Abstract
Listeria monocytogenes has been exploited previously as a vaccine vector for the delivery of heterologous proteins such as tumor-specific antigens for active cancer immunotherapy. However, for effective use of live vector in clinics, safety is a major concern. In the present study, we describe an irreversibly attenuated and highly immunogenic L. monocytogenes platform, the L. monocytogenes dal-, dat-, and actA-deleted strain that expresses the human prostate-specific antigen (PSA) using an antibiotic resistance marker-free plasmid (the dal dat ΔactA 142 strain expressing PSA). Despite limited in vivo survival, the dal dat ΔactA 142 strain was able to elicit efficient immune responses required for tumor clearance. Our results showed that immunization of mice with the dal dat ΔactA 142 strain caused the regression of the tumors established by the prostate adenocarcinoma cell line expressing PSA. An evaluation of immunologic potency indicated that the dal dat ΔactA 142 strain elicits a high frequency of PSA-specific immune responses. Interestingly, immunization with the dal dat ΔactA 142 strain induced significant infiltration of PSA-specific T cells in the intratumoral milieu. Collectively, our data suggest that the dal dat ΔactA 142 strain is a safe and potent vector for clinical use and that this platform may be further exploited as a potential candidate to express other single or multiple antigens for cancer immunotherapy.
Biological and immunological characteristics of Listeria monocytogenes make this gram-positive bacterium an ideal vaccine vector. L. monocytogenes triggers potent cellular immune responses in an infected host due to its ability to survive in both phagocytic and cytosolic compartments. Several groups have shown recombinant L. monocytogenes to be an effective agent for immunotherapy against infection and cancer (2, 16, 19-21, 24, 27, 28). Currently, there are two methods to genetically modify L. monocytogenes to express heterologous antigens in vivo. These include the insertion of a heterologous gene in the bacterial chromosome either by homologous recombination (7, 16) or by phage-specific insertion (13) and the transformation of L. monocytogenes with a plasmid carrying a foreign antigen (7, 26). The plasmid-based strategy has the advantage of multicopy expression but relies on complementation for the maintenance of the plasmid in vivo. To address this, two mechanisms have been described previously for L. monocytogenes. One is based on the complementation of a prfA-deficient L. monocytogenes strain (XFL7) with a copy of episomal prfA (a major gene transcription activator for several virulence genes in L. monocytogenes) (7). This complementation ensures the retention of the plasmid in vivo but requires the presence of antibiotic resistance genes for in vitro selection. The second approach uses complementation with alanine racemase (dal), an enzyme involved in the synthesis of the cell wall component d-alanine (35).
In L. monocytogenes, the d-alanine metabolism is regulated by two genes, dal and dat (34). The complementation of the dal dat strain with either one of these genes is sufficient for restoring the synthesis of d-alanine both in vivo and in vitro (34, 35). Based on this property, Verch et al. designed the shuttle vector pTV3 that is devoid of antibiotic resistance markers but harbors a copy of the L. monocytogenes dal (dalLm) gene (35). This plasmid could complement the growth of both Escherichia coli ala drx (MB2159) and the dal dat mutant strains in vivo and in vitro (35). The dal dat strain contains a dal gene from which 82% of the nucleotides have been deleted; the remaining 18% are 44 bp corresponding to the 5′ end and 158 bp corresponding to the 3′ end (33). The 18% homology between the episomal dalLm gene and the dal dat strain chromosome is low for reverse recombination to create a revertant. However, due to regulatory concerns about using this plasmid in humans, it was safer to replace the dalLm gene in pTV3 with the nonhomologous Bacillus subtilis dal (dalBs) gene. Previously, Zhao et al. (37) showed that the dalBs gene can complement the growth of the L. monocytogenes dal dat strain in vivo and in vitro.
The L. monocytogenes dal dat strain containing pTV3-based plasmid is often attenuated merely due to the metabolic burden caused by the expression of a foreign antigen. Therefore, there is always a probability that these strains might regain their original virulence if the antigen expression is reduced or lost for any reason. This prompted us to construct an attenuated strain that exhibits a reduction in virulence due to an irreversible deletion of a major L. monocytogenes virulence gene, i.e., actA. ActA is a major virulence factor of L. monocytogenes that is involved in actin polymerization, and it is necessary for cytoplasmic movement and the cell-to-cell spread of the organism (12). Here, we tested this new generation of an antibiotic-free, dalBs plasmid antigen expression system (pAdv142) by complementing the attenuated dal dat ΔactA strain. We tested the resulting L. monocytogenes dal dat ΔactA 142 strain (expressing prostate-specific antigen [PSA]) in a previously described mouse model for prostate cancer because of its ability to eradicate established tumors and to induce cell-mediated immunity to PSA (27).
PSA is a kallikrein serine protease (KLK3) secreted by prostatic epithelial cells (36) and is used as a serum marker for prostate cancer (32). The potential of PSA as an antigen in immunotherapy is due to its overexpression in malignant prostate cells and low expression by normal prostate epithelial cells and other organs, such as the small intestine and testes (3). There is a great deal of information regarding the potential of this antigen for the treatment of prostate cancer (1, 10, 15, 17, 25). Previously, we showed that a recombinant L. monocytogenes vaccine expressing PSA (Lm-LLO-PSA) can cause the regression of solid tumors expressing PSA in a murine model for prostate cancer (27). The application of Lm-LLO-PSA is not ideal for a clinical setting due to its virulence and the presence of two chloramphenicol resistance genes (those for CAT) for in vitro selection in gram-negative (E. coli) and gram-positive (L. monocytogenes) bacteria. Here we show that the dalBs-based, antibiotic-free plasmid in an attenuated L. monocytogenes dal dat ΔactA strain backbone is a more efficient delivery vector than our previously described Lm-LLO-PSA vaccine. The expression of the heterologous antigens from the L. monocytogenes dal dat ΔactA strain based on the antibiotic-free shuttle vectors is potentially more compatible with the international regulatory requirements and could be developed further for clinical use.
MATERIALS AND METHODS
Peptides, oligonucleotides, antibodies, and fluorescence-activated cell sorter (FACS) reagents.
Peptides were synthesized by EZBiolabs (Westfield, IN), and oligonucleotides were synthesized by Invitrogen (Carlsbad, CA). The reagents for flow cytometry were obtained from Becton Dickinson (San Diego, CA). The antibodies used for staining in flow cytometry were as follows: CD3e-PerCP-Cy5.5 (clone 142-2C11), CD4-phycoerythrin (PE) (clone RM4-5), CD8-fluorescein isothiocyanate (clone 53-6.7), CD25-allophycocyanin (APC) (clone PC61), CD62L-APC (clone MEL-14), gamma interferon (IFN-γ)-PE (clone XMG1.2), anti-caspase-3-PE (clone C92-605), and anti-FoxP3 (Miltenyi Biotec, Auburn, CA). Culture media and supplements were obtained from Gibco (Carlsbad, CA) or Sigma (St. Louis, MO). Enzyme-linked immunospot (ELISPOT) assay antibodies were obtained from Mabtech (Cincinnati, OH). PSA tetramers were prepared by Emory University (Atlanta, GA). All other reagents, unless indicated, were from Sigma.
Mice, cell lines, and media.
C57BL/6 mice and C57BL/6 Ifngtm1tms (GKO−/−) mice were purchased from Jackson Laboratories (Bar Harbor, ME) and were maintained at the Cook Campus Animal Facility at Rutgers University, New Brunswick, NJ. Experiments with mice were performed after written approval from the Institutional Animal Care and Use Committee at Rutgers University. The construction of the prostate adenocarcinoma cell line expressing human PSA (TPSA23) was described previously (27). TPSA23 cells were grown and maintained in Dulbecco's modified Eagle's medium supplemented with 4 mM glutamine and adjusted to contain 1.5 g/liter sodium bicarbonate, 4.5 g/liter glucose, 5 μg/ml insulin, 10 nM dehydroisoandrosterone, 5% fetal bovine serum (FBS), and 5% NuSerum IV (BD Biosciences, CA) in the presence of 5 μg/ml blasticidin (Invitrogen). MC57G fibrosarcoma cells were maintained in Eagle's minimum essential medium with 2 mM glutamine, 1.5 g/liter sodium bicarbonate, 0.1 mM nonessential amino acids, 1.0 mM sodium pyruvate, and 10% FBS. EL4 lymphoma cells were maintained in Dulbecco's modified Eagle's medium with 4 mM glutamine adjusted to contain 1.5 g/liter sodium bicarbonate, 4.5 g/liter glucose, and 10% FBS. J774A.1, a murine macrophage-like cell line, was maintained in RPMI 1640 medium with 4 mM glutamine, 1.5 g/liter sodium bicarbonate, 4.5 g/liter glucose, and 10% FBS. Complete RPMI (C-RPMI) medium contained RPMI 1640 medium complemented with 2 mM glutamine, 0.1 mM nonessential amino acids, 1.0 mM sodium pyruvate, 10% FBS, penicillin-streptomycin (1%), and HEPES buffer (1 mM). For immunological assays such as intracellular cytokine staining and cytotoxic T-cell assays, 2-mercaptoethanol was added to C-RPMI medium to a final concentration of 55 μM.
Construction of the L. monocytogenes dal dat ΔactA strain.
The dal dat ΔactA strain was constructed using the method of homologous recombination as described previously by Mata et al. (16). The construction of the dal dat strain has been described previously (34). The dal dat strain is based on the L. monocytogenes genetic background 10403S, which contains a streptomycin resistance gene integrated in the chromosome. To delete actA from the dal dat strain, the chromosomal region corresponding to the upstream (657 bp) and downstream (625 bp) regions of actA was amplified and joined by splicing by overlap extension PCR. The primers used for the amplification of DNA region upstream of actA were UActA-F1 (CCGGATCCGCGCCAAATCATTGGTTGATTG) and UActA-R1 (CGTCGTATGGTTCCCTGGGTCGGGGTTAATCGTAATGCAATTGGC), and those used for the downstream region were DActA-F2 (ACGATTAACCCCGACCCAGGGAACCATACGACGTTAATTCTTGC) and DActAR2 (CCGGATCCGCTAGGCCTAATTTATAAAACGC). The resulting 1,282-bp DNA segment was subsequently cloned in the temperature-sensitive shuttle plasmid pKSV7 at its BamHI restriction site (underlined in the primers). The dal dat strain was transformed with the plasmid ΔactA/pKSV7. Repeated passaging of this strain in brain heart infusion (BHI)-d-alanine (100 μg/ml) medium at two different temperatures, 30°C and 42°C, resulted in the desired recombination events that created the dal dat ΔactA strain. The deletion of the actA region was verified by PCR using the primers forward (TGGGATGGCCAAGAAATTC) and reverse (CTACCATGTCTTCCGTTGCTTG) that anneal externally to the recombinant gene.
Construction of the pAdv142 plasmid.
Several modifications were introduced into the original pTV3 plasmid (35) to create the next generation of E. coli/L. monocytogenes antibiotic-free shuttle plasmids. To delete prfA, pTV3 was digested with the restriction enzymes (RE) XmaI and EheI (New England Biolabs). The plasmid pTV3 was first linearized with XmaI, and its ends were filled with Klenow polymerase. The pTV3/XmaI linearized and end-filled fragment was further digested with EheI RE, resulting in the release of a 1,571-bp DNA fragment containing prfA. The remaining 6,000 bp of the plasmid backbone was blunt ligated, resulting in the pTV3ΔprfA plasmid. The cassette for the p60 promoter for dalLm at the NheI/PacI restriction sites was replaced by the p60 promoter for dalBs, resulting in the plasmid pTV3-dalBsΔprfA. The dalBs gene (protein ID no. NP 388345.1) was amplified from the chromosomal DNA of Bacillus subtilis 168 (ATCC) using the forward primer dal F (GAGAGGAGTTTTCCATGAGCACAAAACCTTTTTACAGAGATACGTGGGCGG), which contained a 5′ overhang to join with the p60 promoter by splicing by overlap extension PCR, and the reverse primer dal R (CGCTAGCTTAATTGCTTATATTTACCTGCAATAAAGGATTTC), which contained an NheI site (underlined). The gene klk3 was amplified from our previously described plasmid, pAdv34 (27), using oligonucleotides as follows: F (GTGCTCGAGATTGTGGGAGGCTGGGAGTG) and R (GTTCCCGGGTTAGGGGTTGGCCACGATGG). The RE sites in the oligonucleotides are underlined. The human PSA gene klk3 was cloned in pTV3-dalBsΔprfA at its XhoI/XmaI restriction site, resulting in the plasmid pAdv142.
Construction of dal dat 142 and dal dat ΔactA 142 strains.
The dal dat and dal dat ΔactA strains were transformed with pAdv142 by electroporation, resulting in the dal dat 142 and dal dat ΔactA 142 strains. The expression and secretion of the truncated listeriolysin O (tLLO)-PSA fusion protein was confirmed in the culture supernatants of both of these strains using anti-PSA and anti-LLO antibodies, according to the previously described protocols (27). The dal dat 142 and dal dat ΔactA 142 strains were passaged twice in mice as previously reported (23). The murine macrophage-like cell line J774A.1 was used to examine the ability of dal dat ΔactA 142 to grow intracellularly as described previously (27).
In vitro and in vivo stability studies.
Plasmid maintenance in vitro was determined by serial passages under selective and nonselective conditions. Bacteria were cultured in 10 ml of BHI-streptomycin and subcultured daily at a 1/10,000 dilution into fresh medium in the presence or absence of d-alanine (100 μg/ml). Bacterial titers were determined daily on BHI plates with or without 100 μg/ml d-alanine. Streptomycin (100 μg/ml) was added to the medium as a control to select for L. monocytogenes and to reduce the potential growth of contaminants.
Plasmid maintenance in vivo was determined by intravenous injection of 5 × 107 CFU of dal dat ΔactA 142. Viable bacterial loads were determined in the spleens homogenized in phosphate-buffered saline on days 1, 2, and 3 (two mice/day). The number of CFU was determined at each time point by plating on BHI-streptomycin plates in the presence or absence of 100 μg/ml d-alanine.
In vivo virulence and clearance studies with dal dat 142 and dal dat ΔactA 142 strains.
C57BL/6 (wild-type [WT]) male mice were immunized intraperitoneally with different doses of the dal dat 142 (107 and 108 CFU) and dal dat ΔactA 142 (108 and 109 CFU) strains and were monitored for signs of sickness for a period of 10 days. The in vivo clearance of the strains was examined by immunizing mice (10/group) with 2 × 106 CFU of the dal dat 142 strain (WT) or 1 × 108 CFU of the dal dat ΔactA 142 strain (WT and GKO−/−). Viable CFU in the homogenized spleens and livers were determined from two mice in each group on days 1, 2, 3, 7, and 10 after plating the cell suspension on BHI-streptomycin medium.
Tumor regression study.
The tumor regression study was performed using the murine adenocarcinoma prostate tumor model expressing PSA, TPSA23 (27). Three groups of male C57BL/6 mice (eight/group) had 2 × 106 TPSA23 cells implanted on day 0 and were immunized with 107 CFU of Lm-LLO-PSA (27) or 108 CFU dal dat ΔactA 142 on days 6, 13, and 20 or were untreated (naïve). Tumor growth was monitored once a week using electronic calipers for a period of 8 weeks. The mice were sacrificed when the tumor size was found to be greater than 15 mm.
Immunogenicity studies of mice.
Male C57BL/6 mice (two/group) were immunized twice with dal dat ΔactA 142 (108 CFU) or Lm-LLO-PSA as a positive control (107 CFU) at 1-week intervals or were left untreated (naïve). The immune responses elicited for the PSA H-2Db peptide epitope (65HCIRNKSVIL74) (22) were determined on day 6 after the boost using the ELISPOT assay, PSA tetramer staining and intracellular cytokine staining for IFN-γ, as described previously (27). The data were analyzed for tetramer staining and IFN-γ staining using CellQuest Pro software.
Cytotoxic T-cell assay.
Splenocytes from immunized and naïve mice were stimulated in vitro for 5 days in C-RPMI medium containing 20 U/ml of interleukin 2 (Sigma) in the presence of mitomycin C-treated MC57G cells infected with PSA-vaccinia virus at an effector/stimulator ratio of 20:1 as described previously (27). The cytotoxicity of effector T cells was examined using a FACS assay, which is based on the staining for cleaved caspase-3 and thus measures the number of apoptotic target cells (9). The target cells EL4 were labeled with 0.6 μM Cell Trace Far Red DDAO-succinimidyl ester (SE) (Molecular Probes, Invitrogen) for 20 min at 37°C. Labeled cells were washed once with C-RPMI medium, resuspended at a concentration of 2 × 106/ml, and pulsed with 1 μg/ml of PSA peptide for 1 h. Labeled EL4 cells pulsed with PSA were then washed once in C-RPMI medium and were finally resuspended at a concentration of 1 × 106/ml for use in the cytotoxic T-lymphocyte assay. Effector cells were mixed with the targets at effector/target ratios of 10:1, 3:1, 1:1, 0.3:1, and 0.1:1 and incubated for 3 h at 37°C and 5% CO2. After 3 h, these cells were fixed with paraformaldehyde for 20 min, washed twice in FACS buffer (phosphate-buffered saline, 2% FBS), permeabilized with Perm/Wash (BD), and labeled with anti-caspase-3-PE antibody (BD) for 1 h. The induction of apoptosis in the target cells was measured by determining the number of caspase-positive/DDAO-SE-positive cells at each effector/target ratio. Specific lysis was considered to be directly related to the percentage of caspase-positive cells and was described in the following equation: (% caspase-positive DDAO-SE-positive EL4 PSA peptide-pulsed cells) − (% caspase-positive DDAO-SE-positive EL4 cells).
Analysis of TILs.
Male C57BL/6 mice (three/group) that had TPSA23 tumors implanted on day 0 were immunized with 108 CFU of dal dat ΔactA 142 and 108 CFU of irrelevant L. monocytogenes (Lm-LLO-E7) on day 7 and day 14. On day 20, tumors embedded in Matrigel were excised from the mice. The PSA-specific tumor-infiltrating lymphocytes (TILs) and regulatory T cells were determined in the tumors embedded in Matrigel and spleens using the protocol described previously (27).
Statistical analyses.
The nonparametric Kruskal-Wallis test was applied to compare the tumor sizes among different treatment groups. Tumor sizes were compared on day 40 for statistical analysis, because this was the latest time point with the highest number of mice in each group. The Kaplan-Meier test was applied to compare the survival of mice in different groups. A P value of less than 0.05 was considered statistically significant in these analyses.
RESULTS
Construction of a dal dat ΔactA strain that expresses the tLLO-PSA fusion protein by means of an antibiotic resistance-free plasmid.
To construct an attenuated dal dat ΔactA strain, an in-frame deletion of actA was generated in the dal dat strain background to avoid any polar effects on the expression of the downstream genes. The resulting dal dat ΔactA strain contains the first 19 amino acids at the N terminus and 28 amino acid residues at the C terminus, with a deletion of 591 amino acids of the chromosomal ActA. The deletion of actA was verified by PCR using primers that anneal externally to this region of the chromosome on the genomic DNA isolated from the dal dat or dal dat ΔactA strain (data not shown). The deletion of actA was further confirmed by DNA sequencing.
To increase the stability and safety of the previously constructed pTV3 (35), we introduced two modifications in this plasmid. First, an unnecessary copy of the prfA gene in this plasmid was deleted, and furthermore, the dalLm gene was replaced with the dalBs gene. The new plasmid, pAdv142 (Fig. 1A), expressed dalBs under the control of the L. monocytogenes p60 promoter. The plasmid pAdv142 was able to complement the growth of MB2159 (33) as well as that of the dal dat or dal dat ΔactA strain in the absence of exogenous d-alanine. The antigen expression cassette in pAdv142 consists of the hly promoter from L. monocytogenes and the tLLO-PSA fusion protein.
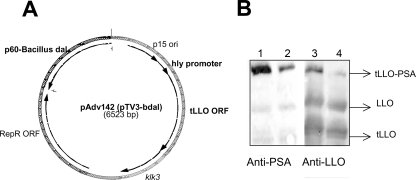
FIG. 1.
(A) Schematic map of the plasmid pAdv142. The antigen expression cassette contains the hly promoter, followed by its signal sequence and 417 amino acids of the N terminus of nonhemolytic protein tLLO fused to human PSA. The PSA protein lacks its secretory signal sequence. (B) Expression and secretion of tLLO-PSA fusion protein by the strains dal dat 142 (lanes 2 and 4) and dal dat ΔactA 142 (lanes 1 and 3). The protein extracts were prepared by precipitating culture supernatants with 10% Trypticase soy agar. The Western blot represents the expression of tLLO-PSA in the total proteins secreted by the dal dat 142 and dal dat ΔactA 142 strains. The blot was stained with a 1:1,000 dilution of both anti-LLO and anti-PSA primary antibodies.
Both the dal dat strain and the dal dat ΔactA strain were transformed with pAdv142, resulting in the strains dal dat 142 and dal dat ΔactA 142. The expression and secretion of the fusion protein tLLO-PSA was confirmed in these strains by Western blotting using both anti-PSA and anti-LLO antibodies (Fig. 1B). These strains were passaged twice in vivo in C57BL/6 mice to select for stable vaccine strains (23). The dal dat 142 and dal dat ΔactA 142 strains retained the expression and secretion of the tLLO-PSA fusion protein after two passages (data not shown), suggesting that both of these constructs were stable in vivo.
ActA deletion results in a 2-log attenuation of the dal dat vaccine strain.
We determined the maximum safe dose of the dal dat 142 and dal dat ΔactA 142 strains in C57BL/6 mice after administering different doses of each construct. Our results indicated that doses of 106 CFU for the dal dat 142 strain and 108 CFU for the dal dat ΔactA 142 strain were 1/10 the minimum dose observed to have adverse effects in mice and thus were used as safe doses for further studies. These virulence studies showed that the dal dat ΔactA 142 strain was at least 2 log more attenuated than the dal dat 142 strain. Additionally, we observed that GKO−/− mice, which are very susceptible to WT L. monocytogenes (8), were also resistant to 108 CFU of the dal dat ΔactA 142 strain, suggesting that high levels of IFN-γ released during L. monocytogenes innate immune responses control infection, possibly by preventing cell-to-cell spread.
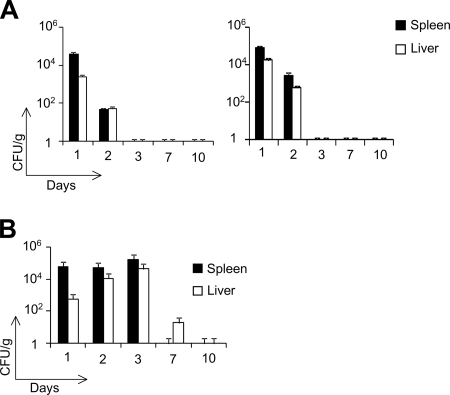
We further examined the in vivo clearance of both of these strains after intraperitoneal administration of the maximum safe doses in C57BL/6 mice and GKO−/− mice. We did not detect any live dal dat ΔactA 142 bacteria in the livers and spleens of either of these mouse strains on day 3 postinjection. This suggests that the dal dat ΔactA 142 strain is completely cleared after 48 to 72 h postinjection (Fig. 2A). In contrast, the dal dat 142 strain, containing the chromosomal actA, could survive in vivo for up to 7 days, reaching a growth peak in the livers and spleens on day 3 postinfection (Fig. 2B). Our data demonstrate that the dal dat ΔactA 142 strain is a safer vaccine construct than its unattenuated counterpart. Therefore, we chose the dal dat ΔactA 142 strain to evaluate its stability and therapeutic efficacy.
FIG. 2.
In vivo clearance of the dal dat ΔactA 142 strain in WT (left) and GKO−/− (right) mice (A) and the dal dat 142 strain in WT mice (B). Mice were immunized intraperitoneally with 2 × 106 CFU of the dal dat 142 strain and 108 CFU of the dal dat ΔactA 142 strain. The bacterial load in the organs, livers, and spleens were determined after days 1, 2, 3, 7, and 10 of immunization. Two mice from each group were sacrificed on different days, and the number of bacterial CFU was determined by plating several dilutions of the homogenized spleens and livers on BHI plates containing 100 μg/ml of streptomycin. The plates were incubated at 37°C for 24 to 48 h for bacterial growth. Columns, mean number of CFU from each mouse; bars, standard deviation (SD).
The L. monocytogenes dal dat ΔactA 142 strain is stable both in vitro and in vivo and can grow intracellularly.
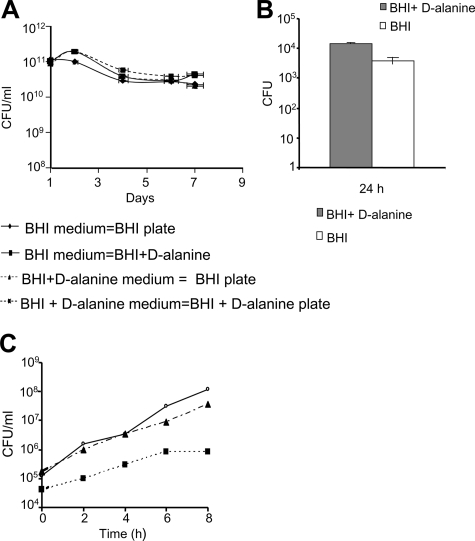
We examined the in vitro stability of the plasmid pAdv142 by passaging the dal dat ΔactA 142 strain in the presence or absence of selective pressure for 7 days. Total CFU counts were determined each day after plating on selective and nonselective media. It was expected that a loss of plasmid would result in higher CFU counts after plating on nonselective medium (BHI plus d-alanine). As depicted in Fig. 3A, there was no significant difference between the numbers of CFU in selective and nonselective media, and this shows that the dal dat ΔactA 142 strain retains the plasmid pAdv142 for at least 60 generations.
FIG. 3.
(A) In vitro stability of the plasmid pAdv142 in the dal dat ΔactA 142 strain after 60 generations. The in vitro stability was determined by subculturing the dal dat ΔactA 142 strain in selective (BHI) and nonselective (BHI plus 100 μg/ml d-alanine) media for 8 days at a 1:10,000 dilution at 37°C and 200 rpm. The average CFU ± SD was determined for each day after plating on selective and nonselective plates in triplicate. Streptomycin (100 μg/ml) was added to the culture medium to restrict the growth of any potential contaminant. (B) In vivo stability was examined by immunizing mice with 5 × 107 CFU of the dal dat ΔactA 142 strain intravenously in the tail vein. The CFU were determined in the homogenized spleens after 24, 48, and 72 h. Viable CFU were determined after plating on both selective and nonselective media. No colonies were recovered at the time points of 48 and 72 h. Columns, mean number of CFU from each mouse; bars, SD. (C) Intracellular growth of the dal dat 142 (▴) and dal dat ΔactA 142 (▪) strains in murine macrophage cells J774A.1. The CFU count was determined by taking duplicate samples at the indicated time points, followed by titration on BHI plates. The data shown are representative of the average CFU at each time point. The experiment was repeated three times, and data are representative of one study. Open circles, the L. monocytogenes 10403S strain.
The in vivo stability of pAdv142 in the dal dat ΔactA 142 strain was tested after immunizing C57BL/6 mice once with this strain intravenously and examining the number of viable bacteria at different time points, such as 24 h, 48 h, and 72 h, by plating bacteria on both selective and nonselective media. We observed no significant differences (P = 0.1566, using paired Student's t test) in CFU counts after 24 h by plating under either condition, suggesting that pAdv142/dal dat ΔactA 142 was stable in vivo (Fig. 3B). However, this does not exclude the possibility of plasmid loss. No colonies were recovered after 48 h and 72 h postinjection, which was due to the rapid in vivo clearance of the dal dat ΔactA 142 strain.
Furthermore, to determine if the deletion of actA caused any deleterious effect on the ability of the dal dat ΔactA 142 strain to infect macrophages and its intracellular growth, we performed a cell infection assay. We observed that both the dal dat 142 and dal dat ΔactA 142 strains were able to infect and grow in this cell line. The more virulent dal dat 142 strain displayed a growth rate similar to that of the WT L. monocytogenes 10403S strain. However, fewer colonies were recovered with the dal dat ΔactA 142 strain at different time points of growth (Fig. 3C). The differences in uptake of the dal dat 142 and dal dat ΔactA 142 strains were three- to fourfold at 0 h but were 37-fold at 8 h. Thus, there is a greater difference at 8 h than at 0 h, suggesting that the loss of cell-to-cell spread in the dal dat ΔactA 142 strain contributes to the reduction in its intracellular growth.
The L. monocytogenes dal dat ΔactA 142 strain mediates the regression of established tumors expressing human PSA.
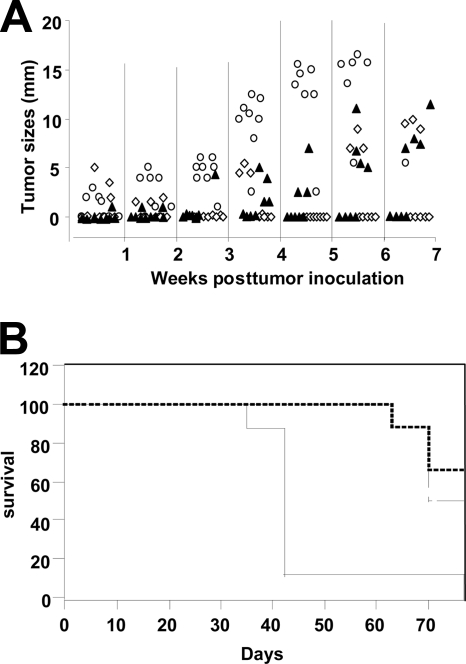
After an initial characterization, we further extended the study to evaluate the therapeutic efficacy of the dal dat ΔactA 142 strain using a murine prostate adenocarcinoma cell line engineered to express human PSA, i.e., TPSA23 (27). Naïve mice developed tumors gradually, and all were sacrificed before day 45 (Fig. 4A). Immunization of mice with Lm-LLO-PSA, which served as a positive control for the study, resulted in the complete regression of three out of eight tumors. In contrast, five out of eight mice immunized with the dal dat ΔactA 142 strain became tumor free and remained in this state until the experiment was terminated on day 70 (Fig. 4A). The statistical differences between each group were examined on day 40 using the nonparametric Kruskal-Wallis test. The results indicate that immunization with the dal dat ΔactA 142 strain makes a significant impact on the TPSA23 tumor growth (P = 0.001). Thus, the dal dat ΔactA 142 strain caused complete tumor regression in 60% of the experimental animals.
FIG. 4.
(A) Tumor regression study using TPSA23 as a transplantable tumor model. Three groups of eight mice were implanted with 2 × 106 tumor cells on day 0, and two groups were immunized with 107 CFU of the Lm-LLO-PSA vaccine (positive control) (▴) and 108 CFU of the dal dat ΔactA 142 strain (⋄) on days 6, 13, and 20. Naïve mice (○) did not receive any treatment. Tumors were monitored weekly, and mice were sacrificed if the average tumor diameter was 14 to 16 mm. Each symbol in the graph represents the tumor size of an individual mouse. The experiment was repeated twice, and similar results were obtained. (B) Percent survival of the naïve mice and immunized mice at different days of the experiment. Solid line, naïve mice; dashed line, mice immunized with the dal dat ΔactA 142 strain; broken line, mice immunized with the Lm-LLO-PSA vaccine.
Additionally, the average survival of mice in each group was determined using the Kaplan-Meier analysis (Fig. 4B). The mean survival of mice immunized with Lm-LLO-PSA (70 days ± 2 days) and the dal dat ΔactA 142 strain (73 days ± 2 days) was twofold higher than that of the naïve mice (42 days ± 2 days), and the overall comparison with the log rank test showed a P value of 0.01. These results suggest that vaccination with the dal dat ΔactA 142 strain significantly impacts the survival of mice.
Immunization with the dal dat ΔactA 142 strain elicits PSA-specific cellular immune responses in mouse spleens.
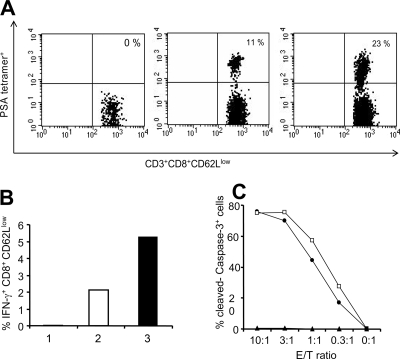
We have shown previously that the ability of recombinant L. monocytogenes-based vaccines to cause regression of the growth of established solid tumors is associated with the generation of antigen-specific T-cell responses. We examined PSA-specific immune responses elicited by the dal dat ΔactA 142 strain in C57BL/6 mice using PSA-specific tetramer staining, intracellular cytokine staining (IFN-γ), and ELISPOT assays. Staining with the PSA-specific tetramer showed that after two immunizations with the dal dat ΔactA 142 strain, 23% of activated CD8+ CD62Llow T cells were PSA specific in the splenocytes (Fig. 5A). This was a twofold-higher PSA tetramer-specific T-cell level compared to that of the mice that received Lm-LLO-PSA.
FIG. 5.
PSA-specific immune responses were examined by tetramer staining and intracellular cytokine staining for IFN-γ. Mice were immunized twice with 107 CFU of Lm-LLO-PSA and 108 CFU of the dal dat ΔactA 142 strain at 1-week intervals. For immune assays, spleens were harvested on day 6 after the boost. Spleens from three mice per group were pooled for this experiment. (A) PSA-specific T cells in the spleens of naïve, Lm-LLO-PSA-, and dal dat ΔactA 142 strain-immunized mice using PSA-specific tetramer staining. Cells were stained with mouse anti-CD8 (fluorescein isothiocyanate), anti-CD3 (Percp-Cy5.5), anti-CD62L (APC), and PSA tetramer-PE and analyzed by FACSCalibur. (B) Intracellular cytokine staining to detect the percentage of IFN-γ-secreting CD8+ CD62Llow cells in the naïve (bar 1), Lm-LLO-PSA- (bar 2), and dal dat ΔactA 142 strain (bar 3)-immunized mice after stimulation with 1 μM of PSA-specific H-2Db peptide for 5 h. (C) PSA-specific cytotoxic responses in mice immunized with the Lm-LLO-PSA vaccine (•) and the dal dat ΔactA 142 strain (□). Mice were immunized twice with 107 CFU of Lm-LLO-PSA and 108 CFU of the dal dat ΔactA 142 strain at a 1-week interval, and spleens were harvested on day 6 after the boost. The pooled splenocytes from each group were stimulated in vitro for 5 days after incubating with PSA-expressing stimulators. The target cells EL4 were pulsed with PSA H-2Db peptide, HCIRNKSVIL, and specific lysis was determined after incubating targets with different ratios of effector cells for 3 h. The experiment was repeated three times, and representative data are shown. Filled triangle, naïve mice; E/T ratio, effector/target ratio.
The results for the intracellular staining for IFN-γ were consistent with those obtained for the tetramer staining. There was a twofold increase in IFN-γ-positive CD8+ CD62Llow cells (5%) in the dal dat ΔactA 142 strain-immunized splenocytes relative to those of the mice immunized with Lm-LLO-PSA (2.2%) (Fig. 5B). Using the ELISPOT assay, we observed that there was a 10-fold increase in the number of IFN-γ-secreting cells in splenocytes from the dal dat ΔactA 142 strain-immunized mice in response to in vitro pulsing with PSA H-2Db peptide compared to that with no peptide (data not shown).
The functional activity of the T cells generated against PSA was determined using an in vitro cytotoxic T-lymphocyte assay that detects the cleavage of caspase-3 in target cells as a function of cell killing (9). At an effector-to-target cell ratio of 10:1, 70% of cleaved caspase-3-positive EL4/H-2Db PSA peptide-pulsed cells were detected when incubated with in vitro-stimulated effector T cells from either L. monocytogenes dal dat ΔactA 142 or Lm-LLO-PSA (Fig. 5C). This response was reduced in proportion to the decrease in the effector/target ratio. Taken together, these assays show that L. monocytogenes dal dat ΔactA 142 is highly immunogenic in mice, as implied by the detection of a high frequency of PSA-specific CD8+ T-cell responses.
Immunization with the dal dat ΔactA 142 strain results in infiltration of tumors by PSA-specific lymphocytes.
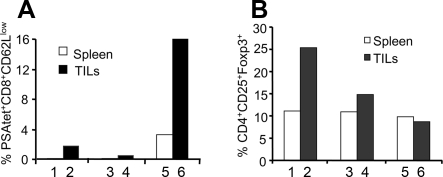
Furthermore, we investigated the ability of PSA-specific CD8+ lymphocytes generated by vaccination with the dal dat ΔactA 142 strain to infiltrate into the tumors. We observed that a very low number of PSA-specific TILs (2%) were present in the tumors harvested from both naïve and irrelevant L. monocytogenes-immunized mice. However, there was about an eightfold increase in the percentage of PSA-specific TILs (16%) in the tumors of mice immunized with the dal dat ΔactA 142 strain compared to those of the naïve mice (Fig. 6A).
FIG. 6.
Effect of vaccination of different Listeria constructs on the presence of PSA-specific TILs (A) and Tregs (B) in the tumors and spleens. Mice were implanted with TPSA23 tumors embedded in Matrigel and immunized with the irrelevant L. monocytogenes strain, the Lm-LLO-E7 vaccine (bars 3 and 4), and the dal dat ΔactA 142 strain (bars 5 and 6) on day 7 and day 14. On day 20, tumors and spleens were harvested from naïve (bars 1 and 2) and immunized mice. The tumors were pooled in each group to obtain an adequate number of cells for the staining of TILs and regulatory T cells. The cells were stained with surface antibodies, CD8, CD3, anti-CD62L, and PSA tetramer. To determine the Treg population in both tumors and spleens, the cells were stained for CD3, CD4, CD25, and Foxp3 antibodies. The experiment was repeated three times, and representative data are shown.
In addition, we determined the presence of CD4+/CD25+/Foxp3+ Tregs in the tumors and spleens of untreated or L. monocytogenes-immunized mice. Interestingly, we observed that immunization with L. monocytogenes (the dal dat ΔactA 142 strain or the irrelevant L. monocytogenes strain) resulted in a two- to threefold decrease in the number of Tregs in the tumors but not in the spleens. However, the dal dat ΔactA 142 strain showed a relatively stronger impact in decreasing the frequency of Tregs in tumors than did the irrelevant L. monocytogenes strain (Fig. 6B).
DISCUSSION
In the present study, we describe the construction of an irreversibly attenuated L. monocytogenes delivery vector, the dal dat ΔactA strain, that harbors an antibiotic resistance-free plasmid to express a tumor-specific antigen. The auxotropic mutant dal dat strain was constructed with the aim of creating an attenuated L. monocytogenes-based platform for clinical use (34). However, the dal dat strain elicited potent immune responses only if administered in the presence of d-alanine, which may not be appropriate for immunotherapy (34). Alternatively, trans-complementation of the dal dat strain with dal restores not only the synthesis of d-alanine but also in vivo virulence. Previously, two mechanisms have been proposed that reduce dal dat strain virulence after dal complementation. These mechanisms involve the use of either a plasmid that expresses the dalBs gene under the control of a tightly regulated inducible promoter (14) or a dalBs-containing suicidal plasmid, which is subsequently sensitive to dilution and degradation in vivo (37). Complementation of the dal dat strain using both of these methods causes the cessation of bacterial growth in vivo after two or three generations, reducing their applicability as potential vaccine platforms. We believe that one of the reasonable approaches to resolving these issues is to attenuate the dal dat strain, preferably by an irreversible deletion of one of its virulence factors. Therefore, we hypothesized that an irreversible in-frame deletion of actA would increase the safety of the dal dat strain as an immunotherapeutic vector.
In addition to the modification of the dal dat strain vaccine, we also generated an improved plasmid (pAdv142) that is retained by the complementation of dalBs. The expression of dalBs in pAdv142 was under the control of the L. monocytogenes promoter p60. The p60 promoter was chosen due to its constitutive mode of expression in L. monocytogenes and its ability to function in E. coli. The complementation of dalBs in E. coli ala drx makes this plasmid more amenable for further genetic manipulations and cloning purposes. We expressed the tumor antigen PSA in the dal dat ΔactA strain to demonstrate its applicability as an antigen delivery system. Initially, we transformed the dal dat and dal dat ΔactA strains with pAdv142, resulting in the dal dat 142 and dal dat ΔactA 142 strains, and confirmed that the recombinant strains dal dat 142 and dal dat ΔactA 142 were expressing and secreting the fusion protein tLLO-PSA using both anti-LLO and anti-PSA antibodies. Furthermore, we established that the dal dat 142 and dal dat ΔactA 142 strains could infect and grow intracellularly by using a cell infection assay. The properties such as expression and secretion of fusion protein and intracellular growth are necessary for successful antigen delivery and presentation when L. monocytogenes is used as an immunotherapeutic vector (7).
To verify the attenuation of the dal dat ΔactA 142 strain, we performed an in vivo virulence and clearance study of WT and IFN-γ−/− mice (GKO−/−). As expected, the dal dat ΔactA 142 strain was at least 2 log more attenuated than the dal dat 142 strain (5). Previous reports demonstrate that GKO−/− mice are extremely sensitive to infection with WT L. monocytogenes 10403S, by 3 orders of magnitude, compared to WT mice (8). A dose of 108 CFU of the dal dat ΔactA 142 strain was easily tolerated by GKO−/− mice, supporting that IFN-γ is not required for the clearance of this strain. The dal dat ΔactA 142 strain was completely cleared from the spleens and livers of the WT and GKO−/− mice by 48 to 72 h postinjection, suggesting that early innate or developing immune responses may not be vital for the in vivo clearance of the dal dat ΔactA 142 strain. On the contrary, immunization with a low dose (2 × 106 CFU) of the virulent dal dat 142 strain resulted in an increased persistence of bacteria in the spleens and livers of C57BL/6 mice for up to 168 h, due to its ability to spread from cell to cell. These observations suggest that the dal dat ΔactA strain may serve as a safer platform for antigen delivery than the dal dat strain.
We extended our study to compare the therapeutic efficacies of the dal dat ΔactA 142 strain and our previously published L. monocytogenes counterpart, Lm-LLO-PSA (27). After immunization with the dal dat ΔactA 142 strain, 60% of tumors were completely eradicated up to day 70. On the other hand, 38% of the mice were tumor free in the group immunized with Lm-LLO-PSA, suggesting that the dal dat ΔactA 142 strain was more efficacious than Lm-LLO-PSA. One of the reasons for this therapeutic effect may be the administration of a 10-fold-higher dose of the dal dat ΔactA 142 strain. This is supported by the observation that antitumor efficacies of the dal dat ΔactA 142 strain and Lm-LLO-PSA were comparable after the administration of similar doses of each immunotherapeutic vector (data not shown). Another live bacterial vaccine, the Salmonella enterica serovar Typhimurium aroA strain expressing PSA, has been shown to confer protection against PSA-expressing tumors (4). However, the therapeutic efficacy for this construct has not been determined. It is well established that the efficacy of active immunotherapy using L. monocytogenes as a vaccine vector is largely dependent on the ability of this bacterium to generate potent innate and adaptive immune responses (2, 18, 30). Our data on immunological assessment showed that the dal dat ΔactA 142 strain elicited high levels of PSA-specific T-cell responses. However, the efficacy of a vaccine is dependent not only on the magnitude of T cells in the periphery but also on creating conditions for them to infiltrate into the tumors. Both the dal dat ΔactA 142 strain and the Lm-LLO-PSA vaccine (27) cause the infiltration of PSA-specific T cells in the tumor microenvironment which are required for TPSA23 tumor regression. Another possible mechanism for tumor regression may involve the down-modulation of intratumoral regulatory T cells (11, 27). Interestingly, immunization with the dal dat ΔactA 142 strain was shown to decrease the population of regulatory T cells in the tumors.
It is noteworthy that PSA is a foreign antigen in mice, and thus no tolerance is expected toward this antigen. Previously we have verified the ability of L. monocytogenes-based vaccines to limit autochthonous tumor growth and break immunologic tolerance in human papillomavirus 16 E6/E7 and Her2/neu transgenic mice using Lm-LLO-E7 and Lm-Her-2/neu vaccines (29, 31). Thus, it is likely that the dal dat ΔactA 142 strain may be able to break tolerance. Moreover, other investigators have shown that tolerance against PSA in humans can be overcome by repeated immunizations (6, 10). As an alternative, male cynomolgus monkeys have been shown to express the PSA protein, which is 89% homologous to human PSA, making this species an attractive candidate for future evaluation of dal dat ΔactA 142 strain immunogenicity as well as evaluation of side effects that might arise upon vaccination against self antigen (15).
Overall, our data show that the dal dat ΔactA strain is superior to the dal dat strain as a vaccine vector due to its attenuation and increased safety, as evident from mouse studies. The challenge in designing an ideal L. monocytogenes vaccine for human use has been to achieve a level of attenuation such that the bacterium is less virulent yet still retains its ability to present antigen to the immune system. Here, we confirmed that the limited in vivo survival of the dal dat ΔactA strain does not compromise its therapeutic potency. Our aim is to move the prototype vaccine described here toward clinical trials in the near future. The broader goal of this study is to extend the application of this vector to designing potential constructs expressing other single or multiple heterologous antigens.
Acknowledgments
We thank the MHC Tetramer Core Facility at the Emory University Vaccine Center for providing us with the PSA tetramer. We thank Fred Frankel, Department of Microbiology, University of Pennsylvania (UPENN), for providing the dal dat strain. We acknowledge John Rothman, Kyla-Driscoll Carroll, and Gurpreet Singh Kapoor (UPENN) for careful reading of the manuscript and constructive comments.
Footnotes
Published ahead of print on 19 November 2008.
REFERENCES
- 1.Arlen, P. M., J. L. Gulley, C. Parker, L. Skarupa, M. Pazdur, D. Panicali, P. Beetham, K. Y. Tsang, D. W. Grosenbach, J. Feldman, S. M. Steinberg, E. Jones, C. Chen, J. Marte, J. Schlom, and W. Dahut. 2006. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin. Cancer Res. 121260-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruhn, K. W., N. Craft, and J. F. Miller. 2007. Listeria as a vaccine vector. Microbes Infect. 91226-1235. [DOI] [PubMed] [Google Scholar]
- 3.Cunha, A. C., B. Weigle, A. Kiessling, M. Bachmann, and E. P. Rieber. 2006. Tissue-specificity of prostate specific antigens: comparative analysis of transcript levels in prostate and non-prostatic tissues. Cancer Lett. 236229-238. [DOI] [PubMed] [Google Scholar]
- 4.Fensterle, J., B. Bergmann, C. L. Yone, C. Hotz, S. R. Meyer, S. Spreng, W. Goebel, U. R. Rapp, and I. Gentschev. 2008. Cancer immunotherapy based on recombinant Salmonella enterica serovar Typhimurium aroA strains secreting prostate-specific antigen and cholera toxin subunit B. Cancer Gene Ther. 1585-93. [DOI] [PubMed] [Google Scholar]
- 5.Goossens, P. L., and G. Milon. 1992. Induction of protective CD8+ T lymphocytes by an attenuated Listeria monocytogenes actA mutant. Int. Immunol. 41413-1418. [DOI] [PubMed] [Google Scholar]
- 6.Gulley, J., A. P. Chen, W. Dahut, P. M. Arlen, A. Bastian, S. M. Steinberg, K. Tsang, D. Panicali, D. Poole, J. Schlom, and J. M. Hamilton. 2002. Phase I study of a vaccine using recombinant vaccinia virus expressing PSA (rV-PSA) in patients with metastatic androgen-independent prostate cancer. Prostate 53109-117. [DOI] [PubMed] [Google Scholar]
- 7.Gunn, G. R., A. Zubair, C. Peters, Z. K. Pan, T. C. Wu, and Y. Paterson. 2001. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J. Immunol. 1676471-6479. [DOI] [PubMed] [Google Scholar]
- 8.Harty, J. T., and M. J. Bevan. 1995. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity 3109-117. [DOI] [PubMed] [Google Scholar]
- 9.He, L., J. Hakimi, D. Salha, I. Miron, P. Dunn, and L. Radvanyi. 2005. A sensitive flow cytometry-based cytotoxic T-lymphocyte assay through detection of cleaved caspase 3 in target cells. J. Immunol. Methods 30443-59. [DOI] [PubMed] [Google Scholar]
- 10.Heiser, A., D. Coleman, J. Dannull, D. Yancey, M. A. Maurice, C. D. Lallas, P. Dahm, D. Niedzwiecki, E. Gilboa, and J. Vieweg. 2002. Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J. Clin. Investig. 109409-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussain, S. F., and Y. Paterson. 2004. CD4+CD25+ regulatory T cells that secrete TGFβ and IL-10 are preferentially induced by a vaccine vector. J. Immunother. 27339-346. [DOI] [PubMed] [Google Scholar]
- 12.Kocks, C., E. Gouin, M. Tabouret, P. Berche, H. Ohayon, and P. Cossart. 1992. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell 68521-531. [DOI] [PubMed] [Google Scholar]
- 13.Lauer, P., M. Y. Chow, M. J. Loessner, D. A. Portnoy, and R. Calendar. 2002. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J. Bacteriol. 1844177-4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li, Z., X. Zhao, C. Zhou, B. Gu, and F. R. Frankel. 2006. A truncated Bacillus subtilis dal gene with a 3′ ssrA gene tag regulates the growth and virulence of racemase-deficient Listeria monocytogenes. Microbiology 1523091-3102. [DOI] [PubMed] [Google Scholar]
- 15.Marshall, D. J., L. R. San Mateo, K. A. Rudnick, S. G. McCarthy, M. C. Harris, C. McCauley, A. Schantz, D. Geng, P. Cawood, and L. A. Snyder. 2005. Induction of Th1-type immunity and tumor protection with a prostate-specific antigen DNA vaccine. Cancer Immunol. Immunother. 541082-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mata, M., Z. J. Yao, A. Zubair, K. Syres, and Y. Paterson. 2001. Evaluation of a recombinant Listeria monocytogenes expressing an HIV protein that protects mice against viral challenge. Vaccine 191435-1445. [DOI] [PubMed] [Google Scholar]
- 17.Noguchi, M., K. Kobayashi, N. Suetsugu, K. Tomiyasu, S. Suekane, A. Yamada, K. Itoh, and S. Noda. 2003. Induction of cellular and humoral immune responses to tumor cells and peptides in HLA-A24 positive hormone-refractory prostate cancer patients by peptide vaccination. Prostate 5780-92. [DOI] [PubMed] [Google Scholar]
- 18.Pamer, E. G. 2004. Immune responses to Listeria monocytogenes. Nat. Rev. Immunol. 4812-823. [DOI] [PubMed] [Google Scholar]
- 19.Pan, Z. K., G. Ikonomidis, A. Lazenby, D. Pardoll, and Y. Paterson. 1995. A recombinant Listeria monocytogenes vaccine expressing a model tumour antigen protects mice against lethal tumour cell challenge and causes regression of established tumours. Nat. Med. 1471-477. [DOI] [PubMed] [Google Scholar]
- 20.Pan, Z. K., G. Ikonomidis, D. Pardoll, and Y. Paterson. 1995. Regression of established tumors in mice mediated by the oral administration of a recombinant Listeria monocytogenes vaccine. Cancer Res. 554776-4779. [PubMed] [Google Scholar]
- 21.Pan, Z. K., L. M. Weiskirch, and Y. Paterson. 1999. Regression of established B16F10 melanoma with a recombinant Listeria monocytogenes vaccine. Cancer Res. 595264-5269. [PubMed] [Google Scholar]
- 22.Pavlenko, M., C. Leder, A. K. Roos, V. Levitsky, and P. Pisa. 2005. Identification of an immunodominant H-2D(b)-restricted CTL epitope of human PSA. Prostate 6450-59. [DOI] [PubMed] [Google Scholar]
- 23.Peters, C., and Y. Paterson. 2003. Enhancing the immunogenicity of bioengineered Listeria monocytogenes by passaging through live animal hosts. Vaccine 211187-1194. [DOI] [PubMed] [Google Scholar]
- 24.Prins, R. M., K. W. Bruhn, N. Craft, J. W. Lin, C. H. Kim, S. K. Odesa, J. F. Miller, and L. M. Liau. 2006. Central nervous system tumor immunity generated by a recombinant Listeria monocytogenes vaccine targeting tyrosinase related protein-2 and real-time imaging of intracranial tumor burden. Neurosurgery 58169-178. [DOI] [PubMed] [Google Scholar]
- 25.Roos, A. K., M. Pavlenko, J. Charo, L. Egevad, and P. Pisa. 2005. Induction of PSA-specific CTLs and anti-tumor immunity by a genetic prostate cancer vaccine. Prostate 62217-223. [DOI] [PubMed] [Google Scholar]
- 26.Sewell, D. A., V. Shahabi, G. R. Gunn III, Z. K. Pan, M. E. Dominiecki, and Y. Paterson. 2004. Recombinant Listeria vaccines containing PEST sequences are potent immune adjuvants for the tumor-associated antigen human papillomavirus-16 E7. Cancer Res. 648821-8825. [DOI] [PubMed] [Google Scholar]
- 27.Shahabi, V., M. Reyes-Reyes, A. Wallecha, S. Rivera, Y. Paterson, and P. Maciag. 2008. Development of a Listeria monocytogenes based vaccine against prostate cancer. Cancer Immunol. Immunother. 571301-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh, R., M. E. Dominiecki, E. M. Jaffee, and Y. Paterson. 2005. Fusion to listeriolysin O and delivery by Listeria monocytogenes enhances the immunogenicity of HER-2/neu and reveals subdominant epitopes in the FVB/N mouse. J. Immunol. 1753663-3673. [DOI] [PubMed] [Google Scholar]
- 29.Singh, R., and Y. Paterson. 2007. In the FVB/N HER-2/neu transgenic mouse both peripheral and central tolerance limit the immune response targeting HER-2/neu induced by Listeria monocytogenes-based vaccines. Cancer Immunol. Immunother. 56927-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh, R., and Y. Paterson. 2006. Listeria monocytogenes as a vector for tumor-associated antigens for cancer immunotherapy. Expert Rev. Vaccines 5541-552. [DOI] [PubMed] [Google Scholar]
- 31.Souders, N. C., D. A. Sewell, Z. K. Pan, S. F. Hussain, A. Rodriguez, A. Wallecha, and Y. Paterson. 2007. Listeria-based vaccines can overcome tolerance by expanding low avidity CD8+ T cells capable of eradicating a solid tumor in a transgenic mouse model of cancer. Cancer Immun. 72. [PMC free article] [PubMed] [Google Scholar]
- 32.Stamey, T. A., N. Yang, A. R. Hay, J. E. McNeal, F. S. Freiha, and E. Redwine. 1987. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N. Engl. J. Med. 317909-916. [DOI] [PubMed] [Google Scholar]
- 33.Strych, U., R. L. Penland, M. Jimenez, K. L. Krause, and M. J. Benedik. 2001. Characterization of the alanine racemases from two mycobacteria. FEMS Microbiol. Lett. 19693-98. [DOI] [PubMed] [Google Scholar]
- 34.Thompson, R. J., H. G. Bouwer, D. A. Portnoy, and F. R. Frankel. 1998. Pathogenicity and immunogenicity of a Listeria monocytogenes strain that requires d-alanine for growth. Infect. Immun. 663552-3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verch, T., Z. K. Pan, and Y. Paterson. 2004. Listeria monocytogenes-based antibiotic resistance gene-free antigen delivery system applicable to other bacterial vectors and DNA vaccines. Infect. Immun. 726418-6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watt, K. W., P. J. Lee, T. M'Timkulu, W. P. Chan, and R. Loor. 1986. Human prostate-specific antigen: structural and functional similarity with serine proteases. Proc. Natl. Acad. Sci. USA 833166-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao, X., Z. Li, B. Gu, and F. R. Frankel. 2005. Pathogenicity and immunogenicity of a vaccine strain of Listeria monocytogenes that relies on a suicide plasmid to supply an essential gene product. Infect. Immun. 735789-5798. [DOI] [PMC free article] [PubMed] [Google Scholar]