Abstract
Cell-mediated immunity plays a major role in conferring protection against tuberculosis (TB) on an individual. It is not known whether the immune status correlates with the bacterial load or whether the immunity improves after treatment. Also, it may be important to monitor treatment by being able to discriminate between active disease and successfully treated TB. The main aim of this study was to investigate the usefulness of a recombinant 32-kDa antigen (r32-kDa Ag) of Mycobacterium bovis BCG (Ag85A-BCG) as a diagnostic marker in patients being treated for TB. Specifically, the in vitro T-cell assays and the release of interleukin-12 (IL-12) (Th1-type cytokine) and IL-10 (Th2-type cytokine) in response to the r32-kDa Ag of BCG were assayed in patients with either pulmonary (sputum positive/negative, n = 74) or extrapulmonary TB (n = 49) and healthy controls. The proliferative responses of stimulated cells at 0, 2 to 4, and 6 months of treatment increased and were highly significant (P < 0.000) compared to the responses in controls. The increase in IL-12 and decrease in IL-10 release suggest that there is cytokine expression modification during different stages of TB, and treatment seems to have an influence on the levels of these cytokines, suggesting an augmentation in the protective responses. The in vitro response to the M. bovis BCG r32-kDa Ag may be useful in monitoring treatment of TB.
Tuberculosis (TB) remains a major global health problem. Mycobacterium tuberculosis infects one-third of the world's population, causing approximately 8 million new cases and 3 million deaths per year (4, 22). Since it is established that cell-mediated immunity plays a major role in conferring protection against TB on an individual and that the immunity of a patient with TB is compromised, an immunological test which reflects the clinical status of an individual is warranted. On the other hand, a test which discriminates between a patient with active disease and a successfully treated person may be useful in monitoring treatment.
We demonstrated earlier that the tuberculin skin test is not a good indicator of immune status (27, 28, 29). The study was conducted in India, a region with environmental mycobacteria where TB is endemic and the Mycobacterium bovis BCG vaccine is routinely administered to children. This endemicity may also influence the sensitivity of an assay which involves measurement of gamma interferon (IFN-γ) concentrations induced by purified protein derivative (27), ESAT-6, and/or CFP-10 (17). The assay appears to be only as sensitive as the tuberculin skin test (17). Furthermore, in the context of the rising prevalence of drug-resistant TB, immunotherapy in conjunction with antibiotics may gain importance in the control of the disease. Therefore, there is a need to identify a mycobacterial immunogenetic protein.
Many cytokines are being evaluated as surrogate markers for successful TB therapy, as the balance between Th1 and Th2 cytokines is likely to determine the fate of the infection (18). The pretreatment biomarkers may perhaps be useful during early treatment, which could help in identifying relapsing patients. Moreover, the biomarkers possibly will also aid in grouping patients for enhanced clinical treatment administration (30). The antigen 85 (Ag85) complex of mycobacteria is a major secretory product and has been found to induce T-cell proliferation and antibody synthesis against TB (20, 32). The results of our earlier studies based on an in vitro T-cell assay using a recombinant 32-kDa Ag (r32-kDa Ag) of Mycobacterium bovis BCG (Ag85A-BCG) revealed high levels of IFN-γ levels in BCG-vaccinated children, indicating its effectiveness both as a booster vaccine (1, 2) and for use in in vitro tests, by virtue of being immunogenic. Hence, the present study investigates the influence of the r32-kDa Ag Ag85A-BCG on the in vitro stimulation and activation of lymphocyte proliferative responses and the production of interleukin-12 (IL-12) (Th1 type) and IL-10 (Th2 type) cytokines in patients with TB.
MATERIALS AND METHODS
Materials.
A total of 153 subjects were included in the study, 74 with pulmonary TB (PTB) and 49 with extrapulmonary TB (EPTB) who attended a Free Chest DOTS Clinic (directly observed therapy—short course) at Mahavir Hospital and Research Center between January 2004 and February 2007 and 30 asymptomatic, healthy volunteers. The criteria used for the diagnosis were sputum smear microscopy and chest X ray for PTB and histopathology examination of tissue biopsy for EPTB. The tuberculin skin test was not performed in all patients and controls (27, 28, 29). All the individuals in the control group had a BCG scar. At the time of preparation of the manuscript, response to anti-TB treatment shown by alleviation of symptoms, sputum conversion, and weight gain further reiterated the diagnosis of TB in these patients. The subjects were stratified into seven groups (numbers of patients in groups are mentioned in parentheses): (i) PTB-0 (34) and (ii) EPTB-0 (33), at the time of diagnosis; (iii) PTB-2/4 (19) and (iv) EPTB-2/4 (10), at between 2 and 4 months of treatment; (v) PTB-6 (21) and (vi) EPTB-6 (6), after completion of treatment (6 months) and cured; and (vii) controls (30). The study was approved by the Institutional Ethics Committee. Peripheral venous blood was collected from all the subjects after an informed consent was obtained from them.
Mycobacterium bovis BCG r32-kDa Ag.
M. bovis r32-kDa Ag was synthesized as described earlier (1, 2).
PBMC proliferation assay.
The peripheral blood mononuclear cell (PBMC) proliferation assay was performed as described earlier (1, 2). Briefly, PBMCs were isolated from heparinized blood by density gradient centrifugation using a Histopaque-1077 (Sigma, St. Louis, MO). Cells were then cultured in RPMI 1640 complete medium (Invitrogen Corporation, Grand Island, NY) at a concentration of 1 × 106 cells/ml (Falcon Products, Becton Dickinson, Oxnard, CA) and stimulated with either 4 μl (3 mg/ml) M. bovis BCG r32-kDa Ag or 30 μl (1 mg/ml) concanavalin A (Sigma Aldrich, St. Louis, MO), the latter as a positive control for cell reactivity. The cells were incubated for 5 days and 3 days, respectively, at 37°C in an atmosphere of 5% CO2. Supernatants were collected and stored at −80°C for further studies. After the addition of MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide], the optical density (OD) was recorded by using an enzyme-linked immunosorbent assay plate reader (Anthos HT II; Anthos Labtec Instruments, Salzburg, Austria) using a dual wavelength of 570 nm with a 620-nm reference filter. Data were expressed as the stimulation index (SI), i.e., the ratio of the mean OD of experimental cultures to the mean OD of control cultures, which was considered positive if the value was >2.
IL-12p40 and IL-10 assay.
To measure the concentrations of IL-12 and IL-10 in culture supernatants with a positive SI, an enzyme-linked immunosorbent assay was performed using kits for cytokine detection (BD Opt EIA for human IL-12, catalogue no. 555171, and for human IL-10, catalogue no. 555157; BD Biosciences, San Diego, CA). The preparation of all reagents and the working standards and protocol were according to the manufacturer's instructions. The antibody pairs used were capture antibody (anti-human IL-12 and IL-10 monoclonal antibodies) and detection antibody (biotinylated anti-human IL-12 and IL-10 monoclonal antibodies).
Statistical analysis.
The data were analyzed using Statistical Package for Social Sciences (SPSS) statistical software (version 11.1). The results are presented as means and standard deviations (SD); the Kruskal-Wallis test and analysis of variance were used to compare the differences between the groups. A difference was considered statistically significant if the P value was <0.05. The median results were also calculated, and they were similar to the mean values.
RESULTS
PBMC proliferative responses induced by the M. bovis BCG r32-kDa Ag.
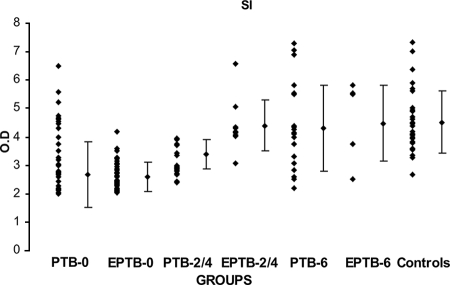
When stimulated with concanavalin A, 86% of patients with both PTB and EPTB and 100% of healthy controls had positive proliferative responses (data not shown). When stimulated with the Ag, 67% of all patients and 100% of the healthy group had positive SI values. The mean values for the PTB-0, EPTB-0, and PTB-2/4 groups (2.66 ± 0.55 [± SD], 2.59 ± 0.43, and 3.38 ± 0.94, respectively) were significantly low (P < 0.000) compared with the mean value for the control group (4.51 ± 1.09) (Fig. 1).
FIG. 1.
SIs of lymphoproliferative assay results for different clinical categories of patients with TB and healthy controls after in vitro stimulation with r32-kDa Ag of M. bovis BCG. Dots represent SIs. Diamonds with vertical bars represent mean ± SD of results for each group. Results are shown for the following groups (with the number of patients in the group in parentheses): PTB-0 (34) and EPTB-0 (33), PTB-2/4 (19) and EPTB-2/4 (10), PTB-6 (21) and EPTB-6 (6), and healthy controls (30). The P value was <0.000 for control group results versus PTB-0, EPTB-0, and PTB-2/4 group results.
IL-12p40 release assays in patients and healthy controls after in vitro stimulation with M. bovis BCG r32-kDa Ag.
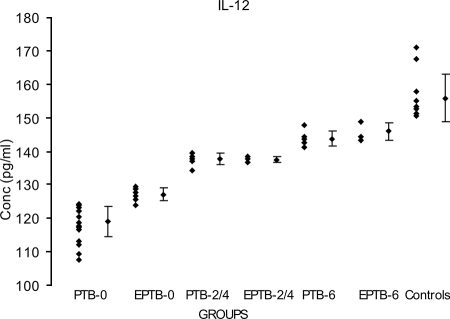
The IL-12p40 levels in the PTB-0 (119 ± 4.62 pg/ml) and EPTB-0 (127.1 ± 1.87 pg/ml) groups were significantly lower than the corresponding value (155.8 ± 7.06 pg/ml) in the control group (P < 0.000). The mean values for the PTB-2/4, EPTB-2/4, PTB-6, and EPTB-6 groups (137.3 ± 1.65 pg/ml, 137.5 ± 0.86 pg/ml, 144.3 ± 2.63 pg/ml, and 145.7 ± 2.17 pg/ml, respectively) were also highly significant compared with the mean value for the control group (155.8 ± 7.06 pg/ml) (for PTB-2/4, P < 0.000; for EPTB-2/4, P < 0.028; for PTB-6, P < 0.001; and for EPTB-6, P < 0.017) (Fig. 2). The lowest mean value observed was for the PTB-0 group, while the highest was for the control group.
FIG. 2.
Mean IL-12 levels in supernatants of r32-kDa Ag-stimulated PBMCs from 65 TB patients in different clinical categories and from healthy controls. Dots represent levels in pg/ml. Diamonds with vertical bars represent mean ± SD of results for each category. Results are shown for the following groups (with the number of patients in the group in parentheses): PTB-0 (24) and EPTB-0 (9), PTB-2/4 (7) and EPTB-2/4 (3), PTB-6 (6) and EPTB-6 (5), and healthy controls (11). P values were <0.000 for control group results versus PTB-0, EPTB-0, and PTB-2/4 group results; <0.028 for control group results versus EPTB-2/4 group results; <0.001 for control group results versus PTB-6 group results; and <0.017 for control group results versus EPTB-6 group results.
IL-10 release assays in patients and healthy controls after in vitro stimulation with M. bovis BCG r32-kDa Ag.
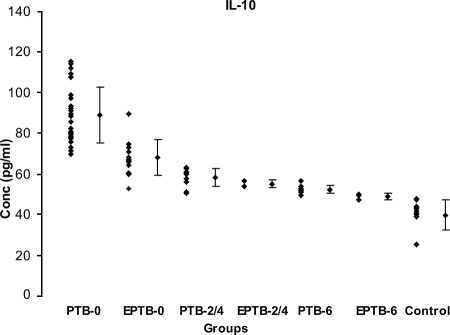
The mean IL-10 level in the controls (39.70 ± 7.36 pg/ml) was significantly low compared to the levels in PTB-0 (88.9 ± 13.8 pg/ml) and EPTB-0 patients (67.1 ± 8.63 pg/ml) (P < 0.000); PTB-2/4 (57.2 ± 4.0 pg/ml) and EPTB-2/4 patients (55 ± 1.97 pg/ml) (P < 0.010); and PTB-6 (49.12 ± 3.82 pg/ml) (P < 0.000) and EPTB-6 patients (48.8 ± 1.44 pg/ml) (P < 0.002) (Fig. 3). Contrary to the proliferation (SI) and IL-12 levels, the lowest level was seen in the control group and the highest in the PTB-0 group.
FIG. 3.
Mean IL-10 levels in supernatants of r32-kDa Ag-stimulated PBMCs from 78 TB patients in different clinical categories and from healthy controls. Dots represent levels in pg/ml. Diamonds with vertical bars represent mean ± SD of results for each category. Results are shown for the following groups (with the number of patients in the group in parentheses): PTB-0 (28) and EPTB-0 (14), PTB-2/4 (10) and EPTB-2/4 (2), PTB-6 (9) and EPTB-6 (3), and healthy controls (12). P values were <0.000 for results for control group versus PTB-0, EPTB-0, and PTB-6 groups; <0.010 for results for control group versus those for PTB-2/4 and EPTB-2/4 groups; and <0.002 for results for control group versus those for EPTB-6 group.
The mean levels of IL-12p40 and IL-10 at the time of diagnosis were 125 ± 6.3 pg/ml and 79 ± 1.6 pg/ml, respectively, for all patients and 156 ± 7.1 pg/ml and 40 ± 7.4 pg/ml, respectively, for controls, and the IL-12p40/IL-10 ratio for patients was 1.58. The ratios increased after 2 to 4 months and after 6 months of treatment (2.36 and 2.8, respectively) (P < 0.05) (Fig. 4).
FIG. 4.
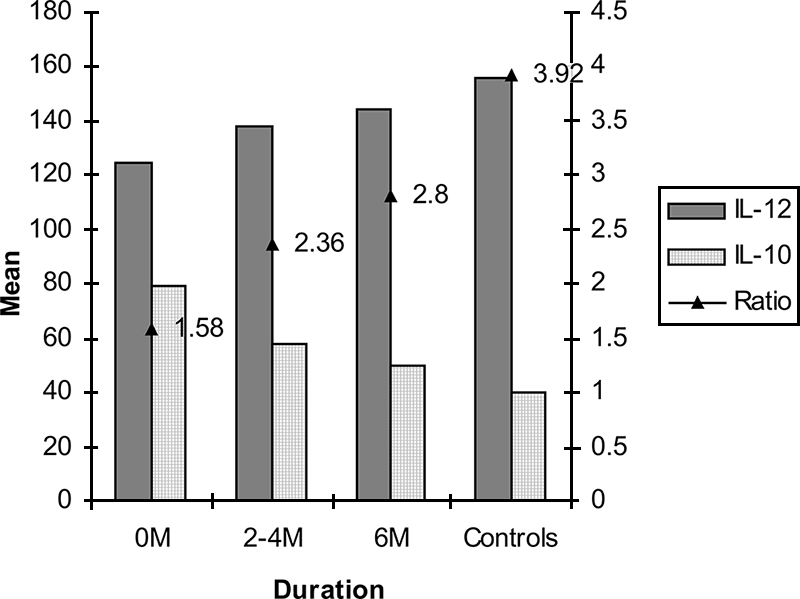
Means and ratios of IL-12/IL-10 levels (pg/ml) in supernatants of M. bovis BCG r32-kDa Ag-stimulated PBMCs over duration of treatment for all the categories of patients in comparison with controls.
DISCUSSION
The Ag85 complex which has been the focus of study over the past several years comprises three closely related proteins, 85A (32 kDa), 85B (30 kDa), and 85C (32.5 kDa) (1, 2, 16, 31, 32). The complex induces T-cell proliferation in PBMCs from healthy tuberculin reactors and might become a candidate for use as a sensitive marker for clinical status of the patient or a novel TB vaccine (7, 9, 25). Indeed, MVA85A, a recombinant modified vaccinia virus Ankara expressing Ag 85A, is the first candidate TB subunit vaccine to enter human trials since BCG was first introduced over 80 years ago. However, its potential use as an in vitro correlate of protection has yet to be established (12). In a study conducted by us with children, the in vitro response seemed to reflect the clinical status of the child (1, 2). It was demonstrated that the response of patients to this 85A Ag was significantly lower than that of healthy controls. This being a region where TB is endemic, the majority of individuals are exposed to environmental mycobacteria. Furthermore, the controls in this study were BCG vaccinated.
The proliferative response toward M. bovis BCG r32-kDa Ag in all the patients with PTB and EPTB was enhanced after completion of treatment in this study, suggesting improvements in cell-mediated immunity. Similarly, Jo et al. (10) reported that the lymphoproliferative response to either the 30- or 32-kDa Ag of M. tuberculosis increased in all patients after 2 months of treatment (10). Furthermore, Boesen et al. reported that patients with active advanced TB exhibit a depressed immune response to mycobacterial Ags (3). In another study, low proliferative responses against recombinant M. tuberculosis 10-kDa, 30-kDa, 32-kDa, and 60-kDa Ags were reported in PBMCs from TB patients (13). The depressed responses may be due to the suppressive factors secreted by monocytes and lymphocytes and may also be due to a shift from the Th1 to the Th2 type of cytokine response (11, 13, 15, 19). Immunosuppression may also be due to the preferential sequestration of Ag-specific T cells into the infected areas, leading to their absence in peripheral blood (24).
Furthermore, augmentation was also observed in the in vitro release of IL-12 in relation to treatment in the present study. IL-12 might play a crucial role by regulating IFN-γ production and the cytotoxic effector function of mycobacterial Ag-specific T cells (26, 33, 34, 35). The results of a study by Fulton et al. (5) suggested that IL-12 released by infected macrophages can in turn further upregulate M. tuberculosis-specific CD4+ T-cell effector function (5). A longitudinal study by Song et al. (23) also reported observations for the 30- or 32-kDa Ag of M. tuberculosis that were similar to those of the present study, suggesting that the early stage of active PTB may be associated with depressed IL-12 expression. Based on these results, Song et al. and Fulton et al. furthermore hypothesized that patients with active TB show increased IL-12 production after therapy due to an afferent feedback signal of IFN-γ and that the resultant IL-12 can upregulate more IFN-γ (6, 23).
The high levels of IL-10 observed in patients in this study decreased in those who were cured, albeit not to the levels seen in healthy subjects. Likewise, Song et al. also illustrated high levels of IL-10 production in some patients stimulated with 30- or 32-kDa Ag, though the levels were not significant compared with those in healthy tuberculin reactors (23). Increased levels of IL-10 in response to 30-kDa Ag of M. tuberculosis in TB patients were demonstrated by Torres et al. (25), suggesting that high levels of IL-10 may be due to decreased blastogenic response and IFN-γ production during active TB (25). In another study, conducted by Hirsch et al. (8), the levels of IL-10 in sera and PBMCs from TB patients in response to M. tuberculosis Ags were high (8). The decrease in IL-10 release suggests that there might be a modification of cytokine expression during different stages of TB, and treatment seems to have an influence on the levels of cytokines, with IL-12, a Th1 cytokine, increasing and IL-10, a Th2 cytokine, decreasing, suggesting an augmentation in the protective responses. These changes are probably related to the Ag load, which decreases after treatment (14, 20). No data for EPTB patients were reported by other studies with respect to the 32-kDa Ag. In the present study, the ratios of the IL-12/IL-10 levels in PTB and EPTB patients increased with treatment. In contrast, in a study conducted by Sahiratmadja et al. (21), the IL-12/IL-10 ratio decreased at the end of the therapy, whereas the IFN-γ/IL-10 ratio showed a slight increase at the end of therapy, suggesting a shift toward a proinflammatory host immune phenotype during control of infection (21).
These results strongly suggest that the balance in the Th1 and Th2 cytokines might play a major role in the clinical outcome for the patient. Among the groups, the sputum-positive group had the lowest immune response (least proliferation, lowest IL-12 levels, and highest IL-10 levels). Follow-up of all patients soon after treatment is completed may help in identifying patients who are probably cured and, more important, those who are likely to return to the clinic to be categorized as “retreatment cases.” In other words, an early diagnosis of the “retreatment cases” may help in monitoring treatment and may have an impact on the national program (category II according to the Revised National Tuberculosis Control Programme [RNTCP] classification; the RNTCP is a comprehensive strategy for TB control in India).
In conclusion, the level of in vitro response to the r32-kDa Ag of M. bovis BCG leading to the release of IL-12 seems to reflect the clinical status of the patient. Further studies may indicate the use of this Ag in in vitro tests to assess the treatment outcome.
Acknowledgments
We are grateful to Bhagwan Mahavir Trust for their financial help in carrying out the study.
We thank Akbar Yazdani, P. S. Raju, and the staff of the TB clinic for the clinical help rendered during the study.
Footnotes
Published ahead of print on 5 November 2008.
REFERENCES
- 1.Anuradha, B., C. M. Santosh, V. Hari Sai Priya, G. Suman Latha, K. J. R. Murthy, and Valluri Vijaya Lakshmi. 2007. Age-related waning of in vitro interferon-γ levels against r32KDaBCG in BCG vaccinated children. J. Immune Based Ther. Vaccines 58. doi: 10.1186/1476-8518-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anuradha, B., S. S. Rakh, M. Ishaq, K. J. R. Murthy, and V. L. Valluri. 2008. Interferon-γ low producer genotype +874 overrepresented in bacillus Calmette-Guerin nonresponding children. Pediatr. Infect. Dis. J. 27325-329. [DOI] [PubMed] [Google Scholar]
- 3.Boesen, H., N. B. Jensen, T. Wilcke, and P. Andersen. 1995. Human T-cell responses to secreted antigen fractions of Mycobacterium tuberculosis. Infect. Immun. 631491-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 282677-686. [DOI] [PubMed] [Google Scholar]
- 5.Fulton, S. A., J. M. Johnsen, S. F. Wolf, D. S. Sieburth, and W. H. Boom. 1996. Interleukin-12 production by human monocytes infected with Mycobacterium tuberculosis: role of phagocytosis. Infect. Immun. 642523-2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fulton, S. A., J. V. Cross, Z. T. Toossi, and W. H. Boom. 1998. Regulation of interleukin-12 by interleukin-10, transforming growth factor-β, tumor necrosis factor-α and interferon-γ in human monocytes infected with Mycobacterium tuberculosis H37Ra. J. Infect. Dis. 1781105-1114. [DOI] [PubMed] [Google Scholar]
- 7.Havlir, D. V., R. S. Wallis, W. H. Boom, T. M. Daniel, K. Chervenak, and J. J. Ellner. 1991. Human immune response to Mycobacterium tuberculosis antigens. Infect. Immun. 59665-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirsch, C. S., J. J. Ellner, R. Blinkhorn, and Z. Toossi. 1997. In vitro restoration of T cell responses in tuberculosis and augmentation of monocyte effector function against mycobacterium tuberculosis by natural inhibitors of transforming growth factor beta. Proc. Natl. Acad. Sci. USA 943926-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huygen, K., J. P. Van Vooren, M. Turneer, R. Bosmans, P. Dierckx, and J. De Bruyn. 1988. Specific lymphoproliferation, gamma interferon production, and serum immunoglobulin G directed against a purified 32 kDa mycobacterial protein antigen (P32) in patients with active tuberculosis. Scand. J. Immunol. 27187-194. [DOI] [PubMed] [Google Scholar]
- 10.Jo, E. K., H. J. Kim, D. Min, Y. Song, C. H. Song, T. H. Paik, J. W. Suhr, and J. K. Park. 2000. Dysregulated production of interferon-gamma, interleukin-4 and interleukin-6 in early tuberculosis patients in response to antigen 85B of Mycobacterium tuberculosis. Scand. J. Immunol. 51209-217. [DOI] [PubMed] [Google Scholar]
- 11.Kleinhenz, M. E., and J. J. Eliner. 1987. Antigen responsiveness during tuberculosis: regulatory interactions of T cell subpopulations and adherent cells. J. Lab. Clin. Med. 11031-36. [PubMed]
- 12.McShane, H., A. A. Pathan, C. R. Sander, S. M. Keating, S. C. Gilbert, K. Huygen, H. A. Fletcher, and A. V. S. Hill. 2004. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat. Med. 101240-1244. [DOI] [PubMed] [Google Scholar]
- 13.Mehra, V., J. Gong, D. V. Iyer, Y. Lin, C. T. Boylen, B. R. Bloom, and P. F. Barnes. 1996. Immune response to recombinant mycobacterial proteins in patients with tuberculosis. J. Infect. Dis. 174431-434. [DOI] [PubMed]
- 14.Moura, E. P., V. P. C. P. Toledo, M. H. P. Oliveira, S. Spíndola-de-Miranda, H. M. Andrade, and T. M. P. D. Guimarães. 2004. Pulmonary tuberculosis: evaluation of interferon-γ levels as an immunological healing marker based on the response to the bacillus Calmette-Guerin. Mem. Inst. Oswaldo Cruz 99283-287. [DOI] [PubMed] [Google Scholar]
- 15.Munk, E. M., J. De Bruyn, H. Gras, and S. H. Kaufmann. 1994. The Mycobacterium bovis 32-kilodalton protein antigen induces human cytotoxic T-cell responses. Infect. Immun. 62726-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagai, S., H. G. Wiker, M. Harboe, and M. Kinomoto. 1991. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect. Immun. 59372-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pai, M. 2005. Alternatives to the tuberculin skin test: interferon-γ assays in the diagnosis of Mycobacterium Tuberculosis infection. Indian J. Med. Microbiol. 23151-158. [DOI] [PubMed] [Google Scholar]
- 18.Raja, A. 2004. Immunology of tuberculosis. Indian J. Med. Res. 120213-232. [PubMed] [Google Scholar]
- 19.Romangnani, S. 1994. Lymphokine production by human T cells in disease states. Annu. Rev. Immunol. 7227-257. [DOI] [PubMed] [Google Scholar]
- 20.Rossi, G. A., B. Balbi, and F. Manca. 1987. Tuberculous pleural effusions. Evidence for selective presence of PPD-specific T-lymphocytes at site of inflammation in the early phase of the infection. Am. Rev. Respir. Dis. 136575-579. [DOI] [PubMed] [Google Scholar]
- 21.Sahiratmadja, E., B. Alisjahbana, T. de Boer, I. Adnan, A. Maya, H. Danusantoso, Ronald H. H. Nelwan, S. Marzuki, J. W. M. van der Meer, R. van Crevel, E. van de Vosse, and T. H. M. Ottenhoff. 2007. Dynamic changes in pro- and anti-inflammatory cytokine profiles and gamma interferon receptor signaling integrity correlate with tuberculosis disease activity and response to curative treatment. Infect. Immun. 75820-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sepkowitz, K. A. 1995. AIDS, tuberculosis, and health care workers. Clin. Infect. Dis. 20232-242. [DOI] [PubMed] [Google Scholar]
- 23.Song, C.-H., H.-J. Kim, J.-K. Park, J.-H. Lim, U.-O. Kim, J.-S. Kim, T.-H. Paik, K.-J. Kim, J.-W. Suhr, and E.-K. Jo. 2000. Depressed interleukin-12 (IL-12), but not IL-18, production in response to a 30- or 32-kilodalton mycobacterial antigen in patients with active pulmonary tuberculosis. Infect. Immun. 684477-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Surcel, M., M. T. Blomberg, and S. Paulie. 1994. Th1/Th2 profiles in tuberculosis based on the proliferation and cytokine response of blood lymphocytes to mycobacterial antigens. Immunology 81171-178. [PMC free article] [PubMed] [Google Scholar]
- 25.Torres, M., T. Herrera, H. Villareal, E. A. Rich, and E. Sada. 1998. Cytokine profiles for peripheral blood lymphocytes from patients with active pulmonary tuberculosis and healthy household contacts in response to the 30-kilodalton antigen of Mycobacterium tuberculosis. Infect. Immun. 66176-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trinchieri, G. 1994. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood 844008-4027. [PubMed] [Google Scholar]
- 27.Vijaya Lakshmi, V., Sunil Kumar, H. Surekha Rani, G. Suman Latha, and K. J. R. Murthy. 2005. Tuberculin specific T cell responses in BCG vaccinated children. Indian Pediatr. 4236-40. [PubMed] [Google Scholar]
- 28.Vijaya, L. V., D. V. Rao, K. J. R. Murthy, and S. N. Jain. 1989. Study of the tuberculin test and its correlation with the in vitro responses. Lung India 263-66. [Google Scholar]
- 29.Vijaya, L. V., K. J. R. Murthy, D. V. Rao, and S. N. Jain. 1990. A study of the tuberculin test II: its correlation with the in vitro humoral responses. Lung India 269-71. [Google Scholar]
- 30.Walzl, G., K. Ronacher, J. F. Djoba Siawaya, and H. M. Dockrell. 2008. Biomarkers for TB treatment response: challenges and future strategies. J. Infect. 57103-109. [DOI] [PubMed] [Google Scholar]
- 31.Wiker, H. G., and M. Harboe. 1992. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol. Rev. 56648-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiker, H. G., M. Harboe, S. Nagai, and J. Bennedsen. 1990. Quantitative and qualitative studies on the major extracellular antigen of M. tuberculosis H37Rv and Mycobacterium bovis BCG. Am. Rev. Respir. Dis. 141830-838. [DOI] [PubMed] [Google Scholar]
- 33.Wong, H. L., D. E. Wilson, J. C. Jenson, P. C. Familletti, D. L. Stremlo, and M. K. Gately. 1988. Characterization of a factor(s) which synergizes with recombinant interleukin 2 in promoting allogeneic human cytolytic T-lymphocyte responses in vitro. Cell. Immunol. 11139-54. [DOI] [PubMed] [Google Scholar]
- 34.Zhang, M., M. K. Gately, E. Wang, J. Gong, S. F. Wolf, S. Lu, R. L. Modlin, and P. F. Barnes. 1994. Interleukin 12 at the site of disease in tuberculosis. J. Clin. Investig. 931733-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang, M., J. Gong, D. H. Presky, W. Xue, and P. F. Barnes. 1999. Expression of the IL-12 receptor beta 1 and beta 2 subunits in human tuberculosis. J. Immunol. 1622441-2447. [PubMed] [Google Scholar]