Abstract
The host determinants of susceptibility to recurrent urinary tract infections (UTI) are poorly understood. We investigated whether the susceptibility is associated with abnormalities in the immunological defense and further explored the linkage to vaginal microbiota. For this purpose, we compared vaginal, urine, and blood samples collected during a disease-free period from 22 women with recurrent UTI and from 17 controls. In UTI-prone women, interleukin-12 (IL-12) production in peripheral monocytes and myeloid dendritic cells (DCs) was significantly (P < 0.05) enhanced whether measured in relative numbers of IL-12-producing cells or in mean IL-12 production per cell. In contrast, no T-cell polarization was observed. Interestingly, it seemed that the cytokine production of DCs and monocytes did not translate into T-cell activation in the UTI-prone group in a manner similar to that seen with the controls. In vaginal mucosa, UTI-prone women had a lower concentration of tissue repair-associated vascular endothelial growth factor (VEGF) (P = 0.006) and less often had detectable amounts of the chief monocyte and DC chemoattractant, monocyte chemotactic protein 1 (P = 0.005), than the controls. The microbiota of UTI-prone women was characterized by a diminished lactobacillus morphotype composition, with an abnormally high (>3) mean Nugent score of 4.6 compared to 1.7 for the controls (P = 0.003). Normal lactobacillus composition was associated with increased IL-17 and VEGF concentrations in vaginal mucosa. In conclusion, immunological defects and a persistently aberrant microbiota, a lack of lactobacilli in particular, may contribute to susceptibility to recurrent UTI. Further studies of antigen-presenting-cell function and T-cell activation in recurrent UTI are called for.
An estimated 50% of women are likely to suffer an episode of urinary tract infections (UTI) at least once in their lifetime, and for a quarter of those the condition recurs (16, 21). Susceptibility is associated with host factors, some of which may be genetic in origin, as well as behavioral and environmental factors (15). One of the initial decisive events leading to UTI is the vaginal colonization by virulent uropathogens (49). For the most part, these organisms originate from the woman's own intestine via the rectal area. In women prone to recurrent UTI, susceptibility to aberrant colonization by uropathogens and gram-negative enterobacteria appears to be increased (33, 36), but it is not known why (15). Attachment sites or defects in the host defenses or an endogenous microbiota conferring colonization resistance could all be explaining factors.
Female members of families of women with recurrent UTI (23) and women who suffered from UTI in childhood are at a higher risk of a repeat infection in adulthood (42). These findings have been taken as indicative of a genetic determinant in the susceptibility to recurrent UTI. The immunological status of UTI-prone women has not previously been well characterized, but there is some indication that genetic polymorphism related to induction of immune responses could contribute to the susceptibility (15, 45). Cell-mediated innate responses (in particular, neutrophil action) form the basis of the defense against genitourinary tract infections (44). One issue is whether the UTI-prone women could have a defect in the translation of these innate immune responses into immunological memory and effective adaptive responses. It is known that antibodies are raised against the uropathogens, but they do not seem to provide efficient protection (44). The role of T-cell-mediated adaptive responses regarding UTI is poorly known.
A healthy vaginal microbiota is characteristically dominated by lactobacilli, which are known to provide colonization resistance and prevent UTI recurrence by several means, including maintenance of normal acidic pH in the vaginal vault and production of hydrogen peroxide (2, 5, 11). The change from a healthy, lactobacillus-dominated microbiota to a complex multispecies microbiota can happen relatively quickly and result in bacterial vaginosis (BV) (18), a condition associated with an increased likelihood of UTI. It may be that in the women prone to UTI, the lactobacillus composition in the “normal” state is aberrant and less resistant to compositional fluctuations (22). Moreover, the vaginal microbiota is a source of continuous microbial stimulation to the host; thus, its composition may define the status of the local defense milieu and influence how the host responds when a pathogenic invader is encountered.
In the current study we addressed these questions for the first time by extensive profiling of the local and peripheral immunological status of UTI-prone women, together with detailed analysis of the vaginal microbiota.
MATERIALS AND METHODS
Study subjects and specimens.
The University of Western Ontario Ethics Review Board approved the protocol, and all volunteers provided written consent. This trial was conducted in accordance with International Conference on Harmonization-Good Clinical Practice guidelines, the international standard for all aspects of clinical trials involving human subjects. These guidelines provide public assurance that the safety and wellbeing of trial subjects are protected and are consistent with the principles that have their origin in the Declaration of Helsinki.
UTI-prone patients and age-matched control subjects were recruited from a tertiary-care teaching hospital within the geographic area of southwestern Ontario, Canada. Urine and blood samples were collected during clinic visits. Vaginal swabs were obtained at the time of a flexible cystoscopy procedure for UTI-prone patients. For UTI-prone subjects, a uroflow analysis using the postvoid residual was performed to rule out the presence of a neurogenic bladder or other urinary tract anomalies, and renal ultrasound procedures were performed to rule out renal calculi or other anatomic abnormalities predisposing to UTIs. Controls did not undergo cystoscopy or other clinical evaluations beyond analysis of the blood, urine, and vaginal samples. Vaginal swabs were obtained from the controls by self-swabbing after instruction by the research staff.
Blinded procedures with respect to the origin of the samples were employed for investigators. UTI-prone subjects were defined as women with more than three previous UTIs, with at least two culture-positive UTIs in the previous 12 months. UTI-prone subjects were excluded if there was evidence of a neurogenic bladder condition, if they had known immunodeficiencies, if they had used antibiotics or steroids within the previous month, if they had known renal calculi by as determined by an ultrasound procedure, if they had a previous history of chemotherapy, if they were found to have an anatomic abnormality on cystoscopic evaluation, or if they were pregnant. None of the subjects had taken any form of probiotics for 1 month prior to the study, and all UTI-prone subjects were infection free for at least 1 month prior to testing. Control subjects had no prior history of UTIs or other urologic abnormalities and had sterile urine cultures. The sample size of 20 per group was calculated on the basis of the expectation that the vaginal microbiota results would differ between the groups by 20%, based upon Nugent scoring.
Urine was cultured to confirm that no subject had active UTI.
Analysis of cytokines and growth-factors.
Cytokine and growth factor concentrations from vaginal swabs, urine, and serum were analyzed using fluorescent microsphere-based multiplexing technology with a Bio-Plex 200 analyzer (Bio-Rad, Inc.). Cytokines, beta interleukin-1 (IL-1β), IL-2, IL-4, IL-6, IL-8, IL-10, IL-12 (p70), IL-13, IL-15, IL-17, basic fibroblast growth factor, gamma interferon (IFN-γ), monocyte chemoattractant protein 1 (MCP-1), platelet-derived growth factor-BB (PDGF-bb), tumor necrosis factor alpha (TNF-α), and vascular endothelial growth factor (VEGF) were analyzed using a premixed multiplex panel (Bio-Rad, San Diego, CA) and transforming growth factor β separately with a human transforming growth factor-β-1 enzyme-linked immunosorbent assay set (BD Biosciences, San Diego, CA). All determinations were performed in duplicate wells according to the manufacturer's instructions. The cytokine concentrations in the samples were calculated by the use of Bio-Plex Manager software and the standard curves derived from the recombinant cytokine standards.
Analysis of intracellular cytokine production in DCs, monocytes, and T cells.
Intracellular cytokine detection was performed by flow cytometry as previously described with some modifications (1, 7). Peripheral blood samples in lithium heparin were supplemented one to one with RPMI 1640 medium (Invitrogen, Burlington, Ontario, Canada) and incubated at 37°C in a 5% CO2 humidified atmosphere with brefeldin A (Sigma, St. Louis, MO) (10 μg/ml) in the presence or absence of lipopolysaccharide from Escherichia coli (serotype 055:B5) (Sigma) (100 ng/ml) plus IFN-γ (R&D Systems, Inc., Minneapolis, MN) (100 U/ml) for stimulation (6 h) of cytokine production by monocytes and dendritic cells (DCs) or of ionomycin (Sigma) (1 μg/ml) plus phorbol 12-myristate 13-acetate (PMA; Sigma) (25 ng/ml) for stimulation (4 h) of cytokine production by T cells. For the identification of the whole DC population (major histocompatibility complex class II positive [MHC II+]/lineage−/CD33+/−), the highly CD33-expressing myeloid (CD33high) and intermediately CD33-expressing myeloid (CD33intermed), and the non- or weakly CD33-expressing plasmocytoid (CD33−/low) subsets and monocytes (MHC II+/CD14+/CD33+), peripheral blood cells were then incubated for 15 min at room temperature with anti-HLA-DR-Cy-chrome, anti-CD33-allophycocyanin, and each of the following fluorescein isothiocyanate (FITC)-labeled lineage marker antibodies: anti-CD3, anti-CD19, anti-CD56, and anti-CD14 (BD Biosciences, San Diego, CA). Stained cells were washed using phosphate-buffered saline (PBS) (pH 7.5) and centrifugation (5 min at 540 × g), fixed, permeabilized, and stained with anti-TNF-α-phycoerythrin (PE) (clone MAb11) and anti-IL-12-PE (C11.5) by the use of Fix & Perm reagent (Caltag, Burlingame, CA) following the manufacturer's instructions. T-cell cytokines were analyzed accordingly, but the cells were identified with anti-CD3-FITC and their cytokines detected with anti-IL-2-PE (clone MQ1-17H12), anti-IFN-γ-PE (B27), anti-IL-4-PE (8D4-8), and anti-IL-10-PE (JES3-19F1). Data acquisition was performed in two consecutive steps with a flow cytometer (FACSCalibur; BD Biosciences). First, 30,000 events/test corresponding to the whole of the peripheral blood cellularity were collected for analysis of cytokines produced by T cells and monocytes. Second, only events in a HLA-DR+/CD3−/CD19−/CD56−/CD14− live gate were stored and a minimum of 300,000 events from the total peripheral blood cellularity were acquired in order to obtain at least 1,000 MHC II+/lineage− cells for the analysis of cytokines produced by DC subsets. CellQuest software (BD Biosciences) was used for data acquisition and analysis.
Analysis of T-cell surface markers.
For analysis of the expression of activation markers CD54 and CD69 on T cells, RPMI medium-diluted peripheral blood was incubated with or without PMA and ionomycin as described above whereas only unstimulated samples were used for regulatory T (Treg) cell analysis. CD4+ CD25+ Treg cells are enriched within the 1 to 2% of peripheral blood CD4+ T cells expressing high levels of CD25, while the population expressing lower levels of CD25 is thought to consist mainly of activated effector T cells (3). Thus, in flow cytometric analyses, CD4+ small lymphocytes were subdivided into bright (CD4+ CD25high/Treg-cell) and intermediate (CD4+ CD25+/activated T-cell) populations based on the CD25 expression. The stimulated and/or unstimulated samples (200 μl each) were stained with 3 μl of anti-CD3-FITC in combination with anti-CD69-PE or anti-CD4-FITC plus anti-CD25-PE (BD Biosciences) for 15 min at room temperature. Data were acquired with flow cytometry (30,000 events/test) and analyzed as described above.
Nugent scoring.
Vaginal swabs were streaked onto glass slides, Gram stained, and scored by the Nugent method (34) and were graded as 0 to 3 (normal vaginal state), 4 to 6 (intermediate), or 7 to 10 (BV).
Extraction of DNA from bacterial strains and swabs.
DNA was extracted using 500 μl of pure bacterial culture and Instagene matrix (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions. Swabs were vigorously agitated in 1 ml of PBS (pH 7.5) to dislodge cells. These were pelleted by centrifugation (10,000 × g for 5 min) and washed once in the PBS before proceeding with the DNA extraction.
PCR amplification of DNA for denaturing gradient gel electrophoresis (DGGE) and identification.
Reactions were carried out in 0.2-ml tubes in an Eppendorf Mastercycler thermocycler. Each PCR mixture (100 μl) consisted of 10 μl of 10× buffer (10 mM Tris-HCl, 2.5 mM MgCl2, 50 mM KCl), 200 μM deoxynucleoside triphosphate (Roche, Germany), 2 μl of glycerol (Sigma), 80 μg of bovine serum albumin, 40 pmol of each primer (GibcoBRL/Life Technologies, Gaithersburg, MD), 5 U of DNA Taq polymerase (Platinum; GibcoBRL), and 10 μl of the DNA preparation and were diluted to the required volume with Milli-Q water. The amplification conditions have been described previously for the various primer sets (9, 12).
DGGE gels.
Preparation of DGGE gel gradients and electrophoresis were carried out according to the manufacturer's guidelines for the D-code universal detection system of Bio-Rad. A 100% solution was defined as a mixture of 7 M urea and 40% formamide. The concentrations of polyacrylamide, denaturant, and Tris-acetate-EDTA buffer (TAE; 40 mM Tris, 20 mM glacial acetic acid, 1 mM EDTA [pH 8.0]) were 8%, 30 to 50%, and 1×, respectively. Solutions were degassed for at least 30 min before the addition of the following polymerization agents: 55 μl of TEMED (N,N,N′,N′-tetramethylethylenediamine; Sigma) and 95 μl of 10% ammonium persulfate (Bio-Rad). Gels were allowed to polymerize overnight. Samples were mixed with 2× loading buffer (0.25 ml bromophenol blue Σ [2%], 0.25 ml of xylene cyanol Σ [2%], 7 ml of glycerol, 2.5 ml of distilled water) and loaded into the wells. Gels were run at 130 V in 1× TAE until the second dye front (xylene cyanol) approached the end of the gel. After electrophoresis, gels were removed, allowed to cool before the removal of the glass plate sandwich, stained for 20 min in 5 μg/ml of ethidium bromide, and destained for 10 min in 1× TAE. Gels were visualized by ultraviolet transillumination, and images were recorded (667 instant film; Polaroid, Bedford, MA).
Band excision from DGGE gels, reamplification, and sequencing.
Fragments of interest were excised from DGGE gels by the use of a sterile scalpel and placed into a single microcentrifuge tube. Gel pieces were washed once in 1× PCR buffer and incubated in 20 μl of the same buffer overnight at 4°C. Five microliters of the buffer solution was used as template for PCR amplification. Reamplification was conducted using either universal eubacterial (HDA1-GC and HDA2) or lactobacillus-specific (LAC1 and LAC2-GC) previously described PCR primer pairs (48). Sequences of the reamplified fragments were determined by the dideoxy chain termination method (Sequencing Facility, John P. Robarts Research Institute, London, Ontario, Canada). Searching of the partial 16S and 18S DNA sequences was conducted using the GenBank DNA database and the BLAST algorithm. Identities of isolates were determined on the basis of the highest score.
Statistical analyses.
The Mann-Whitney U test was used for comparison of the means of continuous data of two independent groups. The two-sided Fisher's exact test was used to determine significant associations between two categorical variables in two-by-two contingency tables. Spearman's rank correlation data were calculated for determination of positive correlations between two continuous variables. Differences were considered to be statistically significant when the P value was less than or equal to 0.05. All the statistical analyses were performed using SPSS 14.0 for Windows, release 14.0.1 (SPSS Inc., Chicago, IL), with the exception of graphs and 95% confidence intervals (CI), which were drawn and calculated using StatView for Windows, version 4.57 (Abacus Concepts, Inc., Berkeley, CA).
RESULTS
A total of 22 UTI-prone subjects (median age, 47.5; range, 19 to 75 years) and 17 health controls (median age, 46; range, 26 to 65 years) were recruited. In the UTI-prone group, 10 patients were premenopausal, 5 perimenopausal, and 7 postmenopausal. In the control group, eight were premenopausal, eight perimenopausal, and one postmenopausal. None of the postmenopausal women were on hormone replacement therapy.
Immunological characteristics of UTI-prone women.
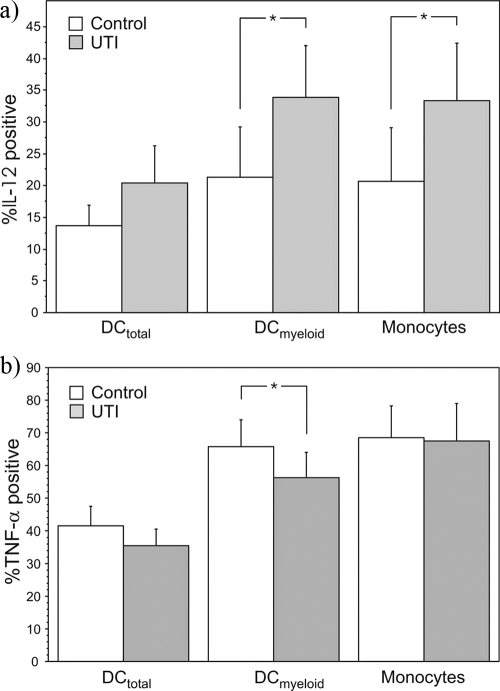
The peripheral immunological milieu in the UTI-prone group was characterized by greater relative numbers of IL-12-producing peripheral DCs and monocytes (Fig. 1a). Also, the mean quantity of IL-12 produced by these cells in response to ex vivo stimulation was significantly enhanced. The mean IL-12 concentrations in mean fluorescent units within the control group and UTI-prone group for DCs in total were 16 (95% CI, 12 to 19) and 38 (95% CI, 13 to 64) (P = 0.047); for the myeloid DC subpopulation, 25 (95% CI, 15 to 34) and 65 (95% CI, 16 to 113) (P = 0.03); and for monocytes, 15 (95% CI, 11 to 19) and 50 (95% CI, 14 to 85) (P = 0.005), respectively. Similar observations were made regarding IL-12 production in CD33 intermediately expressing DCs, but the differences were not statistically significant (data not shown). Other characteristics seen for DCs within the UTI-prone group were a lower percentage of TNF-α-producing myeloid DCs compared to control results (Fig. 1b) and lower relative numbers of plasmacytoid DCs (Table 1). The relative number of monocytes was significantly increased in the UTI-prone group (Table 1).
FIG. 1.
The presence of IL-12 (a)- and TNF-α (b)-producing DCs (total and myeloid) and monocytes in controls and women prone to UTI. The subpopulations of cytokine-producing cells were determined following ex vivo stimulation with IFN-γ and lipopolysaccharide. *, the group means differ significantly at the 95% confidence level.
TABLE 1.
Presence of DCs and their subpopulations as well as of monocytes in peripheral blood of UTI-prone patients and of healthy controls
| Group | No. (95% CI) of DCtotal | No. (95% CI) of DCmyeloid | No. (95% CI) of DCplasmacytoid | No. (95% CI) of DCCD33intermediate | No. (95% CI) of monocytes |
|---|---|---|---|---|---|
| Control | 0.94 (0.77-1.10) | 0.40 (0.29-0.52) | 0.24 (0.14-0.34) | 0.26 (0.17-0.35) | 4.2 (3.4-4.9) |
| UTI | 0.86 (0.67-1.06) | 0.38 (0.29-0.48) | 0.15 (0.07-0.23)a | 0.32 (0.23-0.41) | 5.1 (4.4-5.8)a |
Statistically significant at the 95% confidence level.
Differences within T cells were less evident. The UTI-prone group had a higher mean percentage of T cells spontaneously producing IL-2 (0.21% [95% CI, 0.05 to 0.36] versus 0.32% [95% CI, 0.22 to 0.41] [P = 0.004]), of those producing IL-10 (0.32% [95% CI, 0.18 to 0.49] versus 0.72% [95% CI, 0.44 to 1.00] [P = 0.02]), and of those expressing the early activation marker CD25 (10.2% [95% CI, 6.6 to 18.8] versus 14.7% [95% CI, 11.4 to 18.0] [P = 0.04]). No significant differences were observed in the expression of these markers by T cells in response to polyclonal stimulation with PMA and ionomycin. The production or ratio of Th1 and Th2 signature cytokines IFN-γ and IL-4, respectively, was not significantly different between the UTI-prone group and the controls (data not shown). There were also no significant differences in the mean serum concentrations of these cytokines (Table 2).
TABLE 2.
Presence of cytokines and growth factors in vaginal mucosa, urine, and serum
| Cytokine/growth factor | Mean concn in pg/ml (95% CI) or no. of samples above assay sensitivity limit/total no. of samples (%) ina:
|
|||||
|---|---|---|---|---|---|---|
| Vaginal swab
|
Urine
|
Serum
|
||||
| Control group | UTI group | Control group | UTI group | Control group | UTI group | |
| IL-1β | 45 (0.4-90) | 8.9 (3.2-15) | 2/17 (12) | 4/22 (18) | NDb | ND |
| 9/17 (53) | 13/21 (62) | ND | ND | |||
| IL-2 | 9/17 (53) | 1/21 (4.8)c | 1/17 (5.9) | 1/22 (4.5) | 21 (16-26) | 21 (17-26) |
| IL-4 | 7/17 (41) | 8/21 (38) | 1/17 (5.9) | 2/22 (9.1) | 10/17 (59) | 17/22 (77) |
| IL-6 | 5/17 (29) | 2/21 (9.5) | 2/17 (12) | 3/22 (14) | 5.9 (2.5-9.3) | 6.0 (3.5-8.5) |
| 7/17 (41) | 10/22 (46) | |||||
| IL-8 | 732 (210-1,254) | 361 (148-574) | 36 (16-55) | 91 (0-234) | 24 (0-61) | 14 (4.8-24) |
| 6/17 (35) | 15/22 (68) | |||||
| IFN-γ | 6/17 (35) | 5/21 (24) | 6/17 (35) | 7/22 (32) | 14 (10-17) | 20 (11-30) |
| IL-10 | 1/17 (5.9) | ND | ND | ND | 1/17 (5.9) | 2/22 (9.1) |
| IL-12 | ND | ND | ND | ND | ND | 2/22 (9.1) |
| IL-13 | ND | ND | ND | ND | ND | 2/22 (9.1) |
| IL-15 | ND | ND | ND | ND | 1/17 (5.9) | 2/22 (9.1) |
| IL-17 | 8/17 (47) | 8/22 (36) | ND | ND | 1/17 (5.9) | 4/22 (18) |
| PDGF-bb | 16 (3.7-29) | 5.6 (2.6-8.7) | 2.9 (1.6-4.2) | 2.5 (1.9-3.1) | 8,706 (6,730-10,683) | 9,762 (8,358-11,166) |
| bFGF | 1/17 (5.9) | 1/22 (4.5) | 2/17 (12) | 3/22 (14) | ND | 1/22 (4.5) |
| MCP-1 | 10/17 (59) | 3/22 (14)c | 41 (29-52) | 31 (22-40) | 4/17 (24) | 6/22 (27) |
| TNF-α | 6/17 (35) | 5/21 (24) | ND | ND | 11 (0-23) | 22 (0-47) |
| 2/17 (12) | 5/22 (23) | |||||
| VEGF | 1,118 (21-2,214) | 320 (98-542)c | 200 (95-304) | 168 (112-223) | 160 (101-219) | 159 (120-198) |
| TGF-β | 3.0 (1.9-4.0) | 3.8 (2.3-5.3) | 10.2 (5.7-15) | 9.9 (6.8-13) | 5.5 (4.3-6.7) | 6.1 (4.2-8.1) |
The values represent mean concentrations(95% CI) or prevalence of samples above the assay sensitivity limit(% positive), depending on whether most of the samples were above or below the limit, respectively. Both sets of values are given where relevant.
ND, not detected; i.e., the concentration was below the lower limit of assay sensitivity in all samples.
The concentrations were significantly different between UTI and control subjects at the 99% confidence level.
The samples from the vaginal mucosa of UTI-prone subjects had significantly lower mean concentrations of VEGF than those from healthy controls and tended to contain less PDGH-bb (Table 2). In the UTI-prone group, only one swab sample contained detectable amounts of IL-2 and only three samples contained MCP-1, whereas both of these cytokines were detected in over half of the samples in the control group. A similar trend was observed with MCP-1 concentrations in urine, but the difference was not statistically significant (Table 2). In serum, IL-8 was present in detectable amounts nearly twice as often in the UTI-prone group as in the control group (P = 0.057).
Exploratory analyses for correlations between cytokine production by myeloid DCs and T-cell parameters.
To characterize the potential relevance of the observed differences in cytokine production by the antigen-presenting cells (APCs), we assessed post priori how the production of these cytokines correlated with the T-cell characteristics (Table 3). Relatively weak significant positive correlations were observed between the presence of IL-12-producing myeloid DCs or monocytes and the presence of IL-10-producing T cells, CD69-expressing T cells, CD25-expressing T cells, and putative Treg cells (CD4+ CD25high). The association between the IL-12 production and the presence of CD25-expressing T cells (including the putative Treg cells) appeared to be clearer within or limited to the control group. The presence of IL-12-producing myeloid DC or monocytes did not correlate with the numbers of IFN-γ- or IL-4-producing T cells. On the other hand, relatively strong correlations were observed between the presence of TNF-α-producing myeloid DCs and that of IFN-γ-producing or CD54-expressing T cells within the control group but not within the UTI-prone group. In contrast, higher relative numbers of TNF-α-producing monocytes were associated with a Th2-type cytokine profile with more IL-4- and IL-10-producing T cells.
TABLE 3.
Spearman correlations (ρ) between the percentages of IL-12- or TNF-α-producing myeloid DCs or monocytes and the percentages of specific-cytokine-producing or surface marker-expressing T cells for all subjects and separately within the control group and the UTI-prone group
| T-cell cytokine/activation marker | % IL-12-producing myeloid DCs
|
% TNF-α-producing myeloid DCs
|
% IL-12-producing monocytes
|
% TNF-α-producing monocytes
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All subjects | Control group | UTI-prone group | All subjects | Control group | UTI-prone group | All subjects | Control group | UTI-prone group | All subjects | Control group | UTI-prone group | |
| IL-2 | 0.21 | 0.30 | 0.09 | 0.19 | 0.50b | 0.07 | 0.30a | 0.21 | 0.32 | −0.08 | −0.21 | −0.12 |
| IFN-γ | 0.08 | 0.14 | 0.11 | 0.41b | 0.72d | 0.20 | −0.06 | −0.23 | 0.01 | 0.20 | −0.06 | 0.40a |
| IL-4 | 0.26 | 0.39 | 0.13 | 0.25 | 0.44a | 0.14 | 0.26 | 0.34 | 0.18 | 0.39b | 0.49b | 0.36a |
| IL-10 | 0.49c | 0.33 | 0.42a | 0.12 | 0.17 | 0.27 | 0.49c | 0.29 | 0.33 | 0.39b | 0.45a | 0.34 |
| CD69 | 0.43c | 0.22 | 0.54b | 0.10 | 0.20 | 0.11 | 0.41b | 0.18 | 0.54b | 0.09 | 0.12 | 0.04 |
| CD25 | 0.46c | 0.60b | 0.20 | 0.26 | 0.27 | 0.39a | 0.46c | 0.72d | 0.11 | 0.46c | 0.47a | 0.43b |
| CD54 | 0.11 | −0.07 | 0.29 | 0.17 | 0.62c | −0.12 | −0.07 | −0.34 | 0.13 | −0.10 | −0.27 | 0.06 |
| CD4CD25 | 0.213 | 0.456a | −0.118 | 0.125 | 0.240 | 0.145 | 0.414c | 0.377 | 0.308 | 0.020 | −0.088 | −0.097 |
| Treg celle | 0.37b | 0.60b | 0.09 | 0.23 | 0.40 | 0.23 | 0.46c | 0.43a | 0.29 | 0.35b | 0.26 | 0.43b |
Significant correlation at the 90% confidence level.
Significant correlation at the 95% confidence level.
Significant correlation at the 99% confidence level.
Significant correlation at the 99.9% confidence level.
CD4+ CD25high.
Characteristics of vaginal microbiota in UTI-prone women.
More than 1 in 2 women (10/18 [56%]) with a history of UTI had abnormal vaginal microbiota as defined by a Nugent score greater than 3, while in the control group this was true for fewer than 1 in 7 women (2/15 [13%]) (P = 0.03). This difference was also demonstrated by a higher mean Nugent score (Fig. 2). The abnormal composition was characterized by loss or reduced numbers of lactobacilli and the presence of various potential pathogens not commonly found in the healthy vagina. This finding was also confirmed by PCR-DGGE (Table 4 and Table 5). Nugent scores indicative of BV (score over 7) were more common in members of the UTI-prone group (6/22 [27%]) than in those of the control group (1/17 [6%]) (P = 0.095). No significant differences were observed in the prevalence of specific bacteria between UTI-prone subjects and controls. Lactobacillus iners and L. crispatus were the most commonly detected lactobacillus species in both groups, while L. gasseri was detected only in members of the control group (3/15 versus 0/18; P = 0.08).
FIG. 2.
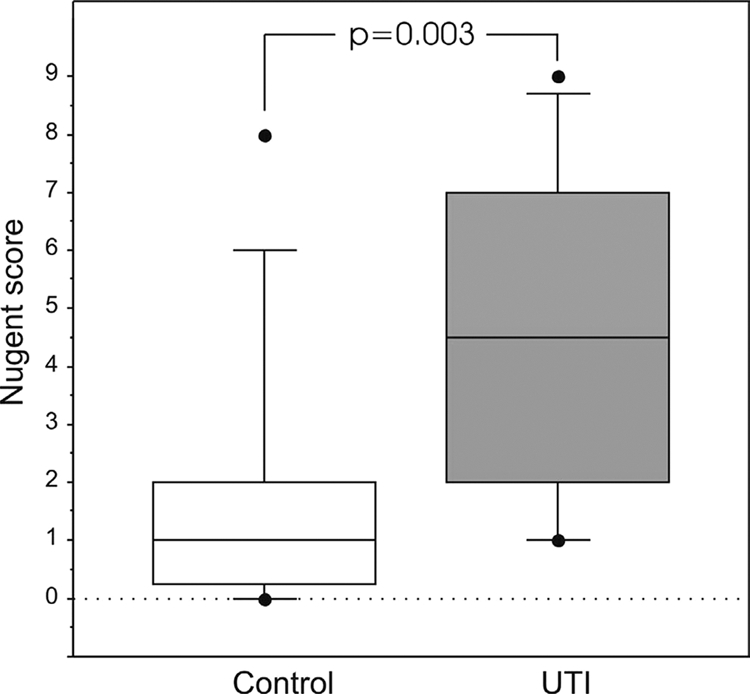
Box plot (median, 75th and 90th percentiles, and outliers) of the Nugent scores for the control group and for women prone to UTI.
TABLE 4.
Composition of vaginal microbiota in samples from control women as determined by Nugent scoring and PCR-DGGEa
| Control group subject no. | Nugent score | Lactobacillus-specific probe result (no. of positive samples)
|
Eubacterium-specific probe result
|
|||||
|---|---|---|---|---|---|---|---|---|
| L. iners | L. gasseri | L. crispatus | Lactobacillus sp. | Staphylococcus | Clostridium | Atopobium | ||
| 14 | 0 | + | ||||||
| 17 | 0 | + | ||||||
| 35 | 0 | + | ||||||
| 37 | 0 | + | ||||||
| 8 | 1 | + | + | |||||
| 9 | 1 | Weak | ||||||
| 12 | 1 | + (3) | ||||||
| 13 | 1 | + | + | |||||
| 16 | 1 | + | ||||||
| 19 | 1 | + | ||||||
| 4 | 2 | + | + | + | ||||
| 10 | 2 | + | ||||||
| 36 | 2 | + | ||||||
| 7 | 6 | + | + | |||||
| 21 | 8 | + | + | |||||
Vaginal swabs from two control subjects were quantitatively inadequate for microbiological analysis.
TABLE 5.
Composition of vaginal microbiota in UTI-prone women as determined by Nugent scoring and PCR-DGGEa
| UTI-prone subject no. | Nugent score | Lactobacillus-specific probe result (no. of positive samples)
|
Eubacterium-specific probe result
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| L. iners | L. mucosae | L. crispatus | Lactobacillus sp. | Staphylococcus | Clostridium | Fusobacterium | Streptococcus | ||
| 1 | 1 | + | + | + | |||||
| 3 | 1 | + (2) | |||||||
| 18 | 1 | + | |||||||
| 29 | 1 | + | |||||||
| 2 | 2 | + (2) | + | ||||||
| 24 | 3 | + | |||||||
| 27 | 3 | + | |||||||
| 33 | 3 | + | |||||||
| 26 | 4 | + | |||||||
| 6 | 5 | + | |||||||
| 32 | 5 | Weak | |||||||
| 34 | 6 | Weak | |||||||
| 11 | 7 | ||||||||
| 20 | 7 | + | + | + | |||||
| 30 | 7 | + | |||||||
| 28 | 8 | + | |||||||
| 5 | 9 | + | + | ||||||
| 39 | 9 | + | |||||||
Vaginal swabs from four UTI-prone subjects were quantitatively inadequate for microbiological analyses.
Exploratory analyses for associations between microbial and immunological characteristics.
Post priori assessments were performed in order to characterize the potential associations between the microbiological and immunological variables. The presence of an abnormal vaginal microbiota did not correlate significantly with the mucosal or urinary concentrations of any determined cytokines or growth factors. On the other hand, those with an abnormal microbiota had higher mean concentrations of VEGF in serum (214 pg/ml [95% CI, 157 to 272] versus 141 pg/ml [95% CI, 94 to 188]) (P = 0.02) and a higher percentage of putative Treg cells (1.30% [95% CI, 0.82 to 1.80] versus 0.88% [95% CI, 0.62 to 1.13] of total cells) (P = 0.01). Because of the low prevalence of abnormal microbiotas in controls (n = 2), it was not possible to account for the group effect but, notably, these trends were also present in the UTI-prone group alone (P = 0.076 and 0.026, respectively, for VEGF and Treg cells).
Because BV was strongly associated with UTI, we investigated the immunological effects of BV within the UTI-prone group alone. The subjects with BV microbiota (Nugent > 6) had a significantly higher percentage of CD69-expressing T cells compared to those with a normal microbiota (Nugent < 4) (94.3% [95% CI, 89.2 to 99.5%] versus 98.2% [95% CI, 96.5 to 99.8%], respectively) (P = 0.045). There were also tendencies (P = 0.05) toward a higher percentage of IL-4-producing T cells (6.1% [95% CI, 0.1 to 12.0%] versus 3.0% [95% CI, 0.5 to 5.4%]) and Treg cells (1.2% [95% CI, 0.4 to 2.0%] versus 0.8% [95% CI, 0.4 to 1.3%]) but a lower presence of DCs in total (0.73% [95% CI, 0.11 to 1.35%] versus 0.93% [95% CI, 0.55 to 1.30%]). Because there was only one BV subject within the control group, it was impossible to determine whether these BV effects were limited to the UTI-prone group.
The detection of at least one of the typical vaginal Lactobacillus species, namely, L. iners, L. crispatus, or L. gasseri, as determined by PCR-DGGE results indicative of dominance of the species was associated with increased detection of IL-17 in vaginal swabs (8/12 [67%] versus 4/18 [33%]) (P = 0.02) and a higher mean concentration of VEGF (1,349 pg/ml [95% CI, 0 to 2,841] versus 289 pg/ml (95% CI, 115 to 462) (P = 0.046). The presence of these lactobacilli was also associated with a higher serum concentration of IL-10 (20.5 pg/ml [95% CI, 0 to 52.2] versus 1.5 pg/ml [95% CI, 0.9 to 2.1]) (P = 0.02) but, in contrast, with a lesser presence of T cells producing IL-10 in response to polyclonal stimulation (1.9% [95% CI, 0.4 to 3.4%] versus 3.8 [95% CI, 2.5 to 5.1%]) (P = 0.04). In those subjects with L. iners as the lone Lactobacillus species, there were significantly higher relative numbers of Treg cells (0.90 [95% CI, 0.61 to 1.19] versus 0.55 [95% CI, 0.34 to 0.76]) and tendencies (within an 85% to 95% CI level) toward enhanced T-cell production of IFN-γ, IL-4, and IL-10, a higher serum concentration of VEGF, a decreased presence of monocytes, and a decreased mean concentration of IFN-γ in urine compared to the results seen with those with L. crispatus alone (data not shown).
DISCUSSION
There were two key characteristics in the immunological profile of UTI-prone women. First, the reduced presence of VEGF, PDGF-bb, and MCP-1 in the vaginal mucosa suggests that the vaginal immune responses may have been defective. VEGF and PDGF-bb are among the first-line factors in tissue repair (17), while MCP-1 is one of the primary chemoattractans of monocytes and DCs. Thereby, the susceptibility to infection may have been enhanced due to a compromised epithelial barrier and ability to clear invading bacteria as well as to the formation of APC-mediated adaptive responses. As VEGF is produced by several different cell types, including leukocytes, a relatively low mucosal concentration may reflect inappropriate recruitment and/or activation of leukocytes such as neutrophils in the vaginal mucosa. Notably, uropathogens as well as BV microbes may be able suppress or evade the induction of a neutrophil response (4, 10). VEGF synthesis may be enhanced by Toll-like-receptor-mediated signals and could thereby also reflect differences in microbial stimuli, for example, in those provided by the aberrant endogenous vaginal microbiota (17). In our study, higher VEGF concentrations in the vagina were seen in subjects predominantly colonized with at least one of the Lactobacillus species typical of a healthy vaginal microbiota. In addition, IL-17 was more commonly found in the samples from these subjects, an important finding considering its central role in the induction of neutrophil-mediated protective immune response against extracellular microbes (31). In contrast to our findings for adults, enhanced VEGF production-associated gene polymorphism (50) and enhanced urinary MCP-1 excretion have previously been linked with UTI in pediatric patients (20).
The second key finding links recurrent UTI with systemically skewed immune system responsiveness and low-grade chronic inflammation. Notwithstanding the increased IL-12 production by APCs, a key cellular response for promoting type 1 T-cell differentiation, there was no evidence of T-cell polarization. It is tempting to speculate that, while APCs have been properly primed to produce IL-12, there is a defect in converting this into Th1 response and memory. This hypothesis is particularly attractive considering the role of Th1 cells in the formation of cell-mediated immune responses, the dominance of such responses in defense against uropathogens, and the apparent ineffectiveness of antibody-mediated protection (15, 44). It has been shown in studies employing animal models of UTI that DCs are recruited to the uroepithelium and capable of uptake of bacterial antigens (14) and that antigen-specific T-cell responses protective of reinfection may be formed (46).
The exploratory bivariate correlation analysis results provided support for the idea that defective T-cell activation may be associated with increased susceptibility to UTI recurrence. It was shown that the T-cell activation marker CD25 may have been improperly induced in response to IL-12 produced by the APCs. Interestingly, it has been shown that signal transducer and activator of transcription 4 (STAT4) is the critical mediator of IL-12-induced expression of CD25 (35) and that STAT4 deficiency in mice is associated with reduced IFN-γ levels, accelerated nephritis, and increased mortality (24). Another characteristic of the UTI-prone subjects studied here was the lack of correlation between CD54 T-cell surface expression and TNF-α production by DCs. Costimulation during antigen presentation via CD54 leads to increased proliferation and production of Th1 cytokines and supports formation of T cells with a memory phenotype (28).
Whether or not the observed tendency for IL-12-skewed cytokine production in APCs is linked with the etiology of UTI, the finding provides a novel perspective in terms of an association between UTI and T-cell-mediated inflammatory diseases, such as rheumatoid arthritis (13) and type 1 diabetes (19). In studies of these diseases, APC-produced IL-12 has been linked with the disease pathogenesis (32, 47).
Recently, the vaginal fluid from women with BV was shown to induce the activation of plasmacytoid DCs (43). It is interesting that despite harboring a BV-resembling microbiota, this cell population was diminished in the UTI-prone women. While the reduction in the size of the peripheral pool of plasmacytoid DCs may reflect the infiltration of these cells to mucosal surfaces, another possibility is that plasmacytoid DC expansion and activation are defective in these women. Notably, such a defect could reduce resistance to viral pathogens, considering that as type I IFN-producing cells, the plasmacytoid DCs play a significant role in antiviral defenses (41).
The tendency to higher serum IL-8 levels in UTI-prone women is also worthy of note, albeit whether or not this represents a true difference needs to be confirmed with a larger study population. As a primary neutrophil chemoattactant, IL-8 is vital for bacterial clearance in cases of UTI (15), and elevated urine and serum levels can be detected during infection, particularly in cases of pyelonephritis (27, 40). Elevated levels during disease-free periods could be indicative of chronic low-rate infection with the uropathogen. Accordingly, a repeat UTI seems typically to be caused by the same endogenous strain rather than by reinfection with a new strain (26).
It is well known that uropathogens are more abundant in the vaginal microbiota of women prone to UTI infection (6, 33) and that BV is associated with active UTI (39). Here, we have confirmed that BV-resembling vaginal microbiota, in which the normal lactobacillus morphotype dominance has been shifted to a more mixed composition, is also typical during a disease-free period for women prone to UTI. While these microbiota were not dominated by the most prominent uropathogens per se, they were likely to be defective in terms of resisting infections because of the lack of colonization resistance enhancing lactobacillus accumulation. This underscores the potential of using probiotic lactobacilli in UTI prevention. Such lactobacilli have been shown to be capable of displacing pathogens and of normalizing the microbiota (8, 37) and to be as effective as antibiotics in prevention of UTI in children with persistent primary vesicoureteral reflux (29). The increasing prevalence of antibiotic-resistant uropathogens as well as the side effects of prolonged use of antibiotics (30, 38) further supports the use of alternative treatment modalities in UTI prevention.
It is possible that the observed aberrance in the microbiota was associated with a history of antibiotic treatments (15), but the reason for the inability of the normal lactobacillus-rich microbiota to reestablish itself remains to be determined. The only observation with respect to any possible abnormality in the type of lactobacilli present was the lack of L. gasseri in the UTI-prone subjects. However, the sample size was too small to make any definitive conclusions.
The abnormal microbiota in the UTI-prone subjects was associated with an increased presence of type 2 T cells and increased numbers of putative Treg cells in the periphery. The Treg cellular response may reflect an attempt of the body to control inflammation associated with the bacterial disturbance, even in cases in which it was at a distant mucosal site. As a bystander effect, predominant Treg cell function can result in inhibition of T-cell activation in the microenvironment. A similar increase in numbers of Treg cells was found in subjects colonized only by L. iners compared to the results seen with those with only L. crispatus. Previously, L. crispatus has been suggested to be more often linked with health and L. iners with disease (25). No clear immunological support was found for this claim in this study population.
In conclusion, the microbiological and immunological characteristics of UTI-prone women demonstrated in this study could translate to compromised defense against uropathogens and thus could explain the repeat infections. The microbiological data underscore the protective importance of Lactobacillus-rich endogenous microbiota. The immunological data, namely, the deficiencies of MCP-1 and VEGF in the vaginal mucosa, suggest compromised local immunity in UTI-prone women. The data also raise the issue of whether susceptibility to repeat infections is associated with defective translation of APC priming into T-cell activation and formation of T-cell memory. The importance of this issue is highlighted by the lack of protective antibody-mediated immunity against UTI. We acknowledge that the limitations of this study included the relatively small sample size, a lack of local cellularity assessments, and a lack of an accounting for the influences of hormonal fluctuations. Studies with larger study populations and aiming to further unravel the relationship between the composition of vaginal microbiota, immunological parameters, and UTI susceptibility are warranted.
Acknowledgments
This project was funded by NSERC of Canada. P.V.K. is funded by the Academy of Finland and K.A. by the Ontario Neurotrauma Foundation.
The technical assistance of Dominque Lam is greatly appreciated.
Footnotes
Published ahead of print on 19 November 2008.
REFERENCES
- 1.Almeida, J., C. Bueno, M. C. Alguero, M. L. Sanchez, M. C. Canizo, M. E. Fernandez, J. M. Vaquero, F. J. Laso, L. Escribano, J. F. San Miguel, and A. Orfao. 1999. Extensive characterization of the immunophenotype and pattern of cytokine production by distinct subpopulations of normal human peripheral blood MHC II+/lineage− cells. Clin. Exp. Immunol. 118392-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aroutcheva, A., D. Gariti, M. Simon, S. Shott, J. Faro, J. A. Simoes, A. Gurguis, and S. Faro. 2001. Defense factors of vaginal lactobacilli. Am. J. Obstet. Gynecol. 185375-379. [DOI] [PubMed] [Google Scholar]
- 3.Baecher-Allan, C., V. Viglietta, and D. A. Hafler. 2004. Human CD4+CD25+regulatory T cells. Semin. Immunol. 1689-98. [DOI] [PubMed] [Google Scholar]
- 4.Billips, B. K., S. G. Forrestal, M. T. Rycyk, J. R. Johnson, D. J. Klumpp, and A. J. Schaeffer. 2007. Modulation of host innate immune response in the bladder by uropathogenic Escherichia coli. Infect. Immun. 755353-5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boskey, E. R., K. M. Telsch, K. J. Whaley, T. R. Moench, and R. A. Cone. 1999. Acid production by vaginal flora in vitro is consistent with the rate and extent of vaginal acidification. Infect. Immun. 675170-5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruce, A. W., P. Chadwick, A. Hassan, and G. F. VanCott. 1973. Recurrent urethritis in women. Can. Med. Assoc. J. 108973-976. [PMC free article] [PubMed] [Google Scholar]
- 7.Bueno, C., J. Almeida, M. C. Alguero, M. L. Sanchez, J. M. Vaquero, F. J. Laso, J. F. San Miguel, L. Escribano, and A. Orfao. 2001. Flow cytometric analysis of cytokine production by normal human peripheral blood dendritic cells and monocytes: comparative analysis of different stimuli, secretion-blocking agents and incubation periods. Cytometry 4633-40. [PubMed] [Google Scholar]
- 8.Burton, J. P., P. A. Cadieux, and G. Reid. 2003. Improved understanding of the bacterial vaginal microbiota of women before and after probiotic instillation. Appl. Environ. Microbiol. 6997-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burton, J. P., and G. Reid. 2002. Evaluation of the bacterial vaginal flora of 20 postmenopausal women by direct (Nugent score) and molecular (polymerase chain reaction and denaturing gradient gel electrophoresis) techniques. J. Infect. Dis. 1861770-1780. [DOI] [PubMed] [Google Scholar]
- 10.Cauci, S., S. Guaschino, D. De Aloysio, S. Driussi, D. De Santo, P. Penacchioni, and F. Quadrifoglio. 2003. Interrelationships of interleukin-8 with interleukin-1beta and neutrophils in vaginal fluid of healthy and bacterial vaginosis positive women. Mol. Hum. Reprod. 953-58. [DOI] [PubMed] [Google Scholar]
- 11.Dahn, A., S. Saunders, K. C. Anukam, J.-A. Hammond, D. Carter, P. Kirjavainen, and G. Reid. 2008. Vaginal gene expression changes and Lactobacillus presence in women treated with oral Premarin estrogen replacement therapy. Microbes Infect. 10620-627. [DOI] [PubMed] [Google Scholar]
- 12.Devillard, E., J. P. Burton, and G. Reid. 2005. Complexity of vaginal microflora as analyzed by PCR denaturing gradient gel electrophoresis in a patient with recurrent bacterial vaginosis. Infect. Dis. Obstet. Gynecol. 1325-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebringer, A., and T. Rashid. 2006. Rheumatoid arthritis is an autoimmune disease triggered by Proteus urinary tract infection. Clin. Dev. Immunol. 1341-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engel, D., U. Dobrindt, A. Tittel, P. Peters, J. Maurer, I. Gutgemann, B. Kaissling, W. Kuziel, S. Jung, and C. Kurts. 2006. Tumor necrosis factor alpha- and inducible nitric oxide synthase-producing dendritic cells are rapidly recruited to the bladder in urinary tract infection but are dispensable for bacterial clearance. Infect. Immun. 746100-6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finer, G., and D. Landau. 2004. Pathogenesis of urinary tract infections with normal female anatomy. Lancet Infect. Dis. 4631-635. [DOI] [PubMed] [Google Scholar]
- 16.Foxman, B. 1990. Recurring urinary tract infection: incidence and risk factors. Am. J. Public Health 80331-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frantz, S., K. A. Vincent, O. Feron, and R. A. Kelly. 2005. Innate immunity and angiogenesis. Circ. Res. 9615-26. [DOI] [PubMed] [Google Scholar]
- 18.Fredricks, D. N., T. L. Fiedler, and J. M. Marrazzo. 2005. Molecular identification of bacteria associated with bacterial vaginosis. N. Engl. J. Med. 3531899-1911. [DOI] [PubMed] [Google Scholar]
- 19.Goswami, R., C. S. Bal, S. Tejaswi, G. V. Punjabi, A. Kapil, and N. Kochupillai. 2001. Prevalence of urinary tract infection and renal scars in patients with diabetes mellitus. Diabetes Res. Clin. Pract. 53181-186. [DOI] [PubMed] [Google Scholar]
- 20.Grandaliano, G., L. Gesualdo, F. Bartoli, E. Ranieri, R. Monno, A. Leggio, G. Paradies, E. Caldarulo, B. Infante, and F. P. Schena. 2000. MCP-1 and EGF renal expression and urine excretion in human congenital obstructive nephropathy. Kidney Int. 58182-192. [DOI] [PubMed] [Google Scholar]
- 21.Griebling, T. L. 2007. Urinary tract infection in women, p. 587-620. In M. S. Litwin and C. S. Saigal (ed.), Urologic diseases in America. U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. U.S. Government Printing Office, Washington, DC.
- 22.Gupta, K., A. E. Stapleton, T. M. Hooton, P. L. Roberts, C. L. Fennell, and W. E. Stamm. 1998. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli colonization in women with recurrent urinary tract infections. J. Infect. Dis. 178446-450. [DOI] [PubMed] [Google Scholar]
- 23.Hopkins, W. J., D. T. Uehling, and D. S. Wargowski. 1999. Evaluation of a familial predisposition to recurrent urinary tract infections in women. Am. J. Med. Genet. 83422-424. [PubMed] [Google Scholar]
- 24.Jacob, C. O., S. Zang, L. Li, V. Ciobanu, F. Quismorio, A. Mizutani, M. Satoh, and M. Koss. 2003. Pivotal role of Stat4 and Stat6 in the pathogenesis of the lupus-like disease in the New Zealand mixed 2328 mice. J. Immunol. 1711564-1571. [DOI] [PubMed] [Google Scholar]
- 25.Jakobsson, T., and U. Forsum. 2007. Lactobacillus iners: a marker of changes in the vaginal flora? J. Clin. Microbiol. 453145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jantunen, M. E., H. Saxen, E. Salo, and A. Siitonen. 2002. Recurrent urinary tract infections in infancy: relapses or reinfections? J. Infect. Dis. 185375-379. [DOI] [PubMed] [Google Scholar]
- 27.Ko, Y. C., N. Mukaida, S. Ishiyama, A. Tokue, T. Kawai, K. Matsushima, and T. Kasahara. 1993. Elevated interleukin-8 levels in the urine of patients with urinary tract infections. Infect. Immun. 611307-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohlmeier, J. E., L. M. Rumsey, M. A. Chan, and S. H. Benedict. 2003. The outcome of T-cell costimulation through intercellular adhesion molecule-1 differs from costimulation through leucocyte function-associated antigen-1. Immunology 108152-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee, S. J., Y. H. Shim, S. J. Cho, and J. W. Lee. 2007. Probiotics prophylaxis in children with persistent primary vesicoureteral reflux. Pediatr. Nephrol. 221315-1320. [DOI] [PubMed] [Google Scholar]
- 30.Manges, A. R., J. R. Johnson, B. Foxman, T. T. O'Bryan, K. E. Fullerton, and L. W. Riley. 2001. Widespread distribution of urinary tract infections caused by a multidrug-resistant Escherichia coli clonal group. N. Engl. J. Med. 3451007-1013. [DOI] [PubMed] [Google Scholar]
- 31.Matsuzaki, G., and M. Umemura. 2007. Interleukin-17 as an effector molecule of innate and acquired immunity against infections. Microbiol. Immunol. 511139-1147. [DOI] [PubMed] [Google Scholar]
- 32.McInnes, I. B., and G. Schett. 2007. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 7429-442. [DOI] [PubMed] [Google Scholar]
- 33.Navas-Nacher, E. L., F. Dardick, M. F. Venegas, B. E. Anderson, A. J. Schaeffer, and J. L. Duncan. 2001. Relatedness of Escherichia coli colonizing women longitudinally. Mol. Urol. 531-36. [DOI] [PubMed] [Google Scholar]
- 34.Nugent, R. P., M. A. Krohn, and S. L. Hillier. 1991. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J. Clin. Microbiol. 29297-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Sullivan, A., H. C. Chang, Q. Yu, and M. H. Kaplan. 2004. STAT4 is required for interleukin-12-induced chromatin remodeling of the CD25 locus. J. Biol. Chem. 2797339-7345. [DOI] [PubMed] [Google Scholar]
- 36.Pfau, A., and T. Sacks. 1981. The bacterial flora of the vaginal vestibule, urethra and vagina in premenopausal women with recurrent urinary tract infections. J. Urol. 126630-634. [DOI] [PubMed] [Google Scholar]
- 37.Saunders, S., A. Bocking, J. Challis, and G. Reid. 2007. Effect of Lactobacillus challenge on Gardnerella vaginalis biofilms. Colloids Surf. B Biointerfaces 55138-142. [DOI] [PubMed] [Google Scholar]
- 38.Schooff, M., and K. Hill. 2005. Antibiotics for recurrent urinary tract infections. Am. Fam. Physician 711301-1302. [PubMed] [Google Scholar]
- 39.Sharami, S. H., M. Afrakhteh, and M. Shakiba. 2007. Urinary tract infections in pregnant women with bacterial vaginosis. J. Obstet. Gynaecol. 27252-254. [DOI] [PubMed] [Google Scholar]
- 40.Sheu, J. N., M. C. Chen, K. H. Lue, S. L. Cheng, I. C. Lee, S. M. Chen, and G. J. Tsay. 2006. Serum and urine levels of interleukin-6 and interleukin-8 in children with acute pyelonephritis. Cytokine 36276-282. [DOI] [PubMed] [Google Scholar]
- 41.Shortman, K., and S. H. Naik. 2007. Steady-state and inflammatory dendritic-cell development. Nat. Rev. Immunol. 719-30. [DOI] [PubMed] [Google Scholar]
- 42.Stamm, W. E., and R. Raz. 1999. Factors contributing to susceptibility of postmenopausal women to recurrent urinary tract infections. Clin. Infect. Dis. 28723-725. [DOI] [PubMed] [Google Scholar]
- 43.St. John, E. P., J. Martinson, J. A. Simoes, A. L. Landay, and G. T. Spear. 2007. Dendritic cell activation and maturation induced by mucosal fluid from women with bacterial vaginosis. Clin. Immunol. 12595-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Svanborg, C., W. Agace, H. Connell, B. Frendeus, G. Godaly, L. Hang, M. Hedlund, P. Rollano, M.-L. Svensson, G. Otto, and B. Wullt. 1999. Urinary tract infections of the mucosal immune system, p. 1381-1393. In P. L. Ogra, J. Mestecky, M. E. Lamm, W. Strober, J. Bienenstock, and J. R. McGhee (ed.), Mucosal immunology, 2nd ed. Academic Press, London, United Kingdom.
- 45.Tabel, Y., A. Berdeli, and S. Mir. 2007. Association of TLR2 gene Arg753Gln polymorphism with urinary tract infection in children. Int. J. Immunogenet. 34399-405. [DOI] [PubMed] [Google Scholar]
- 46.Thumbikat, P., C. Waltenbaugh, A. J. Schaeffer, and D. J. Klumpp. 2006. Antigen-specific responses accelerate bacterial clearance in the bladder. J. Immunol. 1763080-3086. [DOI] [PubMed] [Google Scholar]
- 47.Trembleau, S., G. Penna, S. Gregori, N. Giarratana, and L. Adorini. 2003. IL-12 administration accelerates autoimmune diabetes in both wild-type and IFN-gamma-deficient nonobese diabetic mice, revealing pathogenic and protective effects of IL-12-induced IFN-gamma. J. Immunol. 1705491-5501. [DOI] [PubMed] [Google Scholar]
- 48.Walter, J., C. Hertel, G. W. Tannock, C. M. Lis, K. Munro, and W. P. Hammes. 2001. Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 672578-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xie, J., B. Foxman, L. Zhang, and C. F. Marrs. 2006. Molecular epidemiologic identification of Escherichia coli genes that are potentially involved in movement of the organism from the intestinal tract to the vagina and bladder. J. Clin. Microbiol. 442434-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yim, H. E., I. S. Bae, K. H. Yoo, Y. S. Hong, and J. W. Lee. 2007. Genetic control of VEGF and TGF-beta1 gene polymorphisms in childhood urinary tract infection and vesicoureteral reflux. Pediatr. Res. 62183-187. [DOI] [PubMed] [Google Scholar]