Abstract
Congenital cytomegalovirus (CMV) infection is the leading infectious cause of mental retardation and hearing loss in the developed world. In recent years, there has been an improved understanding of the epidemiology, pathogenesis, and long-term disabilities associated with CMV infection. In this review, current concepts regarding the pathogenesis of neurological injury caused by CMV infections acquired by the developing fetus are summarized. The pathogenesis of CMV-induced disabilities is considered in the context of the epidemiology of CMV infection in pregnant women and newborn infants, and the clinical manifestations of brain injury are reviewed. The prospects for intervention, including antiviral therapies and vaccines, are summarized. Priorities for future research are suggested to improve the understanding of this common and disabling illness of infancy.
INTRODUCTION
Congenital cytomegalovirus (CMV) infection is a major public health concern. CMV causes serious neurodevelopmental sequelae, including mental retardation, cerebral palsy, and sensorineural hearing loss (SNHL). Even with antiviral therapy, these injuries are often irreversible. The pathogenesis of injury to the developing fetal central nervous system (CNS) is unknown. This review focuses on potential pathogenic mechanisms by which CMV injures the CNS. This includes analysis of the cell types infected with CMV, the pattern of injury to the fetal brain, and the long-term neurodevelopmental impact. Multiple mechanisms are proposed to play a potential role(s) in CNS injury. These include the following: CMV acting as a “teratogen,” disrupting normal cellular differentiation and morphogenesis pathways; the impact on apoptosis and antiapoptotic mechanisms; the role of neural stem cells; the critical developmental windows of susceptibility; the role of the inflammatory processes in potentiating CNS injury; and the potential pathogenic impact of CMV on the endovascular system. Insights into the pathogenesis of CNS injury caused by CMV have been obtained from studies in primate, mouse, and guinea pig models. An improved understanding of the pathogenesis of CNS injury should help direct translational vaccine and antiviral interventions that can be applied to the management of infected infants.
Background and Epidemiology of Congenital CMV Infection
Magnitude of the problem.
Congenital CMV infection is the major cause of birth defects and childhood disorders in the United States. It is estimated that about 40,000 children (0.2 to 2% of all deliveries) are born with CMV, resulting in about 400 fatal cases each year (44). Only 10 to 15% of children with congenital CMV infection exhibit clinical signs at birth, although even children who appear asymptomatic at birth are at risk for neurodevelopmental sequelae (34). Most children (60 to 90%) with symptomatic infection, and 10 to 15% of those with asymptomatic infection, develop one or more long-term neurological sequelae, such as mental retardation, psychomotor retardation, SNHL, and ophthalmologic abnormalities (12, 35, 91, 142). Current estimates indicate that approximately 8,000 children are affected each year with some neurological sequelae related to in utero CMV infection. This incidence is far greater than that of better-known childhood disorders, such as Down syndrome (4,000/year), fetal alcohol syndrome (5,000/year), or spinal bifida (3,500/year), making congenital CMV infection the most common cause of birth defects and childhood disabilities in the United States (44). Considering the public health significance of CMV-related long-term neurological disabilities, it is surprising that more attention is not paid to understanding the neuropathogenesis of congenital CMV infection. This review addresses current concepts regarding the epidemiology, pathogenic mechanisms, and intervention strategies being considered for this important clinical problem.
Prevalence and risk factors.
CMV infection is ubiquitous in the human population, and most individuals are eventually infected. Human CMV is a large DNA virus belonging to the family Herpesviridae. Like all herpesviruses, CMV establishes a lifelong latency in the host, with periodic reactivations (180). The overall age-adjusted prevalence of CMV in the United States is about 60%. Although only 0.5 to 1% of children acquire CMV in utero, 40% acquire the infection within the first decade of life. Seroprevalence increases to >80% by the age of 60 (132, 255). Seroprevalence varies among different socioeconomic and ethnic groups and increases among individuals with proximity to infected children or working in childcare facilities (83, 193, 255). It is quite well documented that the risk of congenital CMV is the greatest from a primary infection (i.e., infection in a seronegative individual) of the mother during pregnancy. Transplacental transmission of virus occurs in about one-third of mothers with primary CMV infection (39, 85, 132), and approximately one-half of these infections in utero result in a symptomatic clinical syndrome (6). Epidemiological data suggest that the timing of acquisition of primary infection relative to the establishment of pregnancy is an important factor in establishing the risk to the fetus for in utero transmission (222). Although women who are CMV seropositive preconception are less likely to give birth to an infant with congenital CMV than women who have a primary CMV infection during pregnancy, transplacental transmission with its attendant sequelae still occurs in this setting (fetal infection rate of 1.4% [132]). Transmission in this setting appears to be related to reinfection of seropositive women with new strains of CMV (34, 39). Maternal nonprimary infections account for the major disease burden associated with congenital CMV. It has recently become appreciated that congenitally infected infants born to women with preconception immunity are at substantial risk for long-term neurological sequelae (39, 81, 85, 225). In a study of 300 children with confirmed congenital CMV, Ross et al. observed that in congenitally infected babies born to seropositive women, the incidence of hearing loss and other congenital damage was similar to that observed in congenital infection occurring in the setting of primary gestational infection (226). Better data are needed regarding the incidence of congenital CMV infection, since congenital infection rates have been examined in relatively few populations. The advent of routine newborn screening for congenital CMV could provide a clearer picture of the overall disease burden (224).
Seronegative women of child-bearing age (15 to 44 years of age) undergoing primary infection have the highest risk of transplacental transmission of CMV. For a pregnant women, exposure to CMV-infected children, often her own children who have acquired infection in group day care, is a common primary source of infection (3). Young children shed virus at mucosal surfaces for prolonged periods of time. It is well documented that both symptomatic and asymptomatic infants excrete virus in the urine and saliva for many years after birth. Virus shedding in the urine is often detectable until ∼10 years of age, with the mean shedding interval being ∼4 years (189). In addition, between 15 to 70% of children acquire CMV infection in group day care settings and continue to shed the virus for 6 to 48 months (mean = 18 months) after primary infection (3). Because of chronic nature of CMV infection in young children, they serve as an excellent reservoir for the virus. Pregnant women who have provided care to young children a year before delivery have an increased risk for maternal CMV infection, and this situation increases the risk of transmission of the virus to the fetus (83). CMV infection is readily transmitted to the pregnant mother at mucosal surfaces via infected urine, saliva, or other bodily fluids, but respiratory or aerosol transmission is not common (169). There is also potential for fomite-mediated transmission of CMV (238). Simple hygienic practices such as hand washing can dramatically reduce infection rates in pregnant mothers (44).
Sexual activity is an important mode of virus transmission in women of reproductive age (256). CMV can readily be isolated from the genital tracts of both sexes (42). In young women, a risk factor for congenital CMV infection is the recent onset of sexual activity (83). This study noted that early sexual debut (<16 years), a history of multiple sexual partners, and a history of sexually transmitted infections were not risk factors for transplacental transmission (83), possibly because these activities are associated with seroconversion rates early after the onset of sexual activity but prior to the onset of child-bearing. Since a longer interval between primary infection and pregnancy allows women sufficient time to develop high-avidity antibody to CMV, this in turn may result in a decreased risk of transplacental transmission (248, 255).
In utero infection is believed to be due to maternal viremia with attendant hematogenous spread to the fetus. The rate of materno-fetal transmission is influenced by numerous factors, including trimester of exposure, maternal age, CMV serostatus, character of maternal immunity, and viral loads. The risk of fetal transmission appears to increase with gestational age, but neurological outcomes are more severe when infection occurs during the first trimester (6, 184, 196, 198). However, viral transmission can occur during the entire gestation period, and neurological outcomes may still be seen from infections acquired in late gestation (198, 254). Young maternal age increases the risk for congenital CMV infection. Women who are 20 years of age or less at delivery have a three-times-greater likelihood of delivering an infected infant than older women (42, 84). This increase in age-related risk may be due to a greater probability of primary exposure to the virus in this age group or may be a combination of age-related biological effects on CMV replication (83, 85).
Although is generally accepted that preconception immunity to CMV provides a substantial protective effect against materno-fetal transmission, there have been reports suggesting that maternal antibody titers alone may not be a good indicator of fetal protection (34, 39, 85, 224). The presence of maternal antibodies has been shown to be associated with a decreased incidence of CMV and with improved neurological outcomes in the setting of congenital infection (71, 83). Paradoxically, immunoglobulin G (IgG) antibodies to the viral glycoprotein B (gB) after primary infection are significantly increased in maternal and newborn delivery sera for infants who develop hearing loss (33, 38), suggesting a possible increased exposure to viral antigen. It appears that the qualitative aspects of the antibody response (i.e., presence of neutralizing, high-avidity antibodies) are a critical indicator of fetal protection (33, 148). Antibody-mediated protection against fetal transmission is not absolute. As already noted, the failure of preconception immunity to provide complete protection against congenital CMV infection may be strongly related to reinfection with different strains of CMV with new antibody specificities (39). This observation complicates the development of CMV vaccines based on single proteins such as envelope glycoproteins, since immunity to glycoproteins from one strain may not protect against reinfection with another strain with a different protein-coding sequence in key neutralizing epitopes.
Maternal antibodies are known to effectively cross the maternal-fetal interface, conferring passive immunity to infections. However, antibody may actually facilitate transmission of CMV across the placenta barrier. CMV has been shown to utilize maternal IgG to cross the placenta via transcytosis as IgG-virion complexes utilizing the neonatal Fc receptor (FcRn) that is expressed on the surface of syncytiotrophoblasts (16). It is postulated that IgG-virion complexes formed of high-avidity neutralizing antibodies may be quickly neutralized by villus core macrophages on the fetal side, whereas low-avidity antibody complexes allow virus to escape the macrophages and infect the fetus (161). Thus, in this model, the timing of infection relative to the establishment of pregnancy and the antibody avidity to CMV are critical determinants of protection. Low-avidity antibodies persist for up to 20 weeks after a primary infection (148), and this may be a window of high risk. Once infection of the fetus is established, it is not clear what role antibody plays in ameliorating the risk of injury. In one study, passive administration of CMV antibodies in the postnatal period did not alter the development of certain neurological sequelae, including progressive hearing loss, in the setting of congenital CMV infection (37). The chief benefit of antibody may be its effect on prevention of transplacental transmission. However, there is recent evidence that CMV immune globulin may be therapeutic for a fetus already infected in utero. Therapeutic administration of high-titer CMV Ig during pregnancy in women with evidence of primary infection has been shown in uncontrolled trials to decrease transmission to the fetus, improve ultrasonic abnormalities in the developing fetus, and improve overall placental health (5, 184). Controlled studies are required to confirm these observations and to further examine the potential benefits of immune globulin both in utero and in the newborn.
CMV infection acquired in utero has the potential to result in considerable neurodevelopmental morbidity. Remarkably, hearing loss due to congenital CMV infection can progress through early childhood even when it is clinically unapparent at birth. While it is difficult to accurately predict the severity of congenital CMV infection, several predictive criteria have been suggested (149). Serial ultrasonograms or cranial computed tomography scans are useful to detect overt pathological alterations in the fetal brains of symptomatic children and can accurately predict development of cognitive and motor deficiencies (11, 36, 188). Recently, head ultrasound has also been shown to be of value in predicting the magnitude of injury in the newborn CNS in the setting of congenital infection (11). The lack of detectable lesions in asymptomatic newborns may not preclude them from developing hearing loss later in life (11). Lazzarotto et al. suggested a three-pronged approach for the diagnosis of and prediction of outcomes in congenital CMV infection (149): (i) screening for maternal antibodies to determine primary infection in the mother, including assessment of an avidity index of CMV IgG; (ii) prenatal ultrasound to examine for the presence of fetal abnormalities; and finally (iii) amniocentesis with quantitative PCR analysis for CMV-specific DNA in the amniotic fluid. Thus far, quantitative analysis of viral load has proved to be the best predictor for neurological damage in congenital CMV infection (149). Levels of greater than 1,000 genome copy equivalents in the amniotic fluid were 100% predictive of fetal transmission, and higher loads (>5,000 copies) were predictive of symptomatic infections (149). The increase in viral genomes in the amniotic fluid may reflect the magnitude of the viral load in the fetus, manifest as viral replication in the fetal kidney and excretion via the fetal urine.
PATHOLOGY
Among the primary clinical manifestations associated with congenital CMV infection, the most devastating are those involving the developing CNS, since in contrast to other end-organ injury, CNS injury is generally believed to be irreversible. The most commonly observed symptoms of CMV infection at birth are intrauterine growth retardation, purpura, jaundice, hepatosplenomegaly, microencephaly, hearing impairment, and thrombocytopenia (11, 142). While clinical signs due to abnormalities of the reticuloendothelial system (such as anemia, hepatosplenomegaly, and jaundice) are transient, neurological deficits either are evident at birth and typically persist for life or tend to become evident (as SNHL) in early childhood.
Brain Structural Anomalies, Imaging Abnormalities, and Clinical Correlation
The earliest demonstrated structural brain abnormalities have been observed through fetal imaging studies, as early as 28 weeks of gestation, using either magnetic resonance images or ultrasonograms. T2- and T1-weighted magnetic resonance imaging scans of CMV-infected fetal brains show white matter abnormalities reflective of acute responses, such as loss of intermediate zone layer, focal necrosis, and hemorrhaging. More commonly seen are chronic lesions due to infection, which include ventricular dilatation, white matter gliosis, atrophy (volume loss), parenchymal cysts, ependymal cysts, calcifications, and cortical malformations (most notably polymicrogyria [22]). Fetal sonographic analyses at between 22 and 37 weeks of gestation also detect structural changes in the brain. Transvaginal ultrasonograms show different patterns of abnormal periventricular hyper/hypoechogenicity, ventricular adhesions, cystic formation around the ventricles, ependymal protrusions, abnormal sulcations, and hypoplastic corpus collosum (162). Periventricular cysts develop during the second trimester, cerebellar lesions probably are the result of fetal infection before 18 weeks of gestation, and abnormal sulcations probably are due to injury between 18 and 24 weeks (162). Fetal imaging studies are useful in determining the time and extent of fetal infection, and these findings may in turn help prognosticate neurological outcomes (23).
Neonatal and postnatal imaging of children with symptomatic CMV is almost always associated with structural brain abnormalities similar to those observed in the infected fetus (Fig. 1). The most frequent of these is the presence of intracranial calcifications (50%). Computed tomography scans also show other abnormal changes, such as ventriculomegaly, white matter changes, polymicrogyria, cysts, structural abnormalities, and extensive encephalopathy (36, 188). Other abnormalities observed in the spectrum of neuroimaging/pathological abnormalities include lissencephaly, porencephaly, and schizencephaly (Fig. 1C). Abnormal results from cranial ultrasonograms (showing periventricular or parenchymal calcifications, increased ventricular size, and cerebellar lesions) performed during the first week of life are helpful for symptomatic children in predicting development of some neurological deficit in life (11). Typically, asymptomatic children do not show extensive calcifications or ventriculomegaly (283). The presence of distinct but subtle patterns of white matter lesions with or without polymicrogyria and in combination with anterior temporal lobe cysts is suggestive of CMV infection (274). At least one case of asymptomatic white matter magnetic resonance lesions has been reported in an infant who also developed SNHL (104). The extent to which these white matter lesions seen in magnetic resonance imaging correlate with development of hearing loss or other neurological syndromes is yet to be determined.
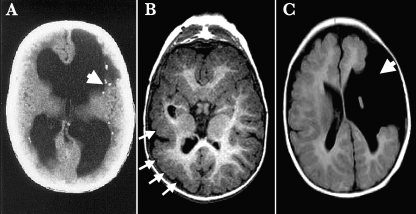
FIG. 1.
Neurological outcomes of congenital CMV infection. Examples of computed tomography (A) and magnetic resonance imaging (B and C) of three infants with severe symptomatic congenital CMV infection with CNS involvement are shown. The classical pattern of injury described with congenital CMV infection involving the CNS is characterized by periventricular calcifications (panel A, arrow). Other consequences of fetal brain infection include abnormalities of neuronal migration, leading to polymicrogyria (panel B, arrows) and, in extreme cases, profound structural defects such as porencephalic cysts with associated schizencephaly (panel C, arrow).
Auditory Abnormalities
CMV is currently the leading cause of nonhereditary SNHL in children (192). CMV infection is associated with 10 to 60% of all SNHL in children. CMV-related SNHL either can be manifested at birth or may be progressive in nature, with deterioration in hearing potentially occurring over the first several years of life. Numerous epidemiological studies have linked congenital CMV infection to the development of SNHL (196), but the viral and/or host inflammatory mechanisms involved in the pathogenesis of auditory dysfunction remain unclear. In the era of routine immunization for Haemophilus influenzae type B infection, CMV has now emerged as the most common infectious disease associated with SNHL in children (196).
The frequency of hearing loss in children due to CMV infection is between 0.2 and 1.3/1,000 live births (196). The risk of SNHL is higher among children with symptomatic CMV infections (30 to 65%) than among those with asymptomatic congenital infection (7 to 15%). Hearing loss is often the only sequela identified in the latter group (61, 115, 283). Various studies define SNHL in children differently, complicating the estimation of the full magnitude of the effect of CMV on the incidence of hearing disabilities. One useful definition, proposed by Fowler et al., defined normal hearing in a child as perception of sounds that range between 0 and 20 dB at frequencies of between 20 and 20,000 Hz. SNHL was defined as an air-bone gap of less than 10 dB and greater than 21-dB thresholds for the affected frequencies (81, 82). Another aspect of CMV-related SNHL, which has only recently been appreciated, is that the rate of hearing loss increases with age. Hearing loss of less than 20-dB threshold was seen in 5.3% of congenitally infected children at birth (among 338), which increased to 6.5% at 3 months, 8.4% by 1 year, and 15% by 6 years of age. Although virtually all states in the United States mandate universal newborn hearing screening, such screening will fail to identify the majority of cases of CMV-associated SNHL, due to the large proportion of affected children who have hearing loss that has its onset in later childhood or progressively increases over time (81).
The progressive nature of SNHL suggests that there may be a chronic infection in the CNS or endolabryrinth that continues to be active throughout early childhood. Alternatively, it may reflect an alteration in developmental gene expression resulting from in utero infection, although the absence of structural anomalies in the endolabyrinth argues against this hypothesis. Viral load appears to correlate with the risk of SNHL. Increased levels and longer duration of urinary excretion of CMV in both symptomatic and asymptomatic congenitally infected children, early in life, are associated with development of hearing loss. Infants with less than 5,000 PFU/ml infectious virus in the urine or less than 10,000 genome copies/ml in peripheral blood may have a lower likelihood of developing progressive hearing loss (13, 35). Furthermore, children with SNHL or progressive SNHL continue to excrete virus in the urine for >4 years, suggesting that the risk of SNHL is related to ongoing active viral replication and high viral burden in congenitally infected children (189).
Pathogenesis of CMV Labyrinthitis
CMV-induced hearing loss is believed to be caused by virus-induced labyrinthitis (259). Inner ear histology from congenitally infected infants shows damage to structures including the vestibular endolymphatic system and the vestibular organs (saccule and utricle) and collapse of the saccular membrane (62). Damage is restricted to the endolymphatic structures, with minor involvement of the cochlea, manifest mainly as hydrops at the basal turn (62, 217). Inclusion-bearing cells are seen in the epithelium of the endolymphatic sac and are positive for CMV antigens (17, 62). It is postulated that CMV enters the endolymph via the stria vascularis (258), and, compatible with this hypothesis, viral DNA can be detected in the perilymph by quantitative PCR analysis (26, 261). To better understand the damage caused by CMV infection of the inner ear, experimental animal models have been extensively used. Elsewhere in this review, the pathogenesis of CMV-induced labyrinthitis in animal models will be discussed.
Cell Types Infected
The neurotropism of CMV is evident from the predominance of CNS abnormalities observed in the setting of symptomatic congenital infection. However, although the brain is a major target of end-organ damage in this setting, the precise cellular targets of infection remain incompletely characterized. Inclusion bodies are detected during postmortem histological analysis of the brain (201, 236), but few or no histological data identifying the different cell types infected during congenital CMV are available. Most of what we know today about susceptibility of brain cells to CMV infection has been afforded by experiments performed on cultured human brain cells and from animal models of brain infection. In primary human cell culture systems or brain-derived cell lines it has been shown that practically all cell types in the brain have some degree of susceptibility to CMV infection. Brain microvascular endothelial cells (EC) (77, 146, 208), astrocytes (157), neuronal cells (208), oligodendroglial cells (253), microglia/macrophages (213, 239), and neural progenitor/stem cells (51, 167) have a propensity for CMV infection. However, these different cell types vary in their ability to support a complete viral replication cycle, which in turn is largely controlled by the transcription factor milieu within the cell during infection.
Astrocytes, the major cell type, constituting about 70% the brain, support CMV replication. Primary human fetal astrocyte cultures show productive and cytopathic viral replication, with immediate-early (IE) gene expression and early gene promoter upregulation. Titers of infectious virus in cell supernatants show an increase of 2 to 3 log10 units over a course of 5 days (157, 168). Different astroglial cell lines support CMV replication to different levels. Some cell lines, such as BHRA, HS-63, and U373-MG, are permissive for complete viral replication, while in some (glioblastoma and T98G cells) replication is aborted at the IE stage (208).
Astrocytes, in association with brain microvasculature EC (BMVEC), form the blood-brain barrier, a structure that maintains the highly regulated solute and cellular microenvironment in the CNS (1). EC derived from the microvasculature also support productive CMV replication (77, 208). Lytic viral replication is supported by BMVEC, whereas, in contrast to BMVEC, EC from the aorta afford persistent viral replication for up to 30 days without cell lysis (77). Three viral genes have been identified as being critical for CMV infection in EC: UL128, UL130, and UL131A. These genes are expressed in the infected cell postentry during the viral replication cycle (2, 146, 245). These genes have a striking tendency to mutate during cell culture propagation, and this may in turn be related to observed differences in tropism among clinical isolates and laboratory strains (9, 106). Interestingly, CMV infection of microvascular EC promotes monocyte activation, migration, and infection, which may be a potential mechanism of viral dissemination into the brain (29).
In contrast to astrocytes, primary differentiated human neurons are refractory to CMV replication. Highly purified primary neuronal cultures (>90% neurons) contain a small percentage of dividing astrocytes that support viral replication, but viral gene products cannot be detected in neurons (157). The block in viral replication is effected at the level of the major IE promoter (MIEP), not during viral entry (51). The human CMV MIEP, one of the first viral transcriptional regulatory elements activated in a susceptible cell, has numerous transcription modulator elements that may be regulated by the state of membrane polarization or cell differentiation (51, 207, 281). The inhibition of MIEP-mediated transcription in resting neurons is effectively reversed by membrane depolarization. The induction of MIEP activity by potassium-mediated depolarization is dependent on activating the cyclic AMP response element binding protein (CREB) binding elements (281). This observation may have relevance for the pathogenesis of CMV-associated labyrinthitis, since the endolymphatic compartment of the cochlea is a high-potassium, low-sodium environment, whereas the perilymphatic compartment consists of a low-potassium, high-sodium milieu (280). Furthermore, a recent study demonstrated that the MIEP block in neurons can be synergistically reversed by activating the cyclic AMP signaling pathway and inhibiting histone deacetylase-mediated viral gene silencing (130, 172). Similar experiments with undifferentiated human oligodendroglioma cells, representative of immature oligodendrocytes, demonstrate that oligodendrocytes, like neurons, may not be fully permissive for CMV infection. However, CMV IE, US11, and gB gene expression is induced in human oligodendroglioma cells upon differentiation with phorbol myristate acetate, without production of viral progeny (253). Taking these findings together, it appears that the state of cell differentiation as well as functional status may modulate permissiveness to CMV brain infection in utero.
Microglia, the end-differentiated resident brain macrophages, also do not support productive CMV infection (157). CMV DNA has been demonstrated in infected microglial cells in the absence of detectable viral IE proteins (212, 213). Although controversial, it is believed that brain microglia may be replenished from bone marrow-derived precursors that migrate into the brain (reviewed in reference 223). It has been shown in many studies that myeloid precursor cells may be a site for CMV latency and a vehicle of viral dissemination in the host (105, 122, 136). Although myeloid precursors and monocytes are not productively infected by CMV (135, 145), they support productive CMV infection at certain stages of differentiation (247). In addition, EC-adapted viral strains have been shown to infect both macrophages and dendritic cells (93, 244). It is not known if brain macrophages are a potential source of viral infection during fetal development, but the proximity of vascular macrophages to CMV-susceptible cells in the CNS could play a major role in viral dissemination into the brain.
PATHOGENESIS
Developmental Biology Paradigm: Is CMV a Teratogen?
Although it is often stated that CMV is a teratogen for the developing fetus, there is in fact little evidence to support a direct teratogenic role in developing fetal tissue. However, several studies suggest that CMV infection may lead to birth defects, either by direct chromosomal injury or by modulation of developmental gene expression. In a study using human fibroblasts, infection with CMV during the S phase of the cell cycle resulted in two specific chromosome 1 breaks at positions 1q42 and 1q21. Purified virions, and not infected cell supernatants alone, were responsible for the effect, which could be blocked by coincubation of virus with neutralizing antibody. UV-inactivated virus was as efficient as untreated virus in inducing specific damage to chromosome 1, suggesting a requirement for viral adsorption/penetration but not de novo viral gene expression (80). Two loci present near this breakpoint may be of particular interest: DFNA7 and USH2A. The DFNA7 gene has been linked to the inheritance of an autosomal dominant, nonsyndromic, progressive form of hearing loss (74). Perturbation of the DFNA7 gene caused by CMV-induced breakage could conceivably be linked to the development of the progressive SNHL. The USH2A gene, which is physically closest to the most prevalent CMV-induced break described by Fortunato et al. (80), encodes a protein important in the pathogenesis of Usher's syndrome type II, an autosomal recessive disorder responsible for both SNHL and blindness (73, 263). The possible relationship between these chromosomal breaks and the CMV-induced sequelae of SNHL, as well as a possible link to the visual impairment caused by CMV, requires further experimental evaluation.
Apoptosis and Cell Cycle Changes
Programmed cell death, or apoptosis, is a mechanism whereby damaged or infected cells are eliminated from the tissue by an “autodestructive pathway” so as to maintain homeostasis. The apoptotic process is essential for the elimination of damaged or poorly developing cells during organogenesis and is also considered a critical defense mechanism to purge virus-infected cells from the host. To sustain a relatively slow replication cycle and the propensity to establish a lifelong infection in the host, the CMV genome has retained gene products that serve as countermeasures against cellular antiviral processes, including apoptosis. Two distinct pathways mediate apoptosis in the mammalian cell. One, the intrinsic pathway, triggers cellular sensor proteins such as p53 and initiates a cascade of biochemical signals leading to the mitochondrial release of cytochrome c. The other is an extrinsic pathway activated by external signals, primarily involving the immune system, and consequent phosphorylation of receptor death domains, such as those in the tumor necrosis factor (TNF) receptor family and FAS, by their respective ligands. Apoptotic signals, both intrinsic and extrinsic, converge to induce the activation of the caspase family of proteases. These eventually lead to proteolysis of the cell architecture, metabolic derangement, genomic fragmentation, and cell death. Viral replication and biosynthesis constitute a cell stressor that results in the inevitable outcome, cell death (reviewed in references 27 and 28).
CMVs have evolved mechanisms to delay the intrinsic apoptotic signaling pathway, presumably to allow time for completion of their relatively slow replication cycles. The viral IE proteins, IE1 and IE2, which are the major transcriptional regulators of viral replication, are known for their ability to inhibit apoptosis (290). When infected with CMV, human astrocytes turn over phosphatidylserine molecules to the extracellular surface, an early cellular alteration that marks apoptotic cells for destruction by macrophages. However, the subsequent nuclear changes in the apoptotic cycle are delayed (i.e., DNA degradation) until later in the viral replication cycle (157). Viral replication in astrocytes and other cells is known to induce the proapoptotic cell sensor p53 (157, 178, 252). Inhibition or delay of late apoptotic events in CMV-infected cells is associated with sequestering of cytoplasmic p53 by viral IE2 (157, 268). However, CMV IE genes by themselves are not sufficient to prevent cell death. Human CMV carries two antiapoptotic genes that suppress virus-induced apoptosis in the late replication phase. The CMV UL36 gene encodes the viral inhibitor of caspase 8 activation (vICA), and UL37 (exon 1) encodes viral mitochondrion-localized inhibitor of apoptosis (vMIA) (97, 219, 246). vICA inhibits apoptosis by binding to procaspase 8 and prevents its activation to an active form (246). Rodent and macaque CMVs also carry a UL36 homolog, but M36 (the murine CMV [MCMV] homolog) appears to be essential for viral replication in vivo (96, 170). On the other hand, vMIA inhibits apoptosis by interacting with Bax (a proapoptotic molecule) and sequestering it within the mitochondrial membrane as an inactive form (97). vMIA physically interacts with cytosolic Bax to form high-molecular-weight oligomers and subsequently prevents the formation of the mitochondrial permeabilization core complex and release of cytochrome c (14, 209). Viral replication requires expression of vMIA, and deletion of the gene can be counteracted by inhibiting cellular apoptotic responses using caspase inhibitors or the adenoviral E1B 19-kDa antiapoptotic protein (219). In addition, vMIA disrupts the mitochondrial reticular network formation (171) and is responsible for the cell architectural changes during infection (210). Additional viral genes, whose mechanisms of action have yet to be elucidated, are known to inhibit apoptosis in infected cells, such as UL38 of CMV, which blocks caspase-dependent apoptosis (267), and the MCMV gene M41, identified as a Golgi apparatus-resident apoptosis inhibitor (43). Given that homologs of some antiapoptotic viral genes have been identified in both rodent and macaque CMVs (170), it has now become possible to experimentally investigate the in vivo relevance of viral functions that subvert cell death processes during congenital CMV brain infection.
While inhibition of cell death may be essential for virus survival in the susceptible host, apoptotic neuronal damage is observed in brains of patients with congenital CMV infection, particularly around the periventricular zone. However, postmortem examination of brain tissue from patients who developed neurological sequelae due to a symptomatic congenital CMV infection indicates that neuronal and glial apoptoses are absent or rare, with spatial and temporal distance from the initial acute viral infection (64). Viral gene products are absent in areas associated with neuronal apoptosis. Similarly, in the mouse model of CMV brain infection, neuronal apoptosis in MCMV-infected brains is seen in areas both close and distal to the infection site, but the dying cells rarely demonstrate the presence of viral antigen (241). This phenomenon, indicative of bystander apoptosis in uninfected cells, has also been observed in CMV infection of the retina (32). The lack of association between viral products of infection and apoptosis in vivo is further substantiated by the resistance of infected neurons to glutamate-induced apoptosis in vitro (141). This suggests an indirect role for virus-induced neuronal loss by apoptosis. One of the mechanisms of indirect neuronal loss can be explained by virus-induced neuroinflammatory responses, involving cytokines, chemokines, and metabolic intermediates that result in neurotoxicity around and distal to the infection site.
The TNF family of ligands, which includes FasL/Apo1L/CD95L and Apo2L/TRAIL (TNF-related apoptosis-inducing ligand), are extrinsic apoptosis signals expressed in response to tissue injury or inflammation. The ligation of death receptors by FasL and TRAIL induces the direct activation of upstream caspases in the apoptosis signaling cascade (15). FasL, but not TRAIL, is expressed in the CNS along with all of its cognate receptors. The expression of FasL is upregulated during inflammation, presumably to “kill” infiltrating activated T cells and prevent irreparable damage to the CNS. Very little is known about the extrinsic apoptotic pathway in the brain, but there is increasing evidence of its role in regulating normal brain development and its compromised state in neurological disorders (reviewed in references 57 and 183). The human eye is yet another immune-privileged site, like the brain, where retinal cells express FasL and TRAIL. CMV infection of retinal pigment epithelial (RPE) cells upregulates FasL expression, via transactivation by the viral IE2 gene product, which in turn induces apoptosis of T lymphocytes (55, 59). Interestingly, cells that are IE2 positive, but not the IE-negative bystander RPE cells, are resistant to FasL-induced apoptosis. The IE2-mediated block in apoptosis in RPE cells is associated with the induction of expression of cellular cFLIP (Fas-associated death domain-like interleukin-1β-converting enzyme-inhibitory protein), an antiapoptotic molecule that inhibits the activation of caspase 8 by FADD (Fas-associated protein with death domain). CMV IE2 expression also inhibits TRAIL-induced apoptosis in retinal cells, indicating that the block is indeed mediated at the level of a common signaling molecule (FADD) in the apoptosis pathway (56). Similar upregulation of FasL and TRAIL is seen with CMV infection of dendritic cells, indicating that the virus may have evolved a multilayered immune evasion strategy, which in turn may result in the apoptotic damage of bystander cells (56, 216).
In addition to inhibiting apoptosis, CMV IE and early gene products IE 72, IE86, pp71, and pUL69 alter cell cycle progression in human fibroblasts by interacting with cell cycle regulatory proteins. In quiescent fibroblasts, CMV infection quickly (in 6 to 12 h) activates cell cycle regulatory proteins and accelerates cell cycle progression from G0/G1 to early S phase. At later stages of infection, when viral DNA replication is initiated, progression of cellular DNA synthesis is inhibited, and the cell cycle is arrested at a pseudomitotic (G2/M) stage, where chromosome segregation and cytokinesis are blocked (41, 114, 123, 159, 249). Inhibition of cellular DNA synthesis is essential for viral replication (202). Modulation of cell cycle progression in infected cells enables the virus to maximize the availability of cellular DNA replication machinery without competition from the cellular genome for the same resources (for a review, see reference (46). In the developing brain, CMV productively infects astrocytes and neural precursor cells, cell types known for their ability to undergo cell division in vivo. However, as noted, neurons, a terminally differentiated cell type, are not permissive to productive viral infection (51, 157, 167). It is likely that cell cycle alterations in the neural stem/precursor cell populations residing in the ventricular regions of the brain are critically important in the neuropathogenesis of congenital CMV infection. However, very little is known about the effects that viral infection has on neural stem cells (see below), except that infection inhibits cell proliferation and alters differentiation profiles (51, 190, 191).
Neural Stem Cells in CMV Infection
Neural stem/precursor cells, located predominantly in the subventricular zone and subgranular zone of the hippocampus in the adult mammalian brain, have taken center stage in medical research because of their ability to migrate, proliferate, and differentiate into neurons, astrocytes, and oligodendrocytes. These cells potentially can repopulate damaged brain cells and aid in the establishment of new neuronal circuits during memory formation (89, 182, 266). In brain infections of both congenitally infected children and adults, CMV preferentially infects cells in the ventricular or subventricular regions (100, 201, 236), indicating the possibility of CMV replication in the neural stem/precursor cells residing in the region. With currently available protocols it is now possible to maintain human neural precursor cells in culture and differentiate them into neurons, astrocytes, and oligodendrocytes (182). At least a few studies have demonstrated that human CMV replicates efficiently in undifferentiated human neural precursor cells in vitro (51, 167, 190). The extent to which these versatile cells are infected in utero may determine the outcome of CNS sequelae associated with congenital CMV infection. During differentiation, susceptibility to CMV infection could then be retained in glial cells but not in differentiated neurons. The apparent refractiveness of differentiated neurons to CMV infection may, at least in part, be explained by expression of the transcription factor C/EBP β, including a dominant negative isoform that retains its DNA binding domain but has lost the transcriptional activation domain (194). The CMV MIEP has C/EBP binding sites immediately downstream from its proximal NF-κB binding sequence. The dominant negative isoform of C/EBP binds to these enhancer regions and inhibits transcription from the CMV MIEP (51, 211). This is yet another possible mechanism for repression of viral gene expression in neurons. CMV infection of human neural precursor cells inhibits their differentiation into both neurons and astrocytes, perhaps due to virus-induced apoptosis in cells undergoing differentiation (190, 191). CMV replication also inhibits neural precursor cell proliferation, possibly by altering cell cycle mechanisms (51, 191). It is possible that disruption of cellular processes in neural precursor cells may indeed account for a large portion of the structural and migratory abnormalities seen during congenital human CMV infection.
The neural stem cell niche in the mouse has been well defined and extensively studied. The subependymal area around the ventricles in murine models of CMV brain infection is selectively predisposed to CMV infection. Ex vivo cultures of thin brain slices, also called organotypic brain cultures, are useful models to determine cell susceptibility to CMV infection while keeping the three-dimensional architecture of the organ intact. MCMV infection of neural stem cells inhibits their growth and decreases their ability to differentiate into neuronal phenotypes. However, like their human counterpart, stem cells differentiated into glia retain their susceptibility to CMV infection in the mouse (139). Virus-infected cells in organotypic brain cultures immunostain for GFAP (an astrocyte marker) and for nestin and Mushashi (stem cell markers). The susceptibility of brain cells to CMV infection in an organ culture system does not differentiate between C57BL/6 (a relatively resistant strain) and BALB/c (a susceptible strain) mouse brains (128), indicating that strain-dependent differences in resistance to CMV infection are not mediated by an altered susceptibility of the brain cells themselves. There is, however, a lack of infection in mature neurons in the murine model. Although neural stem cells are present in relatively small numbers in the adult mouse brain compared to the fetus or neonate, they are also susceptible to CMV infection (Fig. 2). Interestingly, the primordial embryonic stem (ES) cells are refractory to MCMV infection, which is similar to the case for neurons (the prototypic end-differentiated cell). As the ES cells differentiate toward a glial phenotype, they become susceptible to CMV infection (166), indicating that the state of cellular differentiation may play an important role in determining susceptibility to CMV infection, most likely determined by the cellular transcriptional factor milieu.
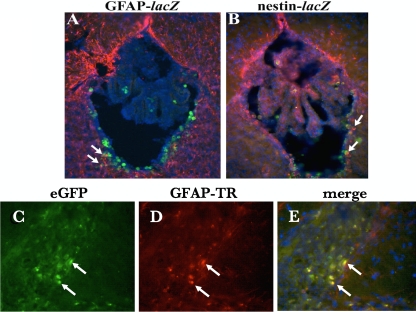
FIG. 2.
Cellular tropism of CMV brain infection in vivo. Coronal murine brain sections immunostained with anti-β-galactosidase antibody display staining (green), indicative of viral infection with the lacZ-containing recombinant MCMV RM461. (A) Double immunohistochemical staining for β-galactosidase and glial fibrilary acidic protein (GFAP) (red) reveals that MCMV has a cellular tropism for astrocytes in the brain (yellow cells). (B) Double immunostaining with nestin (red) indicates that MCMV readily infects neural stem cells, seen just below the ependymal layer of the ventricules of an adult mouse. (C) Coronal section of guinea pig brain following intracerebral inoculation with an enhanced green fluorescent protein (eGFP)-expressing recombinant GPCMV (fluorescein isothiocyanate filter) demonstrating infection of cells in periventricular region. (D) Same section as in panel C following immunohistochemical staining for GFAP (red). (E) Merged image demonstrating eGFP and GFAP colocalization in astrocytes. Blue cells, DAPI (4′,6′-diamidino-2-phenylindole) stain.
Infection of neural stem cells may affect not only prenatal development but also postnatal neuronal development. In mouse studies, fetal brain infection at embryonic day 14.5 (E14.5) to E15.5 shows a dramatic decrease in the numbers of developing neurons (determined by bromodeoxyuridine pulsing) migrating to distal cerebral cortical layers II and III, by half at postnatal day 7. The deeper layers of the cortex (III and IV) were inundated with both infected and immature neuronal cells, indicative of impeded migration of developing neurons to the distal cortical layers (241). CMV infection from the ventricular region is presumed to be carried into the cortical structures by migration of developing neurons. Similar delayed migration of infected neurons is also seen in the cerebellar cortices of CMV-infected neonatal mice. Although postnatal development of the cerebellum is only delayed and not arrested, the delay in development is associated with decreased proliferation and differentiation of granular neuron precursors, potentially mediated by the loss of response to neurotrophins in infected precursors and the induction of inflammatory responses in mononuclear cells (137).
It is interesting to note that infected neural cells retain their ability to migrate, albeit aberrantly (137, 241). CMV-infected migrating neurons express only IE antigens, but glial cells, particularly those seen around the ventricles, express both the IE and late viral proteins (242). The IE viral proteins, IE2 (M128) and IE3 (M122), are expressed in the immature neuronal cells lining the ventricular zone, which decreases as the infection progresses. Additionally, CMV IE expression diverges with differentiation of immature cells in the neonate; IE3 is expressed predominantly in glial cells and IE2 in neurons. Expression of IE2 is concentrated in the cortex and is sustained in cortical neurons up to 2 weeks postinfection (120). It is not clear if MCMV IE2 expression in neurons, which is generally considered to be nonessential for viral replication (163), will alter neuronal physiology.
Developmental Susceptibility to CMV Infection
Neurological symptoms due to CMV infection are relatively unique to congenital infection. In the CMV-infected adult, neurological complications are rare, except in cases of severe immunodeficiency (such as in advanced AIDS and organ transplant patients). The mechanisms that relate to differences in the manifestations and frequency of CMV-induced brain disorders are poorly understood. One explanation for varying disease manifestations may be directly related to the presence of a higher proportion of actively dividing immature neural stem cells in the fetal brain at different fetal ages. The human brain begins development as a thin sheet of neuroepithelial cells that proliferate rapidly to form the neural tube, as early as 4 weeks of gestation. The center of the neural tube ultimately forms the ventricular system, while the neuroepithelial cell divides asymmetrically, along the longitudinal and horizontal axis and in thickness, to form the different brain regions, while sustaining the astrocyte-like neural stem or progenitor cells in the subventricular zone (reviewed in reference 174). The active proliferation of neuroblasts occurs between 5 and 25 weeks of gestation, and the bulk of glial cell development takes place between 20 and 40 weeks. Development of the cortical layer, however, begins as early as 7 weeks, and immature neurons migrate to new regions along a radial axis and form neural networks (reviewed in reference 65).
Neuroepithelial cells (167) and neural progenitor cells (51) isolated from developing human brain tissue are susceptible to CMV infection. In contrast, undifferentiated ES cells do not sustain viral replication (98, 143). The block in viral replication appears to be at the MIEP, with expression being significantly suppressed in human ES cells (143, 286). Given these data, it can be assumed that while the early embryo is not susceptible to CMV infection, the fetus may be infected at as early as 4 weeks of gestation. CMV infections at different gestational ages may have distinct effects on the cellular and developmental patterning of the brain that may ultimately determine neurological outcomes.
While the neuropathogenesis of human CMV infection is not clearly understood, mouse models of congenital CMV infection demonstrate that susceptibility to CMV and outcomes of brain abnormalities are directly related to the gestational age at infection (270). Similar to the case for human ES cells, mouse ES cells do not support productive CMV replication but become susceptible upon differentiation to a glial phenotype (166). In concordance, early blastocysts and postimplantation mouse embryos are not prone to CMV infection, presumably due to a lack of susceptible cells. The mouse embryo acquires permissiveness at E7.5 (125). Infection at the midgastrulation stage (E8.5) results in the development of micropthalmia and cerebral hypoplasia due to infection in the mesodermal tissue (269). Infection of the placenta, at E12.5, results in CMV brain infection, and 25% of the mice showed evidence of microencephaly and growth retardation associated with viral infection in the brain (271). The virus, in animals with brain infection (27%), was located predominantly in the subventricular zone, a region where the neural stem cells also reside (271). Adult mouse brains infused with epidermal growth factor to stimulate neural stem cell proliferation showed higher levels of infected cells and viral titers in the brain (108), suggesting that the susceptibility of adult brains depends on the quantity of neural stem cells present. The numbers of susceptible cells, including neural stem cells and immature glial/neuronal cells, decrease as the brain develops into adulthood and the spatial distribution of susceptible cells becomes more localized to the ventricular and cortical marginal areas (128). It is unclear how the infection of these neural stem cells may affect the neurological outcomes of CMV brain infection.
Second, it is unwise to overlook the possibility that altered fetal immune responses to CMV may explain the increased susceptibility to neuronal abnormalities due to congenital infection. Initial immune responses to viral brain infection are mediated through the nonspecific cellular responses of macrophages, microglia, and NK cells, as well as through the production of cytokines and other soluble mediators by the resident glial cells (astrocytes and microglia). After this initial innate response, adaptive immunity develops and mediates antigen-specific defenses. Both innate and adaptive responses are critical components for defense against viral brain infection (49). In the mouse model, CMV infection in the neonate generates an attenuated interferon (IFN) response compared to a similar infection in the adult (273). Whether the attenuated response is due to a poor glial cell response or proportionally lower numbers of glial cells in the neonate needs to be defined. Neonatal mice infected with CMV in the CNS show evidence of activation of NK cells and macrophages (139). While these local tissue responses are induced by CMV, their involvement in the clearance of viral infection is currently speculative. In addition to the innate responses by resident glial cells, adaptive responses in the fetus may be altered and thus may predispose to CMV infection (see below).
Neuroinflammatory Processes
The traditional view that the brain is immune privileged due to its immunological inert nature and physical separation from the somatic immune system has been dramatically changed by a number of studies in the last decade (90). It is now clear that local CNS cellular responses, mediated largely by astrocytes and microglia, and the somatic immune system interact actively during brain infections. Neuroinflammation has both protective and neurotoxic effects that mediate the outcome of an insult. It is now known that a myriad of CNS-specific responses modulate effector functions of both resident glial cells and infiltrating somatic immune cells, resulting in a specialized response that mediates immune privilege (45).
Ontogeny of the immune response.
The increased susceptibility of the fetus and neonate to many viral infections, including human immunodeficiency virus (HIV), CMV, and herpes simplex virus, is not due to a lack of immune effectors but is associated with their decreased reactivity to antigen compared to adult immunocytes (reviewed in reference 10). Immune cells develop as early as 3 to 4 weeks of gestation, at which time the human embryonic yolk sacs show evidence of granulocyte, macrophage, and erythroid precursors. These primitive immune cells migrate from the yolk sac to the liver by 6 weeks to form the first fetal hematopoeitic organ. The liver provides the niche for further differentiation of primitive precursor cells to macrophages, pro-T and pro-B lymphocytes, and granulocytes. The spleen is fully developed and functional by 18 weeks of gestation, with adequate numbers of functional accessory cells available for antigen presentation. Mature fetal T and B cells are first seen in the fetal circulation as early as 16 weeks of gestation (the development of the immune system in mouse and humans has been extensively reviewed [24, 118, 177]).
Altered immune responses of the fetus may increase susceptibility.
Both clinical and experimental evidence demonstrates that the immune system during pregnancy is skewed to elicit predominantly Th2 responses, thus altering host susceptibility to various pathogens (121). Cytokines, such as interleukin-10 (IL-10), IL-5, and IL-4, predominate at the materno-fetal interface of the placenta, which is essential for maintenance of pregnancy (38, 47). It has been postulated that the microenvironment at the materno-fetal interface selectively downregulates Th1 responses in the fetus, resulting in decreased IFN-γ production, while B cells continue to respond to antigen stimulation to produce IgG and IgM antibodies (118).
Congenitally infected human fetuses can elicit a robust cell-mediated immune response composed predominantly of CD8 lymphocyte effectors, with lower numbers of activated CD4 T cells. Immunophenotypic analysis of the lymphocyte response indicates a switch in circulating T cells toward higher proportions of CMV-specific activated and terminally differentiated effector phenotype (HLA-DR+, CD95+, and CD45RA+ CD28−) as early as 22 to 29 weeks of gestation (72, 164). However these effector CD8+ cells were poor IFN-γ producers in response to CMV antigens (72, 112) and had lower levels of perforin-positive activated cells, although they produced granzyme A (164). The CD8 T-cell response is directed predominantly to two viral proteins, IE1 and pp65. The IE1-specific T cells are detectable for up to 1 year after birth and form the bulk of the T-cell response to CMV later in life (95). The early responses to pp65 and IE1 are elicited by peptides derived from multiple regions of the viral proteins, and the peptide recognition repertoire broadens with age (94). While it is suggested that the CD8 T-cell responses are protective against CMV, it is not clear if decreased cytokine responses or differences in peptide recognition patterns in the fetal response determine the neurological outcome of congenital CMV. Gaining insights into the role of T-cell responses in fetal infection will help in the design of better vaccines for CMV infection. CD8 T cells are critical in protection against MCMV brain infection (21, 49). Interestingly, the lymphocyte response to neonatal brain infection shows a preponderance of CD8+ T cells and is focused against a single immunodominant IE1 epitope (IE1 exon 4168-176) during the acute phase (21). The relevance of immune responses to specific epitopes and their role in protecting against CMV brain infection is still unclear. Nevertheless, investigations such as this in animal models of congenital infection need to devote increased emphasis to the study of the fetal cellular response to CMV infection in utero.
Cytokine-mediated damage.
It is well documented that immune responses in the CNS are mediated by both resident brain cells and immune effectors that infiltrate brain tissue in response to infection or injury. Resident glial cells are the intrinsic sensors in the brain and respond quickly and effectively to neurological insults through the production of soluble mediators, i.e., cytokines and chemokines (reviewed in references 158 and 243). While some of these cytokines have neuroprotective function, overexpression of cytokines may result in neurodegeneration and damage within the CNS (181). Therefore, effective regulation of the innate and adaptive immune responses in the CNS may play a critical role in maintaining the delicate balance between the control of viral infection and immunopathology.
Cultured human glial cells, derived from 16- to 20-week-old fetal brain tissue, respond to CMV infection by expressing a number of immune mediators, including chemokines and cytokines (223). Astroglial cells, which constitute 70% of brain cells, produce chemokines in response to CMV infection. The chemokine response by astrocytes is predominated by the production of CCL2 and less so by the production of CXCL8, CCL3, and CCL5 (53, 54). Interestingly, the cytokine response to CMV infection in astrocytes is restricted to transforming growth factor β, an anti-inflammatory cytokine, which may have an effect on viral replication (138), but none of the proinflammatory cytokines tested (TNF-α, IL-1β, IL-6, IFN-α, IFN-β, and IFN-γ) were found to be induced. On the other hand, microglial cells (resident brain macrophages) respond to CMV infection by producing TNF-α and IL-6 as well as CXCL10, CCL2, CCL3, and CCL5 (53, 54). The proinflammatory cytokines TNF-α and IFN-γ inhibit viral replication in astrocytes by suppressing the CMV MIEP (50). TNF-α induced transcriptional inhibition of the MIEP was mediated at a specific region of the enhancer situated between bp −583 and −242 (Fig. 3). This distal enhancer region is essential for viral replication, but the requirement can be overcome by using a higher multiplicity of infection (173). These findings suggest that proinflammatory cytokine production in the brain may have a protective role in controlling viral spread.
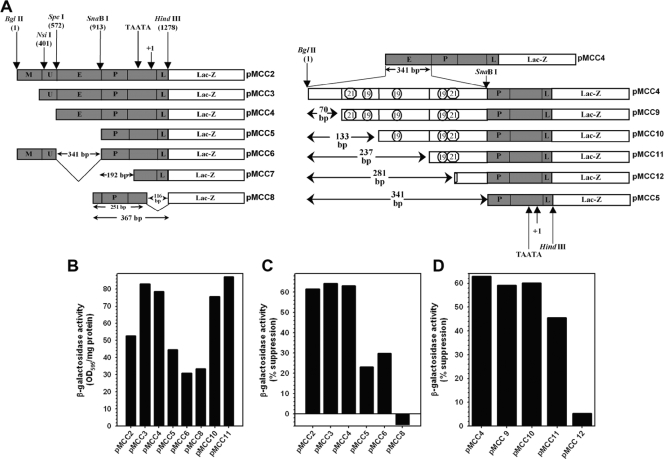
FIG. 3.
TNF-α inhibits CMV MIEP activity in human astrocytes. (A) Schematic description of various truncated MIEP LacZ reporter constructs used to test the effect of cytokines on promoter activity in human astrocytes. M, modulator sequence; U, unique region; E, enhancer sequence; P, basal promoter sequence; L, leader sequence). Numerical positions were assigned relative to the most distal region of the full-length MIEP used. (B to D) Primary human astrocytes, either treated with TNF-α for 48 h or untreated, were transfected with each of the indicated plasmids using Fugene 6 reagent (Roche, Indianapolis, IN). Culture lysates were harvested at 72 h posttransfection and analyzed for β-galactosidase activity, which was normalized to total protein and compared to expression from untreated cells. (B) β-Galactosidase expression from truncated MIEP constructs are expressed as units of optical density at 595 nm (OD595) per mg protein. (C and D) The effect of gross deletions of the MIEP on TNF-α-mediated suppression of promoter activity in astrocytes is expressed as percent suppression, where expression levels from TNF-α-treated astrocytes were compared to the expression of the same plasmid construct in untreated cells. Based on the pattern of reduction of reporter gene expression among various constructs, the effect of TNF-α is mediated largely at the level of the enhancer. Data are representative of at least three experiments performed with astrocytes from different donors.
Chemokines are responsible for recruiting peripheral immune cells into the CNS during infection (119). In a mouse model of CMV brain infection, we have demonstrated that active recruitment of T cells into the CNS is essential for protection. While the initial infection established during the first 3 days is controlled in the absence of peripheral lymphocytes, sustained protection and control of viral spread within the CNS are mediated by a perforin-dependent cytotoxic (CD8+) T-cell response. Viral infection also induces CXCL9 and CXCL10, which are known T-cell chemoattractants that precede lymphocyte infiltration. In addition, the infiltrating lymphocytes, which are a source for IFN-γ in the brain, transiently amplify the virus-initiated CXCL10 response (48, 49). This acute cytokine response, although not critical for protection against CMV brain infection in this model, is regulated by the anti-inflammatory cytokine IL-10. Interestingly, lack of IL-10 expression leads to a severely dysregulated IFN-γ response and renders a benign CMV brain infection lethal. Although lack of IL-10 has little effect on viral clearance, the levels of IL-6, IFN-γ, CXCL10, and CCL2 are dramatically increased. However, not all the cytokines are dysregulated in the absence of IL-10; TNF-α, IL-1β, and CCL5 levels are relatively unaffected (52). Interestingly, human CMV carries its own IL-10 homolog that inhibits CXCL10 production in human microglial cells, which consequently inhibits lymphocyte migration (53). An analogous IL-10 homolog has not been identified in MCMV. The IL-10-mediated control of IFN-γ responses in the infected mouse brain is mediated predominantly by CD45hi CD11b− cells, a phenotype that characterizes infiltrating lymphocytes (52). The brain-infiltrating leukocyte profile in the absence of IL-10 and the mechanisms that regulate cytokine induction in brain cells are currently under investigation. It appears that protection against CMV infection and the regulation of cytokine responses are mediated by distinctly separate mechanisms, both of which are essential for protecting the brain from deleterious consequences of viral infection.
It is known that infiltration of peripheral cells into the brain and the resultant production of the proinflammatory cytokine milieu are the first steps leading to many neurological disorders (195). However, the mechanisms that cause neurotoxicity during CMV brain infection are not fully understood. There is evidence for two possible paradigms for cytokine-induced brain damage: (i) cytokines and their inducible cellular by-products are neurotoxic, or (ii) cytokines produced in response to viral infection alter neural stem cell migration and differentiation. Recent studies of neonatal CMV infection in mice have suggested that the delay in cerebellar development due to infection is associated with the inflammatory response which transiently perturbs the developmental program (137). More studies are needed to determine the contributions of these mechanisms to CMV neuropathogenesis.
Placental Insufficiency: Vascular Damage, Hypoxia, and Altered Permeability
The efficiency of viral transmission at the different stages of placental development may influence fetal infection. The placenta, a six-layer barrier that separates the maternal and fetal circulations, is progressively eroded by the invasion of trophoblasts into the maternal decidua, ultimately fusing together to form syncytiotrophoblasts (107). It is not until the second trimester that the invasion of trophoblasts effectively fenestrates the maternal bloodstream enough to allow exchange of oxygen and nutrients between the maternal and fetal blood to occur. Although there is evidence that CMV-related pathology is mediated by direct infection of the fetus, recent studies have shown that while the placenta serves as an amplifying reservoir and effective conduit for viral transmission, CMV infection of placental cells may also contribute to the pathogenesis of congenital CMV infection by altering placental formation, ultimately resulting in placental insufficiency (6, 200).
CMV infection of placental cytotrophoblasts perturbs their cellular gene expression profile. Of note is the robust repression of genes associated with trophoblast differentiation and invasion and with formation/stabilization of the extracellular matrix (230). CMV infection markedly decreases the expression of α1β1 integrin (laminin/collagen receptor) and other integrin molecules (α9 and β6) on cytotrophoblasts. Consequently, CMV-infected trophoblasts demonstrate impaired cell adhesion and invasion properties (78, 161). In addition, viral infection alters the activity of matrix metalloproteinases (MMP), in particular MMP9, in the placenta and inhibits expression of HLA-G molecules on cytotrophoblasts (78, 287). Upregulation of MMP9, together with the expression of integrin molecules and tissue inhibitor of metalloproteinases, is essential for coordinating placental remodeling and modulating the depth of trophoblast invasion during normal development (25). Human CMV infection of trophoblasts results in the expression of the viral IL-10 homolog and also induces cellular IL-10, both of which inhibit MMP9 expression in placental cells (287). It is interesting to note that cellular proteins dysregulated during CMV infection of placental trophoblasts are similar to those altered in preeclamsia, a condition characterized by poor placentation and intrauterine growth reduction (6, 152).
The timing of trophoblast infection during gestation would determine pregnancy outcomes associated with placental insufficiency. Trophoblast infection seen during the first trimester in chorionic villi (199) could adversely affect placental development. Infection of the trophoblast early in gestation can impair proper implantation and hence contribute to pregnancy loss. In the later stages of pregnancy, improper development of the placenta may result in intrauterine growth reduction and other fetal outcomes resulting from placental pathology. Ultrasound examination of the placenta at between 16 and 36 weeks of gestation showed a significant thickening in pregnant women with primary CMV infection. Placental pathology is strongly associated with fetal and neonatal disease (147). In addition, it is possible that CMV-mediated inhibition of the immunoregulatory major histocompatibility complex molecule HLA-G increases the susceptibility of invading trophoblasts to elimination by the maternal immune response, further endangering placental formation. Hence, it is plausible that some of the clinical features of cytomegalic inclusion disease could be explained by fetal hypoxia resulting from placental insufficiency and hypoperfusion, which could in turn contribute to the pathogenesis of brain abnormalities such as polymicrogryria. Many of these symptoms are resolved after birth, presumably with proper nutrition and adequate oxygenation. Furthermore, postnatal CMV infections are not associated with the symptoms described for placental insufficiency, suggesting that placental insufficiency may play a critical role in the pathogenesis of congenital CMV (6).
ANIMAL MODELS
Although many elegant experiments investigating the pathogenic mechanisms of CMV brain infection have been performed in animal models, an obvious caveat with these animal systems is the strict host specificity of CMVs, requiring the use of viruses that may have a different biology than their human counterparts. However, these systems have been used to model many aspects of congenital infection, including neuropathogenesis and responses to vaccines which could not otherwise be tested for efficacy. The development of CMV cross-species chimeras has also helped bridge some genetic differences among this group of viruses.
Given this caveat, CMVs have comparable genetic makeups, with many genes that have both sequence and functional homologs. They generally have similar pathogenic mechanisms in their host species, which can be used to model human CMV infections. Among the animal models for CMVs (summarized in Table 1), only guinea pig CMV (GPCMV), porcine CMV (PCMV), and rhesus macaque CMV (RhCMV) are known for their ability to cross the placental barrier during a natural infection, resulting in fetal infection (reviewed in reference 228). Anatomically, the guinea pig and the rhesus macaque placentations are classified as hemomonochorial, similar to the case for the human placenta, and are characterized by distinct villous projections, cytotrophoblast invasion, and fenestration of maternal blood vessels that extend into the maternal myometrium. However, anatomical differences in the placental structure, fetal fenestrations into the maternal blood vessels, and extent of fetal-maternal interaction alone do not contribute to in utero transmission. Structurally, the pig placenta forms a more robust placental barrier with a relatively unique epitheliochorial anatomy. During natural infection, PCMV can be transmitted to the fetus, producing disease in multiple fetal organs systems (70). Interestingly, a recently isolated strain of rat CMV (RCMV) strain, ALL-03, has been shown to cause congenital infection in the pregnant rat (156). In addition, the mouse placenta can also be infected with CMV in severely immunodeficient mice, demonstrating pathological outcomes similar to those of human congenital CMV infection (285). Regardless of the mechanisms of transmission into the fetus, the ability of the virus to infect the brain is the single most important contribution to prognosis of CMV infection in the neonate (48, 221, 269) and hence is a critically important experimental end point to keep in perspective in the study of animal models of CMV-associated diseases.
TABLE 1.
Comparison of animal models of CMV infection and pathogenesis
| Animal (order) | CMV | Viral genome size (bp) | Disease models | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Chimpanzee (Primatea) | CCMV | ∼241,000 | Unstudied | Genetic relatedness of CCMV/human CMV; relevant primate model for human CMV | Expense of animals; incompletely defined model |
| Rhesus macaque (Primatea) | RhCMV | ∼221,000 | Congenital and fetal CMV infections; CMV/HIV interactions | Similarity to human CMV pathogenesis; neuropathogenesis, hearing loss; genetic relatedness of RhCMV/human CMV; genome sequenced, mutants available; vaccine studies | Expense of animals; difficulty in maintaining RhCMV-seronegative colonies for congenital infection studies |
| Tupaia (Scandentia) | TCMV | ∼195,000 | Unstudied | Genome sequenced | Lack of reagents; unclear relevance to human CMV disease |
| Pig (Artiodactyla) | PCMV | Incompletely sequenced | Congenital and perinatal transmission | Optimize safety of porcine organs for xenotransplantation; potential model of vascular disease | Lack of disease phenotype; expense of animals |
| Rat (Rodentia) | RCMV | ∼230,000 | Vascular disease; pathogenesis models | Genome sequenced; viral mutants available; immunologic reagents and transgenic animals available | Congenital and placental infections recently described; no clear disease phenotype in pups |
| Mouse (Rodentia) | MCMV | ∼230,000 | Diverse pathogenesis models; viral immunology well defined; vaccine studies feasible | Extensively studied; genome well defined, mutants available; transgenic mice and immunologic reagents available | Lack of placental/fetal infection; less useful for congenital infection studies |
| Guinea pig (Caviomorph) | GPCMV | ∼233,000 | Neurological disease; labyrinthitis and hearing loss; CMV end-organ disease models | Genome sequenced, mutants available; relevant for study of vaccines and antivirals for congenital infection; hemomonochorial placenta | Lack of immunologic reagents for guinea pig studies; lengthy guinea pig pregnancy slows animal studies |
Murine Model
MCMV models have provided tremendous insights into CMV neuropathogenesis and the role of immune responses in controlling infection (48, 221, 271). The advantages of using the murine model are as follows: (i) the fact that the characteristics of CMV infection in the mouse are comparable to those of human CMV infection, in that CMV infection in the immunodeficient host and the fetus renders pathology and symptoms similar to those seen during human infection; (ii) the similarity of the MCMV genome to the human CMV genome at the genetic and nucleotide composition levels, making it a useful model to asses the role of viral genes in disease pathogenesis (218); and (iii) a well-characterized immune system, a relative small animal size, short gestational periods, and the availability of a large number of reagents, including transgenic and knockout animals. In mice, since they are altricial mammals, many developmental processes continue to occur during the postnatal period, enabling the use of neonates to investigate effects of infection on brain development.
With rare exceptions (285), the placental barrier is presumed to be refractory to CMV transmission in the mouse, most likely due to effectiveness of the three-cell-thick trophoblast layer that separates the maternal and fetal circulations. When this placental barrier is circumvented, by direct infection of either the placenta or embryo, the fetal brain becomes susceptible to CMV infection (151, 269). Susceptibility of the brain increases with the age of the fetus. The earliest infection of the embryo can be demonstrated after E7.5, whereas early blastocyst and ES cells are refractory to MCMV infection (125, 166). MCMV-infected blastocysts implanted into mice show neither signs of abnormal implantation nor developmental abnormalities. The embryos tested at E11 are not positive for viral antigens (270). Virus-positive cells are seen predominantly in the ventricular zone and subventricular zone and occasionally in the pyramidal layer of the hippocampus and some cortical regions when infection is at later stages of gestation (151). Embryos that survive late gestational infection often show signs of growth retardation and microencephaly. During the course of maturation from neonate to adult, the susceptibility of brain to CMV infection decreases. It is postulated that this decrease in susceptibility may be due to an age-dependent decrease in the number of susceptible cells in the developing brain (128) or to an age-dependent increase in the ability of the immune response, both innate and adaptive, to protect against infection (139).
The physiological effects of CMV infection on neurons have not been well studied. It is possible that the brain may serve as a site for viral latency, based on the long-lasting expression of MCMV IE genes in the cortices of in utero-infected mice, presumably as a result of maturation of infected neural stem cells into neurons (120). MCMV-infected neurons display altered electrophysiological responses as early as 24 h postinfection (273). The resting potential of infected neurons becomes significantly depolarized (i.e., becomes more positive) and hence attenuates amplitude, duration, and frequency of evoked action potentials. In addition, stepwise enhancement of voltage-evoked electrophysiological responses is significantly attenuated in MCMV-infected neurons (273). The N-methyl-d-aspartate (NMDA) receptor, an ionotropic glutamate receptor subtype seen on both neurons and glial cells, plays a central role in higher brain functions, such as memory and learning, by mediating an influx of Ca2+ ions (276). CMV infection of neonatal brain markedly decreases expression of NMDA receptor subunit 1 in the CA1 neurons of the hippocampus. This decrease in NMDA receptor subunit 1 attenuates neuronal responses to glutamate, in that glutamate-induced excitotoxicity is dampened in infected neurons (140). Whether electrophysiological changes in the infected neuron result from a decrease in ion channels or disruption of other intercellular processes is as yet unknown. Furthermore, the implication of these findings for the pathophysiological outcome of congenital CMV infection needs further investigation.
Guinea Pig Model
Among the small animal models of congenital CMV infection, GPCMV offers some unique advantages compared to rodent models. Chief among these advantages is the fact that GPCMV crosses the guinea pig placenta, causing infection in utero. Thus, the guinea pig model is particularly well suited to the study of vaccines designed to interrupt transplacental transmission of infection. The unique biology of the guinea pig and the use of this model to study congenital CMV infection are briefly summarized below.
The biology of the guinea pig has been the subject of several reviews (69, 187, 214). The guinea pig (Cavia porcellus) had at one time been included as a member of the order Rodentia, but recent molecular phylogenetic analyses indicate that the guinea pig is actually a member of the order Caviomorpha (67). There are three common breeds of guinea pigs: the English (short-haired) breed, the Angora/Peruvian (long-haired) breed, and the Abyssinian breed, which is characterized by its so-called rosette hair pattern. Of the types used in infectious disease research, derivatives of the Dunkin-Hartley line of short-haired guinea pigs are most commonly utilized. Inbred guinea pig strains are also available, including strain 2 and strain 13 animals.
Guinea pigs have relatively lengthy gestational periods, ranging from 65 to 70 days. The average litter size is three newborn pups per pregnant animal. An aspect of guinea pig reproductive biology that makes this model particularly well suited to the study of congenital infection is the structure and histology of the guinea pig placenta. As reviewed above, the guinea pig placenta is hemomonochorial, containing a single trophoblast layer separating maternal and fetal circulations, a feature that is histologically very similar to the human placenta (127, 150). This feature of the guinea pig has enabled the exploration of the pathogenesis of a number of clinically important transplacentally acquired infections, including syphilis (282) and listeriosis (18, 19).
The guinea pig has been also been used for the evaluation of the pathogenesis and prevention of congenital CMV infection. However, human CMV will not infect guinea pigs, so the species-specific CMV indigenous to guinea pigs, GPCMV, is employed in these studies. The history and biology of GPCMV have recently been reviewed (235). Virtually all studies of GPCMV pathogenesis appear to have been conducted with the strain originally isolated by Hartley in 1957 from infected guinea pig salivary glands, which was provided to the American Type Culture Collection (ATCC) as strain 22122 (111). Although it causes a latent, persistent infection in the salivary gland, GPCMV does not commonly cause serious disease in animals in the vivarium. However, there has been one report of two guinea pigs which were housed conventionally in separate animal facilities and had not been experimentally manipulated but were found to have evidence of disseminated CMV disease at necropsy (275).
A summary of features that make the GPCMV model valuable for the study of congenital CMV pathogenesis is included in Table 1. Like congenitally infected infants, congenitally GPCMV-infected guinea pigs exhibit a variety of forms of end-organ disease and injury, including injury to the CNS (101, 129). Importantly, infection of the cochlea, achieved either via direct inoculation or via transplacental transmission in utero, can produced SNHL, mimicking the most important complication of congenital CMV infection in infants. In one study, experimentally infected guinea pigs, inoculated with GPCMV through the round window of the cochlea, developed profound SNHL. These experimentally inoculated cochleas contained inflammatory and cytomegalic inclusion cells and showed various degrees of degenerative changes by histopathological analyses. Of interest, the number of virally infected cells was relatively small, relative to the histopathology observed. Based on this result, it was hypothesized that the histopathology might be mediated by inflammation, in addition to the cytopathic effect of the virus (129). This hypothesis was recently supported by a study of the impact of viral immune modulation genes in which a GPCMV recombinant deleted of a CC chemokine gene was found to have a reduced propensity for producing SNHL following intracochlear inoculation (237). SNHL has also been observed following congenital infection in guinea pigs. In a study of congenital GPCMV labyrinthitis, primary maternal infection in guinea pigs during the first or second trimester of pregnancy resulted in congenital infection in 64% of the offspring. Of the congenitally infected neonates, it was found that 28% had significant auditory deficits. Within the inner ear, histopathological analysis identified the presence of CMV infection localized chiefly in auditory nerve spiral ganglion cells (129). Other studies have documented that viremia in guinea pigs precedes spread of infection to the inner ear, further validating the relevance of this model to the study of CMV SNHL in infants (88). Another study of labyrinthits following congenital transmission resulting from injection of GPCMV into 5-week-pregnant guinea pigs was recently reported (126). Immunohistochemistry detected GPCMV-infected cells in the perilymph area and spinal ganglion but not in the endolymphatic compartment or in the hair cells. These data suggested that virus spreads into the inner ear via the perilymph and neural routes in both models of direct and congenital infections.
Studies with the GPCMV model have shed light on the possible correlates of immune protection against SNHL, observations that in turn may be relevant to exploring novel interventions for infants. In experiments comparing the impact of viral inoculation on viral labyrinthitis and hearing loss in GPCMV-immune and GPCMV-naïve animals, it was shown that systemic immunity to GPCMV could protect against hearing loss following round window challenge (109). These observations were further explored in a study of immunologic and electrophysiologic responses to GPCMV inner ear infection. In that study, guinea pigs all became deaf after inoculation of GPCMV into the cochlear perilymphatic compartment, and they showed severe inflammatory changes. Animals that received inactivated virus maintained normal hearing throughout the observation period and exhibited normal cochlear morphology, indicating that viral replication was required for the effect. The effect of systemic immunity to GPCMV on inner ear viral challenge in animals was extremely significant in this study, since animals with high levels of serum antibodies to GPCMV prior to challenge demonstrated no significant hearing loss following inoculation. Seropositive animals that received live virus demonstrated a significant rise in perilymph antibody titer and showed preservation of normal cochlear morphology. Thus, in this study, systemic and inner ear immunity to GPCMV afforded protection against damage caused after direct perilymphatic viral inoculation (284). The data from these studies provide support for the hypothesis that a vaccine capable of inducing immune responses comparable to those after natural infection could hold promise in the control of CMV-induced SNHL.
Rhesus Macaque Model
Of all of the animal models of CMV pathogenesis, the RhCMV model may have the greatest relevance to the problem of congenital human CMV infection. The pathogenesis of fetal infection in this primate model is remarkably similar to that observed in human infants, and a wide range of injury in the fetus has been described, including injury to the CNS and the cochlea (154, 264). The genome organization and protein-coding content of RhCMV is very similar to those of human CMV, and the immunodominant envelope gB is a target of immune responses in naturally infected rhesus macaques (288). This observation is of particular interest in light of ongoing clinical trials of human CMV gB subunit vaccines (described below). Studies of vaccination with the gB homolog in the RhCMV model for congenital infection of macaques would potentially have great relevance to the field of human CMV vaccines.
Although the RhCMV model is uniquely well suited to the study of CMV pathogenesis and vaccines, there are practical limitations to this model. One drawback is the expense of these primates. Since congenital CMV infection in infants occurs in, at most, 2% of pregnancies and since neurodevelopmental morbidity (including SNHL) ensues in, at most, 15% of these infants (61, 66), large group sizes would be needed in a clinical trial to demonstrate the protective efficacy of vaccination. Assuming similar percentages of transmission in the RhCMV model, the costs of a definitive protection study could be prohibitive. Another challenge is the paucity of RhCMV-seronegative animals, since RhCMV infection is ubiquitous in most colonies. These challenges support the continued development of nonprimate models of congenital CMV infection, as described below.
Other Animal CMVs
As noted in Table 1, a number of other animal CMVs have been described and characterized, to various degrees, in animal models of infection and disease. The chimpanzee CMV (CCMV) has recently been sequenced and has been found to have the highest degree of homology of any animal CMV to the human CMV genome (63). Unfortunately, there are no descriptions of the pathogenesis of CCMV infection, although such studies would likely be of great relevance to human health. RCMV is very well-characterized, in both its molecular biology and pathogenesis. The RCMV genome has been sequenced (278) and has proven amenable to mutagenesis strategies for generation of recombinant viruses for pathogenesis studies in rat models of disease (260). The rat model has emerged as a particularly interesting and valuable model of transplant-associated vascular disease. However, there is little information about the use of RCMV for study of perinatal infection or CNS injury. The recent isolation of a novel strain of RCMV from placental tissue (155) raises the intriguing possibility that RCMV might be transmitted to the fetus, a question that deserves further study. As already noted, PCMV has been shown to cross the placenta in pregnant sows and to infect the fetus (70), but this model has not been explored further in the experimental study of congenital CMV infection. The study of PCMV is important for xenotransplantation, since porcine grafts are being used with increased frequency in human transplantation (87).
INTERVENTIONS
Antiviral Drugs
The currently licensed antivirals for CMV therapy share the feature of inhibition of the viral DNA polymerase, although they differ in their pharmacology, and they are summarized in Table 2. There is limited information about the ability of antiviral therapy to limit the neuropathogenesis of congenital CMV infection, and more studies are greatly needed.
TABLE 2.
CMV antivirals in clinical usage
| Antiviral agent | Mechanism of action | Dose | Clinical use | Major toxicities | Comments |
|---|---|---|---|---|---|
| Ganciclovir | Inhibition of CMV DNA polymerase; requires phosphorylation by virus-specific kinase (UL97 protein) | 10-12 mg/kg/day (intravenously) divided twice daily | CMV retinitis; end-organ disease in immune-compromised patients; congenital infection | Neutropenia, thrombocytopenia; animal studies suggestive of theoretical risks of teratogenicity and inhibition of spermatogenesis | Efficacy against CMV-associated hearing loss in the setting of congenital infection |
| Valganciclovir | Prodrug (valine ester) of ganciclovir; metabolized to ganciclovir following oral dosage | 900 mg (orally) twice daily (for active CMV disease) | CMV retinitis; preemptive therapy in transplant patients | Neutropenia, thrombocytopenia | Orally bioavailable; optimal oral dose for children not established |
| Foscarnet | Inhibition of CMV DNA polymerase; does not require phosphorylation | 180 mg/kg/day (intravenously) in 2-3 divided doses | CMV retinitis, end-organ disease; ganciclovir resistance | Nephrotoxicity; toxicity to developing bones/teeth | Prehydration minimizes renal toxicity |
| Cidofovir | Inhibition of DNA polymerase; does not require phosphorylation | 5 mg/kg (intravenously) once weekly for two wk, then biweekly | CMV end-organ disease in immune-compromised patients; ganciclovir resistance | Renal toxicity | Pretreatment with probenicid, prehydration reduces renal toxicity |
| CMV immune globulin | Uncertain; presumed to be due to direct neutralization of viral infectivity by Ig and/or improvement in antibody avidity | 50-150 mg/kg (intravenously) per schedule (transplant patients); 100-200 U/kg (pregnant patients) | Prophylaxis against CMV (transplant patients); possible value for prevention of transplacental transmission of CMV in pregnancy | Headache; fever and chills; facial flushing; use with caution in patients with IgA deficiency | Efficacy for prevention of fetal CMV infection unknown; randomized trials for congenital infection ongoing |
Ganciclovir.
Ganciclovir was the first compound licensed specifically for treatment of CMV infections. Ganciclovir is a synthetic acyclic nucleoside analog, structurally similar to guanine. Its structure is similar to that of acyclovir, and like acyclovir, it requires phosphorylation for antiviral activity. The enzyme responsible for phosphorylation of GCV is the product of the CMV UL97 gene, a protein kinase and phosphotransferase (102, 153, 262). Following phosphorylation by UL97, cellular enzymes phosphorylate the monophosphate form to di- and triphosphate metabolites; the ganciclovir triphosphate metabolite exerts the antiviral effect in the CMV-infected cell.
Ganciclovir is the treatment of choice for severe CMV disease in immunocompromised adult patients (i.e., bone marrow/solid organ transplant recipients and HIV-infected individuals with CMV end-organ disease). For congenital and perinatal CMV infections, the role of ganciclovir is less well established, although recent evidence suggests that it is of value in ameliorating some of the disabilities seen in these infants. The first reports of the use of ganciclovir therapy for congenital CMV infection date to the late 1980s (75, 206). In subsequent reports (16, 60, 76, 117, 124, 175, 185, 186, 220, 272), ganciclovir has been shown to be generally safe and well tolerated when used in newborns and has appeared to be useful in the management of severe, focal end-organ disease (pneumonitis, hepatitis, etc.) in infants. Based on these reports, the use of ganciclovir is generally indicated in the short-term management of any infant with severe or symptomatic CMV disease, including viremia. It is important to note that no sustained effect on CMV shedding at mucosal sites can be expected; once therapy is completed, infants resume excreting of CMV in urine and saliva cultures.
Although ganciclovir appears to be valuable for short-term management of CMV infection in infants in some settings, it has been less clear whether use of ganciclovir provides any long-term benefit for congenital or perinatally acquired CMV infection. Recently, the results of a phase III randomized double-blind study of parenteral ganciclovir in neonates with symptomatic congenital CMV infection have been reported (134). This study was not a placebo-controlled trial, because of the ethical concerns regarding the prolonged intravenous administration of a placebo through a central venous catheter. Enrollment took place at participating Collaborative Antiviral Study Group sites over a period of nearly 10 years. The primary study end point was an improved brain stem-evoked response (BSER) between baseline and 6-month follow-up or, for those infants with normal hearing at enrollment, maintenance of normal hearing between baseline and 6-month follow-up. Of the 100 patients enrolled in the study, 42 patients had both baseline and 6-month follow-up BSER audiometric examinations. Twenty-one (84%) of 25 ganciclovir recipients either had improved hearing or maintained normal hearing between baseline and 6 months. In contrast, only 10 (59%) of 17 control patients had improved or stable hearing (P = 0.06). Results were even more encouraging when the study and control groups were compared for subsequent maintenance of normal hearing. None (0%) of 25 ganciclovir recipients had worsening of hearing between baseline and 6 month follow-up, compared to 7 (41%) of 17 control patients (P < 0.01). The study further examined whether there was a therapeutic benefit after 12 months of follow-up. Among 43 patients who had a BSER both at baseline and at 1 year or beyond, five (21%) of 24 ganciclovir recipients had worsening of hearing, versus 13 (68%) of 19 control patients (P < 0.01).
Since ganciclovir is associated with a number of drug toxicities (particularly myelosuppression), its use in newborns and infants requires careful consideration. Ganciclovir penetrates well into the CNS (133), an observation of importance for treatment strategies for newborns designed to provide protection against CMV-induced neurodevelopmental injury.
Valganciclovir.
Valganciclovir is the valine ester of ganciclovir. In contrast to oral formulations of ganciclovir, valganciclovir is very well absorbed following oral administration. It is rapidly metabolized into ganciclovir following oral dosing. Valganciclovir is indicated for therapy of CMV retinitis in HIV-positive patients and for the prevention of CMV disease in kidney, heart, and kidney-pancreas transplant patients at high risk for CMV disease (donor CMV seropositive/recipient CMV seronegative). The side effect profile is similar to that of ganciclovir. The commercially available formulation is produced as a tablet; however, there is a suspension formulation available for investigational use (133). There is currently little experience with the use of this agent in infants and children, but the convenience of oral dosage makes this agent attractive for future clinical trial evaluation in the setting of perinatally acquired CMV infection.
Foscarnet.
Foscarnet, an inorganic pyrophosphate analog, is a second-line treatment for CMV infection. The mechanism of action of foscarnet, which is similar to ganciclovir's, is inhibition of the CMV DNA polymerase. However, in contrast to ganciclovir, foscarnet does not require phosphorylation for antiviral activity. The drug directly blocks the pyrophosphate binding site of the DNA polymerase. Resistance to foscarnet may emerge on therapy. Mutations in the CMV UL54 (DNA polymerase) gene which confer resistance to foscarnet have been described (58). Foscarnet has a significant risk of producing nephrotoxicity. Foscarnet has an important toxicity relevant to pediatric practice, which is its ability to affect bone and tooth development, necessitating caution when it is used in children or pregnant women. In contrast to ganciclovir, the risks of myelosupression with foscarnet are minimal, and this agent is the recommended CMV therapy for patients with bone marrow failure. Although consideration should be given to the use of foscarnet in a setting of known or suspected ganciclovir resistance, there are very few data on the use of this agent in newborns or young infants, precluding definitive dosage recommendations in this setting.
Cidofovir.
Another second-line therapy for CMV disease, cidofovir, is, like foscarnet, an inhibitor of the CMV DNA polymerase. Cidofovir is an acyclic phosphonate nucleoside analog, which already has a single phosphate group attached in the formulation used for clinical administration. Hence, it does not require or utilize an initial viral (UL97-mediated) phosphorylation for antiviral activity, but it does undergo additional phosphorylation by cellular kinases to its active form. In its fully phosphorylated form, cidofovir is selectively incorporated into the viral DNA chain, inhibiting viral DNA synthesis. Cidofovir is eliminated by renal clearance, although the drug persists in cells for prolonged periods, and its metabolites have very long intracellular half-lives, which allows for an intermittent dosage schedule. Unfortunately, cidofovir has a wide range of significant toxicities, including nephrotoxicity, neutropenia, and metabolic acidosis. In animal studies, cidofovir is carcinogenic and teratogenic, and it may be associated with gonadal injury in males. Prehydration with saline and treatment with probenecid prior to the infusion are recommended to attempt to prevent the problem of nephrotoxicity. There is no information available about the use of cidofovir in neonates with CMV infection, making dose recommendations in this setting uncertain.
CMV Immune Globulin
CMV immune globulin is a pooled, high-titer intravenous Ig preparation prepared from donors with high titers of CMV antibodies. The presumed mechanism is neutralization of virus infectivity, via interactions with viral envelope glycoproteins. CMV immune globulin is indicated, either alone or in combination with nucleoside analogs, for prophylaxis against CMV disease in solid organ transplant patients. Recently, the potential therapeutic benefit of CMV immune globulin in protection against symptomatic congenital CMV infection has been evaluated. In an uncontrolled study, Nigro and colleagues studied pregnant women with a primary CMV infection, whose amniotic fluid contained either CMV or CMV DNA. These women were offered intravenous CMV hyperimmune globulin (HIG) in two different dose regimens, a “therapy” regimen or a “prevention” regimen (184). In the therapy group, only 1/31 women gave birth to an infant with CMV disease (defined as an infant who was symptomatic at birth and handicapped at 2 years of age or older), compared with 7 of 14 women in an untreated control group. In the prevention group, 6/37 women who received hyperimmune globulin during pregnancy had infants with congenital CMV infection, compared with 19/47 women who did not receive the high-titer CMV globulin. Overall, the CMV HIG therapy was associated with a significantly lower risk of congenital CMV infection (P = 0.04). In a follow-up study (147), ultrasonographic findings that were present in a group of 92 pregnant women with primary CMV infection were compared to those observed in 73 control patients with evidence of preconception immunity to CMV. In the women with evidence of primary infection, at each measurement between 16 and 36 weeks of gestation, CMV infection was associated with significant increases in placental thickness, compared to controls, and placental thickness was further increased in a subgroup of women in the primary infection group who ended up giving birth to an infant with CMV disease. The administration of HIG to women in the primary infection group was associated with significant reductions in placental thickness, suggesting that a major component of the HIG effect was mediated by protection at the placental level (229). Additional randomized controlled trials of HIG are warranted in high-risk pregnancies, to further validate the protective effect of passive immunization.
Vaccines
A number of candidate CMV vaccines have been evaluated in clinical trials. These vaccine candidates are summarized in Table 3. A wide variety of expression strategies have been employed for CMV vaccines, but generally these vaccines can be conceptually subdivided into the categories of live, attenuated vaccines and subunit vaccines that target individual CMV proteins. Ongoing progress in the study of these vaccines is briefly considered below.
TABLE 3.
Candidate CMV vaccines
| Vaccine | Comments |
|---|---|
| Vaccines evaluated in clinical trials | |
| Live virus vaccines | |
| AD169 vaccine | Human CMV-specific antibody responses; no clinical trials active or anticipated |
| Towne vaccine | Elicits humoral and cellular immune responses; reduced human CMV disease in renal transplant recipients; phase I studies with coadministered recombinant IL-12 |
| Towne/Toledo “chimeric” vaccines | Good safety profile; attenuated compared to Toledo strain; no efficacy data available |
| Subunit vaccines | |
| gB/MF59 adjuvant | Neutralizing antibody- and cell-mediated responses; efficacy studies ongoing in phase II/III trials |
| gB/canarypox-vectored vaccine | Suboptimal immunogenicity; prime-boost when administered with Towne vaccine |
| pp65 (UL83)/canarypox-vectored vaccine | Good safety profile; antibody- and cell-mediated immune responses |
| gB/pp65/IE1 trivalent DNA vaccine | DNA adjuvanted with poloxamer/benzalkonium chloride |
| gB/pp65 bivalent DNA vaccine | Phase II studies ongoing in hematopoietic stem cell transplant recipients |
| gB/pp65/IE1 alphavirus replicon vaccine | Replication-deficient VRPs; phase I clinical trial recently initiated |
| Preclinical vaccine approaches | |
| gM/gN (gcII complex) | Major glycoprotein constituent of virion; majority of human sera contain anti-gcII antibodies; gM/gN DNA vaccine immunogenic in mice |
| gH/gL/gO (gcIII complex) | Target of neutralizing antibody responses; protective in murine CMV model |
| Nonstructural genes/novel CTL targets | DNA polymerase (UL54) and helicase (UL105); protective in murine model |
| Prime-boost strategy | Prime with cocktail of plasmid DNA vaccines; boost with formalin-inactivated viral particles; induces “sterilizing immunity” in murine model |
| Bacterial artificial chromosomes | Protective in murine and guinea pig models |
| Peptide vaccines | Effective in murine model; “polyepitope” vaccine approach; requires knowledge of HLA status |
| Dense body vaccines | Noninfectious particles; highly immunogenic in animal models; humoral and cell-mediated immune responses |
Live, attenuated CMV vaccines.
The first live CMV vaccine candidate tested in humans was a laboratory-adapted vaccine based on the attenuated AD169 (179) strain of the virus. Subsequent trials with another live, attenuated vaccine, the Towne strain, confirmed that that this vaccine approach could elicit neutralizing antibodies as well as CMV-specific cell-mediated immune responses, including CD8+ cytotoxic T-lymphocyte (CTL) responses, in immunocompetent individuals (4, 8, 79, 92, 99, 144, 205, 215, 257). The efficacy of Towne vaccine was tested in several studies with kidney transplant recipients (patients at high risk for development of CMV disease), and although the vaccine failed to prevent CMV infection following transplantation, vaccination did provide a favorable, protective impact on severe CMV disease (20, 203, 204, 227). However, a placebo-controlled study of Towne vaccine in seronegative mothers who had children attending group day care indicated that immunization failed to protect these women from CMV infection. Since the lack of efficacy of Towne vaccine may be due to overattenuation of the virus relative to clinical isolates of CMV, a recent vaccine study using “chimeric” vaccines, in which large regions from the genome of the unattenuated Toledo strain of CMV were substituted for the corresponding regions of the Towne genome, was performed (131). Four independent chimeric vaccines were evaluated in a recently completed double-blinded, placebo-controlled phase I trial (113, 234). All of the vaccines were found to be well tolerated, justifying further evaluation of these vaccines in seronegative subjects.
Subunit CMV vaccines.
The leading subunit CMV vaccine candidate is the immunodominant envelope glycoprotein, gB (the UL55 gene product), based on the ability of this protein to elicit high-titer, virus-neutralizing antibody responses in the setting of natural CMV infection. Other viral proteins actively being evaluated as subunit vaccine candidates include the tegument phosphoprotein, pp65 (the UL83 gene product), and IE1 (the UL123 gene product), both of which elicit T-cell responses. The current status of individual subunit vaccine candidates is summarized below (Table 3).
(i) Adjuvanted protein vaccines.
The formulation of CMV gB currently being utilized in clinical vaccine trials is a recombinant protein expressed in Chinese hamster ovary (CHO) cells (250, 251). In a phase I randomized, double-blind, placebo-controlled trial in adults, gB vaccine, combined either with a novel adjuvant, MF59, or alum adjuvant, elicited levels of virus-neutralizing antibody that exceeded those observed in CMV-seropositive control subjects (197). In all subsequent studies to date (86, 176), the safety profile of the vaccine has been very favorable, with injection site discomfort being the only significant adverse event observed. There is currently a phase III efficacy study of gB/MF59 vaccine ongoing in young, CMV-seronegative women who are vaccinated postpartum (289). These women are at high risk for primary CMV infection following delivery. Since these women commonly become pregnant again soon after delivery, this study will be of great importance to the prevention of congenital CMV infection in subsequent deliveries.
(ii) DNA vaccines.
DNA vaccines developed for CMV (265) have focused on the gB, IE1, and pp65 genes as the candidate target immunogens. There are currently phase I clinical trials under way of both a bivalent CMV DNA vaccine candidate, using plasmid DNA encoding pp65 and gB, and a trivalent vaccine candidate, which also includes a third plasmid encoding the IE1 gene product, developed and produced by Vical Vaccines (240, 277). The vaccines are formulated using the poloxamer adjuvant CRL1005 and benzalkonium chloride. A study is currently ongoing with the bivalent DNA vaccine in a hematopoietic stem cell transplant population. Both donors and recipients are being vaccinated, with the goal of reducing CMV disease, viral load, and use of antiviral therapies in the posttransplant period. The long-term role of these vaccines for potential control of congenital CMV infection in women of child-bearing age remains to be determined, as does the ultimate acceptability of DNA vaccines for clinical use.
(iii) Vectored vaccines.
Another approach to CMV subunit vaccination is utilization of a “vectored” vaccine, in which the gene product of interest is expressed in the context of a nonreplicating (usually viral) vector. Once example of such a vaccine vector is a canarypox vector known as ALVAC, an attenuated poxvirus that replicates productively in avian species but abortively in mammalian cells. ALVAC expressing CMV gB has been studied in several clinical trials (7, 30). An ALVAC vaccine expressing pp65, the major CTL target in naturally CMV seropositive persons, has also been evaluated in human trials. The ALVAC expression vector has been found to be safe and well tolerated, although the expression strategy does not appear to offer advantages in immunogenicity compared to other approaches. Other vectored CMV vaccine expression strategies include an approach based on the modified vaccinia virus Ankara (279) and a Venezuelan equine encephalitis virus-vectored CMV vaccine (235), manufactured by AlphaVax Vaccines. Both approaches show promise, and the Venezuelan equine encephalitis virus-vectored approach provided protection in an animal model of congenital CMV infection (see below), but these vaccines have not yet been tested in human clinical trials.
Preclinical vaccine approaches.
Other vaccine approaches have been suggested for development of a CMV vaccine, and these are the subject of a recent review (226). These potential strategies are listed in Table 3. CMV glycoproteins other than gB have been considered for vaccine development, including the gcII complex, consisting of gN (UL73) and gM (UL100), and the gcIII complex, consisting of gH (UL75), gO (UL74), and gL (UL115). Vaccines based on nonstructural proteins that are highly conserved among the CMVs, including the DNA polymerase and helicase genes, show promise in murine models. Dense bodies, which are enveloped, replication-defective particles formed during replication of CMVs in cell culture, are capable of inducing virus-neutralizing antibodies and T-cell responses after immunization of mice (226). The use of synthetic peptides comprising immunodominant cytotoxic T-cell epitopes has been advocated, although such vaccines would have to be tailored for donor-recipient pairs based on HLA genetics. Viral genomes cloned in Escherichia coli as bacterial artificial chromosomes provide the opportunity to generate recombinant, “designer” vaccines with specific genomic deletions or insertions that could modify the immune response or improve the safety profile of the candidate vaccine. A “prime-boost” approach, in which priming with DNA vaccination is followed by boosting with formalin-inactivated viral particles, elicits high levels of neutralizing antibodies as well as CD8+ T-cell responses. These approaches deserve further attention in preclinical study and in phase I clinical trials. The importance of identifying the maternal immune factors that protect the developing fetal CNS cannot be overstated, and this should be a key priority in future vaccine design.
Validation of vaccines for congenital CMV in animal models.
Prioritization of vaccine candidates for clinical trials can be based on results identified in the guinea pig model of congenital CMV infection. In light of the ability of GPCMV to cross the placenta and infect the pup in utero, the guinea pig provides an ideal small animal model for the study of vaccines designed to protect the fetus. A live, attenuated GPCMV vaccine, as well as a partially purified, soluble envelope vaccine, administered with Freund's adjuvant, was able to show protection against acute viremia and death and to reduce the incidence and extent of maternal and fetal infection (31). Adjuvanted glycoprotein vaccines, generated by immunoaffinity purification, have also been shown to protect newborn pups against congenital infection and disease (40, 110). Molecular cloning techniques allowed extension of these studies to generation of cloned, recombinant subunit vaccine candidates in a number of expression systems. Subunit vaccines based on the GPCMV homolog of gB are capable of inducing neutralizing antibody responses when administered either as DNA vaccines or as adjuvanted, purified glcyoprotein vaccines expressed in baculovirus (231, 232). Efficacy analyses against congenital GPCMV have been performed with these vaccines. A DNA gB subunit vaccine was able to reduce congenital infection rates following third-trimester challenge of vaccinated, pregnant guinea pigs. In a group of 26 live-born pups in the control group, the total congenital infection rate was 77% (20/26). In contrast, in the gB-vaccinated group the total congenital infection rate was 41% (11/27) (P < 0.05 versus control group). This was the first study indicating that a DNA vaccine was capable of providing protection against congenital CMV infection (231), and these data provide support for the ongoing development and testing of DNA vaccines for clinical trials. A three-dose series of purified, recombinant gB, expressed and purified from baculovirus, was studied following administration with either Freund's adjuvant or alum-based adjuvant (233). Among 36 live-born pups in the Freund's adjuvant group, 8 (22%) had congenital GPCMV infection, compared to 15/32 pups (47%) with in the alum adjuvant group (P < 0.05 versus Freund's adjuvant). The basis for the improved protection in the Freund's adjuvant group was the induction of higher virus-neutralizing antibody titers, an observation that may be relevant to clinical trials of gB vaccine.
Recently, the UL83 (pp65) homolog of CMV, GP83, was also evaluated as a vaccine against congenital CMV infection and disease in the guinea pig model. This vaccine was developed from a propagation-defective, single-cycle RNA replicon vector system, based on an attenuated strain of the alphavirus Venezuelan equine encephalitis virus. This expression system was used to produce virus-like replicon particles (VRPs) expressing GP83. Subsequent vaccination with VRP-GP83 induced antibodies and CD4+ and CD8+ T-cell responses in GPCMV-seronegative female guinea pigs. Guinea pigs immunized with VRP-GP83 vaccine were bred for pregnancy and subsequent GPCMV challenge during the early third trimester. Dams vaccinated with VRP-GP83 had improved pregnancy outcomes compared with controls, supporting the concept that T-cell-mediated immune responses directed against a CMV matrix protein can protect against congenital CMV infection and disease.
Although these studies shed light on mechanisms that protect the fetus, there is a paucity of information about how vaccination in animal models can protect against neuropathogenesis. This is a high-priority area for future research in the guinea pig model. Assessments of vaccine-mediated protection against SHNL and neuropathology in this and other congenital infection models will be of great importance in clarifying which human CMV vaccines strategies should be emphasized in clinical trials. An important issue to clarify in future clinical trials will be the impact of reinfection in women with preconception immunity on the incidence and morbidity of congenital CMV infection. A fundamental and unresolved quandary is that if vaccines induce immunity but preexisting natural immunity does not fully protect women from CMV reinfection and the fetal consequences of these infections, could vaccines ever be effective in eradication of congenital CMV disease? The answer may lie in how the vaccine would be applied in clinical practice. As suggested by some authorities, the force of infection of CMV is low, and a universal immunization program could eliminate CMV infection from a community, even if the vaccine was only modestly effective (103) Thus, the issue may be not the immunogenicity of the vaccine but the degree to which CMV vaccination could confer herd immunity. If herd immunity in turn decreases the prevalence of CMV infection in the population, then the incidence of congenital CMV infection would be predicted to be substantially reduced. This was the effect observed following adoption of universal immunization against rubella (103), which led to the virtual elimination of congenital rubella syndrome.
SUMMARY AND PERSPECTIVES
Congential CMV infection is the predominant cause of developmental neurological disabilities in the United States. It most frequently begins as a primary infection in the pregnant mother, who may be exposed to several risk factors, including her own CMV-infected children who shed infectious virus. Prior exposure to CMV markedly reduces the possibility of fetal infection but does not preclude the virus from crossing the placenta to infect the fetus. Not only does the infected placenta serve as an amplifying reservoir, but CMV infection may also cause placental insufficiency that result in fetal pathologies. Gestational age at infection greatly influences neurological outcomes in the fetus. However, the combination of factors that render the developing brain susceptible to CMV infection is poorly understood.
CMV infection of the fetus may alter the “normal blueprint” of the developing brain, thus resulting in long-term neurological sequelae. Studies that investigate mechanisms that alter developmental paradigms will help elucidate CMV neuropathogenesis and explain the clinical outcomes of congenital CMV infection. Neural stem cells in the fetal brain appear to be the predominant cell type affected during development by CMV. There is an abundance of neural stem cells in the fetal brain, and their increased susceptibility to viral infection explains the predominance of nerurological sequelae associated with congenital CMV infection. Some possible mechanisms of developmental disruption due to CMV infection include (i) the loss of neural stem cells or intermediate progenitors, the building blocks of the developing brain, due to infection, which may have potential effects on brain size and maturation (165); (ii) alterations in stem cell migration and fate of cell differentiation, processes which are critical for normal pattering and function of neural structures; (iii) infection of astroglia, which may disrupt their normal supportive functions in the brain that are a critical for neural circuitry guidance, synaptic integration, and development of the functionally intergrated mature neuron (160); and (iv) alterations in the microenvironment of the developing brain due to cytokines and soluble factors generated by resident glial cells (microglia and astrocytes) and infiltrating immune cells, which may result in neurotoxicity (68) and altered neuronal physiology (116) and induce abnormal developmental cues in the fetal brain (Fig. 4). All these mechanisms may potentially affect the developmental patterning of the brain.
FIG. 4.
Schematic representation of neuropathogenic mechanisms during congenital CMV infection. CMV brain infection may have multifaceted influences on the developing brain. Neural stem cells (in blue), found along the lateral ventricular wall of the brain, are involved in the development of new neural circuits in both the developing and adult brain. These cells divide symmetrically, to renew the stem cell pool, or asymmetrically, to differentiate into new brain cells (astrocytes, oligodendrocytes, and neurons), either directly or via an intermediate transitional progenitor cell (red cells). The development path for new neural circuitry involves the migration of the early neuronal precursor, neuroblasts (green cells), through a directed path that is supported in part by astroglial cells. CMV brain infection may potentially affect any or all of these stages of development and influence the neurological outcomes of congenital infection. This schematic depicts potential mechanisms by which formation of new neural circuits in the developing brain may be affected by CMV infection. 1, Infection of neural stem cells may disrupt their ability to maintain a self-renewing cycle that would influence subsequent processes involved in brain development. 2, Differentiation of neural stem cells via the transitional cells and eventually neuroblasts may also be disrupted by CMV infection, potentially skewing their end-differentiated fate. 3, It has also been shown that brain infection affects the migratory patterns of neuroblasts, particularly during cortical and cerebellar development, which may involve inhibition of migration to distal brain structures. 4, This might alter the migratory patterning of specific brain structures, such as by causing improper layering of the neocortex. 5, Since glial cells are also susceptible to CMV infection, demonstrated both in vivo and in vitro, one could postulate that important functions of glia in directing neuronal layering patterns may be affected. 6, Finally, CMV infection can induce a myriad of inflammatory mediators, including cytokines, oxidative radicals, and other neurotoxic chemicals. These mediators may potentially affect the immature neuron by directly inducing cytotoxicity or may affect neuronal function by altering the microenvironment that alters normal brain cell physiology. In addition, immune-mediated clearance of infected cells may have an impact on the cellular milieu. Many of these concepts are still speculative, and more research is required to elucidate the neuropathogenesis of CMV infection.
Antiviral drugs are available for treatment of congenital CMV infection, and there is evidence that therapy ameliorates the severity of one of the CNS complications of infection, SNHL. Long-term neurodevelopmental follow-up studies should further clarify the value of antiviral therapy in congenitally infected infants. Uncontrolled studies of therapy in utero with CMV immune globulin have suggested an impact on neuropathogenesis, and controlled trials should be conducted with pregnant women. Finally, CMV vaccines may hold the greatest promise in reducing the neurodevelopmental consequences of congenital infection, although the immune correlates of protection of the fetus remain incompletely defined. Preclinical study with relevant animal models may provide insights into strategies that bear further testing in human clinical trials. Until more-effective interventions are available, better education of women of child-bearing age will be important. Only with increased public awareness of the urgency of this problem can the societal and political forces necessary to effect changes be marshaled. These changes include increased funding for the study of the pathogenesis of congenital CMV and an increased sense of urgency in conducting clinical vaccine trials. Web-based information from both the Centers for Disease Control (http://www.cdc.gov/cmv/) and parent support groups founded by individuals who have children afflicted with congenital CMV (http://www.cmvfoundation.org) can be an effective conduit of knowledge and perspective that help to increase pubic awareness of this urgent problem.
Acknowledgments
This work was supported by NIH grants NS038836, HD038416, and HD044864.
REFERENCES
- 1.Abbott, N. J., L. Ronnback, and E. Hansson. 2006. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 7:41-53. [DOI] [PubMed] [Google Scholar]
- 2.Adler, B., L. Scrivano, Z. Ruzcics, B. Rupp, C. Sinzger, and U. Koszinowski. 2006. Role of human cytomegalovirus UL131A in cell type-specific virus entry and release. J. Gen. Virol. 87:2451-2460. [DOI] [PubMed] [Google Scholar]
- 3.Adler, S. P., J. W. Finney, A. M. Manganello, and A. M. Best. 2004. Prevention of child-to-mother transmission of cytomegalovirus among pregnant women. J. Pediatr. 145:485-491. [DOI] [PubMed] [Google Scholar]
- 4.Adler, S. P., S. H. Hempfling, S. E. Starr, S. A. Plotkin, and S. Riddell. 1998. Safety and immunogenicity of the Towne strain cytomegalovirus vaccine. Pediatr. Infect. Dis. J. 17:200-206. [DOI] [PubMed] [Google Scholar]
- 5.Adler, S. P., and G. Nigro. 2006. Interrupting intrauterine transmission of cytomegalovirus. Rev. Med. Virol. 16:69-71. [DOI] [PubMed] [Google Scholar]
- 6.Adler, S. P., G. Nigro, and L. Pereira. 2007. Recent advances in the prevention and treatment of congenital cytomegalovirus infections. Semin. Perinatol. 31:10-18. [DOI] [PubMed] [Google Scholar]
- 7.Adler, S. P., S. A. Plotkin, E. Gonczol, M. Cadoz, C. Meric, J. B. Wang, P. Dellamonica, A. M. Best, J. Zahradnik, S. Pincus, K. Berencsi, W. I. Cox, and Z. Gyulai. 1999. A canarypox vector expressing cytomegalovirus (CMV) glycoprotein B primes for antibody responses to a live attenuated CMV vaccine (Towne). J. Infect. Dis. 180:843-846. [DOI] [PubMed] [Google Scholar]
- 8.Adler, S. P., S. E. Starr, S. A. Plotkin, S. H. Hempfling, J. Buis, M. L. Manning, and A. M. Best. 1995. Immunity induced by primary human cytomegalovirus infection protects against secondary infection among women of childbearing age. J. Infect. Dis. 171:26-32. [DOI] [PubMed] [Google Scholar]
- 9.Akter, P., C. Cunningham, B. P. McSharry, A. Dolan, C. Addison, D. J. Dargan, A. F. Hassan-Walker, V. C. Emery, P. D. Griffiths, G. W. Wilkinson, and A. J. Davison. 2003. Two novel spliced genes in human cytomegalovirus. J. Gen. Virol. 84:1117-1122. [DOI] [PubMed] [Google Scholar]
- 10.Anastassova-Kristeva, M. 2003. The origin and development of the immune system with a view to stem cell therapy. J. Hematother. Stem Cell Res. 12:137-154. [DOI] [PubMed] [Google Scholar]
- 11.Ancora, G., M. Lanari, T. Lazzarotto, V. Venturi, E. Tridapalli, F. Sandri, M. Menarini, E. Ferretti, and G. Faldella. 2007. Cranial ultrasound scanning and prediction of outcome in newborns with congenital cytomegalovirus infection. J. Pediatr. 150:157-161. [DOI] [PubMed] [Google Scholar]
- 12.Andriesse, G. I., A. J. Weersink, and J. de Boer. 2006. Visual impairment and deafness in young children: consider the diagnosis of congenital infection with cytomegalovirus, even years after birth. Arch. Ophthalmol. 124:743. [DOI] [PubMed] [Google Scholar]
- 13.Arav-Boger, R., C. A. Battaglia, T. Lazzarotto, L. Gabrielli, J. C. Zong, G. S. Hayward, M. Diener-West, and M. P. Landini. 2006. Cytomegalovirus (CMV)-encoded UL144 (truncated tumor necrosis factor receptor) and outcome of congenital CMV infection. J. Infect. Dis. 194:464-473. [DOI] [PubMed] [Google Scholar]
- 14.Arnoult, D., L. M. Bartle, A. Skaletskaya, D. Poncet, N. Zamzami, P. U. Park, J. Sharpe, R. J. Youle, and V. S. Goldmacher. 2004. Cytomegalovirus cell death suppressor vMIA blocks Bax- but not Bak-mediated apoptosis by binding and sequestering Bax at mitochondria. Proc. Natl. Acad. Sci. USA 101:7988-7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashkenazi, A. 2002. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat. Rev. Cancer 2:420-430. [DOI] [PubMed] [Google Scholar]
- 16.Attard-Montalto, S. P., M. C. English, L. Stimmler, and G. J. Snodgrass. 1993. Ganciclovir treatment of congenital cytomegalovirus infection: a report of two cases. Scand. J. Infect. Dis. 25:385-388. [DOI] [PubMed] [Google Scholar]
- 17.Bachor, E., H. Sudhoff, R. Litschel, and C. S. Karmody. 2000. The pathology of the temporal bones of a child with acquired cytomegalovirus infection: studies by light microscopy, immunohistochemistry and polymerase-chain reaction. Int. J. Pediatr. Otorhinolaryngol. 55:215-224. [DOI] [PubMed] [Google Scholar]
- 18.Bakardjiev, A. I., B. A. Stacy, S. J. Fisher, and D. A. Portnoy. 2004. Listeriosis in the pregnant guinea pig: a model of vertical transmission. Infect. Immun. 72:489-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakardjiev, A. I., B. A. Stacy, and D. A. Portnoy. 2005. Growth of Listeria monocytogenes in the guinea pig placenta and role of cell-to-cell spread in fetal infection. J. Infect. Dis. 191:1889-1897. [DOI] [PubMed] [Google Scholar]
- 20.Balfour, H. H., Jr., G. W. Sachs, P. Welo, R. C. Gehrz, R. L. Simmons, and J. S. Najarian. 1984. Cytomegalovirus vaccine in renal transplant candidates: progress report of a randomized, placebo-controlled, double-blind trial. Birth Defects Orig. Artic. Ser. 20:289-304. [PubMed] [Google Scholar]
- 21.Bantug, G. R., D. Cekinovic, R. Bradford, T. Koontz, S. Jonjic, and W. J. Britt. 2008. CD8+ T lymphocytes control murine cytomegalovirus replication in the central nervous system of newborn animals. J. Immunol. 181:2111-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barkovich, A. J., and N. Girard. 2003. Fetal brain infections. Childs Nerv. Syst. 19:501-507. [DOI] [PubMed] [Google Scholar]
- 23.Barkovich, A. J., and C. E. Lindan. 1994. Congenital cytomegalovirus infection of the brain: imaging analysis and embryologic considerations. AJNR Am. J. Neuroradiol. 15:703-715. [PMC free article] [PubMed] [Google Scholar]
- 24.Baron, M. H., and S. T. Fraser. 2005. The specification of early hematopoiesis in the mammal. Curr. Opin. Hematol. 12:217-221. [DOI] [PubMed] [Google Scholar]
- 25.Bass, K. E., H. Li, S. P. Hawkes, E. Howard, E. Bullen, T. K. Vu, M. McMaster, M. Janatpour, and S. J. Fisher. 1997. Tissue inhibitor of metalloproteinase-3 expression is upregulated during human cytotrophoblast invasion in vitro. Dev. Genet. 21:61-67. [DOI] [PubMed] [Google Scholar]
- 26.Bauer, P. W., M. Parizi-Robinson, P. S. Roland, and S. Yegappan. 2005. Cytomegalovirus in the perilymphatic fluid. Laryngoscope 115:223-225. [DOI] [PubMed] [Google Scholar]
- 27.Benedict, C. A. 2003. Viruses and the TNF-related cytokines, an evolving battle. Cytokine Growth Factor Rev. 14:349-357. [DOI] [PubMed] [Google Scholar]
- 28.Benedict, C. A., P. S. Norris, and C. F. Ware. 2002. To kill or be killed: viral evasion of apoptosis. Nat. Immunol. 3:1013-1018. [DOI] [PubMed] [Google Scholar]
- 29.Bentz, G. L., M. Jarquin-Pardo, G. Chan, M. S. Smith, C. Sinzger, and A. D. Yurochko. 2006. Human cytomegalovirus (HCMV) infection of endothelial cells promotes naive monocyte extravasation and transfer of productive virus to enhance hematogenous dissemination of HCMV. J. Virol. 80:11539-11555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernstein, D. I., M. R. Schleiss, K. Berencsi, E. Gonczol, M. Dickey, P. Khoury, M. Cadoz, C. Meric, J. Zahradnik, A. M. Duliege, and S. Plotkin. 2002. Effect of previous or simultaneous immunization with canarypox expressing cytomegalovirus (CMV) glycoprotein B (gB) on response to subunit gB vaccine plus MF59 in healthy CMV-seronegative adults. J. Infect. Dis. 185:686-690. [DOI] [PubMed] [Google Scholar]
- 31.Bia, F. J., B. P. Griffith, M. Tarsio, and G. D. Hsiung. 1980. Vaccination for the prevention of maternal and fetal infection with guinea pig cytomegalovirus. J. Infect. Dis. 142:732-738. [DOI] [PubMed] [Google Scholar]
- 32.Bigger, J. E., M. Tanigawa, M. Zhang, and S. S. Atherton. 2000. Murine cytomegalovirus infection causes apoptosis of uninfected retinal cells. Investig. Ophthalmol. Vis. Sci. 41:2248-2254. [PubMed] [Google Scholar]
- 33.Boppana, S. B., and W. J. Britt. 1995. Antiviral antibody responses and intrauterine transmission after primary maternal cytomegalovirus infection. J. Infect. Dis. 171:1115-1121. [DOI] [PubMed] [Google Scholar]
- 34.Boppana, S. B., K. B. Fowler, W. J. Britt, S. Stagno, and R. F. Pass. 1999. Symptomatic congenital cytomegalovirus infection in infants born to mothers with preexisting immunity to cytomegalovirus. Pediatrics 104:55-60. [DOI] [PubMed] [Google Scholar]
- 35.Boppana, S. B., K. B. Fowler, R. F. Pass, L. B. Rivera, R. D. Bradford, F. D. Lakeman, and W. J. Britt. 2005. Congenital cytomegalovirus infection: association between virus burden in infancy and hearing loss. J. Pediatr. 146:817-823. [DOI] [PubMed] [Google Scholar]
- 36.Boppana, S. B., K. B. Fowler, Y. Vaid, G. Hedlund, S. Stagno, W. J. Britt, and R. F. Pass. 1997. Neuroradiographic findings in the newborn period and long-term outcome in children with symptomatic congenital cytomegalovirus infection. Pediatrics 99:409-414. [DOI] [PubMed] [Google Scholar]
- 37.Boppana, S. B., J. Miller, and W. J. Britt. 1996. Transplacentally acquired antiviral antibodies and outcome in congenital human cytomegalovirus infection. Viral Immunol. 9:211-218. [DOI] [PubMed] [Google Scholar]
- 38.Boppana, S. B., R. F. Pass, and W. J. Britt. 1993. Virus-specific antibody responses in mothers and their newborn infants with asymptomatic congenital cytomegalovirus infections. J. Infect. Dis. 167:72-77. [DOI] [PubMed] [Google Scholar]
- 39.Boppana, S. B., L. B. Rivera, K. B. Fowler, M. Mach, and W. J. Britt. 2001. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N. Engl. J. Med. 344:1366-1371. [DOI] [PubMed] [Google Scholar]
- 40.Bourne, N., M. R. Schleiss, F. J. Bravo, and D. I. Bernstein. 2001. Preconception immunization with a cytomegalovirus (CMV) glycoprotein vaccine improves pregnancy outcome in a guinea pig model of congenital CMV infection. J. Infect. Dis. 183:59-64. [DOI] [PubMed] [Google Scholar]
- 41.Bresnahan, W. A., I. Boldogh, E. A. Thompson, and T. Albrecht. 1996. Human cytomegalovirus inhibits cellular DNA synthesis and arrests productively infected cells in late G1. Virology 224:150-160. [DOI] [PubMed] [Google Scholar]
- 42.Britt, W. J. 1996. Human cytomegalovirus infections during pregnancy, p. 327-343, Baillière's clinical infectious diseases, vol. 3. Baillière Tindall, Oxford, United Kingdom. [Google Scholar]
- 43.Brune, W., M. Nevels, and T. Shenk. 2003. Murine cytomegalovirus m41 open reading frame encodes a Golgi-localized antiapoptotic protein. J. Virol. 77:11633-11643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cannon, M. J., and K. F. Davis. 2005. Washing our hands of the congenital cytomegalovirus disease epidemic. BMC Public Health 5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carson, M. J., J. M. Doose, B. Melchior, C. D. Schmid, and C. C. Ploix. 2006. CNS immune privilege: hiding in plain sight. Immunol. Rev. 213:48-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castillo, J. P., and T. F. Kowalik. 2002. Human cytomegalovirus immediate early proteins and cell growth control. Gene 290:19-34. [DOI] [PubMed] [Google Scholar]
- 47.Chaouat, G., N. Ledee-Bataille, and S. Dubanchet. 2007. Immune cells in uteroplacental tissues throughout pregnancy: a brief review. Reprod. Biomed. Online 14:256-266. [DOI] [PubMed] [Google Scholar]
- 48.Cheeran, M. C., G. Gekker, S. Hu, X. Min, D. Cox, and J. R. Lokensgard. 2004. Intracerebral infection with murine cytomegalovirus induces CXCL10 and is restricted by adoptive transfer of splenocytes. J. Neurovirol. 10:152-162. [DOI] [PubMed] [Google Scholar]
- 49.Cheeran, M. C., G. Gekker, S. Hu, J. M. Palmquist, and J. R. Lokensgard. 2005. T cell-mediated restriction of intracerebral murine cytomegalovirus infection displays dependence upon perforin but not interferon-gamma. J. Neurovirol. 11:274-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheeran, M. C., S. Hu, G. Gekker, and J. R. Lokensgard. 2000. Decreased cytomegalovirus expression following proinflammatory cytokine treatment of primary human astrocytes. J. Immunol. 164:926-933. [DOI] [PubMed] [Google Scholar]
- 51.Cheeran, M. C., S. Hu, H. T. Ni, W. Sheng, J. M. Palmquist, P. K. Peterson, and J. R. Lokensgard. 2005. Neural precursor cell susceptibility to human cytomegalovirus diverges along glial or neuronal differentiation pathways. J. Neurosci. Res. 82:839-850. [DOI] [PubMed] [Google Scholar]
- 52.Cheeran, M. C., S. Hu, J. M. Palmquist, T. Bakken, G. Gekker, and J. R. Lokensgard. 2007. Dysregulated interferon-gamma responses during lethal cytomegalovirus brain infection of IL-10-deficient mice. Virus Res. 130:96-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheeran, M. C., S. Hu, W. S. Sheng, P. K. Peterson, and J. R. Lokensgard. 2003. CXCL10 production from cytomegalovirus-stimulated microglia is regulated by both human and viral interleukin-10. J. Virol. 77:4502-4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheeran, M. C., S. Hu, S. L. Yager, G. Gekker, P. K. Peterson, and J. R. Lokensgard. 2001. Cytomegalovirus induces cytokine and chemokine production differentially in microglia and astrocytes: antiviral implications. J. Neurovirol. 7:135-147. [DOI] [PubMed] [Google Scholar]
- 55.Chiou, S. H., J. H. Liu, W. M. Hsu, S. S. Chen, S. Y. Chang, L. J. Juan, J. C. Lin, Y. T. Yang, W. W. Wong, C. Y. Liu, Y. S. Lin, W. T. Liu, and C. W. Wu. 2001. Up-regulation of Fas ligand expression by human cytomegalovirus immediate-early gene product 2: a novel mechanism in cytomegalovirus-induced apoptosis in human retina. J. Immunol. 167:4098-4103. [DOI] [PubMed] [Google Scholar]
- 56.Chiou, S. H., Y. P. Yang, J. C. Lin, C. H. Hsu, H. C. Jhang, Y. T. Yang, C. H. Lee, L. L. Ho, W. M. Hsu, H. H. Ku, S. J. Chen, S. S. Chen, M. D. Chang, C. W. Wu, and L. J. Juan. 2006. The immediate early 2 protein of human cytomegalovirus (HCMV) mediates the apoptotic control in HCMV retinitis through up-regulation of the cellular FLICE-inhibitory protein expression. J. Immunol. 177:6199-6206. [DOI] [PubMed] [Google Scholar]
- 57.Choi, C., and E. N. Benveniste. 2004. Fas ligand/Fas system in the brain: regulator of immune and apoptotic responses. Brain Res. Brain Res. Rev. 44:65-81. [DOI] [PubMed] [Google Scholar]
- 58.Chou, S., N. S. Lurain, K. D. Thompson, R. C. Miner, and W. L. Drew. 2003. Viral DNA polymerase mutations associated with drug resistance in human cytomegalovirus. J. Infect. Dis. 188:32-39. [DOI] [PubMed] [Google Scholar]
- 59.Cinatl, J., Jr., R. Blaheta, M. Bittoova, M. Scholz, S. Margraf, J. U. Vogel, J. Cinatl, and H. W. Doerr. 2000. Decreased neutrophil adhesion to human cytomegalovirus-infected retinal pigment epithelial cells is mediated by virus-induced up-regulation of Fas ligand independent of neutrophil apoptosis. J. Immunol. 165:4405-4413. [DOI] [PubMed] [Google Scholar]
- 60.Crowley, B. 2002. Ganciclovir treatment of symptomatic congenital cytomegalovirus infection. J. Antimicrob. Chemother. 50:435-436. [DOI] [PubMed] [Google Scholar]
- 61.Dahle, A. J., K. B. Fowler, J. D. Wright, S. B. Boppana, W. J. Britt, and R. F. Pass. 2000. Longitudinal investigation of hearing disorders in children with congenital cytomegalovirus. J. Am. Acad. Audiol. 11:283-290. [PubMed] [Google Scholar]
- 62.Davis, L. E., L. G. Johnsson, and M. Kornfeld. 1981. Cytomegalovirus labyrinthitis in an infant: morphological, virological, and immunofluorescent studies. J. Neuropathol. Exp. Neurol. 40:9-19. [PubMed] [Google Scholar]
- 63.Davison, A. J., A. Dolan, P. Akter, C. Addison, D. J. Dargan, D. J. Alcendor, D. J. McGeoch, and G. S. Hayward. 2003. The human cytomegalovirus genome revisited: comparison with the chimpanzee cytomegalovirus genome. J. Gen. Virol. 84:17-28. [DOI] [PubMed] [Google Scholar]
- 64.DeBiasi, R. L., B. K. Kleinschmidt-DeMasters, S. Richardson-Burns, and K. L. Tyler. 2002. Central nervous system apoptosis in human herpes simplex virus and cytomegalovirus encephalitis. J. Infect. Dis. 186:1547-1557. [DOI] [PubMed] [Google Scholar]
- 65.de Graaf-Peters, V. B., and M. Hadders-Algra. 2006. Ontogeny of the human central nervous system: what is happening when? Early Hum. Dev. 82:257-266. [DOI] [PubMed] [Google Scholar]
- 66.Demmler, G. J. 1994. Congenital cytomegalovirus infection. Semin. Pediatr. Neurol. 1:36-42. [PubMed] [Google Scholar]
- 67.D'Erchia, A. M., C. Gissi, G. Pesole, C. Saccone, and U. Arnason. 1996. The guinea-pig is not a rodent. Nature 381:597-600. [DOI] [PubMed] [Google Scholar]
- 68.Deshpande, M., J. Zheng, K. Borgmann, R. Persidsky, L. Wu, C. Schellpeper, and A. Ghorpade. 2005. Role of activated astrocytes in neuronal damage: potential links to HIV-1-associated dementia. Neurotox. Res. 7:183-192. [DOI] [PubMed] [Google Scholar]
- 69.Donnelly, T. M., and C. J. Brown. 2004. Guinea pig and chinchilla care and husbandry. Vet. Clin. N. Am. Exot. Anim. Pract. 7:351-373. [DOI] [PubMed] [Google Scholar]
- 70.Edington, N., S. Broad, A. E. Wrathall, and J. T. Done. 1988. Superinfection with porcine cytomegalovirus initiating transplacental infection. Vet. Microbiol. 16:189-193. [DOI] [PubMed] [Google Scholar]
- 71.Eggers, M., K. Radsak, G. Enders, and M. Reschke. 2001. Use of recombinant glycoprotein antigens gB and gH for diagnosis of primary human cytomegalovirus infection during pregnancy. J. Med. Virol. 63:135-142. [PubMed] [Google Scholar]
- 72.Elbou Ould, M. A., D. Luton, M. Yadini, B. Pedron, Y. Aujard, E. Jacqz-Aigrain, F. Jacquemard, and G. Sterkers. 2004. Cellular immune response of fetuses to cytomegalovirus. Pediatr. Res. 55:280-286. [DOI] [PubMed] [Google Scholar]
- 73.Eudy, J. D., M. D. Weston, S. Yao, D. M. Hoover, H. L. Rehm, M. Ma-Edmonds, D. Yan, I. Ahmad, J. J. Cheng, C. Ayuso, C. Cremers, S. Davenport, C. Moller, C. B. Talmadge, K. W. Beisel, M. Tamayo, C. C. Morton, A. Swaroop, W. J. Kimberling, and J. Sumegi. 1998. Mutation of a gene encoding a protein with extracellular matrix motifs in Usher syndrome type IIa. Science 280:1753-1757. [DOI] [PubMed] [Google Scholar]
- 74.Fagerheim, T., O. Nilssen, P. Raeymaekers, V. Brox, T. Moum, H. H. Elverland, E. Teig, H. H. Omland, G. K. Fostad, and L. Tranebjaerg. 1996. Identification of a new locus for autosomal dominant non-syndromic hearing impairment (DFNA7) in a large Norwegian family. Hum. Mol. Genet. 5:1187-1191. [DOI] [PubMed] [Google Scholar]
- 75.Fan-Havard, P., M. C. Nahata, and M. T. Brady. 1989. Ganciclovir—a review of pharmacology, therapeutic efficacy and potential use for treatment of congenital cytomegalovirus infections. J. Clin. Pharm. Ther. 14:329-340. [DOI] [PubMed] [Google Scholar]
- 76.Fischler, B., T. H. Casswall, P. Malmborg, and A. Nemeth. 2002. Ganciclovir treatment in infants with cytomegalovirus infection and cholestasis. J. Pediatr. Gastroenterol. Nutr. 34:154-157. [DOI] [PubMed] [Google Scholar]
- 77.Fish, K. N., C. Soderberg-Naucler, L. K. Mills, S. Stenglein, and J. A. Nelson. 1998. Human cytomegalovirus persistently infects aortic endothelial cells. J. Virol. 72:5661-5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fisher, S., O. Genbacev, E. Maidji, and L. Pereira. 2000. Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: implications for transmission and pathogenesis. J. Virol. 74:6808-6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fleisher, G. R., S. E. Starr, H. M. Friedman, and S. A. Plotkin. 1982. Vaccination of pediatric nurses with live attenuated cytomegalovirus. Am. J. Dis. Child. 136:294-296. [DOI] [PubMed] [Google Scholar]
- 80.Fortunato, E. A., M. L. Dell'Aquila, and D. H. Spector. 2000. Specific chromosome 1 breaks induced by human cytomegalovirus. Proc. Natl. Acad. Sci. USA 97:853-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fowler, K. B., A. J. Dahle, S. B. Boppana, and R. F. Pass. 1999. Newborn hearing screening: will children with hearing loss caused by congenital cytomegalovirus infection be missed? J. Pediatr. 135:60-64. [DOI] [PubMed] [Google Scholar]
- 82.Fowler, K. B., F. P. McCollister, A. J. Dahle, S. Boppana, W. J. Britt, and R. F. Pass. 1997. Progressive and fluctuating sensorineural hearing loss in children with asymptomatic congenital cytomegalovirus infection. J. Pediatr. 130:624-630. [DOI] [PubMed] [Google Scholar]
- 83.Fowler, K. B., and R. F. Pass. 2006. Risk factors for congenital cytomegalovirus infection in the offspring of young women: exposure to young children and recent onset of sexual activity. Pediatrics 118:e286-e292. [DOI] [PubMed] [Google Scholar]
- 84.Fowler, K. B., S. Stagno, and R. F. Pass. 1993. Maternal age and congenital cytomegalovirus infection: screening of two diverse newborn populations, 1980-1990. J. Infect. Dis. 168:552-556. [DOI] [PubMed] [Google Scholar]
- 85.Fowler, K. B., S. Stagno, and R. F. Pass. 2003. Maternal immunity and prevention of congenital cytomegalovirus infection. JAMA. 289:1008-1011. [DOI] [PubMed] [Google Scholar]
- 86.Frey, S. E., C. Harrison, R. F. Pass, E. Yang, D. Boken, R. E. Sekulovich, S. Percell, A. E. Izu, S. Hirabayashi, R. L. Burke, and A. M. Duliege. 1999. Effects of antigen dose and immunization regimens on antibody responses to a cytomegalovirus glycoprotein B subunit vaccine. J. Infect. Dis. 180:1700-1703. [DOI] [PubMed] [Google Scholar]
- 87.Fryer, J. F., P. D. Griffiths, V. C. Emery, and D. A. Clark. 2004. Susceptibility of porcine cytomegalovirus to antiviral drugs. J. Antimicrob. Chemother. 53:975-980. [DOI] [PubMed] [Google Scholar]
- 88.Fukuda, S., E. M. Keithley, and J. P. Harris. 1988. Experimental cytomegalovirus infection: viremic spread to the inner ear. Am. J. Otolaryngol. 9:135-141. [DOI] [PubMed] [Google Scholar]
- 89.Gage, F. H. 2000. Mammalian neural stem cells. Science 287:1433-1438. [DOI] [PubMed] [Google Scholar]
- 90.Galea, I., I. Bechmann, and V. H. Perry. 2007. What is immune privilege (not)? Trends Immunol. 28:12-18. [DOI] [PubMed] [Google Scholar]
- 91.Gaytant, M. A., E. A. Steegers, B. A. Semmekrot, H. M. Merkus, and J. M. Galama. 2002. Congenital cytomegalovirus infection: review of the epidemiology and outcome. Obstet. Gynecol. Surv. 57:245-256. [DOI] [PubMed] [Google Scholar]
- 92.Gehrz, R. C., W. R. Christianson, K. M. Linner, K. E. Groth, and H. H. Balfour, Jr. 1980. Cytomegalovirus vaccine. Specific humoral and cellular immune responses in human volunteers. Arch. Intern. Med. 140:936-939. [DOI] [PubMed] [Google Scholar]
- 93.Gerna, G., E. Percivalle, D. Lilleri, L. Lozza, C. Fornara, G. Hahn, F. Baldanti, and M. G. Revello. 2005. Dendritic-cell infection by human cytomegalovirus is restricted to strains carrying functional UL131-128 genes and mediates efficient viral antigen presentation to CD8+ T cells. J. Gen. Virol. 86:275-284. [DOI] [PubMed] [Google Scholar]
- 94.Gibson, L., S. Dooley, S. Trzmielina, M. Somasundaran, D. Fisher, M. G. Revello, and K. Luzuriaga. 2007. Cytomegalovirus (CMV) IE1- and pp65-specific CD8+ T cell responses broaden over time after primary CMV infection in infants. J. Infect. Dis. 195:1789-1798. [DOI] [PubMed] [Google Scholar]
- 95.Gibson, L., G. Piccinini, D. Lilleri, M. G. Revello, Z. Wang, S. Markel, D. J. Diamond, and K. Luzuriaga. 2004. Human cytomegalovirus proteins pp65 and immediate early protein 1 are common targets for CD8+ T cell responses in children with congenital or postnatal human cytomegalovirus infection. J. Immunol. 172:2256-2264. [DOI] [PubMed] [Google Scholar]
- 96.Goldmacher, V. S. 2005. Cell death suppression by cytomegaloviruses. Apoptosis 10:251-265. [DOI] [PubMed] [Google Scholar]
- 97.Goldmacher, V. S., L. M. Bartle, A. Skaletskaya, C. A. Dionne, N. L. Kedersha, C. A. Vater, J. W. Han, R. J. Lutz, S. Watanabe, E. D. Cahir McFarland, E. D. Kieff, E. S. Mocarski, and T. Chittenden. 1999. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc. Natl. Acad. Sci. USA 96:12536-12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gonczol, E., P. W. Andrews, and S. A. Plotkin. 1984. Cytomegalovirus replicates in differentiated but not in undifferentiated human embryonal carcinoma cells. Science 224:159-161. [DOI] [PubMed] [Google Scholar]
- 99.Gonczol, E., J. Ianacone, G. Furlini, W. Ho, and S. A. Plotkin. 1989. Humoral immune response to cytomegalovirus Towne vaccine strain and to Toledo low-passage strain. J. Infect. Dis. 159:851-859. [DOI] [PubMed] [Google Scholar]
- 100.Grassi, M. P., F. Clerici, C. Perin, A. D'Arminio Monforte, L. Vago, M. Borella, R. Boldorini, and A. Mangoni. 1998. Microglial nodular encephalitis and ventriculoencephalitis due to cytomegalovirus infection in patients with AIDS: two distinct clinical patterns. Clin. Infect. Dis. 27:504-508. [DOI] [PubMed] [Google Scholar]
- 101.Griffith, B. P., H. L. Lucia, and G. D. Hsiung. 1982. Brain and visceral involvement during congenital cytomegalovirus infection of guinea pigs. Pediatr. Res. 16:455-459. [DOI] [PubMed] [Google Scholar]
- 102.Griffiths, P. D. 2002. The treatment of cytomegalovirus infection. J. Antimicrob. Chemother. 49:243-253. [DOI] [PubMed] [Google Scholar]
- 103.Griffiths, P. D., A. McLean, and V. C. Emery. 2001. Encouraging prospects for immunisation against primary cytomegalovirus infection. Vaccine 19:1356-1362. [DOI] [PubMed] [Google Scholar]
- 104.Haginoya, K., T. Ohura, K. Kon, T. Yagi, Y. Sawaishi, K. K. Ishii, T. Funato, S. Higano, S. Takahashi, and K. Iinuma. 2002. Abnormal white matter lesions with sensorineural hearing loss caused by congenital cytomegalovirus infection: retrospective diagnosis by PCR using Guthrie cards. Brain Dev. 24:710-714. [DOI] [PubMed] [Google Scholar]
- 105.Hahn, G., R. Jores, and E. S. Mocarski. 1998. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc. Natl. Acad. Sci. USA 95:3937-3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hahn, G., M. G. Revello, M. Patrone, E. Percivalle, G. Campanini, A. Sarasini, M. Wagner, A. Gallina, G. Milanesi, U. Koszinowski, F. Baldanti, and G. Gerna. 2004. Human cytomegalovirus UL131-128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J. Virol. 78:10023-10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Halwachs-Baumann, G. 2006. The congenital cytomegalovirus infection: virus-host interaction for defense and transmission. Curr. Pharm. Biotechnol. 7:303-312. [DOI] [PubMed] [Google Scholar]
- 108.Han, G. P., L. Li, I. Kosugi, H. Kawasaki, T. Tsuchida, K. Miura, and Y. Tsutsui. 2007. Enhancement of susceptibility of adult mouse brain to cytomegalovirus infection by infusion of epidermal growth factor. J. Neurosci. Res. 85:2981-2990. [DOI] [PubMed] [Google Scholar]
- 109.Harris, J. P., N. K. Woolf, A. F. Ryan, D. M. Butler, and D. D. Richman. 1984. Immunologic and electrophysiological response to cytomegaloviral inner ear infection in the guinea pig. J. Infect. Dis. 150:523-530. [DOI] [PubMed] [Google Scholar]
- 110.Harrison, C. J., W. J. Britt, N. M. Chapman, J. Mullican, and S. Tracy. 1995. Reduced congenital cytomegalovirus (CMV) infection after maternal immunization with a guinea pig CMV glycoprotein before gestational primary CMV infection in the guinea pig model. J. Infect. Dis. 172:1212-1220. [DOI] [PubMed] [Google Scholar]
- 111.Hartley, J. W., W. P. Rowe, and R. J. Huebner. 1957. Serial propagation of the guinea pig salivary gland virus in tissue culture. Proc. Soc. Exp. Biol. Med. 96:281-285. [DOI] [PubMed] [Google Scholar]
- 112.Hassan, J., S. Dooley, and W. Hall. 2007. Immunological response to cytomegalovirus in congenitally infected neonates. Clin. Exp. Immunol. 147:465-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Heineman, T. C., M. Schleiss, D. I. Bernstein, R. R. Spaete, L. Yan, G. Duke, M. Prichard, Z. Wang, Q. Yan, M. A. Sharp, N. Klein, A. M. Arvin, and G. Kemble. 2006. A phase 1 study of 4 live, recombinant human cytomegalovirus Towne/Toledo chimeric vaccines. J. Infect. Dis. 193:1350-1360. [DOI] [PubMed] [Google Scholar]
- 114.Hertel, L., S. Chou, and E. S. Mocarski. 2007. Viral and cell cycle-regulated kinases in cytomegalovirus-induced pseudomitosis and replication. PLoS Pathog. 3:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hicks, T., K. Fowler, M. Richardson, A. Dahle, L. Adams, and R. Pass. 1993. Congenital cytomegalovirus infection and neonatal auditory screening. J. Pediatr. 123:779-782. [DOI] [PubMed] [Google Scholar]
- 116.Ho, W. S., and A. N. van den Pol. 2007. Bystander attenuation of neuronal and astrocyte intercellular communication by murine cytomegalovirus infection of glia. J. Virol. 81:7286-7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hocker, J. R., L. N. Cook, G. Adams, and G. P. Rabalais. 1990. Ganciclovir therapy of congenital cytomegalovirus pneumonia. Pediatr. Infect. Dis. J. 9:743-745. [DOI] [PubMed] [Google Scholar]
- 118.Holt, P. G., and C. A. Jones. 2000. The development of the immune system during pregnancy and early life. Allergy 55:688-697. [DOI] [PubMed] [Google Scholar]
- 119.Huang, D., Y. Han, M. R. Rani, A. Glabinski, C. Trebst, T. Sorensen, M. Tani, J. Wang, P. Chien, S. O'Bryan, B. Bielecki, Z. L. Zhou, S. Majumder, and R. M. Ransohoff. 2000. Chemokines and chemokine receptors in inflammation of the nervous system: manifold roles and exquisite regulation. Immunol. Rev. 177:52-67. [DOI] [PubMed] [Google Scholar]
- 120.Ishiwata, M., S. Baba, M. Kawashima, I. Kosugi, H. Kawasaki, M. Kaneta, T. Tsuchida, S. Kozuma, and Y. Tsutsui. 2006. Differential expression of the immediate-early 2 and 3 proteins in developing mouse brains infected with murine cytomegalovirus. Arch. Virol. 151:2181-2196. [DOI] [PubMed] [Google Scholar]
- 121.Jamieson, D. J., J. E. Ellis, D. B. Jernigan, and T. A. Treadwell. 2006. Emerging infectious disease outbreaks: old lessons and new challenges for obstetrician-gynecologists. Am. J. Obstet. Gynecol. 194:1546-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jarvis, M. A., and J. A. Nelson. 2002. Human cytomegalovirus persistence and latency in endothelial cells and macrophages. Curr. Opin. Microbiol. 5:403-407. [DOI] [PubMed] [Google Scholar]
- 123.Jault, F. M., J. M. Jault, F. Ruchti, E. A. Fortunato, C. Clark, J. Corbeil, D. D. Richman, and D. H. Spector. 1995. Cytomegalovirus infection induces high levels of cyclins, phosphorylated Rb, and p53, leading to cell cycle arrest. J. Virol. 69:6697-6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Junker, A. K., D. Matheson, A. J. Tingle, and E. E. Thomas. 1991. Immune responses after ganciclovir and immunoglobulin therapy of infants. Pediatr. Infect. Dis. J. 10:256-258. [DOI] [PubMed] [Google Scholar]
- 125.Kashiwai, A., N. Kawamura, C. Kadota, and Y. Tsutsui. 1992. Susceptibility of mouse embryo to murine cytomegalovirus infection in early and mid-gestation stages. Arch. Virol. 127:37-48. [DOI] [PubMed] [Google Scholar]
- 126.Katano, H., Y. Sato, Y. Tsutsui, T. Sata, A. Maeda, N. Nozawa, N. Inoue, Y. Nomura, and T. Kurata. 2007. Pathogenesis of cytomegalovirus-associated labyrinthitis in a guinea pig model. Microbes Infect. 9:183-191. [DOI] [PubMed] [Google Scholar]
- 127.Kaufmann, P., and M. Davidoff. 1977. The guinea-pig placenta. Adv. Anat. Embryol. Cell Biol. 53:5-91. [DOI] [PubMed] [Google Scholar]
- 128.Kawasaki, H., I. Kosugi, Y. Arai, and Y. Tsutsui. 2002. The amount of immature glial cells in organotypic brain slices determines the susceptibility to murine cytomegalovirus infection. Lab Investig. 82:1347-1358. [DOI] [PubMed] [Google Scholar]
- 129.Keithley, E. M., N. K. Woolf, and J. P. Harris. 1989. Development of morphological and physiological changes in the cochlea induced by cytomegalovirus. Laryngoscope 99:409-414. [DOI] [PubMed] [Google Scholar]
- 130.Keller, M. J., A. W. Wu, J. I. Andrews, P. W. McGonagill, E. E. Tibesar, and J. L. Meier. 2007. Reversal of human cytomegalovirus major immediate-early enhancer/promoter silencing in quiescently infected cells via the cyclic AMP signaling pathway. J. Virol. 81:6669-6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kemble, G., G. Duke, R. Winter, and R. Spaete. 1996. Defined large-scale alterations of the human cytomegalovirus genome constructed by cotransfection of overlapping cosmids. J. Virol. 70:2044-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kenneson, A., and M. J. Cannon. 2007. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 17:253-276. [DOI] [PubMed] [Google Scholar]
- 133.Kimberlin, D. W. 2002. Antiviral therapy for cytomegalovirus infections in pediatric patients. Semin. Pediatr. Infect. Dis. 13:22-30. [DOI] [PubMed] [Google Scholar]
- 134.Kimberlin, D. W., C. Y. Lin, P. J. Sanchez, G. J. Demmler, W. Dankner, M. Shelton, R. F. Jacobs, W. Vaudry, R. F. Pass, J. M. Kiell, S. J. Soong, and R. J. Whitley. 2003. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J. Pediatr. 143:16-25. [DOI] [PubMed] [Google Scholar]
- 135.Kondo, K., H. Kaneshima, and E. S. Mocarski. 1994. Human cytomegalovirus latent infection of granulocyte-macrophage progenitors. Proc. Natl. Acad. Sci. USA 91:11879-11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kondo, K., J. Xu, and E. S. Mocarski. 1996. Human cytomegalovirus latent gene expression in granulocyte-macrophage progenitors in culture and in seropositive individuals. Proc. Natl. Acad. Sci. USA 93:11137-11142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Koontz, T., M. Bralic, J. Tomac, E. Pernjak-Pugel, G. Bantug, S. Jonjic, and W. J. Britt. 2008. Altered development of the brain after focal herpesvirus infection of the central nervous system. J. Exp. Med. 205:423-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kossmann, T., M. C. Morganti-Kossmann, J. M. Orenstein, W. J. Britt, S. M. Wahl, and P. D. Smith. 2003. Cytomegalovirus production by infected astrocytes correlates with transforming growth factor-beta release. J. Infect. Dis. 187:534-541. [DOI] [PubMed] [Google Scholar]
- 139.Kosugi, I., H. Kawasaki, Y. Arai, and Y. Tsutsui. 2002. Innate immune responses to cytomegalovirus infection in the developing mouse brain and their evasion by virus-infected neurons. Am. J. Pathol. 161:919-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kosugi, I., H. Kawasaki, T. Tsuchida, and Y. Tsutsui. 2005. Cytomegalovirus infection inhibits the expression of N-methyl-d-aspartate receptors in the developing mouse hippocampus and primary neuronal cultures. Acta Neuropathol. 109:475-482. [DOI] [PubMed] [Google Scholar]
- 141.Kosugi, I., Y. Shinmura, R. Y. Li, S. Aiba-Masago, S. Baba, K. Miura, and Y. Tsutsui. 1998. Murine cytomegalovirus induces apoptosis in non-infected cells of the developing mouse brain and blocks apoptosis in primary neuronal culture. Acta Neuropathol. 96:239-247. [DOI] [PubMed] [Google Scholar]
- 142.Kylat, R. I., E. N. Kelly, and E. L. Ford-Jones. 2006. Clinical findings and adverse outcome in neonates with symptomatic congenital cytomegalovirus (SCCMV) infection. Eur. J. Pediatr. 165:773-778. [DOI] [PubMed] [Google Scholar]
- 143.LaFemina, R., and G. S. Hayward. 1986. Constitutive and retinoic acid-inducible expression of cytomegalovirus immediate-early genes in human teratocarcinoma cells. J. Virol. 58:434-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.La Rosa, C., Z. Wang, S. F. Lacey, S. F. Markel, M. C. Sharma, J. Martinez, M. M. Lalimarmo, and D. J. Diamond. 2005. Characterization of host immunity to cytomegalovirus pp150 (UL32). Hum. Immunol. 66:116-126. [DOI] [PubMed] [Google Scholar]
- 145.Lathey, J. L., and S. A. Spector. 1991. Unrestricted replication of human cytomegalovirus in hydrocortisone-treated macrophages. J. Virol. 65:6371-6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lathey, J. L., C. A. Wiley, M. A. Verity, and J. A. Nelson. 1990. Cultured human brain capillary endothelial cells are permissive for infection by human cytomegalovirus. Virology 176:266-273. [DOI] [PubMed] [Google Scholar]
- 147.La Torre, R., G. Nigro, M. Mazzocco, A. M. Best, and S. P. Adler. 2006. Placental enlargement in women with primary maternal cytomegalovirus infection is associated with fetal and neonatal disease. Clin. Infect. Dis. 43:994-1000. [DOI] [PubMed] [Google Scholar]
- 148.Lazzarotto, T., P. Spezzacatena, P. Pradelli, D. A. Abate, S. Varani, and M. P. Landini. 1997. Avidity of immunoglobulin G directed against human cytomegalovirus during primary and secondary infections in immunocompetent and immunocompromised subjects. Clin. Diagn. Lab. Immunol. 4:469-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lazzarotto, T., S. Varani, B. Guerra, A. Nicolosi, M. Lanari, and M. P. Landini. 2000. Prenatal indicators of congenital cytomegalovirus infection. J. Pediatr. 137:90-95. [DOI] [PubMed] [Google Scholar]
- 150.Leiser, R., and P. Kaufmann. 1994. Placental structure: in a comparative aspect. Exp. Clin. Endocrinol. 102:122-134. [DOI] [PubMed] [Google Scholar]
- 151.Li, R. Y., and Y. Tsutsui. 2000. Growth retardation and microcephaly induced in mice by placental infection with murine cytomegalovirus. Teratology 62:79-85. [DOI] [PubMed] [Google Scholar]
- 152.Lim, K. H., Y. Zhou, M. Janatpour, M. McMaster, K. Bass, S. H. Chun, and S. J. Fisher. 1997. Human cytotrophoblast differentiation/invasion is abnormal in pre-eclampsia. Am. J. Pathol. 151:1809-1818. [PMC free article] [PubMed] [Google Scholar]
- 153.Littler, E., A. D. Stuart, and M. S. Chee. 1992. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the antiviral nucleoside analogue ganciclovir. Nature 358:160-162. [DOI] [PubMed] [Google Scholar]
- 154.Lockridge, K. M., G. Sequar, S. S. Zhou, Y. Yue, C. P. Mandell, and P. A. Barry. 1999. Pathogenesis of experimental rhesus cytomegalovirus infection. J. Virol. 73:9576-9583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Loh, H. S., M. L. Mohd-Azmi, K. Y. Lai, A. R. Sheikh-Omar, and M. Zamri-Saad. 2003. Characterization of a novel rat cytomegalovirus (RCMV) infecting placenta-uterus of Rattus rattus diardii. Arch. Virol. 148:2353-2367. [DOI] [PubMed] [Google Scholar]
- 156.Loh, H. S., M. A. Mohd-Lila, S. O. Abdul-Rahman, and L. J. Kiew. 2006. Pathogenesis and vertical transmission of a transplacental rat cytomegalovirus. Virol. J. 3:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lokensgard, J. R., M. C. Cheeran, G. Gekker, S. Hu, C. C. Chao, and P. K. Peterson. 1999. Human cytomegalovirus replication and modulation of apoptosis in astrocytes. J. Hum. Virol. 2:91-101. [PubMed] [Google Scholar]
- 158.Lokensgard, J. R., M. C. Cheeran, S. Hu, G. Gekker, and P. K. Peterson. 2002. Glial cell responses to herpesvirus infections: role in defense and immunopathogenesis. J. Infect. Dis. 186(Suppl. 2):S171-S179. [DOI] [PubMed] [Google Scholar]
- 159.Lu, M., and T. Shenk. 1996. Human cytomegalovirus infection inhibits cell cycle progression at multiple points, including the transition from G1 to S. J. Virol. 70:8850-8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ma, D. K., G. L. Ming, and H. Song. 2005. Glial influences on neural stem cell development: cellular niches for adult neurogenesis. Curr. Opin. Neurobiol. 15:514-520. [DOI] [PubMed] [Google Scholar]
- 161.Maidji, E., S. McDonagh, O. Genbacev, T. Tabata, and L. Pereira. 2006. Maternal antibodies enhance or prevent cytomegalovirus infection in the placenta by neonatal Fc receptor-mediated transcytosis. Am. J. Pathol. 168:1210-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Malinger, G., D. Lev, N. Zahalka, Z. Ben Aroia, N. Watemberg, D. Kidron, L. B. Sira, and T. Lerman-Sagie. 2003. Fetal cytomegalovirus infection of the brain: the spectrum of sonographic findings. AJNR Am. J. Neuroradiol. 24:28-32. [PMC free article] [PubMed] [Google Scholar]
- 163.Manning, W. C., and E. S. Mocarski. 1988. Insertional mutagenesis of the murine cytomegalovirus genome: one prominent alpha gene (ie2) is dispensable for growth. Virology 167:477-484. [PubMed] [Google Scholar]
- 164.Marchant, A., V. Appay, M. Van Der Sande, N. Dulphy, C. Liesnard, M. Kidd, S. Kaye, O. Ojuola, G. M. Gillespie, A. L. Vargas Cuero, V. Cerundolo, M. Callan, K. P. McAdam, S. L. Rowland-Jones, C. Donner, A. J. McMichael, and H. Whittle. 2003. Mature CD8(+) T lymphocyte response to viral infection during fetal life. J. Clin. Investig. 111:1747-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Martinez-Cerdeno, V., S. C. Noctor, and A. R. Kriegstein. 2006. The role of intermediate progenitor cells in the evolutionary expansion of the cerebral cortex. Cereb. Cortex 16(Suppl. 1):i152-i161. [DOI] [PubMed] [Google Scholar]
- 166.Matsukage, S., I. Kosugi, H. Kawasaski, K. Miura, H. Kitani, and Y. Tsutsui. 2006. Mouse embryonic stem cells are not susceptible to cytomegalovirus but acquire susceptibility during differentiation. Birth Defects Res. A 76:115-125. [DOI] [PubMed] [Google Scholar]
- 167.McCarthy, M., D. Auger, and S. R. Whittemore. 2000. Human cytomegalovirus causes productive infection and neuronal injury in differentiating fetal human central nervous system neuroepithelial precursor cells. J. Hum. Virol. 3:215-228. [PubMed] [Google Scholar]
- 168.McCarthy, M., C. Wood, L. Fedoseyeva, and S. R. Whittemore. 1995. Media components influence viral gene expression assays in human fetal astrocyte cultures. J. Neurovirol. 1:275-285. [DOI] [PubMed] [Google Scholar]
- 169.McCluskey, R., R. Sandin, and J. Greene. 1996. Detection of airborne cytomegalovirus in hospital rooms of immunocompromised patients. J. Virol. Methods 56:115-118. [DOI] [PubMed] [Google Scholar]
- 170.McCormick, A. L., A. Skaletskaya, P. A. Barry, E. S. Mocarski, and V. S. Goldmacher. 2003. Differential function and expression of the viral inhibitor of caspase 8-induced apoptosis (vICA) and the viral mitochondria-localized inhibitor of apoptosis (vMIA) cell death suppressors conserved in primate and rodent cytomegaloviruses. Virology 316:221-233. [DOI] [PubMed] [Google Scholar]
- 171.McCormick, A. L., V. L. Smith, D. Chow, and E. S. Mocarski. 2003. Disruption of mitochondrial networks by the human cytomegalovirus UL37 gene product viral mitochondrion-localized inhibitor of apoptosis. J. Virol. 77:631-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Meier, J. L. 2001. Reactivation of the human cytomegalovirus major immediate-early regulatory region and viral replication in embryonal NTera2 cells: role of trichostatin A, retinoic acid, and deletion of the 21-base-pair repeats and modulator. J. Virol. 75:1581-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Meier, J. L., and J. A. Pruessner. 2000. The human cytomegalovirus major immediate-early distal enhancer region is required for efficient viral replication and immediate-early gene expression. J. Virol. 74:1602-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Merkle, F. T., and A. Alvarez-Buylla. 2006. Neural stem cells in mammalian development. Curr. Opin. Cell Biol. 18:704-709. [DOI] [PubMed] [Google Scholar]
- 175.Michaels, M. G., D. P. Greenberg, D. L. Sabo, and E. R. Wald. 2003. Treatment of children with congenital cytomegalovirus infection with ganciclovir. Pediatr. Infect. Dis. J. 22:504-509. [DOI] [PubMed] [Google Scholar]
- 176.Mitchell, D. K., S. J. Holmes, R. L. Burke, A. M. Duliege, and S. P. Adler. 2002. Immunogenicity of a recombinant human cytomegalovirus gB vaccine in seronegative toddlers. Pediatr. Infect. Dis. J. 21:133-138. [DOI] [PubMed] [Google Scholar]
- 177.Moore, M. A. 2004. The role of cell migration in the ontogeny of the lymphoid system. Stem Cells Dev. 13:1-21. [DOI] [PubMed] [Google Scholar]
- 178.Muganda, P., O. Mendoza, J. Hernandez, and Q. Qian. 1994. Human cytomegalovirus elevates levels of the cellular protein p53 in infected fibroblasts. J. Virol. 68:8028-8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Neff, B. J., R. E. Weibel, E. B. Buynak, A. A. McLean, and M. R. Hilleman. 1979. Clinical and laboratory studies of live cytomegalovirus vaccine Ad-169. Proc. Soc. Exp. Biol. Med. 160:32-37. [DOI] [PubMed] [Google Scholar]
- 180.Nelson, C. T., and G. J. Demmler. 1997. Cytomegalovirus infection in the pregnant mother, fetus, and newborn infant. Clin. Perinatol. 24:151-160. [PubMed] [Google Scholar]
- 181.Nguyen, M. D., J. P. Julien, and S. Rivest. 2002. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat. Rev. Neurosci. 3:216-227. [DOI] [PubMed] [Google Scholar]
- 182.Ni, H. T., S. Hu, W. S. Sheng, J. M. Olson, M. C. Cheeran, A. S. Chan, J. R. Lokensgard, and P. K. Peterson. 2004. High-level expression of functional chemokine receptor CXCR4 on human neural precursor cells. Brain Res. Dev. Brain Res. 152:159-169. [DOI] [PubMed] [Google Scholar]
- 183.Niederkorn, J. Y. 2006. See no evil, hear no evil, do no evil: the lessons of immune privilege. Nat. Immunol. 7:354-359. [DOI] [PubMed] [Google Scholar]
- 184.Nigro, G., S. P. Adler, R. La Torre, and A. M. Best. 2005. Passive immunization during pregnancy for congenital cytomegalovirus infection. N. Engl. J. Med. 353:1350-1362. [DOI] [PubMed] [Google Scholar]
- 185.Nigro, G., A. Krzysztofiak, U. Bartmann, A. Clerico, E. Properzi, S. Valia, and M. Castello. 1997. Ganciclovir therapy for cytomegalovirus-associated liver disease in immunocompetent or immunocompromised children. Arch. Virol. 142:573-580. [DOI] [PubMed] [Google Scholar]
- 186.Nigro, G., H. Scholz, and U. Bartmann. 1994. Ganciclovir therapy for symptomatic congenital cytomegalovirus infection in infants: a two-regimen experience. J. Pediatr. 124:318-322. [DOI] [PubMed] [Google Scholar]
- 187.Noonan, D. 1994. The guinea pig (Cavia porcellus). ANZCCART News 7:1-8. [Google Scholar]
- 188.Noyola, D. E., G. J. Demmler, C. T. Nelson, C. Griesser, W. D. Williamson, J. T. Atkins, J. Rozelle, M. Turcich, A. M. Llorente, S. Sellers-Vinson, A. Reynolds, J. F. Bale, Jr., P. Gerson, and M. D. Yow. 2001. Early predictors of neurodevelopmental outcome in symptomatic congenital cytomegalovirus infection. J. Pediatr. 138:325-331. [DOI] [PubMed] [Google Scholar]
- 189.Noyola, D. E., G. J. Demmler, W. D. Williamson, C. Griesser, S. Sellers, A. Llorente, T. Littman, S. Williams, L. Jarrett, M. D. Yow, et al. 2000. Cytomegalovirus urinary excretion and long term outcome in children with congenital cytomegalovirus infection. Pediatr. Infect. Dis. J. 19:505-510. [DOI] [PubMed] [Google Scholar]
- 190.Odeberg, J., N. Wolmer, S. Falci, M. Westgren, A. Seiger, and C. Soderberg-Naucler. 2006. Human cytomegalovirus inhibits neuronal differentiation and induces apoptosis in human neural precursor cells. J. Virol. 80:8929-8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Odeberg, J., N. Wolmer, S. Falci, M. Westgren, E. Sundtrom, A. Seiger, and C. Soderberg-Naucler. 2007. Late human cytomegalovirus (HCMV) proteins inhibit differentiation of human neural precursor cells into astrocytes. J. Neurosci. Res. 85:583-593. [DOI] [PubMed] [Google Scholar]
- 192.Ogawa, H., T. Suzutani, Y. Baba, S. Koyano, N. Nozawa, K. Ishibashi, K. Fujieda, N. Inoue, and K. Omori. 2007. Etiology of severe sensorineural hearing loss in children: independent impact of congenital cytomegalovirus infection and GJB2 mutations. J. Infect. Dis. 195:782-788. [DOI] [PubMed] [Google Scholar]
- 193.Ornoy, A., and O. Diav-Citrin. 2006. Fetal effects of primary and secondary cytomegalovirus infection in pregnancy. Reprod. Toxicol. 21:399-409. [DOI] [PubMed] [Google Scholar]
- 194.Ossipow, V., P. Descombes, and U. Schibler. 1993. CCAAT/enhancer-binding protein mRNA is translated into multiple proteins with different transcription activation potentials. Proc. Natl. Acad. Sci. USA 90:8219-8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pachter, J. S., H. E. de Vries, and Z. Fabry. 2003. The blood-brain barrier and its role in immune privilege in the central nervous system. J. Neuropathol. Exp. Neurol. 62:593-604. [DOI] [PubMed] [Google Scholar]
- 196.Pass, R. F. 2005. Congenital cytomegalovirus infection and hearing loss. Herpes 12:50-55. [PubMed] [Google Scholar]
- 197.Pass, R. F., A. M. Duliege, S. Boppana, R. Sekulovich, S. Percell, W. Britt, and R. L. Burke. 1999. A subunit cytomegalovirus vaccine based on recombinant envelope glycoprotein B and a new adjuvant. J. Infect. Dis. 180:970-975. [DOI] [PubMed] [Google Scholar]
- 198.Pass, R. F., K. B. Fowler, S. B. Boppana, W. J. Britt, and S. Stagno. 2006. Congenital cytomegalovirus infection following first trimester maternal infection: symptoms at birth and outcome. J. Clin. Virol. 35:216-220. [DOI] [PubMed] [Google Scholar]
- 199.Pereira, L., E. Maidji, S. McDonagh, O. Genbacev, and S. Fisher. 2003. Human cytomegalovirus transmission from the uterus to the placenta correlates with the presence of pathogenic bacteria and maternal immunity. J. Virol. 77:13301-13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Pereira, L., E. Maidji, S. McDonagh, and T. Tabata. 2005. Insights into viral transmission at the uterine-placental interface. Trends Microbiol. 13:164-174. [DOI] [PubMed] [Google Scholar]
- 201.Perlman, J. M., and C. Argyle. 1992. Lethal cytomegalovirus infection in preterm infants: clinical, radiological, and neuropathological findings. Ann. Neurol. 31:64-68. [DOI] [PubMed] [Google Scholar]
- 202.Petrik, D. T., K. P. Schmitt, and M. F. Stinski. 2006. Inhibition of cellular DNA synthesis by the human cytomegalovirus IE86 protein is necessary for efficient virus replication. J. Virol. 80:3872-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Plotkin, S. A., R. Higgins, J. B. Kurtz, P. J. Morris, D. A. Campbell, Jr., T. C. Shope, S. A. Spector, and W. M. Dankner. 1994. Multicenter trial of Towne strain attenuated virus vaccine in seronegative renal transplant recipients. Transplantation 58:1176-1178. [PubMed] [Google Scholar]
- 204.Plotkin, S. A., S. E. Starr, H. M. Friedman, K. Brayman, S. Harris, S. Jackson, N. B. Tustin, R. Grossman, D. Dafoe, and C. Barker. 1991. Effect of Towne live virus vaccine on cytomegalovirus disease after renal transplant. A controlled trial. Ann. Intern. Med. 114:525-531. [DOI] [PubMed] [Google Scholar]
- 205.Plotkin, S. A., S. E. Starr, H. M. Friedman, E. Gonczol, and R. E. Weibel. 1989. Protective effects of Towne cytomegalovirus vaccine against low-passage cytomegalovirus administered as a challenge. J. Infect. Dis. 159:860-865. [DOI] [PubMed] [Google Scholar]
- 206.Plotkin, S. A., and H. Stetler. 1969. Treatment of congenital cytomegalic inclusion disease with antiviral agents. Antimicrob. Agents Chemother. 9:372-379. [PubMed] [Google Scholar]
- 207.Poland, S. D., L. L. Bambrick, G. A. Dekaban, and G. P. Rice. 1994. The extent of human cytomegalovirus replication in primary neurons is dependent on host cell differentiation. J. Infect. Dis. 170:1267-1271. [DOI] [PubMed] [Google Scholar]
- 208.Poland, S. D., P. Costello, G. A. Dekaban, and G. P. Rice. 1990. Cytomegalovirus in the brain: in vitro infection of human brain-derived cells. J. Infect. Dis. 162:1252-1262. [DOI] [PubMed] [Google Scholar]
- 209.Poncet, D., N. Larochette, A. L. Pauleau, P. Boya, A. A. Jalil, P. F. Cartron, F. Vallette, C. Schnebelen, L. M. Bartle, A. Skaletskaya, D. Boutolleau, J. C. Martinou, V. S. Goldmacher, G. Kroemer, and N. Zamzami. 2004. An anti-apoptotic viral protein that recruits Bax to mitochondria. J. Biol. Chem. 279:22605-22614. [DOI] [PubMed] [Google Scholar]
- 210.Poncet, D., A. L. Pauleau, G. Szabadkai, A. Vozza, S. R. Scholz, M. Le Bras, J. J. Briere, A. Jalil, R. Le Moigne, C. Brenner, G. Hahn, I. Wittig, H. Schagger, C. Lemaire, K. Bianchi, S. Souquere, G. Pierron, P. Rustin, V. S. Goldmacher, R. Rizzuto, F. Palmieri, and G. Kroemer. 2006. Cytopathic effects of the cytomegalovirus-encoded apoptosis inhibitory protein vMIA. J. Cell Biol. 174:985-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Prosch, S., A. K. Heine, H. D. Volk, and D. H. Kruger. 2001. CCAAT/enhancer-binding proteins alpha and beta negatively influence the capacity of tumor necrosis factor alpha to up-regulate the human cytomegalovirus IE1/2 enhancer/promoter by nuclear factor kappaB during monocyte differentiation. J. Biol. Chem. 276:40712-40720. [DOI] [PubMed] [Google Scholar]
- 212.Pulliam, L. 1991. Cytomegalovirus preferentially infects a monocyte derived macrophage/microglial cell in human brain cultures: neuropathology differs between strains. J. Neuropathol. Exp. Neurol. 50:432-440. [DOI] [PubMed] [Google Scholar]
- 213.Pulliam, L., D. Moore, and D. C. West. 1995. Human cytomegalovirus induces IL-6 and TNF alpha from macrophages and microglial cells: possible role in neurotoxicity. J. Neurovirol. 1:219-227. [DOI] [PubMed] [Google Scholar]
- 214.Quesenberry, K. E. 1994. Guinea pigs. Vet. Clin. N. Am. Small Anim. Pract. 24:67-87. [DOI] [PubMed] [Google Scholar]
- 215.Quinnan, G. V., Jr., M. Delery, A. H. Rook, W. R. Frederick, J. S. Epstein, J. F. Manischewitz, L. Jackson, K. M. Ramsey, K. Mittal, S. A. Plotkin, and et al. 1984. Comparative virulence and immunogenicity of the Towne strain and a nonattenuated strain of cytomegalovirus. Ann. Intern. Med. 101:478-483. [DOI] [PubMed] [Google Scholar]
- 216.Raftery, M. J., M. Schwab, S. M. Eibert, Y. Samstag, H. Walczak, and G. Schonrich. 2001. Targeting the function of mature dendritic cells by human cytomegalovirus: a multilayered viral defense strategy. Immunity 15:997-1009. [DOI] [PubMed] [Google Scholar]
- 217.Rarey, K. E., and L. E. Davis. 1993. Temporal bone histopathology 14 years after cytomegalic inclusion disease: a case study. Laryngoscope 103:904-909. [DOI] [PubMed] [Google Scholar]
- 218.Rawlinson, W. D., H. E. Farrell, and B. G. Barrell. 1996. Analysis of the complete DNA sequence of murine cytomegalovirus. J. Virol. 70:8833-8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Reboredo, M., R. F. Greaves, and G. Hahn. 2004. Human cytomegalovirus proteins encoded by UL37 exon 1 protect infected fibroblasts against virus-induced apoptosis and are required for efficient virus replication. J. Gen. Virol. 85:3555-3567. [DOI] [PubMed] [Google Scholar]
- 220.Reigstad, H., R. Bjerknes, T. Markestad, and H. Myrmel. 1992. Ganciclovir therapy of congenital cytomegalovirus disease. Acta Paediatr. 81:707-708. [DOI] [PubMed] [Google Scholar]
- 221.Reuter, J. D., D. L. Gomez, J. H. Wilson, and A. N. Van Den Pol. 2004. Systemic immune deficiency necessary for cytomegalovirus invasion of the mature brain. J. Virol. 78:1473-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Revello, M. G., M. Zavattoni, M. Furione, E. Fabbri, and G. Gerna. 2006. Preconceptional primary human cytomegalovirus infection and risk of congenital infection. J. Infect. Dis. 193:783-787. [DOI] [PubMed] [Google Scholar]
- 223.Rock, R. B., G. Gekker, S. Hu, W. S. Sheng, M. Cheeran, J. R. Lokensgard, and P. K. Peterson. 2004. Role of microglia in central nervous system infections. Clin. Microbiol. Rev. 17:942-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ross, D. S., S. C. Dollard, M. Victor, E. Sumartojo, and M. J. Cannon. 2006. The epidemiology and prevention of congenital cytomegalovirus infection and disease: activities of the Centers for Disease Control and Prevention Workgroup. J. Womens Health (Larchmt.) 15:224-229. [DOI] [PubMed] [Google Scholar]
- 225.Ross, S. A., and S. B. Boppana. 2005. Congenital cytomegalovirus infection: outcome and diagnosis. Semin. Pediatr. Infect. Dis. 16:44-49. [DOI] [PubMed] [Google Scholar]
- 226.Ross, S. A., K. B. Fowler, G. Ashrith, S. Stagno, W. J. Britt, R. F. Pass, and S. B. Boppana. 2006. Hearing loss in children with congenital cytomegalovirus infection born to mothers with preexisting immunity. J. Pediatr. 148:332-336. [DOI] [PubMed] [Google Scholar]
- 227.Sachs, G. W., R. L. Simmons, and H. H. Balfour, Jr. 1984. Cytomegalovirus vaccine: persistence of humoral immunity following immunization of renal transplant candidates. Vaccine 2:215-218. [DOI] [PubMed] [Google Scholar]
- 228.Schleiss, M. R. 2002. Animal models of congenital cytomegalovirus infection: an overview of progress in the characterization of guinea pig cytomegalovirus (GPCMV). J. Clin. Virol. 25(Suppl. 2):S37-S49. [DOI] [PubMed] [Google Scholar]
- 229.Schleiss, M. R. 2006. The role of the placenta in the pathogenesis of congenital cytomegalovirus infection: is the benefit of cytomegalovirus immune globulin for the newborn mediated through improved placental health and function? Clin. Infect. Dis. 43:1001-1003. [DOI] [PubMed] [Google Scholar]
- 230.Schleiss, M. R., B. J. Aronow, and S. Handwerger. 2007. Cytomegalovirus infection of human syncytiotrophoblast cells strongly interferes with expression of genes involved in placental differentiation and tissue integrity. Pediatr. Res. 61:565-571. [DOI] [PubMed] [Google Scholar]
- 231.Schleiss, M. R., N. Bourne, and D. I. Bernstein. 2003. Preconception vaccination with a glycoprotein B (gB) DNA vaccine protects against cytomegalovirus (CMV) transmission in the guinea pig model of congenital CMV infection. J. Infect. Dis. 188:1868-1874. [DOI] [PubMed] [Google Scholar]
- 232.Schleiss, M. R., N. Bourne, N. J. Jensen, F. Bravo, and D. I. Bernstein. 2000. Immunogenicity evaluation of DNA vaccines that target guinea pig cytomegalovirus proteins glycoprotein B and UL83. Viral Immunol. 13:155-167. [DOI] [PubMed] [Google Scholar]
- 233.Schleiss, M. R., N. Bourne, G. Stroup, F. J. Bravo, N. J. Jensen, and D. I. Bernstein. 2004. Protection against congenital cytomegalovirus infection and disease in guinea pigs, conferred by a purified recombinant glycoprotein B vaccine. J. Infect. Dis. 189:1374-1381. [DOI] [PubMed] [Google Scholar]
- 234.Schleiss, M. R., and T. C. Heineman. 2005. Progress toward an elusive goal: current status of cytomegalovirus vaccines. Expert Rev. Vaccines 4:381-406. [DOI] [PubMed] [Google Scholar]
- 235.Schleiss, M. R., J. C. Lacayo, Y. Belkaid, A. McGregor, G. Stroup, J. Rayner, K. Alterson, J. D. Chulay, and J. F. Smith. 2007. Preconceptual administration of an alphavirus replicon UL83 (pp65 homolog) vaccine induces humoral and cellular immunity and improves pregnancy outcome in the guinea pig model of congenital cytomegalovirus infection. J. Infect. Dis. 195:789-798. [DOI] [PubMed] [Google Scholar]
- 236.Schmidbauer, M., H. Budka, W. Ulrich, and P. Ambros. 1989. Cytomegalovirus (CMV) disease of the brain in AIDS and connatal infection: a comparative study by histology, immunocytochemistry and in situ DNA hybridization. Acta Neuropathol. (Berlin). 79:286-293. [DOI] [PubMed] [Google Scholar]
- 237.Schraff, S. A., D. K. Brown, M. R. Schleiss, J. Meinzen-Derr, J. H. Greinwald, and D. I. Choo. 2007. The role of CMV inflammatory genes in hearing loss. Otol. Neurotol. 28:964-969. [DOI] [PubMed] [Google Scholar]
- 238.Schupfer, P. C., J. R. Murph, and J. F. Bale, Jr. 1986. Survival of cytomegalovirus in paper diapers and saliva. Pediatr. Infect. Dis. 5:677-679. [DOI] [PubMed] [Google Scholar]
- 239.Schut, R. L., G. Gekker, S. Hu, C. C. Chao, C. Pomeroy, M. C. Jordan, and P. K. Peterson. 1994. Cytomegalovirus replication in murine microglial cell cultures: suppression of permissive infection by interferon-gamma. J. Infect. Dis. 169:1092-1096. [DOI] [PubMed] [Google Scholar]
- 240.Selinsky, C., C. Luke, M. Wloch, A. Geall, G. Hermanson, D. Kaslow, and T. Evans. 2005. A DNA-based vaccine for the prevention of human cytomegalovirus-associated diseases. Hum. Vaccines 1:16-23. [DOI] [PubMed] [Google Scholar]
- 241.Shinmura, Y., I. Kosugi, S. Aiba-Masago, S. Baba, L. R. Yong, and Y. Tsutsui. 1997. Disordered migration and loss of virus-infected neuronal cells in developing mouse brains infected with murine cytomegalovirus. Acta Neuropathol. 93:551-557. [DOI] [PubMed] [Google Scholar]
- 242.Shinmura, Y., I. Kosugi, M. Kaneta, and Y. Tsutsui. 1999. Migration of virus-infected neuronal cells in cerebral slice cultures of developing mouse brains after in vitro infection with murine cytomegalovirus. Acta Neuropathol. 98:590-596. [DOI] [PubMed] [Google Scholar]
- 243.Simard, A. R., and S. Rivest. 2005. Do pathogen exposure and innate immunity cause brain diseases? Neurol. Res. 27:717-725. [DOI] [PubMed] [Google Scholar]
- 244.Sinzger, C., K. Eberhardt, Y. Cavignac, C. Weinstock, T. Kessler, G. Jahn, and J. L. Davignon. 2006. Macrophage cultures are susceptible to lytic productive infection by endothelial-cell-propagated human cytomegalovirus strains and present viral IE1 protein to CD4+ T cells despite late downregulation of MHC class II molecules. J. Gen. Virol. 87:1853-1862. [DOI] [PubMed] [Google Scholar]
- 245.Sinzger, C., M. Kahl, K. Laib, K. Klingel, P. Rieger, B. Plachter, and G. Jahn. 2000. Tropism of human cytomegalovirus for endothelial cells is determined by a post-entry step dependent on efficient translocation to the nucleus. J. Gen. Virol. 81:3021-3035. [DOI] [PubMed] [Google Scholar]
- 246.Skaletskaya, A., L. M. Bartle, T. Chittenden, A. L. McCormick, E. S. Mocarski, and V. S. Goldmacher. 2001. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc. Natl. Acad. Sci. USA 98:7829-7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Soderberg-Naucler, C., D. N. Streblow, K. N. Fish, J. Allan-Yorke, P. P. Smith, and J. A. Nelson. 2001. Reactivation of latent human cytomegalovirus in CD14+ monocytes is differentiation dependent. J. Virol. 75:7543-7554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Sohn, Y. M., M. K. Oh, K. B. Balcarek, G. A. Cloud, and R. F. Pass. 1991. Cytomegalovirus infection in sexually active adolescents. J. Infect. Dis. 163:460-463. [DOI] [PubMed] [Google Scholar]
- 249.Song, Y. J., and M. F. Stinski. 2002. Effect of the human cytomegalovirus IE86 protein on expression of E2F-responsive genes: a DNA microarray analysis. Proc. Natl. Acad. Sci. USA 99:2836-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Spaete, R. R. 1991. A recombinant subunit vaccine approach to HCMV vaccine development. Transplant. Proc. 23:90-96. [PubMed] [Google Scholar]
- 251.Spaete, R. R., R. M. Thayer, W. S. Probert, F. R. Masiarz, S. H. Chamberlain, L. Rasmussen, T. C. Merigan, and C. Pachl. 1988. Human cytomegalovirus strain Towne glycoprotein B is processed by proteolytic cleavage. Virology 167:207-225. [DOI] [PubMed] [Google Scholar]
- 252.Speir, E., R. Modali, E. S. Huang, M. B. Leon, F. Shawl, T. Finkel, and S. E. Epstein. 1994. Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science 265:391-394. [DOI] [PubMed] [Google Scholar]
- 253.Spiller, O. B., L. K. Borysiewicz, and B. P. Morgan. 1997. Development of a model for cytomegalovirus infection of oligodendrocytes. J. Gen. Virol. 78:3349-3356. [DOI] [PubMed] [Google Scholar]
- 254.Stagno, S., R. F. Pass, G. Cloud, W. J. Britt, R. E. Henderson, P. D. Walton, D. A. Veren, F. Page, and C. A. Alford. 1986. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA 256:1904-1908. [PubMed] [Google Scholar]
- 255.Staras, S. A., S. C. Dollard, K. W. Radford, W. D. Flanders, R. F. Pass, and M. J. Cannon. 2006. Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clin. Infect. Dis. 43:1143-1151. [DOI] [PubMed] [Google Scholar]
- 256.Staras, S. A., W. D. Flanders, S. C. Dollard, R. F. Pass, J. E. McGowan, Jr., and M. J. Cannon. 2008. Influence of sexual activity on cytomegalovirus seroprevalence in the United States, 1988-1994. Sex. Transm. Dis. 35:472-479. [DOI] [PubMed] [Google Scholar]
- 257.Starr, S. E., J. P. Glazer, H. M. Friedman, J. D. Farquhar, and S. A. Plotkin. 1981. Specific cellular and humoral immunity after immunization with live Towne strain cytomegalovirus vaccine. J. Infect. Dis. 143:585-589. [DOI] [PubMed] [Google Scholar]
- 258.Strauss, M. 1985. A clinical pathologic study of hearing loss in congenital cytomegalovirus infection. Laryngoscope 95:951-962. [PubMed] [Google Scholar]
- 259.Strauss, M. 1990. Human cytomegalovirus labyrinthitis. Am. J. Otolaryngol. 11:292-298. [DOI] [PubMed] [Google Scholar]
- 260.Streblow, D. N., C. N. Kreklywich, P. Smith, J. L. Soule, C. Meyer, M. Yin, P. Beisser, C. Vink, J. A. Nelson, and S. L. Orloff. 2005. Rat cytomegalovirus-accelerated transplant vascular sclerosis is reduced with mutation of the chemokine-receptor R33. Am. J. Transplant. 5:436-442. [DOI] [PubMed] [Google Scholar]
- 261.Sugiura, S., T. Yoshikawa, Y. Nishiyama, Y. Morishita, E. Sato, T. Hattori, and T. Nakashima. 2003. Detection of human cytomegalovirus DNA in perilymph of patients with sensorineural hearing loss using real-time PCR. J. Med. Virol. 69:72-75. [DOI] [PubMed] [Google Scholar]
- 262.Sullivan, V., C. L. Talarico, S. C. Stanat, M. Davis, D. M. Coen, and K. K. Biron. 1992. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature 358:162-164. [DOI] [PubMed] [Google Scholar]
- 263.Sumegi, J., J. Y. Wang, D. K. Zhen, J. D. Eudy, C. B. Talmadge, B. F. Li, P. Berglund, M. D. Weston, S. F. Yao, M. Ma-Edmonds, L. Overbeck, P. M. Kelley, E. Zabarovsky, E. Uzvolgyi, E. J. Stanbridge, G. Klein, and W. J. Kimberling. 1996. The construction of a yeast artificial chromosome (YAC) contig in the vicinity of the Usher syndrome type IIa (USH2A) gene in 1q41. Genomics 35:79-86. [DOI] [PubMed] [Google Scholar]
- 264.Tarantal, A. F., M. S. Salamat, W. J. Britt, P. A. Luciw, A. G. Hendrickx, and P. A. Barry. 1998. Neuropathogenesis induced by rhesus cytomegalovirus in fetal rhesus monkeys (Macaca mulatta). J. Infect. Dis. 177:446-450. [DOI] [PubMed] [Google Scholar]
- 265.Temperton, N. J. 2002. DNA vaccines against cytomegalovirus: current progress. Int. J. Antimicrob. Agents 19:169-172. [DOI] [PubMed] [Google Scholar]
- 266.Temple, S. 2001. The development of neural stem cells. Nature 414:112-117. [DOI] [PubMed] [Google Scholar]
- 267.Terhune, S., E. Torigoi, N. Moorman, M. Silva, Z. Qian, T. Shenk, and D. Yu. 2007. Human cytomegalovirus UL38 protein blocks apoptosis. J. Virol. 81:3109-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Tsai, H. L., G. H. Kou, S. C. Chen, C. W. Wu, and Y. S. Lin. 1996. Human cytomegalovirus immediate-early protein IE2 tethers a transcriptional repression domain to p53. J. Biol. Chem. 271:3534-3540. [PubMed] [Google Scholar]
- 269.Tsutsui, Y. 1995. Developmental disorders of the mouse brain induced by murine cytomegalovirus: animal models for congenital cytomegalovirus infection. Pathol. Int. 45:91-102. [DOI] [PubMed] [Google Scholar]
- 270.Tsutsui, Y., A. Kashiwai, N. Kawamura, S. Aiba-Masago, and I. Kosugi. 1995. Prolonged infection of mouse brain neurons with murine cytomegalovirus after pre- and perinatal infection. Arch. Virol. 140:1725-1736. [DOI] [PubMed] [Google Scholar]
- 271.Tsutsui, Y., I. Kosugi, and H. Kawasaki. 2005. Neuropathogenesis in cytomegalovirus infection: indication of the mechanisms using mouse models. Rev. Med. Virol. 15:327-345. [DOI] [PubMed] [Google Scholar]
- 272.Vallejo, J. G., J. A. Englund, J. A. Garcia-Prats, and G. J. Demmler. 1994. Ganciclovir treatment of steroid-associated cytomegalovirus disease in a congenitally infected neonate. Pediatr. Infect. Dis. J. 13:239-241. [DOI] [PubMed] [Google Scholar]
- 273.van den Pol, A. N., M. D. Robek, P. K. Ghosh, K. Ozduman, P. Bandi, M. D. Whim, and G. Wollmann. 2007. Cytomegalovirus induces interferon-stimulated gene expression and is attenuated by interferon in the developing brain. J. Virol. 81:332-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.van der Knaap, M. S., G. Vermeulen, F. Barkhof, A. A. Hart, J. G. Loeber, and J. F. Weel. 2004. Pattern of white matter abnormalities at MR imaging: use of polymerase chain reaction testing of Guthrie cards to link pattern with congenital cytomegalovirus infection. Radiology 230:529-536. [DOI] [PubMed] [Google Scholar]
- 275.Van Hoosier, G. L., Jr., W. E. Giddens, Jr., C. S. Gillett, and H. Davis. 1985. Disseminated cytomegalovirus disease in the guinea pig. Lab. Anim. Sci. 35:81-84. [PubMed] [Google Scholar]
- 276.Verkhratsky, A., and F. Kirchhoff. 2007. NMDA receptors in glia. Neuroscientist 13:28-37. [DOI] [PubMed] [Google Scholar]
- 277.Vilalta, A., R. K. Mahajan, J. Hartikka, D. Rusalov, T. Martin, V. Bozoukova, V. Leamy, K. Hall, P. Lalor, A. Rolland, and D. C. Kaslow. 2005. I. Poloxamer-formulated plasmid DNA-based human cytomegalovirus vaccine: evaluation of plasmid DNA biodistribution/persistence and integration. Hum. Gene Ther. 16:1143-1150. [DOI] [PubMed] [Google Scholar]
- 278.Vink, C., E. Beuken, and C. A. Bruggeman. 2000. Complete DNA sequence of the rat cytomegalovirus genome. J. Virol. 74:7656-7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Wang, Z., C. L. Rosa, Z. Li, H. Ly, A. Krishnan, J. Martinez, W. J. Britt, and D. J. Diamond. 2007. Vaccine properties of a novel marker gene-free recombinant modified vaccinia Ankara expressing immunodominant CMV antigens pp65 and IE1. Vaccine 25:1132-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Wangemann, P. 2002. K(+) cycling and its regulation in the cochlea and the vestibular labyrinth. Audiol. Neurootol. 7:199-205. [DOI] [PubMed] [Google Scholar]
- 281.Wheeler, D. G., and E. Cooper. 2001. Depolarization strongly induces human cytomegalovirus major immediate-early promoter/enhancer activity in neurons. J. Biol. Chem. 276:31978-31985. [DOI] [PubMed] [Google Scholar]
- 282.Wicher, K., V. Wicher, and R. F. Gruhn. 1985. Differences in susceptibility to infection with Treponema pallidum (Nichols) between five strains of guinea pig. Genitourin. Med. 61:21-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Williamson, W. D., A. K. Percy, M. D. Yow, P. Gerson, F. I. Catlin, M. L. Koppelman, and S. Thurber. 1990. Asymptomatic congenital cytomegalovirus infection. Audiologic, neuroradiologic, and neurodevelopmental abnormalities during the first year. Am. J. Dis. Child. 144:1365-1368. [DOI] [PubMed] [Google Scholar]
- 284.Woolf, N. K., J. P. Harris, A. F. Ryan, D. M. Butler, and D. D. Richman. 1985. Hearing loss in experimental cytomegalovirus infection of the guinea pig inner ear: prevention by systemic immunity. Ann. Otol. Rhinol. Laryngol. 94:350-356. [PubMed] [Google Scholar]
- 285.Woolf, N. K., D. V. Jaquish, and F. J. Koehrn. 2007. Transplacental murine cytomegalovirus infection in the brain of SCID mice. Virol. J. 4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Xia, X., Y. Zhang, C. R. Zieth, and S. C. Zhang. 2007. Transgenes delivered by lentiviral vector are suppressed in human embryonic stem cells in a promoter-dependent manner. Stem Cells Dev. 16:167-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Yamamoto-Tabata, T., S. McDonagh, H. T. Chang, S. Fisher, and L. Pereira. 2004. Human cytomegalovirus interleukin-10 downregulates metalloproteinase activity and impairs endothelial cell migration and placental cytotrophoblast invasiveness in vitro. J. Virol. 78:2831-2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Yue, Y., S. S. Zhou, and P. A. Barry. 2003. Antibody responses to rhesus cytomegalovirus glycoprotein B in naturally infected rhesus macaques. J. Gen. Virol. 84:3371-3379. [DOI] [PubMed] [Google Scholar]
- 289.Zhang, C., and R. F. Pass. 2004. Detection of cytomegalovirus infection during clinical trials of glycoprotein B vaccine. Vaccine 23:507-510. [DOI] [PubMed] [Google Scholar]
- 290.Zhu, H., Y. Shen, and T. Shenk. 1995. Human cytomegalovirus IE1 and IE2 proteins block apoptosis. J. Virol. 69:7960-7970. [DOI] [PMC free article] [PubMed] [Google Scholar]