Abstract
We have studied the evolution of the gamma interferon (IFN-γ) and interleukin 10 (IL-10) responses after Mycobacterium ulcerans sonicate stimulation of whole blood from patients with early M. ulcerans lesions during treatment with rifampin and streptomycin for 8 weeks. Among the 26 patients, secretion of IFN-γ increased during treatment, with a significant increase at 4 weeks and a further increase after 8 weeks overall. The increase was more rapid in patients with large or ulcerative lesions, becoming significant by 4 weeks. For small lesions, there was only a minor increase, which did not reach significance. There was no significant change in the median IL-10 response during antibiotic therapy, and there was no inverse correlation between IFN-γ and IL-10 responses. These results demonstrate that an IFN-γ secretory response to M. ulcerans developed, independently of IL-10 secretion, in patients whose M. ulcerans disease healed during antibiotic therapy.
Mycobacterium ulcerans disease is common in parts of the tropics, affecting mainly children below the age of 15, often in remote rural areas with little or no access to health services (24). Early infection manifests as a painless nodule, a firm plaque, or an edematous lesion which breaks down into the chronic and potentially disfiguring ulcers characteristic of M. ulcerans disease (5). The conventional mode of management is surgical excision of lesions with skin grafting. However, treatment of early lesions with rifampin and streptomycin has been shown to kill M. ulcerans in human tissue (4), and increasing clinical experience suggests that such lesions heal after treatment for 8 weeks, with a low recurrence rate (2). This is the treatment, together with appropriate surgery when necessary, that is currently recommended by the WHO.
The pathogenesis of M. ulcerans disease is unusual in that these mycobacteria elaborate mycolactone, a polyketide toxin encoded by a number of genes on a large (174-kb) plasmid (9, 22), and animal studies have shown that it causes the characteristic tissue destruction leading to ulceration (9). Mycolactones induce apoptosis and necrosis of many human cell types in vitro and appear to inhibit recruitment of inflammatory cells to the site of infection (8, 9, 10). In human lesions, clumps of mycobacteria in subcutaneous fat are surrounded by necrotic tissue containing few if any inflammatory cells (9).
We have previously shown that after stimulation with M. ulcerans sonicate of whole blood from patients with M. ulcerans disease, there was significant gamma interferon (IFN-γ) production, which was higher in patients with established ulcers than in those with early lesions, compatible with slow development of a Th1-type immune response. Interleukin 10 (IL-10) production in M. ulcerans-stimulated blood was sustained from an early stage and somewhat nonspecific, being similar to that in patients with active tuberculosis (16).
If development of the immune response is inhibited by mycolactone, antibiotic treatment which kills M. ulcerans and inhibits production of mycolactone would be expected to lead to enhancement of the immune response. Therefore, we have studied the evolution of the IFN-γ and IL-10 responses after M. ulcerans sonicate stimulation of whole blood from patients with early M. ulcerans lesions during antibiotic treatment.
MATERIALS AND METHODS
Patients were recruited by local health workers from villages near Tepa Government Hospital in the Ahafo Ano North District of Ghana, where there is a high prevalence of Buruli ulcer. Patients were recruited if they met the WHO clinical case definition of M. ulcerans disease (1); were not pregnant; were not receiving antibiotic treatment; had no history of tuberculosis, leprosy, or liver, kidney, or hearing impairment; and gave written informed consent (thumb print of parent or guardian in the case of children, depending on literacy). Females older than 15 years were tested for pregnancy before antibiotic therapy was commenced. The study protocol was approved by the ethics review committees at the School of Medical Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana, and St. George's Hospital in London, United Kingdom.
Study protocol. (i) Diagnosis and treatment.
Punch biopsy specimens of 4-mm diameter were taken to confirm the clinical diagnosis, and patients were started on treatment with 10 mg/kg oral rifampin and 15 mg/kg intramuscular streptomycin (RIF-STR) daily, administered at village health posts under direct observation. If the diagnosis was confirmed by PCR for the IS2404 repeat sequence characteristic of M. ulcerans (15), antibiotic treatment was continued for 8 weeks. Biopsy specimens were also stained for acid-fast bacilli (AFB) and cultured on Lowenstein-Jensen slopes. Briefly, homogenized tissue was stained by the Ziehl-Neelsen technique, and 1 ml was decontaminated by the modified Petroff method for 10 min and inoculated on Lowenstein-Jensen slopes. Cultures were incubated at 31°C and examined weekly for 6 months before they were discarded. DNA extraction for PCR was performed by the guanidinium thiocyanate diatoms technique, and PCR was performed targeting the IS2404 insertion sequence as described previously (15).
(ii) Assessment of clinical response.
Patients were categorized into two groups, based on whether the initial lesion size was less than or greater than 10 cm in diameter. Small lesions, less than 10 cm in diameter, were traced onto acetate paper, and surface area was calculated by approximation to a circle. It was impractical to measure large lesions >10 cm in diameter, a few of which were multiple and on uneven body surfaces, in this way, so they were monitored by serial photography until healing occurred. The sizes of lesions were assessed before treatment, at 8 weeks, and at 12 weeks. Patients were reviewed twice weekly up to 12 weeks and monthly thereafter up to 52 weeks. Hearing, renal, and liver function tests were assessed at the baseline and after 4, 8, 12, and 52 weeks.
(iii) Whole-blood assay.
Blood samples were taken at 0, 4, 8, and 12 weeks for cytokine stimulation assays. Twelve milliliters of venous blood was taken in sodium heparin Vacutainer tubes (Becton Dickinson, United Kingdom). The whole-blood assay was performed as described previously (16). One-milliliter aliquots of undiluted blood were distributed in duplicate in 24-well tissue culture plates (Becton-Dickinson, United Kingdom) and incubated with M. ulcerans sonicate, 10 μg per ml phytohemagglutinin or no stimulant. Gentamicin was added to each well at 10 μg per ml (Sigma, United Kingdom). Plates were swirled gently and incubated at 37°C in 5% CO2 for 24 h. Supernatants (200 to 300 μl per well) were stored at −70°C for enzyme-linked immunosorbent assay to measure IL-10 and IFN-γ using OptEIA sets for human IL-10 and human IFN-γ (BD Biosciences, Pharmingen, San Diego, CA).
(iv) Calculation of enzyme-linked immunosorbent assay results and statistical analysis.
The mean absorbance of duplicate standards, samples, and controls was calculated for each plate, and the mean zero standard absorbance was subtracted. Results were analyzed with GraphPad Prism 4 software (GraphPad Software, Inc.) and a standard (best-fit) curve was plotted. Values for unstimulated cultures were subtracted from those for stimulated cultures. The lower detection limits were 4.7 pg/ml for IFN-γ and 7.8 pg/ml for IL-10. Results were validated by a significant response to phytohemagglutinin stimulation. Descriptive results of cytokines were expressed as medians and ranges. Medians for subjects at the various time points of the study were compared using the Mann-Whitney U test. P values less than 0.05 were considered significant.
RESULTS
Patients and clinical response to antibiotic.
Table 1 shows the characteristics of 26 patients with confirmed active M. ulcerans disease (mean age of 12 years). There were 13 preulcerative lesions (4 nodule, 4 plaques, and 5 edemas) and 13 ulcers. A total of 17 lesions were on the upper limbs, 8 on the lower limbs, and 1 on the trunk. The patient-estimated durations of lesions before presentation were longer for ulcers than for preulcerative lesions. PCR for IS2404 was positive in all 26 (100%) patients, compared with 12 (46%) positive cultures and 9 (35%) positive results for AFB staining.
TABLE 1.
Characteristics and diagnostic test results for 26 Buruli ulcer patients
| Lesion form | No. of subjects | Median age in yr (range) | Male to female ratio | Median duration of lesion in wk (range)a | No. of positive results/no. of negative results
|
||
|---|---|---|---|---|---|---|---|
| AFB | Culture | PCR | |||||
| Preulcerative | |||||||
| Nodule | 4 | 16.0 (10.0-25.0) | 4:0 | 4.0 (2.0-26.0) | 2/2 | 2/2 | 4/0 |
| Plaque | 4 | 8.5 (6.0-9.0) | 2:2 | 5.0 (2.0-8.0) | 2/2 | 3/1 | 4/0 |
| Edema | 5 | 13.0 (8.0-15.0) | 3:2 | 3.5 (3.0-8.0) | 2/3 | 3/2 | 5/0 |
| Ulcer | 13 | 10.5 (6.5-52.0) | 4:9 | 8.0 (2.0-52.0) | 3/10 | 4/9 | 13/0 |
| Total | 26 | 11.5 (6.0-52.0) | 13:13 | 4.0 (2.0-52.0) | 9/17 | 12/14 | 26/0 |
The median duration value represents the patient's estimate of the time since the lesion first appeared.
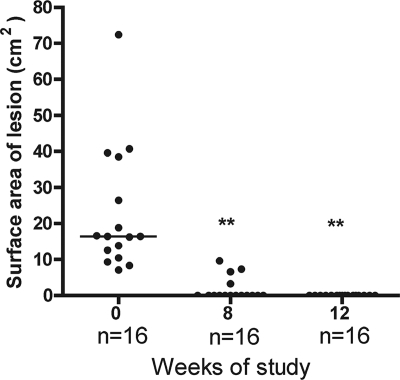
Prior to therapy, 16 patients had small lesions (4 nodules, 4 plaques, 1 edema, and 7 ulcers), with a median surface area of 16.5 cm2 (range of 7.1 to 72.4 cm2). After 8 weeks of antibiotic treatment, 12 patients had healed completely and the other 4 healed 4 weeks later (Fig. 1). Ten patients had large lesions, the surface area of which could not be measured accurately; four lesions were edematous without ulceration, and six were ulcers. None of these had healed after 8 weeks of treatment; but after 12 weeks, three had healed completely and the other seven had reduced in size and contained healthy granulation tissue. All had healed within 24 weeks, and there were no recurrences during 12 months of follow-up. No significant clinical adverse event was observed, and audiometric testing and hepatic and renal function tests were normal before, during, and after treatment.
FIG. 1.
Changes in surface area of small Buruli lesions (widest diameter, ≤10 cm) before and after RIF-STR treatment for 8 weeks. Each dot represents one subject, and the horizontal line represents the median (at 0, 8, and 12 weeks). **, P < 0.05, compared with lesion surface area at 0 weeks.
Whole-blood IFN-γ and IL-10 response to M. ulcerans antigens during antibiotic treatment.
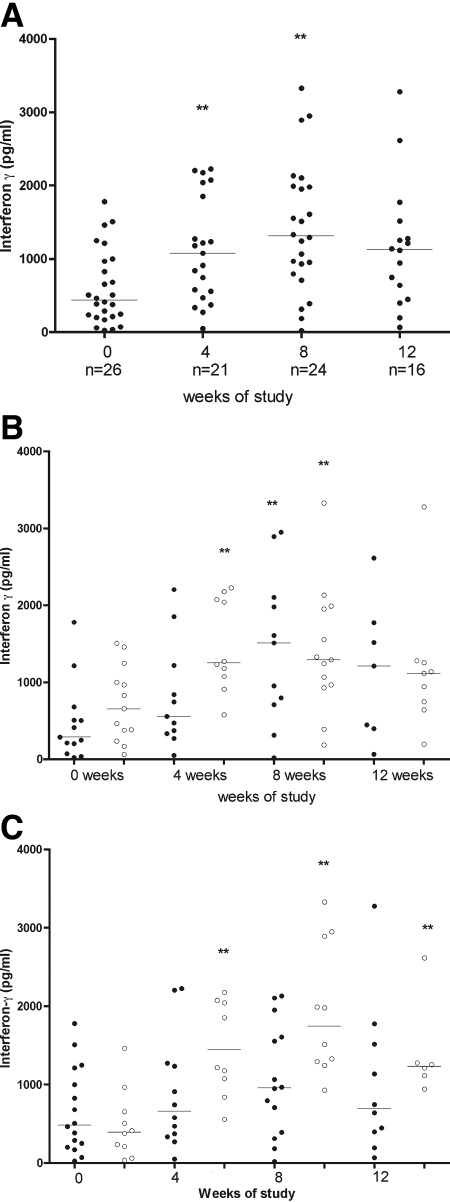
Secretion of IFN-γ after stimulation with M. ulcerans sonicate increased during treatment with RIF-STR (Fig. 2A). There was a significant increase in IFN-γ secretion at 4 weeks (median of 1,128 pg/ml [range of 271 to 2,228 pg/ml]) compared with the baseline (463 pg/ml [range of 37 to 1,780]; P < 0.05) and a further increase after 8 weeks to 1,330 pg/ml (range of 185 to 3,339; P < 0.05, compared to the baseline). At 12 weeks, the level of IFN-γ secretion remained higher than the baseline level at 1,115 pg/ml (range of 66 to 3,279) but not significantly so.
FIG. 2.
(A) IFN-γ production after stimulation with M. ulcerans 1 sonicate of whole blood from patients with Buruli lesions before, during, and after RIF-STR treatment for 8 weeks. Each dot represents one subject, and horizontal lines represent the medians. The incomplete data at 4, 8, and 12 weeks were due either to hemolyzed tissue cultures which could not be harvested for cytokine analysis or to patients missing appointments at the expected time points in the study. (B) IFN-γ production after stimulation with M. ulcerans 1 sonicate of whole blood from patients with preulcerative Buruli lesions compared with that from patients with ulcerative Buruli lesions before, during, and after RIF-STR combination treatment for 8 weeks. Each dot represents one subject. The black (•) and white (○) dots represent subjects with preulcerative and ulcerative lesions, respectively. The horizontal lines represent the medians. The incomplete data at 4, 8, and 12 weeks were due either to hemolyzed tissue cultures which could not be harvested for cytokine analysis or to patients missing appointments at the expected time points in the study. (C) IFN-γ production after stimulation with M. ulcerans 1 sonicate of whole blood from patients with small Buruli lesions (≤10 cm at the widest diameter) compared with that from patients with large Buruli lesions (>10 cm at the widest diameter or for multiple lesions) before, during, and after RIF-STR treatment for 8 weeks. The black (•) and white (○) dots represent subjects with small and large lesions, respectively, and horizontal lines represent the medians. The incomplete data at 4, 8, and 12 weeks were due either to hemolyzed tissue cultures which could not be harvested for cytokine analysis or to patients missing appointments at the expected time points in the study. **, P < 0.05 compared with IFN-γ production at 0 weeks.
In a comparison of patients with preulcerative and ulcerative lesions, median IFN-γ secretion at the baseline was nonsignificantly higher among 13 patients with ulcerative lesions (656 pg/ml [range of 60 to 1,508]) than among 13 patients with preulcerative lesions (289 pg/ml [range of 24 to 1,780]) (Fig. 2B). After treatment for 4 weeks, median IFN-γ secretion increased significantly in patients with ulcerative lesions to 1,253 pg/ml (range of 579 to 2,228) but not in those with preulcerative disease, in whom it rose to 557 pg/ml (range of 49.8 to 2,205) (P < 0.05, compared to ulcerative lesions). After 8 weeks, the difference from the baseline was significant in patients with both ulcerative and preulcerative lesions (median 1,293 pg/ml [range of 185.3 to 3,329] and 1,511 pg/ml [range of 19.9 to 2,949], respectively; [P < 0.05]). A rapid increase was also observed for 10 patients with large lesions, including 4 with edema (Fig. 2C). In this group, median IFN-γ secretion increased from a baseline value of 395 pg/ml (range of 37 to 1,461) to 1,217 pg/ml (range of 557 to 2,178) at 4 weeks, 1,745 pg/ml (range of 930 to 3,329) at 8 weeks, and 1,233 pg/ml (range of 944 to 2,615) at 12 weeks (P < 0.05 at 4, 8, and 12 weeks compared with the baseline). IFN-γ secretion also increased in patients with small lesions, but it did not reach significance.
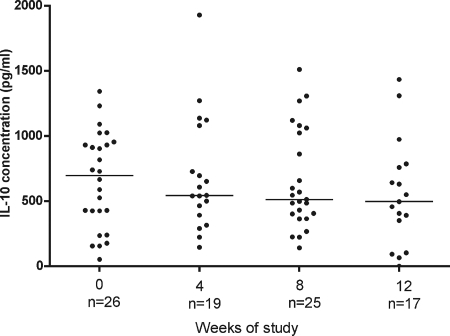
Figure 3 shows that there was a gradual decline in the median IL-10 response during antibiotic therapy, but there were no significant differences between the time points (median of 699 pg/ml at 0 weeks, 545 at 4 weeks, 513 at 8 weeks, and 497 at 12 weeks). There was no inverse correlation between IFN-γ and IL-10 responses.
FIG. 3.
IL-10 production after stimulation with M. ulcerans 1 sonicate of whole blood from patients with Buruli lesions before, during, and after antibiotic treatment. Each dot represents one subject; the horizontal lines represent the medians. The incomplete data at 4, 8, and 12 weeks were due either to hemolyzed tissue cultures which could not be harvested for cytokine analysis or to patients missing appointments at the expected time points in the study.
DISCUSSION
This series of 26 patients with proven M. ulcerans disease responded well to treatment with the antibiotic combination of rifampin and streptomycin, with all lesions healing within 24 weeks with no recurrences during follow-up for 12 months. Large ulcers took longer to reepithelialize, as expected, but the fact that there were no recurrences within 12 months indicates that M. ulcerans infection had been treated adequately. This corresponds with the experience of the same antibiotic treatment in Benin (2), but no patients in the present series required surgery. Some ulcers healed more slowly than others, despite being similar in size initially, and this needs further study. Edematous lesions resolved during antibiotic treatment, a development which has enormous significance for patients, since these lesions extend rapidly, and in the past, they were thought to require wide excision, resulting in large scars.
In the present study, the dynamics of cytokine production after stimulation of peripheral blood cells with M. ulcerans sonicate antigens was investigated during antibiotic treatment, which had a successful outcome. Overall, there was a marked increase in IFN-γ secretion by M. ulcerans-stimulated whole-blood cells after 4 and 8 weeks of antibiotic treatment, with a small falloff by 12 weeks. The greatest increases in IFN-γ secretion were observed in patients with ulcerated or large lesions, some with edema, and the increase occurred earlier in these patients than in those with small preulcerative lesions (Fig. 2B and C).
Secretion of mycolactone by M. ulcerans is likely to influence the development of the IFN-γ response. In untreated human lesions, M. ulcerans is an extracellular pathogen, although there is evidence of a transient intramacrophage growth phase in mice (23). The extracellular location of M. ulcerans has been attributed to the fact that mycolactone kills inflammatory cells by apoptosis and necrosis (10). In both guinea pig and murine models of infection with mycolactone-negative mutant strains of M. ulcerans, there was an initial acute inflammatory response followed by chronic inflammation, including granulomas (10, 14), and recently, it has been shown with tissue samples from Buruli ulcer patients who had received antibiotics for 8 weeks that there were highly organized cellular infiltrates around areas of coagulative necrosis, and mycobacteria were seen within mononuclear phagocytes (19). These findings support the idea that the killing of M. ulcerans with antibiotics permits phagocytosis of organisms and provides macrophages and dendritic cells with more opportunities to present antigens.
It is known that about a third of preulcerative lesions heal spontaneously without any treatment (18), and it is possible that these are ones in which mycolactone production is low, allowing development of a Th1 response. We can speculate that among the patients in the present study there were some with early lesions which would have healed without antibiotic treatment and that they showed a strong IFN-γ response before treatment, while in others M. ulcerans replication was rapid, with high mycolactone production; these would be the subjects whose IFN-γ response was low initially. In the absence of treatment, these lesions would go on to ulcerate, but there would be a gradient of mycolactone concentration from high in the center, where bacterial replication is occurring, to low at the outer margin of the lesion, and with the passage of time, M. ulcerans antigens would interact normally with the immune system and an IFN-γ response would develop. This would explain the finding in this study and reported previously (16) that IFN-γ secretion was higher before treatment in patients with ulcerative lesions than in those with preulcerative lesions. Priming of the immune response in this way would also explain the higher IFN-γ secretion in patients with ulcerative lesions after 4 weeks, shown in Fig. 2B. An alternative explanation is that, because ulcerative lesions had been present for longer and more mycobacterial replication had taken place, larger quantities of antigens in the tissue of the lesions could stimulate a more rapid immune response when antibiotic treatment reduced mycolactone concentration in tissue. Further studies of the concentration of mycolactone in human tissue during treatment may throw more light on this question.
Two earlier studies (11, 17) found that IFN-γ secretion in response to antigen stimulation was lower in ulcerative lesions than in preulcerative lesions, in contrast to the findings in the present study. In both of these studies, there were substantial differences in the assay techniques; in particular, cultures were maintained for periods up to 6 days rather than 24 h, so the cell populations under investigation were different. The long-incubation assays may provide insight into memory and regulatory T-cell responses, whereas the 24-h assay in our study probably focuses on the prevailing effector responses to M. ulcerans antigens.
Using an ex vivo enzyme-linked immunospot assay, recovery of IFN-γ secretion by peripheral blood mononuclear cells from Buruli ulcer patients in response to stimulation with purified protein derivative as well as nonmycobacterial antigens was observed some months after surgical excision of the lesions (25). This was interpreted as showing that mycolactone has a broad immunosuppressive effect, but this aspect was not investigated in the present studies.
The pivotal role of IFN-γ in orchestrating a Th1 response to mycobacterial infection has been demonstrated with animal models of tuberculosis (3, 7). Most patients with active tuberculosis have depressed IFN-γ secretion in response to specific antigen stimulation of peripheral blood mononuclear cells, which significantly improves after successful antituberculous therapy (13). Production of anti-inflammatory cytokines, such as IL-4, IL-10, and TGF-alpha, in response to Mycobacterium tuberculosis downregulates the immune response, thereby limiting tissue injury, but excessive production of these cytokines may result in a failure to control the infection (20). IL-10 is a potent suppressor of IFN-γ synthesis by helper T cells (6) and by NK cells (12), with inhibition of antigen presentation to Th1 cells, and in leprosy it has been found to inhibit T-cell responses as well as IFN-γ release (21). In the present studies, increasing IFN-γ secretion was not correlated with a significant decline in IL-10 secretion, indicating that the Th1 response developed independently of Th2 activation, but it remains possible that IL-10 modulated excess proinflammatory activity.
In conclusion, we have demonstrated that there was recovery of IFN-γ secretion in response to M. ulcerans sonicate stimulation of whole blood in patients with M. ulcerans disease during successful antibiotic therapy, and this occurred independently of IL-10 secretion. These findings support the hypothesis that antibiotic-mediated killing of M. ulcerans results in reduced mycolactone concentration, which enhances the ability of antigen-presenting cells to process antigenic epitopes for typical Th1-type mycobacterial immunity to develop.
Acknowledgments
We are grateful to all the staff at Tepa Government Hospital and Kofi Asare, Regional Director of Health in the Ashanti region of Ghana, for their support.
Footnotes
Published ahead of print on 12 November 2008.
REFERENCES
- 1.Asiedu, K., M. Raviglione, and R. Scherpbier (ed.). 2000. Buruli ulcer: Mycobacterium ulcerans infection. WHO/CDS/CPE/GBUI/2000.1. World Health Organization, Geneva, Switzerland.
- 2.Chauty, A., M. F. Ardant, A. Adeye, H. Euverte, A. Guedenon, C. Johnson, J. Aubry, E. Nuermberger, and J. Grosset. 2007. Promising clinical efficacy of streptomycin-rifampin combination for treatment of buruli ulcer (Mycobacterium ulcerans disease). Antimicrob. Agents Chemother. 514029-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 1782243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Etuaful, S., B. Carbonnelle, J. Grosset, S. Lucas, C. Horsfield, R. Phillips, M. Evans, D. Ofori-Adjei, E. Klustse, J. Owusu-Boateng, G. K. Amedofu, P. Awuah, E. Ampadu, G. Amofah, K. Asiedu, and M. Wansbrough-Jones. 2005. Efficacy of the combination rifampin-streptomycin in preventing growth of Mycobacterium ulcerans in early lesions of Buruli ulcer in humans. Antimicrob. Agents Chemother. 493182-3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans, M. R., R. Phillips, S. N. Etuaful, G. Amofah, J. Adomako, O. Adjei, J. Dennis-Antwi, S. B. Lucas, and M. H. Wansbrough-Jones. 2003. An outreach education and treatment project in Ghana for the early stage of Mycobacterium ulcerans disease. Trans. R. Soc. Trop. Med. Hyg. 97159-160. [DOI] [PubMed] [Google Scholar]
- 6.Fiorentino, D. F., A. Zlotnik, P. Vieira, T. R. Mosmann, M. Howard, K. W. Moore, and A. O'Garra. 1991. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. 1463444-3451. [PubMed] [Google Scholar]
- 7.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 1782249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George, K. M., L. P. Barker, D. M. Welty, and P. L. C. Small. 1998. Partial purification and characterization of biological effects of a lipid toxin produced by Mycobacterium ulcerans. Infect. Immun. 66587-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George, K. M., D. Chatterjee, G. Gunawardana, D. Welty, J. Hayman, R. Lee, and P. L. C. Small. 1999. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science 283854-857. [DOI] [PubMed] [Google Scholar]
- 10.George, K. M., L. Pascopella, D. M. Welty, and P. L. C. Small. 2000. A Mycobacterium ulcerans toxin, mycolactone, causes apoptosis in guinea pig ulcers and tissue culture cells. Infect. Immun. 68877-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gooding, T. M., P. D. Johnson, M. Smith, A. S. Kemp, and R. M. Robins-Browne. 2002. Cytokine profiles of patients infected with Mycobacterium ulcerans and unaffected household contacts. Infect. Immun. 705562-5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu, D. H., K. W. Moore, and H. Spits. 1992. Differential effects of IL-4 and IL-10 on IL-2-induced IFN gamma synthesis and lymphokine-activated killer activity. Int. Immunol. 4563-569. [DOI] [PubMed] [Google Scholar]
- 13.Jo, E. K., J. K. Park, and H. M. Dockrell. 2003. Dynamics of cytokine generation in patients with active pulmonary tuberculosis. Curr. Opin. Infect. Dis. 16205-210. [DOI] [PubMed] [Google Scholar]
- 14.Oliveira, M. S., A. G. Fraga, E. Torrado, A. G. Castro, J. P. Pereira, A. L. Filho, F. Milanezi, F. C. Schmitt, W. M. Meyers, F. Portaels, M. T. Silva, and J. Pedrosa. 2005. Infection with Mycobacterium ulcerans induces persistent inflammatory responses in mice. Infect. Immun. 736299-6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips, R., C. Horsfield, S. Kuijper, A. Lartey, I. Tetteh, S. Etuaful, B. Nyamekye, P. Awuah, K. M. Nyarko, F. Osei-Sarpong, S. Lucas, A. H. Kolk, and M. Wansbrough-Jones. 2005. Sensitivity of PCR targeting the IS2404 insertion sequence of Mycobacterium ulcerans in an assay using punch biopsy specimens for diagnosis of Buruli ulcer. J. Clin. Microbiol. 433650-3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips, R., C. Horsfield, S. Kuijper, S. F. Sarfo, J. Obeng-Baah, S. Etuaful, B. Nyamekye, P. Awuah, K. M. Nyarko, F. Osei-Sarpong, S. Lucas, A. H. Kolk, and M. Wansbrough-Jones. 2006. Cytokine response to antigen stimulation of whole blood from patients with Mycobacterium ulcerans disease compared to that from patients with tuberculosis. Clin. Vaccine Immunol. 13253-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prévot, G., E. Bourreau, H. Pascalis, R. Pradinaud, A. Tanghe, K. Huygen, and P. Launois. 2004. Differential production of systemic and intralesional gamma interferon and interleukin-10 in nodular and ulcerative forms of Buruli disease. Infect. Immun. 72958-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Revill, W. D., R. H. Morrow, M. C. Pike, and J. Ateng. 1973. A controlled trial of the treatment of Mycobacterium ulcerans infection with clofazimine. Lancet ii873-877. [DOI] [PubMed] [Google Scholar]
- 19.Schutte, D., A. Um-Boock, E. Mensah-Quainoo, P. Itin, P. Schmid, and G. Pluschke. 2007. Development of highly organized lymphoid structures in Buruli ulcer lesions after treatment with rifampicin and streptomycin. PLoS Negl. Trop. Dis. 1e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma, S., and M. Bose. 2001. Role of cytokines in immune response to pulmonary tuberculosis. Asian Pac. J. Allergy Immunol. 19213-219. [PubMed] [Google Scholar]
- 21.Sieling, P. A., and R. L. Modlin. 1994. Cytokine patterns at the site of mycobacterial infection. Immunobiology 191378-387. [DOI] [PubMed] [Google Scholar]
- 22.Stinear, T. P., A. Mve-Obiang, P. L. Small, W. Frigui, M. J. Pryor, R. Brosch, G. A. Jenkin, P. D. Johnson, J. K. Davies, R. E. Lee, S. Adusumilli, T. Garnier, S. F. Haydock, P. F. Leadlay, and S. T. Cole. 2004. Giant plasmid-encoded polyketide synthases produce the macrolide toxin of Mycobacterium ulcerans. Proc. Natl. Acad. Sci. USA 1011345-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torrado, E., A. G. Fraga, A. G. Castro, P. Stragier, W. M. Meyers, F. Portaels, M. T. Silva, and J. Pedrosa. 2007. Evidence for an intramacrophage growth phase of Mycobacterium ulcerans. Infect. Immun. 75977-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wansbrough-Jones, M., and R. Phillips. 2006. Buruli ulcer: emerging from obscurity. Lancet 3671849-1858. [DOI] [PubMed] [Google Scholar]
- 25.Yeboah-Manu, D., E. Peduzzi, E. Mensah-Quainoo, A. Asante-Poku, D. Ofori-Adjei, G. Pluschke, and C. A. Daubenberger. 2006. Systemic suppression of interferon-gamma responses in Buruli ulcer patients resolves after surgical excision of the lesions caused by the extracellular pathogen Mycobacterium ulcerans. J. Leukoc. Biol. 791150-1156. [DOI] [PubMed] [Google Scholar]