Abstract
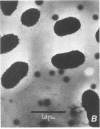
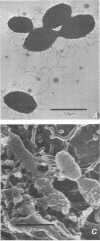
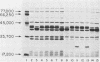
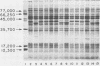
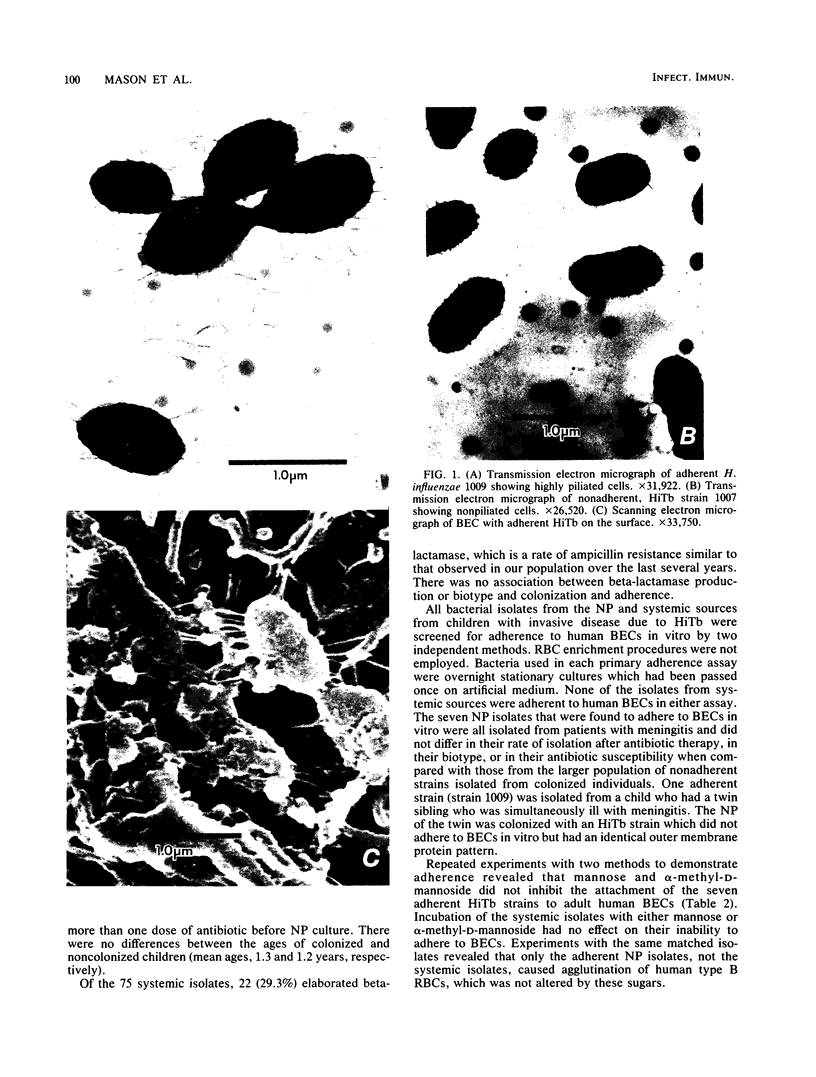
We found that 41 of 75 (55%) children with Haemophilus influenzae type b disease (70 cases of meningitis, 2 of cellulitis, 2 of septic arthritis, and 1 of epiglottitis) and 2 of 120 (1.7%) children with upper respiratory infection were colonized with H. influenzae type b in the nasopharynx (NP). Of these 43 NP strains from children with systemic H. influenzae type b disease, 7 (16%) adhered to human buccal epithelial cells. The strains isolated from the systemic site of all children, including children from whose NP adherent bacteria were isolated, did not adhere to buccal epithelial cells in vitro. Each adherent NP strain had biotype (I), serotype (b), and antibiotic susceptibility (sensitive) similar to that of the corresponding nonadherent systemic isolate. With one exception, all NP-systemic pairs had similar major outer membrane proteins. Six of the seven NP strains had a protein band in the whole cell lysate preparation with a molecular weight between 22,000 and 23,000, which could not be seen in the nonadherent cerebrospinal fluid strains. Electron micrographs of all adherent strains showed that more than 95% of the organisms examined were highly piliated, whereas the nonadherent strains were not piliated. All piliated strains agglutinated human erythrocytes. Adherence to buccal epithelial cells and agglutination of erythrocytes could not be blocked by mannose or alpha-methyl-D-mannoside. We speculate that piliation is not important for NP colonization by H. influenzae type b and that the loss of pili may be required for host invasion.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B., Eriksson B., Falsen E., Fogh A., Hanson L. A., Nylén O., Peterson H., Svanborg Edén C. Adhesion of Streptococcus pneumoniae to human pharyngeal epithelial cells in vitro: differences in adhesive capacity among strains isolated from subjects with otitis media, septicemia, or meningitis or from healthy carriers. Infect Immun. 1981 Apr;32(1):311–317. doi: 10.1128/iai.32.1.311-317.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Subtyping isolates of Haemophilus influenzae type b by outer-membrane protein profiles. J Infect Dis. 1981 May;143(5):668–676. doi: 10.1093/infdis/143.5.668. [DOI] [PubMed] [Google Scholar]
- Craven D. E., Peppler M. S., Frasch C. E., Mocca L. F., McGrath P. P., Washington G. Adherence of isolates of Neisseria meningitidis from patients and carriers to human buccal epithelial cells. J Infect Dis. 1980 Oct;142(4):556–568. doi: 10.1093/infdis/142.4.556. [DOI] [PubMed] [Google Scholar]
- Guerina N. G., Kessler T. W., Guerina V. J., Neutra M. R., Clegg H. W., Langermann S., Scannapieco F. A., Goldmann D. A. The role of pili and capsule in the pathogenesis of neonatal infection with Escherichia coli K1. J Infect Dis. 1983 Sep;148(3):395–405. doi: 10.1093/infdis/148.3.395. [DOI] [PubMed] [Google Scholar]
- Guerina N. G., Langermann S., Clegg H. W., Kessler T. W., Goldman D. A., Gilsdorf J. R. Adherence of piliated Haemophilus influenzae type b to human oropharyngeal cells. J Infect Dis. 1982 Oct;146(4):564–564. doi: 10.1093/infdis/146.4.564. [DOI] [PubMed] [Google Scholar]
- Johanson W. G., Jr, Woods D. E., Chaudhuri T. Association of respiratory tract colonization with adherence of gram-negative bacilli to epithelial cells. J Infect Dis. 1979 Jun;139(6):667–673. doi: 10.1093/infdis/139.6.667. [DOI] [PubMed] [Google Scholar]
- Kahn M. E., Gromkova R. Occurrence of pili on and adhesive properties of Haemophilus parainfluenzae. J Bacteriol. 1981 Feb;145(2):1075–1078. doi: 10.1128/jb.145.2.1075-1078.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan S. L., Mason E. O., Jr, Wiedermann B. L. Role of adherence in the pathogenesis of Haemophilus influenzae type b infection in infant rats. Infect Immun. 1983 Nov;42(2):612–617. doi: 10.1128/iai.42.2.612-617.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M. A taxonomic study of the genus Haemophilus, with the proposal of a new species. J Gen Microbiol. 1976 Mar;93(1):9–62. doi: 10.1099/00221287-93-1-9. [DOI] [PubMed] [Google Scholar]
- Lampe R. M., Mason E. O., Jr, Kaplan S. L., Umstead C. L., Yow M. D., Feigin R. D. Adherence of Haemophilus influenzae to buccal epithelial cells. Infect Immun. 1982 Jan;35(1):166–172. doi: 10.1128/iai.35.1.166-172.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- McGee Z. A., Johnson A. P., Taylor-Robinson D. Pathogenic mechanisms of Neisseria gonorrhoeae: observations on damage to human fallopian tubes in organ culture by gonococci of colony type 1 or type 4. J Infect Dis. 1981 Mar;143(3):413–422. doi: 10.1093/infdis/143.3.413. [DOI] [PubMed] [Google Scholar]
- Mett H., Kloetzlen L., Vosbeck K. Properties of pili from Escherichia coli SS142 that mediate mannose-resistant adhesion to mammalian cells. J Bacteriol. 1983 Feb;153(2):1038–1044. doi: 10.1128/jb.153.2.1038-1044.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels R. H., Stonebraker F. E., Robbins J. B. Use of antiserum agar for detection of Haemophilus influenzae type b in the pharynx. Pediatr Res. 1975 May;9(5):513–516. doi: 10.1203/00006450-197505000-00010. [DOI] [PubMed] [Google Scholar]
- Pichichero M. E., Loeb M., Anderson, Smith D. H. Do pili play a role in pathogenicity of Haemophilus influenzae type B? Lancet. 1982 Oct 30;2(8305):960–962. doi: 10.1016/s0140-6736(82)90161-1. [DOI] [PubMed] [Google Scholar]
- Salit I. E., Vavougios J., Hofmann T. Isolation and characterization of Escherichia coli pili from diverse clinical sources. Infect Immun. 1983 Nov;42(2):755–762. doi: 10.1128/iai.42.2.755-762.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens D. S., McGee Z. A. Attachment of Neisseria meningitidis to human mucosal surfaces: influence of pili and type of receptor cell. J Infect Dis. 1981 Apr;143(4):525–532. doi: 10.1093/infdis/143.4.525. [DOI] [PubMed] [Google Scholar]
- Stull T. L., Mendelman P. M., Haas J. E., Schoenborn M. A., Mack K. D., Smith A. L. Characterization of Haemophilus influenzae type b fimbriae. Infect Immun. 1984 Dec;46(3):787–796. doi: 10.1128/iai.46.3.787-796.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne G. M., Deneke C. F., Gorbach S. L. Hemagglutination and adhesiveness of toxigenic Escherichia coli isolated from humans. Infect Immun. 1979 Mar;23(3):690–699. doi: 10.1128/iai.23.3.690-699.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trust T. J., Gillespie R. M., Bhatti A. R., White L. A. Differences in the adhesive properties of Neisseria meningitidis for human buccal epithelial cells and erythrocytes. Infect Immun. 1983 Jul;41(1):106–113. doi: 10.1128/iai.41.1.106-113.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]