Abstract
We examined the antibody responses of pediatric patients infected with community-associated Staphylococcus aureus isolates. The data show that patients infected with Panton-Valentine leukocidin (PVL)-positive strains developed a dominant immunoglobulin G anti-PVL antibody response that correlates with markers of inflammation.
Staphylococcus aureus is one of the most common bacteria causing skin and soft tissue infections as well as invasive infections, e.g., osteomyelitis, septic arthritis, infective endocarditis, and complicated pneumonia, in humans (14). Over the past decade, the USA300 S. aureus pulsed-field type has become the dominant S. aureus lineage causing community-associated methicillin-resistant S. aureus (CA-MRSA) infections worldwide (3-8, 13, 15, 16, 20); this lineage encodes the Panton-Valentine leukocidin (PVL), a cytotoxin that has been associated with severe S. aureus infections in humans (3-8, 13, 15, 16, 20). We examined the anti-PVL antibody responses in patients diagnosed with S. aureus infections (Table 1) and compared these responses to those against other USA300 virulence factors.
TABLE 1.
Demographic characteristics of children with S. aureus infections
| Characteristic | Result for children with:
|
|
|---|---|---|
| Invasive infectionsa | SSTIb | |
| Mean age ± SE (yr) | 6.58 ± 1.03 | 7.74 ± 0.92 |
| No. (%) of isolates | ||
| pvl positive | 21 (64) | 24 (69) |
| pvl negative | 9 (33) | 2 (6) |
| NDc | 3 (9) | 9 (25) |
| MRSA | 13 (40) | 20 (57) |
| MSSA | 20 (60) | 6 (17) |
| ND | 0 (0) | 9 (26) |
| Previous infection | ||
| Yes | 10 (30) | 19 (54) |
| No | 21 (64) | 7 (20) |
| Unknown | 2 (6) | 9 (26) |
| Race | ||
| Caucasian | 15 (46) | 15 (43) |
| Black | 7 (21) | 11 (31) |
| Hispanic | 6 (18) | 8 (23) |
| Otherd | 5 (15) | 1 (3) |
Invasive infections were defined as infections of the bone (n = 28), myositis (n = 3), and pneumonia (n = 2).
SSTIs were defined as abscesses (n = 26), cellulitis (n = 2), and lymphadenitis (n = 4).
ND, not determined.
Other, three Asian and two unknown.
Sera for antibody analysis were collected at the time of admission from pediatric patients with either skin and soft tissue infections (SSTI), invasive bone infections (osteomyelitis), or pneumonia. Sera from healthy 4- to 6-year-old children (collected in 1991) served as negative controls.
The serum antibody responses to the following S. aureus recombinant factors were measured: extracellular fibrinogen-binding protein from amino acid positions 35 to 165 (Efb35-165) (10, 11), MHCII analog protein from amino acid positions 50 to 237 (Map1950-237) (12), LukF-PV25-325 and LukS-PV29-312 (9), clumping factor B from amino acid positions 201 to 542 (ClfB201-542) (19), collagen-binding adhesin 35 from amino acid positions 29 to 334 (CNA3529-334) (22), or the pGEX-2T (GE Life Sciences, Piscataway, NJ) vector for fibronectin-binding protein A from amino acid positions 620 to 881 (FnbpA620-881) (17). In addition, LukD27-327, LukE40-311, gamma hemolysin A from amino acid positions 30 to 309 (HlgA30-309), HlgB27-325, and HlgC30-315 were cloned and expressed for this study. Alpha toxin was purchased from List Biological Laboratories (Campbell, CA), and the LukS-PV signal peptide (formyl-Met-Val-Lys-Lys-Arg-Leu-Leu-Ala-Ala-Thr-Leu-Ser-Leu-Gly-Ile-Ile-Thr-Pro-Ile-Ala- Thr-Ser-Phe-His-Glu-Ser-Lys-Ala-OH) and α3-phenol soluble modulin (α3-PSM; formyl-Met-Glu-Phe-Val-Ala-Lys-Leu-Phe-Lys-Phe-Phe-Lys-Asp-Leu-Leu-Gly-Lys-Phe-Leu-Gly-Asn-Asn- OH) were synthesized by AnaSpec, Inc. (San José, CA) (21). These factors were chosen as representative members of the adhesin, toxin, and immunomodulator families. The collagen adhesin CNA (not encoded in the USA300 genome) was selected as a negative control.
Specific antibody responses were characterized by using alkaline phosphatase-conjugated (1:5,000) goat anti-human whole immunoglobulin G (IgG) antibodies; IgA antibodies; or mouse isotype-specific anti-human IgG1, IgG2, IgG3, IgG4, or IgM antibodies (Zymed Laboratories, San Francisco, CA). Immulon-1B microtiter plates (Dynatech Laboratories, Chantilly, VA) were coated overnight at 4°C with 1 μg of each recombinant S. aureus antigen, and enzyme-linked immunosorbent assays (ELISAs) were carried out as described previously by using a 1:1,000 dilution of patient serum (2).
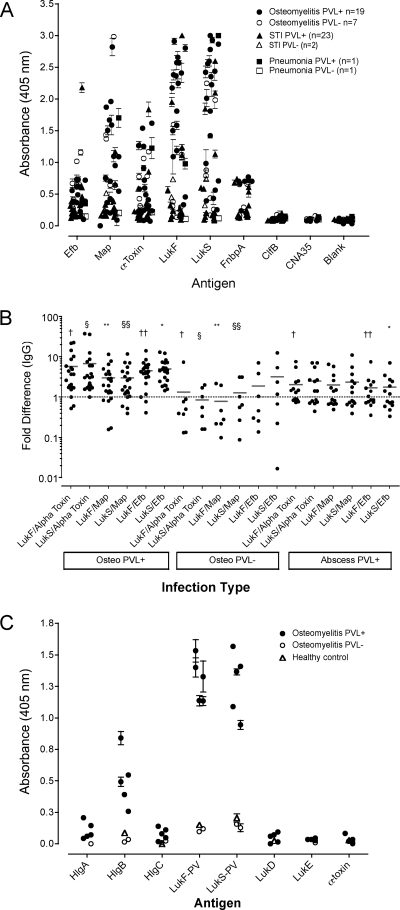
The levels of IgG antibodies to LukS-PV and LukF-PV were significantly higher (P < 0.01) in children with invasive infections (median optical density [OD], 0.98; OD range, 0.12 to 3.00) than in children with SSTI (median OD, 0.47; OD range, 0.09 to 2.60) (Fig. 1A). A similar trend (P = 0.06) for the levels of IgG antibodies to alpha toxin was noted in the two groups, but the levels of antibodies to alpha toxin were lower than those to LukS-PV in each group (Fig. 1A). No reactivity to the LukS-PV signal peptide or α3-PSM was detected. In patients with invasive infections, the levels of IgG antibodies to LukS-PV correlated with the number of days prior to admission that the patient was ill (r = 0.44, P = 0.023) and the C-reactive protein (CRP) values at the time of admission (r = 0.42, P = 0.03).
FIG. 1.
Antibody reactivities to S. aureus virulence proteins. ELISA plate wells coated with S. aureus proteins were incubated with a 1:1,000 dilution of patient serum and were then probed with a 1:5,000 dilution of alkaline phosphatase-conjugated anti-IgG antibody. The plates were developed for 30 min in the dark and then read at 405 nm. The data are expressed as the means ± standard errors for triplicate wells per patient. (A) IgG responses to Efb, Map, alpha toxin, LukF-PV, LukS-PV, ClfB, CNA35, and FnbpA in patients with different disease presentations. (B) Log difference in anti-PVL antibody titers. The data are expressed as the log difference between the mean OD readings obtained for individual patients described above. Statistical differences were determined by the unpaired t test with Welch's correction; and differences between groups are indicated by the corresponding symbols: †, P ≤ 0.02; §, P ≤ 0.02; **, P ≤ 0.004; §§, P ≤ 0.04; ††, P ≤ 0.001; *, P ≤ 0.001. (C) Antibody reactivities to S. aureus toxins. ELISA plate wells were coated with 1 μg/well of either HlgA, HlgB, HlgC, LukF-PV, LukS-PV, LukD, LukE, or alpha toxin. The data are expressed as the means ± standard deviations for triplicate wells per patient. PVL+, PVL positive; PVL−, PVL negative; STI, soft tissue infection; Osteo, osteomyelitis.
There was a trend for the anti-LukS-PV IgG antibody levels to correlate with the erythrocyte sedimentation rate (ESR) at admission (r = 0.35, P = 0.08). In contrast, the levels of antibodies to alpha toxin did not correlate with the duration of illness prior to admission or the CRP value at admission, but a trend for a correlation with the ESRs at admission was noted (r = 0.39, P = 0.09). There was no correlation between the anti-LukS-PV or anti-alpha toxin IgG antibody response and the patient's age or between the anti-LukS-PV and anti-alpha toxin IgG antibody responses.
The anti-Map IgG antibody levels in patients with invasive infections (median OD, 0.64; OD range, 0.09 to 2.98) were also significantly greater (P = 0.002) than those in patients with SSTI (median OD, 0.25; OD range, 0.13 to 1.91). The anti-Efb IgG antibody levels were lower than the levels of the other IgG antibodies and were not different between the patient groups. The levels of IgA antibodies to the protein panel were not significantly different between groups, and IgA antibodies were present at lower levels than the IgG antibodies (data not shown). Antibody responses to CNA and ClfB were not detected in the patients (Fig. 1A).
As described above, patients from all groups had various responses to most of the virulence factors tested. Patients with pvl-positive osteomyelitis had anti-LukF-PV and anti-LukS-PV IgG antibody responses that were more dominant than those of patients diagnosed with pvl-negative osteomyelitis or pvl-positive SSTI. This was examined further by comparing the anti-LukF-PV and anti-LukS-PV IgG antibody responses of each patient to their respective IgG responses to alpha toxin, Map, and Efb (factors that consistently elicited measurable responses). Patients with pvl-positive osteomyelitis on average had statistically higher IgG anti-LukF-PV and anti-LukS-PV responses (approximately fivefold greater than their responses to alpha toxin, Map, or Efb) (Fig. 1B). In contrast, patients infected with pvl-negative isolates had alpha-toxin, Map, and Efb responses higher than their respective anti-LukF-PV and anti-LukS-PV responses (Fig. 1B). These data suggest that patients infected with PVL-positive isolates develop a dominant anti-PVL response at the expense of other factors.
The IgG isotype (i.e., IgG1, IgG2, IgG3, and IgG4) reactivities against the virulence proteins described above were determined to define the differences in Ig responses between groups; however, no significant differences were observed (data not shown).
Since the dominant antibody responses observed were toward LukF-PV and LukS-PV, we examined the possibility that the magnitude of this response was due to the presence of cross-reactive antibodies generated against structurally similar factors, e.g., other pore-forming toxins, by testing patient sera from high and low anti-PVL IgG antibody responders for reactivity to either LukD, LukE, alpha toxin, HlgA, HlgB, or HlgC. Sera from five patients with S. aureus (pvl-positive) osteomyelitis responded exclusively to LukF and LukS and had some reactivity to HlgB (Fig. 1C). Serum obtained from patients with infections caused by pvl-negative isolates or from controls were nonresponsive to the toxins tested (Fig. 1C), indicating that the anti-LukF and anti-LukS responses were primarily due to a specific humoral response to these antigens.
Although the protein panel tested for Ig reactivity represents a small fraction of the total target antigens with the potential of eliciting a humoral response against S. aureus, the antigens selected were chosen because they are virulence factors in humans and in animal models of disease (1, 9, 12, 14, 18). This report is the first describing the antibody responses to selected S. aureus antigens in pediatric patients with either SSTI or invasive infections caused by CA-MRSA isolates of the USA300 lineage. Patients with invasive infections developed more dominant and specific anti-LukF-PV and anti-LukS-PV responses than patients with SSTI. The titers of the antibodies to these determinants correlated with markers of inflammation, the significance of which remains to be understood.
ADDENDUM IN PROOF
After this paper was accepted for publication, we cloned and expressed a new LukE construct (amino acids 29 to 311) that we tested for antibody reactivity as described for Fig. 1C. Sera collected from patients infected with PVL-negative isolates did not react significantly to this construct, LukE29-311. However, serum from patients infected with PVL-positive isolates bound to LukE29-311 at levels similar to those observed for LukS. These observations do not change the conclusions derived from the data presented. Rather since only patients infected with PVL-positive isolates responded to LukE, whereas patients infected with PVL-negative isolates (which also express LukE but not either LukE nor LukS) did not have antibodies reactive to either LukF or LukS, the results obtained with this expanded construct suggest that antibodies raised against LukS cross-reacted with LukE, not vice versa.
Acknowledgments
These studies were supported in part by startup funds to E.L.B., a gift from the Hamill Foundation to M.G.B., and a grant from the Vivian Smith Foundation to S.L.K.
We are grateful to M. Barbu, E. Smeds, S. Prabhakaran, and X. Liang for some of the recombinant proteins used in this study.
Footnotes
Published ahead of print on 12 November 2008.
REFERENCES
- 1.Bodén, M. K., and J. I. Flock. 1994. Cloning and characterization of a gene for a 19 kDa fibrinogen-binding protein from Staphylococcus aureus. Mol. Microbiol. 12599-606. [DOI] [PubMed] [Google Scholar]
- 2.Brown, E. L., E. S. Reisenbichler, J.-H. Kim, and M. Höök. 2005. Multicomponent Lyme vaccine: three is not a crowd. Vaccine 233687-3696. [DOI] [PubMed] [Google Scholar]
- 3.Gillet, Y., B. Issartel, P. Vanhems, J. C. Fournet, G. Lina, M. Bes, F. Vandenesch, Y. Piemont, N. Brousse, D. Floret, and J. Etienne. 2002. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 359753-759. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez, B. E., K. G. Hulten, M. K. Dishop, L. B. Lamberth, W. A. Hammerman, E. O. Mason, Jr., and S. L. Kaplan. 2005. Pulmonary manifestations in children with invasive community-acquired Staphylococcus aureus infection. Clin. Infect. Dis. 41583-590. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez, B. E., G. Martinez-Aguilar, K. G. Hulten, W. A. Hammerman, J. Coss-Bu, A. Avalos-Mishaan, E. O. Mason, Jr., and S. L. Kaplan. 2005. Severe staphylococcal sepsis in adolescents in the era of community-acquired methicillin-resistant Staphylococcus aureus. Pediatrics 115642-648. [DOI] [PubMed] [Google Scholar]
- 6.Hulten, K. G., S. L. Kaplan, B. E. Gonzalez, W. A. Hammerman, L. B. Lamberth, J. Versalovic, and E. O. Mason, Jr. 2006. Three-year surveillance of community onset health care-associated Staphylococcus aureus infections in children. Pediatr. Infect. Dis. J. 25349-353. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan, S. L. 2005. Implications of methicillin-resistant Staphylococcus aureus as a community-acquired pathogen in pediatric patients. Infect. Dis. Clin. N. Am. 19747-757. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan, S. L., K. G. Hulten, B. E. Gonzalez, W. A. Hammerman, L. Lamberth, J. Versalovic, and E. O. Mason, Jr. 2005. Three-year surveillance of community-acquired Staphylococcus aureus infections in children. Clin. Infect. Dis. 401785-1791. [DOI] [PubMed] [Google Scholar]
- 9.Labandeira-Rey, M., F. Couzon, S. Boisset, E. L. Brown, M. Bes, Y. Benito, E. M. Barbu, V. Vazquez, M. Höök, J. Etienne, F. Vandenesh, and M. G. Bowden. 2007. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science 3151082-1085. [DOI] [PubMed] [Google Scholar]
- 10.Lee, L. Y., M. Höök, D. Haviland, R. A. Wetsel, E. O. Yonter, P. Syribeys, J. Vernachio, and E. L. Brown. 2004. A secreted Staphylococcus aureus protein inhibits complement activation. J. Infect. Dis. 190571-579. [DOI] [PubMed] [Google Scholar]
- 11.Lee, L. Y., X. Liang, M. Höök, and E. L. Brown. 2004. Identification and characterization of the C3 binding domain of the Staphylococcus aureus extracellular fibrinogen-binding protein (Efb). J. Biol. Chem. 27950710-50716. [DOI] [PubMed] [Google Scholar]
- 12.Lee, L. Y., Y. J. Miyamoto, B. W. McIntyre, M. Höök, K. W. McCrea, D. McDevitt, and E. L. Brown. 2002. The Staphylococcus aureus Map protein is an immunomodulator that interferes with T cell-mediated responses. J. Clin. Investig. 1101461-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lina, G., Y. Piemont, F. Godail-Gamot, M. Bes, M. O. Peter, V. Gauduchon, F. Vandenesch, and J. Etienne. 1999. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 291128-1132. [DOI] [PubMed] [Google Scholar]
- 14.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339520-532. [DOI] [PubMed] [Google Scholar]
- 15.McCaskill, M. L., E. O. Mason, Jr., S. L. Kaplan, W. Hammerman, L. B. Lamberth, and K. G. Hulten. 2007. Increase of the USA300 clone among community-acquired methicillin-susceptible Staphylococcus aureus causing invasive infections. Pediatr. Infect. Dis. J. 261122-1127. [DOI] [PubMed] [Google Scholar]
- 16.Mishaan, A. M., E. O. Mason, Jr., G. Martinez-Aguilar, W. Hammerman, J. J. Propst, J. R. Lupski, P. Stankiewicz, S. L. Kaplan, and K. Hulten. 2005. Emergence of a predominant clone of community-acquired Staphylococcus aureus among children in Houston, Texas. Pediatr. Infect. Dis. J. 24201-206. [DOI] [PubMed] [Google Scholar]
- 17.Miyamoto, Y. J., E. R. Wann, T. Fowler, E. Duffield, M. Höök, and B. W. McIntyre. 2001. Fibronectin binding protein A of Staphylococcus aureus can mediate human T lymphocyte adhesion and coactivation. J. Immunol. 1665129-5138. [DOI] [PubMed] [Google Scholar]
- 18.Patti, J. M., B. L. Allen, M. J. McGavin, and M. Höök. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 48585-617. [DOI] [PubMed] [Google Scholar]
- 19.Perkins, S., E. J. Walsh, C. C. Deivanayagam, S. V. Narayana, T. J. Foster, and M. Höök. 2001. Structural organization of the fibrinogen-binding region of the clumping factor B MSCRAMM of Staphylococcus aureus. J. Biol. Chem. 27644721-44728. [DOI] [PubMed] [Google Scholar]
- 20.Vandenesch, F., T. Naimi, M. C. Enright, G. Lina, G. R. Nimmo, H. Heffernan, N. Liassine, M. Bes, T. Greenland, M. E. Reverdy, and J. Etienne. 2003. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg. Infect. Dis. 9978-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang, R., K. R. Braughton, D. Kretschmer, T. H. Bach, S. Y. Queck, M. Li, A. D. Kennedy, D. W. Dorward, S. J. Klebanoff, A. Peschel, F. R. DeLeo, and M. Otto. 2007. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 131510-1514. [DOI] [PubMed] [Google Scholar]
- 22.Xu, Y., J. M. Rivas, E. L. Brown, X. Liang, and M. Höök. 2004. Virulence potential of the staphylococcal adhesin CNA in experimental arthritis is determined by its affinity for collagen. J. Infect. Dis. 1892323-2333. [DOI] [PubMed] [Google Scholar]