Abstract
Immature CD4+CD8+ thymocytes expressing T-cell antigen receptors (TCR) are selected by TCR-mediated recognition of peptides associated with major histocompatibility complex molecules on thymic stromal cells. Selection ensures reactivity of the mature cells to foreign antigens and tolerance to self. Although much has been learned about the factors that determine whether a thymocyte with a given specificity will be positively or negatively selected, selection as an aspect of the developmental process as a whole is less well-understood. Here we invoke a model in which thymocytes tune their response characteristics individually and dynamically in the course of development. Cellular development and selection are driven by receptor-mediated metabolic perturbations. Perturbation is a measure of the net intracellular change induced by external stimulation. It results from the integration of several signals and countersignals over time and therefore depends on the environment and the maturation stage of the cell. Individual cell adaptation limits the range of perturbations. Such adaptation renders thymocytes less sensitive to the level of stimulation per se, but responsive to environmental changes in that level. This formulation begins to explain the mechanisms that link developmental and selection events to each other.
The fate of T cell precursors developing in the thymus may be largely determined by the intensity of receptor-mediated interactions with stromal cells (1). In particular, “positive selection” [of functional, major histocompatibility complex (MHC)-restricted, self-tolerant T cells] and “negative selection” (of strongly autoreactive cells) involve encounters with thymic epithelial cells and with antigen-presenting cells of hematopoietic origin, respectively; the outcome of these respective encounters (death by neglect versus survival and differentiation; apoptosis versus survival) depends on the level of stimulation (1). Recently, there have been several efforts to better define quantitative and qualitative aspects of “stimulation level” in the context of thymocyte selection and T-cell activation. We have earlier proposed a model, “the tunable activation-threshold (TAT) model” (2), that ascribes to thymocytes and to mature T cells a degree of adaptability, allowing these cells to tune and adjust their responsiveness to the level and quality of the stimulation which they experience. The concept of a tunable activation threshold has been recently adopted by other investigators to explain their experimental results (3–6). Here we discuss how the model may help us better understand the relationship between development and selection of T cells in the thymus and, in particular, how it explains the remarkable flexibility of the process.
On the Dynamic Relationship Between T-Cell Development and Selection
A common pattern seen during development of nonlymphoid cells is that once expression of a new receptor is induced, the full complement of receptors is expressed on the cell’s membrane within a short time. In contrast, the number of T-cell antigen receptors (TCRs) on maturing, double-positive (CD4+CD8+) thymocytes is initially low and increases only gradually, achieving nearly full expression in time-proximity to a commitment to either the CD4+CD8− or the CD4−CD8+ phenotype. The significance of the gradual-increase phase is not known. It is even more intriguing in light of the finding that the expression of TCR is actively limited during this phase (7).
We propose that the gradual increase in TCR numbers and the flexibility manifested in the selection of thymocytes are related phenomena: the gradual-increase phase is necessary (i) for survival of the immature thymocytes; (ii) for an efficient selection of specificities; and (iii) for a proper regulation of the developmental process itself.
(i) Survival.
The fact that thymic selection begins in immature thymocytes that express only low but increasing levels of TCR allows the cells to become acclimated, and so functionally tolerant, to the level of self antigens present in their environment; when the level of stimulation increases too abruptly, the cell is aberrantly activated and dies. At the same time, the increasing numbers of TCR gradually replace other cell-surface molecules in the role of providing viability-maintaining stimulation. Such replacement parallels the change in the signaling environment as the cells migrate through the thymus. Cells that fail to be recurrently stimulated above a minimal level undergo apoptosis and “die of neglect.”
(ii) Selection.
This acclimation process is geared to the baseline stimulation levels of individual cells with different specificities. Therefore, it cannot be entirely preprogrammed. Instead, it would require an adjustable signal-transduction mechanism that translates different external stimulation levels into relatively uniform intracellular signals. Threshold tuning facilitates just such a mechanism (see below): positive selection is thus accommodated over a broad range of affinities, TCR numbers, ligand concentrations, and other parameters. Moreover, threshold tuning would allow some of the less excessively autoreactive thymocytes to survive longer than others, so that negative selection may occur both early and late in maturation.
(iii) Development.
T-cell development involves a dynamic cross-talk between thymocytes and stromal cells. A hallmark of the developmental process is a progressive acquisition of a greater and better-focused capacity of both kinds of cells to interact with each other. Thymocytes need to be stimulated via CD3/TCR complexes, not only to differentiate and mature, but also to provide inductive signals for maturation and maintenance of the intrathymic environment (8). The induction of developmental modifications and functional activity in maturing thymocytes probably requires well-defined and quantitatively balanced intracellular signals. Again, this would be greatly facilitated by the hypothetical mechanism of sensitivity adjustment. At the same time, the rules of adaptation and inter-signal competition proposed below allow for an ad hoc element in development that can help to explain the diversity and idiosyncrasy of individual developmental pathways.
In summary, thymocyte survival requires successful adaptation to thymic microenvironments. In our view, the gradual change in the numbers of TCR is the distinguishing characteristic of a well-controlled adaptive process. In such a process, thymocytes with a range of different TCRs are able to adjust their responsiveness in a way that results in sustained viability and mutual tolerance vis-à-vis their thymic stromal counterparts.
Activation Thresholds and the Significance of Subthreshold Responses
Signals of different integrated strengths may evoke in lymphocytes qualitatively different cellular responses, implying that different cellular functions have different activation thresholds. The existence of different thresholds for different cellular responses was originally postulated as a novel way to explain self–nonself discrimination (9). The idea was that autoreactivity could be physiologically regulated not only by eliminating or inactivating autoreactive lymphocytes, but also by constraining them, by selection or induction, to the expression of non-destructive cellular responses. Such non-destructive responses have been termed “latent” (9, 10) or “subthreshold” (2). Recent advances, both in the characterization of signaling pathways in T cells and in the recognition of differential outcomes for the same T cell following stimulation by different antigens or different antigen-presenting cells, have largely substantiated these ideas (11–15). The broad significance of subthreshold cellular responses, usually revealed by peptide analogs called “partial agonists” (16), is beginning to be recognized (17).
At a more mechanistic level, the T cell has been viewed as an adaptable integrator of signals. Cell adaptation is the underlying mechanism responsible for the qualitative discrimination of binding events (2, 18). T cells adapt: they act to offset the biochemical changes, referred to collectively as “metabolic excitation,” which are initiated by the ligation and aggregation of TCR and other coreceptors on the cell surface, and which cascade toward the nucleus of the cell. The opposing action of tyrosine kinases and phosphatases may represent, in part, such an excitation–adaptation process (2). Another possible mechanism is the interplay between G protein signaling and its regulators, the RGS proteins (19). It has been postulated that the activation of distinct cellular functions is determined, in a threshold-dependent way, by the size of the metabolic perturbation, a measure of the imbalance (e.g., the difference) between excitation and adaptation. The maximum perturbation achieved upon a particular cell–cell encounter depends on the rate at which excitation is induced; adaptation follows, but not instantaneously. If the excitation process is rapid enough, the intensity of intracellular signals associated with activation (the cellular response) increases before the process is offset, partially or completely, by the counteraction of adaptation signals. A perturbation that exceeds the critical value required for the induction of a given function may then be transiently reached.
The rate of excitation, in turn, depends, in a non-multiplicative way, on TCR affinity for the peptide–MHC molecule complex (the ligand), on the ligand concentration, and on other “avidity”-related factors, including the levels of expression of receptors and coreceptors and of adhesion molecules. The fact that the dependence is non-multiplicative (that is, does not simply depend on the product of the individual factors) means, for example, that higher antigen concentration can compensate for a lower affinity only “up to a point” (9). This departure from a simple receptor occupancy model has important consequences. At the single cell level, it can account for the uncoupling of full activation from other responses to structurally modified peptides, even at high concentrations of these peptides, implying a distinction between quantity and “quality” of antigenic stimulation (17). At the cell population level, selection of cells responding with latent proliferation is predicted to occur under certain conditions, e.g., upon stimulation at high antigenic doses, in the presence of interclonal competition, of a heterogenous population of lymphocytes with a wide range of affinities for the antigen (refs. 9 and 10; see below).
In summary, lymphocyte activation is not an on–off event. The nature and intensity of the response vary with the magnitude of the perturbation. The magnitude of the perturbation, in turn, is dependent upon the relative rates of excitation and short-term adaptation.
The Model of Tunable Activation Threshold (TAT)
The TAT model (2) proposes that the magnitude of the perturbation also depends on the level of adaptation before the encounter. That level of adaptation reflects the interaction history of the cell over several previous encounters and may be called “long-term adaptation.” Stimulatory signals that do not result in full activation nevertheless reset the threshold for activation. This is similar to the differential responsiveness of neurons to light (or to odor) after being exposed to different background levels. The excitation needs to significantly exceed the background level to elicit a full response. Stimuli that fail to do so—“silent excitation” events—nevertheless contribute to the background level and thereby affect the activation threshold.
Because of this cellular memory of adaptation, which may be represented by compounds or structures undergoing storage-degradation cycles in a certain domain of the cell during excitation events, activation thresholds (i.e., excitations that would produce the critical perturbations required for activation) are tunable. Silent excitation redefines these thresholds. Thus, the outcome of recurrent stimulation is sensitive to the kinetics, or time contingency, of the excitation events. In particular, gradually increasing excitation steps associated with development may typically cause perturbations that cannot induce full activation; instead, the associated adaptation processes may lead to desensitization, up-regulation of the threshold for a full activation. This is because each step updates the long-term adaptation level sufficiently to prevent the next perturbation from exceeding the critical value.
The TAT model is schematized in Fig. 1. The sharply oscillating curves describe the changing level of intracellular signals. We call this level “signal intensity.” It peaks during high-avidity cell–cell encounters. The more-smoothly varying, parallel curves depict an activation threshold (Top), and the viability-maintenance threshold (Bottom). Both have been proposed to be directly related to a “moving average” of the signal intensity over time, to which we refer as baseline activity level, and to follow the variation of this baseline level (2).
Figure 1.
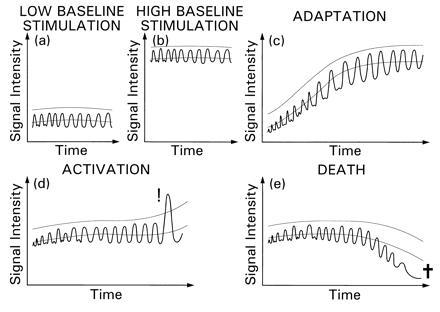
The TAT model. The kinetics of intracellular signal intensity (wavy line), activation threshold (upper smooth line), and viability-maintenance threshold (lower smooth line) are schematically illustrated. Five examples of different cells or of the same cell in different microenvironments are shown. (a) A cell undergoing stationary stimulation of a relatively small intensity. The cell maintains its viability without being fully activated. This is due to the signal intensity remaining below the activation threshold and regularly exceeding the viability-maintenance threshold. (b) A cell undergoing high-intensity stationary stimulation. Activation does not occur since the baseline and the associated thresholds are adjusted to the level of stimulation. (c) A gradual increase in the level of stimulation occurs, driving upward the baseline and the thresholds so that activation does not occur. Threshold tuning has allowed a smooth transition from the level of activity shown in a to that of b. (d) As in a and b, but an increase in the signal intensity occurs abruptly causing a strong perturbation (even though the peak intensity does not exceed intensities occurring in b). The activation threshold is surpassed, resulting in (full) activation. (e) As in a, but the intensity falls abruptly at some point (with the baseline adjusting more slowly), remaining below the viability-maintenance threshold for a sufficiently long time to cause death-by-neglect.
The baseline activity level (previously called also “excitation index”) is a cumulative intracellular memory of the recent excitation events experienced by the cell, to which it has adapted. Therefore, it reflects also the changing level of adaptation. The perturbation is defined as the difference between signal intensity and the baseline level. This difference reflects the net amount of intracellular signals available for cell activation. The activation threshold is the minimum signal intensity required for the probability of activation to be above zero, and it is defined by the addition of a fixed amount (the “critical perturbation”) to the baseline activity level. Activation does not occur in the examples shown in Fig. 1 a and b in spite of the difference in the signal intensities, because the activation threshold is adjusted to the respective baseline levels. A transition from low to high intensities results in activation when the change is abrupt (Fig. 1d), but not when the change is gradual (Fig. 1c).
It has been proposed (2, 18) that subcritical perturbations, namely those below the critical value required for full activation, selectively induce reversible changes in gene expression as well as irreversible, developmental events, and are also required for the inhibition of programmed cell death (apoptosis). These effects too are threshold-dependent. The viability-maintenance threshold, in particular, is also tuned to the baseline activity level, as shown in Fig. 1. Cells will undergo apoptosis if the signal intensity during excitation events falls significantly below the baseline for too long, before the baseline itself is readjusted (Fig. 1e). This can occur when the level of stimulation in the environment changes abruptly from high to low.
Thymic Selection Revisited
The efficacy of stimulation of immature double-positive (DP) thymocytes is gradually enhanced due to increased expression of the TCR. This allows long-term adaptation to keep up with the increased signal intensity (as in Fig. 1c). The result is affinity- and avidity-adjusted desensitization (elevation of the activation threshold) rather than full activation (2). Throughout the maturation process, excitation levels increase substantially, but so does the level of long-term adaptation, so that the perturbations change only modestly. Similarly, diverse binding characteristics correspond to a large range of intensities and baseline activities, but to only a limited range of perturbations. Positive selection of DP thymocytes occurs if the peaks in signal intensity remain, largely, between the rising activation and viability-maintenance thresholds (Fig. 2).
Figure 2.
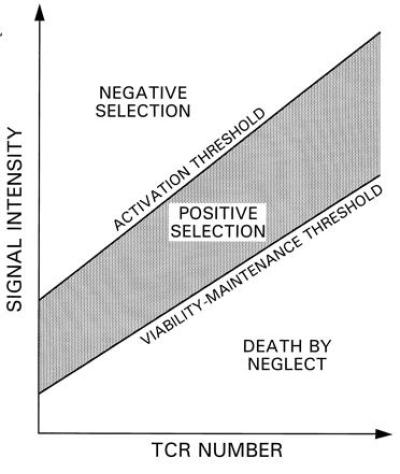
Threshold tuning during gradual increase in TCR expression by DP thymocytes. Illustrated are selection boundaries for the signal intensity, induced in a DP thymocyte, as a function of TCR number. For higher levels of receptor expression, the average signal intensity (i.e., the baseline activity level) is higher, entailing higher activation threshold and viability-maintenance threshold. The thymocyte is not activated as TCR number increases if the fluctuating signal intensity (not shown) does not exceed the activation threshold. At higher intensities, negative selection occurs. If the intensity falls below the viability-maintenance threshold for too long, death-by-neglect may result.
This interpretation is consistent, in particular, with the ability of peptides that manifest very different efficacies in the stimulation of T cells to positively select the corresponding thymocytes. More generally, the ability to downscale the perturbation level endows the necessary precision to thymocyte development and selection.
Fig. 3 a–f illustrates the developmental fates of different prototypical thymocytes, expressing receptors of different affinities for selecting ligands. Fig. 3 b and c show positive selection: thymocytes possessing intermediate affinities, successfully adapting to increasing signal intensities. DP thymocytes that are too weakly stimulated via the TCR die early on, as metabolic perturbations that the microenvironment induces in these cells do not exceed the minimum required for the activation of apoptosis-inhibiting signals (Fig. 3a). This minimum, in turn, is defined by the baseline signaling level during the earlier (double negative) phase of their development. This provides one mode of selection (“death by neglect”). We note that overstimulated cells too can die from a similar cause, according to our model, when they migrate too abruptly from the strongly stimulating environment to a less stimulating one. Such death can be avoided if the viability-maintenance threshold is down-regulated rapidly (viability is enhanced) by some preprogrammed mechanism. This seems to be the case toward the end of the DP developmental phase, upon transition from the cortex into the medulla, as indicated by the rapid increase in the level of expression of bcl-2 at this stage (20).
Figure 3.
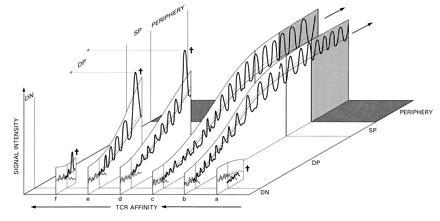
Typical signal intensity profiles of positively and negatively selected cells during thymic development. For positive selection, signal intensity should not exceed the activation threshold (upper curve) and should regularly exceed the viability-maintenance threshold (dotted line). The bottom line represents the baseline activity. Several examples are shown, ordered from right to left by increasing affinities for the selecting MHC–ligand combination. (a) Early death-by-neglect; (b and c) positive selection. (d) Negative selection as a result of a rapid increase in TCR number occurring toward the transition phase from the DP to the single-positive (SP) phenotype. (e) Cortical negative selection, occurring as the rate of increase in signal intensity is accelerated during the DP phase. (f) Negative selection of immature thymocytes upon transition from the double negative (DN) into the DP developmental state (possibly associated with an early receptor-aggregation event).
Strongly autoreactive thymocytes that are negatively selected die as a result of sustaining above-threshold perturbations. When the efficacy of stimulation per additional receptor is large, updating of the baseline can lag considerably behind the peak intensities of intracellular signals reached as the number of TCR increases. This could result in supracritical perturbations (see Fig. 3 d and e).
Thymic epithelial cells and cells of hematopoietic origin are thought to have differential roles, preferentially effecting positive and negative, respectively, selection. In our model, this would suggest that the hematopoietic cells typically induce higher signal intensities. The observation that negative selection occurs both early and late in DP-thymocyte development is consistent with an overlapping distribution of the two types of stromal cells in the thymus.
There are points along the developmental pathway in which the chances for the induction of a supracritical perturbation in strongly autoreactive thymocytes are particularly high, and these points could serve as gating check-points in selection. One such point is just when newly turned DP thymocytes begin to develop their complement of receptors. At that time, only thymocytes whose TCR are engaged by the selecting MHC-peptide molecule combination without aggregation survive (21). Cells with TCR that have high affinity for a ligand can be positively selected if the ligand is presented in sufficiently low numbers on antigen-presenting cells, so that TCR aggregation does not occur; the same is true for TCRs with low affinity for ligands presented at high concentrations. The situation is not necessarily symmetrical, however, as affinity contributes more critically to the stability of the bound TCR aggregates, which is presumably required for an efficient, rapid production of excitation products used for intracellular signaling (17). Stable TCR aggregation probably triggers rapid excitation, which is associated with a strong perturbation in the sense of the present model, distinguishing it clearly from binding without receptor aggregation (Fig. 3f). Cells characterized by weaker autoreactivities are relatively protected at this stage, as aggregation is difficult to accomplish on cells expressing few TCR molecules. As the number of TCR increases, receptor aggregation is more likely to occur, but the perturbation is attenuated by the increased adaptation level, i.e, by the elevation of activation thresholds. The critical perturbation required to induce negative selection may itself increase as well due to increased expression of the protective bcl-2 molecules (20).
Rather than completely preprogrammed, the increase in TCR itself may be seen as an adaptive, perturbation driven response to microenvironmental signals, as thymocytes migrate through the thymus. Survival of immature thymocytes presumably depends in part on stimulation by signals unrelated to those involved in TCR-mediated stimulation; those signals must have been engaged in viability maintenance before TCR started to be expressed. One effect of abundant, relatively low-efficacy signals would be to attenuate the perturbations associated with TCR-mediated, higher-efficacy stimulation. This in turn would slow down the rate of de novo expression of TCR. Indeed, there is evidence for specific intracellular processes that act to limit the rate of TCR expression (7). As the thymocytes migrate, they enter sites where those other signals are no more in abundance, releasing the TCR-expression mechanism from suppression and allowing for a rapid de novo expression. The TCR-mediated signals then replace the other signals in their role of supporting viability. In addition, accelerated increase in TCR might facilitate other developmental and gating functions at such points.
On the whole, the sensitivity-tuning mechanism decrees a considerable degree of uniformity on the processes of negative and positive selection. The outcome of selection is determined by the interplay of this tuning process, microenvironmental signals that guide differentiation, and physiological gating conditions that restrict the admissible autoreactivities (Fig. 3). It is predicted that an overly uniform signaling environment may fail to efficiently induce both negative selection and complete development.
Peripheral Tolerance: Continued Excitation and Adaptation
It is proposed that the activation thresholds of all maturing thymocytes are tuned in the course of their interactions with self antigens, individually and dynamically, resulting in a partial desensitization to activation. Such desensitization could result, for example, from depletion or inactivation of an intracellular kinase or phosphotase involved in the intracellular signaling cascade. The degree of desensitization is directly related to the affinity of the thymocyte for the self antigen. Regardless of its affinity, every positively selected T cell emerges from the thymus with an appropriate level of desensitization, having been adapted to the particular (maximal) level of antigenic stimulation which it had experienced.
If the emerging T cell continues to encounter a similar level of self-antigenic stimulation in the periphery (or a lower one, or even gradually increasing stimulation), it will remain adapted to that level, continuously updating its baseline intracellular activity and its activation threshold. Auto-adjusting desensitization should take care of self tolerance in the periphery, as it had prevented abortive activation in the thymus, while maintaining functional reactivity of T cells to foreign antigens when the latter bind with higher affinity in an immunogenic context.
If threshold tuning alone can account for tolerance to self, what is the role of negative selection? Clonal elimination of T cells with strongly autoreactive receptors is not necessary for maintenance of self tolerance, because those cells, had they not been eliminated, would have adapted to high-level stimulation and would not have been activated in the periphery.
We suggest that negative selection is required primarily to remove useless cells that could not be activated in the periphery, and not to prevent autoimmunity. Since lower-affinity cells are less potently desensitized, they are usually capable of being activated by some combination of foreign peptides and the selecting MHC molecules. Thus, these cells are tolerant, in the conventional sense, to the positively selecting peptide–MHC molecules but not to specific foreign antigens. In contrast, T cells carrying receptors with the highest affinities to self would still be rendered tolerant (anergic), but would be useless (and possibly obstructive). When the baseline level of intracellular signals approaches the maximum signal intensity that can be induced, supracritical perturbations can no longer be generated (i.e., the activation threshold cannot be reached). Such cells could not be activated in their normal environment by any antigen.
We note that, paradoxically, it is cells with intermediate-affinity receptors for self, and not necessarily with the highest affinities, that are more likely to be inappropriately activated. This is because such cells would be more sensitive to abrupt intensification in the level of stimulation due, e.g., to a rapid increase in the local concentration of self peptides or in the local expression of MHC molecules, or to the synergistic effect of inflammatory factors.
Stimulation, antigenic or other, is required also for maintaining the viability of the cells. Thus, it is proposed that naive T cells can be maintained in the periphery in the absence of stimulation by a foreign antigen, but that such maintenance is effected by the microenvironment. Under competitive conditions, the environment will select the fittest clones. This amounts to a continuous “positive selection” also in the periphery. Unlike their DP or single-positive thymocyte precursors, the mature T cells can respond to such stimulation either by the latent mode of activation that we have discussed or by “latent proliferation” (9), i.e., self-renewal division short of the expression of fully developed effector functions. The proliferation threshold is assumed to be lower than the full-activation threshold. Among the totality of positively selected T cells, those specific for abundant self antigens and falling, in terms of affinity, just below the full-activation threshold (i.e., relating to the antigen as a “strong partial agonist”) may gain predominance in a competition against both higher-affinity cells (which upon activation express functions that exclude or interfere with proliferation) and lower-affinity cells. While the affinity threshold can segregate and select the function expressed (e.g., proliferation), high ligand concentration then serves to amplify this expression (9, 10, 17).
For positively selected T cells with relatively high affinities for the selecting molecules, desensitization may appear as “anergy” in conventional assays. That “anergy” is a dynamic and reversible state has gained experimental support (22). In mature T cells, the most direct evidence to date for threshold tuning is the demonstration that the level of “anergy” can be enhanced upon further stimulation (23).
Our hypothesis predicts that those T cells positively selected by relatively high-affinity interactions with self antigens that are confined exclusively to the thymic environment should gradually lose tolerance to the selecting antigens in the periphery. (Thymectomy is perhaps required to exclude recirculation through the original environment.) Another prediction is that such cells would be maintained in the periphery for shorter periods of time as compared with cells whose selecting ligand is found also in the periphery.
Low levels of anergy protect T cells from being activated by self, while maintaining their reactivity to foreign immunogenic antigens. Such low-level anergy is independent of the presence or absence of costimulatory signals. Similarly, it has been proposed (2) that memory T cells are protected from chance activation through a continuing subthreshold interaction with residual antigens and/or with other accessory cells to which they are dynamically coupled, and this protection may be reflected by certain manifestations of “anergy” (24). At the same time, subthreshold interactions maintain the ability of memory cells to respond to a secondary immunogenic challenge. It has also been proposed that important immunological functions are normally mediated by cells engaged in subthreshold interactions, including the control of chronic infection with a minimal damage to the host. Subthreshold autoreactivity, in particular, may be instrumental in the response to various chronic insults (2).
Altogether, the quality of the interactions of positively selected thymocytes with their ligands is comparable to that of mature T cells, when the latter are allowed to adapt and operate in the “subthreshold” mode. This quality is generally not an inherent property of the ligand, neither at the developmental level nor at the mature cell level. Rather, it is a function of the “context”; subthreshold interaction characterizes a relatively stationary stimulation, while full activation results from an acute change in the level of stimulation. This interpretation provides a powerful, unifying approach to a broad range of apparently paradoxical observations in recent immunology (2, 18, 25). One relevant generalization, which has only been hinted above, is interdependent adaptation of both the T cell or thymocyte and the antigen-presenting cell (or thymic epithelium) during subthreshold interactions.
In conclusion, the present model links together development and selection in the thymus. The key idea is that subthreshold interactions have opposing cumulative effects in modifying thymocyte responsiveness. These interactions drive, and are driven by, developmental events, including the increased expression of receptors, accessory molecules and transductions elements. At the same time, the strength of the signal required for functional lymphocyte activation also increases gradually. A critical variable in this model is the relative kinetics of these opposing and interdependent changes, reflecting at any time the stimulation history, state of differentiation and microenvironment of the cell.
Acknowledgments
The work of Z.G. was supported in part by the Harry Palley Endowment Fund of the United Jewish Federation Foundation (Pittsburgh).
Footnotes
Abbreviations: TCR, T-cell receptor; MHC, major histocompatibility complex; DP, double positive; TAT, tunable activation threshold.
References
- 1.Jameson S C, Hogquist K A, Bevan M J. Annu Rev Immunol. 1995;13:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- 2.Grossman Z, Paul W E. Proc Natl Acad Sci USA. 1992;89:10365–10369. doi: 10.1073/pnas.89.21.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawai K, Ohashi P S. Nature (London) 1995;374:68–69. doi: 10.1038/374068a0. , and correction (1995) 378, 419. [DOI] [PubMed] [Google Scholar]
- 4.Rothenberg E. Immunologist. 1995;3:172–175. [Google Scholar]
- 5.Sebzda E, Kundig C D, Thomson C T, Aoki K, Mak S Y, Mayor P J, Zamborelli T, Nathenson S G, Ohashi P S. J Exp Med. 1996;183:1093–1104. doi: 10.1084/jem.183.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodnow C C. Proc Natl Acad Sci USA. 1996;93:2264–2271. doi: 10.1073/pnas.93.6.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakayama T, June C H, Munitz T I, Sheard M, McCarthy S A, Sharrow S O, Samelson L E, Singer A. Science. 1990;249:1558–1561. doi: 10.1126/science.2120773. [DOI] [PubMed] [Google Scholar]
- 8.van Ewijk W, Shores E W, Singer A. Immunol Today. 1994;15:214–217. doi: 10.1016/0167-5699(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 9.Grossman Z. Eur J Immunol. 1982;12:747–756. doi: 10.1002/eji.1830120909. [DOI] [PubMed] [Google Scholar]
- 10.Grossman Z. Immunol Rev. 1984;79:119–138. doi: 10.1111/j.1600-065x.1984.tb00490.x. [DOI] [PubMed] [Google Scholar]
- 11.Sloan L J, Evavold B D, Allen P M. Nature (London) 1993;363:156–159. doi: 10.1038/363156a0. [DOI] [PubMed] [Google Scholar]
- 12.Racioppi L, Ronchese F, Matis L A, Germain R N. J Exp Med. 1993;177:1047–1060. doi: 10.1084/jem.177.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page D M, Alexander J, Snoke K, Apella A, Sette A, Hedrick S M, Grey H M. Proc Natl Acad Sci USA. 1994;91:4057–4061. doi: 10.1073/pnas.91.9.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Speiser D E, Kyburz D, Stubi U, Hengartner H, Zinkernagel R M. J Immunol. 1992;149:972–980. [PubMed] [Google Scholar]
- 15.Bachmann M F, Sebzda E, Kundig T M, Shahinian A, Speiser E D, Mak T W, Ohashi P S. J Exp Med. 1996;183:1093–1104. doi: 10.1084/jem.183.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evavold B D, Allen P M. Science. 1991;252:1308–1310. [PubMed] [Google Scholar]
- 17.Germain R N, Levine E H, Madrenas J. Immunologist. 1995;3:113–121. [Google Scholar]
- 18.Grossman Z. Immunol Rev. 1993;133:45–73. doi: 10.1111/j.1600-065x.1993.tb01509.x. [DOI] [PubMed] [Google Scholar]
- 19.Siderovski D P, Hessel A, Chung S, Mak T W, Tyers M. Curr Biol. 1996;6:211–212. doi: 10.1016/s0960-9822(02)00454-2. [DOI] [PubMed] [Google Scholar]
- 20.Linette G P, Grusby M J, Hedrick S M, Hansen T H, Glimcher M H, Korsmeyer J. Immunity. 1994;1:197–205. doi: 10.1016/1074-7613(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 21.Takahama Y, Suzuki H, Katz K S, Grusby M J, Singer A. Nature (London) 1994;371:67–70. doi: 10.1038/371067a0. [DOI] [PubMed] [Google Scholar]
- 22.Arnold B, Schonrich G, Hammerling G J. Immunol Today. 1993;14:12–14. doi: 10.1016/0167-5699(93)90317-E. [DOI] [PubMed] [Google Scholar]
- 23.Migita K, Ochi A. J Immunol. 1993;150:763–770. [PubMed] [Google Scholar]
- 24.Jenkins M K, Miller R A. FASEB J. 1992;6:2428–2432. doi: 10.1096/fasebj.6.7.1563595. [DOI] [PubMed] [Google Scholar]
- 25.Grossman Z, Bentwich Z, Herberman R B. Clin Immunol Immunopathol. 1993;69:123–135. doi: 10.1006/clin.1993.1160. [DOI] [PubMed] [Google Scholar]


