Abstract
We report the first case of hemophagocytic lymphohistiocytosis (HLH) induced by the monoclonal expansion of Epstein-Barr virus (EBV)-negative NK cells. Consanguinity of the patient's parents made it necessary to discard familial HLH in the patient and her sister with identical HLA markers and demonstrate that no cause other than the expansion of NK cells, which secrete high levels of gamma interferon, was inducing HLH in this patient.
CASE REPORT
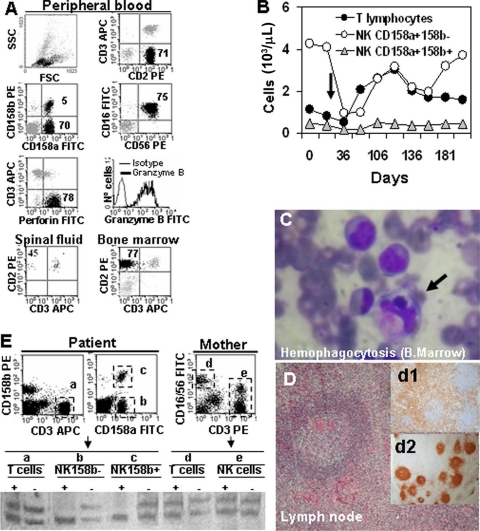
A 17-month-old Ecuadorian girl was admitted to our hospital, Hospital Universitario Virgen de la Arrixaca, Murcia, Spain, in September 2005, because of a fever and cytopenias (leukocytes, 11.6 × 109/liter; hemoglobin, 7.9 g/dl; platelets, 65 × 109/liter) which had persisted for over 3 weeks, splenomegaly (10 cm), hepatomegaly (5 cm), multiple adenopathies, purpura, and edema. The father and mother were cousins and healthy, the patient's gestation and birth had been normal, but recurrent fever episodes were reported from 3 months of age. The girl was vaccinated normally. Biochemical analysis showed normal values for fibrinogen (232 mg/dl; range, 150 to 450 mg/dl) and elevated levels of ferritin (278 ng/ml; normal range, 15 to 150 ng/ml) and triglycerides (311 mg/dl; normal range, 50 to 200 mg/dl). Serology demonstrated anti-cytomegalovirus (anti-CMV) immunoglobulin M (IgM) and IgG, anti-human herpesvirus 6 (anti-HHV-6) IgM and IgG, and anti-Epstein-Barr Virus (anti-EBV) viral capsid antigen (VCA) IgG, but not anti-EBV VCA IgM or anti-early and anti-EBNA EBV antigen IgG. No bacteria, fungi, or genomic evidence of EBV, CMV, HHV-6, HHV-7, HHV-8, varicella-zoster virus, herpes simplex virus, rubella virus, measles virus, parvovirus B19, enterovirus, or adenovirus was found in urine samples, peripheral blood (PB) samples, or lymph node (LN) biopsy specimens by PCR analysis (Table 1). Cytological studies showed an expansion of NK cells both in PB (5,300/μl) and bone marrow (BM) aspirate (7,540/μl) specimens, with normal expression of perforin and granzyme B (Fig. 1A and B), and the NK cells maintained their cytotoxic capacity (Fig. 2A). Evidence of hemophagocytosis, but no other significant abnormality, was observed in the aspirate of the BM (Fig. 1C). Paracortical diffuse hyperplasia in a LN biopsy specimen with no signs of hemophagocytosis was reported (Fig. 1D). Genetic studies including perforin 1 (PRF1) and MUNC13-4 pointed to no genetic alterations, although a clearly skewed X-inactivation pattern in NK cells could be demonstrated (Fig. 1E). High serum concentrations of soluble interleukin 2 receptor (sIL-2R) (36.8 ng/ml) and gamma interferon (IFN-γ) (400 pg/ml) were detected (Fig. 2C). The patient was diagnosed as having HLH, and treatment was set following the 2004 HLH protocol (cyclosporine, etoposide, and dexamethasone) 2 weeks after admission.
TABLE 1.
Results of microbiologic screening at diagnosis
| Microbiologic screening method and infectious agent (antibody)a | Result |
|---|---|
| Serology | |
| Virus | |
| EBV (VCA IgG), CMV (IgM and IgG), and | |
| HHV-6 (IgM and IgG) | Positive |
| EBV (VCA IgM, early IgG, and EBNA IgG), parvovirus B19 (IgM and IgG), HAV, | |
| HBV, HCV, and HIV | Negative |
| Bacteria and parasites | |
| Toxoplasma (IgG), Leishmania (IgG), | |
| Salmonella enterica serovar Typhi (H and O antigens), | |
| Brucella, and Treponema pallidum | Negative |
| PCR on urine, blood, and lymph node specimens | |
| EBV, CMV, HHV-6, HHV-7, HHV-8, herpes | |
| simples virus, varicella-zoster virus, enterovirus, | |
| adenovirus, Bartonella, rubella virus, | |
| measles virus, and parvovirus B19 | Negative |
Abbreviations: EBV, Epstein-Barr Virus; CMV, cytomegalovirus; HHV-6, human herpesvirus 6; HAV, HBV, and HCV, hepatitis A, B, and C virus, respectively: HIV, human immunodeficiency virus.
FIG. 1.
Data on the patient with hemophagocytic lymphohistiocytosis. (A) At diagnosis, NK cells (black dots) represented 75%, 77%, and 45% of total leukocytes from PB, BM, and spinal fluid samples, respectively. NK cells showed the following phenotype: CD2+ CD3− (at the surface and in the cytoplasm) CD7+ CD8+ CD16+ CD56+ CD94+ CD158a+ CD158b−/+ TCR− perforin positive and granzyme B positive. Molecular analysis discarded T-cell receptor (TCR) clonality (data not shown). Two NK cell subsets could be observed: CD158a+ CD158b+ (5% of total leukocytes) and CD158a+ CD158b− (70% of total leukocytes). Abbreviations: FSC, forward scatter; SSC, side scatter; CD2 PE, phycoerythrin-conjugated anti-CD2 antibody; CD3 APC, allophycocyanin-conjugated anti-CD3 antibody; CD16 FITC, fluorescein isothiocyanate-conjugated anti-CD16 antibody. (B) The CD158b− NK cell subset dropped from 4,264/μl to 850/μl on day 36 due to HLH therapy (the black arrow indicates the beginning of HLH treatment) and recovered to pretreatment levels afterwards. The CD158b+ NK cell count showed no change. (C) Bone marrow (B.Marrow) with erythroid, megakaryocytic, and mononuclear-phagocyte hyperplasia and few signs of hemophagocytosis. The black arrow indicates hemophagocytosis. (D) LN with paracortical diffuse hyperplasia and no signs of hemophagocytosis. d1 and d2 inserts, interfollicular/paracortical and follicular details of anti-CD3 and anti-CD20 immunohistochemistry analysis, respectively. (E) X-chromosome inactivation pattern in T and NK cells. T cells (CD3+ [a]) and both NK cell subsets (CD158b− [b] or CD158b+ [c]) from the patient, as well as T cells (CD3+ [d]) and NK cells (CD3− CD16/56+ [e]) from the mother, were purified (>98% purity). DNA was extracted from purified cells and amplified as described in the text. PCR products were digested with HpaII (+) or not digested with HpaII (−) and run on a polyacrylamide gel. A clearly skewed X-chromosome inactivation pattern was observed in both NK cell subsets from the patient (lanes b+ and c+) compared with the patient's T cells (lane a+) and the mother's T and NK cells (lanes d+ and e+).
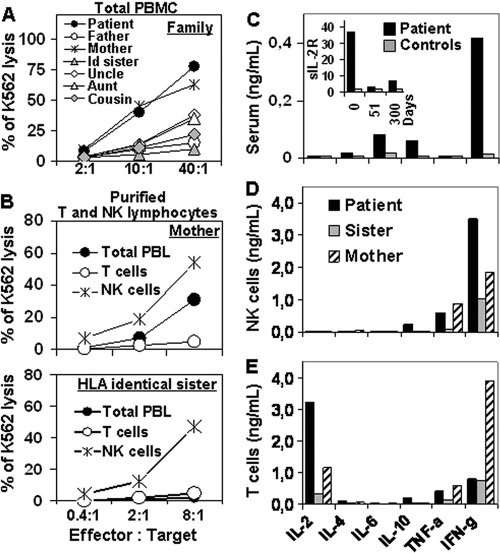
FIG. 2.
Cytotoxic activity and cytokine secretion of NK cells. (A) Cytotoxicity of total PBMC from all family members. Id sister, sister with identical HLA markers. (B) Cytotoxicity of purified NK and T cells from HLA-identical sister and mother. PBL, peripheral blood lymphocytes. (C) Serum levels of cytokines and sIL-2R (insert) in the patient at diagnosis and healthy controls (n = 30). (D and E) On day 51 after diagnosis, NK and T cells were purified (>98% purity) from the patient, mother, and HLA-identical sister, and two million cells per well were stimulated overnight with phorbol myristate acetate (50 ng/ml) and ionomycin (0.5 μg/ml) in a 24-well plate. NK cells (D) from the patient secreted two to three times more IFN-γ than NK cells from the mother or sister, while T cells (E) from the patient secreted mainly IL-2 but low amounts of IFN-γ (IFN-g). TNF-a, tumor necrosis factor alpha.
The patient's sister with identical HLA markers persistently showed reduced NK cell counts and reduced NK cytotoxic activity measured in total peripheral blood mononuclear cells (PBMC) (Fig. 2A). Due to parent consanguinity, NK cell malfunction was suspected in both the patient and sister. However, highly purified NK cells from the sister and mother showed normal NK cell cytotoxic activity (Fig. 2). Bone marrow transplantation from the sister was successfully carried out in mid-September 2006 (values of 6.67 × 109 leukocytes/liter, 14.0 g of hemoglobin/dl, and 370 × 109 platelets/liter in June 2008) at Vall d'Hebron Hospital, Barcelona, Spain. Informed consent was obtained from the parents of the patient and from all members of the family included in the study. The ethical committee of our hospital approved the analysis of results. All procedures are in accordance with the Helsinki Declaration of 1975.
Serum samples were tested for the presence of IgM and IgG antibodies against most common viruses, bacteria, and parasites by means of a competitive chemiluminescence immunoassay (DiaSorin Laboratories, Anthony, France) at the Microbiology Service, University Hospital Virgen Arrixaca (Murcia, Spain). All microbiological molecular studies were performed in the National Centre for Microbiology, Instituto de Salud Carlos-III (Madrid, Spain), using properly validated multiplex reverse transcription-PCR methods (2, 10). Standard methodology was used for flow cytometry, NK cell cytotoxicity (9), cytological and histological analyses, and mutation of perforin (PRF1) and MUNC13-4 (11) analysis. Quantification of sIL-2R (R&D Systems, Abingdon, United Kingdom), IL-2, IL-4, IL-6, IL-10, IFN-γ, and tumor necrosis factor alpha (CBA, BD, San Jose, CA) was performed following the manufacturer's instructions. Cell purification was done in a MoFlo cell sorter (Cytomation Inc., Fort Collins, CO).
X-chromosome inactivation assay was performed in the patient and mother using genomic DNA (DNA blood midi kit; Qiagen, Hilden, Germany) extracted from highly purified NK cells and T lymphocytes. Briefly, this method consisted of the analysis of methylation at the androgen receptor locus. PCR is performed on genomic DNA digested with the methylation-sensitive restriction enzyme HpaII, and only the androgen receptor gene residing in the inactivated X-chromosome is amplified. Conditions were the same as those described previously (4).
According to the criteria of the Histiocyte Society (7), clinical, analytical, and histological evidence, together with parental consanguinity, strongly suggested familial HLH (FHL). Most cases of FHL involve mutations of genes implicated in the NK and cytotoxic T-lymphocyte cytotoxic function (3). Among them, PRF1 is considered to be altered in 20 to 50% of cases worldwide (8, 11). To confirm FHL diagnosis, NK cell counts, perforin expression, and NK cytolytic activity in PBMC were evaluated in all family members (Fig. 2 and data not shown). Normal values for these parameters were observed in all family members, but the patient's sister with identical HLA markers showed a clear reduction of NK cell cytotoxic activity (<3% of K562 lysis at a 40:1 effector/target cell ratio). This last piece of data reinforced the hypothesis of FHL. However, and according to her NK cell counts (71%), the patient showed the highest cytotoxic activity (77% of K562 lysis at a 40:1 effector/target cell ratio). To clarify this point, NK cell function was evaluated in purified NK cells from the sister, using the mother, who had shown normal NK cell function, as a control. Figure 2B clearly shows that the cytolytic capability of purified NK cells was normal in both the mother and sister (54% and 47% of K562 lysis at a 8:1 effector/target cell ratio, respectively). Additional results, such as normal expression of perforin and granzyme B (Fig. 1A), and the absence of mutations in PRF1 and MUNC13-4 reported by Zur Stadt (Hamburg, Germany) (11), led us to conclude that the patient could be affected by a secondary form of HLH, unless other mutations that do not alter NK cell function, were involved.
The two major causes of secondary HLH are infection and lymphoproliferative diseases. The former are mainly represented by viruses and, less frequently, by bacterial or even fungal infections (6). Recent acute CMV and HHV-6 infections in the patient were suspected because of the presence of IgG and IgM antibodies. However, the existence of these infectious agents could not be demonstrated by PCR in urine, blood, or lymph node specimen (Table 1). Additionally, to our knowledge, virus-associated HLH is a rare complication in early CMV infection that, in addition, seems to require active CMV infection (5). Likewise, serology for EBV, i.e., negative anti-EBNA IgG and positive anti-VCA IgG in the absence of IgM could have indicated primary EBV infection, but again PCR analysis for EBV was negative in all samples at diagnosis. Altogether, microbiological analysis seemed to demonstrate a previous exposure to CMV, HHV-6, and EBV, but no clear acute or active infection at diagnosis that could have directly triggered the HLH episode.
Similarly, cytological studies showed no clear evidence of lymphoproliferative disorders (Fig. 1). Nevertheless, at diagnosis, the patient showed severe hepatosplenomegaly and a moderate expansion of monoclonal NK cells (skewed chromosome X-inactivation pattern). Two different NK cell lines could clearly be distinguished. The first, CD158a+ CD158b+, presented constant cell counts in PB, but the second, CD158a+ CD158b−, which showed the highest cell count at diagnosis, was dramatically affected by HLH therapy (Fig. 1B). Both NK cell lines showed a similar X-chromosome inactivation pattern (Fig. 1E), suggesting the possibility that they could have emerged from a common ancestor.
It is important to note that all molecular studies for EBV were negative, so that no possible NK cell transformation induced by EBV could have occurred. After reviewing the literature, we could not find reports describing EBV-negative NK cell proliferative disorders or leukemias associated with HLH. Additionally, it was possible to demonstrate that the patient showed high IFN-γ serum levels (Fig. 2) and, more importantly, highly purified monoclonal NK cells, but not T cells, secreted large amounts of IFN-γ. Consistent with these observations, it has been suggested that IFN-γ may constitute an autocrine survival signal important for the progression of NK cell leukemia, which might eventually lead to the occurrence of secondary HLH (1).
Bone marrow transplantation is the treatment of choice for both FHL and HLH induced by NK lymphoproliferative disease. Fortunately, the patient's sister with identical HLA markers was available and healthy. After transplant, the NK cell population in the patient was restored to levels that could be considered normal and showed a completely different CD158a/CD158b profile (data not shown).
The interesting point of this case, apart from describing for the first time the monoclonal expansion of EBV-negative NK cells associated with HLH, is that the consanguinity of parents made it necessary to discard the familial form of HLH both in the patient and in her HLA-identical sister before bone marrow transplantation was approved. In addition, it was necessary to demonstrate that no cause other than the monoclonal expansion of NK cells, which secrete large amounts of IFN-γ, could be the origin of the HLH. This report describes the long and intricate analytical procedure necessary for the correct diagnosis, a period during which the life of the patient was seriously at risk. This case will hopefully contribute to accelerating the diagnostic procedure in future similar cases and to reducing suffering of the patients involved.
Acknowledgments
We gratefully acknowledge all the clinicians and researchers involved in this case, particularly M. Bermudez (Pediatric Oncology), C. Márquez and A. Menasalva (Microbiology Service) at the University Hospital Virgen de la Arrixaca (Murcia, Spain), and A. de la Loma at the National Centre for Microbiology, Instituto de Salud Carlos-III (Madrid, Spain), L. Allende at 12 de Octubre Hospital (Madrid, Spain), and finally, Udo Zur Stadt of the Hematology and Oncology Unit, Ependorff University (Hamburg, Germany).
This work was supported by Ciberehd, Instituto de Salud Carlos III.
Footnotes
Published ahead of print on 19 November 2008.
REFERENCES
- 1.Akashi, K., and S. I. Mizuno. 2000. Epstein-Barr virus-infected natural killer cell leukemia. Leuk. Lymphoma 4057-66. [DOI] [PubMed] [Google Scholar]
- 2.del Mar Mosquera, M., M., F. de Ory, M. Moreno, and J. E. Echevarría. 2002. Simultaneous detection of measles virus, rubella virus, and parvovirus B19 by using multiplex PCR. J. Clin. Microbiol. 40111-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldmann, J., I. Callebaut, G. Raposo, S. Certain, D. Bacq, C. Dumont, N. Lambert, M. Oauchee-Chardin, G. Chedeville, H. Tamary, V. Minard-Colin, E. Vilmer, S. Blanche, F. Le Deist, A. Fischer, and G. de Saint Basile. 2003. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophahocytic lymphocistiocytosis (FHL3). Cell 115461-473. [DOI] [PubMed] [Google Scholar]
- 4.Heine-Suner, D., L. Torres-Juan, M. Morla, X. Busquets, F. Barcelo, G. Pico, L. Bonilla, N. Govea, M. Bernues, and J. Rosell. 2003. Fragile-X syndrome and skewed X-chromosome inactivation within a family: a female member with complete inactivation of the functional X chromosome. Am. J. Med. Genet. 122108-114. [DOI] [PubMed] [Google Scholar]
- 5.Hot, A., M. H. Madoux, J. P. Viard, B. Coppéré, and J. Ninet. 2008. Successful treatment of cytomegalovirus-associated hemophagocytic syndrome by intravenous immunoglobulins. Am. J. Hematol. 83159-162. [DOI] [PubMed] [Google Scholar]
- 6.Imashuku, S. 2000. Advances in the management of hemophagocytic lymphohistiocytosis. Int. J. Hematol. 721-11. [PubMed] [Google Scholar]
- 7.Janka, G. E., and G. M. Schneider. 2004. Modern management of children with hemophagocytic lymphohistiocytosis. Br. J. Haematol. 1244-14. [DOI] [PubMed] [Google Scholar]
- 8.Katano, H., and J. I. Cohen. 2005. Perforin and lymphohistiocytic proliferative disorders. Br. J. Haematol. 128739-750. [DOI] [PubMed] [Google Scholar]
- 9.Lecoeur, H., M. Fevrier, S. Garcia, Y. Riviere, and M. L. Gougeon. 2001. A novel flow cytometric assay for quantitation and multiparametric characterization of cell-mediated cytotoxicity. J. Immunol. Methods 253177-187. [DOI] [PubMed] [Google Scholar]
- 10.Pozo, F., and A. Tenorio. 1999. Detection and typing of lymphotropic herpesviruses by multiplex polymerase chain reaction. J. Virol. Methods 799-19. [DOI] [PubMed] [Google Scholar]
- 11.Zur Stadt, U., K. Beutel, S. Kolberg, R. Schneppenheim, H. Kabisch, G. E. Janka, and H. C. Hennies. 2006. Mutation spectrum in children with primary hemophagocytic lymphohistiocytosis: molecular and functional analyses of PRF1, UNC13D, STX11, and RAB27A. Hum. Mutat. 2762-68. [DOI] [PubMed] [Google Scholar]