Abstract
The role of papillomaviruses (PVs) in the development of canine cancers is controversial. However, recently a novel canine PV (CPV3) was detected in a dog affected with a condition reminiscent of epidermodysplasia verruciformis (EV). The aim of the present study was to investigate the seroprevalence of CPV3 by using generic enzyme-linked immunosorbent assays (ELISAs) for the detection of antibodies against either canine oral PV (COPV) or CPV3. Therefore, the capsid proteins of both PV types were expressed as glutathione S-transferase fusion protein antigens and adsorbed to glutathione-casein-coated ELISA plates. After showing that PV type-specific antibodies could be detected in the sera from dogs with confirmed COPV or CPV3 infection, CPV3- and COPV-seropositive samples were detected in two sets of canine sera collected in Switzerland and South Africa, respectively. We found specific antibodies against COPV and CPV3 among the tested sera and also a large number that were positive for both antigens. The seroprevalences of PV antibodies of 21.9% (COPV) and 26.9% (CPV3) among the tested dogs from South Africa were higher than those among the dogs from Switzerland at 10.5% (COPV) and 1.3% (CPV3). Our data suggest a need for further CPV-related seroepidemiological surveys in different countries, especially in the context of clinical manifestations and possible breed predispositions. For this purpose, the newly developed ELISAs can be a useful tool.
Papillomaviruses (PVs) are small, icosahedral, nonenveloped DNA viruses with mucosal and skin tropism which are associated with various hyperplastic, dysplastic, and neoplastic conditions in humans and animals (21). More than 100 types of human PVs (HPVs) have been described, and more are continuously being discovered (8). The role of HPVs in the development of human diseases, i.e., cervical carcinomas or epidermodysplasia verruciformis (EV)-associated skin carcinomas, is well established, and pathomechanisms have been the focus of many studies (14, 23, 30, 35, 48). Indeed, both PCR- and enzyme-linked immunosorbent assay (ELISA)-based techniques are commonly used to carry out HPV-related epidemiologic studies (2, 25).
In contrast, the role of PVs in the development of canine cancers remains uncertain, although a clinical counterpart to human EV has been described in dogs with PV infections (28). Until now, only four canine PVs (CPVs) have been unambiguously identified and sequenced (7, 42, 43, 46). The first and best-known CPV, canine oral PV (COPV), affects mostly juvenile dogs and causes warts with a typical cauliflower-like appearance located in the oral cavity and other mucous membranes (32). Since COPV-affected dogs recover spontaneously and specific antibodies protect them from reinfection, COPV infection is not considered a life-threatening condition (32). Although COPV infection is endemic in most dog populations, there never was a great demand for serological surveys, and systematic serological data concerning the prevalence of COPV infection are thus far unavailable. A distinct CPV, designated CPV2, was cloned only recently from a footpad lesion of a golden retriever (46). CPV2 did not induce oral papillomas in immunocompetent animals and caused a type-specific immune response in experimentally inoculated dogs (7, 42, 43, 46). The clinical significance of CPV2 remains to be elucidated. Recently, our laboratory has identified a novel CPV, designated CPV3, and sequenced its genome. This novel CPV was detected in lesions from a dog suffering from canine EV and squamous cell carcinoma (42). Based on a phylogenetic analysis, CPV3 represents the prototype of a new genus and differs significantly from the classical COPV. Finally, an additional CPV, named CPV4, was isolated from the lesions of several pugs suffering from numerous persisting pigmented plaques (28, 43).
Several clinical case reports and some epidemiological evidence suggest that CPVs may play a role in canine cancers (3, 4, 12, 31, 36, 38, 40, 44, 47). Due to its association with canine EV, CPV3 was hypothesized to be a candidate agent for certain forms of canine cancers. However, data concerning the prevalence of CPV3 in the dog population are not yet available and serological tests and reference materials for this purpose are lacking. Therefore, we set out to address these issues.
Typically, PVs do not readily replicate in conventional cell cultures but serological assays can be based on heterologously expressed viral antigens. Structural proteins L1 and L2 are known to assemble into the PV capsid (16). The L1 protein forms pentameric subunits, and 72 of these subunits form one capsid. In conventional viral particles, the L2 protein is present only in minor amounts compared to L1. Of note, heterologously expressed L1 protein is able to form virus-like particles (VLPs) in the absence of L2 (33). L1 is considered to be immunodominant, and L1-based VLPs have been shown to be highly immunogenic in the absence of L2. Therefore, L1 VLPs have been successfully used for vaccination and as antigens in type-specific serological assays (9, 20). Most ELISAs for HPVs are based on the detection of L1-specific antibodies (10, 19, 24, 25, 37). It has been reported that pentameric L1 capsid subunits and whole VLPs may be produced equally well from baculovirus- or Escherichia coli-based expression systems (34).
In the present study, we hypothesized that L1-GST (glutathione S-transferase) fusion proteins, expressed in E. coli, could be used as ELISA antigens in order to detect type-specific antibodies in a collection of dog sera, which would allow us to study the seroprevalence of CPV3 and COPV in dogs. Indeed, our results indicate that CPV3 may be as widely distributed in dogs as COPV in certain geographic areas.
MATERIALS AND METHODS
Plasmid construction and protein expression.
COPV and CPV3 genomic clones (42) in Bluescript vectors (Stratagene, La Jolla, CA) were used as PCR templates. COPV and CPV3 L1 coding sequences, both truncated by 10 codons at the 5′ end, were amplified by PCR with Phusion DNA polymerase (Finzyme, Espoo, Finland). The primers designed for this purpose were COPV L1 forward (5′-GGA ATT CTT TTA CCT TCC ACC ACA GCC C-3′), COPV L1 reverse (5′-GGA ATT CTT ATT TGC GTT TGC GTT TCA C-3′), CPV3 L1 forward (5′-GGA ATT CCT TTT CCT GCC CCC TAA C-3′), and CPV3 L1 reverse (5′-GGA ATT CTT ATT TGT TTT TTT TCC T-3′). EcoRI recognition sites included at the 5′ ends of the primers were used to clone the amplified DNA into the EcoRI site of pGEX-6P-1 (Pharmacia, Uppsala, Sweden). Protein expression was performed with E. coli strain BL21 as described previously, with minor modifications (33). Instead of using a high-pressure homogenizer, we lysed the cells by passing them 10 times through a 25-gauge needle and sonicating them.
GST capture ELISA.
Polysorb 96-well plastic plates (Nunc, Roskilde, Denmark) were coated at 4°C overnight with 200 ng of glutathione-casein (kindly provided by Martin Müller, DKFZ, Heidelberg, Germany) per well in 50 mM sodium carbonate buffer, pH 9.6. After washing, the plates were incubated for 1 h at 37°C with blocking buffer (0.2% [wt/vol] casein-0.3% [vol/vol] Tween 20 in phosphate-buffered saline) and then incubated with the cleared lysates from E. coli expressing GST-tagged proteins diluted in blocking buffer for 1 h at 37°C.
Sera to be tested were diluted 1:500 and incubated with a bacterial culture equivalent of 5 μl of total protein lysate of untransformed E. coli strain BL21 for 1 h at 4°C to block reactions with contaminating E. coli protein (33, 34). The antigen-coated ELISA plates were then incubated with diluted and preincubated sera or, for evaluation purposes, with goat anti-GST antibody (GE Healthcare, Piscataway, NJ) diluted 1:100 in blocking buffer. After washing the plates, we detected primary antibodies with horseradish peroxidase (HRP)-conjugated rabbit anti-dog immunoglobulin G (Sigma, Buchs, Switzerland) diluted 1:10,000 or HRP-conjugated anti-goat antibody diluted 1:1,000 (Southern Biotech, Birmingham, AL) in blocking buffer applied for 1 h at 37°C. Plates were finally washed six times, and substrate [78 mM acetic acid-24 mM CH3COONa · 3H2O-50 mM NaH2PO4 · 1H2O-2 mM 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS; Roche, Rotkreuz, Switzerland) and 1.25 mM H2O2 applied shortly before use] was added. After 45 min, absorbance was measured at 405 nm in a Multiscan RC automated plate reader (Thermo Labsystems, Vantaa, Finland). All washing steps were carried out three times with phosphate-buffered saline containing 0.3% (vol/vol) Tween 20.
All dog sera were tested in duplicate against the antigens COPV L1-GST, CPV3 L1-GST, and GST alone. On every 96-well plate, the same COPV- and CPV3-positive serum was included as a positive control. The reaction of COPV antiserum to COPV antigen served as an overall positive control, while its reaction to CPV3 antigen served as an overall negative control. Reaction of goat anti-GST antibody as the primary antibody and an HRP-conjugated anti-goat secondary antibody against the antigens served as a loading control on all plates. Six wells per plate were used as conjugate controls, which means that they were kept in plain ELISA buffer while the other wells were incubated with primary sera.
The coating of all of the ELISA plates used during the screening was evaluated by testing two wells of each antigen per plate with goat anti-GST antibody as the primary antibody and HRP-conjugated anti-goat antibody as the secondary antibody.
Sera.
Sera from six dogs affected by PV infections were included in the study to serve as controls. Two of these dogs with classical COPV oral lesions, a confirming histopathological diagnosis, and positive PCR and rolling-circle amplification tests were sampled at different time points. The first dog gave blood at the initial presentation (florid clinical lesions, serum 1a) and 3 weeks later (serum 1b). The second was also sampled at the initial presentation (serum 2a) and 3 weeks later (no visible lesions left, serum 2b) but also 5 months after the first presentation (serum 2c). Furthermore, three pugs with CPV4-associated pigmented plaques (sera 3, 4, and 5) and one dog with CPV3-associated pigmented plaques (serum 6) were included. The presence of viral DNA in affected tissues of these dogs was confirmed previously by PCR and rolling-circle amplification (42, 43).
Two large sets of sera were subjected to the ELISA for screening (for additional information, see Table S1 in the supplemental material). The first set, consisting of 229 dog sera, was collected at the veterinary teaching hospital, Vetsuisse Faculty, University of Zurich, Zurich, Switzerland, between 23 January and 1 April 2006. The sera were taken from patients presented for various conditions unrelated to PV infections. The second set containing 315 sera was originally collected at three different veterinary practices in Gauteng Province, South Africa, between May and June 2005 in order to determine the prevalence of antibodies against canine herpesvirus in the serum of dogs older than 1 year in breeding kennels (29). Between 3 and 20 samples from different dogs were available from each of 36 kennels. The mean age of the Swiss dogs was 6.8 ± 4.2 years, while the mean age of the South African dogs was 4.2 ± 2.6 years. All of the sera were stored at −20°C until testing.
Statistical analysis.
To analyze and display the reactions of control sera, standard box plots were constructed by using SPSS software (SPSS Schweiz AG, Zurich, Switzerland). The correlation of loading controls was analyzed and displayed in the form of a linear regression curve with Microsoft Excel software. For determination of the significance of differences among differing prevalences, a Fisher exact test (41) was used. P values below 0.05 were classified as significant.
RESULTS
Antigen coating.
Since CPVs cannot be grown in cell cultures for ELISA antigen production, the major capsid L1 proteins of COPV and CPV3 were prokaryotically expressed as GST fusion proteins. ELISA plates precoated with glutathione-casein were used to capture these GST fusion proteins or GST alone in separate wells of the plate. Serial dilutions of each antigen ranging from 0.2 × 2° to 0.2 × 214 were used to identify the antigen dilution giving equal reactions with anti-GST antibody in all three antigen preparations as a surrogate determinant for identical L1 amounts. Indeed, GST-containing antigen bound to the plate provided a dose-dependent reaction upon testing with goat anti-GST serum and an anti-goat-HRP conjugate. The antigen concentrations corresponding to the highest common reaction were chosen for coating the ELISA plates in all further experiments.
Preliminary characterization of reference sera.
In the absence of commercially available type-specific antisera, samples from dogs with a confirmed history (see Materials and Methods) of COPV, CPV3, or CPV4 infection were used to establish ELISAs for the detection of antibodies against COPV L1 and CPV3 L1. Serum 1a from a dog with fresh oral papillomas yielding COPV DNA barely reacted with any of the antigens and was therefore considered to be negative for both COPV and CPV3. Serum 1b, taken from the same dog 3 weeks later, upon regression of oral warts, gave a clear reaction with the COPV antigen but not with either the CPV3 antigen or GST. Similarly, three consecutive serum samples from a different dog with oral warts (sera 2a, 2b, and 2c) reacted increasingly over time with the COPV antigen but not with CPV3 L1 or GST. Based on case history and availability, serum 2b was used as a preliminary positive reference serum for COPV. Simultaneously, due to its negative reaction against CPV3 antigen, serum 2b served as a negative reference for this antigen. Serum taken from a dog with a known history of CPV3 infection (serum 6) reacted strongly with the CPV3 antigen, weakly with the COPV antigen, and barely with GST alone. This serum was therefore used as a positive reference serum for CPV3. Finally, sera from three dogs with history of CPV4 infection barely reacted with all three antigens, suggesting that these sera did not contain substantial amounts of antibodies against either antigen. Overall, these results indicated that our L1-GST fusion proteins were able to reveal type-specific antibodies against COPV or CPV3. Moreover, a collection of type-specific reference sera was now available for further studies.
Testing of dog sera from two geographically distinct regions.
Two available sets of dog sera were screened for antibodies against either virus, a set of Swiss dog sera and a set of dog sera from South Africa. As shown in Fig. 1A and B, a wide range of reactions was detected in the two dog populations. Controls were included for each single ELISA plate in order to better characterize and establish the ELISA.
FIG. 1.
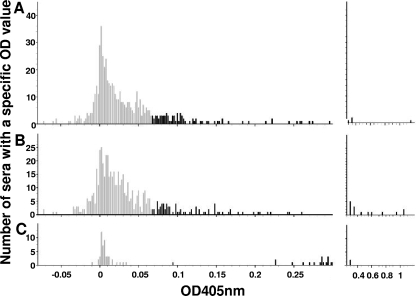
ELISA reactions of dog sera against L1-GST fusion proteins. Panels A (CPV3 L1 antigen) and B (COPV L1 antigen) show the reactions of 229 dog sera from Switzerland and 315 dog sera from South Africa. Each serum is represented twice, once reacting with CPV3 antigen and once reacting with COPV antigen. In panel C, reference serum reactions are shown. On the x axis, the corrected OD values are shown (see Materials and Methods). Sera giving high OD values are presented on the right, where the scale of the x axis is adjusted to fit all of the results. Sera giving ODs below 0.068 (see Table 1) are in gray, and those giving ODs above 0.068 are in black. The y axis provides the number of sera with a specific OD value.
Analysis of antigen coating.
It was important to measure and analyze antigen coating in order to ensure comparison of the results obtained with different ELISA plates. Therefore, a number of wells in each plate were reserved for reactions with an anti-GST antibody. As shown in Fig. 2, the values measured for COPV and CPV3 in each plate were plotted against each other and the linear regression was determined. The regression was 0.9698, the slope of the line was 1.0243, and the abscissa was 0.0029. The slope of nearly 1.0 and the (regression) line through the origin of the coordinate system suggest that we had indeed coated the plates with equal amounts of these two antigens and plate-to-plate comparisons were possible.
FIG. 2.
Plot of reactions (corrected OD) against adsorbed L1-GST fusion proteins (CPV3 on the x axis, COPV on the y axis) measured on each plate with anti-GST monoclonal antibody. Linear regression parameters: y = 1.0243x + 0.0029; R2 = 0.9698.
To compensate for variations associated with plate-to-plate differences in the final evaluation of the dog sera, the optical density (OD) values of individual dog serum samples were corrected by multiplication by a plate correction factor, calculated as the ratio of the individual average plate control (mean of six wells) to the lowest average plate control.
Analysis of reference serum performance.
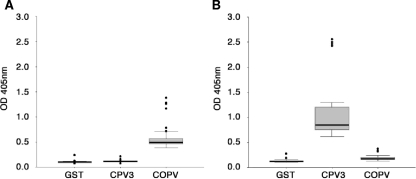
Duplicates of the newly established COPV and CPV3 reference sera were tested on all 49 of the ELISA plates used in the present study and analyzed for reactivity against the antigens GST, COPV L1-GST, and CPV3 L1-GST. The results are shown as box plots in Fig. 3. Both reference sera reacted only slightly with the GST antigen, with median OD values of 0.085 (COPV serum) and 0.099 (CPV3 serum). However, the positive COPV reference serum reacted with a median OD value of 0.408 against COPV antigen and with a median OD value of 0.092 against CPV3 (Fig. 3A). The serum used as a CPV3 positive control produced a median OD value of 0.695 with the CPV3 L1 antigen, while its reaction against COPV produced a median OD of 0.146 (Fig. 3B).
FIG. 3.
Standard box plots, constructed with SPSS, summarizing the reactions of reference sera against different antigens. (A) Summary of reactions (corrected OD, y axis) of COPV reference serum measured in parallel assays against GST, CPV3 L1, and COPV L1. (B) Same assay with CPV3 reference serum.
Thus, the reference sera gave consistent results, suggesting that the cutoff between a positive and a negative reaction could be close to an OD of 0.100. Under these conditions, the CPV3 reference serum appeared slightly positive against the COPV antigen. Therefore, the reactions against GST were considered the background to be subtracted to obtain the final value. Thus, for the final analysis of the data, the plate-corrected GST reaction ODs were subtracted from the corresponding values obtained with the COPV and CPV3 L1 antigens.
Evaluation of cutoff values.
The results of three variants to determine cutoff values are summarized in Table 1. In variant 1, the cutoff was set equal to the highest reaction of the negative reference serum (0.0467), which deemed 84.3% of the Swiss dog sera and 49.8% of the South African dog sera negative. In variant 2, the cutoff was set at the lowest reaction of the positive reference serum (0.0907). Under these conditions, 92.6% of the Swiss dog sera and 78.7% of the South African dog sera were considered negative. In variant 3, the histogram shown in Fig. 1C was applied for the present purpose by using a value midway between the weakest positive control reaction and the strongest negative control reaction as the cutoff (0.0687). Under these conditions, 89.5% of the Swiss dog sera and 68.3% of the South African dog sera were considered negative.
TABLE 1.
Distribution of positive and negative reactions among the groups with alternative cutoff values
| Country (no. of samples) and cutoff | No. (%) of samples
|
|||
|---|---|---|---|---|
| Negative | COPV positive | CPV3 positive | COPV + CPV3 positive | |
| Switzerland (229) | ||||
| 0.0467 | 193 (84.3) | 26 (11.4) | 3 (1.3) | 7 (3.0) |
| 0.0687 | 205 (89.5) | 21 (9.2) | 0 (0.0) | 3 (1.3) |
| 0.0907 | 212 (92.6) | 15 (6.5) | 0 (0.0) | 2 (0.9) |
| South Africa (315) | ||||
| 0.0467 | 157 (49.8) | 15 (4.8) | 39 (12.4) | 104 (33.0) |
| 0.0687 | 215 (68.3) | 15 (4.8) | 31 (9.8) | 54 (17.1) |
| 0.0907 | 248 (78.7) | 14 (4.5) | 24 (7.6) | 29 (9.2) |
COPV and CPV3 prevalence.
COPV and CPV3 seem to circulate both in Switzerland and in South Africa. However, the Swiss dog serum collection was not representative, which prevents a quantitative estimation of the seroprevalence of either COPV or CPV3 in dogs in Switzerland.
In contrast, the pool of South African dog sera was representative for breeding dogs in Gauteng Province and included several samples from the same premises, which allows further interpretations. In Gauteng Province, 27 (75%) of the 36 kennels harbored at least one COPV-positive animal while 32 (89%) harbored at least one CPV3-positive one. The range of positive tested sera among the individual kennels was 0 to 57% for COPV, with an average of 20%, in the entire pool and 0 to 67% for CPV3, with an average of 26%.
Interestingly, a very high percentage of CPV-seropositive dogs was found to be of the Irish setter (>50%) or of the Yorkshire terrier breed (>35%), whereas the bulldog breed seemed to be underrepresented among the dogs seropositive for COPV. To address these observations by statistical methods, the prevalences in two representative breeds from the Gauteng Province group with more than 20 available samples were analyzed with Fisher's exact test. The results are shown in Table 2. Significant deviations in COPV prevalence were found for Yorkshire terriers in comparison to the remaining population and the tested bulldogs. Thus, there may be a breed-specific component in the context of CPVs.
TABLE 2.
Significance of breed-associated seroprevalences among tested bulldogs and Yorkshire terriers from Gauteng Province kennels according to Fisher's exact test
| Breeds comparedb |
P value
|
|
|---|---|---|
| CPV3 seropositivity | COPV seropositivity | |
| Yorkshire terriers vs other dogs | 0.087 | 0.005a |
| Bulldogs vs other dogs | 0.194 | 0.163 |
| Yorkshire terriers vs bulldogs | 0.416 | 0.032a |
Statistically significant difference.
Thirty-one Yorkshire terriers and 21 bulldogs were tested.
DISCUSSION
Although CPVs apart from COPV had been postulated for a long time, novel members of this virus family in dogs, i.e., CPV2, CPV3, and CPV4, have been detected and characterized only recently (42-46). Since oral papilloma is hardly considered a life-threatening disease, there was never a great demand for serological COPV surveys of dogs and corresponding serological data are simply not available. In contrast, some serological data concerning CPVs have originated in the context of experimental immunization (11, 39, 45, 46). Since some of the novel CPVs, i.e., CPV3, have been detected in association with more malignant diseases, there was a need for serological surveys, which may shed light on the prevalence of these viruses among the dog populations of various countries.
Therefore, we set out to establish an ELISA for detecting antibodies against two different types of CPVs, i.e., COPV, the one about which the most is known, and CPV3, the one which is associated with the most malignant disease. However, the difficulties to be overcome included (i) the generation of antigens from nongrowing virus, (ii) coating plates with even amounts of antigen to allow for comparable analysis, (iii) generation and characterization of useful reference sera, (iv) analysis of dog sera and determination of useful cutoff values, and (v) interpretation of data with regard to the prevalence of the viruses of interest.
Difficulties i and ii.
The antigen used in our ELISA was prepared from heterologously expressed CPV capsid protein L1 N terminally fused to GST. This method is frequently used to generate ELISA antigens since the subsequent purification steps are well established (13, 17, 22, 33, 34). Others have shown that GST-tagged L1 proteins spontaneously form pentamers and VLPs (5), and it has been reported that this capture technique equals in its results the alternative system of baculovirus-expressed VLPs (33). Indeed, type-specific results were obtained throughout our work, which implies that the antigen preparations were adequate for the present purpose.
The N-terminal GST tag of the ELISA antigens was not only used for binding of the antigen to the ELISA plate and simultaneous antigen purification but also served as an epitope to measure the amount of bound antigen per well with anti-GST antibodies. Although it is technically not feasible to coat each single well with essentially the same amount of antigen, control measures on each plate provided confidence that comparable amounts of the two different L1 antigens were being used for analysis of the dog sera (Fig. 2). Thus, a comparison between reactions against the different fusion proteins was reasonable.
Difficulty iii.
According to the reactions of our reference sera, the newly developed ELISAs were able to discriminate between dogs that had recently undergone COPV and CPV3 infections, respectively, whereas sera from dogs with CPV4 infections did not react against either COPV L1 or CPV3 L1. These results provided confidence that the ELISA was able to discriminate specific reactions against the two virus types. Therefore, also the newly characterized reference sera were considered type specific. However, CPV3 represents a novel virus and cross-reactivity to any known or unknown PV has to be kept in mind as a possibility. Yet, based on similar experiments, others have concluded that COPV and CPV2 are serologically distinct (46).
Difficulty iv.
Despite the confidence of having established valuable tools for seroepidemiological studies regarding COPV and CPV3, defining widely acceptable cutoff values remained a challenge that could not unambiguously be solved at the present state of knowledge. Therefore, we decided to analyze the reactions of two different sets of dog sera against COPV L1 and CPV3 L1. The results (Fig. 1) show that a certain number of sera within each set overlapped with the reactions of the negative reference serum, whereas a multitude of sera reacted clearly more strongly than the negative sera. These observations support the conclusion that some of these sera reacted specifically against one or both of the L1 proteins whereas other sera reacted negatively.
A simple method to set a cutoff value is to define the highest negative control reaction as the cutoff and consider all higher reactions positive. This choice results in relatively high sensitivity at the cost of specificity. An analogous method with the lowest positive reaction as the cutoff leads to higher specificity at the cost of sensitivity. In contrast to the situation in the dog, ELISAs for detection of antibodies against human PVs are frequently used and several methods to define cutoff values have been suggested. Three of those were also tested in the present work, since they have been subjected to comparative analysis (19). In our case, histogram analysis (variant 3 in our results) seemed to be the most preferable of the methods tested because of its feasibility, simplicity, and balancing influence on specificity and sensitivity.
Difficulty v.
One set of tested sera was obtained from randomly chosen canine patients of a veterinary teaching hospital in Switzerland and is therefore not quantitatively representative of the Swiss dog population. One may conclude, however, that not only COPV but also CPV3 is prevalent in Switzerland. This was not overly surprising, since CPV3 was first detected in Switzerland (42). However, conclusions about the prevalence of CPV3-infected dogs were not possible and further studies in various countries should be done to address this issue.
In contrast, the second set, comprising 315 sera from 36 kennels, consisted of a representative sample of breeding dogs in the South African province of Gauteng. Almost the same set had previously been used for determining the seroprevalence of canine herpesvirus 1 in Gauteng Province (29). In that study, canine herpesvirus 1-positive dogs were found in 45% of the kennels and the prevalence of seropositive dogs in affected kennels was close to 40%. Interestingly, both COPV (75% of kennels) and CPV3 (89% of kennels) seem to be even more prevalent in the same population. In this context, it has to be considered that little is known concerning the duration of seropositivity against various CPVs.
As shown in Table 1, the number of individual CPV-positive dog sera from the Gauteng Province set ranged from 25 to 50%, depending on the cutoff value applied. The largest fraction of the positive sera (10 to 30%) reacted against both COPV L1 and CPV3 L1. These dogs could have had contact with both viruses, might have developed antibodies against viruses closely related to COPV and/or CPV3, or could have cross-reacting antibodies in their serum. Our study does not allow us to completely rule out such possibilities, but surveys of humans have demonstrated that coinfections with more than one PV are very common (15, 25).
It was tempting to analyze our data for statistically significant correlations between antibodies against CPVs and factors such as dog breed, age, gender, and kennel size. For example, as indicated in Table 2, the Yorkshire terrier was found to be significantly overrepresented among the COPV-positive dogs tested. These tested Yorkshire terriers, however, originated from only three kennels with differing prevalences, and thus, possible cohort effects have to be kept in mind (18, 27). The observations nevertheless indicate that breed disposition may actually play a role in the prevalence of various CPVs. Indeed, reports strongly suggest that genetic predisposition should be considered in the context of PV-associated diseases (1, 6, 26, 43). However, our serum pools were not designed to warrant extensive stratification of the data. Therefore, we provide additional data for potential analyses only as supplemental material. For an overview of the breed distribution of the animals in the two sets analyzed, see Table S1 in the supplemental material, and for the distribution of positive tested sera from the perspective of age and gender, see Fig. S2 in the supplemental material. For the number of CPV-seropositive dogs in relation to the individual kennel sizes, see Table S3 in the supplemental material. We consider their presentation important in view of further analyses of these aspects. More studies in this direction are certainly warranted.
In summary, our work provides the first insight into the seroprevalence of CPV3. Serological reactors against this novel PV were detected on two continents. Our data suggest that there is a need for further seroepidemiological surveys in different countries and clinical studies related to the potential diseases caused by CPV3. With the newly developed diagnostic tools at hand, such studies will be feasible.
Supplementary Material
Acknowledgments
This work was supported by the Canton of Zurich and the Dorothea and Robert Wyler donation.
Footnotes
Published ahead of print on 26 November 2008.
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1.Aaltonen, L. M., R. W. Chen, S. Roth, A. A. Makitie, H. Rihkanen, A. Vaheri, and L. A. Aaltonen. 2001. Role of TP53 P72R polymorphism in human papillomavirus associated premalignant laryngeal neoplasm. J. Med. Genet. 38327.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonsson, A., O. Forslund, H. Ekberg, G. Sterner, and B. G. Hansson. 2000. The ubiquity and impressive genomic diversity of human papillomavirus suggest a commensalic nature of these viruses. J. Virol. 7411636-11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bregman, C. L., R. S. Hirth, J. P. Sundberg, and E. F. Christensen. 1987. Cutaneous neoplasms in dogs associated with canine oral papillomavirus vaccine. Vet. Pathol. 24477-487. [DOI] [PubMed] [Google Scholar]
- 4.Callan, M. B., D. Preziosi, and E. Mauldin. 2005. Multiple papillomavirus-associated epidermal hamartomas and squamous cell carcinomas in situ in a dog following chronic treatment with prednisone and cyclosporine. Vet. Dermatol. 16338-345. [DOI] [PubMed] [Google Scholar]
- 5.Chen, X. S., R. L. Garcea, I. Goldberg, G. Casini, and S. C. Harrison. 2000. Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Mol. Cell 5557-567. [DOI] [PubMed] [Google Scholar]
- 6.Coussens, L. M., D. Hanahan, and J. M. Arbeit. 1996. Genetic predisposition and parameters of malignant progression in K14-HPV16 transgenic mice. Am. J. Pathol. 1491899-1917. [PMC free article] [PubMed] [Google Scholar]
- 7.Delius, H., M. A. Van Ranst, A. B. Jenson, H. zur Hausen, and J. P. Sundberg. 1994. Canine oral papillomavirus genomic sequence: a unique 1.5-kb intervening sequence between the E2 and L2 open reading frames. Virology 204447-452. [DOI] [PubMed] [Google Scholar]
- 8.de Villiers, E.-M., C. Fauquet, T. R. Broker, H.-U. Bernard, and H. zur Hausen. 2004. Classification of papillomaviruses. Virology 32417-27. [DOI] [PubMed] [Google Scholar]
- 9.Dillner, J. 1999. The serological response to papillomaviruses. Semin. Cancer Biol. 9423-430. [DOI] [PubMed] [Google Scholar]
- 10.Dunne, E. F., K. L. Karem, M. R. Sternberg, K. M. Stone, E. R. Unger, W. C. Reeves, and L. E. Markowitz. 2005. Seroprevalence of human papillomavirus type 16 in children. J. Infect. Dis. 1911817-1819. [DOI] [PubMed] [Google Scholar]
- 11.Ghim, S., J. Newsome, J. Bell, J. P. Sundberg, R. Schlegel, and A. B. Jenson. 2000. Spontaneously regressing oral papillomas induce systemic antibodies that neutralize canine oral papillomavirus. Exp. Mol. Pathol. 68147-151. [DOI] [PubMed] [Google Scholar]
- 12.Goldschmidt, M. H., J. S. Kennedy, D. R. Kennedy, H. Yuan, D. E. Holt, M. L. Casal, A. M. Traas, E. A. Mauldin, P. F. Moore, P. S. Henthorn, B. J. Hartnett, K. I. Weinberg, R. Schlegel, and P. J. Felsburg. 2006. Severe papillomavirus infection progressing to metastatic squamous cell carcinoma in bone marrow-transplanted X-linked SCID dogs. J. Virol. 806621-6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan, M., H. Y. Chen, S. Y. Foo, Y. J. Tan, P. Y. Goh, and S. H. Wee. 2004. Recombinant protein-based enzyme-linked immunosorbent assay and immunochromatographic tests for detection of immunoglobulin G antibodies to severe acute respiratory syndrome (SARS) coronavirus in SARS patients. Clin. Diagn. Lab. Immunol. 11287-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harwood, C. A., and C. M. Proby. 2002. Human papillomaviruses and non-melanoma skin cancer. Curr. Opin. Infect. Dis. 15101-114. [DOI] [PubMed] [Google Scholar]
- 15.Heim, K., A. Widschwendter, H. Szedenik, A. Geier, N. D. Christensen, A. Bergant, N. Concin, and R. Hopfl. 2005. Specific serologic response to genital human papillomavirus types in patients with vulvar precancerous and cancerous lesions. Am. J. Obstet. Gynecol. 1921073-1083. [DOI] [PubMed] [Google Scholar]
- 16.Howley, P. M., and D. R. Lowy. 2001. Papillomaviruses and their replication, p. 2197-2264. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Baltimore, MD. [Google Scholar]
- 17.Hwang, G. Y., C. Y. Lin, L. M. Huang, Y. H. Wang, J. C. Wang, C. T. Hsu, S. S. Yang, and C. C. Wu. 2003. Detection of the hepatitis B virus X protein (HBx) antigen and anti-HBx antibodies in cases of human hepatocellular carcinoma. J. Clin. Microbiol. 415598-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones, O. R., M. J. Crawley, J. G. Pilkington, and J. M. Pemberton. 2005. Predictors of early survival in Soay sheep: cohort-, maternal- and individual-level variation. Proc. Biol. Sci. 2722619-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karem, K. L., A. C. Poon, C. Bierl, R. Nisenbaum, and E. Unger. 2002. Optimization of a human papillomavirus-specific enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 9577-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirnbauer, R., F. Booy, N. Cheng, D. R. Lowy, and J. T. Schiller. 1992. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Natl. Acad. Sci. USA 8912180-12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lillo, F. B. 2005. Human papillomavirus infection and its role in the genesis of dysplastic and neoplastic lesions of the squamous epithelia. New Microbiol. 28111-118. [PubMed] [Google Scholar]
- 22.Liu, Q., L. Wang, P. Willson, B. O'Connor, J. Keenliside, M. Chirino-Trejo, R. Melendez, and L. Babiuk. 2002. Seroprevalence of porcine circovirus type 2 in swine populations in Canada and Costa Rica. Can. J. Vet. Res. 66225-231. [PMC free article] [PubMed] [Google Scholar]
- 23.Majewski, S., and S. Jablonska. 1995. Epidermodysplasia verruciformis as a model of human papillomavirus-induced genetic cancer of the skin. Arch. Dermatol. 1311312-1318. [PubMed] [Google Scholar]
- 24.Marais, D. J., R. C. Rose, C. Lane, P. Kay, J. Nevin, L. Denny, R. Soeters, C. M. Dehaeck, and A.-L. Williamson. 2000. Seroresponses to human papillomavirus types 16, 18, 31, 33, and 45 virus-like particles in South African women with cervical cancer and cervical intraepithelial neoplasia. J. Med. Virol. 60403-410. [DOI] [PubMed] [Google Scholar]
- 25.Marais, D. J., C. C. Sampson, M. I. Urban, F. Sitas, and A.-L. Williamson. 2007. The seroprevalence of IgG antibodies to human papillomavirus (HPV) types HPV-16, HPV-18, and HPV-11 capsid-antigens in mothers and their children. J. Med. Virol. 791370-1374. [DOI] [PubMed] [Google Scholar]
- 26.Mitra, S., C. Misra, R. K. Singh, C. K. Panda, and S. Roychoudhury. 2005. Association of specific genotype and haplotype of p53 gene with cervical cancer in India. J. Clin. Pathol. 5826-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgenstern, H., and D. Thomas. 1993. Principles of study design in environmental epidemiology. Environ. Health Perspect. 101(Suppl. 4)23-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagata, M., H. Nanko, A. Moriyama, T. Washizu, and T. Ishida. 1995. Pigmented plaques associated with papillomavirus infection in dogs: is this epidermodysplasia verruciformis? Vet. Dermatol. 6179-186. [DOI] [PubMed] [Google Scholar]
- 29.Nöthling, J. O., D. Hussy, D. Steckler, and M. Ackermann. 2008. Seroprevalence of canine herpesvirus in breeding kennels in the Gauteng Province of South Africa. Theriogenology 69276-282. [DOI] [PubMed] [Google Scholar]
- 30.Pfister, H. 2003. Chapter 8: human papillomavirus and skin cancer. J. Natl. Cancer Inst. Monogr. 200352-56. [DOI] [PubMed] [Google Scholar]
- 31.Schwegler, K., J. H. Walter, and R. Rudolph. 1997. Epithelial neoplasms of the skin, the cutaneous mucosa and the transitional epithelium in dogs: an immunolocalization study for papillomavirus antigen. Zentralbl. Veterinaermed. A 44115-123. [DOI] [PubMed] [Google Scholar]
- 32.Scott, D. W., W. H. Miller, and C. E. Griffin. 2001. Viral, rickettsial and protozoal diseases, p. 517-542. In D. W. M. Scott, W. H. Miller, and C. E. Griffin (ed.), Muller & Kirk's small animal dermatology, 6th ed. W. B. Saunders, Philadelphia, PA.
- 33.Sehr, P., M. Muller, R. Hopfl, A. Widschwendter, and M. Pawlita. 2002. HPV antibody detection by ELISA with capsid protein L1 fused to glutathione S-transferase. J. Virol. Methods 10661-70. [DOI] [PubMed] [Google Scholar]
- 34.Sehr, P., K. Zumbach, and M. Pawlita. 2001. A generic capture ELISA for recombinant proteins fused to glutathione S-transferase: validation for HPV serology. J. Immunol. Methods 253153-162. [DOI] [PubMed] [Google Scholar]
- 35.Sterling, J. C. 2005. Human papillomaviruses and skin cancer. J. Clin. Virol. 32(Suppl. 1)S67-S71. [DOI] [PubMed] [Google Scholar]
- 36.Stokking, L. B., E. J. Ehrhart, C. A. Lichtensteiger, and K. L. Campbell. 2004. Pigmented epidermal plaques in three dogs. J. Am. Anim. Hosp. Assoc. 40411-417. [DOI] [PubMed] [Google Scholar]
- 37.Stone, K. M., K. L. Karem, M. R. Sternberg, G. M. McQuillan, A. D. Poon, E. R. Unger, and W. C. Reeves. 2002. Seroprevalence of human papillomavirus type 16 infection in the United States. J. Infect. Dis. 1861396-1402. [DOI] [PubMed] [Google Scholar]
- 38.Sundberg, J. P., R. E. Junge, and W. D. Lancester. 1984. Immunoperoxidase localization of papillomaviruses in hyperplastic and neoplastic epithelial lesions in animals. Am. J. Vet. Res. 451441-1446. [PubMed] [Google Scholar]
- 39.Suzich, J. A., S. J. Ghim, F. J. Palmer-Hill, W. I. White, J. K. Tamura, J. A. Bell, J. A. Newsome, A. B. Jenson, and R. Schlegel. 1995. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc. Natl. Acad. Sci. USA 9211553-11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teifke, J. P., C. V. Löhr, and H. Shirasawa. 1998. Detection of canine oral papillomavirus-DNA in canine oral squamous cell carcinomas and p53 overexpressing skin papillomas of the dog using the polymerase chain reaction and non-radioactive in situ hybridization. Vet. Microbiol. 60119-130. [DOI] [PubMed] [Google Scholar]
- 41.Thrusfield, M. V. 2005. Demonstrating association, p. 247-265. In M. Thrusfield (ed.), Veterinary epidemiology, 3rd ed. Blackwell Publishing Professional, Ames, IA.
- 42.Tobler, K., C. Favrot, G. Nespeca, and M. Ackermann. 2006. Detection of the prototype of a potential novel genus in the family Papillomaviridae in association with canine epidermodysplasia verruciformis. J. Gen. Virol. 873551-3557. [DOI] [PubMed] [Google Scholar]
- 43.Tobler, K., C. Lange, D. N. Carlotti, M. Ackermann, and C. Favrot. 2008. Detection of a novel papillomavirus in pigmented plaques of four pugs. Vet. Dermatol. 1921-25. [DOI] [PubMed] [Google Scholar]
- 44.Watrach, A. M., E. Small, and M. T. Case. 1970. Canine papilloma: progression of oral papilloma to carcinoma. J. Natl. Cancer Inst. 45915-920. [PubMed] [Google Scholar]
- 45.Yuan, H., P. A. Estes, Y. Chen, J. Newsome, V. A. Olcese, R. L. Garcea, and R. Schlegel. 2001. Immunization with a pentameric L1 fusion protein protects against papillomavirus infection. J. Virol. 757848-7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan, H., S. Ghim, J. Newsome, T. Apolinario, V. Olcese, M. Martin, H. Delius, P. Felsburg, B. Jenson, and R. Schlegel. 2007. An epidermotropic canine papillomavirus with malignant potential contains an E5 gene and establishes a unique genus. Virology 35928-36. [DOI] [PubMed] [Google Scholar]
- 47.Zaugg, N., G. Nespeca, B. Hauser, M. Ackermann, and C. Favrot. 2005. Detection of novel papillomaviruses in canine mucosal, cutaneous and in situ squamous cell carcinomas. Vet. Dermatol. 16290-298. [DOI] [PubMed] [Google Scholar]
- 48.zur Hausen, H. 1996. Papillomavirus infections—a major cause of human cancers. Biochim. Biophys. Acta 1288F55-F78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.