Abstract
The combined high pressure and heat resistances of spores of five proteolytic Clostridium botulinum strains and of the nonpathogenic surrogate strain Clostridium sporogenes PA3679 were compared with their heat-only resistances on the basis of equivalent accumulated thermal lethality, expressed as equivalent minutes at a reference temperature of 105°C (F105°C). Comparisons were made with three model (i.e., diluted) products, namely, 30% (wt/wt) Bolognese sauce, 50% (wt/wt) cream sauce, and rice water agar. Pressure was determined to act synergistically with heat during high-pressure thermal (HPT) processing for C. botulinum FRRB 2802 (NCTC 7273) and C. botulinum FRRB 2804 (NCTC 3805 and 62A) in the Bolognese and cream sauces and for C. botulinum FRRB 2807 (213B) in the Bolognese sauce only. No synergy was observed for C. botulinum FRRB 2803 (NCTC 2916) or FRRB 2806 (62A) or C. sporogenes FRRB 2790 (NCTC 8594 and PA3679) in any of the model products. No significant protective effect of pressure against spore inactivation was determined for any Clostridium strain in any product. Because synergy was not consistently observed among strains of C. botulinum or among products, the prediction of inactivation of C. botulinum spores by HPT sterilization (HPTS) for the present must assume a complete lack of synergy. Therefore, any HPTS process for low-acid shelf-stable foods must be at least thermally equivalent to an F0 process of 2.8 min, in line with current good manufacturing practices. The results of this study suggest that the use of C. sporogenes PA3679 as a surrogate organism may risk overestimating inactivation of C. botulinum by HPT processing.
Low-acid shelf-stable products that are microbiologically safe and stable are not obtainable by high-pressure processing (HPP) at near-ambient temperatures, as bacterial spores can survive pressures above 1,500 MPa (3, 6, 16, 35), which far exceed the pressure capabilities of current commercial HPP equipment. Extensive inactivation of bacterial spores by high pressure is likely only to be realized in combination with initial process temperatures that exceed 60°C (16, 20, 23, 24, 31, 37, 39; C. M. Roberts and D. G. Hoover, presented at the Institute of Food Technologists 1996 annual meeting). Of particular interest (and concern) for low-acid shelf-stable foods is the ability of a combined high pressure and heat process to synergistically inactivate spores of the major bacterial spore-forming pathogens of concern, which are proteolytic strains of the neurotoxigenic species Clostridium botulinum.
The combined high pressure and heat resistances of spores of a number of proteolytic C. botulinum strains in a number of different matrices have been investigated. Proteolytic C. botulinum type A BS-A and 62A spores were inactivated by 2 and 3 log10, respectively, in phosphate buffer (0.067 M, pH 7.0) after 20 min at 827 MPa at 75°C and by 3.2 log10 and 2.7 log10, respectively, in a crabmeat blend (pH 7.2 to 7.4) after 15 min (29). For mashed carrot, a 12-min process at 600 MPa and 80°C was required to inactivate a proteolytic type A C. botulinum strain by 5 log10, whereas a proteolytic type B C. botulinum strain was inactivated by <3 log10 by a process of 600 MPa at 80°C for 60 min (18). Meat and carrot broths inoculated with 5 log10 spores/ml Clostridium sporogenes PA 3679, the nonpathogenic surrogate for C. botulinum for thermal processing studies, were sterilized only with pressure treatments of >800 MPa for 5 min at initial temperatures of 80 to 90°C (16).
Most studies comparing the heat-only and high-pressure-plus-heat resistances of bacterial spores have concluded that, in most cases, pressure and heat do act synergistically to deliver lethality (1, 15, 17, 27, 28, 30, 33). Predominantly, the approaches used by others to demonstrate synergy assume log-linear inactivation kinetics during the pressure hold phase of the high-pressure thermal (HPT) process, ignore inactivation during the pressure come-up time (CUT) and decompression, derive decimal reduction times (DT values; the time required at a constant temperature [T] to achieve a decimal reduction in the number of surviving spores) under constant pressure conditions, and compare these with DT values determined at T and ambient pressure. In such a manner, the combination of high pressure and heat has been determined to be more effective than heat only for inactivation of C. sporogenes PA3679 (1, 15, 33), C. botulinum 17B (proteolytic type B) (30), Clostridium tyrobutyricum ATCC 25755 (1), Thermoanaerobacterium thermosaccharolyticum ATCC 27384 (1), Geobacillus stearothermophilus (1, 15, 26, 28), and Bacillus amyloliquefaciens TMW 2.479, TMW 2.482, and ATCC 49763 (1, 27).
In contrast to most other studies, Margosch et al. (17) showed a clear protective effect of pressure against heat-only inactivation with specific pressure and temperature combinations. Their observations of a protective effect were based on spore inactivation under close to isothermal and isobaric conditions, using a specially developed high-pressure microsystem. For C. botulinum TMW 2.357, HPT processes using pressures above 1,000 MPa at 90°C resulted in greater inactivation than those at ambient pressure and the same temperature. At temperatures of 100, 110, and 120°C, however, treatments of 600 and 800 MPa resulted in slower spore inactivation than that at ambient pressure, indicating a protective effect of pressure. A synergistic effect of pressure and heat was observed at >100°C at pressures of 900 to 1,400 MPa. Similarly, for B. amyloliquefaciens TMW 2.479, spore inactivation at 800 to 1,000 MPa at 100 and 110°C was faster than that at ambient pressure; however, at 120°C, a pressure protective effect was observed for pressures of 800 to 1,000 MPa. Spore inactivation in this study was conducted in Tris-His buffer, where the pH is essentially pressure independent. All other published studies of spore inactivation were conducted in phosphate buffer, whose pH is known to be affected by pressure (5, 21; A. Mathys, V. Heinz, and D. Knorr, presented at the 4th International Conference on High Pressure Bioscience and Biotechnology, Tsukuba, Japan, 25 to 29 September 2006), in water, whose ionic dissociation is enhanced under pressure, resulting in a decrease in pH (7), or in model food products, where the pH is also likely to be reduced under pressure (18). While the use of isothermal conditions allowed the identification of processing parameter combinations where high pressure stabilized the bacterial spore against lethal temperatures, this study can be regarded as a “special case” whose results are unlikely to be replicated in commercial HPT equipment or in commercial products. Margosch et al. (17) proposed that due to the observed strain-to-strain variation in response to pressure-temperature combinations that stabilize bacterial spores, a case-by-case approach to demonstrating the safety of HPT-sterilized foods should be taken.
The objective of this study was to investigate the effects of high pressure and high temperatures on spores of five proteolytic strains of C. botulinum and the nonpathogenic thermal processing surrogate C. sporogenes PA3679 in three model products, namely, Bolognese sauce, cream sauce, and rice. Several studies have shown that substantial spore inactivation may occur during pressure CUT and during decompression (1, 15, 17, 26, 27), although such results are frequently ignored during modeling of spore inactivation by HPT processing. Therefore, in our study, the resistances of strains in the three model products to a range of HPT processes were compared to their resistance to a heat-only treatment with an equivalent thermal lethality via comparison of integrated thermal process lethality values (F105°C values). An assessment of apparent synergy was then made on the basis of this comparison, individually for each product and strain combination. The response of C. sporogenes PA3679 was of interest because this strain is generally considered a nonpathogenic surrogate organism for practical assessment of the safety of thermally processed low-acid shelf-stable products with respect to proteolytic C. botulinum.
MATERIALS AND METHODS
Bacterial strains and spore preparations.
Five proteolytic C. botulinum strains were used in this study. C. botulinum FRRB 2807 (213B) and FRRB 2806 (62A) were selected on the basis of their common use in thermal processing studies. C. botulinum FRRB 2802 (NCTC 7273), FRRB 2803 (NCTC 2916), and FRRB 2804 (NCTC 3805; a second isolate of the 62A strain) were selected on the basis of their demonstrated high levels of resistance to combined heat and high pressure in a preliminary screen (data not shown). In the preliminary screening experiments, C. botulinum FRRB 2804 and C. botulinum FRRB 2806 displayed different resistances to combined heat and high-pressure treatment. As previously stated, C. sporogenes FRRB 2790 (NCTC 8594; PA3679) was selected because of its use as a nonpathogenic surrogate for C. botulinum in heat inactivation and high-pressure studies.
Spores were produced in biphasic medium according to the method of Scott and Bernard (36); all cultures and spore crops were incubated in an anaerobic cabinet (gas mix, 96% N2 and 4% H2). Approximately 0.1 ml of a stock culture in cooked meat medium was inoculated into 10 ml of tryptone-peptone-glucose-starch medium. After incubation for 24 h at 30°C, the entire culture was inoculated into 1,000 ml tryptone-peptone-glucose-starch medium and poured over an agar phase in 650-ml tissue culture flasks (Greiner Bio-One GmbH, Frickenhausen, Germany). The biphasic culture was incubated for 5 to 7 days at 30°C. When sufficient spores were present (>70% bright-phase spores by phase-contrast microscopy), spores were harvested by centrifugation (12,000 × g for 10 min at 5°C), washed three times in sterile deionized water, and resuspended in sterile deionized water. Spore suspensions were heated at 80°C for 10 min and enumerated on tryptone soy agar (TSA; Oxoid, Basingstoke, United Kingdom) supplemented with cysteine (0.5 g/liter), egg yolk (0.05 g/ml) and catalase (400 U/g). The pasteurized suspensions were dispensed in 1-ml aliquots and stored at −80°C until use.
Preparation of model products.
Bolognese sauce with minced beef, cream sauce, and long grain rice were rendered commercially sterile by being retorted in pouches (polyethylene terephthalate aluminum oxide [12 μm]-oriented polyamide [15 μm]-white cast polypropylene [100 μm]) and were stored at room temperature until required. Dilution of the products was required in order to reduce their viscosity and/or particle size to facilitate loading and recovery from the small vessel volume of the HPP unit. On each day of experiments, the Bolognese sauce was diluted to 30% (wt/wt) with sterile deionized water and mixed on medium speed (speed setting 5) for 80 s in a Sorvall Omnimix blender (DuPont Company, CT). Similarly, the cream sauce was diluted to 50% (wt/wt) with sterile deionized water and mixed on medium speed for 20 s. The rice was diluted to 40% (wt/wt) with sterile deionized water and mixed on medium speed for 80 s; the blended rice was filtered through a filter stomacher bag (Seward, West Sussex, United Kingdom), and the liquid was extracted by stomaching (Seward) for 20 s. The extracted rice water was decanted into a sterile container, warmed to 50°C, and mixed 1:1 with 3% sterile molten agar. The addition of agar was required to provide greater control of the prepressurization heating process. The final pHs of the model products were 4.74, 5.98, and 6.33 for the diluted Bolognese sauce, diluted cream sauce, and rice water agar, respectively.
Heat-only inactivation of spores in agar and model products.
The heat-only resistances of all C. botulinum strains and C. sporogenes FRRB 2790 were determined in each of the model products. The heat-only resistance of C. botulinum FRRB 2082 was also determined in modified PA3679 agar (MPA3679) (13).
Heat inactivation experiments were conducted with custom-made stainless steel screw-cap test tubes (13-mm inner diameter) fitted with silicon septa, based on the method of Kooiman and Geers (14). Test tubes were filled with 9.9 g of uninoculated agar or model product and fully immersed in an oil bath with a set temperature; a matched, uninoculated tube was used as a temperature control. After the control tube had equilibrated to the test temperature, 100 μl of spore suspension (1.0 × 107 spores/ml) was injected into each test tube via a 22-gauge, 3-in. needle (BD Biosciences, Madrid, Spain) and 1-ml syringe (BD Biosciences, Singapore). Once the desired heating time had elapsed, the test tube was removed; MPA3679 and rice water agar samples were cooled and held at 50°C, and cream and Bolognese model product samples were cooled in room temperature water. For inactivation of C. botulinum FRRB 2802 spores in MPA3679 agar, six time points were examined in duplicate at 105, 110, and 118°C. For spore inactivation in model products, 10 time points were examined in duplicate at 100, 105, and 110°C.
DT values were calculated from the negative reciprocals of the slopes of the regression lines at each temperature, using the linear portions of the survivor curves. The time zero counts (based on the spore count after a standard activation process of 80°C for 10 min) were not used due to the potential for incomplete spore activation for some strains with this treatment. The z value was calculated from the negative reciprocal of the slope of the regression line of log10 DT versus temperature.
HPT inactivation of spores in agar and model products.
The pressure and heat resistances of the five C. botulinum strains and C. sporogenes FRRB 2790 were determined for each of the model products. The pressure and heat resistance of C. botulinum FRRB 2802 was also determined in MPA3679 agar. Samples were pressure treated in a high-pressure multivessel apparatus (model U111; Unipress Equipment, Warsaw, Poland) consisting of five pressure vessels (13-mm inner diameter) separately connected to a silicon oil (Huber GmbH, Offenburg, Germany)-driven intensifier by five high-pressure valves. The pressure vessels were immersed in a silicon oil bath equipped with a thermostat. Pressure vessel temperatures were measured from a K-type thermocouple fitted at the base of each vessel. One pressure vessel of the HPP unit was fitted with a thermocouple assembly (Unipress) for sample temperature measurements. The thermocouple assembly was designed to fit within a stainless steel sample container (1.5 ml; 9.8-mm inner diameter and 12.8-mm outer diameter), which had a piston at the bottom of the container to allow pressure to be transferred to the enclosed sample. The assembly consisted of three K-type thermocouples: the first was positioned in the middle of the sample container, the second was adjacent to the inner wall of the container (3.66 mm from the center thermocouple), and the third was positioned between the first and second thermocouples (2.45 mm from the center thermocouple). HPT experiments were conducted with the temperature profile measured in an uninoculated sample, while a single matched inoculated sample was processed at the same time and under the same conditions in a second stainless steel sample container fitted with a screw cap.
The inoculated and uninoculated samples were chilled to 2°C (to facilitate avoidance of excessive prepressurization heating) in an ice-water slurry prior to being placed simultaneously in the pressure vessels. To achieve an appropriate initial model product sample temperature, the pressure pump of the HPP unit was primed by being pressurized to ∼20 MPa prior to samples being placed into the pressure vessels. Compression was commenced again when the samples had been placed and sealed in the vessels; the valves to the individual pressure vessels were simultaneously opened when the pressure pump reached 50 MPa, thus minimizing the lag in vessel pressurization.
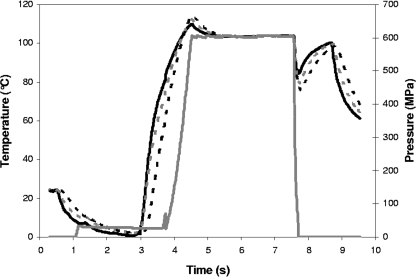
MPA3679 agar samples were processed at 600 MPa for 180 s in pressure vessels preequilibrated to 110°C. The model product samples were processed at 600 MPa with pressure vessels preequilibrated to 102, 104, or 106°C. The compression rate was ∼12 MPa/s, pressure hold times varied from 30 to 300 s, decompression took <10 s, and each HPT process was conducted twice; vessel temperature, sample temperatures, and pressure were measured every 0.42 s (see Fig. 1 for a typical pressure/thermal profile for model product samples and Table 1 for processing parameters). Immediately after the HPT process, the uninoculated and matched inoculated samples were simultaneously removed from the pressure vessels and cooled in a 55°C water bath before immediate enumeration of survivors. Spore inactivation experiments carried out in our HPP unit were therefore nonisothermal within an individual sample, as well as throughout the process (Fig. 1), and the whole inoculated sample was used for enumeration. Given the difficulty of maintaining consistency of control over some process parameters in the HPP unit (e.g., due to manual closure and opening of the two pressure vessels in use, the manual release of pressure, and the manual removal of sample and control tubes from pressure vessels preequilibrated to high temperatures) and the subsequent variations in sample temperatures, we considered each replicate HPT treatment to be an independent process.
FIG. 1.
Typical temperature-pressure profile for a model product. The profile is for 50% (wt/wt) cream sauce in a pressure vessel preequilibrated to 106°C and processed at 600 MPa with a 180-s pressure hold time. The temperature adjacent to the sample container wall (solid black line), temperature at the center of the sample (dashed black line), temperature equidistant from the container wall and the sample center (dashed gray line), and pressure (solid gray line) are shown. The sample was chilled to <2°C prior to being placed in the pressure vessel, the pressure pump was pressurized and held at ∼20 MPa until the sample was loaded and the pressure vessel closed, the pressure pump was pressurized to ∼50 MPa, and the pressure vessel was opened. During compression, the sample temperature increased to a maximum of 110 to 114°C before dropping to ∼103°C for the remainder of the pressure hold time; during decompression, the sample temperature decreased to 77 to 84°C before increasing to 98 to 100°C while the pressure vessel was being opened and the sample removed; the sample was cooled to <70°C in a 55°C water bath.
TABLE 1.
Processing parameters of HPT treatments of Clostridium botulinum strains and Clostridium sporogenes FRRB 2790 in agar and model products at 600 MPaa
| Strain | Model product | Pressure vessel preequilibration temp (°C)b | Hold time at 600 MPa (min) | Initial sample temp (°C)c | Maximum sample temp under pressure (°C)d | Minimum sample temp under hold pressure (°C)e | Sample temp at end of pressure hold time (°C)f | Sample temp when vial removed from pressure vessel (°C)g |
|---|---|---|---|---|---|---|---|---|
| FRRB 2802 | MPA3679 agar | 110 | 180 | 103.1 ± 0.7 | 117.9 ± 0.3 | 105.9 ± 2.4 | 107.5 ± 0.1 | 106.2 ± 0.2 |
| 30% (wt/wt) Bolognese sauce | 102 | 240 | 54.7 ± 6.6 | 107.9 ± 1.5 | 98.2 ± 0.4 | 99.0 ± 0.4 | 93.2 ± 0.1 | |
| 30% (wt/wt) Bolognese sauce | 104 | 240 | 56.1 ± 2.1 | 110.9 ± 0.5 | 100.7 ± 0.1 | 101.7 ± 0.4 | 96.3 ± 0.1 | |
| 30% (wt/wt) Bolognese sauce | 106 | 180 | 58.3 ± 1.5 | 113.6 ± 0.1 | 103.2 ± 0.1 | 103.6 ± 0.4 | 97.8 ± 0.4 | |
| 30% (wt/wt) Bolognese sauce | 106 | 240 | 51.1 ± 0.2 | 109.9 ± 2.3 | 102.8 ± 0.1 | 103.8 ± 0.3 | 97.9 ± 0.3 | |
| 50% (wt/wt) cream sauce | 102 | 300 | 54.5 ± 1.6 | 108.9 ± 0.4 | 98.8 ± 0.1 | 99.9 ± 0.2 | 94.3 ± 0.3 | |
| 50% (wt/wt) cream sauce | 104 | 300 | 54.1 ± 0.6 | 111.4 ± 0.3 | 101.0 ± 0.1 | 102.2 ± 0.0 | 96.5 ± 0.1 | |
| 50% (wt/wt) cream sauce | 106 | 240 | 59.1 ± 1.1 | 114.3 ± 0.3 | 103.2 ± 0.1 | 104.0 ± 0.4 | 98.0 ± 0.9 | |
| 50% (wt/wt) cream sauce | 106 | 300 | 58.4 ± 1.6 | 114.3 ± 0.6 | 103.3 ± 0.1 | 104.2 ± 0.0 | 97.7 ± 0.1 | |
| Rice water agar | 102 | 300 | 55.1 ± 1.1 | 108.1 ± 0.3 | 97.6 ± 0.3 | 99.0 ± 0.2 | 93.6 ± 1.6 | |
| Rice water agar | 104 | 300 | 57.1 ± 1.7 | 110.8 ± 0.0 | 100.0 ± 0.5 | 101.6 ± 0.1 | 96.5 ± 0.8 | |
| Rice water agar | 106 | 240 | 59.0 ± 1.1 | 113.8 ± 0.8 | 103.1 ± 0.0 | 104.2 ± 0.1 | 99.1 ± 0.8 | |
| Rice water agar | 106 | 300 | 59.0 ± 0.2 | 113.7 ± 0.5 | 99.3 ± 5.2 | 104.2 ± 0.1 | 99.9 ± 0.5 | |
| FRRB 2803 | 30% (wt/wt) Bolognese sauce | 102 | 90 | 60.2 ± 1.6 | 109.1 ± 0.7 | 98.3 ± 0.4 | 98.3 ± 0.4 | 93.5 ± 1.3 |
| 30% (wt/wt) Bolognese sauce | 104 | 90 | 59.1 ± 3.3 | 111.5 ± 0.8 | 100.8 ± 0.1 | 100.9 ± 0.1 | 95.6 ± 0.3 | |
| 30% (wt/wt) Bolognese sauce | 106 | 60 | 58.7 ± 3.0 | 113.8 ± 0.5 | 103.5 ± 0.1 | 103.5 ± 0.0 | 97.8 ± 0.5 | |
| 30% (wt/wt) Bolognese sauce | 106 | 90 | 60.0 ± 1.7 | 114.1 ± 0.4 | 103.0 ± 0.0 | 103.1 ± 0.1 | 98.6 ± 0.1 | |
| 50% (wt/wt) cream sauce | 102 | 180 | 79.0 ± 8.2 | 111.8 ± 2.3 | 98.0 ± 0.4 | 98.6 ± 0.4 | 95.3 ± 0.1 | |
| 50% (wt/wt) cream sauce | 104 | 180 | 80.9 ± 1.2 | 114.1 ± 0.2 | 100.6 ± 0.1 | 101.1 ± 0.0 | 97.2 ± 0.4 | |
| 50% (wt/wt) cream sauce | 106 | 90 | 60.5 ± 2.3 | 114.5 ± 0.4 | 102.9 ± 0.2 | 102.9 ± 0.2 | 98.0 ± 0.8 | |
| 50% (wt/wt) cream sauce | 106 | 180 | 61.2 ± 1.3 | 114.9 ± 0.6 | 103.2 ± 0.0 | 103.8 ± 0.1 | 98.5 ± 0.1 | |
| Rice water agar | 102 | 240 | 57.0 ± 0.5 | 110.0 ± 0.1 | 98.7 ± 0.2 | 99.6 ± 0.0 | 97.7 ± 0.6 | |
| Rice water agar | 104 | 240 | 56.5 ± 1.1 | 111.9 ± 0.5 | 100.8 ± 0.0 | 101.7 ± 0.0 | 99.9 ± 0.1 | |
| Rice water agar | 106 | 180 | 59.9 ± 0.1 | 114.2 ± 0.4 | 103.0 ± 0.0 | 103.6 ± 0.1 | 101.5 ± 0.3 | |
| Rice water agar | 106 | 300 | 60.2 ± 5.5 | 114.3 ± 0.6 | 102.9 ± 0.1 | 104.0 ± 0.0 | 101.2 ± 0.4 | |
| FRRB 2804 | 30% (wt/wt) Bolognese sauce | 102 | 180 | 55.9 ± 0.8 | 108.8 ± 0.0 | 98.7 ± 0.3 | 99.4 ± 0.1 | 93.9 ± 0.4 |
| 30% (wt/wt) Bolognese sauce | 104 | 180 | 56.5 ± 1.5 | 110.9 ± 0.3 | 101.0 ± 0.2 | 101.7 ± 0.1 | 95.9 ± 0.1 | |
| 30% (wt/wt) Bolognese sauce | 106 | 90 | 64.0 ± 0.8 | 113.3 ± 0.1 | 102.6 ± 0.4 | 102.7 ± 0.5 | 96.9 ± 0.7 | |
| 30% (wt/wt) Bolognese sauce | 106 | 150 | 58.7 ± 1.6 | 113.3 ± 1.0 | 103.1 ± 0.4 | 103.3 ± 0.2 | 97.9 ± 0.4 | |
| 50% (wt/wt) cream sauce | 102 | 300 | 52.1 ± 1.9 | 108.1 ± 0.4 | 98.3 ± 0.4 | 99.7 ± 0.3 | 94.7 ± 0.0 | |
| 50% (wt/wt) cream sauce | 104 | 300 | 55.6 ± 3.3 | 111.0 ± 1.1 | 100.7 ± 0.2 | 101.8 ± 0.1 | 96.2 ± 0.1 | |
| 50% (wt/wt) cream sauce | 106 | 120 | 57.6 ± 3.2 | 113.7 ± 1.8 | 102.8 ± 0.3 | 103.0 ± 0.1 | 96.8 ± 0.1 | |
| 50% (wt/wt) cream sauce | 106 | 180 | 56.8 ± 0.0 | 113.6 ± 0.0 | 102.9 ± 0.0 | 103.4 ± 0.0 | 97.4 ± 0.0 | |
| 50% (wt/wt) cream sauce | 106 | 240 | 56.4 ± 3.0 | 113.0 ± 0.0 | 102.6 ± 0.0 | 103.6 ± 0.1 | 98.5 ± 0.1 | |
| 50% (wt/wt) cream sauce | 106 | 300 | 58.7 ± 4.0 | 113.5 ± 0.5 | 102.6 ± 0.1 | 104 ± 0.1 | 98.7 ± 0.6 | |
| Rice water agar | 102 | 240 | 56.5 ± 2.3 | 109 ± 0.6 | 98.2 ± 0.1 | 99.1 ± 0.3 | 96.0 ± 0.0 | |
| Rice water agar | 104 | 240 | 56.4 ± 1.4 | 110.6 ± 0.6 | 100.6 ± 0.4 | 101.6 ± 0.1 | 97.4 ± 0.1 | |
| Rice water agar | 106 | 120 | 55.0 ± 5.2 | 112.2 ± 1.7 | 102.1 ± 0.1 | 102.3 ± 0.1 | 99.4 ± 0.5 | |
| Rice water agar | 106 | 180 | 56.7 ± 1.1 | 113.2 ± 0.1 | 102.7 ± 0.0 | 103.3 ± 0.1 | 100.1 ± 0.1 | |
| Rice water agar | 106 | 240 | 58.4 ± 3.3 | 113.7 ± 1.0 | 102.9 ± 0.2 | 103.9 ± 0.1 | 100.7 ± 1.0 | |
| Rice water agar | 106 | 300 | 57.9 ± 1.2 | 113.8 ± 1.1 | 103.0 ± 0.1 | 104.2 ± 0.0 | 99.8 ± 0.4 | |
| FRRB 2807 | 30% (wt/wt) Bolognese sauce | 102 | 60 | 54.0 ± 3.2 | 108.1 ± 0.3 | 99.0 ± 0.1 | 99.0 ± 0.1 | 92.9 ± 1.6 |
| 30% (wt/wt) Bolognese sauce | 104 | 60 | 56.9 ± 0.1 | 110.8 ± 0.4 | 101.1 ± 0.3 | 101.1 ± 0.3 | 95.2 ± 0.3 | |
| 30% (wt/wt) Bolognese sauce | 106 | 30 | 56.9 ± 0.8 | 113.3 ± 0.4 | 105.6 ± 0.6 | 105.6 ± 0.6 | 96.9 ± 0.3 | |
| 30% (wt/wt) Bolognese sauce | 106 | 60 | 62.9 ± 6.1 | 114.4 ± 1.4 | 103.5 ± 0.3 | 103.5 ± 0.3 | 97.3 ± 0.6 | |
| 50% (wt/wt) cream sauce | 102 | 120 | 52.9 ± 0.4 | 109.0 ± 1.0 | 98.2 ± 0.5 | 98.3 ± 0.4 | 92.9 ± 0.4 | |
| 50% (wt/wt) cream sauce | 104 | 120 | 57.5 ± 0.4 | 111.7 ± 0.2 | 100.5 ± 0.1 | 100.8 ± 0.1 | 95.3 ± 0.7 | |
| 50% (wt/wt) cream sauce | 106 | 60 | 56.4 ± 0.9 | 113.9 ± 0.6 | 103.3 ± 0.2 | 103.3 ± 0.2 | 97.0 ± 0.7 | |
| 50% (wt/wt) cream sauce | 106 | 120 | 56.0 ± 1.2 | 113.5 ± 0.3 | 102.9 ± 0.1 | 103.0 ± 0.0 | 97.0 ± 0.0 | |
| Rice water agar | 102 | 120 | 59.5 ± 5.3 | 109.7 ± 0.5 | 97.9 ± 0.3 | 97.9 ± 0.3 | 96.0 ± 0.5 | |
| Rice water agar | 104 | 120 | 57.2 ± 0.3 | 111.3 ± 0.6 | 100.2 ± 0.1 | 100.3 ± 0.3 | 98.8 ± 0.7 | |
| Rice water agar | 106 | 60 | 59.2 ± 2.8 | 114.1 ± 0.6 | 103.0 ± 0.1 | 103.0 ± 0.1 | 100.6 ± 0.4 | |
| Rice water agar | 106 | 120 | 57.9 ± 1.0 | 113.9 ± 0.1 | 102.7 ± 0.1 | 102.9 ± 0.0 | 100.4 ± 0.7 | |
| FRRB 2806 | 30% (wt/wt) Bolognese sauce | 102 | 240 | 53.6 ± 0.3 | 108.6 ± 0.6 | 98.6 ± 0.3 | 99.4 ± 0.3 | 94.4 ± 0.8 |
| 104 | 240 | 56.0 ± 0.4 | 110.8 ± 0.3 | 100.7 ± 0.1 | 101.7 ± 0.1 | 96.7 ± 0.1 | ||
| 106 | 120 | 57.5 ± 0.4 | 113.3 ± 0.4 | 103.2 ± 0.0 | 103.2 ± 0.0 | 97.7 ± 0.5 | ||
| 106 | 240h | 63.9 | 114.1 | 102.7 | 103.8 | 197.9 | ||
| 50% (wt/wt) cream sauce | 102 | 180 | 53.1 ± 1.8 | 108.7 ± 0.1 | 98.6 ± 0.0 | 99.1 ± 0.1 | 93.9 ± 0.1 | |
| 104 | 180 | 53.9 ± 0.2 | 111.6 ± 0.5 | 100.9 ± 0.1 | 101.4 ± 0.0 | 96.5 ± 0.9 | ||
| 106 | 60 | 56.4 ± 3.0 | 113.7 ± 0.6 | 103.3 ± 0.0 | 103.3 ± 0.0 | 97.7 ± 0.1 | ||
| 106 | 120 | 58.9 ± 0.1 | 113.8 ± 0.7 | 102.8 ± 0.0 | 103.1 ± 0.0 | 98.0 ± 0.1 | ||
| Rice water agar | 102 | 180 | 76.5 ± 3.0 | 107.8 ± 0.0 | 97.5 ± 0.1 | 97.9 ± 0.4 | 94.9 ± 0.4 | |
| 104 | 180 | 55.5 ± 0.6 | 110.3 ± 0.7 | 100.1 ± 0.3 | 100.7 ± 0.1 | 97.2 ± 0.1 | ||
| 106 | 60 | 56.7 ± 0.9 | 112.7 ± 0.0 | 103.0 ± 0.0 | 103.0 ± 0.0 | 98.7 ± 0.4 | ||
| 106 | 120 | 57.6 ± 0.1 | 112.6 ± 0.3 | 102.1 ± 0.1 | 102.3 ± 0.1 | 98.2 ± 0.1 | ||
| FRRB 2790 | 30% (wt/wt) Bolognese sauce | 102 | 60 | 59.2 ± 7.0 | 108.6 ± 1.1 | 98.8 ± 0.1 | 98.8 ± 0.1 | 94.1 ± 0.6 |
| 30% (wt/wt) Bolognese sauce | 104 | 60 | 58.6 ± 0.7 | 111.2 ± 0.1 | 101.1 ± 0.1 | 101.3 ± 0.2 | 97.0 ± 0.7 | |
| 30% (wt/wt) Bolognese sauce | 106 | 30h | 59.8 | 112.7 | 105.6 | 105.6 | 97.9 | |
| 30% (wt/wt) Bolognese sauce | 106 | 60 | 65.9 ± 8.4 | 114.0 ± 1.6 | 103.5 ± 0.0 | 103.5 ± 0.0 | 97.9 ± 0.3 | |
| 50% (wt/wt) cream sauce | 102 | 120 | 78.3 ± 8.5 | 110.1 ± 2.5 | 98.7 ± 0.4 | 99.0 ± 0.3 | 95.0 ± 0.3 | |
| 50% (wt/wt) cream sauce | 104 | 120 | 56.9 ± 2.3 | 111.0 ± 1.2 | 100.8 ± 0.1 | 100.9 ± 0.1 | 96.6 ± 0.1 | |
| 50% (wt/wt) cream sauce | 106 | 60 | 66.1 ± 3.0 | 114.9 ± 0.6 | 103.4 ± 0.2 | 103.4 ± 0.3 | 97.9 ± 1.4 | |
| 50% (wt/wt) cream sauce | 106 | 120 | 58.3 ± 2.3 | 113.7 ± 0.4 | 102.9 ± 0.0 | 103.0 ± 0.1 | 99.3 ± 0.6 | |
| Rice water agar | 102 | 120 | 61.2 ± 3.2 | 108.6 ± 1.6 | 97.9 ± 0.5 | 90.3 ± 10.3 | 95.6 ± 0.9 | |
| Rice water agar | 104 | 120 | 64.4 ± 1.5 | 112.2 ± 0.3 | 100.9 ± 0.1 | 100.9 ± 0.0 | 98.4 ± 0.4 | |
| Rice water agar | 106 | 60 | 59.3 ± 0.7 | 113.5 ± 0.0 | 103.1 ± 0.1 | 103.1 ± 0.1 | 99.7 ± 0.6 | |
| Rice water agar | 106 | 120 | 65.0 ± 0.3 | 114.1 ± 0.1 | 103.0 ± 0.1 | 103.2 ± 0.1 | 99.9 ± 0.0 |
Values presented are averages for six replicates ± standard deviations for MPA3679 agar and averages for two replicates ± standard deviations for model products. Sample temperatures were measured at the center of the container.
Equilibration temperature of pressure vessels prior to pressurization cycle.
Temperature of sample at the start of the pressurization cycle.
Maximum temperature of sample attained at the end of the compression phase, when the pressure reached 600 MPa.
Minimum sample temperature during pressure hold time.
Temperature of sample at the end of the pressure hold time.
Temperature of sample when sample vial was removed from the pressure vessel at the end of treatment.
One replicate only for this treatment.
The F105°C value, the integrated lethal rate at 105°C, at the center of the uninoculated sample was calculated for each individual sample by integrating equation 1 (4) using the trapezoidal integration method (11), as follows:
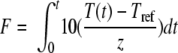 |
(1) |
where z is the temperature difference required to result in a 10-fold change in the DT value of the target organism and Tref is the reference temperature, which in this study was selected as 105°C, since this temperature was the mid-temperature used in the in-product heat-only inactivation trials. The z values used were derived under heat-only conditions for each microorganism in each product. The center of the sample represents the slowest-heating point of the sample during warming and cooling and is the slowest-cooling point during the pressure hold phase (Fig. 1). For a given HPT process, the F105°C value for the center of the sample was consequently higher than that for the outer edge of the sample and therefore represents a worst-case scenario with respect to comparison of inactivation by HPT processing to that by heat-only treatment. For each microorganism in each product, the log10 reduction (i.e., log10 N/N0 MPN/g, where N is the spore count after treatment, N0 is the untreated spore count, and MPN is the most probable number) attained for each HPT process was plotted against the corresponding F105°C value, calculated from the thermocouple in the center of the sample container, for comparison with heat-only inactivation at 105°C.
Enumeration of surviving spores from agar and model products.
A three-tube MPN method, using MPA3679 broth with supplements (0.01% lysozyme, 3.6% sodium carbonate, 6.6% sodium thioglycolate), was used to enumerate surviving spores from model products. Samples were serially diluted in MPA3679 broth with supplements and resazurin (1 μg/ml), and three-1 ml aliquots of each dilution were added to individual wells of 48-well cell culture plates (1.4-ml volume; Becton Dickinson Labware, NJ). The cell culture plates were prereduced in an anaerobic cabinet for at least 3 days prior to use to remove dissolved oxygen in the plastic. Inoculated cell culture plates were incubated anaerobically at 30°C for 7 days prior to turbidity assessment. Replicate wells for each dilution were scored as either turbid (indicating growth) or not turbid (no growth). Turbid wells of the highest dilution were subcultured by being streaked onto supplemented TSA and brain heart infusion agar (Oxoid) to confirm culture purity; plates were incubated anaerobically and aerobically, respectively, for 3 days at 30°C. Wells demonstrating purity, as noted by pure and typical colonies on supplemented TSA and the absence of growth on brain heart infusion agar, were then confirmed as positive for growth of the examined isolate.
The MPN of each sample was calculated using the U.S. Food and Drug Administration method for deriving the MPN from serial dilutions (12), using an Excel spreadsheet tool (available at http://www.cfsan.fda.gov/∼ebam/bam-a2.html [last accessed 15 May 2008]).
Statistical analysis.
Spore inactivation by heat only and HPT was compared on the basis of log10 reduction at equivalent F105°C values. Each strain in each product was considered separately. A regression line (polynomial or logarithmic equation, selected on the basis of the highest r2 value) was fitted, using Microsoft Excel 2002 (Microsoft Corporation), to a plot of the log10 reduction versus F105°C for the heat-only (105°C) data from the first replicate (rep1) data set. The resulting equation was then used to predict the log10 reduction at equivalent F105°C values for the second heat-only replicate (rep2) data set. The difference (i.e., residual) between the observed log10 reduction of each rep2 sample and the predicted log10 reduction was then calculated, and the average and standard deviation of the residuals were determined. Then, using the same regression line, the log10 reduction at each of the observed HPT F105°C values was predicted. The difference between the predicted and observed log10 reductions for each HPT sample was calculated, and the average and standard deviation of the residuals were determined. A two-tailed t test assuming unequal variances (22) was then performed (Microsoft Excel 2002) on the difference between the average heat-only residual and the average HPT residual for each strain in each product. P values of ≤0.05 indicated that the average residuals for the heat-only and HPT data were significantly different at the 95% confidence interval. P values of ≤0.01 indicated that the average residuals for the heat-only and HPT data were significantly different at the 99% confidence interval.
RESULTS
Heat-only inactivation of spores in agar and model products.
The heat-only resistance of C. botulinum FRRB 2802 in MPA3679 agar at 105 and 110°C was slightly lower than its heat-only resistance in 50% (wt/wt) cream sauce and in rice water agar but higher than its heat-only resistance in 30% (wt/wt) Bolognese sauce (Fig. 2; Table 2). The heat-only resistances of C. botulinum FRRB 2802 in the model products were similar at 100°C; however, for the other strains, heat resistance in 30% (wt/wt) Bolognese sauce was substantially lower than that in the other model products at all three temperatures examined (Table 2).
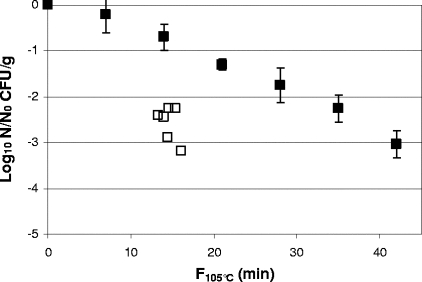
FIG. 2.
Comparison of inactivation of Clostridium botulinum FRRB 2802 in MPA3679 agar by heat-only and HPT processing. ▪, heat-only inactivation at 105°C; □, HPT inactivation at 600 MPa and 110°C for 180 s. F105°C values for HPT data were based on a z value of 10.5°C (r2 = 0.98), derived from heat-only inactivation of C. botulinum FRRB 2802 in MPA3679 agar at 105°C (D105°C = 12.7 min; r2 = 0.94), 110°C (D110°C = 3.0 min; r2 = 0.95), and 118°C (D118°C = 0.7 min; r2 = 0.93). Heat-only inactivation data are means of two replicates, and error bars are standard deviations. HPT inactivation data are for independent samples.
TABLE 2.
DT and z values for C. botulinum strains and C. sporogenes FRRB 2790 in model products under heat-only conditionsa
| Strain | Model product | D100°C (min) | D105°C (min) | D110°C (min) | z value (°C) |
|---|---|---|---|---|---|
| FRRB 2802 | 30% (wt/wt) Bolognese sauce | 42.4 (0.79) | 5.8 (0.86) | 1.4 (0.85) | 6.7 (0.99) |
| 50% (wt/wt) cream sauce | 39.7 (0.86) | 14.0 (0.89) | 3.3 (0.89) | 9.2 (0.99) | |
| Rice water agar | 42.7 (0.89) | 11.3 (0.89) | 4.1 (0.70) | 9.8 (0.99) | |
| FRRB 2803 | 30% (wt/wt) Bolognese sauce | 5.2 (0.95) | 1.9 (0.94) | 0.7 (0.91) | 11.5 (1.00) |
| 50% (wt/wt) cream sauce | 19.3 (0.90) | 5.6 (0.89) | 1.5 (0.88) | 8.9 (1.00) | |
| Rice water agar | 18.8 (0.97) | 6.4 (0.94) | 1.4 (0.90) | 8.9 (0.99) | |
| FRRB 2804 | 30% (wt/wt) Bolognese sauce | 9.6 (0.92) | 2.3 (0.96) | 0.9 (0.88) | 9.9 (0.98) |
| 50% (wt/wt) cream sauce | 19.4 (0.71) | 4.8 (0.87) | 0.9 (0.94) | 7.5 (1.00) | |
| Rice water agar | 21.5 (0.71) | 7.7 (0.76) | 2.2 (0.57) | 10.1 (1.00) | |
| FRRB 2807 | 30% (wt/wt) Bolognese sauce | 6.3 (0.94) | 3.3 (0.89) | 1.0 (0.90) | 12.7 (0.98) |
| 50% (wt/wt) cream sauce | 9.0 (0.93) | 4.2 (0.86) | 1.5 (0.81) | 13.1 (0.99) | |
| Rice water agar | 12.0 (0.83) | 4.4 (0.53)b | 1.7 (0.53)b | 11.8 (1.00) | |
| FRRB 2806 | 30% (wt/wt) Bolognese sauce | 9.4 (0.90) | 2.5 (0.86) | 1.0 (0.93) | 10.1 (0.99) |
| 50% (wt/wt) cream sauce | 26.4 (0.85) | 5.7 (0.96) | 1.4 (0.95) | 7.9 (1.00) | |
| Rice water agar | 20.8 (0.94) | 5.1 (0.98) | 1.8 (0.91) | 9.5 (0.99) | |
| FRRB 2790 | 30% (wt/wt) Bolognese sauce | 5.2 (0.93) | 2.8 (0.89) | 0.9 (0.98) | 13.3 (0.97) |
| 50% (wt/wt) cream sauce | 14.0 (0.86) | 5.1 (0.84) | 2.5 (0.85) | 13.5 (0.99) | |
| Rice water agar | 16.4 (0.88) | 2.2 (0.97) | 1.9 (0.88) | 10.6 (0.80) |
DT values were calculated from the negative reciprocals of the slopes of the regression lines at each temperature, using the linear portions of the survivor curves; z values were determined from the negative reciprocal of the slope of the regression line of log10 DT versus temperature. Values in parentheses are the r2 values of each regression line.
Inactivation curves exhibited strong tailing, but the regression line was fitted through all points of the curve to obtain a suitable trend in DT values and a better fit for the z value.
In all model products, C. botulinum FRRB 2802 was the most heat-resistant strain. In traditional thermal processing, the reference D121.1°C and z values for C. botulinum are 0.2 min and 10°C, respectively (9, 38). Using the experimentally determined DT and zT values (Table 2), a D121.1°C value of 0.33 min was calculated for C. botulinum FRRB 2802 in MPA3679 agar, indicating that this strain is particularly heat resistant. C. botulinum FRRB 2083 and C. sporogenes FRRB 2790 were the least heat-resistant strains in 30% (wt/wt) Bolognese sauce; C. botulinum FRRB 2807 was the least heat-resistant strain in 50% (wt/wt) cream sauce and in rice water agar.
HPT inactivation of spores in agar and model products.
A highly statistically significant difference (P < 0.01) between the average heat-only residual and the average HPT residual was found for C. botulinum FRRB 2802 in MPA3679 agar (Table 3); at equivalent F105°C values, a 1- to 2-log10 greater inactivation was achieved with HPT processing than with heat-only treatment (Fig. 2). The variation in log10 reduction by HPT processing of the six replicate samples can be attributed to the slight variation in the temperature profile of each sample due to small differences in individual sample temperatures when pressure was applied (a result of slight variation in the time taken to manually close the pressure vessels and a lag in the time for pressure build-up in the vessels once pressure was applied).
TABLE 3.
Statistical comparison of heat-only and HPT inactivation at F105°C for C. botulinum strains and C. sporogenes FRRB 2790 in agar and model products
| Strain | Medium | Avg heat-only residual (log10 MPN/g)a | Avg HPT residual (log10 MPN/g)a | P value |
|---|---|---|---|---|
| FRRB 2802 | MPA3679 agar | 0.395 ± 0.137 | −1.629 ± 0.356 | 4.95 × 10−6b |
| 30% (wt/wt) Bolognese sauce | 0.122 ± 0.406 | −1.203 ± 0.514 | 0.042c | |
| 50% (wt/wt) cream sauce | −0.231 ± 0.346 | −1.305 ± 0.507 | 0.050c | |
| Rice water agar | 0.154 ± 0.306 | −0.715 ± 0.292 | 0.069 | |
| FRRB 2803 | 30% (wt/wt) Bolognese sauce | 0.066 ± 0.473 | −0.874 ± 0.518 | 0.193 |
| 50% (wt/wt) cream sauce | 0.190 ± 0.444 | −0.456 ± 0.577 | 0.340 | |
| Rice water agar | 0.083 ± 0.480 | −0.656 ± 0.480 | 0.307 | |
| FRRB 2804 | 30% (wt/wt) Bolognese sauce | 0.018 ± 0.278 | −1.069 ± 0.336 | 0.017c |
| 50% (wt/wt) cream sauce | 0.265 ± 0.305 | −1.394 ± 0.843 | 0.003b | |
| Rice water agar | −0.034 ± 0.462 | −1.262 ± 0.928 | 0.075 | |
| FRRB 2807 | 30% (wt/wt) Bolognese sauce | 0.000 ± 0.223 | −0.754 ± 0.480 | 0.047c |
| 50% (wt/wt) cream sauce | −0.296 ± 0.487 | −0.925 ± 0.581 | 0.368 | |
| Rice water agar | 0.298 ± 0.606 | −0.265 ± 0.622 | 0.533 | |
| FRRB 2806 | 30% (wt/wt) Bolognese sauce | 0.121 ± 0.304 | −0.455 ± 0.568 | 0.252 |
| 50% (wt/wt) cream sauce | 0.005 ± 0.198 | −0.125 ± 0.434 | 0.682 | |
| Rice water agar | 0.001 ± 0.196 | 0.485 ± 0.502 | 0.147 | |
| FRRB 2790 | 30% (wt/wt) Bolognese sauce | −0.166 ± 0.367 | −0.772 ± 0.501 | 0.288 |
| 50% (wt/wt) cream sauce | −0.143 ± 0.268 | −0.064 ± 0.415 | 0.840 | |
| Rice water agar | 0.000 ± 0.461 | 0.723 ± 0.542 | 0.302 |
Data values are averaged residuals ± standard deviations; heat-only and HPT residuals were calculated for each sample as the difference between the actual inactivation and that predicted from a regression line fitted through the rep1 subset of the 105°C heat-only inactivation data.
P values of ≤0.01 indicate a highly statistically significant difference between the average residuals for the heat-only and HPT data at the 99% confidence interval.
P values of ≤0.05 indicate a statistically significant difference between the average residuals for the heat-only and HPT data at the 95% confidence interval.
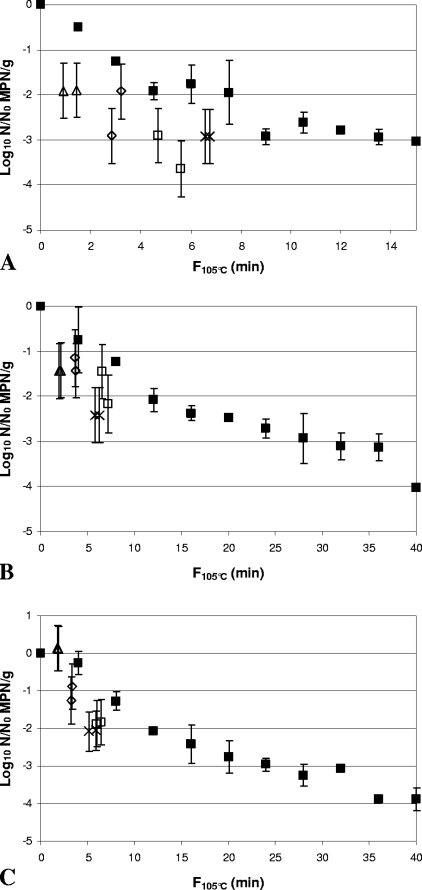
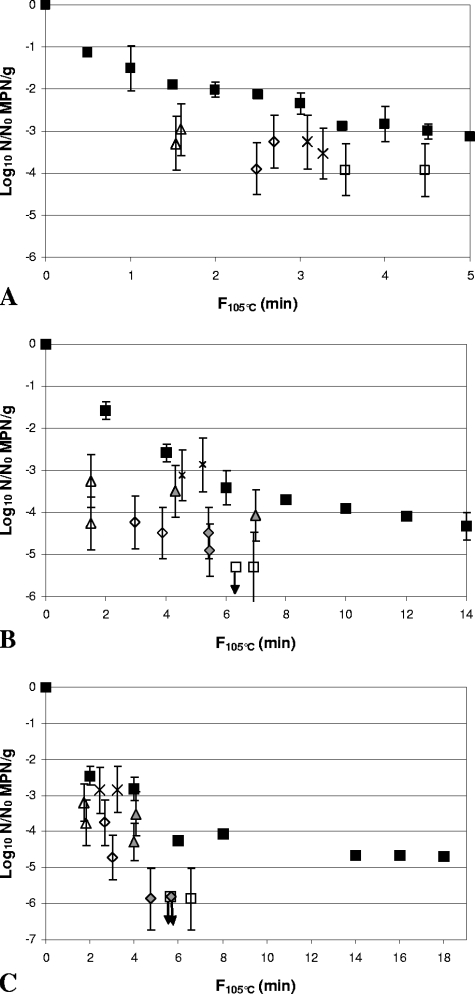
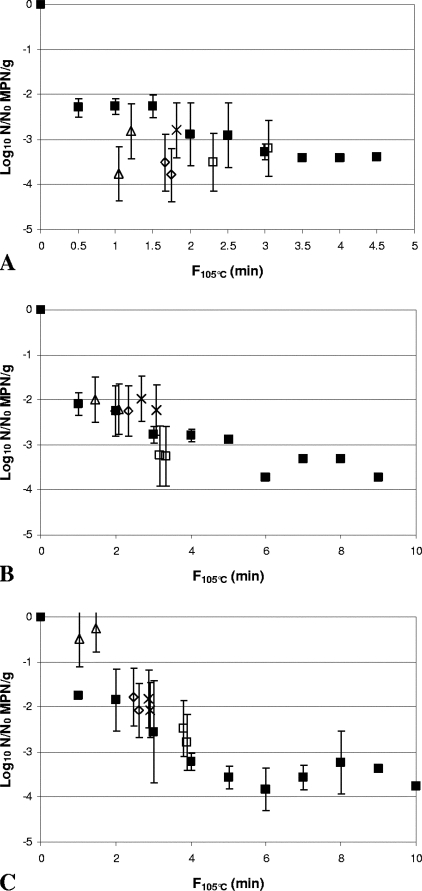
In the 30% (wt/wt) Bolognese sauce, inactivation by HPT processing, with 2- to 4.5-log10 reductions, was observed across an F105°C range of ∼1 to 6.8 min (Fig. 3A, 4A, 5A, 6A, 7A, and 8A). The most pressure- and heat-resistant strain in this medium at the highest F105°C value was C. botulinum FRRB 2802. At lower F105°C values, C. botulinum FRRB 2806 and C. botulinum FRRB 2803 showed similar levels of resistance to that of C. botulinum FRRB 2802. Lower levels of pressure and heat resistance were shown by C. botulinum FRRB 2804, C. sporogenes FRRB 2790, and C. botulinum FRRB 2807, which was generally the most pressure- and heat-sensitive strain in 30% (wt/wt) Bolognese sauce. A statistically significant (P < 0.05) difference between heat-only and HPT inactivation was shown for C. botulinum strains FRRB 2802, 2804, and 2807 (Table 3). Inactivation of C. botulinum FRRB 2802 by HPT processing was greater for all samples in 30% (wt/wt) Bolognese sauce than that by heat-only treatment at equivalent F105°C values, by up to ∼1.5 log10 (Fig. 3A).
FIG. 3.
Comparison of inactivation of Clostridium botulinum FRRB 2802 in 30% (wt/wt) Bolognese sauce (A), 50% (wt/wt) cream sauce (B), and rice water agar (C) by heat-only and HPT processing. ▪, heat-only inactivation at 105°C.). (A) ▵, 600 MPa, 102°C, 240 s; ⋄, 600 MPa, 104°C, 240 s; ×, 600 MPa, 106°C, 180 s; □, 600 MPa, 106°C, 240 s. (B and C) 600 MPa, 102°C, 300 s; ⋄, 600 MPa, 104°C, 300 s; ×, 600 MPa, 106°C, 240 s; □, 600 MPa, 106°C, 300 s. Heat-only inactivation data are means of two replicates, and error bars are standard deviations. HPT inactivation data are for independent samples, and error bars are 95% confidence intervals for the MPN.
FIG. 4.
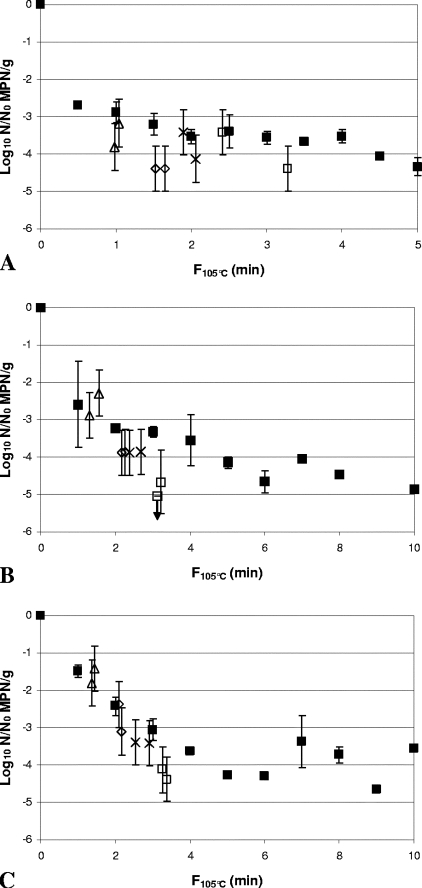
Comparison of inactivation of Clostridium botulinum FRRB 2803 in 30% (wt/wt) Bolognese sauce (A), 50% (wt/wt) cream sauce (B), and rice water agar (C) by heat-only and HPT processing. ▪, heat-only inactivation at 105°C. (A) ▵, 600 MPa, 102°C, 90 s; ⋄, 600 MPa, 104°C, 90 s; ×, 600 MPa, 106°C, 60 s; □, 600 MPa, 106°C, 90 s. (B) ▵, 600 MPa, 102°C, 180 s; ⋄, 600 MPa, 104°C, 180 s; ×, 600 MPa, 106°C, 90 s; □, 600 MPa, 106°C, 180 s. (C) ▵, 600 MPa, 102°C, 240 s; ⋄, 600 MPa, 104°C, 240 s; ×, 600 MPa, 106°C, 180 s; □, 600 MPa, 106°C, 300 s. Heat-only inactivation data are means of two replicates, and error bars are standard deviations. HPT inactivation data are independent samples, and error bars are 95% confidence intervals for the MPN.
FIG. 5.
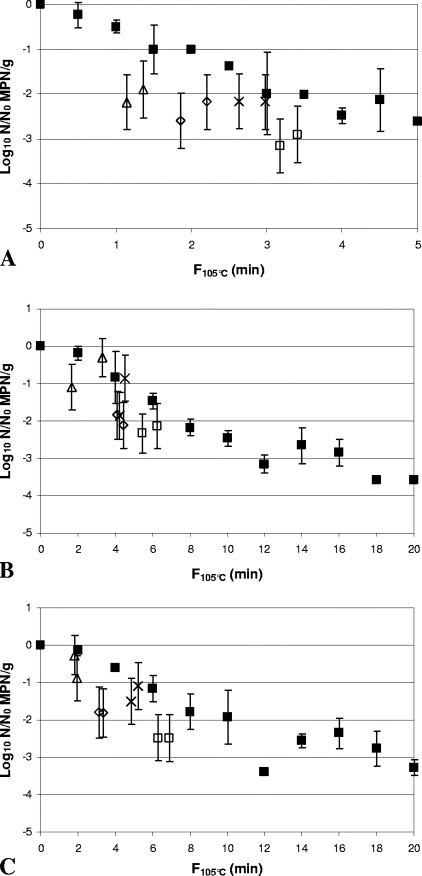
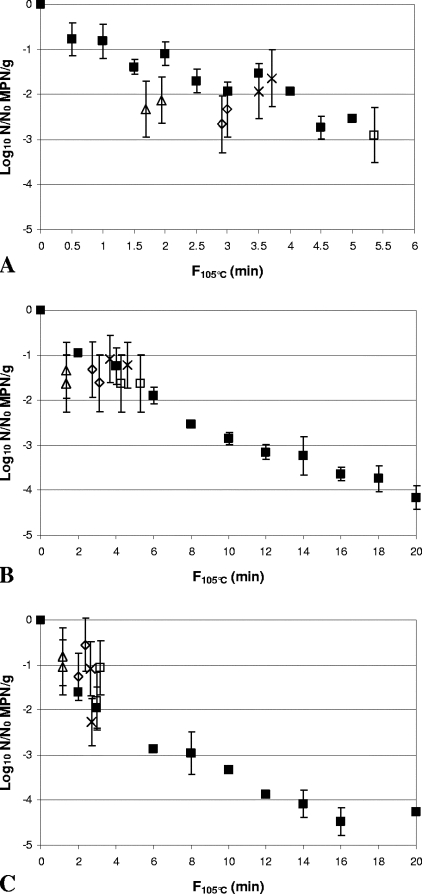
Comparison of inactivation of Clostridium botulinum FRRB 2804 in 30% (wt/wt) Bolognese sauce (A), 50% (wt/wt) cream sauce (B), and rice water agar (C) by heat-only and HPT processing. ▪, heat-only inactivation at 105°C. (A) ▵, 600 MPa, 102°C, 180 s; ⋄, 600 MPa, 104°C, 180 s; ×, 600 MPa, 106°C, 90 s; □, 600 MPa, 106°C, 150 s. (B) ▵, 600 MPa, 102°C, 300 s; ⋄, 600 MPa, 104°C, 300 s; ×, 600 MPa, 106°C, 120 s; ▴, 600 MPa, 106°C, 180 s; ⧫, 600 MPa, 106°C, 240 s; □, 600 MPa, 106°C, 300 s. (C) ▵, 600 MPa, 102°C, 240 s; ⋄, 600 MPa, 104°C, 240 s; ×, 600 MPa, 106°C, 120 s; ▴, 600 MPa, 106°C, 180 s; ⧫, 600 MPa, 106°C, 240 s; □, 600 MPa, 106°C, 300 s. Arrows indicate samples with spore counts below the limit of detection (∼0.4 log10 MPN/g). Heat-only inactivation data are means of two replicates, and error bars are standard deviations. HPT inactivation data are for independent samples, and error bars are 95% confidence intervals for the MPN.
FIG. 6.
Comparison of inactivation of Clostridium botulinum FRRB 2807 in 30% (wt/wt) Bolognese sauce (A), 50% (wt/wt) cream sauce (B), and rice water agar (C) by heat-only and HPT processing. ▪, heat-only inactivation at 105°C (▪). (A) ▵, 600 MPa, 102°C, 60 s; ⋄, 600 MPa, 104°C, 60 s; ×, 600 MPa, 106°C, 30 s; □, 600 MPa, 106°C, 60 s. (B and C) ▵, 600 MPa, 102°C, 120 s; ⋄, 600 MPa, 104°C, 120 s; ×, 600 MPa, 106°C, 60 s; □, 600 MPa, 106°C, 120 s. Arrows indicate samples with spore count below the limit of detection (∼0.4 log10 MPN/g). Heat-only inactivation data are means of two replicates, and error bars are standard deviations. HPT inactivation data are for independent samples, and error bars are 95% confidence intervals for the MPN.
FIG. 7.
Comparison of inactivation of Clostridium botulinum FRRB 2806 in 30% (wt/wt) Bolognese sauce (A), 50% (wt/wt) cream sauce (B), and rice water agar (C) by heat-only and HPT processing. ▪, heat-only inactivation at 105°C. (A) ▵, 600 MPa, 102°C, 240 s; ⋄, 600 MPa, 104°C, 240 s; ×, 600 MPa, 106°C, 120 s; □, 600 MPa, 106°C, 240 s. (B and C) ▵, 600 MPa, 102°C, 180 s; ⋄, 600 MPa, 104°C, 180 s; ×, 600 MPa, 106°C, 60 s; □, 600 MPa, 106°C, 120 s. Heat-only inactivation data are means of two replicates, and error bars are standard deviations. HPT inactivation data are for independent samples, and error bars are 95% confidence intervals for the MPN.
FIG. 8.
Comparison of inactivation of Clostridium sporogenes FRRB 2790 in 30% (wt/wt) Bolognese sauce (A), 50% (wt/wt) cream sauce (B), and rice water agar (C) by heat-only and HPT processing. ▪, heat-only inactivation at 105°C. (A) ▵, 600 MPa, 102°C, 60 s; ⋄, 600 MPa, 104°C, 60 s; ×, 600 MPa, 106°C, 30 s; □, 600 MPa, 106°C, 60 s. (B and C) ▵, 600 MPa, 102°C, 120 s; ⋄, 600 MPa, 104°C, 120 s; ×, 600 MPa, 106°C, 60 s; □, 600 MPa, 106°C, 120 s. Heat-only inactivation data are means of two replicates, and error bars are standard deviations. HPT inactivation data are for independent samples, and error bars are 95% confidence intervals for the MPN.
For 50% (wt/wt) cream sauce, a broad range of pressure and heat resistances was observed for the Clostridium strains, with log10 reductions of <1 to >5 log10 at equivalent F105°C values (Fig. 3B, 4B, 5B, 6B, 7B, and 8B). At the maximum F105°C value, ∼ 7 min, the most pressure- and heat-resistant strain was again C. botulinum FRRB 2802. At lower F105°C values, C. botulinum FRRB 2806 and C. botulinum FRRB 2803 again showed similar levels of resistance to that of C. botulinum FRRB 2802 at equivalent F105°C values. At F105°C values of <4 min, C. sporogenes FRRB 2790 was the next most pressure- and heat-resistant microorganism, followed by C. botulinum FRRB 2807 and C. botulinum FRRB 2804. However, at F105°C values between 4 and ∼5.5 min, C. botulinum FRRB 2804 sometimes appeared more pressure and heat resistant than either C. sporogenes FRRB 2790 or C. botulinum FRRB 2807. In 50% (wt/wt) cream sauce, a statistically significant difference between the average heat-only and average HPT residuals was determined for C. botulinum FRRB 2802 (P < 0.05) and a highly significant difference was determined for C. botulinum FRRB 2804 (P < 0.01) (Table 3). Again, inactivation of C. botulinum FRRB 2802 by HPT processing was greater for all samples in 50% (wt/wt) cream sauce than that by heat-only treatment at equivalent F105°C values, by up to ∼1.5 log10 (Fig. 3B).
As observed for the 50% (wt/wt) cream sauce, the range of pressure and heat resistances of the Clostridium strains in rice water agar was broad, with up to a 5-log10 range at the higher F105°C values (Fig. 3C, 4C, 5C, 6C, 7C, and 8C). In rice water agar, C. botulinum FRRB 2802 was generally the most pressure- and heat-resistant Clostridium strain across the F105°C range studied. The next most resistant strains were C. botulinum FRRB 2803, C. botulinum FRRB 2806, and C. sporogenes FRRB 2790, showing similar log10 reductions at equivalent F105°C values of <3.5 min. However, at F105°C values of >3.5 min, C. botulinum FRRB 2803 sometimes appeared more pressure and heat resistant than C. botulinum FRRB 2806 and C. sporogenes FRRB 2790. The least pressure- and heat-resistant strains in rice water agar were C. botulinum FRRB 2807 and then C. botulinum FRRB 2804. There was no significant effect of pressure during HPT processing for any C. botulinum strain or for C. sporogenes FRRB 2790 in rice water agar under the conditions studied (Table 3). For C. botulinum FRRB 2806 and C. sporogenes FRRB 2790 in rice water agar, inactivation by some HPT processes was up to ∼1.5-log10 less effective than that by heat-only treatment (Fig. 7C and 8C), but although the values for the average HPT residuals for these strains were greater than the respective average heat-only residuals, the differences were not statistically significantly different at the 95% confidence interval (Table 3).
DISCUSSION
The effect of pressure during HPT processing may be characterized as being synergistic, not significantly different, or protective compared to heat-only inactivation. A synergistic effect of HPT was observed for C. botulinum FRRB 2802, 2804, and 2807 in 30% (wt/wt) Bolognese sauce and for C. botulinum FRRB 2802 and 2804 in 50% (wt/wt) cream sauce. There was no significant difference between HPT processing and heat-only inactivation for C. botulinum FRRB 2803, C. botulinum FRRB 2806, or C. sporogenes FRRB 2790 in any product. Our results are in agreement with several studies that have shown that pressure can act synergistically with heat (1, 15, 17, 27, 28, 30, 33, 34; A. Mathys, V. Heinz, and D. Knorr, presented at the 4th International Conference on High Pressure Bioscience and Biotechnology, Tsukuba, Japan, 25 to 29 September 2006), except that unlike others (1, 15, 33, 34), we did not observe a synergistic effect of pressure and heat on C. sporogenes FRRB 2790, a PA3679 strain, during HPT processing. No protective effect of pressure against heat inactivation was identified for C. botulinum (or C. sporogenes) for the HPT processes undertaken in the model products. Our results are therefore also in contrast to the unique observations of Margosch et al. (17) of pressure-mediated protection of C. botulinum TMW 2.357 (and B. amyloliquefaciens TMW 2.479) with specific isothermal and isobaric HPT processes.
Comparison of heat-only DT values with those derived during only the isothermal or isobaric phase of HPT processes may be inadequate to fully explain the effect of high pressure on thermal inactivation (1). Spore inactivation under nonisothermal HPT conditions has not been well characterized, yet the results of others strongly suggest that the CUT phase of the HPT process should not be ignored. Margosch et al. (17) reported a reduction of 1.5 log10 for proteolytic C. botulinum TMW 2.357 in mashed carrots during the pressure CUT (5 min) for an HPT process of 600 MPa at 80°C, and Rajan et al. (27) showed a reduction of 1.2 log10 for B. amyloliquefaciens TMW 2.479 in egg patties during the pressure CUT for an HPT process of 700 MPa at 121°C. Ahn et al. (1) found inactivation of C. sporogenes PA3679 (ATCC 7955) increased from a 3.3- to 6.3-log10 reduction during a pressure CUT of 0.58 min to reach 700 MPa as the process temperature increased from 105 to 121°C. G. stearothermophilus was found to be reduced by an average of 3.7 log10 CFU/ml at zero holding time after reaching 700 MPa at 111°C (26). In a commercial setting, it is likely that a large-volume pressure unit will have an extended pressure CUT, and spore inactivation during this phase should be incorporated into the overall reduction (26).
In the current study, our approach was to consider each HPT sample as being derived from a distinct nonisothermal process and to compare spore inactivation levels with those achieved by heat-only processes via an integrated process lethality value (i.e., F105°C) which incorporated thermal lethality delivered in all process phases. The application of F values derived from heat-only inactivation studies to HPT inactivation calculations relies on the assumption that the zT value (the temperature change required at constant pressure to achieve a 10-fold change in the DT value) derived under heat-only conditions does not change when pressure is applied. Studies with C. sporogenes PA3679 (15, 33) found that zT values were similar under constant pressures of 600, 700, and 800 MPa but that zT values under pressure were larger than that at ambient pressure, indicating that the spores were less sensitive overall to temperature changes under pressure. Spores of B. amyloliquefaciens TMW 2.479 were shown to become less sensitive to temperature changes as pressure increased, with zT values increasing from 16.7 to 26.8°C as pressure increased from 500 to 700 MPa, compared with a zT value of 8.2°C at ambient pressure (27). Ahn et al. (1) found that for various strains, including strains of C. sporogenes, C. tyrobutyricum, T. thermosaccharolyticum, and B. amyloliquefaciens, pressure greatly accelerated spore inactivation at 105°C; however, at 121°C, the synergistic effect between pressure and heat was reduced. Nevertheless, the relative simplicity, and hence utility, of the F value approach in determining the efficacy of HPT processing cannot be overlooked in this very labor-intensive area of process validation.
Application of the F value approach to comparison of inactivation by HPT and heat-only processes also assumes that the lethality delivered during a thermal process is not affected by variations in the shape of the thermal profile but is determined only by the area under the curve (by integration). This means that given two thermal profiles with identical areas, the lethality of the process is predicted to be the same. It is known, however, that the shape of the thermal profile, particularly the exceeding of a critical temperature (CT), is also an important factor in determining lethality in some cases; for example, it has been noted that zT values derived for Bacillus subtilis under high-temperature conditions do not apply to ultra-high-temperature conditions (8). It is possible that the shape of the thermal profile may dictate the mechanism by which spores are inactivated by both heat-only and HPT processes, and it is also possible that CT values under high pressures may be different from CT values under atmospheric pressure conditions.
The effect of the intrinsic properties of food on spore inactivation by HPT processing has been explored by several research groups (2, 18, 21, 28, 29, 30). Criteria for choosing the model products for this work were based partially on their various intrinsic properties, namely, pH and fat and starch contents; although these factors were not assessed systematically, it is worth considering the effects of the various products on spore inactivation, since no published inactivation studies have been performed with similar products. More C. botulinum strains showed a significant synergistic effect of pressure and heat in 30% (wt/wt) Bolognese sauce than in the 50% cream sauce or in the rice water agar, and these differences may be attributable to the lower pH of the 30% (wt/wt) Bolognese sauce. Margosch et al. (18) found that inactivation of C. botulinum TMW 2.357 was not affected by decreasing the pH from 6.0 to 5.15. However, the inactivation rate was increased by a shift from pH 5.15 to 4.0.
The 50% (wt/wt) cream sauce had the highest fat content of the model sauces, ∼7.4%, and spores exhibited greater resistance to heat-only inactivation in the 50% (wt/wt) cream sauce than that in the 30% Bolognese sauce. A substantial increase in spore resistance to HPT processing in the 50% (wt/wt) cream sauce compared with that in the 30% (wt/wt) Bolognese sauce or in rice water agar was not observed. Ananta et al. (2) found that Bacillus stearothermophilus ATCC 7953 was protected from inactivation when it was inoculated into cocoa mass with 10% water and subjected to HPT treatments of 600 MPa and up to 90°C for 60 min but that the same protective effect was not observed in the presence of 20 or 30% water. They suggested that the protective effect was due to the low water activity of the fat-rich product due to the concentration of solutes in the aqueous phase rather than to the fat itself (2).
For the C. botulinum strains and C. sporogenes FRRB 2790 used in this study, spore resistance to heat-only treatments was generally greatest in rice water agar. Similarly, spore resistance to HPT treatments was higher in rice water agar than in the other model products, and in this medium a significant effect of pressure and heat was not shown for any strains. The higher starch content of the rice water agar may have contributed to the higher spore resistance, as starch is known to aid in the recovery of injured spores through the absorption of inhibitory agents (10), hence its addition to spore recovery and enumeration media (11, 25).
Interestingly, in all three model products, the order of pressure and heat resistances of the Clostridium strains did not change dramatically. In summarized form, in 30% (wt/wt) Bolognese sauce and 50% (wt/wt) cream sauce the order of most to least pressure- and heat-resistant strains was the same, as follows: C. botulinum FRRB 2802 (NCTC 7273) > C. botulinum FRRB 2803 (NCTC 2916) = C. botulinum FRRB 2806 (62A) > C. botulinum FRRB 2804 (NCTC 3805; 62A) > C. sporogenes FRRB 2790 (NCTC 8594; PA3679) > C. botulinum FRRB 2807 (213B). In rice water agar, the order of most to least pressure- and heat-resistant strains was C. botulinum FRRB 2802 > C. botulinum FRRB 2803 > C. botulinum FRRB 2806 = C. sporogenes > C. botulinum FRRB 2807 > C. botulinum FRRB 2804. Clearly, C. sporogenes PA3679 does not seem to be an appropriate nonpathogenic surrogate for C. botulinum for HPT process validation experiments because C. sporogenes FRRB 2790 was less pressure and heat resistant than at least half of the C. botulinum strains in all three model products. The use of C. sporogenes PA3679 as a surrogate organism may risk overestimating inactivation of C. botulinum.
Acknowledgments
We gratefully acknowledge C. M. Stewart for assistance with initial C. botulinum strain selection, J. Smelt for technical assistance with C. botulinum methodologies and handling, S. W. White and K. Sultana for their technical assistance, A. Mathys for his advice on operation of the high-pressure unit, and T. Ross for his guidance with statistical analyses.
Footnotes
Published ahead of print on 14 November 2008.
REFERENCES
- 1.Ahn, J., V. M. Balasubramaniam, and A. E. Yousef. 2007. Inactivation kinetics of selected aerobic and anaerobic bacterial spores by pressure-assisted thermal processing. Int. J. Food Microbiol. 113:321-329. [DOI] [PubMed] [Google Scholar]
- 2.Ananta, E., V. Heinz, O. Schlueter, and D. Knorr. 2001. Kinetic studies on high-pressure inactivation of Bacillus stearothermophilus spores suspended in food matrices. Innov. Food Sci. Emerg. Technol. 2:261-272. [Google Scholar]
- 3.Arroyo, G., P. D. Sanz, and G. Prestamo. 1999. Response to high-pressure, low-temperature treatment in vegetables: determination of survival rates of microbial populations using flow cytometry and detection of peroxidase activity using confocal microscopy. J. Appl. Microbiol. 86:544-556. [DOI] [PubMed] [Google Scholar]
- 4.Bakalis, S., P. W. Cox, and P. J. Fryer. 2001. Modelling particular thermal technologies, p. 113-137. In P. Richardson (ed.), Thermal technologies in food processing. Woodhead Publishing, Cambridge, England.
- 5.Bruins, M. E., A. M. Matser, A. E. M. Janssen, and R. M. Boom. 2007. Buffer selection for HP treatment of biomaterials and its consequences for enzyme inactivation studies. High Press. Res. 27:101-107. [Google Scholar]
- 6.Cheftel, J.-C. 1992. Effects of high hydrostatic pressure on food constituents: an overview, p. 195-209. In C. Balny, R. Hayashi, K. Heremans, and P. Masson (ed.), High pressure and biotechnology. INSERM/John Libbey Eurotext, Paris, France.
- 7.Cheftel, J.-C. 1995. High-pressure, microbial inactivation and food preservation. Food Sci. Technol. Int. 7:75-90. [Google Scholar]
- 8.Edwards, J. L., F. F. Busta, and M. L. Speck. 1965. Thermal inactivation characteristics of Bacillus subtilis spores at ultrahigh temperatures. Appl. Microbiol. 13:851-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esty, J. R., and K. F. Meyer. 1922. The heat resistance of the spores of B. botulinus and allied anaerobes. J. Infect. Dis. 31:650-663. [Google Scholar]
- 10.Foegeding, P. M., and F. F. Busta. 1981. Bacterial spore injury—an update. J. Food Prot. 44:776-786. [DOI] [PubMed] [Google Scholar]
- 11.Food Science Australia and D. Warne. 2005. Australian Quarantine Inspection Service approved persons course for thermal processing of low-acid foods. Food Science Australia, Werribee, Australia.
- 12.Garthright, W. E., and R. J. Blodgett. 2003. FDA's preferred MPN methods for standard, large or unusual tests, with a spreadsheet. Food Microbiol. 20:439-445. [Google Scholar]
- 13.Grischy, R. O., R. V. Speck, and D. M. Adams. 1983. New media for enumeration and detection of Clostridium sporogenes (PA3679) spores. J. Food Sci. 48:1466-1469. [Google Scholar]
- 14.Kooiman, W. J., and J. M. Geers. 1975. Simple and accurate technique for the determination of heat resistance of bacterial spores. J. Appl. Bacteriol. 38:185-189. [DOI] [PubMed] [Google Scholar]
- 15.Koutchma, T., B. Guo, E. Patazca, and B. Parisi. 2005. High pressure-high temperature sterilization: from kinetic analysis to process verification. J. Food Proc. Eng. 28:610-629. [Google Scholar]
- 16.Maggi, A., S. Gola, P. Rovere, L. Miglioli, G. Dall'Aglio, and N. G. Lonneborg. 1996. Effects of combined high pressure-temperature treatments on Clostridium sporogenes spores in liquid media. Ind. Conserv. 71:8-14. [Google Scholar]
- 17.Margosch, D., M. A. Ehrmann, R. Buckow, V. Heinz, R. F. Vogel, and M. G. Ganzle. 2006. High-pressure-mediated survival of Clostridium botulinum and Bacillus amyloliquefaciens endospores at high temperature. Appl. Environ. Microbiol. 72:3476-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Margosch, D., M. A. Ehrmann, M. G. Gänzle, and R. F. Vogel. 2004. Comparison of pressure and heat resistance of Clostridium botulinum and other endospores in mashed carrots. J. Food Prot. 67:2530-2537. [DOI] [PubMed] [Google Scholar]
- 19.Reference deleted.
- 20.Mills, G., R. Earnshaw, and M. F. Patterson. 1998. Effects of high hydrostatic pressure on Clostridium sporogenes spores. Lett. Appl. Microbiol. 26:227-230. [DOI] [PubMed] [Google Scholar]
- 21.Molina-Gutierrez, A., V. Stippl, A. Delgado, M. G. Ganzle, and R. F. Vogel. 2002. In situ determination of the intracellular pH of Lactococcus lactis and Lactobacillus plantarum during pressure treatment. Appl. Environ. Microbiol. 68:4399-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Motulsky, H. 1995. Intuitive biostatistics. Oxford University Press, New York, NY.
- 23.Nakayama, A., Y. Yano, S. Kobayashi, M. Ishikawa, and K. Sakai. 1996. Comparison of pressure resistances of spores of six Bacillus strains with their heat resistances. Appl. Environ. Microbiol. 62:3897-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okazaki, T., K. Kakugawa, S. Yamauchi, T. Yoneda, and K. Susuki. 1996. Combined effects of temperature and pressure on inactivation of heat-resistant bacteria, p. 415-418. In R. Hayashi and C. Balny (ed.), High pressure bioscience and biotechnology: proceedings of the International Conference on High Pressure Bioscience and Biotechnology. Elsevier, New York, NY.
- 25.Olsen, A. M., and W. J. Scott. 1950. The enumeration of heated bacterial spores. I. Experiments with Clostridium botulinum and other species of Clostridium. Aust. J. Sci. Res. Ser. B 3:219-233. [Google Scholar]
- 26.Patazca, E., T. Koutchma, and H. S. Ramaswamy. 2006. Inactivation kinetics of Geobacillus stearothermophilus spores in water using high-pressure processing at elevated temperatures. J. Food Sci. 71:110-116. [Google Scholar]
- 27.Rajan, S., J. Ahn, V. M. Balasubramaniam, and A. E. Yousef. 2006. Combined pressure-thermal inactivation kinetics of Bacillus amyloliquefaciens spores in egg patty mince. J. Food Prot. 69:853-860. [DOI] [PubMed] [Google Scholar]
- 28.Rajan, S., S. Pandrangi, V. M. Balasubramaniam, and A. E. Yousef. 2006. Inactivation of Bacillus stearothermophilus spores in egg patties by pressure-assisted thermal processing. Lebensm.-Wiss. Technol. 39:844-851. [Google Scholar]
- 29.Reddy, N. R., H. M. Solomon, R. C. Tetzloff, and E. J. Rhodehamel. 2003. Inactivation of Clostridium botulinum type A spores by high-pressure processing at elevated temperatures. J. Food Prot. 66:1402-1407. [DOI] [PubMed] [Google Scholar]
- 30.Reddy, N. R., R. C. Tetzloff, H. M. Solomon, and J. W. Larkin. 2006. Inactivation of Clostridium botulinum nonproteolytic type B spores by high pressure processing at moderate to elevated high temperatures. Innov. Food Sci. Emerg. Technol. 7:169-175. [Google Scholar]
- 31.Roberts, C. M., and D. G. Hoover. 1996. Sensitivity of Bacillus coagulans spores to combinations of high hydrostatic pressure, heat, acidity and nisin. J. Appl. Bacteriol. 81:363-368. [Google Scholar]
- 32.Reference deleted.
- 33.Rovere, P., A. Maggi, N. Scaramuzza, S. Gola, L. Miglioli, G. Dall Carpi, and G. Dall'Aglio. 1996. High-pressure heat treatments: evaluation of the sterilizing effect and of thermal damage. Ind. Conserv. 71:473-483. [Google Scholar]
- 34.Rovere, P., L. Miglioli, N. G. Lonneborg, N. Scaramuzza, and S. Gola. 1998. Modelling and calculation of the sterilising effect in high pressure heat-treatments. Ind. Conserv. 73:303-315. [Google Scholar]
- 35.Sale, A. J., G. W. Gould, and W. A. Hamilton. 1970. Inactivation of bacterial spores by hydrostatic pressure. J. Gen. Microbiol. 60:323-334. [DOI] [PubMed] [Google Scholar]
- 36.Scott, V. N., and D. T. Bernard. 1982. Heat resistance of spores of nonproteolytic type B Clostridium botulinum. J. Food Prot. 45:909-912. [DOI] [PubMed] [Google Scholar]
- 37.Stewart, C. M., P. C. Dunne, A. Sikes, and D. G. Hoover. 2000. Sensitivity of spores of Bacillus subtilis and Clostridium sporogenes PA 3679 to combinations of high hydrostatic pressure and other processing parameters. Innov. Food Sci. Emerg. Technol. 1:49-56. [Google Scholar]
- 38.Townsend, C. T., J. R. Esty, and F. C. Baselt. 1938. Heat resistance in relation to the determination of safe processes for canned foods. Food Res. 3:323-346. [Google Scholar]
- 39.Wuytack, E. Y., S. Boven, and C. W. Michiels. 1998. Comparative study of pressure-induced germination of Bacillus subtilis spores at low and high pressures. Appl. Environ. Microbiol. 64:3220-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]