Abstract
Pneumonia in cattle is an important disease both economically and in terms of animal welfare. Recent evidence in other species has shown ATP to be an important modulator of inflammation in the lung, where it is released by activated alveolar macrophages and damaged lung cells. Whether ATP serves a similar process during infection in the bovine lung is unknown. In the present study, we examined the effects of ATP treatment on the morphology, apoptosis, and permeability of bovine pulmonary epithelial (BPE) cells and bovine pulmonary microvascular endothelial cells (BPMEC). Monolayers of BPE cells underwent striking morphological changes when exposed to ATP that included separation of the cells. Neither BPE cells nor BPMEC exhibited increased apoptosis in response to ATP. BPE cell and BPMEC monolayers displayed virtually identical increases in permeability when exposed to ATP, with a 50% change occurring within the first hour of exposure. Both cell types contained mRNA for the P2X7 receptor, a known receptor for ATP. In BPE cells, but not BPMEC, the change in permeability in response to ATP was reversed by the addition of a P2X7 receptor antagonist. If similar permeability changes occur in vivo, they could be a factor in vascular leakage into lung airspaces during pneumonia.
Several infectious agents are involved in the bovine respiratory disease complex. Among the bacteria involved in this complex, Mannheimia haemolytica is associated with particularly severe lung inflammation that culminates in extensive lung pathology. One of the hallmark pathological changes associated with M. haemolytica pneumonia is the extensive leakage of vascular products into the lung interstitium and air spaces (24, 30). We have previously demonstrated that lipopolysaccharide (LPS) can induce permeability changes in endothelial cells in vitro that would be consistent with vascular leakage. However, the effects are slow, requiring well over 12 h to cause measurable effects on permeability (13). It is possible that the host releases substances in the early pathological response that contribute to the extensive leakage of vascular products into the lung. One such possible substance is ATP.
The involvement of extracellular ATP in lung inflammation is a relatively new area of research. Patients with cystic fibrosis, a chronic inflammatory lung disease in humans, have increased levels of ATP in both their sputum and lungs (4). This has been correlated with increased neutrophil numbers in the lung. In a model of asthma, extracellular ATP activated dendritic cells in the lungs, which then stimulated airway inflammation (6). Airway smooth muscle contraction, another physiologic change associated with asthma, is also stimulated by ATP (16). The effects of ATP are not always detrimental. ATP appears to have a protective effect in acute inflammation. Kolosova et al. demonstrated that a long-lasting, nonhydrolyzed form of ATP (ATPγS) given to mice after transtracheal administration of LPS prevented increases in bronchoalveolar lavage protein and white blood cell counts, Evans blue leakage into the lung, and decreased transendothelial cell electrical resistance (11). ATP has also been shown to induce anion secretion in airway epithelial cells and surfactant secretion in alveolar epithelial cells, both of which help to remove noxious particles from the lung (10, 22, 25). In addition, extracellular ATP appears to downregulate the human monocyte response to LPS through Toll-like receptor 4 (8).
There are many sources of ATP during lung inflammation, including bacteria, apoptotic and necrotic host cells, activated epithelial cells, and macrophages (1, 5, 23, 26). The effects of increased ATP in the bovine lung and its involvement in inflammation have not been studied previously. The purpose of the present study was to investigate the effects of ATP on bovine lung epithelial and endothelial cells in vitro.
MATERIALS AND METHODS
Cell culture.
Bovine pulmonary epithelial (BPE) cells were isolated from a yearling Holstein-cross heifer calf at the time of slaughter, using a procedure described previously (13). Briefly, the lungs were aseptically removed from the thoracic cavity, and strips of tissue from the distal portion of the accessory lung lobe were removed, washed three times, minced, and then digested in a solution containing 1% protease (Sigma, St. Louis, MO). After digestion, the filtrate was passed through a series of filters with decreasing pore sizes. The final filtrate was then placed on a Percoll gradient (Amersham Bioscience, Piscataway, NJ) and centrifuged (1,000 × g). After the cells were recovered from the Percoll, they were washed three times, resuspended in RMPI (Cellgro; Mediatech, Inc., Herndon, VA), and placed in several wells of a six-well tissue culture plate. After 1 h at 37°C, the nonadherent cells were removed and transferred to a second plate. This process was repeated twice. After 1 week, all wells were examined, and areas of cells with typical epithelial morphology were recovered by the use of cloning cylinders (Sigma) and trypsin-EDTA (Cambrex, East Rutherford, NJ). The recovered cells were transferred to new six-well plates and, after the cell monolayers became confluent, a second round of selective trypsinization with cloning cylinders was performed. After several promising cell lines were expanded, their epithelial origin was confirmed by performing immunohistochemistry staining with an anti-cytokeratin antibody (AE1/AE3; Dako, Carpinteria, CA).
Bovine pulmonary microvascular endothelial cells (BPMEC) were purchased from CS-C Cell Systems (Kirkland, WA). Both cell types were maintained in media consisting of Dulbecco modified Eagle medium-Ham F-12 mix (Cellgro) with 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), a penicillin (100 IU)-streptomycin (100 μg/ml) mix (Cellgro), 2 mM glutamine (Cellgro), and 1 μg of insulin (Sigma)/ml. In addition, 1 ng of epithelial growth factor (Sigma)/ml was added to the medium for the BPE cells. The cells were grown in tissue culture plates (Falcon; BD Biosciences, Franklin Lakes, NJ), and when the cells were confluent they were passaged using trypsin.
BPE cell and BPMEC morphology, apoptosis, and necrosis assays.
BPE cells and BPMEC were grown on six-well plates (Falcon). When the cells were confluent, either diluent alone or 0.1, 1, or 5 mM ATP (Sigma) was added to the wells, and the plate incubated for 6, 12, or 24 h. At the indicated time points, the cells were examined by using an inverted light microscope (Diaspot; Nikon, Japan) and photographed. Adherent cells were recovered by trypsinization and washed twice with Dulbecco phosphate-buffered salt solution (Fisher, Fair Lawn, NJ). A commercial kit (BD Biosciences) that uses annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) staining was used to determine the level of apoptosis and necrosis in the cells using the protocol provided by the commercial supplier. For analysis, a FACSCalibur (BD Biosciences) flow cytometer was used to determine the two-color fluorescence of the cells. A four-quadrant region was created for each cell type, using cells stained with either annexin V-FITC or PI. This region setup was then used to analyze 10,000 events of either the diluent or the ATP-treated cells that had been stained with both dyes. The percentage of cells in the upper right quadrant (both annexin V-FITC and PI positive; late apoptotic or necrotic cells) and in the lower right quadrant (only annexin V-FITC positive; early apoptotic cells) was determined. The experiment was repeated three times.
BPE cell and BPMEC permeability.
Permeability of the BPE cell and BPMEC monolayers was determined by measuring the Transwell electrical resistance (TEER) of the cell monolayers. The BPE cells or BPMEC were grown to confluence on 8.0-μm-pore-size Transwell culture inserts (BD Biosciences). Prior to the use of an insert in an experiment, an initial electrical resistance reading was performed to ensure that the monolayer had minimum resistances of 75 Ω2 for the BPMEC and 150 Ω2 for the BPE cells. The inserts were then aseptically transferred to an insert chamber (EndOhm-6; World Precision Instruments, Sarasota, FL) that consisted of an upper electrode in the lid that extended into the medium of the insert and a lower electrode, which sat beneath the insert in 1 ml of medium. Resistance readings between the electrodes were measured by using an ohmmeter (EVOM; World Precision Instruments). Inserts were incubated at 37°C with 5.0% CO2, and TEER measurements were performed immediately after the addition of ATP (0.1 to 5 mM) and then hourly afterward for up to 6 h. All treatment groups were run in triplicate, and all experiments were performed five times. The results for replicate samples were averaged, and the percent change from the no-treatment group at the same time point was calculated.
In a similar set of experiments, the P2X7 receptor antagonist periodate-oxidized 2′,3′-dialdehyde ATP (oATP; Sigma) was added at a concentration of 100 μM to the inserts 30 min prior to the addition of 1 mM ATP. As controls, some inserts received only oATP, ATP, or diluent prior to the resistance measurements. The TEER of the inserts was measured immediately after the addition of and 0.5, 1, 2, and 3 h after the addition of ATP.
P2X7 expression.
P2X7 expression was determined in the BPE cells and BPMEC by measuring mRNA expression for this receptor using endpoint PCR. Monolayers of BPE cells and BPMEC were grown to confluence in six-well plates. To some of the wells, 1 μg of Escherichia coli-derived LPS (Sigma)/ml was added 3 h prior to harvesting mRNA by using an RNeasy kit (Qiagen, Valencia, CA) according to the supplied protocol. The concentration and purity of the sample was checked by measuring the absorbances of the sample at 240 and 260 nm with a spectrophotometer (SmartSpec 300; Bio-Rad). mRNA (1.5 μg) was converted to cDNA by using reverse transcriptase (Reverse Transcriptase Systems; Promega, Madison, WI) according to the supplied protocol. A fixed volume of each sample cDNA was added to a Taq polymerase master mix (Promega), along with 5 μM concentrations of the forward and reverse primers for bovine P2X7 or β-actin. Primers were designed by performing a PubMed (National Center for Biotechnology Information, Bethesda, MD) search for coding sequences for these bovine genes. Using these sequences, the software program PrimerExpress (version 3; AB Biosciences, Foster City, CA) was used to design primers that had a melting point of 60°C and an amplicon length of 400 to 500 bp. Primers were manufactured at Integrated DNA Technologies (Coralville, IA). The primers were as follows: P2X7 forward, TGGATCCAAAGCAAGACTTATGG; P2X7 reverse, TACACCTGCCGGTCTGGATT; β-actin forward, CGGGCAGGTCATCACCAT; and β-actin reverse, TGCGCAAGTTAGGTTTTGTCA.
Amplification was performed by using a thermocycler (PTC-200; MJ Research, Waltham, MA). After amplification, samples were electrophoresed on a 1.5% agarose gel (power supply [PS500X2; Hoeffer Scientific], gel apparatus [Bio-Rad]). DNA bands were visualized by staining the gels in ethidium bromide (Sigma) and viewing them with a UV light source (Fotodyne, Hartford, WI). Images were captured by using a camera (Fotodyne).
Statistical analysis.
Comparisons between different treatment groups were accomplished by calculating the analysis of variance using a software program (StatView SE+; Abacus Concepts, Berkeley, CA). The Tukey-Kramer test was then used to determine which means were significantly different. Statistical significance was set at P ≤ 0.05.
RESULTS
Cell morphology of ATP-treated cells.
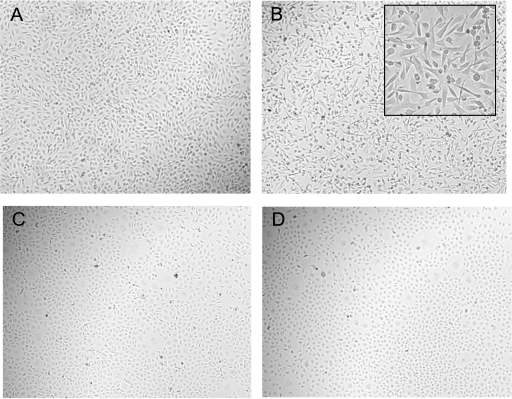
The influence of ATP on BPE cell and BPMEC morphology was analyzed by examining the cell monolayers by light microscopy. BPE cells underwent distinctive morphological changes after ATP exposure that included loss of cell-to-cell contact and a more spindlelike conformation of individual cells (Fig. 1A and B). These changes in BPE cell morphology were greatest at 6 h; at later time points the BPE cells reverted back to a morphology similar to that of untreated control cells (data not shown). In contrast, BPMEC exhibited no discernible changes in morphology after ATP treatment (Fig. 1C and D).
FIG. 1.
Shape change occurred in BPE cells but not BPMEC treated with ATP. BPE cells (A and B) and BPMEC (C and D) were exposed to diluent (A and C) or 5 mM ATP (B and D) for 6 h and then photographed (magnification, ×100). The inset in panel B shows a higher-power magnification of BPE cells treated with ATP (magnification, ×400).
Apoptosis and necrosis levels in ATP-treated cells.
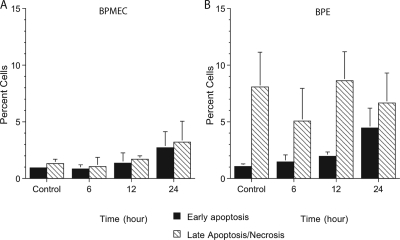
To determine whether the observed changes in the ATP-treated BPE cells were a manifestation of cell death, we assessed the uptake of annexin IV and PI to estimate the levels of necrotic or apoptotic cell death. BPE cells and BPMEC incubated with ATP for 6 to 24 h displayed a slight, but not statistically significant, increase in the numbers of early apoptotic or late apoptotic or necrotic cells compared to the untreated controls (Fig. 2).
FIG. 2.
Neither BPMEC (A) nor BPE cells (B) exhibited significant increases in cell death when treated with ATP. Cells were harvested at the times indicated, stained with annexin-FITC and PI, and then examined by flow cytometry. Gates were established based on staining with the two fluorochromes, and cells were counted based on single or double positive staining. The values shown represent the means ± the standard errors of the mean (SEM) of three separate experiments.
Permeability changes in ATP-treated cells.
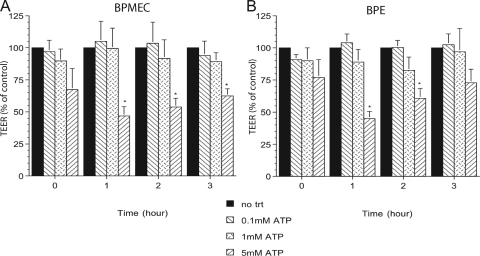
Because the morphological changes of ATP-treated BPE cells could lead to changes in monolayer permeability, we decided to examine this further by measuring the trans-endothelial or -epithelial electrical resistance (TEER). Both BPE cells and BPMEC exhibited a rapid decrease in electrical resistance almost immediately after the addition of 5 mM ATP compared to untreated cells or cells treated with 0.1 mM ATP (Fig. 3, time zero). By 1 h, BPE cell and BPMEC monolayers treated with 5 mM ATP exhibited a >50% decrease in electrical resistance. This was reversed somewhat at 2 and 3 h, although TEER was still significantly decreased compared to untreated control cells. At later time points, the TEER for ATP-treated BPE cells and BPMEC had recovered and was similar to that for untreated control cells (data not shown). Incubation with a lower concentration of ATP (1 mM) also decreased the TEER for monolayers of both cell types, although the decrease was smaller (10 and 20% in the BPMEC and BPE cells, respectively).
FIG. 3.
BPE cells and BPMEC incubated with 5 mM ATP exhibited decreased permeability (TEER). BPMEC (A) or BPE cell (B) monolayers in Transwell inserts were incubated with diluent or ATP. The electrical resistance of the monolayers was measured immediately after the addition of ATP (time zero) and at hourly intervals thereafter. The percent change in the TEER was calculated by comparing the resistance for the treated monolayers versus the time-matched control monolayers. The values shown represent the means ± the SEM of three separate experiments. *, P ≤ 0.05 compared to the diluent-treated control monolayers at the same time points.
P2X7 receptor mRNA levels.
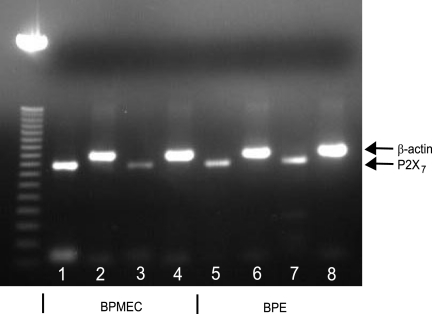
The need to use relatively high concentrations of ATP to induce changes in TEER in both cell types suggested the possible involvement of the P2X7 receptor. To determine its role in this process, we first measured P2X7 mRNA expression in BPE cells and BPMEC by using endpoint PCR. Both cell types contained mRNA for the P2X7 receptor (Fig. 4). The effects of incubation with LPS on P2X7 mRNA in BPE cells was negligible; however, there was a decrease in P2X7 mRNA levels in LPS-treated BPMEC compared to untreated control cells.
FIG. 4.
BPE cells and BPMEC express mRNA for the purinergic receptor P2X7. BPE cells and BPMEC were incubated for 3 h at 37°C with medium alone or medium containing 1 μg of LPS/ml. mRNA was collected from the BPMEC (lanes 1 to 4) and BPE cells (lanes 5 to 8), converted to cDNA, and then amplified using the primers sets for P2X7 (lanes 1, 3, 5, and 7) or β-actin (lanes 2, 4, 6, and 8). Lane 3 shows the results with BPMEC treated with LPS; lane 7 shows the results for BPE cells treated with LPS.
P2X7 receptor involvement in permeability.
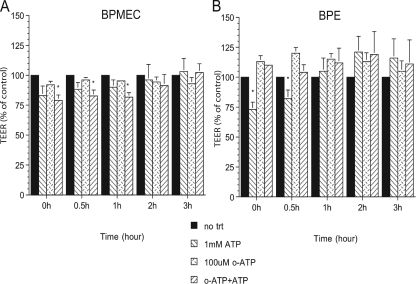
To determine whether the P2X7 receptor was involved in the altered permeability of the ATP-treated BPE cell and BPMEC monolayers, the P2X7 receptor antagonist oATP was added to each monolayer before the addition of ATP. Addition of oATP to the inserts containing BPMEC, prior to the addition of ATP, did not prevent the decrease in electrical resistance compared to monolayers treated with ATP alone (Fig. 5A). In contrast, addition of oATP to the inserts containing BPE cells, prior to adding ATP (Fig. 5B), prevented the decrease in electrical resistance seen with BPE cell monolayers incubated with ATP alone.
FIG. 5.
The permeability changes in BPE cells (A) but not BPMEC (B) incubated with ATP were prevented by preincubating the cells with the P2X7 receptor antagonist oATP. A total of 100 μM oATP was added to some inserts 30 min prior to the addition of diluent or 1 mM ATP. The electrical resistance of the monolayers was then measured immediately after the addition of ATP (time zero) and at hourly intervals afterward. The percent change in the TEER was calculated by comparing the resistance in the treated monolayers with the time-matched control monolayers. Values shown represent the mean ± the SEM of three separate experiments. *, P ≤ 0.05 compared to the diluent-treated control monolayers at the same time points.
DISCUSSION
We examined here the effects of extracellular ATP on BPE cells and BPMEC. We demonstrated that, although only epithelial cells exhibited morphological changes after ATP exposure, both endothelial and epithelial cell monolayers underwent reversible permeability (TEER) changes. These reductions in TEER were not associated with detectable increases in the numbers of apoptotic or necrotic cells. Most importantly, the changes in monolayer permeability were rapid (within minutes of adding ATP). If similar events occur in vivo, they would provide a possible explanation for the rapid leakage of vascular products into the airways of the bovine lung during the early stages of pneumonia caused by respiratory pathogens such as M. haemolytica.
The lack of correlation between morphological changes in the cell monolayers, as well as the increased permeability after ATP incubation, were unexpected. To the best of our knowledge, the effects of extracellular ATP on bovine lung endothelial and epithelial cell permeability have not been reported previously. The >50% reduction in electrical resistance (TEER) of the BPE cell monolayers after incubation with ATP was not surprising in light of the intercellular gaps that were evident by light microscopy. In contrast, BPMEC had neither visible morphological changes nor intercellular gaps, and yet they exhibited a similar 50% decrease in electrical resistance in response to ATP.
There are several potential mechanisms to explain the changes in the permeability of cell monolayers. At a cellular level, changes in the actin cytoskeleton may be critical in influencing cell permeability, since actin-myosin contraction likely creates intercellular gaps in both endothelial and epithelial cell monolayers (2, 12). In one study, actin disruption by mechanical stretching of lung epithelial cells led to decreased cell-cell attachment but did not affect concentrations of the proteins making up the tight junctions (2). A possible molecular mechanism to explain the permeability change is modification of the proteins that constitute the tight junctions between cell membranes. This process has been best described during the migration of leukocytes through endothelial cell monolayers, which requires the release of proteases by the migrating leukocytes (29). Tight and adherens junction protein modifications can also occur without protease release. For example, overexpression of Raf kinase inhibitor protein in epithelial cells decreases cell-to-cell adhesion by affecting proteins that form the adherens and tight junctions between cells (15). In regard to endothelial cells, increased intracellular Ca2+, both released from internal stores and by movement of extracellular Ca2+ into the cell, induces contraction of the cells via myosin light chain-dependent cytoskeleton retraction and the disassembly of interendothelial junctions (28). In the present study, the morphological changes observed in the ATP-treated epithelial cells were consistent with cytoskeletal changes in the cells. In contrast, morphological changes were not observed in the ATP-treated endothelial cells, although the monolayers did undergo permeability changes. Perhaps these seemingly conflicting observations reflect (i) minute cytoskeletal changes that were not detectable by light microscopy or (ii) modifications in the proteins making up the tight and adherens junctions between the cells. The decreased endothelial cell permeability we observed is contrary to a previous report by Verin and coworkers (11) of enhanced barrier protection in endothelial cells treated with ATP. The differences between that study and ours may be due to the use of mouse and human endothelial cells in the previous study, which also used a long-lasting ATP preparation (ATPγS).
Lung endothelial and epithelial cells have several receptors that could interact with extracellular ATP, including those of the P2Y and P2X families (19, 20, 27). Particular attention has been paid to the role of P2X7 in the response to ATP. One distinguishing feature of P2X7 receptor activation is that it requires relatively large (usually greater than 100 μM) concentrations of ATP (19). In the present study, 100 μM ATP had no measurable effect on monolayer permeability for either cell type, whereas an effect on monolayer permeability was observed with 1 and 5 mM ATP. Piper and coworkers previously demonstrated decreased permeability of bovine aortic endothelial cells exposed to 10 μM ATP (18), a dose that should not engage the P2X7 receptor but could potentially activate other P2Y and P2X receptors (9, 19). In the present study, we observed increased permeability at an ATP concentration that should have engaged the P2X7 receptor. Further support for P2X7 involvement in the ATP response of epithelial cells was provided by the ability of the P2X7 receptor antagonist, oATP, to block the effects of ATP on monolayer permeability. However, oATP will also interact with P2X1 and P2X2 receptors and possibly other receptors outside the P2X family (3, 19). Other P2X7 receptor antagonists also suffer from this limitation. More specific inhibitors are not commercially available at this time (17, 19). Based on our observations, including evidence that epithelial and endothelial cells express the P2X7 receptor, we infer that the P2X7 receptor was involved in the monolayer permeability changes that occurred in response to ATP. However, we cannot exclude the possibility that other receptors were also involved.
To the best of our knowledge, this is the first study to examine the effects of extracellular ATP on bovine lung endothelial and epithelial cells. With both cell types, high concentrations of ATP (≥1 mM) induced rapid and reversible changes in the permeability of the cell monolayers that did not involve increased cell necrosis or apoptosis. Several experimental studies with calves have demonstrated the leakage of blood components into the interstitial spaces and airways of the lung within the first couple of hours after inoculation with M. haemolytica (14, 24, 30). The in vitro permeability changes observed in the present study mirror the changes in vascular integrity that have been observed in these previous studies. It is still unknown at this time whether ATP levels can reach millimolar concentrations in the lung under any circumstances. It has been postulated that levels near leaking or lysed cells may get into the millimolar range, given the fact that levels of ATP in normal intact cells can be that high (7). This theory has some support, since a recent study looking at ATP levels in the interstitium of a tumor cell line found levels of ATP in the tumor to be in the hundreds of micromolar range (21). If levels of ATP can reach millimolar levels in the bovine lung, especially during infection, this would warrant additional study of extracellular ATP as a mediator in the pathogenesis of bovine respiratory disease.
Acknowledgments
This study was supported by a grant (1 K01 RR020793-01) from the National Center for Research Resources, Division of Comparative Medicine, National Institutes of Health.
Footnotes
Published ahead of print on 5 November 2008.
REFERENCES
- 1.Button, B., M. Picher, and R. C. Boucher. 2007. Differential effects of cyclic and constant stress on ATP release and mucociliary transport by human airway epithelia. J. Physiol. 580577-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cavanaugh, K. J., Jr., J. Oswari, and S. S. Margulies. 2001. Role of stretch on tight junction structure in alveolar epithelial cells. Am. J. Respir. Cell Mol. Biol. 25584-591. [DOI] [PubMed] [Google Scholar]
- 3.Chen, H. B., Y. H. Lin, and S. H. Sun. 2006. Oxidized ATP decreases beta-actin expression and intracellular superoxide concentrations in RBA-2 type-2 astrocytes independently of P2X7 receptor. Eur. J. Pharmacol. 5501-7. [DOI] [PubMed] [Google Scholar]
- 4.Esther, C. R., Jr., N. E. Alexis, M. L. Clas, E. R. Lazarowski, S. H. Donaldson, C. M. Ribeiro, C. G. Moore, S. D. Davis, and R. C. Boucher. 2008. Extracellular purine samples are biomarkers of neutrophilic airway inflammation. Eur. Respir. J. 31949-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrari, D., P. Chiozzi, S. Falzoni, S. Hanau, and F. Di Virgilio. 1997. Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. J. Exp. Med. 185579-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Idzko, M., H. Hammad, M. van Nimwegen, M. Kool, M. A. Willart, F. Muskens, H. C. Hoogsteden, W. Luttmann, D. Ferrari, F. Di Virgilio, J. C. Virchow, Jr., and B. N. Lambrecht. 2007. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat. Med. 13913-919. [DOI] [PubMed] [Google Scholar]
- 7.Jun, D. J., J. Kim, S. Y. Jung, R. Song, J. H. Noh, Y. S. Park, S. H. Ryu, J. H. Kim, Y. Y. Kong, J. M. Chung, and K. T. Kim. 2007. Extracellular ATP mediates necrotic cell swelling in SN4741 dopaminergic neurons through P2X7 receptors. J. Biol. Chem. 28237350-37358. [DOI] [PubMed] [Google Scholar]
- 8.Kaufmann, A., B. Musset, S. H. Limberg, V. Renigunta, R. Sus, A. H. Dalpke, K. M. Heeg, B. Robaye, and P. J. Hanley. 2005. “Host tissue damage” signal ATP promotes non-directional migration and negatively regulates Toll-like receptor signaling in human monocytes. J. Biol. Chem. 28032459-32467. [DOI] [PubMed] [Google Scholar]
- 9.Khakh, B. S., and R. A. North. 2006. P2X receptors as cell-surface ATP sensors in health and disease. Nature 442527-532. [DOI] [PubMed] [Google Scholar]
- 10.Knowles, M. R., L. L. Clarke, and R. C. Boucher. 1991. Activation by extracellular nucleotides of chloride secretion in the airway epithelia of patients with cystic fibrosis. N. Engl. J. Med. 325533-538. [DOI] [PubMed] [Google Scholar]
- 11.Kolosova, I. A., T. Mirzapoiazova, L. Moreno-Vinasco, S. Sammani, J. G. Garcia, and A. D. Verin. 2008. Protective effect of purinergic agonist ATPγS against acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 294L319-L324. [DOI] [PubMed] [Google Scholar]
- 12.Maniatis, N. A., and S. E. Orfanos. 2008. The endothelium in acute lung injury/acute respiratory distress syndrome. Curr. Opin. Crit. Care. 1422-30. [DOI] [PubMed] [Google Scholar]
- 13.McClenahan, D., K. Hellenbrand, D. Atapattu, N. Aulik, D. Carlton, A. Kapur, and C. Czuprynski. 2008. Effects of lipopolysaccharide and Mannheimia haemolytica leukotoxin on bovine lung microvascular endothelial cells and alveolar epithelial cells. Clin. Vaccine Immunol. 15338-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McClenahan, D. J., J. J. Fagliari, O. A. Evanson, and D. J. Weiss. 2000. Role of platelet-activating factor in alveolar septal injury associated with experimentally induced pneumonic pasteurellosis in calves. Am. J. Vet. Res. 61248-254. [DOI] [PubMed] [Google Scholar]
- 15.McHenry, K. T., R. Montesano, S. Zhu, A. B. Beshir, H. H. Tang, K. C. Yeung, and G. Fenteany. 2008. Raf kinase inhibitor protein positively regulates cell-substratum adhesion while negatively regulating cell-cell adhesion. J. Cell Biochem. 103972-985. [DOI] [PubMed] [Google Scholar]
- 16.Mounkaila, B., R. Marthan, and E. Roux. 2005. Biphasic effect of extracellular ATP on human and rat airways is due to multiple P2 purinoceptor activation. Respir. Res. 6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson, D. W., K. Sarris, D. M. Kalvin, M. T. Namovic, G. Grayson, D. L. Donnelly-Roberts, R. Harris, P. Honore, M. F. Jarvis, C. R. Faltynek, and W. A. Carroll. 2008. Structure-activity relationship studies on N′-aryl carbohydrazide P2X7 antagonists. J. Med. Chem. 513030-3034. [DOI] [PubMed] [Google Scholar]
- 18.Noll, T., H. Holschermann, K. Koprek, D. Gunduz, W. Haberbosch, H. Tillmanns, and H. M. Piper. 1999. ATP reduces macromolecule permeability of endothelial monolayers despite increasing [Ca2+]i. Am. J. Physiol. 276H1892-H1901. [DOI] [PubMed] [Google Scholar]
- 19.North, R. A. 2002. Molecular physiology of P2X receptors. Physiol. Rev. 821013-1067. [DOI] [PubMed] [Google Scholar]
- 20.Oguma, T., S. Ito, M. Kondo, Y. Makino, K. Shimokata, H. Honjo, K. Kamiya, and H. Kume. 2007. Roles of P2X receptors and Ca2+ sensitization in extracellular adenosine triphosphate-induced hyper-responsiveness in airway smooth muscle. Clin. Exp. Allergy 37893-900. [DOI] [PubMed] [Google Scholar]
- 21.Pellegatti, P., L. Raffaghello, G. Bianchi, F. Piccardi, V. Pistoia, and F. Di Virgilio. 2008. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS ONE 3e2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rice, W. R. 1990. Effects of extracellular ATP on surfactant secretion. Ann. N. Y. Acad. Sci. 60364-75. [DOI] [PubMed] [Google Scholar]
- 23.Schwiebert, E. M., and A. Zsembery. 2003. Extracellular ATP as a signaling molecule for epithelial cells. Biochim. Biophys. Acta 16157-32. [DOI] [PubMed] [Google Scholar]
- 24.Slocombe, R. F., J. Malark, R. Ingersoll, F. J. Derksen, and N. E. Robinson. 1985. Importance of neutrophils in the pathogenesis of acute pneumonic pasteurellosis in calves. Am. J. Vet. Res. 462253-2258. [PubMed] [Google Scholar]
- 25.Son, M., Y. Ito, S. Sato, T. Ishikawa, M. Kondo, S. Nakayama, K. Shimokata, and H. Kume. 2004. Apical and basolateral ATP-induced anion secretion in polarized human airway epithelia. Am. J. Respir. Cell Mol. Biol. 30411-419. [DOI] [PubMed] [Google Scholar]
- 26.Tatur, S., N. Groulx, S. N. Orlov, and R. Grygorczyk. 2007. Ca2+-dependent ATP release from A549 cells involves synergistic autocrine stimulation by coreleased uridine nucleotides. J. Physiol. 584419-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor, A. L., L. M. Schwiebert, J. J. Smith, C. King, J. R. Jones, E. J. Sorscher, and E. M. Schwiebert. 1999. Epithelial P2X purinergic receptor channel expression and function. J. Clin. Investig. 104875-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vandenbroucke, E., D. Mehta, R. Minshall, and A. B. Malik. 2008. Regulation of endothelial junctional permeability. Ann. N. Y. Acad. Sci. 1123134-145. [DOI] [PubMed] [Google Scholar]
- 29.Wagner, J. G., K. E. Driscoll, and R. A. Roth. 1999. Inhibition of pulmonary neutrophil trafficking during endotoxemia is dependent on the stimulus for migration. Am. J. Respir. Cell Mol. Biol. 20769-776. [DOI] [PubMed] [Google Scholar]
- 30.Weiss, D. J., M. C. Bauer, L. O. Whiteley, S. K. Maheswaran, and T. R. Ames. 1991. Changes in blood and bronchoalveolar lavage fluid components in calves with experimentally induced pneumonic pasteurellosis. Am. J. Vet. Res. 52337-344. [PubMed] [Google Scholar]