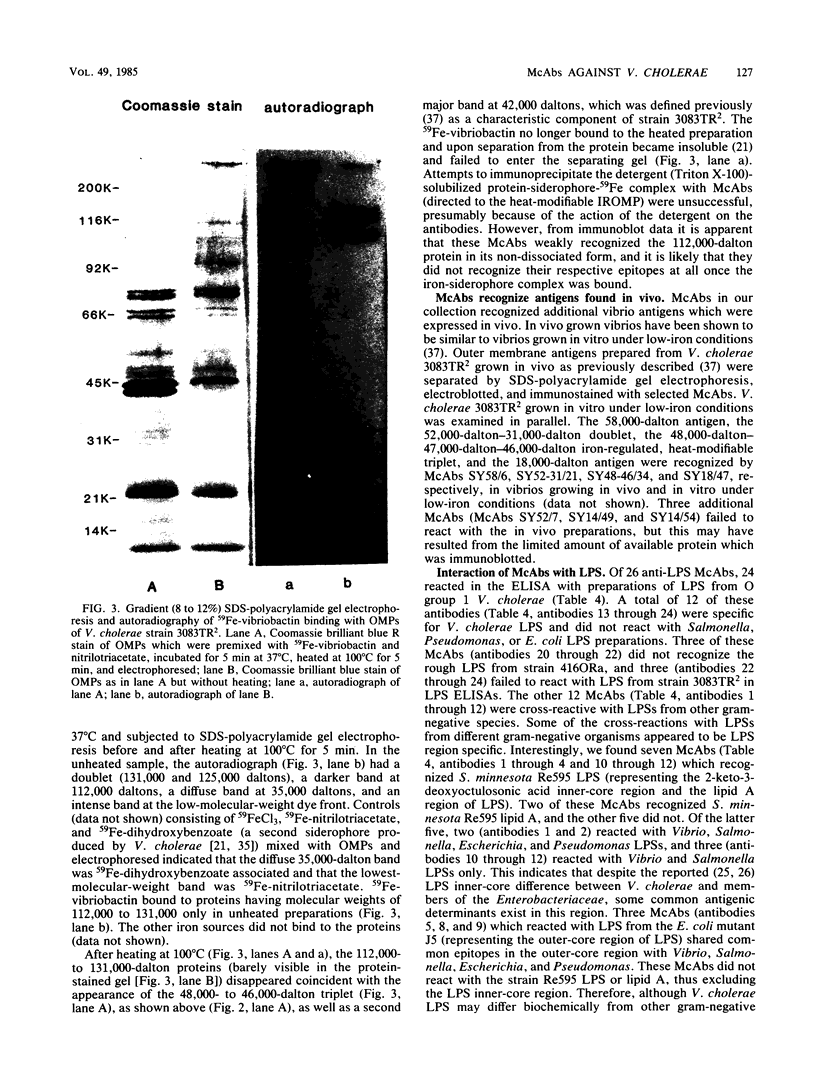
Abstract
Hybridoma-derived monoclonal antibodies were prepared against outer membrane antigens of four strains of Vibrio cholerae that were cultivated under iron-limited conditions, and these antibodies were partially characterized. We established a library of 66 hybridomas which produced monoclonal antibodies defining 16 different V. cholerae antigens. Two antigens (molecular weights, 18,000 and 112,000) were heat modifiable, whereas the reacting epitope of a third antigen (40,000-dalton-18,000-dalton doublet) was completely destroyed when it was heated at 100 degrees C. The 112,000-dalton heat-modifiable protein was an iron-regulated outer membrane protein. This protein bound 59Fe in vitro when it was combined with the V. cholerae siderophore-iron complex 59Fe-vibriobactin; it was also found in in vivo grown V. cholerae, as were three other antigens. A total of 26 hybridomas produced antibody to V. cholerae lipopolysaccharide. Of these, 12 were cross-reactive with lipopolysaccharides of other gram-negative bacteria, including 2 which recognized lipid A. Several of these anti-lipopolysaccharide monoclonal antibodies appeared to be lipopolysaccharide region specific. Some membrane antigens were strain specific, whereas others were common to both O group 1 and non-O group 1 vibrios.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banoub J. H., Shaw D. H., Michon F. Hydrolytic release, and identification by g.l.c.-m.s., of 3-deoxy-D-manno-2-octulosonic acid in the lipopolysaccharides isolated from bacteria of the Vibrionaceae. Carbohydr Res. 1983 Nov 11;123(1):117–122. doi: 10.1016/0008-6215(83)88386-4. [DOI] [PubMed] [Google Scholar]
- Blake P. A., Weaver R. E., Hollis D. G. Diseases of humans (other than cholera) caused by vibrios. Annu Rev Microbiol. 1980;34:341–367. doi: 10.1146/annurev.mi.34.100180.002013. [DOI] [PubMed] [Google Scholar]
- Brade H., Galanos C., Lüderitz O. Differential determination of the 3-Deoxy-D-mannooctulosonic acid residues in lipopolysaccharides of Salmonella minnesota rough mutants. Eur J Biochem. 1983 Mar 1;131(1):195–200. doi: 10.1111/j.1432-1033.1983.tb07249.x. [DOI] [PubMed] [Google Scholar]
- Brenner D. J., Davis B. R., Kudoh Y., Ohashi M., Sakazaki R., Shimada T., Smith H. L., Jr Serological comparison of two collections of Vibrio cholerae non O1. J Clin Microbiol. 1982 Aug;16(2):319–323. doi: 10.1128/jcm.16.2.319-323.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T. M., Eschenbach D. A., Knapp J. S., Holmes K. K. Gonococcal salpingitis is less likely to recur with Neisseria gonorrhoeae of the same principal outer membrane protein antigenic type. Am J Obstet Gynecol. 1980 Dec 1;138(7 Pt 2):978–980. doi: 10.1016/0002-9378(80)91091-1. [DOI] [PubMed] [Google Scholar]
- Craven D. E., Frasch C. E. Protection against group B meningococcal disease: evaluation of serotype 2 protein vaccines in a mouse bacteremia model. Infect Immun. 1979 Oct;26(1):110–117. doi: 10.1128/iai.26.1.110-117.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Fernandes P. B., Kim C., Cundy K. R., Haung N. N. Antibodies to cell envelope proteins of Pseudomonas aeruginosa in cystic fibrosis patients. Infect Immun. 1981 Aug;33(2):527–532. doi: 10.1128/iai.33.2.527-532.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A. Immunology of cholera. Curr Top Microbiol Immunol. 1975;69:138–196. [PubMed] [Google Scholar]
- Finkelstein R. A., Sciortino C. V., McIntosh M. A. Role of iron in microbe-host interactions. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S759–S777. doi: 10.1093/clinids/5.supplement_4.s759. [DOI] [PubMed] [Google Scholar]
- Finn T. M., Arbuthnott J. P., Dougan G. Properties of Escherichia coli grown in vivo using a chamber implant system. J Gen Microbiol. 1982 Dec;128(12):3083–3091. doi: 10.1099/00221287-128-12-3083. [DOI] [PubMed] [Google Scholar]
- Goding J. W. Antibody production by hybridomas. J Immunol Methods. 1980;39(4):285–308. doi: 10.1016/0022-1759(80)90230-6. [DOI] [PubMed] [Google Scholar]
- Griffiths G. L., Sigel S. P., Payne S. M., Neilands J. B. Vibriobactin, a siderophore from Vibrio cholerae. J Biol Chem. 1984 Jan 10;259(1):383–385. [PubMed] [Google Scholar]
- Gulig P. A., McCracken G. H., Jr, Frisch C. F., Johnston K. H., Hansen E. J. Antibody response of infants to cell surface-exposed outer membrane proteins of Haemophilus influenzae type b after systemic Haemophilus disease. Infect Immun. 1982 Jul;37(1):82–88. doi: 10.1128/iai.37.1.82-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B., Holme T. Monoclonal antibodies against group- and type-specific lipopolysaccharide antigens of Vibrio cholerae O:1. J Clin Microbiol. 1983 Sep;18(3):480–485. doi: 10.1128/jcm.18.3.480-485.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Frisch C. F., McDade R. L., Jr, Johnston K. H. Identification of immunogenic outer membrane proteins of Haemophilus influenzae type b in the infant rat model system. Infect Immun. 1981 Jun;32(3):1084–1092. doi: 10.1128/iai.32.3.1084-1092.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisatsune K., Kondo S., Iguchi T., Machida M., Asou S., Inaguma M., Yamamoto F. Sugar composition of lipopolysaccharides of family Vibrionaceae. Absence of 2-keto-3-deoxyoctonate (KDO) except in Vibrio parahaemolyticus O6. Microbiol Immunol. 1982;26(8):649–664. doi: 10.1111/j.1348-0421.1982.tb00209.x. [DOI] [PubMed] [Google Scholar]
- Jackson G. D.F., Redmond J. W. Immunochemical studies of the O-antigens of Vibrio cholerae. The constitution of a lipopolysaccharide from V. cholerae 569B (Inaba). FEBS Lett. 1971 Feb 19;13(2):117–120. doi: 10.1016/0014-5793(71)80213-2. [DOI] [PubMed] [Google Scholar]
- Kabir S., Ali S. Characterization of surface properties of Vibrio cholerae. Infect Immun. 1983 Mar;39(3):1048–1058. doi: 10.1128/iai.39.3.1048-1058.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Black R. E., Clements M. L., Cisneros L., Nalin D. R., Young C. R. Duration of infection-derived immunity to cholera. J Infect Dis. 1981 Jun;143(6):818–820. doi: 10.1093/infdis/143.6.818. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Black R. E., Clements M. L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983 Dec;47(4):510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Outer membrane protein composition in disease isolates of Haemophilus influenzae: pathogenic and epidemiological implications. Infect Immun. 1980 Dec;30(3):709–717. doi: 10.1128/iai.30.3.709-717.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutharia L. M., Crockford G., Bogard W. C., Jr, Hancock R. E. Monoclonal antibodies specific for Escherichia coli J5 lipopolysaccharide: cross-reaction with other gram-negative bacterial species. Infect Immun. 1984 Sep;45(3):631–636. doi: 10.1128/iai.45.3.631-636.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. Siderophore production by Vibrio cholerae. Infect Immun. 1978 Apr;20(1):310–311. doi: 10.1128/iai.20.1.310-311.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIBI E., MILNER K. C., PERRINE T. D. Endotoxic and antigenic fractions from the cell wall of Salmonella enteritidis; methods for separation and some biologic activities. J Immunol. 1959 Jan;82(1):75–84. [PubMed] [Google Scholar]
- SIMON E. H., TESSMAN I. THYMIDINE-REQUIRING MUTANTS OF PHAGE T4. Proc Natl Acad Sci U S A. 1963 Sep;50:526–532. doi: 10.1073/pnas.50.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciortino C. V., Finkelstein R. A. Vibrio cholerae expresses iron-regulated outer membrane proteins in vivo. Infect Immun. 1983 Dec;42(3):990–996. doi: 10.1128/iai.42.3.990-996.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears S. D., Richardson K., Young C., Parker C. D., Levine M. M. Evaluation of the human immune response to outer membrane proteins of Vibrio cholerae. Infect Immun. 1984 May;44(2):439–444. doi: 10.1128/iai.44.2.439-444.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel S. P., Payne S. M. Effect of iron limitation on growth, siderophore production, and expression of outer membrane proteins of Vibrio cholerae. J Bacteriol. 1982 Apr;150(1):148–155. doi: 10.1128/jb.150.1.148-155.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol P. A., Woods D. E. Demonstration of an iron-siderophore-binding protein in the outer membrane of Pseudomonas aeruginosa. Infect Immun. 1983 May;40(2):665–669. doi: 10.1128/iai.40.2.665-669.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson S. B., Nurminen M., Lindberg A. A. Artificial Salmonella vaccines: O-antigenic oligosaccharide-protein conjugates induce protection against infection with Salmonella typhimurium. Infect Immun. 1979 Sep;25(3):863–872. doi: 10.1128/iai.25.3.863-872.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Surface-exposed protein antigens of the gonococcal outer membrane. Infect Immun. 1981 Dec;34(3):804–816. doi: 10.1128/iai.34.3.804-816.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal O., Jann K., Himmelspach K. Chemistry and immunochemistry of bacterial lipopolysaccharides as cell wall antigens and endotoxins. Prog Allergy. 1983;33:9–39. [PubMed] [Google Scholar]