asexual parasitemia (116, 221, 302, 303). High-density parasitemia constitutes a significant risk factor for a poor clinical outcome. In areas of holoendemicity, the prevalence of hyperparasitemia correlates with an exaggerated risk of cerebral malaria, severe anemia, hypoglycemia, lactic acidosis, and respiratory distress (321), all linked to high risk of death (189). However, that correlation often fails among individuals as opposed to populations. Parasitemia among patients with severe malarial anemia tends to be low compared to that among less anemic patients. Nonetheless, prior bouts of hyperparasitemia in such patients very likely account for the severe anemia despite low parasitemia concurrent with anemia (210). In areas of endemicity, the rate of exposure to infected mosquitoes (EIR) has been correlated with the density but not the prevalence of parasitemia (202), although as stated above, the density of parasites in the peripheral circulation does not account for those parasites that may be sequestered. Nonetheless, it has been proposed that parasite density may be used as a surrogate marker for morbidity and mortality associated with malaria (27, 202). It follows from the correlation between density of parasitemia and the EIR (202) that the degree of exposure to biting infectious mosquitoes may determine the risk of death. This is a vitally important point that may help define technical objectives in malaria intervention strategies in areas of holoendemicity, including vaccination. If decreasing parasite loads in the human host, by any means, creates a corresponding decrease in the risk of death, striving to do so merits attention.
Several sets of experimental data support the hypothesis (27, 202, 203) that interventions that reduce P. falciparum transmission intensity will reduce high-density parasitemia and malaria-associated morbidity and mortality. Insecticide-treated bed nets (ITNs) provide protection against morbidity and mortality attributable to malaria (4, 34, 239, 299). This is also true with regard to the risk of fever per se (5, 26, 278) and the prevalence or incidence of parasitemia (26, 97, 278, 291).
Three proposed explanations for the association between intensity of transmission and parasite density seem likely (27). First, decreased parasitemia as measured in low-transmission seasons may be due to acquired immunity, since such children would have recently experienced high-transmission exposure. Second, a higher intensity of exposure to sporozoites may increase the likelihood of being exposed to a strain of parasite to which one has not yet acquired protective immunity. Finally, increased exposure to infected mosquitoes may result in more sporozoites reaching the liver and consequently more parasites developing to mature liver-stage schizonts, which then rupture, resulting in more infected erythrocytes. An inverse correlation between the number of infectious bites and the prepatent period has been reported in experimental challenge studies (66, 242).
In The Gambia and Kenya, the risks of severe malaria (cerebral malaria or severe malaria anemia) in childhood were reported to be lowest among populations with the highest transmission intensities, and the highest disease risks were observed among populations exposed to low to moderate intensities of transmission (195, 196, 202, 203, 292). Snow et al. (292) argued that interventions that diminish the risk of infection may actually increase the risk of poor clinical outcomes. That interpretation provoked some controversy (96, 220). The difficulty was in grappling with an apparent paradox: a greater risk of infection yields a lower risk of disease, and attacking the risk of infection yields a lower risk of disease. We assert that no paradox exists and that the viewpoints are reconcilable when put in the context of quantitative risk of infection and disease and of NAI. The thresholds of exposure leading to clinical immunity or to a high risk of severe disease are not necessarily superimposed. An intervention that pushes the attack rate below the threshold of risk of severe disease does not necessarily cross below the threshold of exposure needed to sustain acquired clinical immunity. However, there must be a threshold of exposure to sustain clinical immunity, and it can be crossed by interventions that diminish the risk of infection. If this threshold is crossed, an increase in susceptibility to less frequent episodes of infection may occur. This may be visualized by the hypothetical relationship illustrated in Fig. 1. The obvious solution is recognizing these thresholds and applying interventions appropriately. However, as discussed below, age is a critical factor that compounds the complexity of such solutions.
FIG. 1.
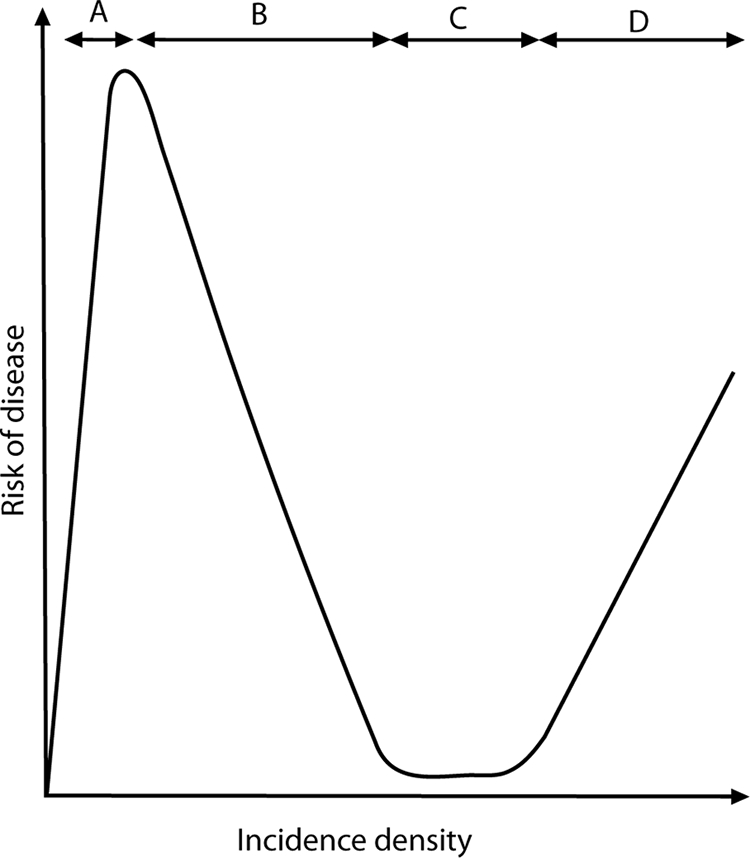
Hypothetical immunity-exposure curve, showing the hypothesized rise and fall of host susceptibility to severe disease with falciparum malaria. Segment A shows an increasing risk of death, principally from hyperparasitemia and cerebral malaria (and perhaps from respiratory and renal failure), rising with an increasing risk of exposure to infection. Segment B shows a declining risk of death with the onset of sufficient exposure to induce NAI. Segment C represents the threshold of exposure that maintains maximum NAI. Segment D shows an intensity of exposure that overwhelms NAI and where the risk of disease states such as severe anemia becomes predominant. (Reproduced from reference 13 with permission of the publisher.)
The results of a recent modeling exercise to develop an age-structured mathematical model of malaria transmission to investigate the processes driving NAI (117) support this threshold hypothesis. The authors of that study concluded that the epidemiological age-prevalence curves seen empirically are best reproduced by a model comprising a form of clinical immunity that reduces susceptibility to clinical disease, develops with age and exposure, and is relatively short lived (half-life of ∼5 years) as well as a form of antiparasite immunity that reduces parasitemia, is acquired later in life, and is relatively long lived (half-life of >20 years). Other data on the effect of intermittent preventive treatment (IPT) and ITNs on rebound of malaria, or the lack thereof, are also consistent with this hypothesis; specifically, it has been proposed (132, 214, 295) that the extended period of protection observed in some (269, 270) but not all (8, 132, 214) studies following cessation of intervention may be due to a situation (such as a partially effective drug) which allows for low-level and persistent parasitemia and, consequently, prolonged stimulation of the immune system. It is also possible that the extended protective effect noted with the RTS,S vaccine in the field (3, 6, 309) may be due to the induction of blood-stage immunity in some vaccinees as a result of leaky RTS,S-elicited protection (295, 319). An alternative explanation to an extended protective efficacy following cessation of intervention (IPT, ITN, or vaccine) may be a decrease in the malaria transmission rate during the study period as a result of a very successful intervention (129). A recent meta-analysis of hospital data from areas with differing transmission intensities in Africa found that the mean age of individuals experiencing clinical attacks of malaria increased with decreasing transmission intensity but that the total number of clinical episodes was similar until transmission dropped below a certain threshold (233).
Effect of Age
The risk of disease hinges upon both the age of the host and the intensity of exposure to the parasite. Certain manifestations of clinical malaria (e.g., anemia) become less severe with age, and others (e.g., cerebral malaria) become more severe with age (193). Age is a significant risk factor for the prevalence and density, but not the incidence, of parasitemia (27, 203). The main determinant of the age distribution of morbidity is the development of antiparasite immunity that restricts the density of asexual parasitemia. In areas of high endemicity, the prevalence and density of P. falciparum parasitemia and the incidence of overall fevers and of malaria-associated fevers increase with age for the first 6 months of life and then gradually decline. Parasitemia peaks in children less than 5 years old and subsequently declines in an age-dependent manner. A significant association between age and parasite density among children between 6 months and 6 years has been demonstrated, with younger children more likely to have a density of ≥5,000 parasites/μl (27, 202). This association is even more pronounced with parasite densities of ≥20,000/μl. Where the attack rate is lower, peak prevalence occurs in older individuals. Paradoxically, a pathological response is triggered at a lower parasite density threshold among older individuals, and the absolute risk of clinical disease at a given parasite density is higher in older individuals (237, 272, 288).
In populations living permanently under heavy exposure to infection, separating the possibly independent effects of age and of cumulative exposure is very difficult. The key determinant of the conspicuous differences in their relative susceptibilities would appear to be their cumulative exposure to infection. Protection among adults may be presumed to be the cumulative product of many dozens of infections, and the susceptibility of children would be due to their relative paucity of experience with infection, accumulating at a rate of about 5 to 10 per year. However, what if only five infections within a single year were sufficient for onset of solid clinical immunity, as occurred in the neurosyphilis patients? The relative failure of children to develop immunity would likely be the product of innate differences in how their acquired immune systems function compared to those of adults. A population having abrupt and continuous exposure to infection is required to see such effects.
Cross-sectional studies of malaria-naive transmigrants in Indonesian Papua suggest that intrinsic features of the immune system that change with age may be key determinants of NAI against falciparum malaria (12). A relatively rapid acquisition of protective immunity against parasitemia and mild disease caused by P. falciparum, but not P. vivax, was observed in Indonesia (15, 20). The data shown in Fig. 2 illustrate those findings. In malaria-naive individuals abruptly and permanently exposed to the hyper- to holoendemic malaria of Papua (formerly known as Irian Jaya), the age-specific prevalence of parasitemia was at first uniform among age groups, but after 18 to 24 months a distinct age-dependent pattern emerged. This pattern paralleled that seen in lifelong residents of the area. Acquired immunity in the transmigrants was apparently not the cumulative product of many years of heavy exposure but was the product of recent exposure and intrinsic characteristics of the acquired immune response that change with age.
FIG. 2.
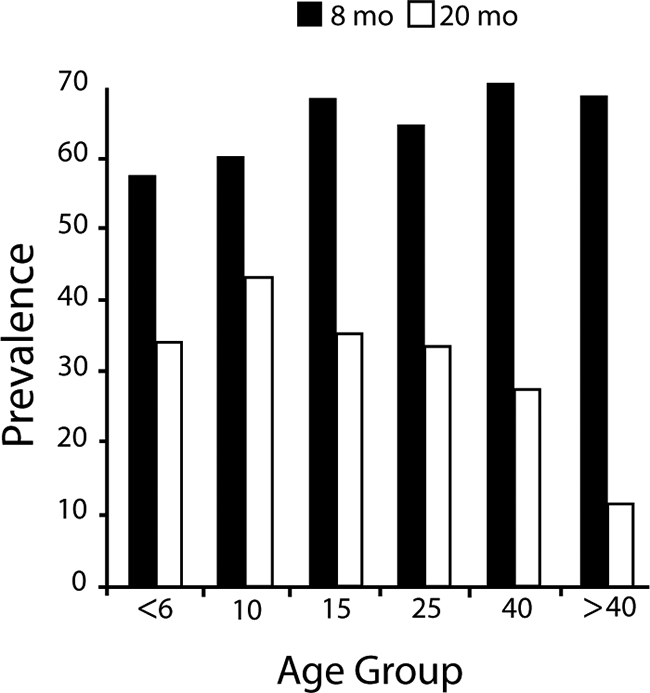
Onset of age-dependent NAI. The graph illustrates the prevalence of parasitemia (percent) across age groups (years) among malaria-naive newcomers to Indonesian New Guinea. After 8 months of exposure, all ages appear to be equally susceptible to parasitemia detectable by microscopic diagnosis. One year later (20 months), a distinct age-dependent pattern of susceptibility to parasitemia has appeared. These data revealed the onset of NAI to be dependent upon intrinsic age-related factors independent of lifelong, chronic exposure. (Reproduced from reference 11 with permission of the Liverpool School of Tropical Medicine.)
More recently, a longitudinal cohort of 243 transmigrants was followed from the day of their arrival in Papua in August 1996 until July 1999 (176). The key findings from that study include the demonstration of quantitatively equal risks of infection for children and adults (24) and the identification of four infections within any 24-month period as the threshold for onset of clinical immunity (16). Protection from fever occurred only among subjects having low-density parasitemia. This longitudinal study corroborated the results and assumptions from the earlier cross-sectional studies in the same region. In all of the studies in Indonesian New Guinea, coinfection with P. vivax routinely occurred, and confounding of the onset of NAI by that infection cannot be ruled out. Nonetheless, studies of the neurosyphilis patients demonstrated that prior immunity against one species offered no advantage in terms of onset of immunity against the subsequent species. Moreover, Barcus et al. (24) demonstrated that among the Indonesian migrants, prior infection with either species did not mitigate the risk or clinical course of immediately subsequent infection by the other species.
Another finding in the studies of Javanese transmigrants in Papua highlights the importance of innate age-dependent (and cumulative exposure-independent) differences between immune responses to acute versus chronic exposure to infection. Within 3 months of arriving in Papua, an epidemic of falciparum malaria occurred among transmigrants (15). In this group up to 74% were infected, and nearly all residents reported clinical symptoms of malaria. During this period, the number of emergency medical evacuations sharply increased among adults but not children (18). During the peak month of the epidemic, 48 adults were evacuated, compared to just 7 children (comprising 60% and 40% of the population of that month, respectively). These numbers provided incidence density of emergency evacuation values of 1.3 events per person year among adults and 0.23 event per person year among children (relative risk = 2.7; P < 0.0001; 95% confidence interval = 1.9 to 3.8). The incidence of evacuation among adults fell as sharply as it rose and was indistinguishable from the rate among children for the next 2 years. Another transmigration village in the same region has been similarly retrospectively analyzed, with essentially the same findings: adults were initially at a greatly exaggerated risk of severe disease compared to equally exposed children (14). The longitudinal cohort in Papua did not yield information on the risk of severe disease because the close follow-up and prompt therapy virtually precluded the possibility of severe disease outcomes. However, among the eight cases of malaria severe enough to prompt on-site intravenous quinine therapy, only one was in a child (176).
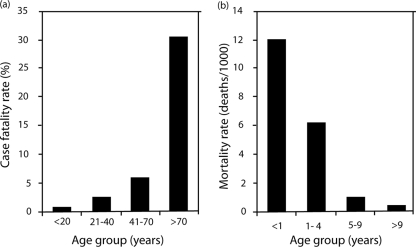
A review of the literature regarding epidemic malaria has corroborated the susceptibility of adults to severe disease caused by P. falciparum that was observed in the transmigrants. Table 2 summarizes the absolute or relative rates of death or severe disease among children and adults under conditions of acute exposure to P. falciparum (i.e., epidemic or travel). In all instances of epidemic malaria where epidemiologic data revealed age-specific rates of severe morbidity or mortality, the data consistently showed higher rates among adults than children. The data illustrated in Fig. 3 contrast patterns of age-dependent immunity under conditions of chronic versus acute exposure. A recent report by Dondorp et al. (103) also corroborated the conclusions drawn from the studies of Indonesian migrants; i.e., hospitalized nonimmune adults were at a higher risk of severe disease and death caused by falciparum malaria. All of these findings underscore the apparently dominant effect on clinical course of disease exerted by intrinsic age-related distinctions, about which we know very little in the context of malaria.
TABLE 2.
Age-dependent risk of death with falciparum malaria
| Locality | Population | Old vs young cutoff age (yr) | Case-fatality rate (%) for:
|
Odds ratio (95% CI) | Reference | |
|---|---|---|---|---|---|---|
| Old | Young | |||||
| United States | Travelers | 40 | 7.0 | 1.7 | 4.28 (2.5-7.4) | 58 |
| Italy | Travelers | 40 | 2.6 | 0.8 | 3.15 (0.28-35) | 58 |
| Italy | Travelers | 40 | 3.6 | 1.2 | 2.96 (1.2-7.6) | 265 |
| Israel | Travelers | 40 | 5.8 | 0 | >4 (2.5-∞) | 275 |
| Germany | Travelers | 60 | 16 | 1-4 | >4 | 58 |
| Indonesia | Migrants | 20 | 2.5 | 0.6 | 4.2 (2.7-7.3) | 18 |
| South Africa | Hospital ICU patients | 12 | 15% | 0 | >4 | 265 |
| Senegal | Hospital ICU patients | 15 | 41 | 16 | 2.6 (1.4-5.8) | 265 |
| Sri Lanka | Epidemic | 10 | 7.7 | 0.4 | 19 (4.8-36) | 265 |
| Tanzania | Residents | <1/>15 | 6.24 (3.5-11) | 247 | ||
FIG. 3.
Age- and exposure-dependent inversion of susceptibility to disease. These graphs illustrate the apparent age-dependent inversion of susceptibility to death caused by P. falciparum with acute (a) versus chronic (b) exposure. Malaria-naive travelers experiencing acute exposure to infection show a sharp increase in the risk of death (odds ratio) with increasing age, whereas the mortality rate for malaria among people living in an area of holoendemic transmission shows the opposite trend. (Reproduced from reference 11 with permission of the Liverpool School of Tropical Medicine.)
Thus, age-dependent immunity occurs in both acute and chronic exposure to infection, with apparently critical effects upon the course of infection that differ sharply between children and adults: adults apparently are better able to withstand chronic exposure, whereas children fare better with acute exposure. Under conditions of chronic exposure, adults apparently develop antiparasite immunity and clinical immunity more rapidly and completely than children, such that after about 18 months they have fewer and lower-density parasitemias with reduced clinical symptoms. However, under conditions of acute exposure, there is an inverse pattern of relative susceptibility, i.e., a higher risk of severe disease among adults than among children. Figure 4 illustrates a possible explanation for this inversion of susceptibility/resistance. The immune mechanisms underlying this inversion are unclear, but data in a rodent model may shed some light. In one study, adult rats were shown to have a higher degree of sensitivity to lipopolysaccharide than young rats (68), and this feature was correlated to sensitivity to infection by P. berghei. Thus, a relatively exaggerated production of tumor necrosis factor alpha by adult humans in response to a primary infection by P. falciparum that wanes with continued exposure could explain these differences.
FIG. 4.
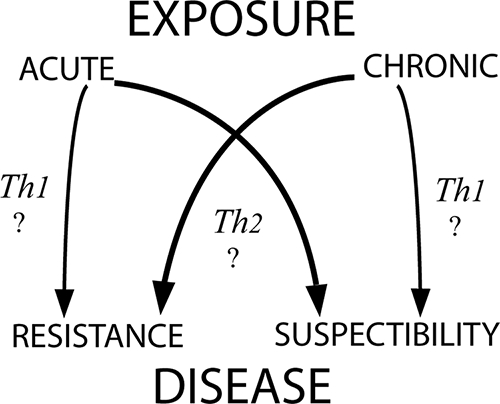
Hypothetical basis of age-dependent inversion of susceptibility to disease with acute versus chronic exposure in children and adults. Consider Th1- and Th2-type immune responses as surrogates for any immune response that changes with age independent of exposure and plays a critical role in infection outcomes. Th1-driven effectors may dominate the immune response of children (hollow arrows), whereas Th2-driven effectors may dominate the adult immune response (solid arrows). These distinct, age- and exposure-dependent responses cause harm or benefit to the host. (Reproduced from reference 11 with permission of the Liverpool School of Tropical Medicine.)
Insights from Intervention Studies
The use of intermittent treatment or prophylaxis to control morbidity and mortality among youngsters in sub-Saharan Africa represents another good example of the importance of understanding NAI and the consequences of interventions that affect exposure. Some have argued that interventions such as intermittent treatment or prophylaxis would set up children for more severe (cerebral) malaria when the intervention was ultimately withdrawn (182, 290). Intervention could perhaps interrupt the accumulation of sufficient antigenic diversity on the way to children becoming naturally immune. On the other hand, what if the intervention simply allowed the children to pass through an age window in which their susceptibility to severe disease and death, being driven by innate age-related factors, peaked? An examination of outcomes with such interventions indeed favors the former interpretation. Rebound morbidity was not observed in several large-scale placebo-controlled trials (186, 194), although one such trial noted relatively slight rebound anemia among those treated (170). A recently published study examined this question in detail (8). A 4-year follow up of Tanzanian children receiving weekly prophylaxis with Deltaprim or a placebo between ages 2 and 12 months suggested that the reduction of exposure to P. falciparum early in life delayed the acquisition of immunity. However, those authors argued that,
Our data do not support this conclusion [that intermittent therapy sets up children for more severe disease later in life], but instead suggest that a delayed acquisition of immunity may lead to a small, but not significant, increase in the cumulative number of malaria episodes and, importantly, to a lower CR [cumulative rate] of severe malaria. A similar pattern was observed for severe anaemia, which is the other major life-threatening complication of malaria in young children. In summary, shifting the age pattern of disease to older age groups does not markedly affect the overall number of mild uncomplicated febrile episodes or lead to an increase in severe malaria, and it is associated with an overall decreased CR of severe anaemia (8).
We agree with those authors and point to age-dependent (versus cumulative exposure-dependent) NAI as the likely basis of their key observation.
ACQUISITION OF NAI
The molecular and cellular events that drive the onset of immunity against malaria constitute the crux of efforts to obtain an understanding of sufficient depth for rational exploitation in the development of vaccines. Two fundamentally distinct hypotheses have emerged (Table 3). The most widely accepted hypothesis explains the slow onset of clinical immunity in populations where the disease is holoendemic on the basis of parasite diversity. In brief, NAI is viewed as the cumulative product of exposure to multiple parasite infections over time, yielding a sufficiently diverse repertoire of strain-specific immune responses. In areas of holoendemicity in Africa, the onset of clinical immunity requires 10 to 15 years of roughly five infections per year. The alternative hypothesis, based largely on the observations of transmigrants in Indonesian Papua, attributes the onset of clinical immunity to recent heavy exposure and development of cross-reactive, strain-transcending immune responses governed predominantly by intrinsic characteristics that change with age independent of lifelong parasite exposure. At the core of the disagreement between these hypotheses is the basis of susceptibility/resistance to falciparum malaria: it is driven by the extrinsic factor of antigenic diversity on the one hand or by the intrinsically appropriate/inappropriate age-dependent immune responses on the other. The distinction is not simply academic; it defines vaccine development strategies that seek to overcome the perceived inadequate exposure to antigenic diversity or to overcome an intrinsically inappropriate immune response to sufficient exposure to antigenic diversity. In the context of modeling NAI in vaccine development, this is a crucial point.
TABLE 3.
Strain-specific and strain-transcending hypotheses for development of NAI
| Hypothesis | Onset | Exposure | Determinant of protection | Basis of susceptibility | Evidence of basis of susceptibility | Heterologous immunity | Characteristics | Vaccine strategy |
|---|---|---|---|---|---|---|---|---|
| Strain specific | Slow | >30 infections in 5-15 yr | Cumulative exposure | Antigenic variation (extrinsic) | Early childhood morbidity and mortality in areas of holoendemicity | Relative susceptibility to heterologous strain challenge of a homologous immunity | Strain-specific agglutination (possible confounding by IgM, a superior agglutinin) | Defeat antigenic variation |
| Strain transcending | Rapid | 3-5 infections in 1-2 yr | Recent exposure | Age of host (intrinsic) | Onset of NAI among nonimmune adult migrants to areas of hyperendemicity areas within 1-2 yr | Relative resistance to heterologous strain challenge of a broader immunity | Strain-transcending protection mediated by IgG, a poor agglutinin | Overcome age-dependent inappropriate immune response |
Parasite Strains
Within a single species of Plasmodium, allelic polymorphisms result in the coexistence of different genotypes, i.e., clones or so-called “strains” (167, 315). The definition of the term “strain” has recently been extensively reviewed (209). Allelic polymorphisms or genetic polymorphisms in certain protein loci give rise to antigenically distinct forms of the protein in different parasite clones or strains. This type of diversity (antigenic diversity/antigenic polymorphism/strain heterogeneity) underlies the concept of strain-specific immunity (98, 125). According to this hypothesis, immunity to P. falciparum is essentially strain specific (49, 318), and thus an individual becomes immune after being exposed to a large number of strains circulating in the community. Evidence for strain-specific immunity has accumulated from animal studies, in particular experimental infections in monkeys (57, 164), as well as induced infections in humans, including the treatment of syphilis patients by inoculations of Plasmodium (67). In experimental malaria in humans, a primary infection by one parasite strain elicited an immune response which was capable of protecting against that strain but not against infection by a different strain (161). In Aotus monkeys, repeated infections induced increasingly more rapid sterile immunity to homologous challenge (164).
Such studies revealed an important difference between the clinic and the field: the onset of immunity was apparently rapid in the neurosyphilis patients and slow in the field. The rapid onset of experimentally induced immunity was attributed to the use of homologous strains of parasites, and the slow onset in areas of endemicity was attributed to heterologous challenge. The immune system was thought to require continuous exposure to many heterologous strains in order to expand the repertoire of relevant memory effector cells required to effectively suppress subsequent challenges with antigenically diverse parasites in natural populations. However, the neurosyphilis patients who were repeatedly challenged did not include children, for obvious reasons. If the onset of clinical immunity in a hypothetical set of serially challenged children was not rapid, antigenic variation would not explain a slow versus rapid onset of clinical immunity. Likewise, if naturally exposed nonimmune adults rapidly acquired clinical immunity, a strain-transcending immune response would seem the most likely explanation. This was the key analytical insight offered by the populations of transmigrants in Papua.
Antigenic Variation
The concept of phenotypic antigenic variation is distinct from that of strain-specific immunity. Broadly, the term antigenic variation refers to changes in antigenic phenotype by regulated expression of different genes of a clonal population of parasites over the natural course of an infection. Indirect evidence for antigenic differences between parasite populations was first reported by Cox (91), when mice harboring chronic infections with P. berghei were demonstrated to be more susceptible to challenge with relapse parasites than to challenge with the original parasite population. The classic demonstration of antigenic variation in P. knowlesi by Brown and Brown in 1965 (47), when chronic erythrocytic infections of P. knowlesi were shown to consist of a succession of antigenic variants, provided the foundation for studies of antigenic variation and evasion of the host immune response as an explanation for the apparently slow onset of NAI. In this scenario, the Plasmodium parasite evades host immunity by varying the antigenic character of infected erythrocytes. Consistent with this, a large and extremely diverse family of P. falciparum genes, known as the var genes, have been described (25, 287, 294). These encode an antigenically diverse parasite-derived protein of 200 to 350 kDa, P. falciparum erythrocyte membrane protein 1 (PfEMP1), on the surface of parasitized erythrocytes with the expected properties of antigenically variant adhesion molecules. PfEMP1 has been implicated as the key target antigen involved in NAI to malaria (108, 152, 175). More recently, two other larger families of clonally variant surface molecules called rifins (115, 179) and STEVOR (166) have been described, although their role in acquired immunity remains unknown. Collectively, these proteins, expressed at the infected red blood cell membrane, are referred to as variant surface antigens (VSA), and the immunity directed against these antigens is termed variant-specific immunity.
It has been demonstrated that VSA of P. falciparum can undergo clonal variation in vitro to a variety of antigenic and adhesive phenotypes in the absence of immune pressure (255). Furthermore, it has been demonstrated that parasites of a given strain can undergo antigenic variation in vivo, as shown in monkey models by a switch in the antigens exposed on the erythrocyte surface following transfer of erythrocytes infected with a given P. falciparum strain from a splenectomized into an intact Saimiri monkey (150). Following challenge, previously infected spleen-intact Saimiri monkeys demonstrated sterile immunity to the homologous parasite strain but not to a heterologous strain (148), although the peak parasitemia was perhaps lower and of shorter duration following heterologous challenge (244). In other nonhuman primate studies, passive transfer of malaria-specific antibodies to a naive splenectomized Saimiri monkey infected with P. falciparum resulted in the emergence of parasites resistant to the transferred antibodies, but monkeys primed with original parasites were fully susceptible to challenge by the resistant ones, and vice versa (114).
A number of studies have examined the role of antibody responses to VSA in the development of NAI, by using traditional (agglutination) (191) as well as novel (flow cytometry) immunological assays. VSA expressed during episodes of clinical malaria in Kenyan children were less likely to be recognized by the preexisting antibodies in the same child than that by other children, as assessed by agglutination (52), and agglutination by diverse plasma was associated with severe disease and young host age (51). Anti-VSA IgG levels have been correlated with protection from clinical malaria in Ghana (102, 232), Kenya (169), Gabon (324), and Tanzania (187). A recent study with malaria-naive humans experiencing a single P. falciparum infection demonstrated antibody reactive with up to six P. falciparum lines expressing different heterologous PfEMP1 variants (112). Taken together, these studies give support to the hypothesis that anti-VSA antibodies may provide variant-specific protective immunity (154-156), specifically immunity against severe disease (163), and VSA antigens have been proposed for malaria vaccine development (63).
Strain-Specific versus Cross-Reactive (Strain-Transcending) Immune Responses
Both strain-specific immunity and cross-reactive immunity have been documented in mice, monkeys, and humans in the laboratory as well as the field.
Extensive antigenic diversity has been revealed using monoclonal antibodies assayed by the indirect fluorescent-antibody test on laboratory and clinical isolates of P. falciparum schizonts and merozoites from distinct geographical areas (85, 86, 94, 197-199). Significant strain-specific and cross-reactive inhibition of parasite multiplication was observed when homologous and heterologous sera from Gambian children were assessed for any inhibitory effects on parasite growth (318). Similarly, in nonhuman primates, it was demonstrated that immune sera from Aotus monkeys contained antibodies that blocked or reversed cytoadherence in vitro and that were isolate specific (305). Strain specificity of antibody recognition and growth-inhibitory activity has been particularly well documented for one of the leading blood-stage vaccine candidate antigens, P. falciparum AMA1 (77, 141, 143, 168, 219).
Like antigenic variation, antigenic diversity has been revealed using agglutination assays. In humans, studies using both field isolates and laboratory clones demonstrated that the predominant agglutinating antibody response in humans was variant specific, and antibodies which cross-react between different serotypes were rare (229). In The Gambia (190) and in Papua New Guinea (118), sera from children in the convalescent stage of infection reacted with autologous but, with few exceptions, not with heterologous infected cells in the antibody-mediated agglutination assay. No two isolates with the same agglutinating phenotype could be identified in a group of 20 P. falciparum isolates from children in Papua New Guinea (246) using children's convalescent-phase sera or adult immune sera. In nonhuman primates, chronic erythrocytic infections of P. knowlesi were shown to consist of a succession of antigenically distinct strains (47), sera from rhesus macaque monkeys immune to one P. knowlesi strain did not agglutinate cells infected with another strain (46), P. knowlesi schizont-infected erythrocytes did not agglutinate with sera from monkeys suffering a chronic infection with another strain (46), and antibodies in sera from P. falciparum-infected Aotus monkeys recognized antigenically diverse determinants, rather than conserved epitopes, on the surface of infected erythrocytes (151).
Despite in vitro and in vivo support for the strain specificity of immune responses, there is evidence that naturally exposed individuals develop cross-reactive antibodies which recognize an increasingly broad array of P. falciparum isolates with increasing age or exposure. In The Gambia (190) and in Papua New Guinea (118), although sera from children failed to agglutinate heterologous infected cells in the agglutination assay, adult immune sera contained antibodies that recognized by agglutination the majority or all of the isolates and reacted with the surface of infected cells from most children. Chattopadhyay et al. (62) demonstrated cross-reactive antibodies against VSA among adults in an area of hyperendemicity in India. An almost total lack of geographical specificity in such agglutination assays was reported for both African and South American adults (2). These data are consistent with the acquisition of immunity against antigenically diverse strains with exposure.
It should be noted, however, that methodology may be a potentially important confounder of the agglutination data. None of the studies discussed here has used purified IgG, which is thought to be the immunoglobulin of key importance to NAI. Since IgM titers increase in responses to natural infection and quickly wane (83) and IgG is a poor agglutinin relative to IgM, the reported agglutination studies may be biased by strain-specific IgMs that may have little relevance to protective immunity. Moreover, it should be recognized that IgG cannot act as an agglutinin in red cell suspensions that lack sufficient concentrations of albumin to neutralize the zeta potential of the surface of red blood cells. The zeta potential is a negative charge that repels red blood cells at very close proximity, and IgM but not IgG can bridge that distance. This phenomenon would further favor IgM- over IgG-mediated agglutination. Agglutination studies using purified IgG are needed to corroborate the studies described above. Reeder et al. (245) questioned the relevance of whole-serum agglutination studies with the finding that sera from transmigrant adults and children exhibited restricted red cell agglutination profiles relative to those of people native to Papua. The parasitologically demonstrated protection of the adult transmigrants was not reflected in the results of the assays.
Strain-Specific versus Cross-Reactive (Strain-Transcending) Protection
With regard to protective immunity, there are a number of reports documenting a significant isolate-specific component in the induced immunity against Plasmodium spp. both in nonhuman primates and in humans.
In chimpanzees, it was shown that infection with a West African strain of P. falciparum could protect against challenge with the homologous strain of P. falciparum but was much less effective against heterologous challenge (267). In splenectomized gibbons, infection with P. falciparum isolates of Thai origin conferred significant protection against homologous challenge, and some isolates were able to stimulate a degree of cross-protection similar to that conferred by the homologous strain. However, other strains stimulated little or no cross-protection (57). Likewise, Brown et al. (46) reported partial or complete homologous variant immunity to P. knowlesi in rhesus monkeys following infection (P. knowlesi-infected erythrocytes) and drug cure which was not as effective against a heterologous variant challenge. Voller and Rossan (313) tested heterologous antigenic variants (from a homologous strain) and demonstrated complete protection despite antigenic dissimilarity; i.e., variant-transcending immunity appears to be the rule. Fandeur and Chalvet (113) demonstrated both variant- and strain-specific immunity against P. falciparum in Aotus monkeys but also demonstrated the apparently strict requirement for having only a single precedent infection. When animals had more than a single variant or strain infection experience, immunity to challenge proved to be both variant and strain transcending. In all models, the degree of protection against heterologous challenge increased following multiple reinfection with a homologous strain (57, 286).
In humans, it was demonstrated that protective immunity against heterologous challenge with distinct strains of P. vivax (39, 67, 158, 161), P. cynamolgi (227), and P. falciparum (38, 160, 161) was less effective than that against homologous strains. Furthermore, the degree of protective immunity induced by malariatherapy against challenge with heterologous strains was almost always less effective than that against homologous challenge (38, 41, 161, 243). Nonetheless, almost all of these studies demonstrated a conspicuous partial protection with homologous immunity to heterologous challenge. The data from Powell et al. (243) seem typical, where the mean percent reductions in days with relatively high parasitemia, mild fever, or high fever between the first and second challenge with either homologous (14 human subjects) or heterologous (5 human subjects) strains were barely distinguishable (Fig. 5). Although homologous immunity performed “better,” with slightly higher reductions in markers of infection susceptibility, clinical protection from heterologous challenge was, as the authors said, “unequivocal.” Among subjects challenged repeatedly with homologous strains and then challenged with a heterologous strain, parasitemia initially behaved as if the person was nonimmune, complete with high parasite counts and fever (243). However, after 9 days the parasitemia spontaneously fell and fever subsided. These subjects retained control over these parasitemias and remained asymptomatic for a month or more until therapy was finally administered (243). The onset of strain-transcending protective immunity was, as the authors pointed out, “rapidly acquired.”
FIG. 5.
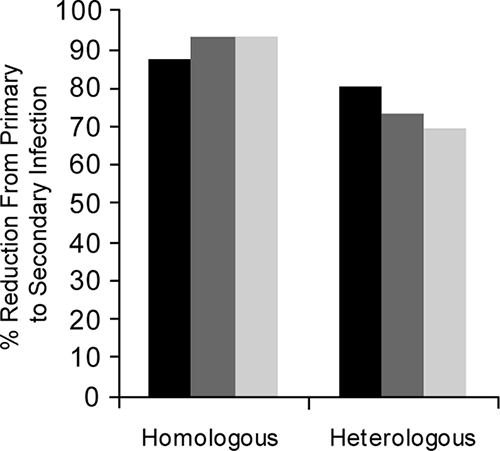
Onset of clinical immunity to falciparum malaria in a second experimental challenge with homologous versus heterologous strains. Solid bars show the percent reduction in number of days with greater than 1,000 parasites per μl after challenge with a homologous (left, data from 14 human subjects) or heterologous (right, data from 5 human subjects) strain. Gray bars show the percent reduction in mean number of days with a fever higher than 100°F. Light bars show the same for a fever higher than 102°F. (Based on data from reference 243.)
Other evidence of strain-transcending clinical immunity comes from a wide range of other experiments. No difference was observed between the infections developed by residents of an area of hyperendemicity in Liberia experimentally infected with P. falciparum sporozoites from a local strain and those infected with a strain from geographically distinct areas, although there was no evidence that these strains were in fact distinct (43). When neurosyphilis patients who had been infected with P. falciparum-parasitized erythrocytes of Colombian origin 18 months previously were infected with a Thai strain, parasitemia spontaneously cleared, concomitant with a marked increase in antibody specific for the Colombian strain and a much slower induction of lower-titer antibodies specific for the Thai strain (81). A marked reduction in parasitemia was observed following passive administration of IgG from immune adult West Africans to children resident in East Africa (205) or Thailand (266), to Aotus monkeys infected with an Asian isolate of P. falciparum (101), or to Saimiri monkeys acutely infected with P. falciparum strains of African or French Guyana origin (137). Volunteers immunized with irradiated P. falciparum sporozoites were protected against challenge with heterologous P. falciparum sporozoites (69, 70, 250). Finally, in the landmark study of clonal antigenic variation in P. knowlesi, Brown and Brown (47) recognized a significant strain-transcending immune response. This was also noted for P. fragile in toque monkeys (139). The relevance of strain-specific versus strain-transcending aspects of the immune response to NAI has not been elucidated.
The burden upon advocates of strain-specific immunity is the demonstration that a less pronounced protection following heterologous challenge in hosts with homologous immunity has relevance to an immune system that sees a dozen or more infections in just 2 years. Interpreting slightly less effective protective responses to heterologous challenge after a single exposure to infection (Fig. 5) as evidence of the strain specificity of NAI seems a narrow view. Validation of strain specificity as the dominant factor governing the seemingly slow onset of NAI surely requires stronger and more direct evidence. For example, do serial challenges with five distinct strains leave the subject susceptible to challenge with a sixth strain? This has not been demonstrated to be true and seems doubtful in light of a broader view of the data from the heterologous variant and strain challenge studies in human and animal subjects cited above. No direct evidence supports the supposition that strain-specific immunity represents a dominant factor in host susceptibility to infection by P. falciparum as occurs in areas of heavy exposure.
Indeed, a very persuasive argument that the protective immunity against malaria is predominantly strain transcending rather than strain specific may be made. Intuitively, the number of episodes of clinical malaria experienced by a given individual even in areas of high endemicity must be significantly smaller than the repertoire of antigenic types. This may be especially true in light of the apparently vast potential for variation contained in the var genes of a single clone of P. falciparum. The issue of persistent variant- or strain-specific immunity must also be considered: if even just strain-specific NAI accumulates through the decade of childhood, what accounts for the apparent persistence of immunity to strains seen long ago? The requirement for uninterrupted exposure for maintenance of NAI has long been and is still widely acknowledged, and recent studies tend to affirm the view. Although Africans taking up long-term residence in France showed less susceptibility to falciparum malaria than natives of Europe, these were all people reporting to hospital with illness, 4.4% of whom had severe disease (35). A similar study in the United Kingdom showed that 31% of Africans reporting to hospital with illness required parenteral treatment and that 4.3% required management in an intensive care unit (ICU) (compared to 41% and 15% of native French, respectively) (53). Jennings et al. (162) found that “… severe disease was no less common among patients who might be assumed to have a degree of protective immunity than among previously malaria-naïve patents.” Given the apparently short-lived nature of protection afforded by NAI, it seems unlikely that protection against a given strain seen in childhood could effectively persist through adult life.
A relatively rapidly acquired strain-transcending immunity in older children and adults would explain much that appears to be mysterious about the accumulation of a strain-specific immunity over years. How is it that adults lose NAI, apparently quickly and at least temporarily, when they leave areas of endemicity when they are supposedly carrying protective immunity to strains experienced in early childhood? How did Javanese adults, who were demonstrably susceptible to severe disease and death at the onset of exposure, develop clinical immunity to heterologous (wild-type) strains after just four infections over 2 years? Why do humans and animals exposed to serial challenge almost uniformly exhibit variant- and strain-transcending immunity and at least suppress heterologous variants or strains even with just a single exposure? Given what we now understand of VSA in P. falciparum, how is that neurosyphilis patients undergoing malaria therapy developed solid clinical immunity after just four challenges with allegedly homologous strains? Is the repertoire of variants within strains so limited, or are these switches simply defeated by host immunity with relative ease? We consider these questions incompatible with the model of cumulative strain-specific immunity and consistent with an immunity driven by only relatively recent exposure and the intrinsic nature of the immune response in the physiologically mature. Thus, we consider the basis of age-dependent and chronic-exposure-independent NAI the key to developing an understanding of NAI sufficient to exploit in rational vaccine development, as well as safely attacking holoendemic malaria transmission.
EMPIRICAL OBSERVATIONS OF PREERYTHROCYTIC-STAGE IMMUNITY
The induction of stage-specific immunity against the preerythrocytic stage of Plasmodium parasites was first demonstrated in 1941 by Mulligan and coworkers (226). Repeated injections of UV radiation-inactivated P. gallinaceum sporozoites rendered chickens partially immune to sporozoite challenge, as assessed by a decrease in mortality and an increase in the proportion of animals that spontaneously recovered from infection. In 1948, a neurosyphilis patient previously treated with malaria therapy was bitten by 2,000 infectious P. vivax-infected mosquitoes and intravenously injected with the salivary gland emulsion of 200 others and failed to develop detectable blood-stage parasitemia or clinical disease; preerythrocytic forms of the parasite were readily detected in the liver (89). In 1966, Richards (248) demonstrated that protective immunity against P. gallinaceum sporozoite challenge could be induced in chickens by active immunization with sporozoites inactivated by either UV irradiation, drying, freeze-thawing, or formalin treatment. Holbrook et al. (147) obtained some protection in mice against P. berghei by immunization with formalin-treated exoerythrocytic stages of P. fallax.
Recent studies have focused on the induction of whole-organism immunity by immunization with Plasmodium sporozoites attenuated by radiation. Gamma irradiation of Plasmodium-infected mosquitoes attenuates the parasite such that it can invade the host hepatocyte but fails to differentiate into erythrocytic stages (281). It is now well established that immunization with radiation-attenuated sporozoites induces sterile protective immunity against malaria in every model studied, including humans, nonhuman primates, and rodents. Immunization with irradiated P. berghei, P. chabaudi, or P. yoelii sporozoites protects against sporozoite challenge in rats and mice (145, 230, 273, 316). Immunization with irradiated P. cynamolgi or P. knowlesi sporozoites protects monkeys (79, 84, 135), and immunization with irradiated P. falciparum or P. vivax sporozoites protects humans (69-71, 109, 110, 142, 144, 250, 251). Protection is species specific but not strain specific (110, 144) and may last for up to 9 months in humans (based on n = 1) (109).
Protection against malaria liver stages has also been demonstrated after vaccination with live sporozoites under chloroquine treatment in mice (33), with heat-killed sporozoites (138), and with genetically attenuated sporozoites (223, 224, 308).
EMPIRICAL OBSERVATIONS OF ASEXUAL ERYTHROCYTIC-STAGE IMMUNITY
The induction of asexual erythrocytic-stage immunity in all species tested, including humans, monkeys, and mice, has also been demonstrated. It was first reported in 1929 (296) that a massive dose of parasitized erythrocytes injected into birds with a latent or low-grade P. cathemerium blood-stage infection entirely or almost completely cleared the infection within 1 day, whereas the same dose injected into a normal bird frequently was fatal. In 1936, it was reported that whole blood from highly immune subjects produced beneficial results when injected into patients suffering from acute attacks of malaria (293). In 1945, Freund et al. (120) induced strong protection against P. lophurae in ducks by immunization with formalin-killed erythrocytic stages in adjuvant. Formalin-killed merozoites in adjuvant were shown to induce stage-specific protection against P. fallax in turkeys (147), and McGhee et al. (204) obtained complete protection in chickens with P. gallinaceum erythrocytic stages. In mice, partial protection was achieved by vaccination with inactivated (cryopreserved and homogenized) P. chabaudi merozoites in complete Freund's adjuvant (149).
The success of vaccination against the erythrocytic stages of Plasmodium was extended to the nonhuman primate model. Rhesus macaque monkeys were vaccinated with whole formalin-killed P. knowlesi schizonts (121, 297) or fractions of these parasites (282) in complete Freund's adjuvant. Brown et al. then demonstrated that immunization of rhesus monkeys with freeze-thawed schizonts in adjuvant conferred protection against homologous as well as heterologous P. knowlesi challenge (48). It was shown that such immunity could be induced after as few as two immunizations (271). Subsequently, immunization of rhesus monkeys with P. knowlesi merozoites emulsified in adjuvant induced sterilizing immunity which was species specific but not strain specific (54, 56, 72, 215, 216). One Kra macaque monkey immunized with P. knowlesi merozoites in adjuvant was completely protected in four of five challenges, while a monkey immunized in parallel with P. knowlesi schizonts in adjuvant was not protected (215). With P. falciparum, Voller and Richards observed a significant delay in the prepatent period following immunization of Aotus monkeys with formalin-treated schizonts of P. falciparum in adjuvant, but the induced immunity was not sufficient to protect the monkeys against death (312). Partial protection against homologous as well as heterologous P. falciparum challenge was induced in Aotus monkeys by immunization with P. falciparum merozoites in adjuvant (279, 280). Immunization with mature schizont stages of P. falciparum conferred strong protection against homologous challenge in Saimiri monkeys (106), and Saimiri monkeys develop significant, long-lasting protection (up to 7 months) against challenge with homologous as well as heterologous strains of P. falciparum following infection with merozoites/schizonts (262).
Irradiated erythrocytic-stage parasites, in the absence of adjuvant, induced partial protection against P. gallinaceum in chickens (61, 248), against P. lophurae in ducks and chickens (253), and against P. yoelii nigeriensis in mice (314). Richards also demonstrated that protective immunity against homologous P. gallinaceum challenge in chickens could be induced by active immunization with erythrocytic-stage parasites inactivated by either UV irradiation, freeze-thawing, or formalin treatment but not drying (248).
The partial protection observed with irradiated blood-stage parasites contrasted with the complete sterile immunity induced by immunization with irradiated sporozoites in rats and mice (231, 317) and in humans (70, 71, 250). Clearance of parasites passively transferred into a neurosyphilis patient suffering an acute attack of P. vivax (39) nevertheless demonstrated that protective immune responses against asexual erythrocytic-stage parasites can be rapidly activated.
More recently, a new whole-cell vaccination strategy using ultra-low doses of nonattenuated Plasmodium-infected erythrocytes (128, 322) has proven to induce protective immunity which correlates with Th1 cellular immune responses rather than antibody responses in mice (P. chabaudi) (111) and with robust T-cell-mediated immune responses in humans (P. falciparum) (240).
EMPIRICAL OBSERVATIONS OF TRANSMISSION-BLOCKING IMMUNITY
It is the sexual stages of the malaria parasite, known as gametocytes, which are responsible for the transmission of the parasite to the mosquito vector. Effective transmission-blocking immunity was first demonstrated by immunization with whole killed P. gallinaceum parasites (a mixture of asexual- and sexual-stage parasites) in chickens or with P. fallax parasites in turkeys (153). These studies were subsequently confirmed using purified sexual-stage gametes of P. gallinaceum in chickens (59), P. knowlesi in rhesus monkeys (136), and P. yoelii in mice (211). Other studies showed that gametocyte infectivity and oocyst development of P. gallinaceum could be reduced or eliminated in mosquitoes by immunizing the chickens on which the mosquitoes feed with infected red blood cells that have been inactivated with formalin or X rays (134). Effective transmission-blocking immunity eliminating or reducing the infectivity of malarial parasites for mosquitoes has been observed following natural infections in humans, during infections in animals, and following artificial immunization of animals with sexual-stage malaria parasites (36, 60, 180). Recently, for example, sera from Macaca mulatta monkeys (78, 218) or Aotus lemurinus griseimembra monkeys (9) immunized with a leading candidate for a transmission-blocking vaccine, recombinant Pvs25, or from rabbits immunized with recombinant Pfs25 (218) were shown to have transmission-blocking activity in a mosquito membrane-feeding assay, and this activity could be correlated with the antigen-specific antibody titer by enzyme-linked immunosorbent assay.
Naturally acquired sexual-stage immune responses (105), the role of sexual-stage parasites and transmission-blocking immunity in the development of NAI (36), and the public health implications of transmission-blocking vaccines (268) have been recently reviewed. It is apparent that immune responses to gametocytes do develop, which presumably reflects the fact that only a relatively small proportion of gametocytes are taken up by the mosquito vector, the majority being cleared by the human host (36), but there are few data to indicate whether or not sexual-stage specific immunity increases with age (105). Epidemiological data show that the prevalence of gametocytes declines with age (228), and this was originally interpreted to be a direct consequence of increasing immunity against asexual blood-stage parasites (184). More recent data, however, show that the prevalence of gametocytes decreases more rapidly with age than that of asexual-stage parasites (reviewed in reference 105), indicating that immunity to gametocytes develops more quickly than immunity to asexual blood-stage parasites. NAI does not appear to inhibit gametocytogenesis, since immune adults retain the capacity to infect mosquitoes and constitute a sizeable and important part of the available gametocyte reservoir (225). Conversely, sexual-stage-specific immune responses do not play a significant role in NAI. In the classic antibody transfer experiments (75, 76), there was no apparent effect of gamma globulin on gametocytes, unlike the dramatic effect on asexual blood-stage parasites.
STAGE SPECIFICITY OF NAI
The underlying mechanisms and antigenic specificity of protective immunity against malaria are not well understood; our current knowledge base will be reviewed elsewhere. Epidemiological data indicate that natural exposure to sporozoites does not induce complete (sterilizing) antiparasite and antidisease immunity (146) and that naturally acquired immune mechanisms responsible for the acquisition of clinical protection in areas of endemicity affect the prevalence of P. falciparum parasitemia, limiting the density of parasitemia and thereby decreasing the malaria-associated morbidity and mortality. The widespread persistence of patent parasitemia in asymptomatic individuals resident in areas where malaria is endemic and the ability of hyperimmune serum to transfer protection suggest that NAI is directed predominantly against the parasite in its asexual erythrocytic stage and that it does not effectively block sporozoite invasion or intrahepatic development. More specifically, the primary targets of this protective immunity appear to be the extracellular merozoites in circulation; the reduction in parasitemia conferred by the passive transfer of immune serum occurs following schizogony, and the serum does not appear to be effective against developing or mature trophozoites or against developing or mature gametocytes (73, 76, 100, 206). These in vivo observations are confirmed by in vitro studies with P. knowlesi (74) and P. falciparum (217, 238).
Although the preerythrocytic stage is an effective target of induced immunity against malaria (104), we nonetheless believe it to be highly likely that this stage is also targeted by protective immune responses during the development of NAI. In the malariatherapy studies, infection was introduced either by the bites of infected mosquitoes or by injection of infected blood, and the prepatent period was dependent upon the infectious dose. The immunity was thought to be stage specific and directed only against asexual blood-stage parasites, regardless of whether the infection was induced by sporozoites or blood-stage parasites since “the immunity does not prevent an appreciable although subclinical increase in the density of the trophozoites” (40). Immunity nevertheless “limits the effective multiplication [of the parasite] so they rarely, if ever, attain a density sufficient to produce more than a slight clinical attack” (40). However, it was concluded from earlier studies of induced immunity (320) that immunity to P. cathemerium in canaries was probably effective against sporozoites as well as trophozoites, since canaries experiencing latent P. cathemerium blood-stage infections were able to completely clear the parasites following inoculation with infectious sporozoites. This inhibition of blood-stage parasitemia, together with the delay in patency noted with some patients, is consistent with acquired preerythrocytic immunity targeted at the infected hepatocyte. Indeed, Sinton (284, 285), studying the induction of protective immunity against sporozoites by immunization with P. ovale in humans, concluded that immunity developed by sporozoite immunization was more effective than immunity developed as a result of blood inoculation. This view was supported by Russell et al. (263, 264), who observed that the agglutinating antibody titer in chickens is often higher after repeated infections with inactivated sporozoites than that in acutely or chronically infected animals induced by blood inoculation. These data led those authors to speculate that it is possible “that sporozoites are antigenically more potent than trophozoites” (226).
FUTURE PERSPECTIVES
Almost any endeavor in the field of immunity to malaria represents a step in the journey to a practical and effective vaccine that serves to diminish the enormous losses of life, health, and prosperity imposed by these parasites. As with any journey to an unfamiliar destination, the traveler must stop, take bearings, and ask, “Am I going in the right direction?” We consider the endeavor represented by this review to be such a pause. The long chronological reach of this paper was not a matter of academics. Our assessment of bearings reached the point of origin by the necessity of addressing this question: how did parasite antigenic variation come to dominate strategic thinking in vaccine development? The answer, we believe, is found primarily in three sets of observations: (i) the seemingly slow onset of acquired immunity in areas of heavy transmission, where the effects of age and cumulative exposure could not be separated; (ii) the rapid onset of acquired immunity in neurosyphilis patients being reconciled to the slow onset in settings of endemicity by the invocation of strain-specific immunity; and (iii) the discoveries of variant antigenic types, var genes, and PfEMP1 proteins being held as the molecular basis of the slow onset of strain-specific NAI in zones of heavy endemicity.
Against this backdrop, the goal of vaccine development became discovery of an antigen or set of antigens that could mount a strain-transcending immune response capable of defeating the array of antigenic variation defenses mounted by the parasite. A dominant role for antigenic variation in host susceptibility to the parasite lies at the core of this strategic thinking, based largely on the three sets of observations listed above.
In this review we have made an argument for an alternative hypothesis for the basis of host susceptibility to falciparum malaria. That hypothesis represents an almost complete departure from the conventional view, placing intrinsic differences in how acquired immunity operates in immature and mature immune systems as the basis of host susceptibility to malaria. The genesis of this hypothesis may be found in observations that challenged the earliest assumptions regarding the onset of NAI. When a population of nonimmune migrants of all ages became abruptly exposed to hyper- to holoendemic transmission of malaria, the adults developed clinical immunity after three or four infections within 12 to 24 months. Their children did not. These data upset two of the three foundations of antigenic variation as the basis of host susceptibility. Onset of immunity was not slow; it only seemed that way when age and cumulative exposure could not be separated. A reexamination of the strain-specific immunity described for the neurosyphilis patients revealed substantial evidence of potent strain-transcending immunity. The focus on the often relatively slight differences between homologous and heterologous strain immunity in that body of work began to resemble a forced fit to the hypothesis of antigenic variation as basis of host susceptibility. Indeed, the onset of immunity in the Indonesian migrants very closely resembled that among the neurosyphilis patients.
We consider the body of evidence supporting intrinsic age-related factors as the key determinants of host susceptibility to falciparum malaria to be compelling. However, it is also incomplete in terms of mechanistic detail and, therefore, conclusive proofs. The literature demonstrating age-related differences in host responses and susceptibility to infectious diseases is as vast as it is heterogeneous. None of the many systems studied, including falciparum malaria in humans, provides a testable hypothesis of cellular and molecular mechanisms that could account for the age-related phenomena observed in organisms and populations. There remains no understanding of what may be at work in establishing the often very conspicuous differences between immature and mature animals coping with challenge by infection.
One likely starting point for more firmly establishing age-dependent, strain-transcending immunity in malaria and for beginning to sort through the myriad possible mechanisms at work may be a nonhuman primate model. In particular, the system of P. knowlesi in rhesus macaques offers important advantages. The course and consequence of P. knowlesi infection in that monkey species resemble the hyperparasitemia and death caused by P. falciparum in humans. Breeding colonies of rhesus macaques may offer a sufficient cross-section of ages, and the available immunological reagents for that animal would permit relatively inexpensive, sophisticated, and immediate work. If adult animals in that model prove to be more susceptible to acute infection than younger animals and if older animals experience more rapid and complete onset of protective acquired immunity to P. knowlesi than younger animals, the ground may be laid for penetrating analyses of similar immune processes at work in humans.
Human populations offering the same analytical insights rarely appear. In areas of heavy exposure, separation of the cumulative effects of heavy exposure from those of intrinsic age-related factors will be difficult. Nonetheless, Kurtis et al. (178) achieved this by examining susceptibility to fever with malaria relative to physiological markers of onset of puberty; demonstrating the event (signaling physiological age) rather than calendar age as the principal determinant of resistance to fever with parasitemia. It may be possible to conduct similar analyses by applying other physiological markers of age among younger people. The work of Reyburn et al. (247), conducted along a gradient of elevation and intensity of transmission pressure, also offers analytical leverage against age versus exposure. Another approach to teasing out the relative effect of age versus exposure on the development of NAI in areas of holoendemicity where both are superimposed is to design and conduct intervention studies using targeted malaria tools, such as IPT or continuous chemoprophylaxis, in defined age groups or age-stratified cohorts (8).
Migration of nonimmune people of all ages into areas of heavy endemicity occurs under some circumstances, as in the studies from Indonesian New Guinea discussed here. The increasing demand for natural resources and people striving for improvement of their economic welfare will continue to push development into less and less hospitable surroundings. These migrations occur despite the risks to life from many causes, including malaria. What is a normal hazard in the pursuit of life hinges upon what is necessary to secure essential needs, and people in a London suburb will have different standards from those at the edge of an Amazonian forest. Provided that a research agenda did not prompt the migration, the study of the people who embark on journeys of high risk of their own free will (298) imposes no ethical obstacles beyond those of international standards for research involving human subjects. Indeed, avoiding health research in such populations may be ethically dubious without a compelling justification. A dislike of the consequences of human migrations does not adequately support assertions of ethical compromise in the conduct of medical research within such populations.
Acknowledgments
This work was supported in part by a Pfizer Australia Research Fellowship (to D.L.D.), as well as by support from the Wellcome Trust and the Southeast Asian Clinical Research Network (to J.K.B.).
We express our sincere gratitude to the anonymous peer reviewers of this work for their constructive criticisms.
We report no conflicts of interest with regard to this paper.
REFERENCES
- 1.Achidi, E. A., H. Perlmann, L. S. Salimonu, P. Perlmann, O. Walker, and M. C. Asuzu. 1995. A longitudinal study of seroreactivities to Plasmodium falciparum antigens in Nigerian infants during their first year of life. Acta Trop. 59:173-183. [DOI] [PubMed] [Google Scholar]
- 2.Aguiar, J. C., G. R. Albrecht, P. Cegielski, B. M. Greenwood, J. B. Jensen, G. Lallinger, A. Martinez, I. A. McGregor, J. N. Minjas, J. Neequaye, M. E. Patarroyo, J. A. Sherwood, and R. J. Howard. 1992. Agglutination of Plasmodium falciparum-infected erythrocytes from East and West African isolates by human sera from distant geographic regions. Am. J. Trop. Med. Hyg. 47:621-632. [DOI] [PubMed] [Google Scholar]
- 3.Alonso, P. L. 2006. Malaria: deploying a candidate vaccine (RTS,S/AS02A) for an old scourge of humankind. Int. Microbiol. 9:83-93. [PubMed] [Google Scholar]
- 4.Alonso, P. L., S. W. Lindsay, J. R. Armstrong, M. Conteh, A. G. Hill, P. H. David, G. Fegan, A. de Francisco, A. J. Hall, F. C. Shenton, K. Cham, and B. M. Greenwood. 1991. The effect of insecticide-treated bed nets on mortality of Gambian children. Lancet 337:1499-1502. [DOI] [PubMed] [Google Scholar]
- 5.Alonso, P. L., S. W. Lindsay, J. R. Armstrong Schellenberg, K. Keita, P. Gomez, F. C. Shenton, A. G. Hill, P. H. David, G. Fegan, K. Cham, and B. M. Greenwood. 1993. A malaria control trial using insecticide-treated bed nets and targeted chemoprophylaxis in a rural area of The Gambia, West Africa. 6. The impact of the interventions on mortality and morbidity from malaria. Trans. R. Soc. Trop. Med. Hyg. 87(Suppl. 2):37-44. [DOI] [PubMed] [Google Scholar]
- 6.Alonso, P. L., J. Sacarlal, J. J. Aponte, A. Leach, E. Macete, P. Aide, B. Sigauque, J. Milman, I. Mandomando, Q. Bassat, C. Guinovart, M. Espasa, S. Corachan, M. Lievens, M. M. Navia, M. C. Dubois, C. Menendez, F. Dubovsky, J. Cohen, R. Thompson, and W. R. Ballou. 2005. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet 366:2012-2018. [DOI] [PubMed] [Google Scholar]
- 7.Amino, R., S. Thiberge, B. Martin, S. Celli, S. Shorte, F. Frischknecht, and R. Menard. 2006. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat. Med. 12:220-224. [DOI] [PubMed] [Google Scholar]
- 8.Aponte, J. J., C. Menendez, D. Schellenberg, E. Kahigwa, H. Mshinda, P. Vountasou, M. Tanner, and P. L. Alonso. 2007. Age interactions in the development of naturally acquired immunity to Plasmodium falciparum and its clinical presentation. PLoS Med. 4:1259-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arevalo-Herrera, M., Y. Solarte, M. F. Yasnot, A. Castellanos, A. Rincon, A. Saul, J. Mu, C. Long, L. Miller, and S. Herrera. 2005. Induction of transmission-blocking immunity in Aotus monkeys by vaccination with a Plasmodium vivax clinical grade PVS25 recombinant protein. Am. J. Trop. Med. Hyg. 73:32-37. [DOI] [PubMed] [Google Scholar]
- 10.Baer, K., C. Klotz, S. H. Kappe, T. Schnieder, and U. Frevert. 2007. Release of hepatic Plasmodium yoelii merozoites into the pulmonary microvasculature. PLoS Pathog. 3:1651-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baird, J. K. 1998. Age-dependent characteristics of protection versus susceptibility to Plasmodium falciparum. Ann. Trop. Med. Parasitol. 92:367-390. [DOI] [PubMed] [Google Scholar]
- 12.Baird, J. K. 1995. Host age as a determinant of naturally acquired immunity to Plasmodium falciparum. Parasitol. Today 11:105-111. [DOI] [PubMed] [Google Scholar]
- 13.Baird, J. K. 2000. Resurgent malaria at the millennium: control strategies in crisis. Drugs 59:719-743. [DOI] [PubMed] [Google Scholar]
- 14.Baird, J. K., H. Basri, P. Weina, J. D. MaGuire, M. J. Barcus, H. Picarema, I. R. Elyazar, E. Ayomi, and Sekartuti. 2003. Adult Javanese migrants to Indonesian Papua at high risk of severe disease caused by malaria. Epidemiol. Infect. 131:791-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baird, J. K., T. R. Jones, E. W. Danudirgo, B. A. Annis, M. J. Bangs, H. Basri, Purnomo, and S. Masbar. 1991. Age-dependent acquired protection against Plasmodium falciparum in people having two years exposure to hyperendemic malaria. Am. J. Trop. Med. Hyg. 45:65-76. [DOI] [PubMed] [Google Scholar]
- 16.Baird, J. K., Krisin, M. J. Barcus, I. R. Elyazar, M. J. Bangs, J. D. Maguire, D. J. Fryauff, T. L. Richie, Sekartuti, and W. Kalalo. 2003. Onset of clinical immunity to Plasmodium falciparum among Javanese migrants to Indonesian Papua. Ann. Trop. Med. Parasitol. 97:557-564. [DOI] [PubMed] [Google Scholar]
- 17.Baird, J. K., B. Leksana, S. Masbar, D. J. Fryauff, M. A. Sutanihardja, Suradi, F. S. Wignall, and S. L. Hoffman. 1997. Diagnosis of resistance to chloroquine by Plasmodium vivax: timing of recurrence and whole blood chloroquine levels. Am. J. Trop. Med. Hyg. 56:621-626. [DOI] [PubMed] [Google Scholar]
- 18.Baird, J. K., S. Masbar, H. Basri, S. Tirtokusumo, B. Subianto, and S. L. Hoffman. 1998. Age-dependent susceptibility to severe disease with primary exposure to Plasmodium falciparum. J. Infect. Dis. 178:592-595. [DOI] [PubMed] [Google Scholar]
- 19.Baird, J. K., S. Owusu Agyei, G. C. Utz, K. Koram, M. J. Barcus, T. R. Jones, D. J. Fryauff, F. N. Binka, S. L. Hoffman, and F. N. Nkrumah. 2002. Seasonal malaria attack rates in infants and young children in northern Ghana. Am. J. Trop. Med. Hyg. 66:280-286. [DOI] [PubMed] [Google Scholar]
- 20.Baird, J. K., Purnomo, H. Basri, M. J. Bangs, E. M. Andersen, T. R. Jones, S. Masbar, S. Harjosuwarno, B. Subianto, and P. R. Arbani. 1993. Age-specific prevalence of Plasmodium falciparum among six populations with limited histories of exposure to endemic malaria. Am. J. Trop. Med. Hyg. 49:707-719. [DOI] [PubMed] [Google Scholar]
- 21.Baird, J. K., and K. H. Rieckmann. 2003. Can. primaquine therapy for vivax malaria be improved? Trends Parasitol. 19:115-120. [DOI] [PubMed] [Google Scholar]
- 22.Baird, J. K., I. Wiady, D. J. Fryauff, M. A. Sutanihardja, B. Leksana, H. Widjaya, Kysdarmanto, and B. Subianto. 1997. In vivo resistance to chloroquine by Plasmodium vivax and Plasmodium falciparum at Nabire, Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 56:627-631. [DOI] [PubMed] [Google Scholar]
- 23.Barcus, M. J., H. Basri, H. Picarima, C. Manyakori, Sekartuti, I. Elyazar, M. J. Bangs, J. D. Maguire, and J. K. Baird. 2007. Demographic risk factors for severe and fatal vivax and falciparum malaria among hospital admissions in northeastern Indonesian Papua. Am. J. Trop. Med. Hyg. 77:984-991. [PubMed] [Google Scholar]
- 24.Barcus, M. J., Krisin, I. R. Elyazar, H. Marwoto, T. L. Richie, H. Basri, I. Wiady, D. J. Fryauff, J. D. Maguire, M. J. Bangs, and J. K. Baird. 2003. Primary infection by Plasmodium falciparum or P. vivax in a cohort of Javanese migrants to Indonesian Papua. Ann. Trop. Med. Parasitol. 97:565-574. [DOI] [PubMed] [Google Scholar]
- 25.Baruch, D. I., B. L. Pasloske, H. B. Singh, X. Bi, X. C. Ma, M. Feldman, T. F. Taraschi, and R. J. Howard. 1995. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell 82:77-87. [DOI] [PubMed] [Google Scholar]
- 26.Beach, R. F., T. K. Ruebush, 2nd, J. D. Sexton, P. L. Bright, A. W. Hightower, J. G. Breman, D. L. Mount, and A. J. Oloo. 1993. Effectiveness of permethrin-impregnated bed nets and curtains for malaria control in a holoendemic area of western Kenya. Am. J. Trop. Med. Hyg. 49:290-300. [DOI] [PubMed] [Google Scholar]
- 27.Beadle, C., P. D. McElroy, C. N. Oster, J. C. Beier, A. J. Oloo, F. K. Onyango, D. K. Chumo, J. D. Bales, J. A. Sherwood, and S. L. Hoffman. 1995. Impact of transmission intensity and age on Plasmodium falciparum density and associated fever: implications for malaria vaccine trial design. J. Infect. Dis. 172:1047-1054. [DOI] [PubMed] [Google Scholar]
- 28.Beeson, J. G., N. Amin, M. Kanjala, and S. J. Rogerson. 2002. Selective accumulation of mature asexual stages of Plasmodium falciparum-infected erythrocytes in the placenta. Infect. Immun. 70:5412-5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beeson, J. G., and P. E. Duffy. 2005. The immunology and pathogenesis of malaria during pregnancy. Curr. Top. Microbiol. Immunol. 297:187-227. [DOI] [PubMed] [Google Scholar]
- 30.Beier, J. C., G. F. Killeen, and J. I. Githure. 1999. Entomologic inoculation rates and Plasmodium falciparum malaria prevalence in Africa. Am. J. Trop. Med. Hyg. 61:109-113. [DOI] [PubMed] [Google Scholar]
- 31.Beier, J. C., C. N. Oster, F. K. Onyango, J. D. Bales, J. A. Sherwood, P. V. Perkins, D. K. Chumo, D. V. Koech, R. E. Whitmire, C. R. Roberts, C. L. Diggs, and S. L. Hoffman. 1994. Plasmodium falciparum incidence relative to entomologic inoculation rates at a site proposed for testing malaria vaccines in western Kenya. Am. J. Trop. Med. Hyg. 50:529-536. [DOI] [PubMed] [Google Scholar]
- 32.Belmonte, M., T. R. Jones, M. Lu, R. Arcilla, T. Smalls, A. Belmonte, J. Rosenbloom, D. J. Carucci, and M. Sedegah. 2003. The infectivity of Plasmodium yoelii in different strains of mice. J. Parasitol. 89:602-603. [DOI] [PubMed] [Google Scholar]
- 33.Belnoue, E., F. T. Costa, T. Frankenberg, A. M. Vigario, T. Voza, N. Leroy, M. M. Rodrigues, I. Landau, G. Snounou, and L. Renia. 2004. Protective T cell immunity against malaria liver stage after vaccination with live sporozoites under chloroquine treatment. J. Immunol. 172:2487-2495. [DOI] [PubMed] [Google Scholar]
- 34.Binka, F. N., A. Kubaje, M. Adjuik, L. A. Williams, C. Lengeler, G. H. Maude, G. E. Armah, B. Kajihara, J. H. Adiamah, and P. G. Smith. 1996. Impact of permethrin impregnated bednets on child mortality in Kassena-Nankana district, Ghana: a randomized controlled trial. Trop. Med. Int. Health 1:147-154. [DOI] [PubMed] [Google Scholar]
- 35.Bouchaud, O., M. Cot, S. Kony, R. Durand, R. Schiemann, P. Ralaimazava, J. P. Coulaud, J. Le Bras, and P. Deloron. 2005. Do African immigrants living in France have long-term malarial immunity? Am. J. Trop. Med. Hyg. 72:21-25. [PubMed] [Google Scholar]
- 36.Bousema, J. T., C. J. Drakeley, and R. W. Sauerwein. 2006. Sexual-stage antibody responses to P. falciparum in endemic populations. Curr. Mol. Med. 6:223-229. [DOI] [PubMed] [Google Scholar]
- 37.Bouyou-Akotet, M. K., A. A. Adegnika, S. T. Agnandji, E. Ngou-Milama, M. Kombila, P. G. Kremsner, and E. Mavoungou. 2005. Cortisol and susceptibility to malaria during pregnancy. Microbes Infect. 7:1217-1223. [DOI] [PubMed] [Google Scholar]
- 38.Boyd, M. F., and S. F. Kitchen. 1945. On the heterologous value of acquired immunity to Plasmodium falciparum J. Natl. Mal. Soc. 4:301-306. [PubMed] [Google Scholar]
- 39.Boyd, M. F., and C. B. Matthews. 1939. Further observations of the duration of immunity to the homologous strain of Plasmodium vivax. Am. J. Trop. Med. Hyg. 19:63-67. [Google Scholar]
- 40.Boyd, M. F., W. K. Stratman-Thomas, and S. F. Kitchen. 1936. On acquired immunity to Plasmodium falciparum. Am. J. Trop. Med. Hyg. 16:139-145. [Google Scholar]
- 41.Boyd, M. F., W. K. Stratman-Thomas, and H. Muench. 1934. Studies on benign tertian malaria. 6. On heterologous tolerance. Am. J. Trop. Med. Hyg. 20:481. [Google Scholar]
- 42.Branch, O., W. M. Casapia, D. V. Gamboa, J. N. Hernandez, F. F. Alava, N. Roncal, E. Alvarez, E. J. Perez, and E. Gotuzzo. 2005. Clustered local transmission and asymptomatic Plasmodium falciparum and Plasmodium vivax malaria infections in a recently emerged, hypoendemic Peruvian Amazon community. Malar. J. 4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bray, R. S. 1962. The inoculation of semi-immune Africans with sporozoites of Laverania falcipara (Plasmodium falciparum) in Liberia. Riv. Malariol. 41:199-210. [PubMed] [Google Scholar]
- 44.Breman, J. G., M. S. Alilio, and A. Mills. 2004. Conquering the intolerable burden of malaria: what's new, what's needed: a summary. Am. J. Trop. Med. Hyg. 71:1-15. [PubMed] [Google Scholar]
- 45.Broen, K., K. Brustoski, I. Engelmann, and A. J. Luty. 2007. Placental Plasmodium falciparum infection: causes and consequences of in utero sensitization to parasite antigens. Mol. Biochem. Parasitol. 151:1-8. [DOI] [PubMed] [Google Scholar]
- 46.Brown, I. N., K. N. Brown, and L. A. Hills. 1968. Immunity to malaria: the antibody response to antigenic variation by Plasmodium knowlesi. Immunology 14:127-138. [PMC free article] [PubMed] [Google Scholar]
- 47.Brown, K. N., and I. N. Brown. 1965. Immunity to malaria: antigenic variation in chronic infections of Plasmodium knowlesi. Nature 208:1286-1288. [DOI] [PubMed] [Google Scholar]
- 48.Brown, K. N., I. N. Brown, and L. A. Hills. 1970. Immunity to malaria. I. Protection against Plasmodium knowlesi shown by monkeys sensitized with drug-suppressed infections or by dead parasites in Freund's adjuvant. Exp. Parasitol. 28:304-317. [DOI] [PubMed] [Google Scholar]
- 49.Bruce, M. C., M. R. Galinski, J. W. Barnwell, C. A. Donnelly, M. Walmsley, M. P. Alpers, D. Walliker, and K. P. Day. 2000. Genetic diversity and dynamics of Plasmodium falciparum and P. vivax populations in multiply infected children with asymptomatic malaria infections in Papua New Guinea. Parasitology 121:257-272. [DOI] [PubMed] [Google Scholar]
- 50.Bruce-Chwatt, L. J. 1980. Essential malariology, vol. 354. William Heinemann Medical Books Ltd., London, United Kingdom.
- 51.Bull, P. C., M. Kortok, O. Kai, F. Ndungu, A. Ross, B. S. Lowe, C. I. Newbold, and K. Marsh. 2000. Plasmodium falciparum-infected erythrocytes: agglutination by diverse Kenyan plasma is associated with severe disease and young host age. J. Infect. Dis. 182:252-259. [DOI] [PubMed] [Google Scholar]
- 52.Bull, P. C., B. S. Lowe, M. Kortok, C. S. Molyneux, C. I. Newbold, and K. Marsh. 1998. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat. Med. 4:358-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bunn, A., R. Escombe, M. Armstrong, C. J. Whitty, and J. F. Doherty. 2004. Falciparum malaria in malaria-naive travellers and African visitors. Q. J. M. 97:645-649. [DOI] [PubMed] [Google Scholar]
- 54.Butcher, G. A., G. H. Mitchell, and S. Cohen. 1978. Antibody mediated mechanisms of immunity to malaria induced by vaccination with Plasmodium knowlesi merozoites. Immunology 34:77-86. [PMC free article] [PubMed] [Google Scholar]
- 55.Byam, W., and R. G. Archibald. 1921. The practice of medicine in the tropics, p.1513-1514. Henry Frowde and Hodder and Stoughton, London, United Kingdom.
- 56.Cabrera, E. J., M. L. Barr, and P. H. Silverman. 1977. Long-term studies on rhesus monkeys (Macaca mulatta) immunized against Plasmodium knowlesi. Infect. Immun. 15:461-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cadigan, F. C., Jr., and V. Chaicumpa. 1969. Plasmodium falciparum in the white-handed gibbon: protection afforded by previous infection with homologous and heterologous strains obtained in Thailand. Mil. Med. 134:1135-1139. [PubMed] [Google Scholar]
- 58.Calleri, G., F. Lipani, A. Macor, S. Belloro, G. Riva, and P. Caramello. 1998. Severe and complicated Falciparum malaria in Italian travelers. J. Travel Med. 5:39-41. [DOI] [PubMed] [Google Scholar]
- 59.Carter, R., and D. H. Chen. 1976. Malaria transmission blocked by immunisation with gametes of the malaria parasite. Nature 263:57-60. [DOI] [PubMed] [Google Scholar]
- 60.Carter, R., and K. N. Mendis. 1992. Transmission immunity in malaria: reflections on the underlying immune mechanisms during natural infections and following artificial immunization. Mem. Inst. Oswaldo Cruz 87:169-173. [DOI] [PubMed] [Google Scholar]
- 61.Ceithaml, J., and E. A. J. Evans. 1946. The biochemistry of the malaria parasite. IV. The in vitro effects of X-rays upon Plasmodium gallinaceum. J. Infect. Dis. 78:190-197. [DOI] [PubMed] [Google Scholar]
- 62.Chattopadhyay, R., A. Sharma, V. K. Srivastava, S. S. Pati, S. K. Sharma, B. S. Das, and C. E. Chitnis. 2003. Plasmodium falciparum infection elicits both variant-specific and cross-reactive antibodies against variant surface antigens. Infect. Immun. 71:597-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen, Q. 2007. The naturally acquired immunity in severe malaria and its implication for a PfEMP-1 based vaccine. Microbes Infect. 9:777-783. [DOI] [PubMed] [Google Scholar]
- 64.Christophers, S. R. 1911. Malaria in the Punjab. Scientific memoirs by officers of the Medical and Sanitary Departments of the Government of India. Government of India, Calcutta, India.
- 65.Christophers, S. R. 1924. The mechanism of immunity against malaria in communities living under hyperendemic conditions. Indian J. Med. Res. 12:273-294. [Google Scholar]
- 66.Church, L. W. P., T. P. Le, J. P. Bryan, D. M. Gordon, R. Edelman, L. Fries, J. R. Davis, D. A. Herrington, D. F. Clyde, M. J. Shmuklarsky, I. Schneider, T. W. McGovern, J. D. Chulay, W. R. Ballou, and S. L. Hoffman. 1997. Clinical manifestations of Plasmodium falciparum malaria experimentally induced by mosquito challenge. J. Infect. Dis. 175:915-920. [DOI] [PubMed] [Google Scholar]
- 67.Ciuca, M., L. Ballif, and M. Chelarescu-Vireu. 1934. Immunity to malaria. Trans. R. Soc. Trop. Med. Hyg. 27:619-622. [Google Scholar]
- 68.Clark, I. A. 1982. Correlation between susceptibility to malaria and babesia parasites and to endotoxicity. Trans. R. Soc. Trop. Med. Hyg. 76:4-7. [DOI] [PubMed] [Google Scholar]
- 69.Clyde, D. F., V. C. McCarthy, R. M. Miller, and R. B. Hornick. 1973. Specificity of protection of man immunized against sporozoite-induced falciparum malaria. Am. J. Med. Sci. 266:398-401. [DOI] [PubMed] [Google Scholar]
- 70.Clyde, D. F., V. C. McCarthy, R. M. Miller, and W. E. Woodward. 1975. Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. Am. J. Trop. Med. Hyg. 24:397-401. [DOI] [PubMed] [Google Scholar]
- 71.Clyde, D. F., H. Most, V. C. McCarthy, and J. P. Vanderberg. 1973. Immunization of man against sporozoite-induced falciparum malaria. Am.J. Med. Sci. 266:169-177. [DOI] [PubMed] [Google Scholar]
- 72.Cohen, S. 1977. Mechanisms of malarial immunity. Trans. R. Soc. Trop. Med. Hyg. 71:283-286. [DOI] [PubMed] [Google Scholar]
- 73.Cohen, S., and G. A. Butcher. 1971. Serum antibody in acquired malarial immunity. Trans. R. Soc. Trop. Med. Hyg. 65:125-135. [DOI] [PubMed] [Google Scholar]
- 74.Cohen, S., G. A. Butcher, and R. B. Crandall. 1969. Action of malarial antibody in vitro. Nature 223:368-371. [DOI] [PubMed] [Google Scholar]
- 75.Cohen, S., and I. A. McGregor. 1963. Gamma globulin and acquired immunity to malaria, p. 123-159. In P. C. Garnham, A. E. Pierce, and I. Roitt (ed.), Immunity to protozoa. Blackwell Scientific Publications Ltd., Oxford, United Kingdom.
- 76.Cohen, S., I. A. McGregor, and S. Carrington. 1961. Gamma-globulin and acquired immunity to human malaria. Nature 192:733-737. [DOI] [PubMed] [Google Scholar]
- 77.Coley, A. M., K. Parisi, R. Masciantonio, J. Hoeck, J. L. Casey, V. J. Murphy, K. S. Harris, A. H. Batchelor, R. F. Anders, and M. Foley. 2006. The most polymorphic residue on Plasmodium falciparum apical membrane antigen 1 determines binding of an invasion-inhibitory antibody. Infect. Immun. 74:2628-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Collins, W. E., J. W. Barnwell, J. S. Sullivan, D. Nace, T. Williams, A. Bounngaseng, J. Roberts, E. Strobert, H. McClure, A. Saul, and C. A. Long. 2006. Assessment of transmission-blocking activity of candidate Pvs25 vaccine using gametocytes from chimpanzees. Am. J. Trop. Med. Hyg. 74:215-221. [PubMed] [Google Scholar]
- 79.Collins, W. E., and P. G. Contacos. 1972. Immunization of monkeys against Plasmodium cynomolgi by X-irradiated sporozoites. Nat. New Biol. 236:176-177. [DOI] [PubMed] [Google Scholar]
- 80.Collins, W. E., and G. M. Jeffery. 1999. A retrospective examination of sporozoite- and trophozoite-induced infections with Plasmodium falciparum: development of parasitologic and clinical immunity during primary infection. Am. J. Trop. Med. Hyg. 61:4-19. [DOI] [PubMed] [Google Scholar]
- 81.Collins, W. E., G. M. Jeffery, E. Guinn, and J. C. Skinner. 1966. Fluorescent antibody studies in human malaria. IV. Cross-reactions between human and simian malaria. Am. J. Trop. Med. Hyg. 15:11-15. [DOI] [PubMed] [Google Scholar]
- 82.Collins, W. E., G. M. Jeffery, and J. M. Roberts. 2004. A retrospective examination of reinfection of humans with Plasmodium vivax. Am. J. Trop. Med. Hyg. 70:642-644. [PubMed] [Google Scholar]
- 83.Collins, W. E., J. C. Skinner, and R. E. Coifman. 1967. Fluorescent antibody studies in human malaria. V. Response of sera from Nigerians to five Plasmodium antigens. Am. J. Trop. Med. Hyg. 16:568-571. [DOI] [PubMed] [Google Scholar]
- 84.Collins, W. E., J. C. Skinner, P. Millet, J. R. Broderson, V. K. Filipski, C. L. Morris, P. P. Wilkins, G. H. Campbell, P. S. Stanfill, B. B. Richardson, and J. Sullivan. 1992. Reinforcement of immunity in Saimiri monkeys following immunization with irradiated sporozoites of Plasmodium vivax. Am. J. Trop. Med. Hyg. 46:327-334. [DOI] [PubMed] [Google Scholar]
- 85.Conway, D. J., B. M. Greenwood, and J. S. McBride. 1992. Longitudinal study of Plasmodium falciparum polymorphic antigens in a malaria-endemic population. Infect. Immun. 60:1122-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Conway, D. J., and J. S. McBride. 1991. Population genetics of Plasmodium falciparum within a malaria hyperendemic area. Parasitology 103:7-16. [DOI] [PubMed] [Google Scholar]
- 87.Cot, M., L. Abel, A. Roisin, D. Barro, A. Yada, P. Carnevale, and J. Feingold. 1993. Risk factors of malaria infection during pregnancy in Burkina Faso: suggestion of a genetic influence. Am. J. Trop. Med. Hyg. 48:358-364. [DOI] [PubMed] [Google Scholar]
- 88.Covell, G., and J. D. Baily. 1932. The study of a regional epidemic of malaria in northern Sind. Rec. Mal. Surv. India 3:279-322. [Google Scholar]
- 89.Covell, G., and W. D. Nicol. 1951. Clinical, chemotherapeutic and immunological studies on induced malaria. Br. Med. Bull. 8:51-55. [DOI] [PubMed] [Google Scholar]
- 90.Cowman, A. F., and B. S. Crabb. 2006. Invasion of red blood cells by malaria parasites. Cell 124:755-766. [DOI] [PubMed] [Google Scholar]
- 91.Cox, H. W. 1959. A study of relapse Plasmodium berghei infections isolated from white mice. J. Immunol. 82:209-214. [PubMed] [Google Scholar]
- 92.Cox-Singh, J., T. M. Davis, K. S. Lee, S. S. Shamsul, A. Matusop, S. Ratnam, H. A. Rahman, D. J. Conway, and B. Singh. 2008. Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin. Infect. Dis. 46:165-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cox-Singh, J., and B. Singh. 2008. Knowlesi malaria: newly emergent and of public health importance? Trends Parasitol. 24:406-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Creasey, A. M., L. C. Ranford-Cartwright, D. J. Moore, D. H. Williamson, R. J. Wilson, D. Walliker, and R. Carter. 1993. Uniparental inheritance of the mitochondrial gene cytochrome b in Plasmodium falciparum. Curr. Genet. 23:360-364. [DOI] [PubMed] [Google Scholar]
- 95.Curtin, P. D. 1994. Malarial immunities in nineteenth-century west Africa and the Caribbean. Parassitologia 36:69-82. [PubMed] [Google Scholar]
- 96.D'Alessandro, U. 1997. Severity of malaria and level of Plasmodium falciparum transmission. Lancet 350:362. [DOI] [PubMed] [Google Scholar]
- 97.D'Alessandro, U., B. O. Olaleye, W. McGuire, M. C. Thomson, P. Langerock, S. Bennett, and B. M. Greenwood. 1995. A comparison of the efficacy of insecticide-treated and untreated bed nets in preventing malaria in Gambian children. Trans. R. Soc. Trop. Med. Hyg. 89:596-598. [DOI] [PubMed] [Google Scholar]
- 98.Day, K. P., and K. Marsh. 1991. Naturally acquired immunity to Plasmodium falciparum. Immunol. Today 12:A68-71. [DOI] [PubMed] [Google Scholar]
- 99.Desowitz, R. S., J. Elm, and M. P. Alpers. 1993. Plasmodium falciparum-specific immunoglobulin G (IgG), IgM, and IgE antibodies in paired maternal-cord sera from east Sepik Province, Papua New Guinea. Infect. Immun. 61:988-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Diggs, C. L., and A. G. Osler. 1975. Humoral immunity in rodent malaria. III. Studies on the site of antibody action. J. Immunol. 114:1243-1247. [PubMed] [Google Scholar]
- 101.Diggs, C. L., B. T. Wellde, J. S. Anderson, R. M. Weber, and E. Rodriguez. 1972. The protective effect of African human immunoglobulin G in Aotus trivirgatus infected with Asian Plasmodium falciparum. Proc. Helm. Soc. Wash. 39:449-456. [Google Scholar]
- 102.Dodoo, D., T. Staalsoe, H. Giha, J. A. Kurtzhals, B. D. Akanmori, K. Koram, S. Dunyo, F. K. Nkrumah, L. Hviid, and T. G. Theander. 2001. Antibodies to variant antigens on the surfaces of infected erythrocytes are associated with protection from malaria in Ghanaian children. Infect. Immun. 69:3713-3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dondorp, A. M., S. J. Lee, M. A. Faiz, S. Mishra, R. Price, E. Tjitra, M. Than, Y. Htut, S. Mohanty, E. B. Yunus, R. Rahman, F. Nosten, N. M. Anstey, N. P. Day, and N. J. White. 2008. The relationship between age and the manifestations of and mortality associated with severe malaria. Clin. Infect. Dis. 47:151-157. [DOI] [PubMed] [Google Scholar]
- 104.Doolan, D. L., and N. Martinez-Alier. 2006. Immune response to pre-erythrocytic stages of malaria parasites. Curr. Mol. Med. 6:169-185. [DOI] [PubMed] [Google Scholar]
- 105.Drakeley, C., C. Sutherland, J. T. Bousema, R. W. Sauerwein, and G. A. Targett. 2006. The epidemiology of Plasmodium falciparum gametocytes: weapons of mass dispersion. Trends Parasitol. 22:424-430. [DOI] [PubMed] [Google Scholar]
- 106.Dubois, P., J. P. Dedet, T. Fandeur, C. Roussilhon, M. Jendoubi, S. Pauillac, O. Mercereau-Puijalon, and L. Pereira Da Silva. 1984. Protective immunization of the squirrel monkey against asexual blood stages of Plasmodium falciparum by use of parasite protein fractions. Proc. Natl. Acad. Sci. USA 81:229-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Duffy, P. E. 2007. Plasmodium in the placenta: parasites, parity, protection, prevention and possibly preeclampsia. Parasitology 134:1877-1881. [DOI] [PubMed] [Google Scholar]
- 108.Duffy, P. E., A. G. Craig, and D. I. Baruch. 2001. Variant proteins on the surface of malaria-infected erythrocytes—developing vaccines. Trends Parasitol. 17:354-356. [DOI] [PubMed] [Google Scholar]
- 109.Edelman, R., S. L. Hoffman, J. R. Davis, M. Beier, M. B. Sztein, G. Losonsky, D. A. Herrington, H. A. Eddy, M. R. Hollingdale, D. M. Gordon, and D. F. Clyde. 1993. Long-term persistence of sterile immunity in a volunteer immunized with X-irradiated Plasmodium falciparum sporozoites. J. Infect. Dis. 168:1066-1070. [DOI] [PubMed] [Google Scholar]
- 110.Egan, J. E., S. L. Hoffman, J. D. Haynes, J. C. Sadoff, I. Schneider, G. E. Grau, M. R. Hollingdale, W. R. Ballou, and D. M. Gordon. 1993. Humoral immune response in volunteers immunized with irradiated Plasmodium falciparum sporozoites. Am. J. Trop. Med. Hyg. 49:166-173. [DOI] [PubMed] [Google Scholar]
- 111.Elliott, S. R., R. D. Kuns, and M. F. Good. 2005. Heterologous immunity in the absence of variant-specific antibodies after exposure to subpatent infection with blood-stage malaria. Infect. Immun. 73:2478-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Elliott, S. R., P. D. Payne, M. F. Duffy, T. J. Byrne, W. H. Tham, S. J. Rogerson, G. V. Brown, and D. P. Eisen. 2007. Antibody recognition of heterologous variant surface antigens after a single Plasmodium falciparum infection in previously naive adults. Am. J. Trop. Med. Hyg. 76:860-864. [PubMed] [Google Scholar]
- 113.Fandeur, T., and W. Chalvet. 1998. Variant- and strain-specific immunity in Saimiri infected with Plasmodium falciparum. Am. J. Trop. Med. Hyg. 58:225-231. [DOI] [PubMed] [Google Scholar]
- 114.Fandeur, T., C. Le Scanf, B. Bonnemains, C. Slomianny, and O. Mercereau-Puijalon. 1995. Immune pressure selects for Plasmodium falciparum parasites presenting distinct red blood cell surface antigens and inducing strain-specific protection in Saimiri sciureus monkeys. J. Exp. Med. 181:283-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fernandez, V., M. Hommel, Q. Chen, P. Hagblom, and M. Wahlgren. 1999. Small, clonally variant antigens expressed on the surface of the Plasmodium falciparum-infected erythrocyte are encoded by the rif gene family and are the target of human immune responses. J. Exp. Med. 190:1393-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Field, J. W., and J. C. Niven. 1937. A note on prognosis in relation to parasite counts in acute subtertian malaria. Trans. R. Soc. Trop. Med. Hyg. 30:569-574. [Google Scholar]
- 117.Filipe, J. A., E. M. Riley, C. J. Drakeley, C. J. Sutherland, and A. C. Ghani. 2007. Determination of the processes driving the acquisition of immunity to malaria using a mathematical transmission model. PLoS Comput. Biol. 3:2569-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Forsyth, K. P., G. Philip, T. Smith, E. Kum, B. Southwell, and G. V. Brown. 1989. Diversity of antigens expressed on the surface of erythrocytes infected with mature Plasmodium falciparum parasites in Papua New Guinea. Am. J. Trop. Med. Hyg. 41:259-265. [PubMed] [Google Scholar]
- 119.Fouda, G. G., R. F. Leke, C. Long, P. Druilhe, A. Zhou, D. W. Taylor, and A. H. Johnson. 2006. Multiplex assay for simultaneous measurement of antibodies to multiple Plasmodium falciparum antigens. Clin. Vaccine Immunol. 13:1307-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Freund, J., H. E. Sommer, and A. W. Walter. 1945. Immunization against malaria: vaccination of ducks with killed parasites incorporated with adjuvants. Science 102:200-202. [DOI] [PubMed] [Google Scholar]
- 121.Freund, J., K. J. Thomson, H. E. Sommer, A. W. Walter, and T. M. Pisani. 1948. Immunization of monkeys against malaria by means of killed parasites with adjuvants. Am. J. Trop. Med. Hyg. s1-28:1-22. [DOI] [PubMed] [Google Scholar]
- 122.Frevert, U., S. Engelmann, S. Zougbede, J. Stange, B. Ng, K. Matuschewski, L. Liebes, and H. Yee. 2005. Intravital observation of Plasmodium berghei sporozoite infection of the liver. PLoS Biol. 3:1034-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gallup, J. L., and J. D. Sachs. 2001. The economic burden of malaria. Am. J. Trop. Med. Hyg. 64:85-96. [DOI] [PubMed] [Google Scholar]
- 124.Gamain, B., J. D. Smith, N. K. Viebig, J. Gysin, and A. Scherf. 2007. Pregnancy-associated malaria: parasite binding, natural immunity and vaccine development. Int. J. Parasitol. 37:273-283. [DOI] [PubMed] [Google Scholar]
- 125.Garnham, P. C. C. 1966. Malaria parasites and other haemosporidia. Blackwell Scientific Publications, Oxford, United Kingdom.
- 126.Genton, B., V. D'Acremont, L. Rare, K. Baea, J. C. Reeder, M. P. Alpers, and I. Muller. 2008. Plasmodium vivax and mixed infections are associated with severe malaria in children: a prospective cohort study from Papua New Guinea. PLoS Med. 5:881-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gill, C. A. 1914. Epidemic or fulminant malaria together with a preliminary study of the part played by immunity to malaria. Indian J. Med. Res. 2:268-315. [Google Scholar]
- 128.Good, M. F. 2005. Genetically modified Plasmodium highlights the potential of whole parasite vaccine strategies. Trends Immunol. 26:295-297. [DOI] [PubMed] [Google Scholar]
- 129.Gosling, R. D., A. C. Ghani, J. L. Deen, L. von Seidlein, B. M. Greenwood, and D. Chandramohan. 2008. Can. changes in malaria transmission intensity explain prolonged protection and contribute to high protective efficacy of intermittent preventive treatment for malaria in infants? Malar. J. 7:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Grassi, B., A. Bignami, and G. Bastianelli. 1899. Ulteriori ricerche sul ciclo dei parassiti malarici umani nel corpo del zanzarone. Atti Reale Accad. Lincei 5:8-21. [Google Scholar]
- 131.Greenwood, B., K. Marsh, and R. Snow. 1991. Why do some African children develop severe malaria? Parasitol. Today 7:277-281. [DOI] [PubMed] [Google Scholar]
- 132.Greenwood, B. M., P. H. David, L. N. Otoo-Forbes, S. J. Allen, P. L. Alonso, J. R. Armstrong Schellenberg, P. Byass, M. Hurwitz, A. Menon, and R. W. Snow. 1995. Mortality and morbidity from malaria after stopping malaria chemoprophylaxis. Trans. R. Soc. Trop. Med. Hyg. 89:629-633. [DOI] [PubMed] [Google Scholar]
- 133.Guerra, C. A., R. W. Snow, and S. I. Hay. 2006. Mapping the global extent of malaria in 2005. Trends Parasitol. 22:353-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133a.Gupta, S., R. W. Snow, C. A. Donnelly, K. Marsh, and C. Newbold. 1999. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat. Med. 5:340-343. [DOI] [PubMed] [Google Scholar]
- 134.Gwadz, R. W. 1976. Successful immunization against the sexual stages of Plasmodium gallinaceum. Science 193:1150-1151. [DOI] [PubMed] [Google Scholar]
- 135.Gwadz, R. W., A. H. Cochrane, V. Nussenzweig, and R. S. Nussenzweig. 1979. Preliminary studies on vaccination of rhesus monkeys with irradiated sporozoites of Plasmodium knowlesi and characterization of surface antigens of these parasites. Bull. W. H. O. 57(Suppl. 1):165-173. [PMC free article] [PubMed] [Google Scholar]
- 136.Gwadz, R. W., and I. Green. 1978. Malaria immunization in Rhesus monkeys. A vaccine effective against both the sexual and asexual stages of Plasmodium knowlesi. J. Exp. Med. 148:1311-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gysin, J., P. Moisson, L. Pereira da Silva, and P. Druilhe. 1996. Antibodies from immune African donors with a protective effect in Plasmodium falciparum human infection are also able to control asexual blood forms of the parasite in Saimiri monkeys. Res. Immunol. 147:397-401. [DOI] [PubMed] [Google Scholar]
- 138.Hafalla, J. C., U. Rai, A. Morrot, D. Bernal-Rubio, F. Zavala, and A. Rodriguez. 2006. Priming of CD8+ T cell responses following immunization with heat-killed Plasmodium sporozoites. Eur. J. Immunol. 36:1179-1186. [DOI] [PubMed] [Google Scholar]
- 139.Handunnetti, S. M., K. N. Mendis, and P. H. David. 1987. Antigenic variation of cloned Plasmodium fragile in its natural host Macaca sinica. Sequential appearance of successive variant antigenic types. J. Exp. Med. 165:1269-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Harrison, G. A. 1978. Mosquitoes, malaria & man: a history of the hostilities since 1880, p.5. E. P. Dutton, New York, NY.
- 141.Healer, J., V. Murphy, A. N. Hodder, R. Masciantonio, A. W. Gemmill, R. F. Anders, A. F. Cowman, and A. Batchelor. 2004. Allelic polymorphisms in apical membrane antigen-1 are responsible for evasion of antibody-mediated inhibition in Plasmodium falciparum. Mol. Microbiol. 52:159-168. [DOI] [PubMed] [Google Scholar]
- 142.Herrington, D., J. Davis, E. Nardin, M. Beier, J. Cortese, H. Eddy, G. Losonsky, M. Hollingdale, M. Sztein, M. Levine, R. S. Nussenzweig, D. Clyde, and R. Edelman. 1991. Successful immunization of humans with irradiated sporozoites: humoral and cellular responses of the protected individuals. Am. J. Trop. Med. Hyg. 45:539-547. [DOI] [PubMed] [Google Scholar]
- 143.Hodder, A. N., P. E. Crewther, and R. F. Anders. 2001. Specificity of the protective antibody response to apical membrane antigen 1. Infect. Immun. 69:3286-3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hoffman, S. L., L. M. Goh, T. C. Luke, I. Schneider, T. P. Le, D. L. Doolan, J. Sacci, P. de La Vega, M. Dowler, C. Paul, D. M. Gordon, J. A. Stoute, L. W. Church, M. Sedegah, D. G. Heppner, W. R. Ballou, and T. L. Richie. 2002. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparumsporozoites. J. Infect. Dis. 185:1155-1164. [DOI] [PubMed] [Google Scholar]
- 145.Hoffman, S. L., D. Isenbarger, G. W. Long, M. Sedegah, A. Szarfman, L. Waters, M. R. Hollingdale, P. H. van der Miede, D. S. Finbloom, and W. R. Ballou. 1989. Sporozoite vaccine induces genetically restricted T cell elimination of malaria from hepatocytes. Science 244:1078-1081. [DOI] [PubMed] [Google Scholar]
- 146.Hoffman, S. L., C. N. Oster, C. V. Plowe, G. R. Woollett, J. C. Beier, J. D. Chulay, R. A. Wirtz, M. R. Hollingdale, and M. Mugambi. 1987. Naturally acquired antibodies to sporozoites do not prevent malaria: vaccine development implications. Science 237:639-642. [DOI] [PubMed] [Google Scholar]
- 147.Holbrook, T. W., N. C. Palczuk, and L. A. Stauber. 1974. Immunity to exoerythrocytic forms of malaria. 3. Stage-specific immunization of turkeys against exoerythrocytic forms of Plasmodium fallax. J. Parasitol. 60:348-354. [PubMed] [Google Scholar]
- 148.Hommel, M. 1985. The role of variant antigens in acquired immunity to Plasmodium falciparum. Ann. Soc. Belg. Med. Trop. 65(Suppl. 2):57-67. [PubMed] [Google Scholar]
- 149.Hommel, M., P. H. David, M. Guillotte, and L. Pereira da Silva. 1982. Protection against Plasmodium chabaudi malaria. I. Vaccination of mice with merozoites and Freund's adjuvants. Ann. Immunol. 133C:57-67. [DOI] [PubMed] [Google Scholar]
- 150.Hommel, M., P. H. David, and L. D. Oligino. 1983. Surface alterations of erythrocytes in Plasmodium falciparum malaria. Antigenic variation, antigenic diversity, and the role of the spleen. J. Exp. Med. 157:1137-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hommel, M., P. H. David, L. D. Oligino, and J. R. David. 1982. Expression of strain-specific surface antigens on Plasmodium falciparum-infected erythrocytes. Parasite Immunol. 4:409-419. [DOI] [PubMed] [Google Scholar]
- 152.Horrocks, P., R. Pinches, Z. Christodoulou, S. A. Kyes, and C. I. Newbold. 2004. Variable var transition rates underlie antigenic variation in malaria. Proc. Natl. Acad. Sci. USA 101:11129-11134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Huff, C. G., D. F. Marchbank, and T. Shiroishi. 1958. Changes in infectiousness of malarial gametocytes. II. Analysis of the possible causative factors. Exp. Parasitol. 7:399-417. [DOI] [PubMed] [Google Scholar]
- 154.Hviid, L. 2004. The immuno-epidemiology of pregnancy-associated Plasmodium falciparum malaria: a variant surface antigen-specific perspective. Parasite Immunol. 26:477-486. [DOI] [PubMed] [Google Scholar]
- 155.Hviid, L. 2005. Naturally acquired immunity to Plasmodium falciparum malaria in Africa. Acta Trop. 95:270-275. [DOI] [PubMed] [Google Scholar]
- 156.Hviid, L., and A. Salanti. 2007. VAR2CSA and protective immunity against pregnancy-associated Plasmodium falciparum malaria. Parasitology 134:1871-1876. [DOI] [PubMed] [Google Scholar]
- 157.Ishino, T., K. Yano, Y. Chinzei, and M. Yuda. 2004. Cell-passage activity is required for the malarial parasite to cross the liver sinusoidal cell layer. PLoS Biol. 2:77-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.James, S. P. 1931. Some general results of a study of induced malaria in England. Trans. R. Soc. Trop. Med. Hyg. 24:477-538. [Google Scholar]
- 159.James, S. P., and M. Ciuca. 1938. Acta Conventus Tertii de Tropicis atque Malariae Morbis. 2:269-281. [Google Scholar]
- 160.James, S. P., W. D. Nicol, and P. D. Shute. 1932. A study of induced malignant tertian malaria. Proc. R. Soc. Med. 25:1153-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jeffery, G. M. 1966. Epidemiological significance of repeated infections with homologous and heterologous strains and species of Plasmodium. Bull. W. H. O. 35:873-882. [PMC free article] [PubMed] [Google Scholar]
- 162.Jennings, R. M., J. B. De Souza, J. E. Todd, M. Armstrong, K. L. Flanagan, E. M. Riley, and J. F. Doherty. 2006. Imported Plasmodium falciparum malaria: are patients originating from disease-endemic areas less likely to develop severe disease? A prospective, observational study. Am. J. Trop. Med. Hyg. 75:1195-1199. [PubMed] [Google Scholar]
- 163.Jensen, A. T., P. Magistrado, S. Sharp, L. Joergensen, T. Lavstsen, A. Chiucchiuini, A. Salanti, L. S. Vestergaard, J. P. Lusingu, R. Hermsen, R. Sauerwein, J. Christensen, M. A. Nielsen, L. Hviid, C. Sutherland, T. Staalsoe, and T. G. Theander. 2004. Plasmodium falciparum associated with severe childhood malaria preferentially expresses PfEMP1 encoded by group A var genes. J. Exp. Med. 199:1179-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jones, T. R., N. Obaldia III, R. A. Gramzinski, and S. L. Hoffman. 2000. Repeated infection of Aotus monkeys with Plasmodium falciparum induces protection against subsequent challenge with homologous and heterologous strains of parasite. Am. J. Trop. Med. Hyg. 62:675-680. [DOI] [PubMed] [Google Scholar]
- 165.Kassim, O. O., K. A. Ako-Anai, S. E. Torimiro, G. P. Hollowell, V. C. Okoye, and S. K. Martin. 2000. Inhibitory factors in breastmilk, maternal and infant sera against in vitro growth of Plasmodium falciparum malaria parasite. J. Trop. Pediatr. 46:92-96. [DOI] [PubMed] [Google Scholar]
- 166.Kaviratne, M., S. M. Khan, W. Jarra, and P. R. Preiser. 2002. Small variant STEVOR antigen is uniquely located within Maurer's clefts in Plasmodium falciparum-infected red blood cells. Eukaryot. Cell 1:926-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kemp, D. J., A. F. Cowman, and D. Walliker. 1990. Genetic diversity in Plasmodium falciparum. Adv. Parasitol. 29:75-149. [DOI] [PubMed] [Google Scholar]
- 168.Kennedy, M. C., J. Wang, Y. Zhang, A. P. Miles, F. Chitsaz, A. Saul, C. A. Long, L. H. Miller, and A. W. Stowers. 2002. In vitro studies with recombinant Plasmodium falciparum apical membrane antigen 1 (AMA1): production and activity of an AMA1 vaccine and generation of a multiallelic response. Infect. Immun. 70:6948-6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kinyanjui, S. M., T. Mwangi, P. C. Bull, C. I. Newbold, and K. Marsh. 2004. Protection against clinical malaria by heterologous immunoglobulin G antibodies against malaria-infected erythrocyte variant surface antigens requires interaction with asymptomatic infections. J. Infect. Dis. 190:1527-1533. [DOI] [PubMed] [Google Scholar]
- 170.Kobbe, R., C. Kreuzberg, S. Adjei, B. Thompson, I. Langefeld, P. A. Thompson, H. H. Abruquah, B. Kreuels, M. Ayim, W. Busch, F. Marks, K. Amoah, E. Opoku, C. G. Meyer, O. Adjei, and J. May. 2007. A randomized controlled trial of extended intermittent preventive antimalarial treatment in infants. Clin. Infect. Dis. 45:16-25. [DOI] [PubMed] [Google Scholar]
- 171.Koch, R. 1900. Dritter bericht uber di thatigkeit der malaria-expedition. Dtsch. Med. Wochenschr. 1900 26:296-297. [Google Scholar]
- 172.Koch, R. 1900. Funfter bericht uber di thatigkeit der malaria-expedition. Dtsch. Med. Wochenschr. 1900 26:541-542. [Google Scholar]
- 173.Koch, R. 1900. Vierter bericht uber di thatigkeit der malaria-expedition. Dtsch. Med. Wochenschr. 1900 26:397-398. [Google Scholar]
- 174.Koch, R. 1900. Zweiter bericht uber di thatigkeit der malaria-expedition. Dtsch. Med. Wochenschr. 1900 26:88-90. [Google Scholar]
- 175.Kraemer, S. M., and J. D. Smith. 2006. A family affair: var genes, PfEMP1 binding, and malaria disease. Curr. Opin. Microbiol. 9:374-380. [DOI] [PubMed] [Google Scholar]
- 176.Krisin, H. Basri, D. J. Fryauff, M. J. Barcus, M. J. Bangs, E. Ayomi, H. Marwoto, I. R. Elyazar, T. L. Richie, and J. K. Baird. 2003. Malaria in a cohort of Javanese migrants to Indonesian Papua. Ann. Trop. Med. Parasitol. 97:543-556. [DOI] [PubMed] [Google Scholar]
- 177.Krotoski, W. A., W. E. Collins, R. S. Bray, P. C. Garnham, F. B. Cogswell, R. W. Gwadz, R. Killick-Kendrick, R. Wolf, R. Sinden, L. C. Koontz, and P. S. Stanfill. 1982. Demonstration of hypnozoites in sporozoite-transmitted Plasmodium vivax infection. Am. J. Trop. Med. Hyg. 31:1291-1293. [DOI] [PubMed] [Google Scholar]
- 178.Kurtis, J. D., R. Mtalib, F. K. Onyango, and P. E. Duffy. 2001. Human resistance to Plasmodium falciparum increases during puberty and is predicted by dehydroepiandrosterone sulfate levels. Infect. Immun. 69:123-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kyes, S. A., J. A. Rowe, N. Kriek, and C. I. Newbold. 1999. Rifins: a second family of clonally variant proteins expressed on the surface of red cells infected with Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 96:9333-9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lavazec, C., and C. Bourgouin. 2008. Mosquito-based transmission blocking vaccines for interrupting Plasmodium development. Microbes Infect. 10:845-849. [DOI] [PubMed] [Google Scholar]
- 181.Laveran, A. 1880. A new parasite found in the blood of malarial patients. Parasitic origin of malarial attacks. Bull. Mem. Soc. Med. Hosp. Paris 17:158-164. [Google Scholar]
- 182.Lengeler, C., J. A. Schellenberg, and U. D'Alessandro. 1995. Will reducing Plasmodium falciparum malaria transmission alter malaria mortality among African children? Parasitol. Today 11:425. (Author reply 11:426.) [DOI] [PubMed] [Google Scholar]
- 183.Luty, A. J., B. Lell, R. Schmidt-Ott, L. G. Lehman, D. Luckner, B. Greve, P. Matousek, K. Herbich, D. Schmid, F. Migot-Nabias, P. Deloron, R. S. Nussenzweig, and P. G. Kremsner. 1999. Interferon-gamma responses are associated with resistance to reinfection with Plasmodium falciparum in young African children. J. Infect. Dis. 179:980-988. [DOI] [PubMed] [Google Scholar]
- 184.Macdonald, G. 1951. Community aspects of immunity to malaria. Br. Med. Bull. 8:33-36. [DOI] [PubMed] [Google Scholar]
- 185.Macdonald, G. 1957. The epidemiology and control of malaria. Oxford University Press, London, United Kingdom.
- 186.Macete, E., P. Aide, J. J. Aponte, S. Sanz, I. Mandomando, M. Espasa, B. Sigauque, C. Dobano, S. Mabunda, M. DgeDge, P. Alonso, and C. Menendez. 2006. Intermittent preventive treatment for malaria control administered at the time of routine vaccinations in Mozambican infants: a randomized, placebo-controlled trial. J. Infect. Dis. 194:276-285. [DOI] [PubMed] [Google Scholar]
- 187.Magistrado, P. A., J. Lusingu, L. S. Vestergaard, M. Lemnge, T. Lavstsen, L. Turner, L. Hviid, A. T. Jensen, and T. G. Theander. 2007. Immunoglobulin G antibody reactivity to a group A Plasmodium falciparum erythrocyte membrane protein 1 and protection from P. falciparum malaria. Infect. Immun. 75:2415-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Maguire, J. D., I. W. Sumawinata, S. Masbar, B. Laksana, P. Prodjodipuro, I. Susanti, P. Sismadi, N. Mahmud, M. J. Bangs, and J. K. Baird. 2002. Chloroquine-resistant Plasmodium malariae in south Sumatra, Indonesia. Lancet 360:58-60. [DOI] [PubMed] [Google Scholar]
- 189.Marsh, K., D. Forster, C. Waruiru, I. Mwangi, M. Winstanley, V. Marsh, C. Newton, P. Winstanley, P. Warn, N. Peshu, G. Pasvol, and R. Snow. 1995. Indicators of life-threatening malaria in African children. N. Engl. J. Med. 332:1399-1404. [DOI] [PubMed] [Google Scholar]
- 190.Marsh, K., and R. J. Howard. 1986. Antigens induced on erythrocytes by P. falciparum: expression of diverse and conserved determinants. Science 231:150-153. [DOI] [PubMed] [Google Scholar]
- 191.Marsh, K., L. Otoo, R. J. Hayes, D. C. Carson, and B. M. Greenwood. 1989. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans. R. Soc. Trop. Med. Hyg. 83:293-303. [DOI] [PubMed] [Google Scholar]
- 192.Marsh, K., and R. W. Snow. 1997. Host-parasite interaction and morbidity in malaria endemic areas. Philos. Trans. R. Soc. London B 352:1385-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Marsh, K., and R. W. Snow. 1999. Malaria transmission and morbidity. Parassitologia 41:241-246. [PubMed] [Google Scholar]
- 194.Massaga, J. J., A. Y. Kitua, M. M. Lemnge, J. A. Akida, L. N. Malle, A. M. Ronn, T. G. Theander, and I. C. Bygbjerg. 2003. Effect of intermittent treatment with amodiaquine on anaemia and malarial fevers in infants in Tanzania: a randomised placebo-controlled trial. Lancet 361:1853-1860. [DOI] [PubMed] [Google Scholar]
- 195.Mbogo, C. N., R. W. Snow, E. W. Kabiru, J. H. Ouma, J. I. Githure, K. Marsh, and J. C. Beier. 1993. Low-level Plasmodium falciparum transmission and the incidence of severe malaria infections on the Kenyan coast. Am. J. Trop. Med. Hyg. 49:245-253. [DOI] [PubMed] [Google Scholar]
- 196.Mbogo, C. N., R. W. Snow, C. P. Khamala, E. W. Kabiru, J. H. Ouma, J. I. Githure, K. Marsh, and J. C. Beier. 1995. Relationships between Plasmodium falciparum transmission by vector populations and the incidence of severe disease at nine sites on the Kenyan coast. Am. J. Trop. Med. Hyg. 52:201-206. [DOI] [PubMed] [Google Scholar]
- 197.McBride, J. S., C. I. Newbold, and R. Anand. 1985. Polymorphism of a high molecular weight schizont antigen of the human malaria parasite Plasmodium falciparum. J. Exp. Med. 161:160-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.McBride, J. S., D. Walliker, and G. Morgan. 1982. Antigenic diversity in the human malaria parasite Plasmodium falciparum. Science 217:254-257. [DOI] [PubMed] [Google Scholar]
- 199.McBride, J. S., P. D. Welsby, and D. Walliker. 1984. Serotyping Plasmodium falciparum from acute human infections using monoclonal antibodies. Trans. R. Soc. Trop. Med. Hyg. 78:32-34. [DOI] [PubMed] [Google Scholar]
- 200.McCallum, W. 1897. On the flagellated form of the malarial parasite. Lancet ii:240-1241. [Google Scholar]
- 201.McCallum, W. 1898. On the haematozoan infections of birds J. Exp. Med. 3:117-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.McElroy, P. D., J. C. Beier, C. N. Oster, C. Beadle, J. A. Sherwood, A. J. Oloo, and S. L. Hoffman. 1994. Predicting outcome in malaria: correlation between rate of exposure to infected mosquitoes and level of Plasmodium falciparum parasitemia. Am. J. Trop. Med. Hyg. 51:523-532. [PubMed] [Google Scholar]
- 203.McElroy, P. D., J. C. Beier, C. N. Oster, F. K. Onyango, A. J. Oloo, X. Lin, C. Beadle, and S. L. Hoffman. 1997. Dose- and time-dependent relations between infective Anopheles inoculation and outcomes of Plasmodium falciparum parasitemia among children in western Kenya. Am. J. Epidemiol. 145:945-956. [DOI] [PubMed] [Google Scholar]
- 204.McGhee, R. B., S. D. Singh, and A. B. Weathersby. 1977. Plasmodium gallinaceum: vaccination in chickens. Exp. Parasitol. 43:231-238. [DOI] [PubMed] [Google Scholar]
- 205.McGregor, I. A., and S. P. Carrington. 1963. Treatment of East African P. falciparum malaria with West African human gamma-globulin. Trans. R. Soc. Trop. Med. Hyg. 57:170-175. [Google Scholar]
- 206.McGregor, I. A. 1964. The passive transfer of human malarial immunity. Am. J. Trop. Med. Hyg. 13(Suppl.):237-239. [DOI] [PubMed] [Google Scholar]
- 207.McGregor, I. A. 1993. Towards a vaccine against malaria. Br. J. Biomed. Sci. 50:35-42. [PubMed] [Google Scholar]
- 208.McGregor, I. A., and R. J. M. Wilson. 1988. Specific immunity: acquired in man, p. 559-619. In W. H. Wernsdorfer and I. McGregor (ed.), Malaria. Principles and practice of malariology., vol. 1. Churchill Livingstone, Edinburgh, United Kingdom. [Google Scholar]
- 209.McKenzie, F. E., D. L. Smith, W. P. O'Meara, and E. M. Riley. 2008. Strain theory of malaria: the first 50 years. Adv. Parasitol. 66:1-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Meerman, L., R. Ord, J. T. Bousema, M. van Niekerk, E. Osman, R. Hallett, M. Pinder, G. Walraven, and C. J. Sutherland. 2005. Carriage of chloroquine-resistant parasites and delay of effective treatment increase the risk of severe malaria in Gambian children. J. Infect. Dis. 192:1651-1657. [DOI] [PubMed] [Google Scholar]
- 211.Mendis, K. N., and G. A. Targett. 1979. Immunisation against gametes and asexual erythrocytic stages of a rodent malaria parasite. Nature 277:389-391. [DOI] [PubMed] [Google Scholar]
- 212.Menendez, C. 2006. Malaria during pregnancy. Curr. Mol. Med. 6:269-273. [DOI] [PubMed] [Google Scholar]
- 213.Menendez, C. 1995. Malaria during pregnancy: a priority area of malaria research and control. Parasitol. Today 11:178-183. [DOI] [PubMed] [Google Scholar]
- 214.Menendez, C., E. Kahigwa, R. Hirt, P. Vounatsou, J. J. Aponte, F. Font, C. J. Acosta, D. M. Schellenberg, C. M. Galindo, J. Kimario, H. Urassa, B. Brabin, T. A. Smith, A. Y. Kitua, M. Tanner, and P. L. Alonso. 1997. Randomised placebo-controlled trial of iron supplementation and malaria chemoprophylaxis for prevention of severe anaemia and malaria in Tanzanian infants. Lancet 350:844-850. [DOI] [PubMed] [Google Scholar]
- 215.Mitchell, G. H., G. A. Butcher, and S. Cohen. 1975. Merozoite vaccination against Plasmodium knowlesi malaria. Immunology 29:397-407. [PMC free article] [PubMed] [Google Scholar]
- 216.Mitchell, G. H., G. A. Butcher, and S. Cohen. 1974. A merozoite vaccine effective against Plasmodium knowlesi malaria. Nature 252:311-313. [DOI] [PubMed] [Google Scholar]
- 217.Mitchell, G. H., G. A. Butcher, A. Voller, and S. Cohen. 1976. The effect of human immune IgG on the in vitro development of Plasmodium falciparum. Parasitology 72:149-162. [DOI] [PubMed] [Google Scholar]
- 218.Miura, K., D. B. Keister, O. V. Muratova, J. Sattabongkot, C. A. Long, and A. Saul. 2007. Transmission-blocking activity induced by malaria vaccine candidates Pfs25/Pvs25 is a direct and predictable function of antibody titer. Malar. J. 6:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Miura, K., H. Zhou, O. V. Muratova, A. C. Orcutt, B. Giersing, L. H. Miller, and C. A. Long. 2007. In immunization with Plasmodium falciparum apical membrane antigen 1, the specificity of antibodies depends on the species immunized. Infect. Immun. 75:5827-5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Molineaux, L. 1997. Nature's experiment: what implications for malaria prevention? Lancet 349:1636-1637. [DOI] [PubMed] [Google Scholar]
- 221.Molyneux, M. E., T. E. Taylor, J. J. Wirima, and A. Borgstein. 1989. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children Q. J. Med. 71:441-459. [PubMed] [Google Scholar]
- 222.Mota, M. M., J. C. Hafalla, and A. Rodriguez. 2002. Migration through host cells activates Plasmodium sporozoites for infection. Nat. Med. 8:1318-1322. [DOI] [PubMed] [Google Scholar]
- 223.Mueller, A. K., N. Camargo, K. Kaiser, C. Andorfer, U. Frevert, K. Matuschewski, and S. H. Kappe. 2005. Plasmodium liver stage developmental arrest by depletion of a protein at the parasite-host interface. Proc. Natl. Acad. Sci. USA 102:3022-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mueller, A. K., M. Labaied, S. H. Kappe, and K. Matuschewski. 2005. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature 433:164-167. [DOI] [PubMed] [Google Scholar]
- 225.Muirhead-Thomson, R. C. 1957. The malarial infectivity of an African village population to mosquitoes (Anopheles Gambiae): a random xenodiagnostic survey. Am. J. Trop. Med. Hyg. 6:971-979. [DOI] [PubMed] [Google Scholar]
- 226.Mulligan, H. W., P. Russell, and B. N. Mohan. 1941. Active immunization of fowls against Plasmodium gallinaceum by injections of killed homologous sporozoites. J. Mal. Inst. India 4:25-34. [Google Scholar]
- 227.Mulligan, H. W., and J. A. Sinton. 1933. Studies in immunity in malaria. II. Superinfection with various strains of monkey malarial parasites. Rec. Mal. Surv. India 3:529-568. [Google Scholar]
- 228.Nedelman, J. 1989. Gametocytaemia and infectiousness in falciparum malaria: observations and models. Adv. Dis. Vector Res. 6:59-89. [Google Scholar]
- 229.Newbold, C. I., R. Pinches, D. J. Roberts, and K. Marsh. 1992. Plasmodium falciparum: the human agglutinating antibody response to the infected red cell surface is predominantly variant specific. Exp. Parasitol. 75:281-292. [DOI] [PubMed] [Google Scholar]
- 230.Nussenzweig, R., J. Vanderberg, and H. Most. 1969. Protective immunity produced by the injection of X-irradiated sporozoites of Plasmodium berghei. IV. Dose response, specificity and humoral immunity. Mil. Med. 134:1176-1182. [PubMed] [Google Scholar]
- 231.Nussenzweig, R. S., J. Vanderberg, H. Most, and C. Orton. 1967. Protective immunity produced by the injection of X-irradiated sporozoites of Plasmodium berghei. Nature 216:160-162. [DOI] [PubMed] [Google Scholar]
- 232.Ofori, M. F., D. Dodoo, T. Staalsoe, J. A. Kurtzhals, K. Koram, T. G. Theander, B. D. Akanmori, and L. Hviid. 2002. Malaria-induced acquisition of antibodies to Plasmodium falciparum variant surface antigens. Infect. Immun. 70:2982-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.O'Meara, W. P., T. W. Mwangi, T. N. Williams, F. E. McKenzie, R. W. Snow, and K. Marsh. 2008. Relationship between exposure, clinical malaria, and age in an area of changing transmission intensity. Am. J. Trop. Med. Hyg. 79:185-191. [PMC free article] [PubMed] [Google Scholar]
- 234.Reference deleted.
- 235.Reference deleted.
- 236.Owusu-Agyei, S., K. A. Koram, J. K. Baird, G. C. Utz, F. N. Binka, F. K. Nkrumah, D. J. Fryauff, and S. L. Hoffman. 2001. Incidence of symptomatic and asymptomatic Plasmodium falciparum infection following curative therapy in adult residents of northern Ghana. Am. J. Trop. Med. Hyg. 65:197-203. [DOI] [PubMed] [Google Scholar]
- 237.Petersen, E., B. Hogh, N. T. Marbiah, K. David, and A. P. Hanson. 1991. Development of immunity against Plasmodium falciparum malaria: clinical and parasitologic immunity cannot be separated. J. Infect. Dis. 164:949-953. [DOI] [PubMed] [Google Scholar]
- 238.Phillips, R. S., P. I. Trigg, T. J. Scott-Finnigan, and R. K. Bartholomew. 1972. Culture of Plasmodium falciparum in vitro: a subculture technique used for demonstrating antiplasmodial activity in serum from some Gambians, resident in an endemic malarious area. Parasitology 65:525-535. [DOI] [PubMed] [Google Scholar]
- 239.Phillips-Howard, P. A., B. L. Nahlen, M. S. Kolczak, A. W. Hightower, F. O. ter Kuile, J. A. Alaii, J. E. Gimnig, J. Arudo, J. M. Vulule, A. Odhacha, S. P. Kachur, E. Schoute, D. H. Rosen, J. D. Sexton, A. J. Oloo, and W. A. Hawley. 2003. Efficacy of permethrin-treated bed nets in the prevention of mortality in young children in an area of high perennial malaria transmission in western Kenya. Am. J. Trop. Med. Hyg. 68:23-29. [PubMed] [Google Scholar]
- 240.Pombo, D. J., G. Lawrence, C. Hirunpetcharat, C. Rzepczyk, M. Bryden, N. Cloonan, K. Anderson, Y. Mahakunkijcharoen, L. B. Martin, D. Wilson, S. Elliott, S. Elliott, D. P. Eisen, J. B. Weinberg, A. Saul, and M. F. Good. 2002. Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum. Lancet 360:610-617. [DOI] [PubMed] [Google Scholar]
- 241.Ponnudurai, T., A. H. W. Lensen, G. J. Van Gemert, M. G. Bolmer, and J. H. Meuwissen. 1991. Feeding behaviour and sporozoite ejection by infected Anopheles stephensi. Trans. R. Soc. Trop. Med. Hyg. 85:175-180. [DOI] [PubMed] [Google Scholar]
- 242.Powell, R. D., and J. V. McNamara. 1970. Infection with chloroquine-resistant Plasmodium falciparum in man: prepatent periods, incubation periods, and relationships between parasitemia and the onset of fever in nonimmune persons. Ann. N. Y. Acad. Sci. 174:1027-1041. [DOI] [PubMed] [Google Scholar]
- 243.Powell, R. D., J. V. McNamara, and K. H. Rieckmann. 1972. Clinical aspects of acquisition of immunity to falciparum malaria. Proc. Helminthol. Soc. Wash. 39:51-66. [Google Scholar]
- 244.Pye, D., C. M. O'Brien, P. Franchina, C. Monger, and R. F. Anders. 1994. Plasmodium falciparum infection of splenectomized and intact Guyanan Saimiri monkeys. J. Parasitol. 80:558-562. [PubMed] [Google Scholar]
- 245.Reeder, J. C., K. M. Davern, J. K. Baird, S. J. Rogerson, and G. V. Brown. 1997. The age-specific prevalence of Plasmodium falciparum in migrants to Irian Jaya is not attributable to agglutinating antibody repertoire. Acta Trop. 65:163-173. [DOI] [PubMed] [Google Scholar]
- 246.Reeder, J. C., S. J. Rogerson, F. al-Yaman, R. F. Anders, R. L. Coppel, S. Novakovic, M. P. Alpers, and G. V. Brown. 1994. Diversity of agglutinating phenotype, cytoadherence, and rosette-forming characteristics of Plasmodium falciparum isolates from Papua New Guinean children. Am. J. Trop. Med. Hyg. 51:45-55. [DOI] [PubMed] [Google Scholar]
- 247.Reyburn, H., R. Mbatia, C. Drakeley, J. Bruce, I. Carneiro, R. Olomi, J. Cox, W. M. Nkya, M. Lemnge, B. M. Greenwood, and E. M. Riley. 2005. Association of transmission intensity and age with clinical manifestations and case fatality of severe Plasmodium falciparum malaria. JAMA 293:1461-1470. [DOI] [PubMed] [Google Scholar]
- 248.Richards, W. G. H. 1966. Active immunization of chicks against Plasmodium gallinacium by inactivated homologous sporozoites and erythrocytic parasites. Nature 212:1492-1494. [DOI] [PubMed] [Google Scholar]
- 249.Rickman, L. S., T. R. Jones, G. W. Long, S. Paparello, I. Schneider, C. F. Paul, R. L. Beaudoin, and S. L. Hoffman. 1990. Plasmodium falciparum-infected Anopheles stephensi inconsistently transmit malaria to humans. Am. J. Trop. Med. Hyg. 43:441-445. [DOI] [PubMed] [Google Scholar]
- 250.Rieckmann, K. H., R. L. Beaudoin, J. S. Cassells, and D. W. Sell. 1979. Use of attenuated sporozoites in the immunization of human volunteers against falciparum malaria. Bull. W. H. O. 57:261-265. [PMC free article] [PubMed] [Google Scholar]
- 251.Rieckmann, K. H., P. E. Carson, R. L. Beaudoin, J. S. Cassells, and K. W. Sell. 1974. Sporozoite induced immunity in man against an Ethiopian strain of Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 68:258-259. [DOI] [PubMed] [Google Scholar]
- 252.Rieckmann, K. H., D. R. Davis, and D. C. Hutton. 1989. Plasmodium vivax resistance to chloroquine? Lancet ii:1183-1184. [DOI] [PubMed] [Google Scholar]
- 253.Rigdon, R. H., and H. R. Rudicell. 1945. Effect of radiation on malaria. An experimental study in the chick and duck. Proc. Soc. Exp. Biol. Med. 59:167-170. [Google Scholar]
- 254.Riley, E. M., G. E. Wagner, M. F. Ofori, J. G. Wheeler, B. D. Akanmori, K. Tetteh, D. McGuinness, S. Bennett, F. K. Nkrumah, R. F. Anders, and K. A. Koram. 2000. Lack of association between maternal antibody and protection of African infants from malaria infection. Infect. Immun. 68:5856-5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Roberts, D. J., A. G. Craig, A. R. Berendt, R. Pinches, G. Nash, K. Marsh, and C. I. Newbold. 1992. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature 357:689-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Rogerson, S. J., L. Hviid, P. E. Duffy, R. F. Leke, and D. W. Taylor. 2007. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect. Dis. 7:105-117. [DOI] [PubMed] [Google Scholar]
- 257.Rogerson, S. J., V. Mwapasa, and S. R. Meshnick. 2007. Malaria in pregnancy: linking immunity and pathogenesis to prevention. Am. J. Trop. Med. Hyg. 77:14-22. [PubMed] [Google Scholar]
- 258.Rogerson, S. J., E. Pollina, A. Getachew, E. Tadesse, V. M. Lema, and M. E. Molyneux. 2003. Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am. J. Trop. Med. Hyg. 68:115-119. [PubMed] [Google Scholar]
- 259.Romi, R., M. C. Razaiarimanga, R. Raharimanga, E. M. Rakotondraibe, L. H. Ranaivo, V. Pietra, A. Raveloson, and G. Majori. 2002. Impact of the malaria control campaign (1993-1998) in the highlands of Madagascar: parasitological and entomological data. Am. J. Trop. Med. Hyg. 66:2-6. [DOI] [PubMed] [Google Scholar]
- 260.Rosenberg, R., R. A. Wirtz, I. Schneider, and R. Burge. 1990. An estimation of the number of malaria sporozoites ejected by a feeding mosquito. Trans. R. Soc. Trop. Med. Hyg. 84:209-212. [DOI] [PubMed] [Google Scholar]
- 261.Ross, R. 1899. Mosquitoes and malaria. Br. Med. J. 432-433.
- 262.Roussilhon, C., T. Fandeur, and J. P. Dedet. 1988. Long-term protection of squirrel monkeys (Saimiri sciureus) against Plasmodium falciparum challenge inoculations after various time intervals. Parasitol. Res. 75:118-122. [DOI] [PubMed] [Google Scholar]
- 263.Russell, P. F., and B. N. Mohan. 1942. The immunization of fowls against mosquito-borne Plasmodium gallinaceum by injections of serum and of inactiviated homologous sporozoites. J. Exp. Med. 76:477-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Russell, P. F., H. W. Mulligan, and B. N. Mohan. 1941. Specific agglutinating properties of inactivated sporozoites of P. gallinaceum. J. Malar. Inst. India 4:15-24. [Google Scholar]
- 265.Sabatinelli, G., G. Majori, F. D'Ancona, and R. Romi. 1994. Malaria epidemiological trends in Italy. Eur. J. Epidemiol. 10:399-403. [DOI] [PubMed] [Google Scholar]
- 266.Sabchareon, A., T. Burnouf, D. Ouattara, P. Attanath, H. Bouharoun Tayoun, P. Chantavanich, C. Foucault, T. Chongsuphajaisiddhi, and P. Druilhe. 1991. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am. J. Trop. Med. Hyg. 45:297-308. [DOI] [PubMed] [Google Scholar]
- 267.Sadun, E. H., R. L. Hickman, B. T. Wellde, A. P. Moon, and I. O. Udeozo. 1966. Active and passive immunization of chimpanzees infected with West African and Southeast Asian strains of Plasmodium falciparum. Mil. Med. 131(Suppl.):1250-1262. [PubMed] [Google Scholar]
- 268.Sauerwein, R. W. 2007. Malaria transmission-blocking vaccines: the bonus of effective malaria control. Microbes Infect. 9:792-795. [DOI] [PubMed] [Google Scholar]
- 269.Schellenberg, D., C. Menendez, J. J. Aponte, E. Kahigwa, M. Tanner, H. Mshinda, and P. Alonso. 2005. Intermittent preventive antimalarial treatment for Tanzanian infants: follow-up to age 2 years of a randomised, placebo-controlled trial. Lancet 365:1481-1483. [DOI] [PubMed] [Google Scholar]
- 270.Schellenberg, D., C. Menendez, E. Kahigwa, J. Aponte, J. Vidal, M. Tanner, H. Mshinda, and P. Alonso. 2001. Intermittent treatment for malaria and anaemia control at time of routine vaccinations in Tanzanian infants: a randomised, placebo-controlled trial. Lancet 357:1471-1477. [DOI] [PubMed] [Google Scholar]
- 271.Schenkel, R. H., G. L. Simpson, and P. H. Silverman. 1973. Vaccination of Rhesus monkeys (Macaca mulatta) against Plasmodium knowlesi by the use of nonviable antigen. Bull. W. H. O. 48:597-604. [PMC free article] [PubMed] [Google Scholar]
- 272.Schofield, L., and I. Mueller. 2006. Clinical immunity to malaria. Curr. Mol. Med. 6:205-221. [DOI] [PubMed] [Google Scholar]
- 273.Schofield, L., J. Villaquiran, A. Ferreira, H. Schellekens, R. S. Nussenzweig, and V. Nussenzweig. 1987. Gamma-interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature 330:664-666. [DOI] [PubMed] [Google Scholar]
- 274.Schuffner, W. A. P. 1919. Two subjects relating to the epidemiology of malaria. Mededeelingen van der Burgerlijken Geneeskundingen Dienst in Nederlandsche-Indie 9:1-34. [Google Scholar]
- 275.Schwartz, E., S. Sadetzki, H. Murad, and D. Raveh. 2001. Age as a risk factor for severe Plasmodium falciparum malaria in nonimmune patients. Clin. Infect. Dis. 33:1774-1777. [DOI] [PubMed] [Google Scholar]
- 276.Sergent, E., and L. Parrot. 1935. L'immunite, la premunition et la resistance innee. Arch. Inst. Pasteur Algerie 13:279-319. [Google Scholar]
- 277.Sergente, T., and D. Sergente. 1910. Sur l'immunité dans le paludisme des oiseaux. Conservation in vitro de sporozoïtes de Plasmodium relictum. Immunité relative obtenue par inoculation de ces sporozoïtes. C. R. Acad. Sci. 151:407. [Google Scholar]
- 278.Sexton, J. D., T. K. Ruebush II, A. D. Brandling-Bennett, J. G. Breman, J. M. Roberts, J. S. Odera, and J. B. Were. 1990. Permethrin-impregnated curtains and bed-nets prevent malaria in western Kenya. Am. J. Trop. Med. Hyg. 43:11-18. [DOI] [PubMed] [Google Scholar]
- 279.Siddiqui, W. A. 1977. An effective immunization of experimental monkeys against a human malaria parasite, Plasmodium falciparum. Science 197:388-389. [DOI] [PubMed] [Google Scholar]
- 280.Siddiqui, W. A., D. W. Taylor, S. C. Kan, K. Kramer, and S. Richmond-Crum. 1978. Partial protection of Plasmodium falciparum-vaccinated Aotus trivirgatus against a challenge of a heterologous strain. Am. J. Trop. Med. Hyg. 27:1277-1278. [DOI] [PubMed] [Google Scholar]
- 281.Sigler, C. L., P. Leland, and M. R. Hollingdale. 1984. In vitro infectivity of irradiated Plasmodium berghei sporozoites to cultured hepatoma cells. Am. J. Trop. Med. Hyg. 33:544-547. [DOI] [PubMed] [Google Scholar]
- 282.Simpson, G. L., R. H. Schenkel, and P. H. Silverman. 1974. Vaccination of rhesus monkeys against malaria by use of sucrose density gradient fractions of Plasmodium knowlesi antigens. Nature 247:304-305. [DOI] [PubMed] [Google Scholar]
- 283.Singh, B., L. Kim Sung, A. Matusop, A. Radhakrishnan, S. S. Shamsul, J. Cox-Singh, A. Thomas, and D. J. Conway. 2004. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet 363:1017-1024. [DOI] [PubMed] [Google Scholar]
- 284.Sinton, J. A. 1940. Studies of infections with Plasmodium ovale. IV. The efficacy and nature of immunity acquired as a result of infections induces by sporozoite inoculations as compared with those by trophozoite injections. Trans. R. Soc. Trop. Med. Hyg. 33:439-446. [Google Scholar]
- 285.Sinton, J. A. 1939. A summary of our present knowledge of the mechanisms of immunity in malaria. J. Mal. Inst. India 2:71-83. [Google Scholar]
- 286.Sinton, J. A., and J. Harbhagwan. 1935. Studies in immunity in malaria. IV. The results of multiple heterologous superinfections, with a discussion of their relationship to some epidemiological problems and to the general principles of treatment. Rec. Mal. Surv. India 5:307-334. [Google Scholar]
- 287.Smith, J. D., C. E. Chitnis, A. G. Craig, D. J. Roberts, D. E. Hudson-Taylor, D. S. Peterson, R. Pinches, C. I. Newbold, and L. H. Miller. 1995. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell 82:101-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Smith, T., B. Genton, K. Baea, N. Gibson, J. Taime, A. Narara, F. Al-Yaman, H. P. Beck, J. Hii, and M. Alpers. 1994. Relationships between Plasmodium falciparum infection and morbidity in a highly endemic area. Parasitology 109:539-549. [DOI] [PubMed] [Google Scholar]
- 289.Snow, R. W., C. A. Guerra, A. M. Noor, H. Y. Myint, and S. I. Hay. 2005. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434:214-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Snow, R. W., and K. Marsh. 2002. The consequences of reducing transmission of Plasmodium falciparum in Africa. Adv. Parasitol. 52:235-264. [DOI] [PubMed] [Google Scholar]
- 291.Snow, R. W., C. S. Molyneux, P. A. Warn, J. Omumbo, C. G. Nevill, S. Gupta, and K. Marsh. 1996. Infant parasite rates and immunoglobulin M seroprevalence as a measure of exposure to Plasmodium falciparum during a randomized controlled trial of insecticide-treated bed nets on the Kenyan coast. Am. J. Trop. Med. Hyg. 55:144-149. [PubMed] [Google Scholar]
- 292.Snow, R. W., J. A. Omumbo, B. Lowe, C. S. Molyneux, J. O. Obiero, A. Palmer, M. W. Weber, M. Pinder, B. Nahlen, C. Obonyo, C. Newbold, S. Gupta, and K. Marsh. 1997. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet 349:1650-1654. [DOI] [PubMed] [Google Scholar]
- 293.Sotiriades, D. 1936. Passive immunity in experimental and natural malaria. J. Trop. Med. 39:257. [Google Scholar]
- 294.Su, X. Z., V. M. Heatwole, S. P. Wertheimer, F. Guinet, J. A. Herrfeldt, D. S. Peterson, J. A. Ravetch, and T. E. Wellems. 1995. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell 82:89-100. [DOI] [PubMed] [Google Scholar]
- 295.Sutherland, C. J., C. J. Drakeley, and D. Schellenberg. 2007. How is childhood development of immunity to Plasmodium falciparum enhanced by certain antimalarial interventions? Malar. J. 6:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Taliaferro, W. H., and L. G. Taliaferro. 1929. Acquired immunity in avian malaria. II. The absence of protective antibodies in immunity to superinfection. J. Prev. Med. 3:197. [Google Scholar]
- 297.Targett, G. A., and J. D. Fulton. 1965. Immunization of rhesus monkeys against Plasmodium knowlesi malaria. Exp. Parasitol. 17:180-193. [DOI] [PubMed] [Google Scholar]
- 298.Taylor-Robinson, A. 2001. Population migration and malaria: terms of reference. Trends Parasitol. 17:315. [DOI] [PubMed] [Google Scholar]
- 299.ter Kuile, F. O., D. J. Terlouw, P. A. Phillips-Howard, W. A. Hawley, J. F. Friedman, M. S. Kolczak, S. K. Kariuki, Y. P. Shi, A. M. Kwena, J. M. Vulule, and B. L. Nahlen. 2003. Impact of permethrin-treated bed nets on malaria and all-cause morbidity in young children in an area of intense perennial malaria transmission in western Kenya: cross-sectional survey. Am. J. Trop. Med. Hyg. 68:100-107. [PubMed] [Google Scholar]
- 300.Tjitra, E., N. M. Anstey, P. Sugiarto, N. Warikar, E. Kenangalem, M. Karyana, D. A. Lampah, and R. N. Price. 2008. Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. PLoS Med. 5:890-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Trape, J. F., E. Lefebvre-Zante, F. Legros, P. Druilhe, C. Rogier, H. Bouganali, and G. Salem. 1993. Malaria morbidity among children exposed to low seasonal transmission in Dakar, Senegal and its implications for malaria control in tropical Africa. Am. J. Trop. Med. Hyg. 48:748-756. [DOI] [PubMed] [Google Scholar]
- 302.Trape, J. F., P. Peelman, and B. Morault-Peelman. 1985. Criteria for diagnosing clinical malaria among a semi-immune population exposed to intense and perennial transmission. Trans. R. Soc. Trop. Med. Hyg. 79:435-442. [DOI] [PubMed] [Google Scholar]
- 303.Trape, J. F., and C. Rogier. 1996. Combating malaria morbidity and mortality by reducing transmission. Parasitol. Today 12:236-240. [DOI] [PubMed] [Google Scholar]
- 304.Trape, J. F., C. Rogier, L. Konate, N. Diagne, H. Bouganali, B. Canque, F. Legros, A. Badji, G. Ndiaye, P. Ndiaye, K. Brahimi, O. Faye, P. Druilhe, and L. P. Da Silva. 1994. The Dielmo project: a longitudinal study of natural malaria infection and the mechanisms of protective immunity in a community living in a holoendemic area of Senegal. Am. J. Trop. Med. Hyg. 51:123-137. [DOI] [PubMed] [Google Scholar]
- 305.Udeinya, I. J., L. H. Miller, I. A. McGregor, and J. B. Jensen. 1983. Plasmodium falciparum strain-specific antibody blocks binding of infected erythrocytes to amelanotic melanoma cells. Nature 303:429-431. [DOI] [PubMed] [Google Scholar]
- 306.Vanderberg, J. P. 1977. Plasmodium berghei: quantitation of sporozoites injected by mosquitoes feeding on a rodent host. Exp. Parasitol. 42:169-181. [DOI] [PubMed] [Google Scholar]
- 307.Vanderberg, J. P., and U. Frevert. 2004. Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int. J. Parasitol. 34:991-996. [DOI] [PubMed] [Google Scholar]
- 308.van Dijk, M. R., B. Douradinha, B. Franke-Fayard, V. Heussler, M. W. van Dooren, B. van Schaijk, G. J. van Gemert, R. W. Sauerwein, M. M. Mota, A. P. Waters, and C. J. Janse. 2005. Genetically attenuated, P36p-deficient malarial sporozoites induce protective immunity and apoptosis of infected liver cells. Proc. Natl. Acad. Sci. USA 102:12194-12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Vekemans, J., and W. R. Ballou. 2008. Plasmodium falciparum malaria vaccines in development. Expert Rev. Vaccines 7:223-240. [DOI] [PubMed] [Google Scholar]
- 310.Verhage, D. F., D. S. Telgt, J. T. Bousema, C. C. Hermsen, G. J. van Gemert, J. W. van der Meer, and R. W. Sauerwein. 2005. Clinical outcome of experimental human malaria induced by Plasmodium falciparum-infected mosquitoes. Neth. J. Med. 63:52-58. [PubMed] [Google Scholar]
- 311.Vleugels, M. P., W. M. Eling, R. Rolland, and R. de Graaf. 1987. Cortisol and loss of malaria immunity in human pregnancy. Br. J. Obstet. Gynaecol. 94:758-764. [DOI] [PubMed] [Google Scholar]
- 312.Voller, A., and W. H. Richards. 1968. An attempt to vaccinate owl monkeys (Aotus trivirgatus) against falciparum malaria. Lancet ii:1172-1174. [DOI] [PubMed] [Google Scholar]
- 313.Voller, A., and R. N. Rossan. 1969. Immunological studies on simian malaria. III. Immunity to challenge and antigenic variation in P. knowlesi. Trans. R. Soc. Trop. Med. Hyg. 63:507-523. [PubMed] [Google Scholar]
- 314.Waki, S., I. Yonome, and M. Suzuki. 1986. Plasmodium yoelii: induction of attenuated mutants by irradiation. Exp. Parasitol. 62:316-321. [DOI] [PubMed] [Google Scholar]
- 315.Walliker, D. 1991. Malaria parasites: randomly interbreeding or ‘clonal’ populations? Parasitol. Today 7:232-235. [DOI] [PubMed] [Google Scholar]
- 316.Weiss, W. R., M. Sedegah, R. L. Beaudoin, L. H. Miller, and M. F. Good. 1988. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc. Natl. Acad. Sci. USA 85:573-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Wellde, B. T., and E. H. Sadun. 1967. Resistance produced in rats and mice by exposure to irradiated Plasmodium berghei. Exp. Parasitol. 21:310-324. [DOI] [PubMed] [Google Scholar]
- 318.Wilson, R. J., and R. S. Phillips. 1976. Method to test inhibitory antibodies in human sera to wild populations of Plasmodium falciparum. Nature 263:132-134. [DOI] [PubMed] [Google Scholar]
- 319.Wipasa, J., and E. M. Riley. 2007. The immunological challenges of malaria vaccine development. Expert Opin. Biol. Ther. 7:1841-1852. [DOI] [PubMed] [Google Scholar]
- 320.Wolfson, F., and O. R. Causey. 1939. Immunity to superinfection with sporozoites in two strains of Plasmodium cathemerium. J. Parasitol. 25:510-511. [Google Scholar]
- 321.World Health Organization. 2000. Severe falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 94:S1-S90. [PubMed] [Google Scholar]
- 321a.World Health Organization. 2008. Global malaria control and elimination: report of a technical review. www.who.int/malaria/docs/elimination/MalariaControlEliminationMeeting.pdf.
- 321b.World Health Organization. 2005. World malaria report 2005. http://rbm.who.int/wmr2005/index.html.
- 322.Wykes, M., and M. F. Good. 2007. A case for whole-parasite malaria vaccines. Int. J. Parasitol. 37:705-712. [DOI] [PubMed] [Google Scholar]
- 323.Yamauchi, L. M., A. Coppi, G. Snounou, and P. Sinnis. 2007. Plasmodium sporozoites trickle out of the injection site. Cell. Microbiol. 9:1215-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Yone, C. L., P. G. Kremsner, and A. J. Luty. 2005. Immunoglobulin G isotype responses to erythrocyte surface-expressed variant antigens of Plasmodium falciparum predict protection from malaria in African children. Infect. Immun. 73:2281-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Yorke, W., and J. W. S. Macfie. 1924. Observations of malaria made during treatment of general paralysis. Trans. R. Soc. Trop. Med. Hyg. 18:13-44. [Google Scholar]
- 326.Yount, E. H., and L. T. Coggeshall. 1949. Status of immunity following cure of recurrent vivax malaria. Am. J. Trop. Med. Hyg. 29:701-705. [DOI] [PubMed] [Google Scholar]