Abstract
In this study, the early pulmonary cytokine and chemokine responses in mice immunized with either BCG vaccine, a ΔsecA2 mutant of Mycobacterium tuberculosis, or a DNA vaccine expressing an ESAT6-antigen 85B fusion protein and then aerogenically challenged with a low dose of M. tuberculosis were evaluated by PCR array. The cellular immune responses at day 10 postchallenge were essentially equivalent in the lungs of mice immunized with either the highly immunogenic BCG vaccine or the ΔsecA2 M. tuberculosis mutant strain. Specifically, 12 immune biomolecules (including gamma interferon [IFN-γ], interleukin-21 [IL-21], IL-27, IL-17f, CXCL9, CXCL10, and CXCL11) were differentially regulated, relative to the levels for naïve controls, in the lungs of vaccinated mice at this time point. Although the vaccine-related immune responses evoked in mice immunized with the DNA vaccine were relatively limited at 10 days postinfection, upregulation of IFN-γ RNA synthesis as well as increased expression levels of CXCL9, CXCL10, and CXCL11 chemokines were detected.
Infections with Mycobacterium tuberculosis are a leading cause of morbidity and mortality worldwide, with 9 million new cases and 2 million deaths attributed to this disease annually (28). To improve control and to eventually decrease the global burden of tuberculosis (TB), more-effective immunization strategies are desperately needed. Although the current TB vaccine, M. bovis BCG, has been used to prevent TB for more than 70 years, its efficacy in controlled clinical trials has been highly variable (2). While BCG clearly reduces disseminated disease in children, it is relatively ineffective in preventing adult pulmonary TB. Because of the inability of BCG immunization to limit the global TB epidemic, the development of novel vaccines has become a focus for TB research. At present, clinical testing of at least five new TB vaccines has been initiated and preliminary data on the safety and immunogenicity of these preparations are being collected (29). Despite these recent advances, the mechanisms of vaccine-induced protection for TB still have not been adequately defined and a precise profile of vaccine-induced immune responses needed to generate optimal anti-TB protective immunity has not been established. Although studies with mice and humans have shown that Th1-type T-cell responses are critical for controlling tuberculous infections, the immune biomolecules that mediate TB vaccine activity have not been fully elucidated (6, 7, 21, 26). Clearly, the identification of biomarkers which could serve as correlates of protective immunity for TB could provide early indications of vaccine effectiveness in clinical studies and would facilitate decision-making processes in designing complex and expensive phase III vaccine trials.
To identify a pattern of biomarker expression which correlates with the induction of vaccine-induced anti-TB protective immunity, we evaluated early immune responses in the lungs of mice which had been vaccinated and then challenged by the aerosol route with a low dose of virulent M. tuberculosis. In mice, the immunologic events occurring soon after a tuberculous challenge seem to be critical for determining the extent of disease progression. Significant reductions of M. tuberculosis growth (relative to the levels for naïve controls) in the lungs of vaccinated mice during the first month after an aerosol infection often lead to substantially increased survival periods (3, 4). Since different types of TB vaccines will likely evoke various immune responses, we assessed the postchallenge cytokine and chemokine responses induced in the lungs of mice immunized with three different vaccines: BCG, a ΔsecA2 M. tuberculosis attenuated strain, and the M. tuberculosis SD1 DNA vaccine. The ΔsecA2 strain is a highly protective proapoptotic deletion mutant of M. tuberculosis (11). The SD1 DNA vaccine construct expresses a ESAT6-antigen 85B fusion protein which has been shown to boost BCG-induced immune responses and to protect against primary M. tuberculosis infection (5).
MATERIALS AND METHODS
Evaluation of vaccine effectiveness after a low-dose aerogenic challenge with M. tuberculosis.
In these experiments, C57BL/6 mice were immunized once subcutaneously with 106 CFU of either BCG or the ΔsecA2 mutant. For the DNA vaccine studies, mice were immunized three times, 3 weeks apart, with 200 μg of the SD1 DNA vaccine or the pVax DNA vector (Invitrogen, Carlsbad CA) preparation. The vaccinated mice were challenged about 3 months later by the aerosol route with 100 to 200 CFU of M. tuberculosis Erdman, using a Middlebrook-type inhalation chamber (5). To assess vaccine effectiveness, the growth of M. tuberculosis in relevant organs in naïve and BCG-vaccinated mice was assessed at 7, 10, 14, and 28 days postchallenge by plating organ homogenates on Middlebrook 7H11 plates as described earlier (4, 5). Mice immunized with the ΔsecA2 mutant and the SD1 DNA vaccine were sacrificed at 28 days postchallenge. The level of protection was defined as the difference in organ bacterial burden between naïve and vaccinated animals at the various time points after the M. tuberculosis challenge. The data were analyzed for statistical significance by using the t test analysis in the Graph Pad Prism version 4 software program.
Evaluation of pulmonary cytokine and chemokine responses.
To evaluate postchallenge immune responses, which may contribute to anti-TB protective immunity, pulmonary transcriptional responses in vaccinated and naïve mice were assessed after a low-dose aerogenic infection with M. tuberculosis Erdman. Briefly, at 7 to 14 days postchallenge, five infected mice per group were sacrificed and lung cells were harvested by shredding the lung tissues with razor blades before treatment with 2 mg/ml dispase (Invitrogen) for 1 h at 37°C. A single cell suspension of lymphocytes was achieved by passing the homogenate through a 70-μm strainer, followed by treatment with ACK lysing buffer and washing with phosphate-buffered saline with fetal calf serum. Total RNA was isolated from the lung cell suspensions by using an RNeasy minikit protocol (Qiagen, Valencia, CA). Equal amounts of RNA from each sample were then reverse transcribed to cDNA by using a Superscript first-strand synthesis kit (Invitrogen). The quality of the cDNA conversion was confirmed by doing PCR on individual samples, using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) primers. To quantitate lung transcriptional responses, the expression levels of 84 cytokines and 84 chemokine-like molecules were evaluated using the RT2 profiler PCR array system (SABiosciences, Frederick, MD) and an ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA). The specific genes evaluated are listed in Tables S1 and S2 in the supplemental material. The mRNA expression levels obtained for each gene were normalized to the expression of the GAPDH housekeeping gene by using the following equation: relative mRNA expression = 2−(Ct of cytokine −Ct of GAPDH) (where Ct is the threshold cycle). To determine whether the relative levels of gene expression in vaccinated animals were significantly different from the expression levels in naïve mice, the PCR array results were compared using the Wilcoxon matched-pair test (GraphPad Prism 4 software). Finally, the normalized gene expression levels in experimental samples (relative to the levels for naïve controls) were determined by dividing the relative gene expression values in vaccinated animals by the expression levels of naïve controls. Each reported cytokine or chemokine value represents the mean increase (or decrease) of mRNA expression relative to the levels for naïve controls for four to six experiments.
RESULTS
Vaccine-induced protection after a low-dose M. tuberculosis aerogenic challenge.
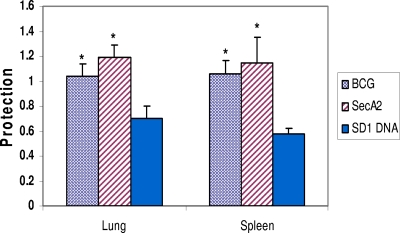
Initially, the effectiveness of the BCG vaccine, the ΔsecA2 M. tuberculosis mutant, and the SD1 DNA vaccine was confirmed in vaccination/challenge studies. In these experiments, vaccine-induced protection was defined as the relative reduction in organ bacterial burdens in vaccinated compared to naïve mice. Figure 1 shows the mean protective responses in the murine lungs and spleens at 28 days after the aerosol M. tuberculosis challenge. In these studies, the growth of the TB infection strain was reduced significantly in all vaccinated animals compared to the levels for naïve controls (P < 0.05). In contrast, injection of the DNA vector did not evoke significant protective responses (data not shown). It should be emphasized that BCG and ΔsecA2 immunization evoked stronger protective responses than vaccination with the SD1 DNA vaccine at this early time point (P < 0.05). Also, although the anti-TB protection evoked by vaccination with the ΔsecA2 mutant was frequently increased relative to the BCG-induced protective responses, these differences were not statistically significant at 4 weeks after an aerogenic challenge with M. tuberculosis Erdman. Importantly, for all three vaccines, the protection detected at 28 days postchallenge is consistent with the significantly enhanced survival periods (relative to the levels for naïve controls) seen in previous studies (5, 11).
FIG. 1.
Protective responses in vaccinated mice at 28 days postchallenge. Mice were vaccinated with either BCG, the ΔsecA2 mutant of M. tuberculosis, or the SD1 DNA vaccine expressing an ESAT6-antigen 85B fusion protein, as described in Materials and Methods; challenged by the aerosol route with a low dose of M. tuberculosis Erdman; and then sacrificed at 28 days postchallenge. Vaccine-induced protection was calculated as follows: number of organ bacterial CFU in naïve mice − number of CFU in vaccinated mice. These results represent the mean protective results for three or four experiments. The mean lung and spleen CFU values at day 28 postinfection for all vaccinated mice were significantly lower than the CFU values for naïve controls (P < 0.05; t tests). The protective responses in the BCG- and the ΔsecA2-vaccinated mice were significantly increased relative to the levels for SD1 DNA-vaccinated mice (*, P < 0.05).
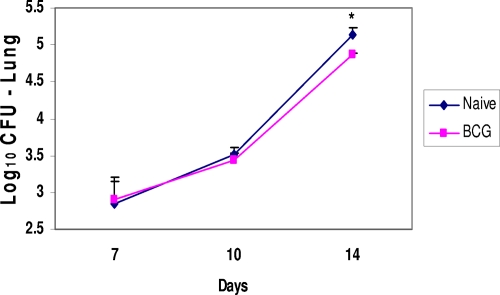
While protective responses are often measured at 28 days after the M. tuberculosis infection in this mouse model, the minimal period needed to detect significant decreases in pulmonary growth in vaccinated animals is less certain. To better define when protective responses can be initially detected in vaccinated animals, BCG-immunized mice and naïve controls were challenged with virulent M. tuberculosis by the aerosol route and then sacrificed to determine relative lung bacterial burdens 7, 10, and 14 days later. As seen in Fig. 2, the M. tuberculosis infection progressed similarly in the lungs of immunized and control mice for 10 days. However, at 2 weeks postchallenge, significant reductions in pulmonary bacterial CFU values were detected in vaccinated animals relative to the levels for naïve controls (P < 0.05).
FIG. 2.
Protective responses in the lungs of BCG-vaccinated mice during the first 2 weeks after the aerogenic infection. The mean numbers of CFU in the lungs of the BCG-vaccinated mice were significantly lower than the mean numbers of lung CFU for the naïve controls at day 14 postchallenge (*, P < 0.05).
Early postinfection pulmonary cytokine and chemokine responses in vaccinated mice.
Since vaccine-induced protection against virulent M. tuberculosis was seen by 2 weeks postinfection, we decided to evaluate early pulmonary immune responses in vaccinated and naïve animals between 7 and 14 days after the aerogenic challenge. Because M. tuberculosis infections are largely controlled by cellular immune responses, we focused on assessing early postchallenge cytokine and chemokine responses in vaccinated and control mice. In initial studies, BCG-immunized and naïve mice were infected with a low dose of M. tuberculosis Erdman, and then pulmonary cellular responses were evaluated at 7, 10, and 14 days postchallenge by PCR array. In a previous study, we had shown that the differences in pulmonary immune response between vaccinated and naïve mice were maximal at 10 to 14 days postchallenge but that by 21 days, the cytokine responses in naïve animals exceeded those detected in immunized mice, probably because of the increasing bacterial burdens in naïve animals (9). As seen in Table 1, modest changes in the transcriptional responses (relative to the levels for naïve controls) were found in the lungs of vaccinated mice for only 5 of 84 cytokines evaluated at day 7 postinfection. Only Gdf3, gamma interferon (IFN-γ), interleukin-7 (IL-7), and IL-21 expression levels were upregulated at this early time point, while Gdf10 expression was consistently downregulated. At day 10 postinfection, the expression levels of five cytokine-related genes (the Gdf3, IFN-γ, IL-17f, IL-21, and IL-27 genes) had increased 7- to 24-fold relative to the levels for naïve controls. Surprisingly, IL-4 expression was also significantly increased (a modest threefold increase) in vaccinated animals at day 10. In contrast, the expression levels of three immune response genes (the Gdf10, Bmp3, and IL-11 genes) were decreased about threefold at this time point. By day 14 after the challenge, mRNA levels of eight cytokine-related genes were increased in the lungs of BCG-vaccinated mice, including the IFN-γ, IFN-β, IL-17f, IL-27, and tumor necrosis factor alpha genes.
TABLE 1.
Normalized values for early pulmonary cytokine responses in vaccinated mice following an aerosol infection with M. tuberculosis
| Cytokine | Fold expression level (mean ± SEM) induced by vaccinea
|
||||
|---|---|---|---|---|---|
| Day 7b | Day 10
|
Day 14b | |||
| BCG | ΔsecA2 | SD1 DNA | |||
| Gdf3 | 2.2 ± 0.4 | 7.7 ± 1.8 | 9.8 ± 4.1 | 6.7 ± 0.9 | |
| IFN-γ | 2.6 ± 0.5 | 7.3 ± 2.0 | 8.3 ± 2.4 | 2.5 ± 0.8 | 6.8 ± 1.5 |
| IL-7 | 2.3 ± 0.4 | 2.0 ± 0.2 | |||
| IL-21 | 6.5 ± 2.0 | 7.9 ± 0.8 | 11.5 ± 3.0 | ||
| IL-4 | 3.4 ± 0.8 | 2.9 ± 0.8 | 2.8 ± 0.7 | ||
| IL-17f | 24.1 ± 12 | 13.0 ± 8.2 | 3.7 ± 0.2 | ||
| IL-27 | 7.2 ± 2.2 | 4.8 ± 0.2 | 4.2 ± 1.6 | ||
| IFN-β | 4.8 ± 1.6 | ||||
| IL-1b | 2.3 ± 0.2 | ||||
| TNF-α | 2.2 ± 0.1 | ||||
| Gdf10 | 0.3 ± 0.05c | 0.3 ± 0.1 | 0.4 ± 0.1 | ||
| Bmp3 | 0.3 ± 0.1 | 0.3 ± 0.1 | |||
| IL-11 | 0.3 ± 0.1 | 0.3 ± 0.1 | |||
Each reported value represents the mean increase (or decrease) in cytokine expression (relative to the level for naïve controls) ± the standard error of the mean for four to six experiments. “Day” refers to the number of days after the low-dose aerogenic infection with M. tuberculosis Erdman.
Only BCG reactions were measured on this day.
Normalized values of <1 represent expression levels that are downregulated relative to levels for naïve controls.
Since early chemokine synthesis is critical for directing infiltrating cells to the site of infection, we also evaluated whether the expression levels of chemokine genes were elevated in the lungs of vaccinated mice following an aerosol infection with virulent M. tuberculosis. As seen in Table 2, the expression levels of only 3 of 84 chemokine genes evaluated were upregulated at day 10 compared to naïve controls. The expression levels of IFN-γ inducible T-cell chemoattractant molecules CXCL9 (41.7-fold increase), CXCL10 (10.1-fold), and CXCL11 (17.6-fold) were highly increased in BCG-vaccinated mice.
TABLE 2.
Normalized values for pulmonary chemokine responses in vaccinated mice 10 days after an aerosol infection with M. tuberculosis
| Chemokine | Fold expression level (mean ± SEM) induced by vaccinea
|
||
|---|---|---|---|
| BCG | ΔsecA2 | SD1 DNA | |
| CXCL9 | 41.7 ± 12 | 36.1 ± 2.9 | 37.6 ± 2.5 |
| CXCL10 | 10.1 ± 2.1 | 5.7 ± 2.9 | 5.5 ± 0.6 |
| CXCL11 | 17.6 ± 7.2 | 12.3 ± 1.2 | 6.9 ± 0.1 |
Each reported value represents the mean increase in chemokine expression (relative to the level for naïve controls) ± the standard error of the mean for four to six experiments.
To analyze the cellular immune responses induced by other TB vaccines, mice were immunized with the ΔsecA2 mutant or the SD1 DNA vaccine, and then pulmonary cytokine and chemokine expression levels were evaluated 10 days after a low-dose aerosol Μ. tuberculosis infection. Interestingly, the early cytokine and chemokine responses in the lungs of infected mice immunized with the ΔsecA2 mutant strain were nearly identical to the BCG results, with no significant differences detected in the levels of expression of upregulated and downregulated genes (Tables 1 and 2). In particular, the expression levels of the Gdf3, IFN-γ, IL-17f, IL-21, and IL-27 cytokine genes as well as the CXCL9, CXCL10, and CXCL11 chemokine genes were highly upregulated in mice vaccinated with the ΔsecA2 mutant 10 days after the aerosol M. tuberculosis infection. In contrast, the overall cytokine expression responses at day 10 postinfection in animals immunized with the SD1 DNA vaccine were substantially lower than the responses detected in mice vaccinated with live, attenuated vaccines. Among the 84 cytokines evaluated by PCR array, only the expression levels of IFN-γ and IL-4 were upregulated in the lungs of the SD1 DNA-vaccinated mice at this early time point. Furthermore, the level of IFN-γ expression was reduced about threefold relative to levels seen in mice immunized with BCG or the ΔsecA2 mutant strain. Surprisingly, despite the modest IFN-γ responses in SD1 DNA-vaccinated mice, the postinfection pulmonary IFN-γ-inducible CXCL9, CXCL10, and CXCL11 chemokine responses in these mice were not significantly different from those in the other vaccinated animals. At 10 days postinfection, the average increases in lung mRNA expression for these chemokines were 37.6-fold for CXCL9, 5.5-fold for CXCL10, and 6.9-fold for CXCL11 in mice vaccinated with the SD1 DNA vaccine.
DISCUSSION
Taken together, our PCR array data have verified the complexity of the early immune responses following vaccination and challenge with Mycobacterium tuberculosis. Although IFN-γ responses are frequently being used as a correlate of vaccine-induced protective immunity in preclinical experiments and clinical studies, our results are consistent with previous data which indicate that TB vaccine-induced immunity involves the expression of many genes rather than just a few genes (8, 12, 20, 25, 27). In our studies, we showed that vaccination with the highly immunogenic attenuated strains followed by an aerosol tuberculous infection induced differential regulation of expression of at least 15 genes encoding immune mediators at 7 to 14 days postchallenge. While the precise biological significance of the up- or downregulation of cytokine expression in the lungs of immunized animals remains uncertain, these early changes do correspond with decreased mycobacterial organ burdens at 1 month postchallenge and increased survival periods for vaccinated mice relative to the levels for naïve controls (5, 11). Interestingly, the early cytokine and chemokine responses after vaccination with BCG or the ΔsecA2 mutant of M. tuberculosis were essentially equivalent. These results are consistent with our 4-week postinfection bacterial burden data, which showed that the protection induced by these vaccines against M. tuberculosis Erdman was statistically indistinguishable in this mouse model. It should be noted that in an earlier study, we demonstrated that mice immunized with the ΔsecA2 mutant survived significantly longer than BCG-vaccinated mice after an aerosol M. tuberculosis infection (11). This absence of a direct linear correlation between the day 28 protection data and the survival results is likely due to the enhanced capacity of the ΔsecA2 mutant to induce CD8 T-cell responses. In this mouse model, early pulmonary anti-TB cellular immunity is dominated by CD4 T-cell responses, while antigen-specific CD8 T-cell responses are likely more important during the later phases of the infection (25). Clearly, this CD4 T-cell dominance is a limitation in assessing early immune responses in mice. To adequately evaluate protective responses evoked by vaccines designed to induce CD8 responses, new models which can assess vaccine-induced immunity at later phases of the infection are needed.
While the live, attenuated strains are clearly highly immunogenic, immunization with the SD1 DNA vaccine also induced anti-TB protection in the lung and modest pulmonary cytokine responses in this mouse model. Despite decreased relative IFN-γ responses in the SD1 DNA-vaccinated mice (compared to the levels for the attenuated vaccine strains), the expression levels of the CXCL9, CXCL10, and CXCL11 chemokines were still substantially upregulated. These results, coupled with previous data, suggest that to generate a modest protective response, early postinfection IFN-γ expression (probably from memory T cells) is needed to activate macrophages and to induce early synthesis of the CXCL9, CXCL10, and CXCL11 chemokines. Interestingly, these IFN-γ-inducible chemokines possess biological activities associated with both innate and adaptive immune mechanisms. It is well known that CXC chemokines serve as adaptive immune response signals in the recruitment of lymphocytes to infected tissues (23, 24). Additionally, these peptides have innate defensin-like activities and can inactivate microbial pathogens, such as Escherichia coli, Neisseria gonorrhoeae, and Listeria monocytogenes (3, 19). Clearly, further studies are needed to determine whether the CXCL9, CXCL10, and CXCL11 chemokines possess antimycobacterial activity and whether these bactericidal properties may contribute to early control of M. tuberculosis infections.
The roles of the other up (or down)-regulated cytokines (including Gdf3, IL-17f, IL-21, and IL-27) in the generation of enhanced anti-TB protection are also uncertain. For example, IL-17 expression has been shown to be critical in the host pulmonary response to organisms such as Klebsiella pneumoniae and Bordetella pertussis as well as M. tuberculosis (1, 10, 14). Khader et al. proposed that vaccination against M. tuberculosis induces IL-17-producing CD4 T cells that evoke chemokine production, which ultimately leads to recruitment of IFN-γ-producing CD4 cells to the lung (14). Although the induction of chemokine expression is directly relevant to the anti-TB protective response, it is unclear whether other well-known activities of IL-17, including neutrophil recruitment and maintenance of granulopoietic responses, are important for limiting M. tuberculosis growth in vaccinated mice (16). Moreover, the anti-TB protective roles of IL-21 and IL-27 need to be clarified. Although the upregulation of IL-21 and IL-27 responses have been shown to stimulate T-cell proliferation and promote Th1 responses, these molecules can also regulate pathogen-induced immune responses by suppressing effector T-cell cytokine production (13, 17, 22). Whether IL-21 and IL-27 amplify Th1-type responses or limit potentially dangerous inflammatory responses in vaccinated mice after infection is unclear. It has recently been shown that the synergistic combination of IL-21 and transforming growth factor β can initiate an alternative pathway for inducing the proinflammatory Th17 cells (15). It would be of interest to assess whether Gdf3, a transforming growth factor β family member that was shown to be upregulated in this study, has similar Th17-promoting activities when combined with IL-21 (18).
In sum, by characterizing the pulmonary cytokine and chemokine responses in the lungs of vaccinated mice after a M. tuberculosis aerosol infection, we have identified several immune mediators which may contribute to the anti-TB protective responses induced by TB vaccines. Clearly, these data suggest novel experiments for better understanding the cellular mechanisms of vaccine-induced protection against M. tuberculosis and for further identification of the potential correlates of anti-TB protective immunity.
Supplementary Material
Footnotes
Published ahead of print on 26 November 2008.
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1.Aujla, S. J., P. J. Dubin, and J. K. Kolls. 2007. Interleukin-17 in pulmonary host defense. Exp. Lung Res. 33507-518. [DOI] [PubMed] [Google Scholar]
- 2.Colditz, G. A., T. F. Brewer, C. S. Berkey, M. E. Wilson, E. Burdick, H. V. Fineberg, and F. Mosteller. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271698-702. [PubMed] [Google Scholar]
- 3.Cole, A. M., T. Ganz, A. M. Liese, M. D. Burdick, L. Liu, and R. M. Strieter. 2001. Cutting edge: IFN-inducible ELR-CXC chemokines display defensin-like antimicrobial activity. J. Immunol. 167623-627. [DOI] [PubMed] [Google Scholar]
- 4.Delogu, G., A. Li, C. Repique, F. Collins, and S. L. Morris. 2002. DNA vaccine combinations expressing either tissue plasminogen activator signal sequence fusion proteins or ubiquitin-conjugated antigens induce sustained protective immunity in a mouse model of pulmonary tuberculosis. Infect. Immun. 70292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Derrick, S. C., A. L. Yang, and S. L. Morris. 2004. A polyvalent DNA vaccine expressing an ESAT6-Ag85B fusion protein protects mice against a primary infection with Mycobacterium tuberculosis and boosts BCG-induced protective immunity. Vaccine 23780-788. [DOI] [PubMed] [Google Scholar]
- 6.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 1993-129. [DOI] [PubMed] [Google Scholar]
- 7.Flynn, J. L. 2004. Immunology of tuberculosis and implications in vaccine development. Tuberculosis (Edinburgh) 8493-101. [DOI] [PubMed] [Google Scholar]
- 8.Fuller, C. L., J. L. Flynn, and T. A. Reinhart. 2003. In situ study of abundant expression of proinflammatory chemokines and cytokines in pulmonary granulomas that develop in cynomolgus macaques experimentally infected with Mycobacterium tuberculosis. Infect. Immun. 717023-7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goter-Robinson, C., S. C. Derrick, A. L. Yang, B. Y. Jeon, and S. L. Morris. 2006. Protection against an aerogenic Mycobacterium tuberculosis infection in BCG-immunized and DNA-vaccinated mice is associated with early type I cytokine responses. Vaccine 243522-3529. [DOI] [PubMed] [Google Scholar]
- 10.Higgins, S. C., A. G. Janecki, E. C. Lavelle, and K. H. Mills. 2006. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J. Immunol. 1777980-7989. [DOI] [PubMed] [Google Scholar]
- 11.Hinchey, J., S. Lee, B. Y. Jeon, R. J. Basaraba, M. M. Venkataswamy, B. Chen, J. Chan, M. Braunstein, I. M. Orme, S. C. Derrick, S. L. Morris, W. R. Jacobs, Jr., and S. A. Porcelli. 2007. Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. J. Clin. Investig. 1172279-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang, D., L. Qiu, R. Wang, X. Lai, G. Du, P. Seghal, Y. Shen, L. Shao, L. Halliday, J. Fortman, L. Shen, N. L. Letvin, and Z. W. Chen. 2007. Immune gene networks of mycobacterial vaccine-elicited cellular responses and immunity. J. Infect. Dis. 19555-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunter, C. A. 2005. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat. Rev. Immunol. 5521-531. [DOI] [PubMed] [Google Scholar]
- 14.Khader, S. A., G. K. Bell, J. E. Pearl, J. J. Fountain, J. Rangel-Moreno, G. E. Gilley, F. Shen, S. M. Eaton, S. I. Gaffen, S. L. Swain, R. M. Locksley, L. Haynes, T. D. Randall, and A. M. Cooper. 2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 8369-377. [DOI] [PubMed] [Google Scholar]
- 15.Korn, T., E. Bettelli, W. Gao, J. Awasthi, A. Jäger, T. B. Strom, M. Oukka, and V. K. Kuchroo. 2007. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 448484-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laan, M., Z. H. Cui, H. Hoshino, J. Lotvall, M. Sjostrand, D. C. Gruenert, B. E. Skough, and A. Linde. 1999. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J. Immunol. 1622347-2352. [PubMed] [Google Scholar]
- 17.Leonard, W. J., and R. Spolski. 2005. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat. Rev. Immunol. 5688-698. [DOI] [PubMed] [Google Scholar]
- 18.Levine, A. J., and A. H. Brivanlou. 2006. GDF3 at the crossroads of TGF-beta signaling. Cell Cycle 51069-1073. [DOI] [PubMed] [Google Scholar]
- 19.Linge, H. M., M. Collin, A. Giwercman, J. Milan, A. Bjartell, and A. Egesten. 2008. The antibacterial chemokine MIG/CXCL9 is constitutively expressed in epithelial cells of the male urogenital tract and is present in seminal plasma. J. Interferon Cytokine Res. 28191-196. [DOI] [PubMed] [Google Scholar]
- 20.Mollenkopf, H. J., K. Hahnke, and S. H. E. Kaufmann. 2006. Transcriptional responses in mouse lungs induced by vaccination with Mycobacterium bovis BCG and infection with Mycobacterium tuberculosis. Microbes Infect. 8136-144. [DOI] [PubMed] [Google Scholar]
- 21.Ottenhoff, T. H., D. Kumararatne, and J. L. Casanova. 1998. Novel human immunodeficiencies reveal the essential role of type-I cytokines in immunity to intracellular bacteria. Immunol. Today 19491-494. [DOI] [PubMed] [Google Scholar]
- 22.Pearl, J. E., S. A. Khader, A. Solache, L. Gilmartin, N. Ghilardi, F. deSauvage, and A. M. Cooper. 2004. IL-27 signaling compromises control of bacterial growth in Mycobacteria-infected mice. J. Immunol. 1737490-7496. [DOI] [PubMed] [Google Scholar]
- 23.Qiu, B., K. A. Frait, F. Reich, E. Komuniecki, and S. W. Chensue. 2001. Chemokine expression dynamics in mycobacterial (type-1) and schistosomal (type-2) antigen-elicited pulmonary granuloma formation. Am. J. Pathol. 1581503-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhoades, E. R., A. M. Cooper, and I. M. Orme. 1995. Chemokine response in mice infected with Mycobacterium tuberculosis. Infect. Immun. 633871-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodgers, A., K. M. Whitmore, and K. B. Walker. 2006. Potential correlates of BCG induced protection against tuberculosis detected in a mouse aerosol model using gene expression profiling. Tuberculosis 86255-262. [DOI] [PubMed] [Google Scholar]
- 26.Soares, A. P., T. J. Scriba, S. Joseph, R. Harbacheuski, R. A. Murray, S. J. Gelderbloem, A. Hawkridge, G. D. Hussey, H. Maecker, G. Kaplan, and W. A. Hanekom. 2008. Bacillus Calmette-Guérin vaccination of human newborns induces T cells with complex cytokine and phenotypic profiles. J. Immunol. 1803569-3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Pinxteren, L. A., J. P. Cassidy, B. H. Smedegaard, E. M. Agger, and P. Andersen. 2000. Control of latent Mycobacterium tuberculosis infection is dependent on CD8 T cells. Eur. J. Immunol. 303689-3698. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. 2008. Global tuberculosis control—surveillance, planning, financing. World Health Organization, Geneva, Switzerland.
- 29.Young, D. B., M. D. Perkins, K. Duncan, and C. E. Barry III. 2008. Confronting the scientific obstacles to global control of tuberculosis. J. Clin. Investig. 1181255-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.