Abstract
A number of studies have clearly demonstrated that flagellin is a potent adjuvant that promotes robust immune responses when it is given with a protein antigen. In view of the potential biological and practical benefits of a recombinant protein vaccine composed of a single fusion protein containing flagellin and antigen, we have evaluated the efficacy of a fusion protein composed of flagellin and two protective antigens of Yersinia pestis (F1 and V) in eliciting protection against respiratory challenge with Y. pestis. Flagellin-F1-V was produced and purified in high yield under good manufacturing practices conditions. The fusion protein retains full Toll-like receptor 5-stimulating activity in vitro. Using a prime-boost immunization protocol, we found that flagellin-F1-V elicits robust antigen-specific humoral immunity in mice and two species of nonhuman primates. Immune mice were fully protected against intranasal challenge with 150 mean tolerated doses of Y. pestis CO92. In immune mice, the bacteria were completely cleared within 3 days after challenge. Flagellin-F1-V exhibited full stability for at least 297 days at 4°C and at least 168 days at 25°C. At between 29 and 84 days at 37°C, the protein exhibited a loss of biological activity that appeared to be associated with a substantial change in protein diameter, possibly due to oligomerization. On the basis of our results, we believe that flagellin-F1-V is an outstanding candidate for evaluation in studies with humans.
Yersinia pestis, a gram-negative coccobacillus, causes an acute and often fatal disease that may appear in one of three major manifestations: bubonic, septicemic, or pneumonic disease. Transmission to humans most commonly occurs via a bite by infectious fleas and is associated with regional lymphadenopathy or bubo. In the United States, bubonic plague accounts for 80 to 90% of the cases of Y. pestis infection. Pneumonic plague is the most dangerous form of the disease and is generally fatal if an individual does not receive appropriate treatment within 18 h after the onset of respiratory symptoms. Pneumonic plague would be the most likely outcome in the case of a bioterrorism attack.
In view of the seriousness of infection with Y. pestis, a substantial effort has been focused on the development of protective vaccines. Killed bacteria have been used in plague vaccines for over 100 years, and a formalin-inactivated bacterial vaccine has been licensed in the United States. However, its efficacy has not been properly evaluated in well-controlled studies. Nonetheless, a retrospective study of armed services personnel who served in Vietnam provided indirect evidence for efficacy in the prevention of bubonic plague (8). However, no evidence in support of a protective effect against the pneumonic form of the disease was obtained. Although a live attenuated strain of Y. pestis (strain EV76) has been evaluated (31), more recent studies have focused on the development of acellular vaccines, in particular, vaccines containing the fraction 1 capsular antigen (the F1 antigen) and LcrV (also termed the V antigen). Baker et al. (3) and later others (2, 34) demonstrated that immunization with the F1 antigen provided protection against a lethal challenge with Y. pestis in mice. A number of laboratories have demonstrated that immunization with V antigen also provides protection against challenge in mice (1, 21, 29). Subsequent studies established that a combination of F1 and V or a fusion protein containing F1 and V also provided complete protection in mice (14, 20). In each case in which F1 and V, or the F1-V fusion protein, was tested, the induction of a protective adaptive immune response was dependent on the concomitant use of an adjuvant, either alum, mutant forms of cholera toxin, or the heat-labile toxin from Escherichia coli.
Flagellin, the major structural protein of the flagellar filament of gram-negative bacteria, is an extraordinarily potent inducer of innate immunity in vitro and in vivo (for a review, see reference 16). The actions of flagellin on innate immunity result from a direct interaction with Toll-like receptor 5 (TLR5) (11, 13, 28) on a variety of cell types, including macrophages, endothelial cells, and epithelial cells. Studies by Arnon and colleagues (5-7, 19, 23, 26) provided the first evidence that flagellin is also a highly effective adjuvant. More recently, we and others have provided additional data in support of the conclusion that flagellin is a highly effective mucosal and systemic adjuvant (10, 17, 18, 22, 25, 27, 30). Our studies have focused on the adjuvant activity of flagellin in the context of a vaccine against Y. pestis (4, 17). When mice were immunized on two occasions with flagellin and the Y. pestis F1 antigen, the mice exhibited a robust humoral response against the F1 antigen and were fully protected against intranasal (i.n.) challenge with Y. pestis CO92 (17). F1 by itself elicited only a low level of antibody production and provided no protection against challenge. In addition, immunization of cynomolgus monkeys with flagellin and a fusion protein containing F1 and an additional protective antigen (antigen V) also elicited very high titers of anti-F1 and anti-V immunoglobulin G (IgG) antibodies. As with F1 alone, F1-V elicited markedly lower levels of antibody production.
With the idea in mind that a single fusion protein containing flagellin and the amino acid sequences for the F1 and the V antigens would efficiently target antigens and adjuvant to the same antigen-presenting cells and would be a more cost-effective and practical vaccine for use in humans, we generated an expression plasmid encoding a fusion protein in which the majority of the hypervariable region of flagellin was replaced by F1 and V. The protein was induced and purified under good manufacturing practices (GMP) conditions and was evaluated for its ability to promote protective immune responses in mice and two species of nonhuman primates.
MATERIALS AND METHODS
Generation of a flagellin-F1-V expression plasmid.
The genes encoding the Y. pestis F1 and V antigens were kindly provided by J. B. Bliska (State University of New York, Stony Brook). A DNA encoding an F1-V fusion was generated by a two-step PCR. The F1 and V sequences were separated by a linker encoding a 6-amino-acid sequence (GSIEGR). The F1-V DNA was digested with NdeI and SalI and cloned into NdeI- and XhoI-digested pET29a. A Salmonella enterica serovar Enteritidis FliC (flagellin) product was generated in which nucleotides 586 to 1134 were deleted. This region covers a significant portion of the hypervariable domain of flagellin. The resultant flagellin fragment containing an internal BglII site and KpnI and XhoI sites at the 5′ and 3′ termini, respectively, was then cloned into KpnI- and XhoI-digested pET29a. The F1-V fragment was amplified by PCR with primers containing BglII sites to allow its insertion into the BglII site of the flagellin fragment. The resultant flagellin-F1-V protein was amplified by PCR, such that NcoI and BamHI were present at the 5′ and 3′ termini, respectively, which allowed cloning into pET16b digested with NcoI and BamHI. The resultant flagellin-F1-V protein does not have any tags (e.g., a six-histidine tag). Escherichia coli BL21(DE) (Invitrogen) was transformed with the flagellin-F1-V in pET16b. A single clone was obtained by multiple rounds of plating and had the following properties: ampicillin resistance; sensitivity to chloramphenicol, gentamicin, kanamycin, and tetracycline; and growth on glucose medium but not galactose medium. Master and production cell banks were prepared according to current GMP at the Walter Reed Bioproduction Facility (Silver Spring, MD).
Induction of flagellin-F1-V.
According to current GMP, a production cell bank vial of BL21(DE) containing pET16b-flagellin-F1-V was thawed, inoculated into 3,000 ml of Select APS Super broth medium (BD Difco)-0.4% glycerol (Spectrum), and grown until the optical density (OD) at 600 nm reached a value greater than 1 OD unit. The culture was then transferred to a fermentor containing 300 liters of medium, grown to an OD of 1 to 2, and then induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG; Spectrum) for 3 h. The cells were then harvested. No antibiotics were used at any time during the culturing of the bacteria.
Purification of flagellin-F1-V.
All purification steps were carried out under currently accepted GMP conditions. Approximately 260 g of cell paste was resuspended at 4 to 8°C in approximately 2.1 liters of 50 mM Tris HCl, pH 8.0-5 mM EDTA. The bacteria were lysed with a microfluidizer (average running pressure, 17,500 lb/in2) cooled to 4 to 8°C. The resultant lysate was centrifuged at approximately 3,000 × g for 15 min at 4°C to remove large cell debris. The supernatant was removed and centrifuged at 27,000 × g for 30 min at 2 to 8°C to pellet the inclusion bodies. The pellet was resuspended in the same volume used to lyse the bacteria, and the suspension was centrifuged a second time at 27,000 × g for 30 min at 2 to 8°C. The supernatant was discarded, and then the pellet was resuspended in 50 mM Tris HCl, pH 8.0-5 mM EDTA-1% Triton X-100 (TX-100) and then centrifuged at 27,000 × g for 30 min at 2 to 8°C. After a second round of washing with the buffer containing TX-100, the pellet was resuspended in 50 mM Tris HCl, pH 8.0-5 mM EDTA-1.75 M guanidine HCl. The resuspended material was then incubated at 20 ± 5°C for 10 to 20 min to solubilize the flagellin-F1-V (see Fig. 1 and Results). The supernatant containing flagellin-F1-V was then diluted 10-fold with 50 mM Tris HCl, pH 7.5-150 mM NaCl-8 M urea. The resultant solution was concentrated to approximately 200 ml with a 50-kDa-pore-size Pellicon 2 Mini BioMax-50 ultrafiltration cartridge. The concentrated material was then applied to a Sephacryl S400 HR column (70 by 9 cm; GE Healthcare) equilibrated in 50 mM Tris HCl, pH 7.5-150 mM NaCl-8 M urea, and the column was run at 21 to 32 ml/min (20 to 30 cm/h). Peak fractions containing flagellin-F1-V were collected (see Results), concentrated, and reapplied to the Sephacryl S400 column. The peak of flagellin-F1-V from the second column was relatively symmetrical. Peak fractions were collected and then concentrated and dialyzed into phosphate-buffered saline (PBS; pH 6.2). Endotoxin and nucleic acids were removed by using an Acrodisc Mustang Q capsule (Pall Corporation).
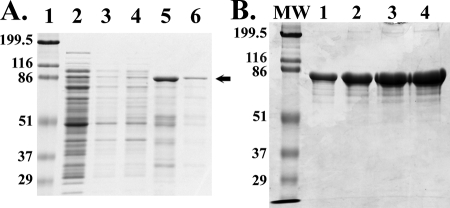
FIG. 1.
Extraction and purification of flagellin-F1-V. (A) Induced bacteria were lysed in a microfluidizer with a buffer containing 50 mM Tris-HCl, pH 8, and 5 mM EDTA, and the inclusion bodies were enriched by centrifugation and then sequentially washed with TX-100, 1.75 M guanidine HCl, and then 5 M guanidine. Samples were then prepared for SDS-PAGE. Lane 1, molecular weight markers; lane 2, inclusion bodies; lane 3, starting buffer wash; lane 4, 1% TX-100 wash; lane 5, 1.75 M guanidine HCl; lane 6, 5 M guanidine HCl. The arrow marks the position of flagellin-F1-V (approximately 90 kDa). (B) SDS-PAGE of the purified flagellin-F1-V. Lane MW, molecular weight markers; lane 1, 1 μg flagellin-F1-V; lane 2, 5 μg flagellin-F1-V, lane 3, 10 μg flagellin-F1-V; lane 4, 20 μg flagellin-F1-V.
Amino acid sequence analysis.
Protein sequence analyses were performed on an Applied Biosystems model 492 Procise automated protein sequencer equipped with an online high-performance liquid chromatography (HPLC) system. The phenylthiohydantoin-amino acid released after each cycle of Edman degradation was quantified by comparison of the integrated peak areas to the peaks obtained after injections of authentic phenylthiohydantoin-amino acid standards.
Dynamic light scattering.
The size distributions for flagellin-F1-V were determined by dynamic light scattering with a Malvern ZEN 1600 instrument, as described previously (12). Data from at least five replicate runs with each sample were collected at 25°C, with each run yielding an autocorrelation function that was analyzed by use of the CONTIN algorithm to obtain an intensity-weighted size distribution profile. The resultant data were averaged to obtain the size distributions.
Determination of protein carbamylation.
To determine if purified flagellin-F1-V contains carbamylated lysine residues (N6-carbamoyl-l-lysine [homocitrulline]), quantitative amino acid composition analysis was performed by use of the Pico-Tag method developed by Waters Associates (32). The protein was hydrolyzed in 6 N HCl in vacuo at 110°C for 24 h. Amino acids were derivatized with phenylisothiocyanate to form the phenylthiocarbamyl amino acids (PTC-AAs). The PTC-AAs were then separated by HPLC with a reverse-phase HPLC column. N6-carbamoyl-l-lysine was purchased from Sigma-Aldrich and was used to prepare standards for use for the detection and quantification of carbamyl-lysine. The homocitrulline was derivatized with phenylisothiocyanate to form PTC-homocitrulline, which was then used as a standard. The lower limit of detection of PTC-homocitrulline was estimated to be 5 pmol.
Determination of residual guanidine and IPTG.
Intact protein was analyzed with a Waters Q-TOF ApCI US quadrupole/time-of-flight mass spectrometer interfaced to a CapLC liquid chromatography apparatus and equipped with a nanoelectrospray source. A 10-μl aliquot of sample was diluted with 90 μl of solvent A (water and acetonitrile containing 0.1% formic acid; 97:3). One microliter of this mixture was injected and retained on a trapping column (3-μm-diameter PLRP with a 300-Å pore diameter). After the salts were washed out for 4 min, the trapped protein was back flushed onto the reverse-phase analytical nanocolumn (a column of 0.1 by 50 mm containing 3-μm-diameter PLRP with 100-Å pores [Varian, Inc.]) eluted at 450 nl/min. The 90-min gradient profile started with 100% solvent A and ended with 90% solvent B (water and acetonitrile containing 0.1% formic acid; 3:97). Data were acquired from 600 to 1,700 m/z and were transformed with MassLynx software (version 4.0). The acquisition parameters were optimized for proteins.
Small molecules were analyzed on a Waters Micromass Quattro II triple-quadrupole mass spectrometer interfaced to an Agilent model 1100 high-performance liquid chromatograph through an electrospray source. To improve the sensitivity of selectivity, the data were recorded in the selected reaction monitoring mode with MassLynx software (version 3.5). Four selected reaction monitoring m/z pairs were monitored: for IPTG, 238.9 m/z > 220.9 m/z (collision energy [CE]= 8 V); for urea, 61.2 m/z > 44.2 m/z (CE = 15 V) and 120.9 m/z > 61.0 m/z (CE = 8 V); and for guanidine, 60.2 m/z > 43.4 m/z (CE = 15 V). Component separations were performed on a Vydac C8 reverse-phase column (1.0 by 250 mm) packed with 5-μm particles. Gradient analysis was required to remove proteins and to prevent interference between analyses. The 30-min gradient elution started at 0% solvent B, which was held for 1 min and which was then ramped up in a linear gradient to 90% solvent B by 15 min, held at 90% solvent B to 18 min, dropped to 0% solvent B in 0.5 min, and then held at 0% solvent B to the end of the 30-min analysis. The analysis was conducted at 70 μl/min. Solvent A was 100% water, and solvent B was acetonitrile containing 0.1% formic acid. The response curves for IPTG and guanidine were prepared by diluting 1 mM stock solutions of each into PBS. The responses were obtained for 10, 50, 100, and 20 μM concentrations of each component. The lower limit of quantitation in PBS was found to be 10 μM for IPTG and guanidine.
Determination of residual endotoxin.
Endotoxin levels were measured with a pyrochrome Limulus amebocyte lysate chromogenic kit (Associates of Cape Cod, Inc.). Since this is a kinetic assay, it provides a very accurate assessment of endotoxin levels. Using this assay, we have found that 1 endotoxin unit (EU) equals approximately 0.1 ng of endotoxin.
In vitro bioassay of TLR5-specific signaling.
To evaluate the ability of flagellin-F1-V to induce TLR5-specific signaling, we used two cell lines that differ only in the expression of TLR5. RAW 264.7 cells, a murine macrophage cell line, do not express TLR5 and, thus, do not produce tumor necrosis factor alpha (TNF-α) in response to flagellin (33). However, RAW 264.7 cells stably transfected with TLR5 are fully responsive to flagellin (33). TLR5-specific signaling is defined on the basis of the amount of TNF-α produced by TLR5-positive RAW 264.7 cells but not by untransfected RAW 264.7 cells. The two cell lines were plated at 4 × 105 to 5 × 105 cells per well in 24-well tissue culture dishes in 1 ml of RPMI 1640 containing 10% fetal bovine serum. After overnight incubation, increasing concentrations of flagellin-F1-V or flagellin (as a positive control) were added to each culture. After 4 h, the culture medium from each well was harvested and analyzed for its TNF-α content by using a commercial enzyme-linked immunosorbent assay (ELISA) kit (BD OptEIA kit; BD Pharmingen).
Immunization of BALB/c mice with flagellin-F1-V.
All studies with mice complied with federal and institutional guidelines set by the Wake Forest University Health Sciences Animal Care and Use Committee. Groups of 7 to 10 female BALB/c mice ages 5 to 7 weeks were immunized with flagellin-F1-V i.n. in a volume ≤15 μl, as described previously (4, 15, 17), or with 20 μl in a hind muscle. Twenty-eight days later, the mice were boosted with the protein by using the same amounts and volumes. Control mice received only PBS. After an additional 10 to 14 days, the mice were bled by nicking the tail vein or by using a Goldenrod animal lancet (MEDIpoint, Inc.) to access the vascular bundle located at the rear of the jaw bone. Plasma was prepared and analyzed for F1- and V-specific IgG by a conventional ELISA. The titer of antibody was defined as the lowest dilution resulting in an absorbance of 0.1 unit at an OD at 450 nm above the value achieved with naïve plasma.
Respiratory challenge with Yersinia pestis CO92.
All challenge studies were done according to CDC-approved standard operating procedures for the biosafety level 3 and animal biosafety level 3 facility at the Infectious Disease Unit at Virginia Tech (CDC approval no. C20031120-0016). Immune and control mice were challenge with approximately 150 50% maximally tolerated doses (MTD50s; equivalent to lethal doses) of Y. pestis CO92, as described previously (17). The mice were monitored twice daily for signs of morbidity and were euthanized if they exhibited obvious signs of illness, such as lethargy, difficulty in breathing, or a 30% weight loss. CFU/ml values were determined by plating serial dilutions of tissue homogenates or blood onto tryptose blood agar plates.
Immunization of African green monkeys with flagellin-F1-V.
Immunization studies with adult male African green monkeys (Chlorocebus aethiops SK) (weight, 5 to 6 kg) were conducted at the Behavioral Science Foundation, St. Kitts, by using a protocol approved by the foundation-associated Animal Care and Use Committee. Plasma from the monkeys was prepared several days before initiation of the immunization protocol. The monkeys were immunized with 200 μg flagellin-F1-V in a volume of approximately 160 μl in the gluteus maximus muscle on two occasions separated by 28 days. Fourteen days later, blood was collected and plasma was prepared for analysis of anti-F1 and -V IgG antibody titers.
Young African green monkeys (age, 4 to 6 months) were immunized with flagellin-F1-V at doses ranging from 25 to 100 μg on two occasions and were evaluated for anti-F1 and -V IgG antibody titers, as outlined in the preceding paragraph.
Immunization of cynomolgus monkeys (Macaca fascicularis) with flagellin-F1-V.
Immunization studies with young cynomolgus monkeys (weight, approximately 1 kg) were conducted at the Wake Forest University Health Sciences Primate Center by using a protocol approved by the institutional Animal Care and Use Committee. The animals were immunized with 125 μg flagellin-F1-V intramuscularly (i.m.), as described above, and analyzed for anti-F1 and -V IgG antibody titers 10 days after the boost.
RESULTS
Purification and characterization of flagellin-F1-V.
The production and purification of flagellin-F1-V were done according to currently accepted GMP. An initial analysis revealed that flagellin-F1-V accumulates in inclusion bodies and can be solubilized in the presence of 1.75 M guanidine HCl (Fig. 1A). By washing the inclusion bodies with 50 mM Tris HCl, pH 8.0-5 mM EDTA and then washing them with the same buffer containing TX-100 (twice) and the starting buffer-1.75 M guanidine HCl, it is possible to obtain a highly enriched preparation of flagellin-F1-V. The flagellin-F1-V solubilized from the inclusion bodies was subjected to two rounds of size-exclusion chromatography on a Sephacryl S-400 HR column with a buffer containing 50 mM Tris HCl, pH 7.5, 150 mM NaCl, and 8 M urea. Fractions were collected beginning after the exclusion volume. The final product was a clear, colorless solution with a protein concentration of 1.25 mg/ml and an endotoxin content of 24 EU/ml (19.2 EU/mg protein or 1.92 ng endotoxin/mg protein). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis of the final product revealed a major band at approximately 90 kDa and two minor bands at 70 to 80 kDa (Fig. 1B). Western blot analysis with rabbit antiserum against flagellin revealed that the major band as well as the minor bands contained flagellin (data not shown). Furthermore, a single N-terminal amino acid sequence that was identical to that of S. enterica serovar Enteritidis FliC was obtained (data not shown). The final product contained proteins of 87,836, 87,891, and 87,942 Da in a ratio of 68%:25%:7%. Two minor peptides of 5,574 and 5,331 Da were present at levels of 1% or less. Consistent with the findings of those analyses, two-dimensional gel electrophoresis revealed the presence of three proteins of similar molecular masses and pI values in the range of 5.5 to 6 (data not shown). A hydrolyzed sample of flagellin-F1-V containing 1.54 nmol of PTC-lysine did not contain detectable PTC-homocitrulline, a finding that is consistent with the absence of carbamylation. Additional analyses demonstrated that the final product contained less than 10 μM guanidine and IPTG.
In vitro TLR5-specific signaling activity of flagellin-F1-V.
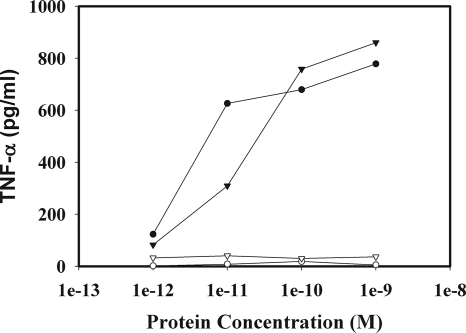
To evaluate the TLR5-specific signaling activity of the purified flagellin-F1-V, we incubated TLR5-negative and TLR5-positive RAW 264.7 cells with increasing concentrations of flagellin-F1-V or flagellin (as a positive control) and determined the amount of TNF-α produced over 4 h. As shown in Fig. 2, flagellin-F1-V, like flagellin, induced TNF-α production in a TLR5-specific manner. The potencies of the two proteins were quite similar, indicating that the addition of the F1 and V sequences to the flagellin did not have a significant effect on the TLR5 binding domain of flagellin.
FIG. 2.
Concentration-dependent stimulation of TNF-α production by flagellin-F1-V in cultures of TLR5-positive RAW 264.7 cells. TLR5-positive RAW 264.7 cells (closed circles and closed triangles) and TLR5-negative RAW 264.7 cells (open circles and open triangles) were stimulated with either flagellin (open and closed circles) or flagellin-F1-V (open and closed triangles) for 4 h, and the level of TNF-α in the culture supernatants was determined by ELISA.
Induction of an antigen-specific humoral immune response using flagellin-F1-V.
To evaluate the ability of flagellin-F1-V to induce a humoral immune response against the F1 and V antigens, groups of seven female BALB/c mice were immunized i.m. with 0.3, 1, 3, or 10 μg of flagellin-F1-V. Twenty-eight days later, the mice were boosted by use of the same route and doses and were then bled 14 days later. Plasma was prepared and assayed for anti-F1 and -V IgG antibodies by ELISA. As shown in Fig. 3, flagellin-F1-V induced a very robust humoral immune response against the F1 and V antigens, with titers approaching 3 × 106. Similar results were obtained when the flagellin-F1-V was administered i.n. (data not shown).
FIG. 3.
Flagellin-F1-V immunization of BALB/c mice results in a robust antigen-specific IgG response against F1 and V. Groups of seven BALB/c mice were immunized twice i.m. with increasing doses of flagellin-F1-V. Ten days later, the animals were bled and the plasma was evaluated for the levels of anti-F1 and V IgG antibodies by ELISA. (A) Anti-F1 antibody titers; (B) anti-V antibody titers.
Y. pestis challenge of mice.
To assess the protective effect of the flagellin-F1-V vaccine against respiratory challenge with Y. pestis CO92, groups of BALB/c mice were given flagellin-F1-V or PBS i.m., as described above, and were then challenged i.n. with approximately 150 MTD50s of Y. pestis CO92. The PBS-treated mice succumbed within 3 days after challenge, whereas all of the mice immunized with flagellin-F1-V survived with few, if any, signs of morbidity (Fig. 4). Complete protection was also obtained with mice immunized i.n. (data not shown).
FIG. 4.
Immunization of BALB/c mice with flagellin-F1-V protects against a lethal respiratory challenge with Y. pestis. Groups of 10 BALB/c mice were immunized twice i.m. with 10 μg flagellin-F1-V (open circles) or PBS only (closed circles). Two weeks later the mice were challenged i.n. with approximately 150 MTD50s of Y. pestis CO92 (in 15 μl).
In a subsequent challenge study, immune and control mice were challenged with approximately 150 MTD50s of Y. pestis CO92 and were then killed after 3 days to assess the levels of Y. pestis in their blood, liver, lungs, and spleen. As shown in Table 1, control mice had very large numbers of CFU in all of the sites. However, there was no detectable CFU at any site in mice immunized with flagellin-F1-V. Taken together, the challenge studies clearly demonstrate that flagellin-F1-V is a highly protective vaccine and promotes sterile immunity against Y. pestis given i.n.
TABLE 1.
Bacterial burden in BALB/c mice challenged with Y. pestisa
| Groupb |
Y. pestis count (CFU/ml) at 3 days
|
|||
|---|---|---|---|---|
| Blood | Liver | Lungs | Spleen | |
| Control | 3.4 × 105 | |||
| Control | 7 × 106 | 3.2 × 107 | 1.3 × 106 | 8.2 × 107 |
| Control | 2.2 × 103 | 2.1 × 104 | 1.1 × 106 | 2.1 × 104 |
| Control | 6 × 102 | 6 × 105 | 2.8 × 105 | 8.4 × 105 |
| Control | 9.6 × 107 | 2.1 × 107 | 9.8 × 108 | 2.1 × 109 |
| Flagellin-F1-V | 0 | 0 | 0 | 0 |
| Flagellin-F1-V | 0 | 0 | 0 | 0 |
| Flagellin-F1-V | 0 | 0 | 0 | 0 |
| Flagellin-F1-V | 0 | 0 | 0 | 0 |
| Flagellin-F1-V | 0 | 0 | 0 | 0 |
| Flagellin-F1-V | 0 | 0 | 0 | 0 |
Groups of five mice each were given PBS or 10 μg flagellin-F1-V on two occasions and were then challenged with approximately 150 MTD50s of Y. pestis i.n. After 3 days, the mice were killed and the numbers of CFU in the blood, liver, lungs, and spleen were determined.
Each line represents the results from a single mouse (PBS or flagellin-F1-V treated).
Stability of flagellin-F1-V.
To assess the stability of flagellin-F1-V, samples of the protein were incubated at 4°C, 25°C, or 37°C; and at various times aliquots were assayed for in vitro TLR5-stimulating activity, in vivo adjuvant activity, and protective effect against i.n. challenge with Y. pestis. As shown in Table 2, the TLR5-stimulating activity of flagellin-F1-V (as measured by the RAW 264.7 cell assay) was stable for at least 297 days when it was stored at 4°C and at least 168 days when it was stored at 25°C. In contrast, the TLR5-stimulating activity decreased significantly when flagellin-F1-V was stored at 37°C for greater than 29 days and was most evident after 168 days. Although the activity was reduced by approximately 80% at 168 days, the half-maximal response was still obtained at 1.5 × 10−10 M. The stability observed at 4°C and 25°C was also observed when the samples were assayed for the ability to promote an antigen-specific IgG response against the F1 and V antigens in BALB/c mice (Table 2). As with the TLR5-stimulating activity of material stored at 37°C, flagellin-F1-V lost significant activity at the higher temperature. By day 168, the efficacy of the vaccine was approximately 15% of that for the day 0 sample. It is of interest that the drop in the anti-V IgG titer was markedly greater than that of the anti-F1 titer. This finding may indicate that the V antigen in the fusion protein is more sensitive to temperature than the F1 antigen.
TABLE 2.
Temperature stability of flagellin-F1-Va
| Assay temp (°C) | Day | TNF-α inductionb | Adjuvant activityc |
|---|---|---|---|
| 4 | 158 | 1.8 | 205/86 |
| 213 | 3.2 | 112/95 | |
| 297 | 0.9 | 193/79 | |
| 25 | 29 | 1.8 | 149/110 |
| 84 | 4 | 115/93 | |
| 168 | 2 | 167/77 | |
| 37 | 29 | 2.5 | 107/93 |
| 84 | 6.2d | 71/86 | |
| 168 | 11d | 70/15d |
Aliquots of flagellin-F1-V were stored at 4°C, 25°C, or 37° for the indicated periods and were then analyzed for residual biologic activities.
Samples at each time point were titrated in the in vitro RAW 264.7 cell assay for determination of the level of TNF-α production. The concentrations (10−11 M) required for 50% of the maximal response are shown.
Samples were used at each time point to immunize BALB/c mice, as described in Materials and Methods. The levels of anti-F1 and -V in plasma were determined and compared to those in a day 0 control. The number before the slash is the F1 titer as a percentage of that for the day 0 control, and the number after the slash is the V titer as a percentage of that for the day 0 control.
P < 0.001 by Mann-Whitney rank-sum test.
We also examined the effect of temperature on the ability of flagellin-F1-V to promote a protective response against respiratory challenge with Y. pestis. When BALB/c mice were immunized twice with 10 μg flagellin-F1-V stored at 4°C, 25°C, or 37°C for 56 or 112 days, all of the mice were fully protected (data not shown). This finding is consistent with the results of the dose-response experiment presented in Fig. 3, in which we observed that 1 μg of flagellin-F1-V was as effective as 10 μg in eliciting a maximal humoral response.
As a first step in developing an understanding of the physical changes in flagellin-F1-V that were responsible for the loss of bioactivity at 37°C, we used dynamic light scattering to assess any potential changes in the overall structure of the fusion protein that might be associated with a decrease in adjuvant activity or immunogenicity. As shown in Fig. 5, flagellin-F1-V appeared to assume a coil-like monomeric structure of approximately 25 nm that was maintained at 4°C and 25°C for at least 168 days. However, at 37°C, the protein diameter increased to approximately 40 nm by day 29 and remained at this diameter over the next 139 days. This change may reflect oligomerization of the protein. Since the full biologic function at 37°C was retained for 29 days, it is likely that this change may be a precursor to other changes (not detected by dynamic light scattering) that ultimately reduce bioactivity.
FIG. 5.
Dynamic light-scattering analysis of flagellin-F1-V incubated at 4°C, 25°C, and 37°C. Samples of flagellin-F1-V incubated at 4°C (—), 25°C (- - -), or 37°C (-·- ·) were subjected to dynamic light-scattering analysis at days 0, 29, 84, and 168.
Induction of an antigen-specific humoral immune response in African green monkeys immunized with flagellin-F1-V.
In a prior study (17), we demonstrated that flagellin is a highly effective adjuvant in cynomolgus monkeys, promoting a robust humoral immune response against the F1 and V antigens of Y. pestis. In the current study, we examined the effectiveness of the flagellin-F1-V vaccine in adult male African green monkeys (average weight, approximately 6 kg). Prior to immunization, these animals had relatively low titers against the F1 and V antigens (Table 3). However, two immunizations with flagellin-F1-V generated a dramatic increase in the titers of anti-F1 and -V IgG antibodies in these animals. The mean anti-F1 IgG antibody titers increased from 3.7 × 103 ± 3.6 × 103 to 4.39 × 105 ± 1.68 × 105, and the anti-V IgG titers from 8.51 × 103 ± 14.6 × 103 to 2.73 × 106 ± 2.14 × 106.
TABLE 3.
Flagellin-F1-V induces an antigen-specific adaptive immune response in nonhuman primatesa
| Species | Animal no. | Preimmunization IgG titer
|
Postimmunization IgG titer
|
||
|---|---|---|---|---|---|
| Anti-F1 | Anti-V | Anti-F1 | Anti-V | ||
| Adult African green monkeysb | 1 | <1,000 | <1,000 | 4.9 × 105 | 6.5 × 106 |
| 2 | 9.2 × 103 | 7.2 × 103 | 4.2 × 105 | 4.8 × 106 | |
| 3 | <1,000 | <1,000 | 1.5 × 105 | 8.4 × 105 | |
| 4 | 5.2 × 103 | 6.0 × 103 | 6.0 × 105 | 9.2 × 105 | |
| 5 | <1,000 | 4.1 × 104 | 5.7 × 105 | 2.0 × 106 | |
| 6 | 7.6 × 103 | 2.3 × 103 | 5.7 × 105 | 2.8 × 106 | |
| 7 | <1,000 | <1,000 | 2.8 × 105 | 1.4 × 106 | |
| Young African green monkeysc | 1 | 3.3 × 103 | <1,000 | 7.0 × 104 | 1.5 × 105 |
| 2 | <1,000 | <1,000 | 3.6 × 105 | 8.6 × 105 | |
| 3 | <1,000 | 4.6 × 104 | 9.1 × 104 | 8.7 × 105 | |
| 4 | <1,000 | <1,000 | 3.1 × 105 | 8.5 × 105 | |
| 5 | 4.9 × 103 | <1,000 | 8.6 × 104 | 5.6 × 105 | |
| 6 | <1,000 | <1,000 | 1.0 × 105 | 6.6 × 105 | |
| 7 | <1,000 | <1,000 | 2.1 × 105 | 6.8 × 105 | |
| Cynomolgus monkeysd | 1 | <1,000 | <1,000 | 1.6 × 106 | 1.0 × 107 |
| 2 | <1,000 | 6.2 × 103 | 1.7 × 106 | 9.4 × 106 | |
| 3 | <1,000 | <1,000 | 9.1 × 105 | 8.2 × 106 | |
| 4 | 9.5 × 103 | <1,000 | 9.4 × 104 | 6.0 × 105 | |
| 5 | <1,000 | <1,000 | 7.5 × 105 | 6.1 × 106 | |
All of the monkeys were immunized i.m. twice with flagellin-F1-V and were then evaluated 2 weeks after the boost for the titers of anti-F1 and -V IgG antibodies in plasma.
Adult monkeys immunized with 200 μg flagellin-F1-V.
Monkeys ages 4 to 6 months immunized with 25 μg flagellin-F1-V.
Immunized with 125 μg flagellin-F1-V.
In an initial experiment, we immunized 4- to 6-month-old African green monkeys (weight, approximately 1 kg) with the dose of flagellin-F1-V given to the adults (200 μg) and found that they, like the adults, also exhibited a robust antigen-specific response to F1 and V (data not shown). In a subsequent experiment, we evaluated lower doses in the range of 25 to 100 μg and found that the monkeys responded as well to 25 μg as they did to 100 μg (data for 25 μg are shown in Table 3).
Finally, we examined the effect of flagellin-F1-V in cynomolgus monkeys (age, approximately 1 year). As shown in Table 3, all of the cynomolgus monkeys exhibited robust F1- and V-specific IgG responses to flagellin-F1-V.
DISCUSSION
Our results clearly demonstrate that flagellin-F1-V promotes a robust antigen-specific adaptive immune response in mice that affords complete protection against a respiratory challenge with Y. pestis (Fig. 4). Immune mice possessed the ability to completely clear the bacteria within 3 days after challenge and exhibited no significant signs of morbidity (Table 1). It is important to note that i.n. and i.m. immunization with flagellin-F1-V provided equally effective protection against respiratory challenge with Y. pestis. Although we have previously demonstrated that flagellin promotes a robust secretory IgA (sIgA) response against F1 when flagellin and F1 were given i.n. (4), it is likely that sIgA is not required for the protective response to respiratory challenge since i.m. administration would not result in the production of anti-F1 and -V sIgA antibodies. The lack of a requirement for sIgA is also evidenced by our observation that passive immunization of mice with plasma from cynomolgus monkeys immunized with flagellin-F1 antigen provides complete protection against respiratory challenge with Y. pestis (A. N. Honko and S. B. Mizel, unpublished observations).
In addition to mice, flagellin-F1-V also elicited robust responses in young and adult African green monkeys and cynomolgus monkeys (Table 3). In preliminary studies, we found that young African green monkeys but not adult African green monkeys exhibited a dose-dependent acute fever in response to immunization with flagellin-F1-V. Doses less than 100 μg elicited an approximately 3°C increase in body temperature, whereas higher doses induced a more substantial increase in body temperature. It should be noted that a 3°C increase in body temperature following immunization is quite common in young children. To avoid large increases in body temperature, we used lower doses of flagellin-F1-V in the young African green monkeys than in the adult animals. Thus, part of the observed difference in antibody response may simply be due to the differences in dosage and not actual vaccine efficacy. With regard to the protective potential of flagellin-F1-V in nonhuman primates, we believe that the titers observed are of a sufficient magnitude to provide complete protection against challenge in these animals. This conclusion is based on the fact that the protective plasma from cynomolgus monkeys that was used for passive protection in mice had anti-F1 IgG antibody titers in the same range as the those for the samples listed in Table 3. We plan on directly testing the protective effect of flagellin-F1-V in an upcoming challenge study with both species of nonhuman primates.
In a prior study (17), we demonstrated that flagellin-F1 or F1-V is also effective in eliciting a protective response. However, 1 μg of flagellin and 10 μg of antigen were required to elicit a maximal response in mice. In contrast, a dose of flagellin-F1-V as low as 0.3 μg was sufficient to generate an adaptive response that was quite close to maximal (Fig. 3). Recent results from our laboratory (J. T. Bates and S. B. Mizel, submitted for publication) demonstrate that TLR5-positive dendritic cells are essential for the adjuvant effect of flagellin. On the basis of this finding and the observed potency of the flagellin fusion proteins, we have concluded that fusion proteins are dramatically more potent than flagellin plus an antigen (at least 10-fold) because the high-affinity association of flagellin with TLR5 (24, 28) enhances not only cellular activation but also the uptake of the associated antigen by TLR5-positive dendritic cells. The enhanced efficacy of fusion proteins is clearly of practical importance, since the production of a fusion protein is far more cost-effective than the production of separate proteins and less vaccine is required to elicit a protective response.
A number of other features of flagellin-F1-V make it a very promising vaccine for use in humans. The protein is effective when it is given i.n. or i.m. and does not appear to elicit any significant adverse reactions, for example, inflammation at the site of injection or a type I hypersensitivity response. In addition, prior immunity to flagellin itself does not have a serious effect on its adjuvant activity (5, 17). With regard to type I hypersensitivity, it is important to note that flagellin does not promote antigen-specific IgE responses in mice or nonhuman primates (17). The fusion protein can be produced in large amounts in bacteria and can be purified by a very simple process involving inclusion body extraction and size-exclusion chromatography (Fig. 1). Due to the need for denaturing agents such as urea or guanidine to solubilize a protein present in inclusion bodies, the yield of protein in its native configuration is all too often quite low. Our results clearly demonstrate that the potency of flagellin-F1-V extracted from inclusion bodies is extraordinarily high (i.e., it has activity in the picomolar range; Fig. 2). Indeed, its presence in inclusion bodies provides for a very effective purification step (Fig. 1). Flagellin-F1-V also exhibits a relatively high degree of stability at 4°C or 25°C, as evidenced by retention of in vitro TLR5 signaling and in vivo adjuvant activity (Table 2). However, activity is lost at some point between 29 and 84 days at 37°C. In view of the loss of TLR5 signaling activity (Table 2), it is clear that temperature-dependent changes must occur in flagellin-F1-V that affect its ability to interact with TLR5. In addition, changes may also occur in the F1 and V domains that reduce the number of fusion protein molecules with F1 and V in their native conformations. On the basis of our results (Table 2), we hypothesize that more profound changes may occur in V as opposed to F1. Data from dynamic light-scattering analysis of the protein at different temperatures clearly demonstrate that a significant change in the average diameter of flagellin-F1-V occurs at 37°C but not at lower temperatures (Fig. 5). As noted earlier, this change may reflect oligomerization of the protein. Since the change in diameter precedes the loss of TLR5-stimulating activity and the in vivo adjuvant activity and immunogenicity, it is likely that the change in diameter is a prerequisite to other changes that ultimately affect the efficacy of the fusion protein. In future studies, we plan on assessing the temperature stability of flagellin itself, since it is possible that temperature-dependent changes in F1 and V but not flagellin may be responsible for the loss of activity. In this regard, it is of interest to note that our early studies with flagellin (9) revealed that the bioactivity of flagellin was retained after short-term incubation at 100°C.
On the completion of a toxicology study with rabbits that is currently under way, it is our intent to submit an investigational new drug application to the FDA with the intent of initiating a phase I clinical trial of flagellin-F1-V. On the basis of its efficacy in two species of nonhuman primates, there is a very high probability that this vaccine will be effective in humans. In view of the results obtained with flagellin-F1-V, it is quite likely that flagellin fusions may be appropriate for use in a broad range of recombinant protein-based vaccines against bacteria and viruses.
Acknowledgments
This work was supported by NIH grant U01 AI070440 to S.B.M.
Footnotes
Published ahead of print on 5 November 2008.
REFERENCES
- 1.Anderson, G. W., Jr., S. E. C. Leary, E. D. Williamson, R. W. Titball, S. L. Welkos, P. L. Worsham, and A. M. Friedlander. 1996. Recombinant V antigen protects mice against pneumonic and bubonic plague caused by F1-capsule-positive and -negative strains of Yersinia pestis. Infect. Immun. 644580-4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews, G. P., D. G. Heath, G. W. Anderson, Jr., S. L. Welkos, and A. M. Friedlander. 1996. Fraction 1 capsular antigen (F1) purification from Yersinia pestis CO92 and from an Escherichia coli recombinant strain and efficacy against lethal plague challenge. Infect. Immun. 642180-2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker, E. E., H. Sommer, L. E. Foster, E. Meyer, and K. F. Meyer. 1952. Studies on immunization against plague. I. The isolation and characterization of the soluble antigen of Pasteurella pestis. J. Immunol. 68131-145. [PubMed] [Google Scholar]
- 4.Bates, J. T., A. N. Honko, A. H. Graff, N. Kock, and S. B. Mizel. 2008. Mucosal adjuvant activity of flagellin in aged mice. Mech. Ageing Dev. 129271-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ben-Yedidia, T., and R. Arnon. 1998. Effect of pre-existing carrier immunity on the efficacy of synthetic influenza virus. Immunol. Lett. 649-15. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Yedidia, T., H. Marcus, Y. Reisner, and R. Arnon. 1999. Intranasal administration of peptide vaccine protects human/mouse radiation chimera from influenza infection. Int. Immunol. 111043-1051. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Yedidia, T., R. Tarrab-Hazdai, D. Schechtman, and R. Arnon. 1999. Intranasal administration of synthetic recombinant peptide-based vaccine protects mice from infection by Schistosoma mansoni. Infect. Immun. 674360-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavanaugh, D. C., B. L. Elisberg, C. H. Llewllyn, J. D. J. Marshall, J. H. J. Rust, J. E. Williams, and K. F. Meyer. 1974. Plague immunisation. V. Indirect evidence for the efficacy of plague vaccine. J. Infect. Dis. 129S40. [DOI] [PubMed] [Google Scholar]
- 9.Ciacci-Woolwine, F., L. S. Kucera, S. H. Richardson, N. P. Iyer, and S. B. Mizel. 1997. Salmonellae activate tumor necrosis factor alpha production in a human promonocytic cell line via a released polypeptide. Infect. Immun. 654624-4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuadros, C., F. J. Lopez-Hernandez, A. L. Dominguez, M. McClelland, and J. Lustgarten. 2004. Flagellin fusion proteins as adjuvants or vaccines induce specific immune responses. Infect. Immun. 722810-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gewirtz, A. T., T. A. Navas, S. Lyons, P. J. Godowski, and J. L. Madara. 2001. Bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 1671882-1885. [DOI] [PubMed] [Google Scholar]
- 12.Hantgan, R. R., M. C. Stahle, J. H. Connor, D. S. Lyles, D. A. Horita, M. Rocco, C. Nagawami, J. W. Weisel, and M. A. McLane. 2004. The disintegrin echistatin stabilizes integrin alphaIIbbeta3′s open conformation and promotes its oligomerization. J. Mol. Biol. 3421625-1636. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 4101099-1103. [DOI] [PubMed] [Google Scholar]
- 14.Heath, D. G., Anderson, G. W., Jr., J. M. Mauro, S. L. Welkos, G. P. Andrews, J. Adamovicz, and A. M. Friedlander. 1998. Protection against experimental bubonic and pneumonic plague by a recombinant capsular F1-V fusion protein vaccine. Vaccine 161131-1137. [DOI] [PubMed] [Google Scholar]
- 15.Honko, A. N., and S. B. Mizel. 2004. Mucosal administration of flagellin induces innate immunity in the mouse lung. Infect. Immun. 726676-6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honko, A. N., and S. B. Mizel. 2005. Effects of flagellin on innate and adaptive immunity. Immunol. Res. 3383-102. [DOI] [PubMed] [Google Scholar]
- 17.Honko, A. N., N. Sriranganathan, C. J. Lees, and S. B. Mizel. 2006. Flagellin is an effective adjuvant for immunization against lethal respiratory challenge with Yersinia pestis. Infect. Immun. 741113-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huleatt, J. W., A. R. Jacobs, J. Tang, P. Desai, E. B. Kopp, Y. Huang, L. Song, V. Nakaar, and T. J. Powell. 2007. Vaccination with recombinant fusion proteins incorporating Toll-like receptor ligands induces rapid cellular and humoral immunity. Vaccine 25763-775. [DOI] [PubMed] [Google Scholar]
- 19.Jeon, S. H., T. Ben-Yedidia, and R. Arnon. 2002. Intranasal immunization with synthetic recombinant vaccine containing multiple epitopes of influenza virus. Vaccine 202772-2780. [DOI] [PubMed] [Google Scholar]
- 20.Leary, S. E. C., K. F. Griffin, H. S. Garmory, E. D. Williamson, and R. W. Titball. 1997. Expression of an F1/V fusion protein in attenuated Salmonella typhimurium and protection of mice against plague. Microb. Pathog. 23167-179. [DOI] [PubMed] [Google Scholar]
- 21.Leary, S. E. C., E. D. Williamson, K. F. Griffin, P. Russell, S. M. Eley, and R. W. Titball. 1995. Active immunization with recombinant V antigen from Yersinia pestis protects mice against plague. Infect. Immun. 632854-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee, S. E., S. Y. Kim, B. C. Jeong, Y. R. Kim, S. J. Bae, O. S. Ahn, J. J. Lee, H. C. Song, J. M. Kim, H. E. Choy, S. S. Chung, M. N. Kweon, and J. H. Rhee. 2006. A bacterial flagellin, Vibrio vulnificus FlaB, has a strong mucosal adjuvant activity to induce protective immunity. Infect. Immun. 74694-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levi, R., and R. Arnon. 1996. Synthetic recombinant influenza vaccine induces efficient long-term immunity and cross-strain protection. Vaccine 1485-92. [DOI] [PubMed] [Google Scholar]
- 24.McDermott, P. F., F. Ciacci-Woolwine, J. A. Snipes, and S. B. Mizel. 2000. High-affinity interaction between gram-negative flagellin and a cell surface polypeptide results in human monocyte activation. Infect. Immun. 685525-5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonald, W. F., J. W. Huleatt, H. G. Foellmer, D. Hewitt, J. Tang, P. Desai, A. Price, A. Jacobs, V. N. Takahashi, Y. Hunag, V. Nakaar, L. Alexopoulou, E. Fikrig, and T. J. Powell. 2007. A West Nile virus recombinant protein vaccine that coactivates innate and adaptive immunity. J. Infect. Dis. 1951607-1617. [DOI] [PubMed] [Google Scholar]
- 26.McEwen, J., R. Levi, R. J. Horwitz, and R. Arnon. 1992. Synthetic recombinant vaccine expressing influenza haemagglutinin epitope in Salmonella leads to partial protection in mice. Vaccine 10405-411. [DOI] [PubMed] [Google Scholar]
- 27.McSorley, S. J., B. D. Ehst, Y. Yu, and A. T. Gewirtz. 2002. Bacterial flagellin is an effective adjuvant for CD4+ T cells in vivo. J. Immunol. 1693914-3919. [DOI] [PubMed] [Google Scholar]
- 28.Mizel, S. B., A. P. West, and R. R. Hantgan. 2003. Identification of a sequence in human Toll-like receptor 5 required for the binding of gram-negative flagellin. J. Biol. Chem. 27823624-23629. [DOI] [PubMed] [Google Scholar]
- 29.Motin, V. L., R. Nakajima, G. B. Smirvov, and R. R. Brubaker. 1994. Passive immunity to yersiniae mediated by anti-recombinant V antigen and protein A-V antigen fusion peptide. Infect. Immun. 62192-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pino, O., M. Martin, and S. M. Michalek. 2005. Cellular mechanisms of the adjuvant activity of the flagellin component FljB of Salmonella enterica serovar Typhimurium to potentiate mucosal and systemic responses. Infect. Immun. 736763-6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell, P., S. M. Eley, S. E. Hibbs, R. J. Manchee, A. J. Stagg, and R. W. Titball. 1995. A comparison of plague vaccine, USP and EV76 vaccine induced protection against Yersinia pestis in a murine model. Vaccine 131551-1556. [DOI] [PubMed] [Google Scholar]
- 32.Strydom, D. J., and S. A. Cohen. 1994. Comparison of amino acid analyses by phenyisothiocyanate and 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate precolumn derivatization. Anal. Biochem. 22219-28. [DOI] [PubMed] [Google Scholar]
- 33.West, A. P., B. A. Dancho, and S. B. Mizel. 2005. Gangliosides inhibit flagellin signaling in the absence of an effect on flagellin binding to Toll-like receptor 5. J. Biol. Chem. 2809482-9488. [DOI] [PubMed] [Google Scholar]
- 34.Williamson, E. D., S. M. Eley, A. J. Stagg, M. Green, P. Russell, and R. W. Titball. 1997. A subunit vaccine elicits IgG in serum, spleen cell cultures and bronchial washings and protects immunized animals against pneumonic plague. Vaccine 151079-1084. [DOI] [PubMed] [Google Scholar]