Abstract
Summary: The human gingival niche is a unique microbial habitat. In this habitat, biofilm organisms exist in harmony, attached to either enamel or cemental surfaces of the tooth as well as to the crevicular epithelium, subjacent to a rich vascular plexus underneath. Due to this extraordinary anatomical juxtaposition, plaque biofilm bacteria have a ready portal of ingress into the systemic circulation in both health and disease. Yet the frequency, magnitude, and etiology of bacteremias due to oral origin and the consequent end organ infections are not clear and have not recently been evaluated. In this comprehensive review, we address the available literature on triggering events, incidence, and diversity of odontogenic bacteremias. The nature of the infective agents and end organ infections (other than endocarditis) is also described, with an emphasis on the challenge of establishing the link between odontogenic infections and related systemic, focal infections.
INTRODUCTION
Recent advances in bacterial identification methods, particularly culture independent approaches such as 16S rRNA gene sequencing, have shown that oral niches are inhabited by more than 6 billion bacteria representing in excess of 700 species belonging to at least nine different phyla (1, 33, 36, 70, 96). Since some 30 to 40% of the bacteria normally residing in the human mouth remain to be identified, the 16S rRNA sequencing approach also provides a tool to arbitrarily estimate the diversity of these organisms (24). The bacterial flora of the mouth, like most other resident floras of the human body, such as those of the skin and the gut, exhibits commensalism, a survival mechanism that benefits the microbes without harming the host. Yet these largely innocuous commensal residents of the human body have the propensity to become pathogenic in the event of translocation to a different niche (58).
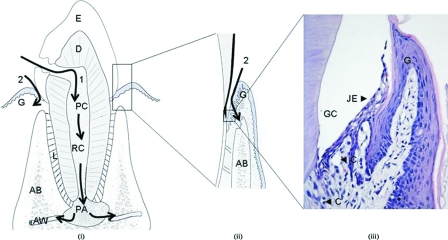
Oral commensals, particularly those residing in periodontal niches, commonly exist in the form of biofilm communities on either nonshedding surfaces, such as the teeth or prostheses, or shedding surfaces, such as the epithelial linings of gingival crevices or periodontal pockets. A feature that is unique to the oral bacterial biofilm, particularly the subgingival plaque biofilm, is its close proximity to a highly vascularized milieu (90). This environment is different from other sites where bacteria commonly reside in the human body. For example, the ingress of cutaneous flora into the circulation is prevented by the relatively thick and impermeable keratinized layers of the skin, while the mucosal flora of the gastrointestinal and genitourinary tracts is commandeered by the rich submucosal lymphatics, which keep microorganisms under constant check. Their covering epithelia are continuously shed at a quick rate, denying prolonged colonization by the bacterial flora. Although innate defense by polymorphonuclear neutrophils is highly developed at the dentogingival junction and backed up by a highly organized lymphatic system, the oral biofilms, if left undisturbed, can establish themselves permanently on nonshedding tooth surfaces subjacent to the dentogingival junction. Under these circumstances, any disruption of the natural integrity between the biofilm and the subgingival epithelium, which is at most about 10 cell layers thick, could lead to a bacteremic state (Fig. 1) (116).
FIG. 1.
Possible routes of bacterial entry from teeth into the systemic circulation. Pathway 1, entry via the root canal (RC) or from periapical lesions (PA) into the alveolar blood vessels (AW); pathway 2, entry from the periodontium, where bacteria in the gingival crevices (GC) translocate to the capillaries (C) in the gingival connective tissues, possibly through the junctional epithelium (JE). E, enamel; D, dentine; L, periodontal ligament; and AB, alveolar bone. (Panels i and ii are modified from reference 116 with permission from Elsevier. Panel iii is courtesy of Lu Qian [Oral Bio-Sciences, Faculty of Dentistry].) Note that the junctional epithelium was detached from the enamel due to processing for microscopy.
All too often, in common inflammatory conditions such as gingivitis and chronic periodontitis, which are precipitated by the buildup of plaque biofilms, the periodontal vasculature proliferates and dilates, providing an even greater surface area that facilitates the entry of microorganisms into the bloodstream. Often, these bacteremias are short-lived and transient, with the highest intensity limited to the first 30 min after a trigger episode, as discussed in the following sections (Fig. 2). On occasions, this may lead to seeding of organisms in different target organs, resulting in subclinical, acute, or chronic infections. Bacterial endocarditis is a well-known complication of such bacteremias of odontogenic origin and has been a matter of great concern for dentists, cardiologists, and microbiologists alike for many a decade. The literature and guidelines on the prevention and management of bacteremia of odontogenic origin have been under constant review, particularly with regard to prophylactic antibiotics and dental procedures, with differing opinions expressed on both sides of the Atlantic, particularly in recent months (51, 108, 143). Yet there are a number of other organs and body sites that may be affected by focal bacteremic spread from the oral cavity. Hence, the focus of this review is on issues that are unrelated to the connectivity between bacterial endocarditis and odontogenic bacteremias, with emphasis on other local and systemic morbidities due to such bacteremias.
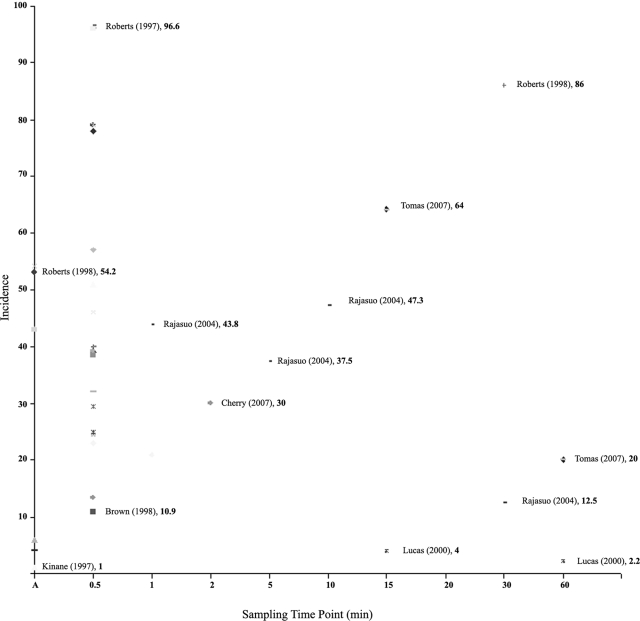
FIG. 2.
Chart indicating the time course distribution of the incidence of odontogenic bacteremias from 50 studies reported in the literature. The lowest and highest incidences reported at a given time point are annotated in the chart, with the percentage shown in bold. (Note that at time point A the sampling was done during the intervention or immediately thereafter.)
Bacterial Entry into the Bloodstream
Based on the current evidence, it is likely that bacteria gain entry into the bloodstream from oral niches through a number of mechanisms and a variety of portals. First, and most commonly, when there is tissue trauma induced by procedures such as periodontal probing, scaling, instrumentation beyond the root apex, and tooth extractions, a breakage in capillaries and other small blood vessels that are located in the vicinity of the plaque biofilms may lead to spillage of bacteria into the systemic circulation (Fig. 1). Obviously, a higher microbial load would facilitate such dissemination, as it is known that individuals with poor oral hygiene are at a higher risk of developing bacteremias during oral manipulative procedures (48). Second, innate microbial factors may play a role in the latter phenomenon, as only a few species are detected in experimental bacteremias despite the multitude of diverse bacteria residing within the periodontal biofilm. Species that are commonly found in the bloodstream have virulence attributes that could be linked to vascular invasion (Table 1). Of particular note are attributes such as endothelial adhesion of Streptococcus spp., degradation of intercellular matrices by Porphyromonas gingivalis, and impedance of phagocytic activity by Aggregatibacter actinomycetemcomitans and Fusobacterium nucleatum.
TABLE 1.
Major bacterial species recovered from odontogenic bacteremias and putative virulence factors thought to facilitate their entry and survival in blood
| Organism | Factor, function, and/or characteristic | Reference |
|---|---|---|
| Streptococcus mutans | Ability to switch peripheral blood monocytes to dendritic cells which exhibit large numbers of adhesion molecules, such as ICAM-1, ICAM-2, and LFA-1, contributing to adhesion to the injured endothelium and to fibrinogen in blood clots | 52 |
| Porphyromonas gingivalis | Cysteine proteinase (gingipain); degrades extracellular matrix proteins, such as laminin, fibronectin, and type IV collagen | 6 |
| Abiotrophia defectiva | Strains associated with endocarditis in humans show a higher fibronectin-binding ability than that of related species | 122 |
| Aggregatibacter actinomycetemcomitans and Fusobacterium nucleatum | Inhibits binding of chemotactic peptides to neutrophils | 136 |
| A. actinomycetemcomitans | EmaA (extracellular matrix protein adhesin A); forms antenna-like protrusions associated with the surface and mediates adhesion to collagen | 115 |
| Streptococcus intermedius | Hyaluronidase and chondroitin sulfate depolymerase break the ground substance in connective tissues, facilitating spread | 123 |
| Streptococcus anginosus | Binds to the endothelium, the basement membrane, and collagen | 4 |
| Streptococcus sanguis | Platelet aggregation-associated protein; enhances platelet accumulation | 52 |
| Porphyromonas gingivalis | Porin-like activity of the outer membrane molecules (probably lipopolysaccharide) can depolarize neutrophils and immobilize polymorphonuclear leukocyte responses | 91 |
| Viridans streptococci | Secreted factors could increase the production of interleukin-8, which is particularly important in lung pathology, such as acute respiratory distress syndrome (ARDS) | 125 |
OBJECTIVES
The focus of this review is threefold. The first aim is to evaluate the evidence base on triggering mechanisms and underlying predisposing factors that lead to odontogenic bacteremias, the second aim is to determine the diversity and frequency of causative bacterial species in relation to various oral manipulative procedures, and the final aim is to assess the role of odontogenic bacteremia as a causative factor in systemic and end organ infections other than endocardial infections. We have particularly focused on systemic or end organ affections other than bacterial endocarditis, primarily because the pathogenesis of endocarditis secondary to odontogenic bacteremia has been reviewed extensively elsewhere (for a recent review, see reference 40).
Search Strategy
We searched the PubMed database for articles on bacteremia in human subjects, limiting the search to articles published in English. In order to elicit the events that lead to bacteremia of oral origin, the search query was worded “bacteremia,” coupled with “procedure” and the Boolean operator “and.” Bacterial diversity in the mouth was studied by analyzing the records cited in the database that had adopted the rRNA sequence-based phylogenetic approach, while diversity of oral bacteremias was appraised by combining the search for “bacterial genera” with the term “bacteremia.” On all occasions, records were sorted according to relevance, and if it was deemed necessary, references cited in a given record were further analyzed. In total, 170 citations were derived for the search “bacteremia and procedure.” By relevance, 6 citations in relation to physiological and personal hygiene activities, 13 regarding periodontal procedures, 19 regarding different modalities of tooth extractions, 5 regarding orthodontic appliances, 3 regarding endodontic procedures, and 5 regarding miscellaneous procedures were further appraised. In relation to systemic affections consequent to odontogenic bacteremia, 27 citations were evaluated based on relevance.
HISTORICAL PERSPECTIVES
The link between dental procedures and resultant bacteremias has been under scrutiny for more than half a century. Some early work dates back to the 1930s, when it was found that the extent of gum disease has a confounding effect on the development of bacteremia following dental extractions. In one of the earliest reports, in 1939, Elliot (41) noted in an article to the Royal Society of Medicine in the United Kingdom that the incidence of postextraction bacteremia due to streptococci was increased when the condition of the periodontal health declined from minimally detectable gum disease to moderate and marked periodontitis. Furthermore, the intensity of the bacteremia was also observed to be highest among subjects with periodontal disease (41). In subsequent studies, the decline in incidence and the efficacy of antimicrobials, such as sulfanilamide, that were in use during the period in reducing bacteremias were evaluated, and the higher incidence of viridans group streptococcal bacteremias was reported (10). In 1954, Cobe evaluated the degree of dental and periodontal trauma that resulted in bacteremia in a cohort of 1,350 subjects assigned to five groups based on operative or surgical procedure. His findings in this landmark study indicated the highest incidence of bacteremia with periodontal cleaning (40%), followed by exodontia (tooth extraction) (35%), brushing (24%), and hard mastication (chewing hard candy) (17%) (25). The more “innovative” approach of other early investigators, who inoculated hapless subjects with bacteria and lytic enzymes to evaluate the degree of bacteremia, cannot even be contemplated in the present-day context of medical ethics (17, 25)! Nevertheless, these studies helped to highlight the significance and importance of the gingival crevice as a portal of bacterial entry into the bloodstream.
TRIGGERING FACTORS IN ODONTOGENIC BACTEREMIA
Any disruption of the finely and harmoniously balanced bacterial biofilm within the gingival tissue niche leads to dissemination of organisms into the bloodstream (116). Not only oral procedures such as periodontal probing, scaling, root planing, and tooth extractions but also routine oral hygiene activities such as brushing, flossing, and other physiological phenomena, such as chewing, can be assumed to be disruptive to the delicate anatomical and functional barrier between the oral biofilm and the host tissues. For these reasons, it can be anticipated that the presence of inflammation in situations such as periodontitis, gingivitis, pulpal or root canal infections, or oral trauma by poorly fabricated appliances poses an increased risk of bacteremia, especially following mechanical manipulations of the oral cavity.
The literature reveals a vast number of studies on the factors triggering odontogenic bacteremia, varying from prospective controlled studies to clinical trials and case reports. As described in detail below, these fall essentially into bacteremias related to the following: chewing, personal oral hygiene measures such as toothbrushing, periodontal procedures such as scaling and root planing, tooth extractions, insertion of orthodontic appliances, endodontic procedures, and miscellaneous procedures such as sialography (Table 2).
TABLE 2.
Summary of clinical trials and controlled studies on odontogenic bacteremias from 1966 to 2007 (in chronological order)
| Procedure studied and parameters under investigation (yr, author [reference]) | No. and description of subjects | Sampling time (vol) | Culture method/identification technique | Incidence (%) | Intensity (no. of colonies), diversity (no. of species), and/or predominant species | Remarks |
|---|---|---|---|---|---|---|
| Physical maneuvers and cleaning procedures | ||||||
| Brushing performed by the investigator, using an electric tooth brush (1973, Sconyers [120]) | 30 subjects who were physically healthy and had periodontitis | Baseline and at the fourth min of brushing (10 ml) | Broth culture with standard identification tests | 16.7 | 4 of 5 positive cultures were Streptococcus mitis | Anaerobic cultures yielded a higher rate of recovery of organisms than aerobic cultures did |
| Routine dental cleaning and prophylaxis (1974, de Leo [32]) | 39 children between 7 and 12 yr of age | Pre- and postprocedure (6 to 12 ml) | Standard broth cultures and identification methods | Preprophylaxis, 5.1; postprophylaxis, 28.2 | 28 positive cultures and 8 different strains with 11 diphtheroid strains | While diphtheroids comprise oral commensals, these could be skin contaminants |
| Different tooth cleaning procedures (2000, Lucas [80]) | 155 (brushing, 52; cleaning, 53; scaling, 50) | Baseline and 30 s after cleaning (8 ml) | Automated broth cultures, lysis centrifugation, standard API biochemical methods | Brushing, 39; cleaning, 25; scaling, 40 | S. mitis, S. sanguis, and coagulase-negative staphylococci | All three predominant isolates are known agents of odontogenic endocarditis |
| Comparison of electric and manual toothbrushes used under general anesthesia (2002, Bhanji [12]) | 50 healthy children (manual toothbrush, 24; Sonicare [electric] toothbrush, 23; 3 were excluded) | After induction and 30 s after brushing (10 ml) | Automated (Bactec 9240) aerobic and anaerobic culture system; standard biochemical tests | Manual, 46; Sonicare, 78 | 32 isolates; 23 were Streptococcus spp. | Sonicare toothbrushing induced a higher degree of bacteremia, indicating that the degree of mechanical disruption is an important determinant of bacteremia |
| Brushing with soft-bristled toothbrush (2005, Hartzell [56]) | 30 physically healthy adults | Baseline and 30 s and 20 min after brushing | Blood culture bottles, standard identification methods | 0 | 3 of the 180 blood cultures were positive for Propionibacterium acnes | Brushing in healthy individuals is not an important cause of bacteremia; the positive cultures were regarded as skin contaminants |
| Chewing, toothbrushing, and scaling (2006, Forner [48]) | 60 (healthy individuals, 20; patients with gingivitis, 20; patients with periodontitis, 20) | Baseline and at 0.5, 10, and 30 min (9 ml) | Lysis filtration, anaerobic culture, standard biochemical tests, gdh and 16S rRNA gene partial sequencing | Healthy patients (scaling), 10; patients with gingivitis (scaling), 20; patients with periodontitis (scaling), 75 (brushing), 5 (chewing) 20 | 163 positive cultures, 23 different species; 36 of the cultures were streptococci | Periodontitis is a more important predictor of bacteremia than gingivitis with these procedures |
| Periodontal procedures | ||||||
| Split mouth expt to study the effects of gingival irrigation with sterile water (control) and tap water (test) on bacteremia following ultrasonic scaling (1982, Reinhardt [106]) | 30 healthy adults | Baseline and immediately postoperative (10 ml) | Broth cultures with subcultures on differential media and pour plate method to quantify the intensity | Control, 50; test, 53.3 (the difference is not statistically significant) | Mostly gram-positive aerobic and anaerobic cocci; identification of individual species was not mentioned | The intensity of the bacteremia was <1 CFU/ml for both groups |
| Effects of subgingival irrigation with chlorhexidine on bacteremia following scaling and root planing (1990, Waki [141]) | 60 patients on periodontal maintenance treatment | Baseline and 2 min after starting the procedure (15 ml) | Initial culture in Septi-Check slide system with subcultures in different enriched media | Pretreatment, 3.7; posttreatment, 18.5 | Propionobacterium acnes (predominant isolate), Eubacterium lentes, Streptococcus spp., Neisseria spp. | Species other than streptococci were isolated as the predominant species |
| Subgingival irrigation and scaling and root planing (1991, Lofthus [77]) | 30 subjects who were on periodontal maintenance therapy | Baseline, after irrigation, before and after scaling/planing (15 ml) | Standard and Septi-Check blood culture bottles and subculture onto differential media | 50 (overall); no statistically significant difference in irrigation with water and chlorhexidine | No data available on the exact identification of the isolates; the majority of the isolates were facultative gram-positive cocci | Chlorhexidine irrigation does not reduce the incidence of bacteremia |
| Effects of intraoperative and preoperative irrigation with Prosol-chlorhexidine (1993, Allison [5]) | 12 subjects in whom no dental therapy had been started | Baseline, within 1 min of scaling of each quadrant, and 10 min after the whole procedure (15 ml) | Aerobic and anaerobic cultures in Bactec culture bottles | Placebo rinse, 9/12 patients; Prosol-chlorhexidine (test), 3/12 patients | 36 positive cultures, 13 of which were viridans group streptococci | Initial and continuous use of chlorhexidine reduces the incidence of bacteremia |
| Subgingival irrigation and rinsing with an antiseptic mouth rinse (1996, Fine [46]) | 18 subjects who were considered bacteremia prone in the initial assessment | Baseline and postscaling with placebo and antiseptic (10 ml) | Spread plating after mixing with thioglycolate broth | All 18 patients had aerobic and anaerobic bacteremia | No species-level identification was mentioned | Intensity of bacteremia is significantly lower with antiseptic |
| Effects of bleeding severity and extent of lesions in bacteremia associated with probing (1997, Daly [27]) | 30 subjects at the beginning of treatment for periodontitis | Before and immediately after probing (20 ml) | Automated cultures, standard identification methods | 43 | 45% of the isolates were viridans group streptococci | No significant correlations were found between bacteremia and the severity of periodontitis or extent of bleeding upon probing |
| Effects of gingivitis and periodontitis on probing (2001, Daly [28]) | 40 (20 patients with periodontitis and 20 patients with gingivitis, based primarily on the radiographic evidence) | Prior to and after probing (20 ml) | Aerobic and anaerobic automated cultures and identification at the genus level | Gingivitis patients, 10; periodontitis patients, 40 | 4 of 9 aerobic/facultative isolates were streptococci | Risk of bacteremia is higher with periodontal inflammation than with gingivitis alone, as noted by a higher odds ratios |
| Effects of periodontal probing (P), brushing (B), and scaling (S) (2005, Kinane [68]) | 30 subjects with untreated periodontal disease at the commencement of treatment | Baseline (BL1 and BL2), at probing (P), 2 wk later, at brushing (B), at scaling (S) (28 ml) | Automated cultures, standard identification methods, 16S PCR using bacterial DNA in blood | Culture: BL1, 2; P, 6; BL2, 0; B, 1; S, 4; PCR: BL1, 3; P, 5; BL2, 1; B, 4; S, 7 | A mixture of organisms, where Actinomyces naeslundii and Streptococcus spp. were found in more than two subjects | No species-level identification was done by PCR; in general, the incidence was lower than that reported previously |
| Effects of povidone-iodine rinse (test) on bacteremia induced by ultrasonic scaling (2007, Cherry [68]) | 60 patients who had plaque-induced gingivitis at the commencement of treatment (30 each in control and test groups) | Before treatment, 30 s and 2 min after procedure (10 ml) | Lysis centrifugation with differential aerobic and anaerobic cultures, biochemical tests | At 30 s: control group, 13.3; test group, 3.3; at 2 min: control group, 30; test group, 6.7 | In controls, Prevotella, Actinomyces, and Streptococcus; in povidone-iodine group, Enterobacteriaceae spp. | Rinsing with povidone-iodine eliminates viridans group streptococci |
| Efficacy of diode lasers in reducing bacteremia associated with ultrasonic scaling (2007, Assaf [8]) | 22 patients with plaque-induced generalized periodontitis at the commencement of treatment | For control group, at baseline, 3 min after initiation of scaling, and 30 min later; for test group, just before and 3 min after initiation of scaling (10 ml) | Automated culture with indicator system | Control group, 68; diode laser group, 36 | Of the 28 positive cultures, 61% were streptococci, which included S. mitis, S. salivarius, and S. sanguis; other oral species included Prevotella and Capnocytophaga | Diode lasers were shown to reduce the incidence in this split mouth expt, but the time lapse between the two experiments allowed a possible carryover effect |
| Identity of periodontopathogens in blood after scaling and root planning (2007, Lafaurie [71]) | 42 (27 with generalized severe chronic periodontitis and 15 with generalized aggressive periodontitis) | Baseline and immediately, 15 min, and 30 min after the procedure (5 ml) | Culture in Ruiz-Castaneda medium for 15 days, with subcultures at different intervals for identification | Highest incidence (at 30 min) for generalized aggressive periodontitis, 93.7 (14/15 patients); that for generalized severe chronic periodontitis, 74.1 (20/27 patients) (P > 0.05) | Of the 14 different species isolated, P. gingivalis was predominant, followed by (28.6%) Actinomyces spp. and Micromonas micros | No significant difference in incidence was seen between chronic periodontitis and aggressive periodontitis |
| Dental extractions | ||||||
| Incidence, nature, and antibiotic sensitivity of anaerobic bacteria isolated in postextraction bacteremia (1966, Khairat [66]) | 100 subjects undergoing dental extractions, irrespective of periodontal health status | Pre- and postprocedure (30 ml) | Differential aerobic and anaerobic broth and pour plate cultures, with and without CO2 | Postextraction, 64 | 155 strains; 46 were strict anaerobes, 44 of the isolates were viridans group streptococci | Periodontal health status has no influence on the causation of postextraction bacteremia, and the need for use of correct anaerobic cultures was highlighted |
| Effects of povidone-iodine irrigation on postextraction bacteremia (1971, Scopp [121]) | 64 treated with povidone-iodine, 30 treated with placebo | Baseline, 3 min after dental extraction (unknown vol) | Conventional culture and standard identification tests | Povidone-iodine group, 28; placebo group, 56 | Identities of isolates were not mentioned | Reduction or no growth in gingival cultures was observed in the povidone-iodine group |
| Effects of topical application of antiseptics prior to extraction (1978, Sweet [127]) | 100 patients divided into four groups (no antiseptics, chloramine T, chloramine T with electric brush, or irrigation with Lugol's solution) | Baseline, 5 min after antiseptic, 1 min, 30 min, 1 h, and 6 h after extractions | Primary and differential culture, morphology, chromatography, Limulus amoebocyte lysate test for endotoxin | Control group, 84; chloramine T rinse group, 48; brushing with chloramine T, 48; irrigation with Lugol's solution, 80 | 290 positive cultures, among which 29 were α-hemolytic or nonhemolytic Streptococcus spp.; of the 300 Limulus tests, 7 were positive | One study where the duration of bacteremia has been studied beyond 1 h; results for the incidence of bacteremia at 6 h are not shown |
| Efficacy of povidone-iodine and chlorhexidine in postextraction bacteremia (1984, Macfarlane [84]) | 60 patients undergoing uncomplicated dental extractions under local anesthesia (placebo group, 20 patients; chlorhexidine group, 20 patients; povidone-iodine group, 20 patients) | Immediately prior to and 30 s after extraction (10 ml) | Incubation in thioglycolate broth and biphasic media, with subsequent aerobic and anaerobic cultures and standard identification methods | Placebo group, 16/20 patients; chlorhexidine group, 5/20 patients; povidone-iodine group, 8/20 patients | Of 46 positive cultures, 39 were streptococci, including eight Streptococcus sanguis strains and six S. mutans strains | Antimicrobial sensitivity tests revealed that all 46 isolates were sensitive for ampicillin, cephaloridine, and vancomycin |
| Effect of macrolide antibiotics in postextraction bacteremia (1991, Cannell [18]) | 60 (placebo group, 20 patients; erythromycin group, 20 patients; josamycin group, 20 patients) | Prior to prophylaxis and 2 min after extraction (20 ml) | Conventional aerobic and anaerobic cultures, with identification of presumptive streptococci by API system | Placebo group, 65; erythromycin group, 60; josamycin group, 70 (not statistically significant) | No data available on the number of isolates identified | No significant effects of these two macrolide antibiotics in reducing postextraction bacteremia were seen |
| Effects of povidone-iodine, hydrogen peroxide, and chlorhexidine on postextraction bacteremia (1992, Yamalik [144]) | 80 patients with medium-degree oral hygiene indices (placebo group, 20 patients; hydrogen peroxide group, 20 patients; povidone-iodine group, 20 patients; chlorhexidine group, 20 patients | Pre- and postprocedure (10 ml) | Conventional culture and standard identification tests | Placebo group, 70; hydrogen peroxide group, 50; povidone- iodine group, 35; chlorhexidine group, 40 | Of 61 isolates in all groups, 16 were viridans group streptococci | Use of antiseptics alone is not enough to eliminate bacteremia, despite the significant reduction of the incidence |
| Effects of oral administration of erythromycin and clindamycin in postextraction bacteremia (1995, Aitken [2]) | 40 patients who required emergency dental extractions (erythromycin group, 20 patients; clindamycin group, 20 patients) | Baseline, 1 to 1.5 h after the oral drug was taken (10 ml), 2 to 3 min after extraction (20 ml) | Conventional broth cultures and subculture on differential agar media and standard identification tests | Clindamycin group, 40; erythromycin group, 60 | Streptococcus oralis was the most frequent isolate | There is no placebo group in this study for comparison of the true effects of the two antimicrobials that were used |
| Comparison of the efficacy of povidone-iodine and chlorhexidine irrigation in preventing posttreatment bacteremia (1995, Rahn [102]) | 120 (control group, 40 patients; povidone-iodine group, 40 patients; chlorhexidine group, 40 patients) | Baseline, 2,4, and 6 min postprocedure (10 ml) | Culture in Bactec bottles and standard identification | Control group, 52.5; povidone-iodine group, 27.5; chlorhexidine group, 45 | Viridans group streptococci were the predominant isolates in all three groups | The two treatments, i.e., intraligamentary injection and elective extractions, have not been evaluated separately for the development of bacteremia |
| Effects of prophylactic cefaclor on postextraction bacteremia (1996, Hall [54]) | 39 (cefaclor group, 20 patients; placebo group, 19 patients) | Before, during, and 10 min after extraction (8.3 ml) | Lysis filtration, standard biochemical methods | Cefaclor group, 53 to 79; placebo group,47 to 85 | 432 isolates, of which 29 were viridans group streptococci | No significant reduction of postextraction bacteremia by oral administration of cefaclor |
| Different modalities of extractions are compared (1998, Roberts [111]) | 207 children undergoing dental extractions under general anesthesia | After anesthesia (baseline), during the procedure, and after the procedure (8 ml) | Broth culture, lysis centrifugation, standard biochemical testing, and Streptococcus-specific API testing | Baseline, 11.3; single extraction, 43.2; multiple extraction, 54.2; flap, 43.1 | 113 different isolates, 57% of which were streptococci | Highest intensity of bacteremia (63 CFU/ml) was observed with mucoperiosteal flaps |
| Antimicrobial rinse (chlorhexidine) upon intraoral suture removal after extraction of the third molar (1998, Brown [16]) | 55 patients followed up after third molar surgery (chlorhexidine group, 31 patients; control group, 24 patients) | Before and 1.5 min after the removal of sutures (10 ml) | Lysis centrifugation and culture on differential media, standard tests and biochemical reactions | 10.9 (no significant difference in incidence between the two groups) | Predominant isolates from both groups were streptococci | Use of antiseptic rinse does not reduce the incidence of bacteremia and also does not eliminate oral streptococci from blood |
| Effect of single dose of cefuroxime on multiple tooth extractions (1999, Wahlmann [140]) | 59 patients with oral cancer undergoing preradiotherapy multiple extractions (placebo group, 29 patients) | Start of procedure and 30 min postprocedure | Automated culture system | Placebo group, 79 to 86; cefuroxime group, 20 to 33 | Of 73 isolates, 40 were viridans group streptococci | Although 9/10 streptococcal isolates were sensitive to cefuroxime, the drug does not eliminate streptococci from the bloodstream |
| Comparison of topical and oral antibiotic prophylaxis on postextraction bacteremia (2001, Vergis [137]) | 36 (control group, 9 patients; oral amoxicillin group, 10 patients; topical amoxicillin group, 10 patients) | After extraction (8.3 ml) | Automated Bactec cultures under aerobic and anaerobic conditions | Control group, 89; oral amoxicillin group,10; topical amoxicillin group, 60 | Altogether, 23 species were isolated; 61% were viridans group streptococci, followed by Lactobacillus (13%) | There was no statistically significant difference between the incidence of bacteremia in the control group and the topical amoxicillin group |
| Effects of amoxicillin prophylaxis on dental extractions under general anesthesia (2004, Lockhart [76]) | 100 children undergoing extraction under general anesthesia (placebo group, 51 patients; amoxicillin group, 49 patients) | 2 min after intubation, before extraction, at different time intervals (6 ml) | Automated aerobic and anaerobic cultures, standard biochemical tests | Placebo group, 84; amoxicillin group, 33 | 152 positive cultures of 29 different species, with viridans group streptococci being the most frequent isolates in both groups | Incidence of bacteremia was highest (in both groups) during and immediately after the extraction procedure |
| Extraction of partly erupted mandibular third molars (2004, Rajasuo [103]) | 16 healthy military recruits | 1 min after incision, 1, 5, 10, 15, and 30 min after extraction (16 ml) | Standard cultures with subculture on differential media | Overall incidence, 88 (14/16 recruits) | 31 different bacterial species (8 aerobic, 23 anaerobic); viridans group streptococci and S. milleri were the commonest | Organisms with similar phenotypic profiles were detected in 93% of pericoronal pockets and 43% of extraction sockets |
| Duration, prevalence, and intensity of bacteremia following extractions (2006, Roberts [110]) | 500 (divided into 10 time groups) | 10 s, 30 s, 1 min, 2 min, 4 min, 7.5 min, 15 min, 30 min, 45 min, and 60 min after extraction (13 ml) | Aerobic and anaerobic Bactec culture lysis filtration, PCR analysis, comparative 16S rRNA and sodA gene sequencing | Highest number of CFU was measured at 1 min | 45 different species isolated; Streptococcus > Actinomyces > Staphylococcus | Intensity of bacteremia rose to a peak at 1 min and then gradually declined toward 60 min |
| Efficacies of amoxicillin, clindamycin, and moxifloxacin on postextraction bacteremia (2006, Diz Dios [39]) | 221 Patients undergoing dental extractions under general anesthesia (control group, 53 patients; amoxicillin group, 56 patients; clindamycin group, 54 patients; moxifloxacin group, 58 patients | Before the dental work and 30 s, 15 min, and 60 min after the final extraction | Aerobic and anaerobic culture in Bactec 9240 tubes, biochemical tests provided with the Vitek system | Control group, 20 to 96; clindamycin group, 22 to 85; amoxicillin group, 7 to 57; moxifloxacin group, 4 to 46 | Control group, 139 isolates; amoxicillin group, 36 isolates; clindamycin group, 135 isolates; moxifloxacin group, 62 isolates (Streptococcus > Staphylococcus > Neisseria) | Amoxicillin is the most effective antimicrobial in reducing the incidence and intensity of bacteremia |
| Factors affecting the development of postextraction bacteremia (2007, Tomas [132]) | 53 patients undergoing extraction under general anesthesia for behavioral reasons | Baseline and at 30 s, 15 min, and 1 h | Aerobic and anaerobic culture in Bactec 9240 system, biochemical tests provided by Vitek system | 30 s, 96.2; 15 min, 64.2; 60 min, 20 | Of the 133 isolates, 63.6% were Streptococcus spp. | Occurrence of bacteremia is not influenced by oral health status |
| Effects of chlorhexidine mouthwash on postextraction bacteremia (2007, Tomas [132]) | 106 patients undergoing extractions under general anesthesia for behavioral reasons | Baseline, 30 s, 15 min, and 1 h postextraction (10 ml) | Conventional culture and standard identification tests | Control group at 30 s, 96; at 15 min, 64; at 60 min, 20; chlorhexidine group at 30 s, 79; at 15 min, 30; at 60 min, 2 | Streptococcus spp. were the predominant isolates in all groups | Although the incidence was reduced, chlorhexidine did not eliminate postextraction bacteremia altogether |
| Orthodontic procedures | ||||||
| Orthodontic debanding and debonding (2000, Erverdi [43]) | 30 patients with fixed appliances in both jaws | Before and after the removal of bands (10 ml) | Pour plate and automated blood culture system | Baseline, 6.6; after procedure, 6.6 | All four isolates were Streptococcus spp. | No significant increase in incidence was associated with the procedure |
| Effect of chlorhexidine mouthwash on bacteremia following orthodontic banding and debanding (2001, Erverdi [42]) | 80 (banding procedure group, 40 patients; debanding and debonding group,40 patients) | Pretreatment and posttreatment (10 ml) | Automated blood culture system, pour plate method, standard biochemical methods | No significant increase in bacteremia compared to baseline | Banding group, single isolate of Bacteroides oralis; debanding and debonding group, Staphylococcus aureus, S. sanguis | No statistically significant bacteremia in the two groups studied |
| Bacteremia following different orthodontic procedures (2002, Lucas [83]) | 85 (alginate impression group, 39 patients; separator placement group, 42 patients; banding group, 25 patients; arch wire adjustment group, 36 patients) | Preprocedure and 30 s after procedure (6 ml) | Pour plate cultures after lysis, biochemical methods | No significant increase in bacteremia compared to baseline | 161 discrete colonies of 15 different species; coagulase-negative staphylococci were the most frequent isolates (69.9% of the colonies) | The studied orthodontic procedures do not seem to contribute to significant odontogenic bacteremia |
| Removal of Haas palatal expanders (2005, Rosa [113]) | 8 | Before and 10 min after removal (10 ml) | Standard blood cultures and identification methods | 4 of 8 patients developed bacteremia after removal of the expanders | All strains isolated were Streptococcus oralis | Although half of the patients who underwent the procedure developed bacteremia, a larger cohort is necessary to arrive at definitive conclusions |
| Debanding and gold chain adjustment (2007, Lucas [81]) | 49 (upper deband group, 42 patients; gold chain adjustment group, 7 patients) | Before treatment and 30 s after procedure (6 ml) | Lysis filtration | Deband group, 26; gold chain adjustment group, 57 | 39 isolates representing 15 different species; coagulase-negative staphylococci were the predominant isolates | No significant difference in bacteremia compared to baseline |
| Endodontic procedures | ||||||
| Bacteremia following endodontic therapy for apical periodontitis (1995, Debelian [31]) | 26 (group with reamers passed beyond the apical foramen, 13 patients; group with reamers within the root canal, 13 patients) | During and 10 min after treatment (8.3 ml) | Standard cultures, biochemical and antimicrobial susceptibility tests | Group with reamers passed beyond the apical foramen, 7/13 patients; group with reamers within the root canal, 4/13 patients | Six different species; Propionibacterium acnes was isolated from blood of three patients | Incidence of bacteremia was higher when the reamers passed beyond the apices, and identical profiles were demonstrated for blood and root canals |
| Comparison of endodontic manipulation within and beyond the root canal (2003, Bender [11]) | 50 (group with manipulation within root canal, 26 patients; group with manipulation beyond the root canal, 24 patients) | Before, soon after, and 10 min after the procedure (16 ml) | Standard aerobic and anaerobic cultures | Group with manipulation beyond the root canal, 12 | Six isolates; four were viridans group streptococci | The same organisms were isolated from root canals |
| Nonsurgical root canal treatment (2005, Savarrio [118]) | 30 | Preoperative, perioperative, and postoperative (45 ml) | Standard cultures and identification, genomic DNA detection with 16S rRNA PCR, pulsed-field gel electrophoresis to compare genetic homogeneity | Broth culture, 20; pour plate, 12; PCR, 40 | 46 anaerobic isolates (14 species) and 30 aerobic isolates (14 species); Streptococcus spp. were the predominant isolates | Some isolates showed genetic similarity with isolates from the root canal |
| Miscellaneous and aesthetic procedures | ||||||
| Prophylaxis and fluoride application in pediatric patients (1971, Hurwitz [60]) | 32 | 6 min after finishing the procedure (6 ml) | Standard cultures | No bacteremia detected | Whether aerobic and anaerobic culture methods were attempted is not known | |
| Occurrence of bacteremia with sialography (1985, Lamey [72]) | 30 patients with possible nonneoplastic salivary gland disease | For 20 patients, at baseline and after 1 min and 5 min; for 10 patients, at 30-s intervals up to 5 min (10 ml) | Initial cultures on broth medium, followed by aerobic and anaerobic subcultures on enriched media | 30% developed bacteremia at different stages of the procedure | 5/7 isolates were Staphylococcus spp. | Usually Staphylococcus spp. are not detected in odontogenic bacteremias; it is possible that salivary bacteria ingress through the ductal system into the circulation |
| Air polishing (1989, Hunter [59]) | 40 (rotating rubber cup [control] group, 20 patients; air polishing group, 20 patients) | Before and 5 min after the procedure (20 ml) | Aerobic and anaerobic broth cultures | Control group, 35; air polishing group, 15 | 6/12 isolates were viridans group streptococci | Those patients who developed bacteremia in both groups had higher plaque or gingivitis scores |
| Aspiration incision and drainage versus incision and drainage of dentoalveolar abscesses (1990, Flood [47]) | 25 (needle aspiration group, 13 patients; incision-and-drainage-only group, 12 patients) | Baseline and at 1-min intervals until 5 min periprocedure (10 ml) | Initial cultures on broth medium, followed by aerobic and anaerobic subcultures on enriched media | Precedent aspiration group, 0/13 patients; incision-and-drainage-only group, 3/12 patients | All three isolates were Streptococcus spp. | Some isolates recovered from blood had identical phenotypic profiles to those for isolates recovered from pus drained from the abscesses |
| Routine dental procedures in pediatric dentistry with general anesthesia (1997, Roberts [109]) | 735 children in 15 different subgroups according to the procedure being investigated | Baseline and 30 s after procedure (8 ml) | Automated aerobic and anaerobic cultures, routine biochemical testing | Brushing group, 38.5; polishing group, 24.5; scaling group, 40.0; injection group, 96.6; rubber dam group, 29.4; matrix band group, 32.1; extraction group, 38.7 to 50.9; flap group, 39.2 | 357 isolates representing 31 different species, with viridans group streptococci making up >50% of the isolates | One of the most detailed studies of odontogenic bacteremia in children |
| Effects of amoxicillin on bacteremia following nonsurgical dental treatment in children under general anesthesia (2007, Brennan [15]) | 100 (placebo group, 51 patients; amoxicillin group, 49 patients) | 8 draws (before treatment, during treatment and after treatment) (6 ml) | Automated aerobic and anaerobic cultures | From draw 2, placebo group, 20; amoxicillin group, 6 | Species-level identifications are not mentioned | Gingival disease has an impact on bacteremia, and antibiotics do not eliminate it altogether |
Chewing
The normal physiological act of chewing has marked individual variations, making it one of the most difficult aspects to control in an experimental setting. Despite the theoretical possibility that in susceptible individuals chewing could give rise to a bacteremic state, the data available in the literature so far are inconclusive. In one of the earlier studies of odontogenic bacteremia, it was shown that 17% of the subjects who chewed hard candy (hard mastication) developed bacteremia, while none was detected with gentle mastication (25). Similar results were observed in a later study where postchewing bacteremia was detected in 20% of subjects who had evidence of periodontal inflammation, while none was detected in periodontally healthy subjects and subjects with gingivitis (i.e., superficial inflammation of the gums but not the deep periodontal tissues) (48). However, Murphy and coworkers (89) showed in a controlled study that chewing failed to seed detectable numbers of bacterial species into the bloodstream at different time intervals, irrespective of periodontal health status. Interestingly, in a parallel approach, Geerts et al. (49) noted significantly raised levels of bacterial endotoxins in subjects with chronic periodontitis, as assessed by the Limulus amoebocyte lysate assay. Whether this is a sequel of odontogenic bacteremia or endotoxemia from the inflamed periodontal niches remains to be determined.
Personal Oral Hygiene Measures
The effect of routine personal oral hygiene measures on the development of bacteremia is also difficult to evaluate. Therefore, procedures such as toothbrushing, flossing, and tooth picking and their relationship to bacteremia have been evaluated under controlled conditions, although in reality such exercises could be deemed rather artificial. Nevertheless, Forner and coworkers (48) found that toothbrushing did not cause significant bacteremia of oral origin in either healthy subjects or those with gingivitis, as no positive blood cultures were found in their controlled clinical trial of 60 subjects. Although the latter findings need to be confirmed by further work, these data indicate that mild gingivitis may not cause bacteremia during oral manipulation. Also, in another study of 30 healthy military recruits, it was shown that the true incidence of bacteremia was zero after brushing with soft-bristled toothbrushes. Of the 180 aerobic and anaerobic cultures that were analyzed, only 3 yielded detectable growth, and even these were identified as Propionibacterium acnes, a well-known skin contaminant of blood cultures (56). In early studies, detectable levels of bacteremia ranging from 17 to 44% were noted following toothbrushing in healthy subjects when brushing was performed by the investigator (25, 120). Thus, it appears that at least in adults with a healthy periodontium, brushing is not a significant factor contributing to systemic bacterial dissemination from the mouth.
On the other hand, subjects wearing orthodontic appliances demonstrated bacteremias of odontogenic origin following toothbrushing, with an incidence of 25% (119). For children, there is some evidence to indicate that bacteremias ensue upon toothbrushing performed under general anesthesia and nasotracheal intubation. Furthermore, the incidence was higher when electric sonicating toothbrushes rather than conventional tooth brushes were used (12). The latter finding on bacteremia under general anesthesia needs to be interpreted cautiously, as prolonged intubation in itself causes bacteremia and the magnitude of bacteremia in terms of incidence and diversity increases with the duration of the intubation (93).
With regard to dental flossing, a procedure that could mechanically disturb the periodontal plaque biofilms, bacteremia was associated with sporadic flossing, while no significant bacteremia was detected with daily flossing, even in subjects with gingivitis (19). However, due to the paucity of data and given the fact that flossing is an oral hygiene measure widely advocated for those with gingivitis and periodontitis, prospective randomized controlled studies with larger numbers of subjects are necessary to arrive at a definitive conclusion.
Periodontal Procedures
Various degrees of bacterial dissemination are associated with periodontal probing (27, 28), scaling (22, 68), root planing, and subgingival irrigation (5, 77, 106, 141). The presence of inflammation in the periodontal site is an important contributory factor to bacterial dissemination, as shown by the increased incidence of bacteremia in subjects with periodontitis compared to those with gingivitis (where the degree of inflammation is considered less severe) and those with healthy periodontium (48). For instance, the incidence of bacteremia varies from 5 to 75% for subjects with periodontitis, as opposed to 5 to 20% for subjects with gingivitis (27, 48). Despite the foregoing, the inflammation associated with gingivitis can be less than that with periodontitis, but not always. In very severe cases of gingivitis, the intensity and inflammation can be equal to or exceed that associated with some cases of periodontitis. In addition, there is no uniform agreement on a positive association between various indices used to measure the degree of periodontal inflammation, such as the presence of bleeding upon probing, gingival index, and plaque index, and systemic bacterial dissemination (Table 2).
Subgingival irrigation with antiseptics such as chlorhexidine prior to scaling and root planing is practiced by some dental surgeons. Waki and his group (141) found no significant decline in the incidence of bacteremia due to this supplementary procedure, nor was there a significant relationship with the quality of the irrigation fluid used, whether sterile water, clean water, or antiseptics (77, 106). However, there is some evidence that the bacteremic incidence does drop with continuous and regular rinsing with chlorhexidine or povidone-iodine irrigation of gingival sulci (5, 102).
In laboratory microbiological terms, although there is no significant influence on reducing the incidence of bacteremia, some workers found the intensity of bacteremia, measured by the number of CFU per milliliter, to be significantly lower when antiseptic mouth rinses were used prior to ultrasonic scaling (46). Taken together, the foregoing data imply that when periodontal procedures are performed on individuals with poor oral hygiene (as indicated by increased plaque indices), there is a higher risk of developing a bacteremia.
As for methods that reduce the incidence and intensity of bacteremia during periodontal manipulations, promising results have been shown with the use of a diode laser as an adjunct to ultrasonic scaling, and this could be attributed to the lesser degree of tissue trauma inflicted. In addition, lasers may also possess a degree of antibacterial activity (8). However, large-scale and well-controlled studies are necessary to confirm the latter finding.
Tooth Extraction
Tooth extraction, or exodontia, and associated tissue trauma cause bacteremia, and this is by far the most-studied oral surgical procedure evaluated to assess odontogenic bacteremias. According to a number of studies, the incidence of bacteremia ranges from 13 to 96%, depending on the evaluated time intervals as well as other experimental variables studied (Table 2). It has been shown that bacteremias can follow simple dental extractions as well as extractions of impacted teeth that entail minor surgical intervention (76, 103, 109, 110, 132, 137, 140). In such circumstances, the bacteremic incidence appears to be influenced positively by the presence of gingivitis, periodontitis, and other odontogenic infections, such as dentoalveolar abscesses, suggesting a direct relationship between an increased bacterial biofilm burden and bacteremia (15, 26, 128, 132). Other contributory factors for the phenomenon are the extent and duration of the surgical period and the magnitude of blood loss (92). When surgical incisions were made to facilitate the extraction of teeth, particularly impacted third molars, with subsequent insertion of sutures, nearly 10% of individuals had a bacteremia following the removal of sutures, and the incidence was not reduced by the use of preoperative antiseptic rinses (16).
Despite the fact that intubation is associated with bacteremia (93), no significant change in bacteremic incidence was observed when extractions were performed under general anesthesia (128). In addition, there is no apparent change in the incidence of bacteremia with increased numbers of teeth extracted or the use of mucoperiosteal elevators (103, 111, 133). Similarly, pre- and perioperative administration of antimicrobial agents, such as clindamycin, erythromycin, josamycin, and cefaclor, appears to have no significant effect in reducing the incidence of bacteremia (18, 53, 54). Similar results have been observed with the use of perioperative topical antibiotic applications (137).
Only a few procedures related to exodontia appear to reduce surgical bacteremias, and these include preoperative administration of antimicrobial agents, such as amoxicillin, cefuroxime, and moxifloxacin, that significantly reduce the incidence of such bacteremias (39, 140). Moreover, broad-spectrum degerming rinses, such as povidone-iodine, chloramine-T, and chlorhexidine, have been shown to reduce the incidence of bacteremia when administered as a rinse or irrigation prior to the extraction procedure or instrumentation, and povidone-iodine was shown to be the most potent in reducing the incidence (84, 121, 127, 144).
Orthodontic Procedures
Insertion of fixed orthodontic appliances involves manipulation of the oral tissues, particularly the teeth and the gingivae, and this may lead to systemic bacterial dissemination. However, the evidence to date shows that significant bacteremia is associated only with the insertion of separators that mechanically push teeth apart with significant force to create space for unerupted teeth (83). Other orthodontic procedures, such as taking alginate impressions, banding, debonding, removal of fixed appliances, and gold chain adjustments, appear to produce no significant bacteremia (42, 43, 83, 113).
Endodontic Procedures
It is highly likely that endodontic procedures that entail instrumentation of pulp chambers of either single or multiple root canals of teeth with reamers and broaches may introduce bacteria into the periapical vasculature of the teeth, with consequent bacteremic episodes. One important determinant of the onset of bacteremia in such situations appears to be the degree of tissue trauma caused by the instrumentation. Not surprisingly, therefore, the incidence of bacteremia was reportedly higher when the reamers reached beyond the confines of the root canal than in atraumatic endodontic procedures (11, 30, 31).
Miscellaneous Oral Procedures
Apart from the routine therapeutic and preventive dental procedures such as extractions and periodontal surgery discussed above, elective esthetic procedures, such as tooth polishing and whitening, which are now very popular and which are known to induce gingival epithelial abrasion and trauma, have not been shown to produce bacteremia (59). There is, however, a paucity of literature in this area and a need for large-scale controlled clinical trials.
Sialography, a procedure commonly carried out by specialists in oral medicine for imaging of the major salivary glands, entails injection of radio-opaque dyes into the ductal system of the glands. Bacteremias of streptococcal origin have been observed by some workers during different stages of such procedures (72). One reason for this could be the translocation of mucosal flora into the salivary duct system due to the pressure of the injecting dye.
Dentoalveolar abscesses are known to induce inflammation and vascular proliferation essentially at the periapical region of the tooth. Bacteremias have been reported when the purulent content of such lesions was drained by simple incisions. However, needle aspiration of pus prior to incision and drainage of the abscess was shown to reduce or totally eliminate the bacterial dissemination, possibly by reduction of the pressure inside the abscess before the surgical manipulation of the tissues (47).
To conclude, then, with reference to all of the dental treatment procedures discussed above, it appears that the following two major parameters dictate bacteremic episodes: first, and most importantly, the degree of inflammation present at the site, which is often directly related to the microbial load of the biofilm, as seen in generalized aggressive periodontitis (7, 29, 94); and second, the amount of tissue trauma that is inflicted. As for measures to eradicate odontogenic bacteremia, no single method (either topical or systemic antimicrobials or degerming antiseptic agents) has been effective, despite the fact that some measures may reduce the incidence significantly relative to others.
TEMPORAL NATURE OF ODONTOGENIC BACTEREMIA
It is generally accepted that odontogenic bacteremias are transient in nature. Furthermore, it has been surmised that at least in healthy individuals, the bacteria are scavenged from the bloodstream relatively quickly by the innate and adaptive defense mechanisms (20, 100). Despite this general contention, some studies indicate that an episode of odontogenic bacteremia could last as long as 60 min (39, 110, 132), and most studies report the presence of bacterial species in blood for up to 30 min (Table 2; Fig. 2). This incidence profile can be observed in all forms of odontogenic bacteremias irrespective of the triggering factors, underlying predisposing conditions, or detection methods. A schematic illustration compiled from the data in the literature on the relationship between the incidence of bacteremia and the postprocedure time is given in Fig. 2. From the cumulative data, it can be deduced that the bacteremic incidence peaks within the first few minutes and then gradually declines after 10 to 20 min. Furthermore, depending on the threshold sensitivity of the detection method, the total reduction in the bacteremic load may vary from 10 to 90% at 30 s and from 2 to 20% at 60 min. It is important, however, that in spite of an initial steep fall, a few bacteria survive in the circulation after a bacteremic challenge from the oral cavity. The role of these persisters and how they survive host defenses need to be evaluated further, as they may well be the ones that evade the initial host immune burst and have the propensity to seed target organs and cause systemic and distant infections (34, 73).
SYSTEMIC OR END ORGAN INFECTIONS WITH ORAL BACTERIA
The relationship between focal infection in the oral cavity, such as chronic periodontitis and gingivitis, and systemic diseases, including heart disease, diabetes, adverse pregnancy outcomes, and stroke, has aroused much concern lately (104). Apart from the seeding infection as a direct consequence of transient bacteremia, such oral-systemic interactions and outcomes could be due to other, indirect reasons, such as metastatic inflammation as a result of circulating macromolecular complexes and/or metastatic injury due to soluble toxins and bacterial lipopolysaccharide (50, 58, 74).
Although virtually any system or tissue can be affected by disseminated infection from the oral niches, endocarditis is the most well known and highly evaluated such affection. As stated earlier, literature on the latter subject is vast and extensive, and we make no attempt to review this aspect herein. Available data in the literature on other systemic and end organ infections due to oral origin are summarized in Table 3.
TABLE 3.
Summary table annotating the literature on systemic infections (apart from endocarditis) due to oral bacteria
| Phylum, yr, and author (reference) | Genus or species | Systemic infection | Putative oral source | Diagnostic method | Remarks/comments on isolates |
|---|---|---|---|---|---|
| Firmicutes | |||||
| 1985, Lindqvist (75) | Viridans streptococci | Hip arthroplasty infection | Antecedent dental procedures | Culture and biochemical tests | Detailed identification to the species level not performed |
| 1990, Bisiaux-Salauze (13) | Selenomonas (S. artemidis and S. infelix) | Lung infections leading to ARDS | Poor oral hygiene | Culture and biochemical tests with gas chromatography of metabolic products and electron microscopy | Two cases; Selenomonas spp. are members of the oral commensal flora |
| 1993, Christensen (23) | Streptococcus intermedius | Septic pulmonary embolism | Recent history of toothache | Culture and biochemical tests | A predominant member of the oral commensal flora also implicated in bacterial endocarditis |
| 2000, Kaar (62) | Streptococcus intermedius | Infection of a revision total hip arthroplasty | Supragingival cleaning ∼30 h before the onset of bacteremia | Culture and biochemical tests | The bacteria might have disseminated from periodontal plaque biofilm |
| 2000, Michelow (88) | Abiotrophia spp. | Cerebral abscess | Severe mucositis with oral ulceration | Culture and biochemical tests with RapID ANA II system | A definitive pathogenic role within the oral cavity has not been determined for this organism |
| 2002, Rousee (114) | Dialister pneumosintes | Brain abscess | Gingivitis and periodontitis | Phenotypic characterization and 16S rRNA gene sequencing | No evidence hitherto of concomitant systemic affections |
| 2004, Dietl (38) | Viridans streptococci | Infection of postpneumonectomy space | Recent dental work (gum surgery) | Culture and biochemical tests | Viridans group streptococci are commonly implicated in endocarditis, but in this case, they caused lung infection in a cancer patient |
| 2004, Marques da Silva (86) | Streptococcus constellatus | Brain abscess | Presence of deep periodontal pockets, bleeding, tooth mobility, and radiographic signs of alveolar bone loss | Restriction fragment length polymorphism analysis, ribotyping, and random amplification of cDNA ends showed identical profiles with the oral isolates | One of the few instances where genetic homogeneity has been determined for blood/tissue and oral isolates |
| 2005, Wilhelm (142) | Abiotrophia defectiva | Multiple discitis and sacroiliitis | Recent series of dental surgical procedures | Culture and biochemical tests with 16S rRNA gene sequencing | Previously known as nutritionally variant streptococci, this organism is increasingly isolated from immunocompromised patients |
| 2005, Kazanci (63) | Streptococcus oralis | Middle cerebral artery thrombosis | Dental abscess | Culture and biochemical tests | It is difficult to determine whether the infection is a result of local spread or hematogenous dissemination |
| 2006, Detry (35) | Solobacterium moorei | Septicemia in a patient with multiple myeloma | Apical dental abscesses | Cultures, biochemical tests, and chromatography | This organism is also considered to be associated with halitosis |
| Fusobacteria | |||||
| 1985, Reig (105) | Leptotrichia buccalis | Septicemia (two cases) | Oral mucosal ulceration | Culture, biochemical tests, GC content of cellular organic acids | Both patients were undergoing chemotherapy for malignancy |
| 1999, Patel (97) | Novel Leptotrichia spp. | Septicemia | Oral herpes simplex lesions | Culture, biochemical tests, GC content of cellular organic acids, and 16S rRNA gene sequencing | The patient had acute myeloid leukemia |
| 2001, Tee (131) | Leptotrichia trevisanii | Septicemia | Gingivitis, mucositis, and oral ulceration | Anaerobic culture, RapID ANA profiles, and 16S rRNA gene sequencing | Infection in a patient with acute myeloid leukemia |
| 2003, Heckmann (57) | Fusobacterium nucleatum | Multiple brain abscesses | Poor oral hygiene | Presence of fusobacterial DNA in the cerebrospinal fluid | F. nucleatum is a well-recognized periodontal pathogen |
| 2006, Ulstrup (135) | Leptotrichia buccalis | Septicemia | Periodontitis | Culture and biochemical tests | 12/19 patients had oral lesions |
| 2006, Khouzam (67) | Fusobacterium spp. | Brain abscess | Poor oral hygiene, with numerous cavities and gingivitis | Culture, biochemical tests | Mixed microbial infection with Streptococcus spp. |
| 2006, Ewald (44) | Fusobacterium nucleatum | Brain abscess in the right occipital lobe | One or more of periapical osteitis, caries in teeth, gingivitis, and retained tooth | Isolation of the organism from the lesion through culture | Series of six cases; Streptococcus, Bacillus, and Actinomyces spp. were also isolated |
| Proteobacteria | |||||
| 1993, Pedersen (98) | Neisseria meningitidis | Septicemia | Recent tooth extraction | Culture and biochemical tests | Although the normal route of infection is through nasopharyngeal colonization, this case is likely to be odontogenic |
| 1997, Roiz (112) | Kingella kingae | Septicemia | Recent dental procedure | Culture and biochemical tests | Well-known etiological agent of odontogenic endocarditis (i.e., the HACEK group) |
| 1997, Samuel (117) | Haemophilus paraphrophilus | Paraspinal abscess | Recent periodontal surgery | Specialized cultures | Members of the HACEK group of organisms and oral commensals |
| 1999, Dewire (37) | Haemophilus aphrophilus | Acute osteomyelitis of the humeral shaft | Recent dental cleaning and ultrasonic scaling | Culture and antibiotic sensitivity tests | Members of the HACEK group of organisms and oral commensals; rare cause of bone infections |
| 2004, Iida (61) | Porphyromonas gingivalis | Brain abscess | Presence of tooth infection | Recovery of the organism from the cerebrospinal fluid | Well-confirmed etiological agent of periodontitis |
| 2008, Pasqualini (95) | Aggregatibacter aphrophilus | Spinal epidural abscess with cervical spondylodiscitis | Apical periodontitis | Standard cultures biochemical tests and API index | No underlying predisposing conditions except periodontitis |
| 2005, Stepanovic (126) | Aggregatibacter actinomycetemcomitans | Brain abscess | Poor dentition, poor oral hygiene, with cariotic teeth | Culture and biochemical tests | Previously known as Actinomyces actinomycetemcomitans, associated with a number of oral lesions |
| Actinobacteria | |||||
| 2003, Takiguchi (129) | Actinomyces odontolyticus | Lung abscess | Periodontitis | Culture and biochemical tests | Known oral pathogen |
| Bacteroidetes | |||||
| 2003, Mantadakis (85) | Capnocytophaga gingivalis | Septicemia | Dental caries and gingivitis | Quantitative culture by lysis centrifugation | Acute lymphoblastic leukemia |
CNS
The central nervous system (CNS), principally the brain and the spinal cord, is encased in an anatomical and functional covering. Thus, circulation to the brain is dependent upon a meshwork of blood vessels in juxtaposition with specialized brain cells known as the blood-brain barrier. Bacteria circulating in the bloodstream could ingress into the CNS through the blood-brain barrier. In addition, because of anatomical proximity, organisms could directly ascend to the brain or the upper part of the spinal cord from oral niches. For these reasons, it is apparent that the CNS could be a vulnerable relocation site for oral bacteria. In a review of cases of pyogenic infections in the brain and the spinal cord, Ewald and coworkers (44) pointed out that predominant oral bacterial species, such as Fusobacterium nucleatum, Veillonella, Streptococcus anginosus, Streptococcus oralis, and Actinomyces meyeri, could be detected in both the primary lesions and either cerebrospinal fluid or the blood. In addition, most subjects demonstrated one or more intraoral pathologies, such as periodontitis, gingivitis, caries, or periapical osteitis. Arguably, these lesions could serve as the primary infective focus for the dissemination and subsequent seeding of bacteria into an end organ site. For a majority of episodes, the absence of other detectable infective foci led researchers to conclude that the oral cavity, by default, is the primary source of infection (57, 61, 63, 67, 86, 88, 114, 126, 139). However, these studies, mainly case reports, suggest a relationship between pyogenic CNS infections and an oral origin of bacteria such as Abiotrophia spp., Dalister pneumosintes, Streptococcus intermedius, S. oralis, Streptococcus constellatus, Fusobacterium spp., F. nucleatum, Aggregatibacter actinomycetemcomitans, and Porphyromonas gingivalis (57, 61, 63, 67, 86, 88, 114, 126, 139). These organisms are all members of the oral resident flora, particularly that of the subgingival niche. In most reports, there is concomitant circumstantial evidence of poor oral hygiene, periodontitis, dental abscesses, mucositis with oral ulcerations, and the CNS lesions. Interestingly, in a few reports, phenotypic or genotypic homogeneity of the CNS and oral isolates was also traced (86, 139).
Skeletal Infections
As evident from the data presented in Table 3, odontogenic infections of the bones and joints are not as frequent as CNS infections. Yet there is clear evidence of oral bacteria causing multiple discitis and sacroiliitis, prosthetic hip joint infections, acute osteomyelitis, and paraspinal abscesses (Table 3). Apart from the presence of chronic oral infections such as periodontitis, recent invasive oral procedures, such as tooth extraction and ultrasonic scaling, have also been implicated as potential triggering factors for these infections. Common oral bacterial species such as Abiotrophia defectiva, Streptococcus intermedius, viridans group streptococci, Haemophilus aphrophilus, and Haemophilus paraphrophilus have all been implicated in such infections (37, 62, 75, 117, 142).
Respiratory Infections
Pulmonary infections consequential to odontogenic bacteremia have been described, although this is controversial because it can always be argued that bacteria could translocate into the lungs through direct anatomical routes. However, involvement of more than one lung, the multiplicity of lesions, and the nature of the organisms favor an oral-hematogenous spread. It has therefore been argued that such episodes are a result of spread from an oral niche due to the presence of poor oral hygiene or recent orogingival manipulation. Selenomonas spp., viridans group streptococci, Streptococcus intermedius, and Actinomyces odontolyticus have been implicated in acute respiratory distress syndrome, infection of the postpneumonectomy space, septic pulmonary embolism, and lung abscesses, respectively (13, 23, 38, 129). Interestingly, however, almost all subjects with such pulmonary infections were shown to have a compromised immune status due to advanced malignancies.
Septicemia
Similarly, there are many reports implicating odontogenic bacteremia as a causal factor for septicemia, particularly in individuals with hematological malignancies (35, 64, 97, 98, 105, 131, 138). In a recent study, even among otherwise healthy subjects, a majority of subjects with Leptotrichia buccalis septicemia were shown to have some form of intraoral infective focus (135). Yet in a vast majority of these cases, the connectivity between the oral cavity and the systemic focus is tenuous and, at best, circumstantial.
Infections in Pregnancy
Although a recent well-controlled randomized trial by Michalowicz and coworkers (87) found no association between periodontal indices and pregnancy outcomes, there is increasing evidence to indicate that adverse pregnancy outcomes, such as preterm birth and premature rupture of the membranes, are associated with poor maternal oral health, particularly chronic periodontitis (14, 69, 78, 79, 130). This could be due to a low level of chronic bacteremia as well as to chronic inflammation, which upregulates the levels of cytokines and other inflammatory modulators.
Furthermore, periodontopathic bacteria could also be detected in placentas of pre-eclamptic women (9). Despite the fact that most infections of the gravid uterus, fetus, newborn, or postpartum uterus originate from the reproductive tract, records indicate at least one instance where oral bacterial species with identical genetic homogeneity were recovered from both the in utero infection and the oral cavity (55). Given the well-known historical fact that the syphilitic spirochete Treponema pallidum crosses the fetoplacental barrier, resulting in neonatal syphilis and associated dental abnormalities (e.g., mulberry molars and Hutchinson's incisors), it is tempting to speculate that oral bacteria, including the spirochete Treponema microdentium and others, may affect the fetus, possibly through fetoplacental passage. Immunological studies have also revealed higher levels of periodontopathogen-specific immunoglobulin M in umbilical cord blood in babies born preterm (14). Whether this is a result of fetoplacental bacterial passage and dissemination or a generalized immune response has yet to be determined. Therefore, much more research is warranted to establish a link between odontogenic bacteremias and fetal and maternal health.
DIAGNOSING THE SOURCE OF BACTEREMIA
The traditional gold standard for the detection of bacteremia is the use of in vitro cultures. For this purpose, liquid, solid, or biphasic culture media have been used with various incubation periods (124). In clinical microbiology laboratories, automated blood culture systems such as the Bactec system are now routinely used for this purpose (21). These automated systems enable the rapid recognition of bacterial growth by emitting fluorescence or a color change of the sample. Once growth is detected, aliquots are subcultured onto different agar media for further definitive identification of the offending organism(s). These automated methods are rapid, yet quantification of bacteremia is difficult, and for this purpose, the lysis filtration method is generally used. Briefly, lysis filtration involves treatment of a defined volume of blood with a medium that can digest the cellular contents and filtering through a membrane of defined pore size to capture the bacteria. The membrane is then inoculated in a growth medium to evaluate bacterial growth. The data yielded are quantitative and thus are more sensitive than those obtained with conventional and automated culture techniques (82, 145).
Once cultured, the bacteria are usually identified using phenotypic characteristics, such as the presence or absence of metabolic pathways, alterations in metabolic pathways, the utilization of substrates, and end product profiles. These tests are miniaturized and packaged into standardized, commercially available kits that allow comparison of the activity profile indices (e.g., API tests). One drawback of these identification methods is that they are exclusively dependent on the growth of the organism in either a liquid or solid culture medium. Given the fact that a large number of oral bacterial species are now known to be extremely slow growing or uncultivable, the value of standard culture methods and growth-dependent identification profiling has recently been questioned (3). As a partial solution to this problem, molecular probe-based identification methods are used and are gaining popularity (45, 99). Unfortunately, though, most of the latter methods are also dependent upon the successful isolation of the bacterial species in the primary culture. The latest approaches in detecting bloodstream infections, such as PCR-single-strand conformation polymorphism and direct amplification and sequencing of the 16S rRNA genes, which do not rely on in vitro culture, appear to be the solution to this impasse (101, 134). Nevertheless, such approaches have yet to be used and validated in prospective trials of experimental odontogenic bacteremias.
Whenever a bacterial species is isolated from a clinical blood sample, the next issue that needs to be addressed is its origin. A vast number of studies on odontogenic bacteremias and systemic infections of odontogenic origins are based on circumstantial evidence that relates the oral organism and those of the infective focus. These include factors such as poor oral hygiene in the affected individual, a history of dental treatment in the immediate past, or a traumatic event related to the oral cavity (Table 3). The veracity of these assumptions and the significance of the information can be ascertained only when the same phenotype is isolated, in particular when the isolates from the two niches are genotypically identical. Phenotypic similarities between the isolates from the two sites have been demonstrated by biochemical profiles, chromatographic evidence of cellular macromolecules, sodium dodecyl sulfate-polyacrylamide gel electrophoresis of cellular proteins, and antibiotic sensitivity patterns (11, 31, 103). However, only a few workers have attempted to determine the genetic homogeneity of the isolates, despite the greater robustness of this method. For the latter purpose, pulsed-field gel electrophoresis of genomic DNA and ribotyping have commonly been used, although more recently, the use of 16S rRNA gene sequence homogeneity and bioinformatics-based methods has shown promising results in assessing clonality (30, 55, 64, 65, 86, 118).
Also notable is the fact that molecular sequence-based approaches have higher sensitivities than do cultivation-based approaches. In addition, sequence-based detection methods based on universal 16S rRNA genes or other specific bacterial gene markers could be employed as markers of a treatment response when cultures fail to show bacterial growth. One drawback of these rapid and sensitive molecular diagnostic methods, however, is that they do not lend themselves to antimicrobial sensitivity testing, as the organism cannot be physically grown in culture. This information is important for clinicians, who rely on laboratory antimicrobial sensitivity tests to initiate or modify treatment (107). In clinical terms, the molecular typing methods to determine clonality should be considered adjuncts in situations where the clinical presentation is atypical or when there is no or a poor response to antimicrobial therapy.
BACTERIAL DIVERSITY IN ODONTOGENIC BACTEREMIA
As opposed to the more than 700 bacterial species that inhabit the oral cavity, relatively fewer species have been isolated from blood cultures for odontogenic bacteremias. As shown in Table 4, there are at least eight bacterial taxonomic families that are represented in different oral niches. Of these, members of the family Firmicutes occupy the bulk of the microbiome in terms of the numbers of genera and bacterial species (1, 36, 70). Phylogenetic studies of the oral microbiome have shown that a large proportion of the oral bacteria comprise the genus Streptococcus. This holds true for the isolates in experimental odontogenic bacteremias as well (Table 2). In a number of controlled clinical trials, streptococci were the predominant organisms isolated, ranging from 40 to 65% of isolates (39, 48, 109, 110, 132, 140). Other genera of the Firmicutes family have been isolated from the bloodstream, to a lesser extent than streptococci, and include species of the Abiotrophia, Dialister, Selenomonas, and Solobacterium genera, in descending order. The Fusobacteria family is the second most commonly isolated bacterial family from the bloodstream and includes genera such as Leptotrichia and Fusobacteria, while other families, such as Actinobacteria, Synergistes, and Bacteroidetes, are far less common. Finally, there are no data in the literature describing the dissemination of either Spirochaetes or recently detected families, such as the Obsidian pool and TM7 bacteria, from the mouth in experimental odontogenic bacteremias or systemic infections secondary to dental treatment procedures. The last two families are not known to possess any cultivable species thus far.
TABLE 4.
Bacterial families and genera that inhabit oral nichesa
| Family | Genus |
|---|---|
| Firmicutes | Abiotrophia |
| Anaerococcus | |
| Anaeroglobus | |
| Bulleidia | |
| Butyrivibrio | |
| Catonella | |
| Centipeda | |
| Dialister | |
| Eubacterium | |
| Filifactor | |
| Gemella | |
| Granulicatella | |
| Johnsonella | |
| Lactobacillus | |
| Megasphaera | |
| Micromonas | |
| Mogibacterium | |
| Paenibacillus | |
| Peptococcus | |
| Peptostreptococcus | |
| Schwartzia | |
| Selenomonas | |
| Solobacterium | |
| Staphylococcus | |
| Streptococcus | |
| Veillonella | |
| Fusobacteria | Leptotrichia |
| Fusobacterium | |
| Actinobacteria | Actinobaculum |
| Actinomyces | |
| Atopobium | |
| Corynebacterium | |
| Rothia | |
| Synergistes | Synergistes |
| Spirochaetes | Treponema |
| Proteobacteria | Kingella |
| Lautropia | |
| Neisseria | |
| Haemophilus | |
| Desulfobulbus | |
| Cardiobacterium | |
| Campylobacter | |
| TM7 | TM7 clone |
| Bacteroidetes | Capnocytophaga |
| Porphyromonas | |
| Tannerella | |
| Prevotella | |
| Bacteroides |
Data are from various studies. Note that apart from the domain Bacteria, members of the domain Archaea have also been isolated from different oral niches. However, their role in systemic infections has yet to be evaluated.
CONCLUDING REMARKS
On appraising the literature on odontogenic bacteremia, it is clear that a number of oral manipulative procedures can give rise to bacteremias of oral origin. The highest incidence of bacteremia results from tooth extractions. Periodontal manipulations are also shown to produce a long-lasting (>30 min) bacteremia. This is probably a reflection of the bacterial load and tissue trauma or associated inflammation at these niches. Other oral procedures are not as significant, at least for individuals with healthy oral cavities. Routine oral hygiene measures such as brushing or flossing are unlikely to cause a significant degree of bacteremia, but sporadic cleaning after accumulation of a heavy plaque load should be considered a potential risk factor for a bacteremic state.
As evident from Tables 2 and 3, bacteria of potential oral origin detected in odontogenic bacteremias show some degree of diversity, but to a much lesser extent than that of the oral cavity. It is probable that this lack of diversity of blood-borne bacterial species is due to (i) their virulence attributes that facilitate entry into the bloodstream, (ii) the rapid clearance of the bacteria by host defenses, and (iii) the low threshold of the current detection methods used in clinical laboratories. However, as in the oral niche, the predominant bacterial genus isolated from the blood is Streptococcus. More than half of systemic affections are of streptococcal etiology, indicating that the predominance of streptococci in the oral niche could be a factor in determining their entry into the bloodstream (136).
A vast majority of bacteremias have been detected by cultivation and subsequent phenotypic characterization. Different forms of aerobic and anaerobic culture methods have been utilized. Comparison of different experimental bacteremia studies is difficult because there is no uniform agreement between timing of sampling, volume of blood drawn, and bacteriological identification methods. However, irrespective of the culture method, a larger volume of blood drawn does not necessarily concur with a higher incidence of bacteremia. Quantification of bacteria has often been done using lysis filtration of a defined volume of blood. Novel, more sensitive bacterial detection and identification methods, such as 16S rRNA gene sequencing, have not been used on a large scale. Strangely, though, the two studies that employed PCR of 16S rRNA genes showed lower incidences of bacteremia due to oral manipulations than that reported by culture techniques (68, 118). These differences could possibly be overcome by increasing the template volume as well as the sample size.
Most of the bacteremias considered odontogenic in the literature are based on circumstantial evidence. Hence, it is imperative that future workers study the genetic relatedness of organisms, rather than their phenotypes, in evaluating experimental odontogenic bacteremias.
Apart from bacterial endocarditis, there are a number of other systemic affections caused by bacteria residing in the oral cavity. However, most of the records on such infections are presented in the literature in the form of case reports or as a summary of a few cases. Since case reports are not the preferred mode of editorial boards due to a lack of originality, it is safe to assume that a large number of these go unreported.
It is safe to conclude, then, that odontogenic bacteremias are likely to cause systemic and end organ infections, but it is likely that most such infections are occult and dealt with swiftly by body defenses. The available data provide us only a glimpse of the reality, and much more work, using sophisticated sampling, detection, and analytical techniques, is required to draw firm conclusions. It is hoped that the availability of novel molecular and genomic tests will help to demystify the connection between odontogenic bacteremia and end organ infections in the not too distant future.
REFERENCES
- 1.Aas, J. A., B. J. Paster, L. N. Stokes, I. Olsen, and F. E. Dewhirst. 2005. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 43:5721-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aitken, C., H. Cannell, A. M. Sefton, C. Kerawala, A. Seymour, M. Murphy, R. A. Whiley, and J. D. Williams. 1995. Comparative efficacy of oral doses of clindamycin and erythromycin in the prevention of bacteraemia. Br. Dent. J. 178:418-422. [DOI] [PubMed] [Google Scholar]
- 3.Al Amri, A., A. C. Senok, A. Y. Ismaeel, A. E. Al-Mahmeed, and G. A. Botta. 2007. Multiplex PCR for direct identification of Campylobacter spp. in human and chicken stools. J. Med. Microbiol. 56:1350-1355. [DOI] [PubMed] [Google Scholar]
- 4.Allen, B. L., B. Katz, and M. Hook. 2002. Streptococcus anginosus adheres to vascular endothelium basement membrane and purified extracellular matrix proteins. Microb. Pathog. 32:191-204. [DOI] [PubMed] [Google Scholar]
- 5.Allison, C., A. E. Simor, D. Mock, and H. C. Tenenbaum. 1993. Prosol-chlorhexidine irrigation reduces the incidence of bacteremia during ultrasonic scaling with the Cavi-Med: a pilot investigation. J. Can. Dent. Assoc. 59:673, 676-682. [PubMed] [Google Scholar]
- 6.Andrian, E., D. Grenier, and M. Rouabhia. 2004. In vitro models of tissue penetration and destruction by Porphyromonas gingivalis. Infect. Immun. 72:4689-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armitage, G. C. 1999. Development of a classification system for periodontal diseases and conditions. Ann. Periodontol. 4:1-6. [DOI] [PubMed] [Google Scholar]
- 8.Assaf, M., S. Yilmaz, B. Kuru, S. D. Ipci, U. Noyun, and T. Kadir. 2007. Effect of the diode laser on bacteremia associated with dental ultrasonic scaling: a clinical and microbiological study. Photomed. Laser Surg. 25:250-256. [DOI] [PubMed] [Google Scholar]
- 9.Barak, S., O. Oettinger-Barak, E. E. Machtei, H. Sprecher, and G. Ohel. 2007. Evidence of periopathogenic microorganisms in placentas of women with preeclampsia. J. Periodontol. 78:670-676. [DOI] [PubMed] [Google Scholar]
- 10.Bender, I. B., and A. B. Pressman. 1945. Factors in dental bacteraemia. J. Am. Dent. Assoc. 32:836-853. [Google Scholar]
- 11.Bender, I. B., S. Seltzer, and M. Yermish. 2003. The incidence of bacteremia in endodontic manipulation: preliminary report. 1960. J. Endod. 29:697-700. [DOI] [PubMed] [Google Scholar]
- 12.Bhanji, S., B. Williams, B. Sheller, T. Elwood, and L. Mancl. 2002. Transient bacteremia induced by toothbrushing: a comparison of the Sonicare toothbrush with a conventional toothbrush. Pediatr. Dent. 24:295-299. [PubMed] [Google Scholar]
- 13.Bisiaux-Salauze, B., C. Perez, M. Sebald, and J. C. Petit. 1990. Bacteremias caused by Selenomonas artemidis and Selenomonas infelix. J. Clin. Microbiol. 28:140-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boggess, K. A. 2005. Pathophysiology of preterm birth: emerging concepts of maternal infection. Clin. Perinatol. 32:561-569. [DOI] [PubMed] [Google Scholar]
- 15.Brennan, M. T., M. L. Kent, P. C. Fox, H. J. Norton, and P. B. Lockhart. 2007. The impact of oral disease and nonsurgical treatment on bacteremia in children. J. Am. Dent. Assoc. 138:80-85. [DOI] [PubMed] [Google Scholar]
- 16.Brown, A. R., C. J. Papasian, P. Shultz, F. C. Theisen, and R. E. Shultz. 1998. Bacteremia and intraoral suture removal: can an antimicrobial rinse help? J. Am. Dent. Assoc. 129:1455-1461. [DOI] [PubMed] [Google Scholar]
- 17.Burket, L. W., and M. D. Burn. 1937. Bacteraemias following dental extraction. Demonstration of source of bacteria by means of a non pathogen (Serratia marcescens). J. Dent. Res. 16:521-530. [Google Scholar]
- 18.Cannell, H., C. Kerawala, A. M. Sefton, J. P. Maskell, A. Seymour, Z. M. Sun, and J. D. Williams. 1991. Failure of two macrolide antibiotics to prevent post-extraction bacteraemia. Br. Dent. J. 171:170-173. [DOI] [PubMed] [Google Scholar]
- 19.Carroll, G. C., and R. J. Sebor. 1980. Dental flossing and its relationship to transient bacteremia. J. Periodontol. 51:691-692. [DOI] [PubMed] [Google Scholar]
- 20.Cates, K. L. 1983. Host factors in bacteremia. Am. J. Med. 75:19-25. [DOI] [PubMed] [Google Scholar]
- 21.Cetin, E. S., S. Kaya, M. Demirci, and B. C. Aridogan. 2007. Comparison of the BACTEC blood culture system versus conventional methods for culture of normally sterile body fluids. Adv. Ther. 24:1271-1277. [DOI] [PubMed] [Google Scholar]
- 22.Cherry, M., C. G. Daly, D. Mitchell, and J. Highfield. 2007. Effect of rinsing with povidone-iodine on bacteraemia due to scaling: a randomized-controlled trial. J. Clin. Periodontol. 34:148-155. [DOI] [PubMed] [Google Scholar]
- 23.Christensen, P. J., K. Kutty, R. T. Adlam, T. A. Taft, and B. H. Kampschroer. 1993. Septic pulmonary embolism due to periodontal disease. Chest 104:1927-1929. [DOI] [PubMed] [Google Scholar]
- 24.Clarridge, J. E., III. 2004. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin. Microbiol. Rev. 17:840-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cobe, H. M. 1954. Transitory bacteremia. Oral Surg. Oral Med. Oral Pathol. 7:609-615. [DOI] [PubMed] [Google Scholar]
- 26.Coulter, W. A., A. Coffey, I. D. Saunders, and A. M. Emmerson. 1990. Bacteremia in children following dental extraction. J. Dent. Res. 69:1691-1695. [DOI] [PubMed] [Google Scholar]
- 27.Daly, C., D. Mitchell, D. Grossberg, J. Highfield, and D. Stewart. 1997. Bacteraemia caused by periodontal probing. Aust. Dent. J. 42:77-80. [DOI] [PubMed] [Google Scholar]
- 28.Daly, C. G., D. H. Mitchell, J. E. Highfield, D. E. Grossberg, and D. Stewart. 2001. Bacteremia due to periodontal probing: a clinical and microbiological investigation. J. Periodontol. 72:210-214. [DOI] [PubMed] [Google Scholar]
- 29.Darveau, R. P., A. Tanner, and R. C. Page. 1997. The microbial challenge in periodontitis. Periodontol. 2000 14:12-32. [DOI] [PubMed] [Google Scholar]
- 30.Debelian, G. J., I. Olsen, and L. Tronstad. 1998. Anaerobic bacteremia and fungemia in patients undergoing endodontic therapy: an overview. Ann. Periodontol. 3:281-287. [DOI] [PubMed] [Google Scholar]
- 31.Debelian, G. J., I. Olsen, and L. Tronstad. 1995. Bacteremia in conjunction with endodontic therapy. Endod. Dent. Traumatol. 11:142-149. [DOI] [PubMed] [Google Scholar]
- 32.De Leo, A. A., F. D. Schoenknecht, M. W. Anderson, and J. C. Peterson. 1974. The incidence of bacteremia following oral prophylaxis on pediatric patients. Oral Surg. Oral Med. Oral Pathol. 37:36-45. [DOI] [PubMed] [Google Scholar]
- 33.de Lillo, A., F. P. Ashley, R. M. Palmer, M. A. Munson, L. Kyriacou, A. J. Weightman, and W. G. Wade. 2006. Novel subgingival bacterial phylotypes detected using multiple universal polymerase chain reaction primer sets. Oral Microbiol. Immunol. 21:61-68. [DOI] [PubMed] [Google Scholar]
- 34.del Pozo, J. L., and R. Patel. 2007. The challenge of treating biofilm-associated bacterial infections. Clin. Pharmacol. Ther. 82:204-209. [DOI] [PubMed] [Google Scholar]
- 35.Detry, G., D. Pierard, K. Vandoorslaer, G. Wauters, V. Avesani, and Y. Glupczynski. 2006. Septicemia due to Solobacterium moorei in a patient with multiple myeloma. Anaerobe 12:160-162. [DOI] [PubMed] [Google Scholar]
- 36.Dewhirst, F. E., M. A. Tamer, R. E. Ericson, C. N. Lau, V. A. Levanos, S. K. Boches, J. L. Galvin, and B. J. Paster. 2000. The diversity of periodontal spirochetes by 16S rRNA analysis. Oral Microbiol. Immunol. 15:196-202. [DOI] [PubMed] [Google Scholar]
- 37.Dewire, P., B. E. McGrath, and C. Brass. 1999. Haemophilus aphrophilus osteomyelitis after dental prophylaxis. A case report. Clin. Orthop. Relat. Res. 1999:196-202. [PubMed] [Google Scholar]
- 38.Dietl, C. A., and R. J. Mehran. 2004. Infection of the post-pneumonectomy space after dental procedures. J. Cardiovasc. Surg. (Torino) 45:521-522. [PubMed] [Google Scholar]
- 39.Diz Dios, P., I. Tomas Carmona, J. Limeres Posse, J. Medina Henriquez, J. Fernandez Feijoo, and M. Alvarez Fernandez. 2006. Comparative efficacies of amoxicillin, clindamycin, and moxifloxacin in prevention of bacteremia following dental extractions. Antimicrob. Agents Chemother. 50:2996-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duval, X., and C. Leport. 2008. Prophylaxis of infective endocarditis: current tendencies, continuing controversies. Lancet Infect. Dis. 8:225-232. [DOI] [PubMed] [Google Scholar]
- 41.Elliott, M. B. 1939. Bacteraemia and oral sepsis. Proc. R. Soc. Med. 32:57-64. [PMC free article] [PubMed] [Google Scholar]
- 42.Erverdi, N., A. Acar, B. Isguden, and T. Kadir. 2001. Investigation of bacteremia after orthodontic banding and debanding following chlorhexidine mouth wash application. Angle Orthod. 71:190-194. [DOI] [PubMed] [Google Scholar]
- 43.Erverdi, N., S. Biren, T. Kadir, and A. Acar. 2000. Investigation of bacteremia following orthodontic debanding. Angle Orthod. 70:11-14. [DOI] [PubMed] [Google Scholar]
- 44.Ewald, C., S. Kuhn, and R. Kalff. 2006. Pyogenic infections of the central nervous system secondary to dental affections—a report of six cases. Neurosurg. Rev. 29:163-166. [DOI] [PubMed] [Google Scholar]
- 45.Fenollar, F., and D. Raoult. 2007. Molecular diagnosis of bloodstream infections caused by non-cultivable bacteria. Int. J. Antimicrob. Agents 30(Suppl. 1):S7-S15. [DOI] [PubMed] [Google Scholar]
- 46.Fine, D. H., I. Korik, D. Furgang, R. Myers, A. Olshan, M. L. Barnett, and J. Vincent. 1996. Assessing pre-procedural subgingival irrigation and rinsing with an antiseptic mouthrinse to reduce bacteremia. J. Am. Dent. Assoc. 127:641-646. [DOI] [PubMed] [Google Scholar]
- 47.Flood, T. R., L. P. Samaranayake, T. W. MacFarlane, A. McLennan, D. MacKenzie, and F. Carmichael. 1990. Bacteraemia following incision and drainage of dento-alveolar abscesses. Br. Dent. J. 169:51-53. [DOI] [PubMed] [Google Scholar]
- 48.Forner, L., T. Larsen, M. Kilian, and P. Holmstrup. 2006. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J. Clin. Periodontol. 33:401-407. [DOI] [PubMed] [Google Scholar]
- 49.Geerts, S. O., M. Nys, M. P. De, J. Charpentier, A. Albert, V. Legrand, and E. H. Rompen. 2002. Systemic release of endotoxins induced by gentle mastication: association with periodontitis severity. J. Periodontol. 73:73-78. [DOI] [PubMed] [Google Scholar]
- 50.Gendron, R., D. Grenier, and L. Maheu-Robert. 2000. The oral cavity as a reservoir of bacterial pathogens for focal infections. Microbes Infect. 2:897-906. [DOI] [PubMed] [Google Scholar]
- 51.Gould, F. K., T. S. Elliott, J. Foweraker, M. Fulford, J. D. Perry, G. J. Roberts, J. A. Sandoe, R. W. Watkin, et al. 2006. Guidelines for the prevention of endocarditis: report of the Working Party of the British Society for Antimicrobial Chemotherapy. J. Antimicrob. Chemother. 57:1035-1042. [DOI] [PubMed] [Google Scholar]
- 52.Hahn, C. L., H. A. Schenkein, and J. G. Tew. 2005. Endocarditis-associated oral streptococci promote rapid differentiation of monocytes into mature dendritic cells. Infect. Immun. 73:5015-5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hall, G., A. Heimdahl, and C. E. Nord. 1996. Effects of prophylactic administration of cefaclor on transient bacteremia after dental extraction. Eur. J. Clin. Microbiol. Infect. Dis. 15:646-649. [DOI] [PubMed] [Google Scholar]
- 54.Hall, G., C. E. Nord, and A. Heimdahl. 1996. Elimination of bacteraemia after dental extraction: comparison of erythromycin and clindamycin for prophylaxis of infective endocarditis. J. Antimicrob. Chemother. 37:783-795. [DOI] [PubMed] [Google Scholar]
- 55.Han, Y. W., A. Ikegami, N. F. Bissada, M. Herbst, R. W. Redline, and G. G. Ashmead. 2006. Transmission of an uncultivated Bergeyella strain from the oral cavity to amniotic fluid in a case of preterm birth. J. Clin. Microbiol. 44:1475-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hartzell, J. D., D. Torres, P. Kim, and G. Wortmann. 2005. Incidence of bacteremia after routine tooth brushing. Am. J. Med. Sci. 329:178-180. [DOI] [PubMed] [Google Scholar]
- 57.Heckmann, J. G., C. J. Lang, H. Hartl, and B. Tomandl. 2003. Multiple brain abscesses caused by Fusobacterium nucleatum treated conservatively. Can. J. Neurol. Sci. 30:266-268. [DOI] [PubMed] [Google Scholar]
- 58.Henderson, B., and M. Wilson. 1998. Commensal communism and the oral cavity. J. Dent. Res. 77:1674-1683. [DOI] [PubMed] [Google Scholar]
- 59.Hunter, K. M., D. W. Holborow, T. B. Kardos, C. T. Lee-Knight, and M. M. Ferguson. 1989. Bacteraemia and tissue damage resulting from air polishing. Br. Dent. J. 167:275-278. [DOI] [PubMed] [Google Scholar]
- 60.Hurwitz, G. A., W. T. Speck, and G. B. Keller. 1971. Absence of bacteremia in children after prophylaxis. Oral Surg. Oral Med. Oral Pathol. 32:891-894. [DOI] [PubMed] [Google Scholar]
- 61.Iida, Y., K. Honda, T. Suzuki, S. Matsukawa, T. Kawai, T. Shimahara, and H. Chiba. 2004. Brain abscess in which Porphyromonas gingivalis was detected in cerebrospinal fluid. Br. J. Oral Maxillofac. Surg. 42:180. [DOI] [PubMed] [Google Scholar]
- 62.Kaar, T. K., E. R. Bogoch, and H. R. Devlin. 2000. Acute metastatic infection of a revision total hip arthroplasty with oral bacteria after noninvasive dental treatment. J. Arthroplasty 15:675-678. [DOI] [PubMed] [Google Scholar]
- 63.Kazanci, E., K. K. Oguz, A. Gurgey, and M. Topcu. 2005. Streptococcus oralis as a risk factor for middle cerebral artery thrombosis. J. Child. Neurol. 20:611-613. [DOI] [PubMed] [Google Scholar]
- 64.Kennedy, H. F., D. Morrison, M. E. Kaufmann, M. S. Jackson, J. Bagg, B. E. Gibson, C. G. Gemmell, and J. R. Michie. 2000. Origins of Staphylococcus epidermidis and Streptococcus oralis causing bacteraemia in a bone marrow transplant patient. J. Med. Microbiol. 49:367-370. [DOI] [PubMed] [Google Scholar]
- 65.Kennedy, H. F., D. Morrison, D. Tomlinson, B. E. Gibson, J. Bagg, and C. G. Gemmell. 2003. Gingivitis and toothbrushes: potential roles in viridans streptococcal bacteraemia. J. Infect. 46:67-70. [DOI] [PubMed] [Google Scholar]
- 66.Khairat, O. 1966. The non-aerobes of post-extraction bacteremia. J. Dent. Res. 45:1191-1197. [DOI] [PubMed] [Google Scholar]
- 67.Khouzam, R. N., A. M. El-Dokla, and D. L. Menkes. 2006. Undiagnosed patent foramen ovale presenting as a cryptogenic brain abscess: case report and review of the literature. Heart Lung 35:108-111. [DOI] [PubMed] [Google Scholar]
- 68.Kinane, D. F., M. P. Riggio, K. F. Walker, D. MacKenzie, and B. Shearer. 2005. Bacteraemia following periodontal procedures. J. Clin. Periodontol. 32:708-713. [DOI] [PubMed] [Google Scholar]
- 69.Klebanoff, M., and K. Searle. 2006. The role of inflammation in preterm birth—focus on periodontitis. BJOG 113(Suppl. 3):43-45. [DOI] [PubMed] [Google Scholar]
- 70.Kroes, I., P. W. Lepp, and D. A. Relman. 1999. Bacterial diversity within the human subgingival crevice. Proc. Natl. Acad. Sci. USA 96:14547-14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lafaurie, G. I., I. Mayorga-Fayad, M. F. Torres, D. M. Castillo, M. R. Aya, A. Baron, and P. A. Hurtado. 2007. Periodontopathic microorganisms in peripheric blood after scaling and root planing. J. Clin. Periodontol. 34:873-879. [DOI] [PubMed] [Google Scholar]
- 72.Lamey, P. J., T. W. Macfarlane, D. W. Patton, L. P. Samaranayake, and M. M. Ferguson. 1985. Bacteraemia consequential to sialography. Br. Dent. J. 158:218-220. [DOI] [PubMed] [Google Scholar]
- 73.Lewis, K. 2007. Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol. 5:48-56. [DOI] [PubMed] [Google Scholar]
- 74.Li, X., K. M. Kolltveit, L. Tronstad, and I. Olsen. 2000. Systemic diseases caused by oral infection. Clin. Microbiol. Rev. 13:547-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lindqvist, C., and P. Slatis. 1985. Dental bacteremia—a neglected cause of arthroplasty infections? Three hip cases. Acta Orthop. Scand. 56:506-508. [DOI] [PubMed] [Google Scholar]
- 76.Lockhart, P. B., M. T. Brennan, M. L. Kent, H. J. Norton, and D. A. Weinrib. 2004. Impact of amoxicillin prophylaxis on the incidence, nature, and duration of bacteremia in children after intubation and dental procedures. Circulation 109:2878-2884. [DOI] [PubMed] [Google Scholar]
- 77.Lofthus, J. E., M. Y. Waki, D. L. Jolkovsky, J. Otomo-Corgel, M. G. Newman, T. Flemmig, and S. Nachnani. 1991. Bacteremia following subgingival irrigation and scaling and root planing. J. Periodontol. 62:602-607. [DOI] [PubMed] [Google Scholar]
- 78.Lopez, N. J., I. Da Silva, J. Ipinza, and J. Gutierrez. 2005. Periodontal therapy reduces the rate of preterm low birth weight in women with pregnancy-associated gingivitis. J. Periodontol. 76:2144-2153. [DOI] [PubMed] [Google Scholar]
- 79.Lopez, N. J., P. C. Smith, and J. Gutierrez. 2002. Periodontal therapy may reduce the risk of preterm low birth weight in women with periodontal disease: a randomized controlled trial. J. Periodontol. 73:911-924. [DOI] [PubMed] [Google Scholar]
- 80.Lucas, V., and G. J. Roberts. 2000. Odontogenic bacteremia following tooth cleaning procedures in children. Pediatr. Dent. 22:96-100. [PubMed] [Google Scholar]
- 81.Lucas, V. S., A. Kyriazidou, M. Gelbier, and G. J. Roberts. 2007. Bacteraemia following debanding and gold chain adjustment. Eur. J. Orthod. 29:161-165. [DOI] [PubMed] [Google Scholar]
- 82.Lucas, V. S., V. Lytra, T. Hassan, H. Tatham, M. Wilson, and G. J. Roberts. 2002. Comparison of lysis filtration and an automated blood culture system (BACTEC) for detection, quantification, and identification of odontogenic bacteremia in children. J. Clin. Microbiol. 40:3416-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lucas, V. S., J. Omar, A. Vieira, and G. J. Roberts. 2002. The relationship between odontogenic bacteraemia and orthodontic treatment procedures. Eur. J. Orthod. 24:293-301. [DOI] [PubMed] [Google Scholar]
- 84.Macfarlane, T. W., M. M. Ferguson, and C. J. Mulgrew. 1984. Post-extraction bacteraemia: role of antiseptics and antibiotics. Br. Dent. J. 156:179-181. [DOI] [PubMed] [Google Scholar]
- 85.Mantadakis, E., V. Danilatou, A. Christidou, E. Stiakaki, and M. Kalmanti. 2003. Capnocytophaga gingivalis bacteremia detected only on quantitative blood cultures in a child with leukemia. Pediatr. Infect. Dis. J. 22:202-204. [PubMed] [Google Scholar]
- 86.Marques da Silva, R., D. A. Caugant, R. Josefsen, L. Tronstad, and I. Olsen. 2004. Characterization of Streptococcus constellatus strains recovered from a brain abscess and periodontal pockets in an immunocompromised patient. J. Periodontol. 75:1720-1723. [DOI] [PubMed] [Google Scholar]
- 87.Michalowicz, B. S., J. S. Hodges, A. J. DiAngelis, V. R. Lupo, M. J. Novak, J. E. Ferguson, W. Buchanan, J. Bofill, P. N. Papapanou, D. A. Mitchell, S. Matseoane, and P. A. Tschida. 2006. Treatment of periodontal disease and the risk of preterm birth. N. Engl. J. Med. 355:1885-1894. [DOI] [PubMed] [Google Scholar]
- 88.Michelow, I. C., G. H. McCracken, Jr., P. M. Luckett, and K. Krisher. 2000. Abiotrophia spp. brain abscess in a child with Down's syndrome. Pediatr. Infect. Dis. J. 19:760-763. [DOI] [PubMed] [Google Scholar]
- 89.Murphy, A. M., C. G. Daly, D. H. Mitchell, D. Stewart, and B. H. Curtis. 2006. Chewing fails to induce oral bacteraemia in patients with periodontal disease. J. Clin. Periodontol. 33:730-736. [DOI] [PubMed] [Google Scholar]
- 90.Nanci, A., and D. D. Bosshardt. 2006. Structure of periodontal tissues in health and disease. Periodontol. 2000 40:11-28. [DOI] [PubMed] [Google Scholar]
- 91.Novak, M. J., and H. J. Cohen. 1991. Depolarization of polymorphonuclear leukocytes by Porphyromonas (Bacteroides) gingivalis 381 in the absence of respiratory burst activation. Infect. Immun. 59:3134-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Okabe, K., K. Nakagawa, and E. Yamamoto. 1995. Factors affecting the occurrence of bacteremia associated with tooth extraction. Int. J. Oral Maxillofac. Surg. 24:239-242. [DOI] [PubMed] [Google Scholar]
- 93.Oncag, O., B. Cokmez, S. Aydemir, and T. Balcioglu. 2005. Investigation of bacteremia following nasotracheal intubation. Paediatr. Anaesth. 15:194-198. [DOI] [PubMed] [Google Scholar]
- 94.Page, R. C., S. Offenbacher, H. E. Schroeder, G. J. Seymour, and K. S. Kornman. 1997. Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Periodontol. 2000 14:216-248. [DOI] [PubMed] [Google Scholar]
- 95.Pasqualini, L., A. Mencacci, A. M. Scarponi, C. Leli, G. Fabbriciani, L. Callarelli, G. Schillaci, F. Bistoni, and E. Mannarino. 2008. Cervical spondylodiscitis with spinal epidural abscess caused by Aggregatibacter aphrophilus. J. Med. Microbiol. 57:652-655. [DOI] [PubMed] [Google Scholar]
- 96.Paster, B. J., S. K. Boches, J. L. Galvin, R. E. Ericson, C. N. Lau, V. A. Levanos, A. Sahasrabudhe, and F. E. Dewhirst. 2001. Bacterial diversity in human subgingival plaque. J. Bacteriol. 183:3770-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Patel, J. B., J. Clarridge, M. S. Schuster, M. Waddington, J. Osborne, and I. Nachamkin. 1999. Bacteremia caused by a novel isolate resembling Leptotrichia species in a neutropenic patient. J. Clin. Microbiol. 37:2064-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pedersen, L. M., O. R. Madsen, and E. Gutschik. 1993. Septicaemia caused by an unusual Neisseria meningitidis species following dental extraction. Scand. J. Infect. Dis. 25:137-139. [PubMed] [Google Scholar]
- 99.Peters, R. P., M. A. van Agtmael, S. A. Danner, P. H. Savelkoul, and C. M. Vandenbroucke-Grauls. 2004. New developments in the diagnosis of bloodstream infections. Lancet Infect. Dis. 4:751-760. [DOI] [PubMed] [Google Scholar]
- 100.Petrunov, B., S. Marinova, R. Markova, P. Nenkov, S. Nikolaeva, M. Nikolova, H. Taskov, and J. Cvetanov. 2006. Cellular and humoral systemic and mucosal immune responses stimulated in volunteers by an oral polybacterial immunomodulator “Dentavax.” Int. Immunopharmacol. 6:1181-1193. [DOI] [PubMed] [Google Scholar]
- 101.Qian, Q., Y. W. Tang, C. P. Kolbert, C. A. Torgerson, J. G. Hughes, E. A. Vetter, W. S. Harmsen, S. O. Montgomery, F. R. Cockerill III, and D. H. Persing. 2001. Direct identification of bacteria from positive blood cultures by amplification and sequencing of the 16S rRNA gene: evaluation of BACTEC 9240 instrument true-positive and false-positive results. J. Clin. Microbiol. 39:3578-3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rahn, R., S. Schneider, O. Diehl, V. Schafer, and P. M. Shah. 1995. Preventing post-treatment bacteremia: comparing topical povidone-iodine and chlorhexidine. J. Am. Dent. Assoc. 126:1145-1149. [DOI] [PubMed] [Google Scholar]
- 103.Rajasuo, A., K. Perkki, S. Nyfors, H. Jousimies-Somer, and J. H. Meurman. 2004. Bacteremia following surgical dental extraction with an emphasis on anaerobic strains. J. Dent. Res. 83:170-174. [DOI] [PubMed] [Google Scholar]
- 104.Rautemaa, R., A. Lauhio, M. P. Cullinan, and G. J. Seymour. 2007. Oral infections and systemic disease—an emerging problem in medicine. Clin. Microbiol. Infect. 13:1041-1047. [DOI] [PubMed] [Google Scholar]
- 105.Reig, M., F. Baquero, M. Garcia-Campello, and E. Loza. 1985. Leptotrichia buccalis bacteremia in neutropenic children. J. Clin. Microbiol. 22:320-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Reinhardt, R. A., R. W. Bolton, and G. Hlava. 1982. Effect of nonsterile versus sterile water irrigation with ultrasonic scaling on postoperative bacteremias. J. Periodontol. 53:96-100. [DOI] [PubMed] [Google Scholar]
- 107.Rice, P. A., and G. E. Madico. 2005. Polymerase chain reaction to diagnose infective endocarditis: will it replace blood cultures? Circulation 111:1352-1354. [DOI] [PubMed] [Google Scholar]
- 108.Richey, R., D. Wray, and T. Stokes. 2008. Prophylaxis against infective endocarditis: summary of NICE guidance. BMJ 336:770-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Roberts, G. J., H. S. Holzel, M. R. Sury, N. A. Simmons, P. Gardner, and P. Longhurst. 1997. Dental bacteremia in children. Pediatr. Cardiol. 18:24-27. [DOI] [PubMed] [Google Scholar]
- 110.Roberts, G. J., E. C. Jaffray, D. A. Spratt, A. Petrie, C. Greville, M. Wilson, and V. S. Lucas. 2006. Duration, prevalence and intensity of bacteraemia after dental extractions in children. Heart 92:1274-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Roberts, G. J., R. Watts, P. Longhurst, and P. Gardner. 1998. Bacteremia of dental origin and antimicrobial sensitivity following oral surgical procedures in children. Pediatr. Dent. 20:28-36. [PubMed] [Google Scholar]
- 112.Roiz, M. P., F. G. Peralta, and R. Arjona. 1997. Kingella kingae bacteremia in an immunocompetent adult host. J. Clin. Microbiol. 35:1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rosa, E. A., R. N. Rached, O. Tanaka, F. Fronza, and R. Araujo Assad. 2005. Preliminary investigation of bacteremia incidence after removal of the Haas palatal expander. Am. J. Orthod. Dentofacial Orthop. 127:64-66. [DOI] [PubMed] [Google Scholar]
- 114.Rousee, J. M., D. Bermond, Y. Piemont, C. Tournoud, R. Heller, P. Kehrli, M. L. Harlay, H. Monteil, and B. Jaulhac. 2002. Dialister pneumosintes associated with human brain abscesses. J. Clin. Microbiol. 40:3871-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ruiz, T., C. Lenox, M. Radermacher, and K. P. Mintz. 2006. Novel surface structures are associated with the adhesion of Actinobacillus actinomycetemcomitans to collagen. Infect. Immun. 74:6163-6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Samaranayake, L. P. 2006. Microbiology of periodontal disease, p. 275-285. In Essential microbiology for dentistry, 3rd ed. Elsevier, Philadelphia, PA.
- 117.Samuel, W., M. Dryden, M. Sampson, A. Page, and H. Shepherd. 1997. Spinal abscess of Haemophilus paraphrophilus. A case report. Spine 22:2763-2765. [DOI] [PubMed] [Google Scholar]
- 118.Savarrio, L., D. Mackenzie, M. Riggio, W. P. Saunders, and J. Bagg. 2005. Detection of bacteraemias during non-surgical root canal treatment. J. Dent. 33:293-303. [DOI] [PubMed] [Google Scholar]
- 119.Schlein, R. A., E. M. Kudlick, C. A. Reindorf, J. Gregory, and G. C. Royal. 1991. Toothbrushing and transient bacteremia in patients undergoing orthodontic treatment. Am. J. Orthod. Dentofacial Orthop. 99:466-472. [DOI] [PubMed] [Google Scholar]
- 120.Sconyers, J. R., J. J. Crawford, and J. D. Moriarty. 1973. Relationship of bacteremia to toothbrushing in patients with periodontitis. J. Am. Dent. Assoc. 87:616-622. [DOI] [PubMed] [Google Scholar]
- 121.Scopp, I. W., and L. D. Orvieto. 1971. Gingival degerming by povidone-iodine irrigation: bacteremia reduction in extraction procedures. J. Am. Dent. Assoc. 83:1294-1296. [DOI] [PubMed] [Google Scholar]
- 122.Senn, L., J. M. Entenza, and G. Prod'hom. 2006. Adherence of Abiotrophia defectiva and Granulicatella species to fibronectin: is there a link with endovascular infections? FEMS Immunol. Med. Microbiol. 48:215-217. [DOI] [PubMed] [Google Scholar]
- 123.Shain, H., K. A. Homer, and D. Beighton. 1996. Degradation and utilisation of chondroitin sulphate by Streptococcus intermedius. J. Med. Microbiol. 44:372-380. [DOI] [PubMed] [Google Scholar]
- 124.Shanson, D. C., I. Moule, and M. Tadayon. 1985. Clinical comparison of anaerobic blood-culture media for detecting bacteraemia due to viridans streptococci and oral anaerobes. J. Med. Microbiol. 19:187-193. [DOI] [PubMed] [Google Scholar]
- 125.Soto, A., P. H. McWhinney, C. C. Kibbler, and J. Cohen. 1998. Cytokine release and mitogenic activity in the viridans streptococcal shock syndrome. Cytokine 10:370-376. [DOI] [PubMed] [Google Scholar]
- 126.Stepanovic, S., T. Tosic, B. Savic, M. Jovanovic, G. K'Ouas, and J. P. Carlier. 2005. Brain abscess due to Actinobacillus actinomycetemcomitans. APMIS 113:225-228. [DOI] [PubMed] [Google Scholar]
- 127.Sweet, J. B., V. J. Gill, M. J. Chusid, and R. J. Elin. 1978. Nitroblue tetrazolium and Limulus assays for bacteremia after dental extraction: effect of topical antiseptics. J. Am. Dent. Assoc. 96:276-281. [DOI] [PubMed] [Google Scholar]
- 128.Takai, S., T. Kuriyama, M. Yanagisawa, K. Nakagawa, and T. Karasawa. 2005. Incidence and bacteriology of bacteremia associated with various oral and maxillofacial surgical procedures. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 99:292-298. [DOI] [PubMed] [Google Scholar]
- 129.Takiguchi, Y., T. Terano, and A. Hirai. 2003. Lung abscess caused by Actinomyces odontolyticus. Intern. Med. 42:723-725. [DOI] [PubMed] [Google Scholar]
- 130.Tarannum, F., and M. Faizuddin. 2007. Effect of periodontal therapy on pregnancy outcome in women affected by periodontitis. J. Periodontol. 78:2095-2103. [DOI] [PubMed] [Google Scholar]
- 131.Tee, W., P. Midolo, P. H. Janssen, T. Kerr, and M. L. Dyall-Smith. 2001. Bacteremia due to Leptotrichia trevisanii sp. nov. Eur. J. Clin. Microbiol. Infect. Dis. 20:765-769. [DOI] [PubMed] [Google Scholar]
- 132.Tomas, I., M. Alvarez, J. Limeres, C. Potel, J. Medina, and P. Diz. 2007. Prevalence, duration and aetiology of bacteraemia following dental extractions. Oral Dis. 13:56-62. [DOI] [PubMed] [Google Scholar]
- 133.Tomas, I., F. Pereira, R. Llucian, R. Poveda, P. Diz, and J. V. Bagan. 2008. Prevalence of bacteraemia following third molar surgery. Oral Dis. 14:89-94. [DOI] [PubMed] [Google Scholar]
- 134.Turenne, C. Y., E. Witwicki, D. J. Hoban, J. A. Karlowsky, and A. M. Kabani. 2000. Rapid identification of bacteria from positive blood cultures by fluorescence-based PCR-single-strand conformation polymorphism analysis of the 16S rRNA gene. J. Clin. Microbiol. 38:513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ulstrup, A. K., and S. H. Hartzen. 2006. Leptotrichia buccalis: a rare cause of bacteraemia in non-neutropenic patients. Scand. J. Infect. Dis. 38:712-716. [DOI] [PubMed] [Google Scholar]
- 136.Van Dyke, T. E., E. Bartholomew, R. J. Genco, J. Slots, and M. J. Levine. 1982. Inhibition of neutrophil chemotaxis by soluble bacterial products. J. Periodontol. 53:502-508. [DOI] [PubMed] [Google Scholar]
- 137.Vergis, E. N., P. N. Demas, S. J. Vaccarello, and V. L. Yu. 2001. Topical antibiotic prophylaxis for bacteremia after dental extractions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 91:162-165. [DOI] [PubMed] [Google Scholar]
- 138.Vidal, A. M., J. C. Sarria, R. C. Kimbrough III, and Y. K. Keung. 2000. Anaerobic bacteremia in a neutropenic patient with oral mucositis. Am. J. Med. Sci. 319:189-190. [DOI] [PubMed] [Google Scholar]
- 139.Wagner, K. W., R. Schon, M. Schumacher, R. Schmelzeisen, and D. Schulze. 2006. Case report: brain and liver abscesses caused by oral infection with Streptococcus intermedius. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 102:e21-e23. [DOI] [PubMed] [Google Scholar]
- 140.Wahlmann, U., B. Al-Nawas, M. Jutte, and W. Wagner. 1999. Clinical and microbiological efficacy of single dose cefuroxime prophylaxis for dental surgical procedures. Int. J. Antimicrob. Agents 12:253-256. [DOI] [PubMed] [Google Scholar]
- 141.Waki, M. Y., D. L. Jolkovsky, J. Otomo-Corgel, J. E. Lofthus, S. Nachnani, M. G. Newman, and T. F. Flemmig. 1990. Effects of subgingival irrigation on bacteremia following scaling and root planing. J. Periodontol. 61:405-411. [DOI] [PubMed] [Google Scholar]
- 142.Wilhelm, N., S. Sire, A. Le Coustumier, J. Loubinoux, M. Beljerd, and A. Bouvet. 2005. First case of multiple discitis and sacroiliitis due to Abiotrophia defectiva. Eur. J. Clin. Microbiol. Infect. Dis. 24:76-78. [DOI] [PubMed] [Google Scholar]
- 143.Wilson, W., K. A. Taubert, M. Gewitz, P. B. Lockhart, L. M. Baddour, M. Levison, A. Bolger, C. H. Cabell, M. Takahashi, R. S. Baltimore, J. W. Newburger, B. L. Strom, L. Y. Tani, M. Gerber, R. O. Bonow, T. Pallasch, S. T. Shulman, A. H. Rowley, J. C. Burns, P. Ferrieri, T. Gardner, D. Goff, and D. T. Durack. 2007. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 116:1736-1754. [DOI] [PubMed] [Google Scholar]
- 144.Yamalik, M. K., S. Yucetas, and U. Abbasoglu. 1992. Effects of various antiseptics on bacteremia following tooth extraction. J. Nihon Univ. Sch. Dent. 34:28-33. [DOI] [PubMed] [Google Scholar]
- 145.Zierdt, C. H., D. L. Peterson, J. C. Swan, and J. D. MacLowry. 1982. Lysis-filtration blood culture versus conventional blood culture in a bacteremic rabbit model. J. Clin. Microbiol. 15:74-77. [DOI] [PMC free article] [PubMed] [Google Scholar]