Abstract
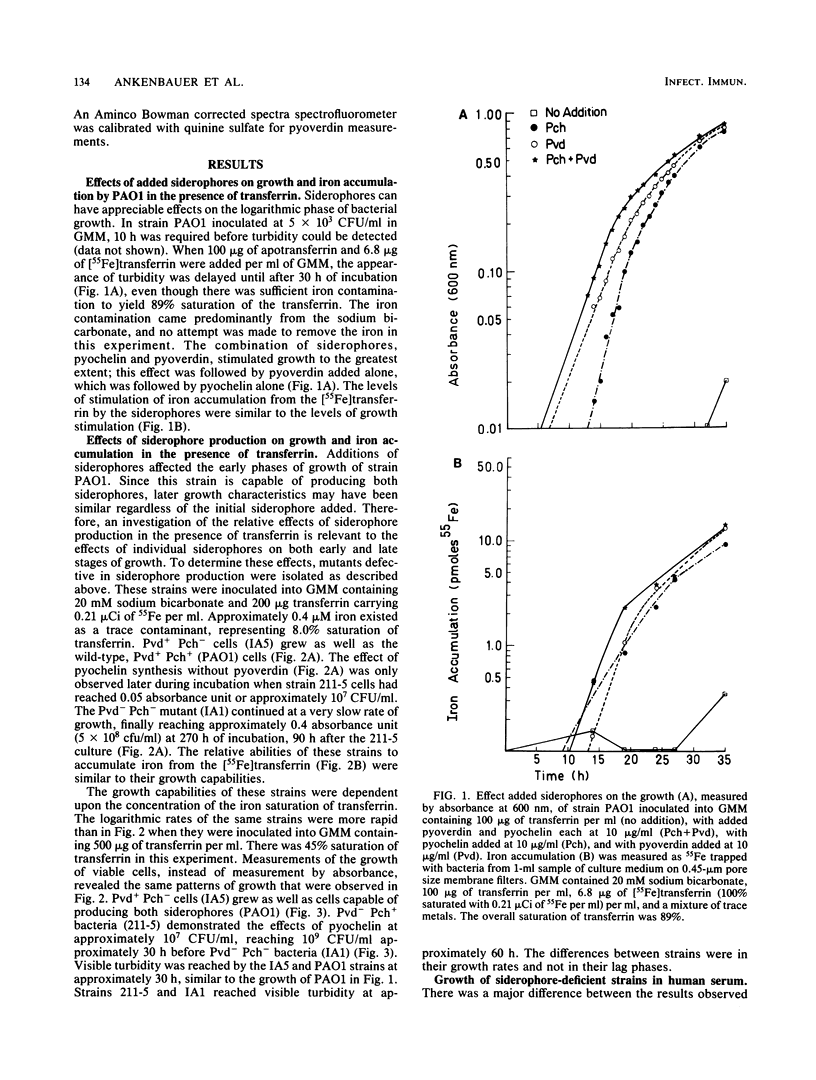
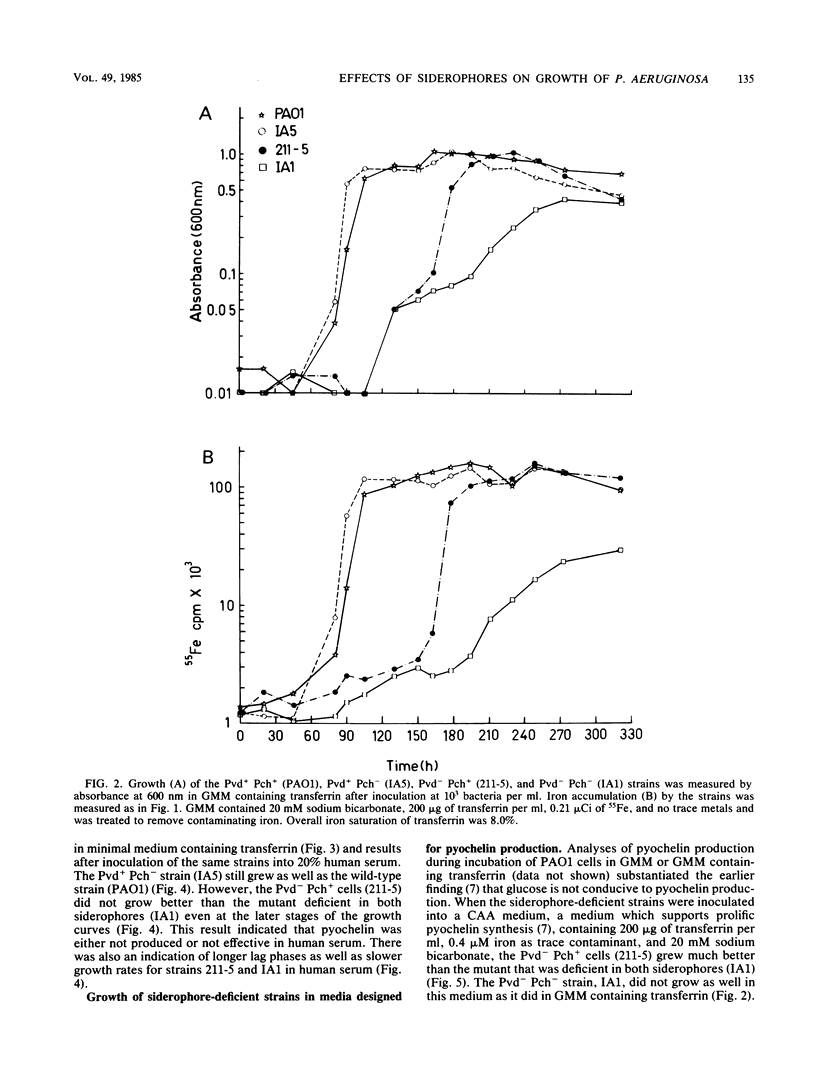
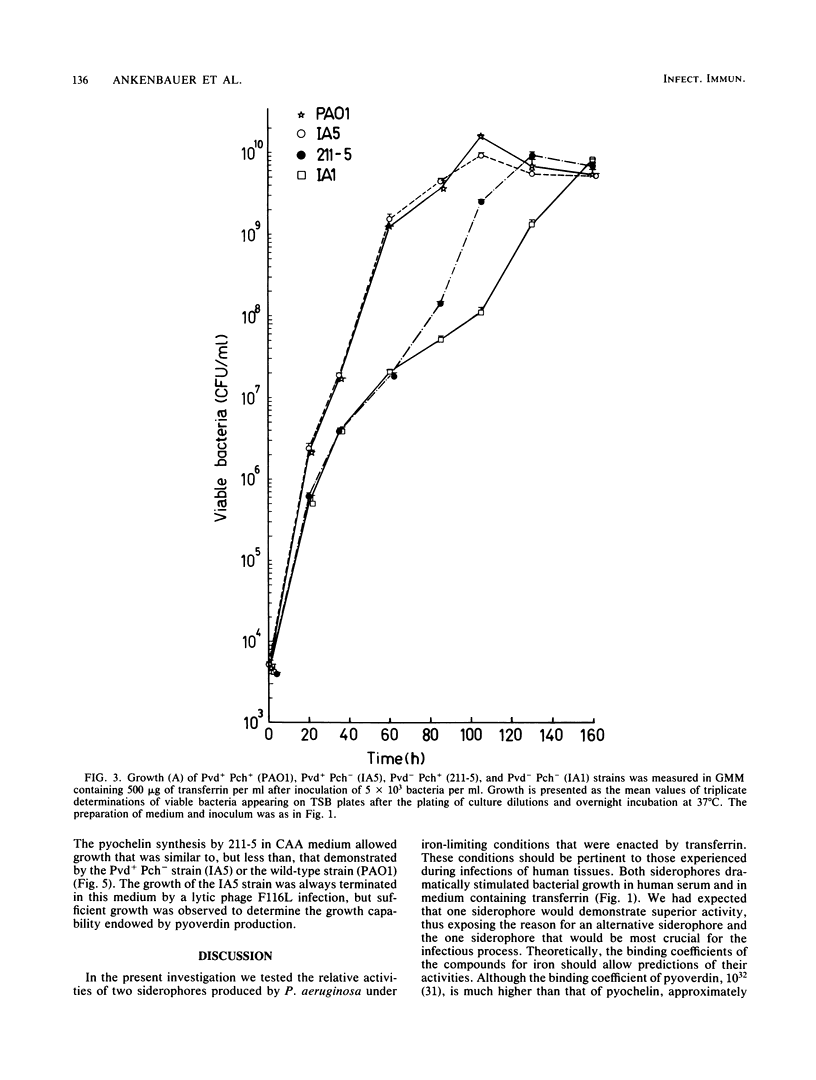
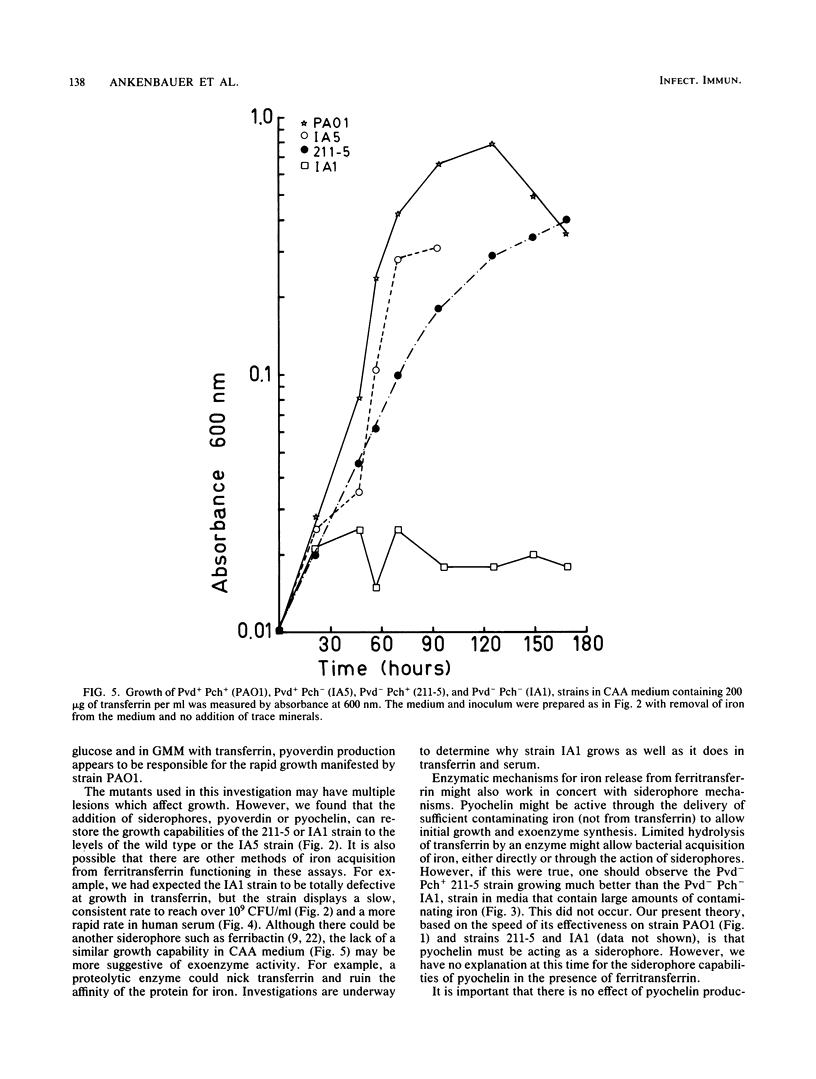
A combination of the siderophores produced by Pseudomonas aeruginosa, pyochelin and pyoverdin, dramatically stimulates the growth of this bacterium in medium containing human transferrin. The amount of growth stimulation observed when each siderophore was added alone was only slightly less than the amount observed with the combination. Siderophore-defective mutants of strain PAO1 were isolated to test the effects of siderophore production on growth in transferrin and human serum. The pyoverdin-proficient (Pvd+), pyochelin-deficient (Pch-) strain (IA5) grows just as well as the parent (PAO1), which produces both siderophores. On the other hand, the Pvd- Pch+ strain (211-5) has severely retarded growth, similar to that demonstrated by a mutant lacking production of both siderophores (IA1), but has an accelerated log phase compared with strain IA1 at the later stages of the growth curve. However, the Pvd- Pch+ strain (211-5) had no observable advantage over the Pvd- Pch- strain, IA1, during incubation in human serum. The inability of P. aeruginosa strains to produce pyochelin in glucose-minimal medium may explain the poor growth of 211-5 in this medium and in human serum. The 211-5 strain grows much better than the IA1 strain in the medium that allows pyochelin synthesis, but it still does not grow as well as the Pvd+ Pch- strain (IA5). Therefore, pyoverdin appears to be the most important siderophore for growth in human serum.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Listowsky I. Iron transport and storage proteins. Annu Rev Biochem. 1980;49:357–393. doi: 10.1146/annurev.bi.49.070180.002041. [DOI] [PubMed] [Google Scholar]
- Bullen J. J. The significance of iron in infection. Rev Infect Dis. 1981 Nov-Dec;3(6):1127–1138. doi: 10.1093/clinids/3.6.1127. [DOI] [PubMed] [Google Scholar]
- Carhart G., Hegeman G. Improved method of selection for mutants of Pseudomonas putida. Appl Microbiol. 1975 Dec;30(6):1046–1047. doi: 10.1128/am.30.6.1046-1047.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine). Anal Biochem. 1971 Apr;40(2):450–458. doi: 10.1016/0003-2697(71)90405-2. [DOI] [PubMed] [Google Scholar]
- Corbin J. L., Bulen W. A. The isolation and identification of 2,3-dihydroxybenzoic acid and 2-N,6-N-di-92,3-dihydroxybenzoyl)-L-lysine formed by iron-deficient Azotobacter vinelandii. Biochemistry. 1969 Mar;8(3):757–762. doi: 10.1021/bi00831a002. [DOI] [PubMed] [Google Scholar]
- Cox C. D., Adams P. Siderophore activity of pyoverdin for Pseudomonas aeruginosa. Infect Immun. 1985 Apr;48(1):130–138. doi: 10.1128/iai.48.1.130-138.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D. Iron uptake with ferripyochelin and ferric citrate by Pseudomonas aeruginosa. J Bacteriol. 1980 May;142(2):581–587. doi: 10.1128/jb.142.2.581-587.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D., Rinehart K. L., Jr, Moore M. L., Cook J. C., Jr Pyochelin: novel structure of an iron-chelating growth promoter for Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4256–4260. doi: 10.1073/pnas.78.7.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald S. P., Rogers H. J. Bacteriostatic effect of serum: role of antibody to lipopolysaccharide. Infect Immun. 1980 Feb;27(2):302–308. doi: 10.1128/iai.27.2.302-308.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka K., Neilands J. B. Effect of serum albumin on siderophore-mediated utilization of transferrin iron. Biochemistry. 1984 May 8;23(10):2122–2127. doi: 10.1021/bi00305a003. [DOI] [PubMed] [Google Scholar]
- Krishnapillai V. A novel transducing phage. Its role in recognition of a possible new host-controlled modification system in Pseudomonas aeruginosa. Mol Gen Genet. 1972;114(2):134–143. doi: 10.1007/BF00332784. [DOI] [PubMed] [Google Scholar]
- LIN E. C., LERNER S. A., JORGENSEN S. E. A method for isolating constitutive mutants for carbohydrate-catabolizing enzymes. Biochim Biophys Acta. 1962 Jul 2;60:422–424. doi: 10.1016/0006-3002(62)90423-7. [DOI] [PubMed] [Google Scholar]
- Liu P. V., Shokrani F. Biological activities of pyochelins: iron-chelating agents of Pseudomonas aeruginosa. Infect Immun. 1978 Dec;22(3):878–890. doi: 10.1128/iai.22.3.878-890.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. G., Yancey R. J., Lankford C. E., Earhart C. F. Bacteriostatic enterochelin-specific immunoglobulin from normal human serum. Infect Immun. 1980 Feb;27(2):418–423. doi: 10.1128/iai.27.2.418-423.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- O'Brien I. G., Gibson F. The structure of enterochelin and related 2,3-dihydroxy-N-benzoylserine conjugates from Escherichia coli. Biochim Biophys Acta. 1970 Aug 14;215(2):393–402. doi: 10.1016/0304-4165(70)90038-3. [DOI] [PubMed] [Google Scholar]
- Page W. J., Huyer M. Derepression of the Azotobacter vinelandii siderophore system, using iron-containing minerals to limit iron repletion. J Bacteriol. 1984 May;158(2):496–502. doi: 10.1128/jb.158.2.496-502.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philson S. B., Llinás M. Siderochromes from Pseudomonas fluorescens. I. Isolation and characterization. J Biol Chem. 1982 Jul 25;257(14):8081–8085. [PubMed] [Google Scholar]
- Philson S. B., Llinás M. Siderochromes from Pseudomonas fluorescens. II. Structural homology as revealed by NMR spectroscopy. J Biol Chem. 1982 Jul 25;257(14):8086–8090. [PubMed] [Google Scholar]
- Rogers H. J. Ferric iron and the antibacterial effects of horse 7S antibodies to Escherichia coli O111. Immunology. 1976 Mar;30(3):425–433. [PMC free article] [PubMed] [Google Scholar]
- Smith H. W. A search for transmissible pathogenic characters in invasive strains of Escherichia coli: the discovery of a plasmid-controlled toxin and a plasmid-controlled lethal character closely associated, or identical, with colicine V. J Gen Microbiol. 1974 Jul;83(0):95–111. doi: 10.1099/00221287-83-1-95. [DOI] [PubMed] [Google Scholar]
- Stuart S. J., Greenwood K. T., Luke R. K. Hydroxamate-mediated transport of iron controlled by ColV plasmids. J Bacteriol. 1980 Jul;143(1):35–42. doi: 10.1128/jb.143.1.35-42.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teintze M., Hossain M. B., Barnes C. L., Leong J., van der Helm D. Structure of ferric pseudobactin, a siderophore from a plant growth promoting Pseudomonas. Biochemistry. 1981 Oct 27;20(22):6446–6457. doi: 10.1021/bi00525a025. [DOI] [PubMed] [Google Scholar]
- Warner P. J., Williams P. H., Bindereif A., Neilands J. B. ColV plasmid-specific aerobactin synthesis by invasive strains of Escherichia coli. Infect Immun. 1981 Aug;33(2):540–545. doi: 10.1128/iai.33.2.540-545.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams H. L., Conrad M. E. A one-tube method for measuring the serum iron concentration and unsaturated iron-binding capacity. J Lab Clin Med. 1966 Jan;67(1):171–176. [PubMed] [Google Scholar]
- Young G. Pigment Production and Antibiotic Activity in Cultures of Pseudomonas aeruginosa. J Bacteriol. 1947 Aug;54(2):109–117. doi: 10.1128/jb.54.2.109-117.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]


