Abstract
This study developed models to predict lactic acid concentration, dipping time, and storage temperature combinations determining growth/no-growth interfaces of Listeria monocytogenes at desired probabilities on bologna and frankfurters. L. monocytogenes was inoculated on bologna and frankfurters, and 75 combinations of lactic acid concentrations, dipping times, and storage temperatures were tested. Samples were stored in vacuum packages for up to 60 days, and bacterial populations were enumerated on tryptic soy agar plus 0.6% yeast extract and Palcam agar on day zero and at the end point of storage. The combinations that allowed L. monocytogenes increases of ≥1 log CFU/cm2 were assigned the value of 1 (growth), and the combinations that had increases of <l log CFU/cm2 were given the value of 0 (no growth). These binary growth response data were fitted to logistic regression to develop a model predicting probabilities of growth. Validation with existing data and various indices showed acceptable model performance. Thus, the models developed in this study may be useful in determining probabilities of growth and in selecting lactic acid concentrations and dipping times to control L. monocytogenes growth on bologna and frankfurters, while the procedures followed may also be used to develop models for other products, conditions, or pathogens.
Food-borne illness caused by Listeria monocytogenes is estimated to cause 2,493 infections and 499 deaths annually in the United States (17). Although processed meats may contain antimicrobials to inhibit L. monocytogenes growth, their effectiveness may vary due to the type of product and other factors (7, 14). Nuñez de Gonzalez et al. (18) suggested that most of the L. monocytogenes contamination on ready-to-eat (RTE) products occurs after processing. The U.S. Department of Agriculture Food Safety and Inspection Service (26) established a new listeriosis control regulation for RTE meat and poultry products that may be exposed to postprocessing contamination and allow L. monocytogenes multiplication during storage. According to this regulation, processors must improve L. monocytogenes control by including one of three alternative approaches in their hazard analysis critical control point plans or prerequisite programs. Alternative 1 requires processing and chemical antimicrobial interventions that inactivate and inhibit L. monocytogenes growth. Alternative 2 involves the use of either processes or antimicrobial agents that inactivate or inhibit growth of the pathogen. Alternative 3 focuses on documented sanitation, intensive sampling/testing, and product-holding programs to control L. monocytogenes growth; sampling/testing intensity decreases under alternative 2 and especially under alternative 1. Thus, the industry needs information on antimicrobial treatments that may be considered for use to meet these regulatory requirements (5, 6). In response to such need, studies have evaluated the effectiveness of dipping or spraying RTE meat and poultry products with antimicrobial solutions including lactic acid (1, 5, 6, 8, 9, 10, 18, 19, 23). Palumbo and Williams (19) suggested that the application of a secondary lethal treatment would provide additional protection and safety to the processor and consumer. In addition, storage temperature may influence not only pathogen growth but also the antimicrobial effects of the secondary inhibitory treatments during storage. According to the U.S. listeriosis risk assessment (29), discontinuing use of refrigerators operating above 7°C and 5°C could reduce the number of estimated listeriosis cases in the United States from 2,105 to 656 (69% reduction) and 28 (>98% reduction) per year, respectively.
Although currently RTE foods must comply with a “zero tolerance” policy for presence of the pathogen in the United States, other countries, such as those in the European Union (3), have variable tolerances depending on product properties and intended consumers. Such variable tolerances may also be considered in the United States in the future (28). In instances where certain growth levels may be allowed in a product, means of predicting storage time and conditions for such growth under various probability levels would be necessary.
Mathematical models have been developed to predict the fate of pathogens in foods, based on experimental data from studies evaluating the effects of combinations of factors that influence their growth (25). Kinetic mathematical models calculate the microbiological shelf life of food products, while probabilistic models are used to predict the probability of growth of a microorganism under various storage conditions (11, 25). The concept of determining the probability of food-borne-pathogen growth was introduced by Genigeorgis et al. (4) for the prediction of combinations of factors that prevented growth and toxin production by Staphylococcus aureus. In addition, Ratkowsky and Ross (21) introduced the use of logistic regression for modeling the boundary between growth and no growth. Mathematical modeling of growth limits has been considered to be a critical component of modern predictive microbiology (16). However, even though the majority of foods are solid, most models have been developed with data from studies with liquid laboratory media (30). Thus, modeling the boundaries between growth and no growth of food-borne pathogens by using results of studies conducted with foods should be useful. Thus, the objective of this study was to develop models to predict growth/no-growth boundaries of L. monocytogenes contamination on bologna and frankfurters at desired probabilities as a function of lactic acid concentration, dipping time and storage temperature.
MATERIALS AND METHODS
Preparation of inoculum.
The 10-strain mixed inoculum of L. monocytogenes bacteria (Scott A, NA-3, NA-19, 101 M, 103 M, 558, PVM1, PVM2, PVM3, and PVM4) used in this study was prepared as described by Bedie et al. (2) and Samelis et al. (22, 23). Stationary-phase cells were harvested by centrifugation (4,629 × g, 15 min, 4°C), washed, resuspended in phosphate-buffered saline (pH 7.4; 0.2 g of KH2PO4, 1.5 g of Na2HPO4·7H2O, 8.0 g of NaCl, and 0.2 g of KCl in 1 liter of distilled water), and diluted appropriately in phosphate-buffered saline to obtain approximately 2 log CFU/cm2 on product samples after inoculation.
Preparation of samples and inoculation.
Bologna samples (including no antimicrobials) were prepared according to procedures described by Bedie et al. (2) and Samelis et al. (22). Commercial frankfurters (including no antimicrobials) were also used in this study. The formulation of frankfurters consisted of pork, water, modified food starch, hydrolyzed soy and potato proteins, corn syrup, salt, dextrose, flavorings, potassium chloride, autolyzed yeast, sodium phosphates, smoke flavoring, paprika, sodium erythorbate, and sodium nitrite. A 0.1-ml portion of the inoculum was spread over one side of each bologna slice with a sterile, bent glass rod, left to stand at 4°C for 15 min to allow bacterial attachment, and then inoculated on the second side, using the same procedure. A 0.25-ml portion of the inoculum was deposited over two frankfurters in a vacuum bag (15-cm by 20-cm, 3 mil standard barrier, nylon/polyethylene vacuum pouch; Koch, Kansas City, MO). The frankfurters were then massaged in the bag to spread the inoculum over the entire surface of the product. The inoculated frankfurters were stored at 4°C for 30 min to allow for bacterial attachment.
Application of treatments.
Following inoculation, bologna (14 slices/300 ml of lactic acid solution) and frankfurters (20 frankfurters/800 ml of lactic acid solution) were completely immersed into lactic acid solutions (0, 1, 2, 3, or 4%; Purac, Lincolnshire, IL) (4°C) for 0, 1, 2, 3, or 4 min. Two bologna slices or frankfurters were then placed into vacuum bags (15 by 20 cm; Koch), vacuum packaged (Hollymatic Corp., Countryside, IL), and stored at 4°C (bologna, 60 days; frankfurters, 40 days), 7°C (bologna, 50 days; frankfurters, 35 days), and 10°C (bologna, 30 days) or 12°C (frankfurters, 15 days). For model development, it was assumed that if L. monocytogenes growth has not been observed within 30 days and 15 days at 10°C and 12°C, respectively, then it will not happen later.
Microbiological analyses.
Samples were aseptically transferred into a Whirl-Pak bag (Nasco, Modesto, CA) containing 50 ml of maximum recovery diluent (0.1% peptone and 0. 85% NaCl) (13) and shaken 30 times (1). After serial dilution of the rinsate, 0.1-ml portions of selected dilutions were plated onto tryptic soy agar (Difco, Becton Dickinson and Company, Sparks, MD) plus 0.6% yeast extract (Acumedia, Lansing, MI) (TSAYE) and Palcam agar (Difco) to enumerate total bacterial populations and cell counts of L. monocytogenes, respectively, at time zero and the end point of storage. All plates were incubated at 30°C for 48 h (Palcam agar) or at 25°C for 72 h (TSAYE), and colonies were recorded as the number of CFU/cm2. The pH values of sample homogenates in maximum recovery diluent were determined using a pH meter with a glass pH electrode (Denver Instruments, Arvada, CO).
Evaluation of growth response.
A total of 75 combinations of storage temperatures (4, 7, 10, or 12°C), lactic acid concentrations (0 to 4%), and dipping times (0 to 4 min) in two replicates (two samples/replicate) for each combination were studied. Microbiological data (CFU/cm2) were converted into log10 CFU/cm2 values before being analyzed. Combinations that had ≥l-log CFU/cm2 increases in cell counts of L. monocytogenes bacteria were assigned the value of 1 (growth), while combinations that had <l-log CFU/cm2 increases were given the value of 0 (no growth). The threshold level of pathogen growth may be changed according to a company's product standards or other considerations, including regulatory standards if different than “zero tolerance.” Koutsoumanis et al. (11) also used a threshold level of 1-log growth to determine growth or no growth for the evaluation of a model's performance. The U.S. Department of Agriculture Food Safety and Inspection Service determines the degree of inspection frequency of sampling in commercial plants based on whether the treatments applied (26) allow 1- or 2-log units of growth (27). Thus, there are situations where it is useful to predict probabilities of a certain level of pathogen growth rather than to predict magnitudes of bacterial populations during storage. This can be accomplished with a probabilistic model based on results obtained from studies with foods, considering the effects of food structure and microbial interactions on pathogen behavior (11, 20). Moreover, the results using visible changes (e.g., turbidity) in broth media could be used to develop the probabilistic model; however, it may result in underestimating the risk of L. monocytogenes growth because the visible changes may happen after growth of the pathogen by several log CFU. Because of these reasons, the binary data were created from log counts as described above.
Model development for growth/no-growth boundaries at desired probabilities.
The growth response data were used in logistic regression analysis (PROC LOGISTIC) using SAS version 9.1 (SAS Institute Inc., Cary, NC) for the development of models to predict the boundaries between growth and no growth (21). Equation 1 was derived using the logistic regression analysis and the automatic variable selection option (P < 0.05) with a stepwise selection method to determine estimates of significant parameters (11, 12, 15). For the selection of parameters, a parameter was left in a model, even though it was not significant (P ≥ 0.05), if the parameter was involved in an interaction which was significant.
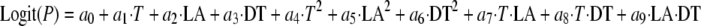 |
(1) |
where Logit(P) is an abbreviation for ln [P/(1 − P)], P is the probability of growth (in the range of 0 to 1), ai values are estimates, T is storage temperature, LA is lactic acid concentration, and DT is dipping time. To produce the predicted boundaries between growth and no growth for probabilities of 0.1, 0.5, and 0.9, Microsoft Excel Solver was used (11, 12, 15).
Model validation.
The predicted probabilities of growth from the models were compared with observed data from the limited literature data available on RTE meat products stored in vacuum packages. If a >1-log CFU/cm2 increase was observed during storage, it was defined as “growth,” and if a <1-log CFU/cm2 increase in growth or a decline was observed, it was defined as “no growth” (11). In literature data, growth response at 10°C was determined within 15 days (frankfurters) and 30 days (bologna) of the storage time.
RESULTS AND DISCUSSION
The average pH value of untreated (0% lactic acid) bologna was 6.22, and it decreased to within the ranges of 5.27 to 5.66 (1%), 4.71 to 5.28 (2%), 4.27 to 4.88 (3%), and 4.19 to 4.73 (4%) after dipping samples into lactic acid solutions. Differences in pH values within an acid level range were due to dipping times; longer dipping times caused larger decreases in the pHs of samples. Corresponding pH values at the end point of storage were 4.83 to 5.03 (0%), 4.83 to 5.28 (1%), 4.68 to 5.26 (2%), 4.31 to 5.04 (3%), and 4.13 to 4.82 (4%). The pH value of untreated frankfurters (0% lactic acid) was 5.86, while after immersion of samples into lactic acid solutions, pH values decreased to 5.67 to 5.75 (1%), 5.52 to 5.62 (2%), 5.45 to 5.62 (3%), and 5.30 to 5.51 (4%). Corresponding pH values at the end point of storage were 5.44 to 6.02 (0%), 5.41 to 5.92 (1%), 5.32 to 5.75 (2%), 5.20 to 5.76 (3%), and 5.01 to 5.70 (4%).
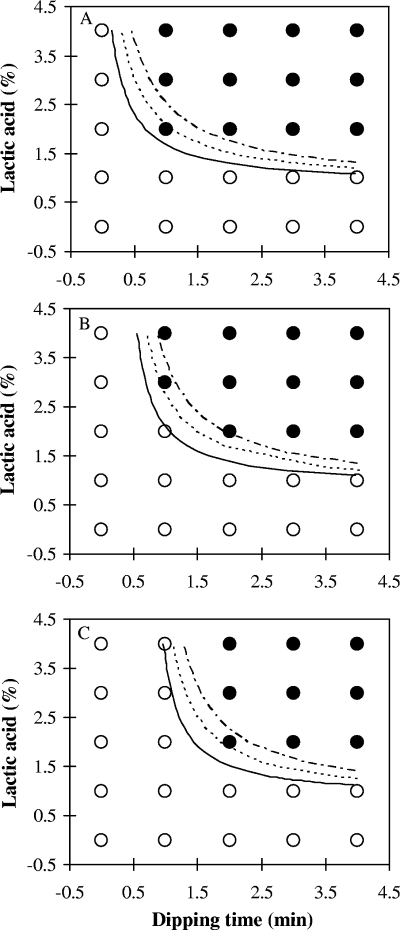
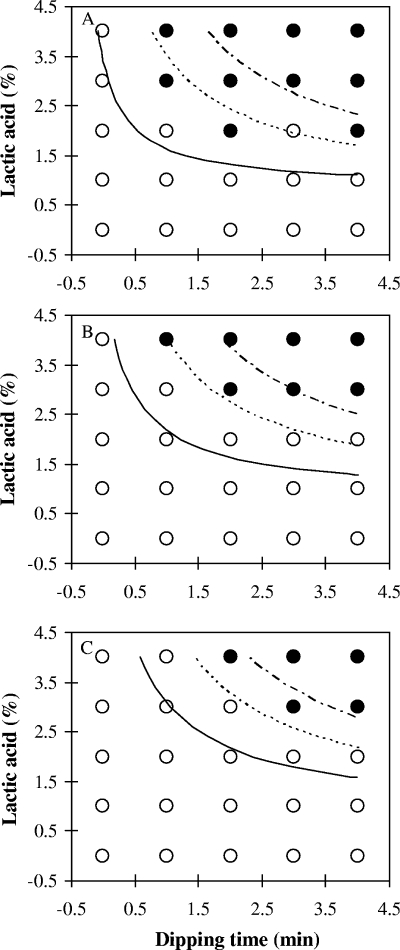
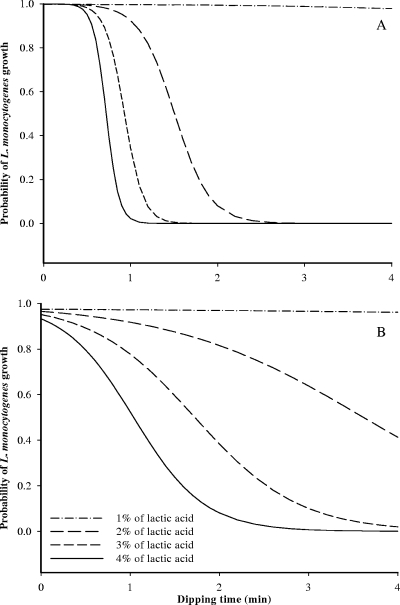
In general, total bacterial populations recovered with TSAYE were similar to those determined with Palcam agar (data not shown), and thus, bacterial populations recovered with Palcam agar were used to develop models. The estimates of parameters selected from logistic regression analysis are presented in Table 1 and were used to generate boundaries between growth and no growth of L. monocytogenes at probabilities of 10, 50, and 90% for bologna and frankfurters (Fig. 1 and 2). In Fig. 1 and 2, observed growth responses were determined according to the averages of differences in cell counts of L. monocytogenes between day zero and the end point of storage; an average of ≥1 log CFU/cm2 indicates growth, and an average <1 log CFU/cm2 indicates no growth. As expected, the MICs of lactic acid solutions decreased with increasing dipping times for both bologna and frankfurters (Fig. 1 and 2). This finding is also supported by results in Fig. 3 which show that lower concentrations of lactic acid needed longer dipping times and that use of 1% lactic acid solution was not sufficient to inhibit L. monocytogenes growth on either bologna or frankfurters.
TABLE 1.
Estimates of parameters selected from the logistic regression analysis by the automatic variable selection option with a stepwise selection method to predict boundaries between growth and no growth of Listeria monocytogenes at desired probabilities
| Variable | Parameter value fora:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bologna
|
Frankfurters
|
||||||||||
| Intercept | Temp | LA concn | DT | Temp × LA | LA × DT | Intercept | Temp | LA concn | DT | LA × DT | |
| Estimate | 9.216 | −0.587 | −3.109 | 3.681 | 0.61 | −4.307 | 2.548 | 0.21 | −0.348 | 0.692 | −0.805 |
| SE | 4.912 | 0.519 | 2.134 | 1.171 | 0.24 | 0.967 | 1.161 | 0.071 | 0.373 | 0.49 | 0.206 |
| P value | 0.0607 | 0.2584 | 0.1452 | 0.0017 | 0.0109 | <0.0001 | 0.0283 | 0.0031 | 0.3516 | 0.1574 | <0.0001 |
LA, lactic acid; DT, dipping time.
FIG. 1.
Observed growth response (growth [○] and no growth [•]) and predicted boundaries between growth and no growth of Listeria monocytogenes on bologna stored at 4°C (A), 7°C (B), and 10°C (C), with respect to lactic acid concentrations and dipping times at growth probabilities of 0.1 (upper line), 0.5 (middle line), and 0.9 (lower line).
FIG. 2.
Observed growth response (growth [○] and no growth [•]) and predicted boundaries between growth and no growth of Listeria monocytogenes on frankfurters stored at 4°C (A), 7°C (B), and 12°C (C), with respect to lactic acid concentrations and dipping times at growth probabilities of 0.1 (upper line), 0.5 (middle line), and 0.9 (lower line).
FIG. 3.
The probability of Listeria monocytogenes growth on bologna (A) and on frankfurters (B) at 7°C for different lactic acid levels and dipping times.
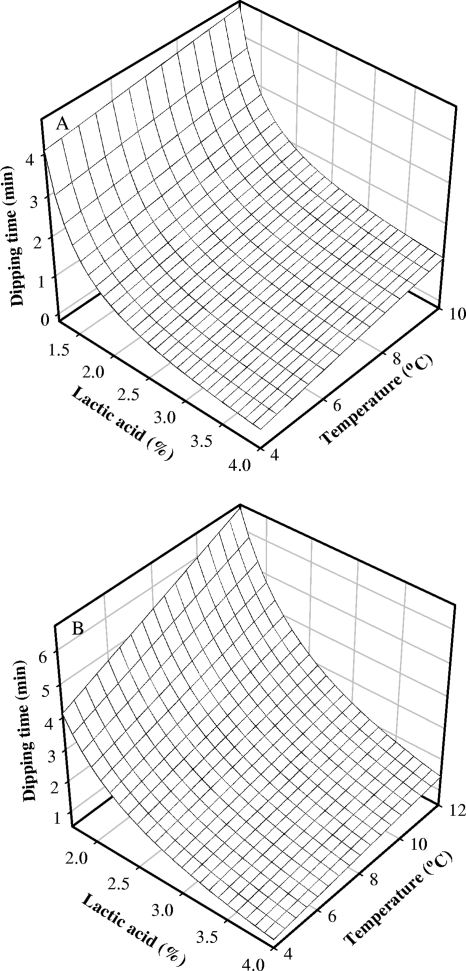
Probability of L. monocytogenes growth on bologna reached approximately 0 after dipping for 1 to 2 min in 2%, 3%, and 4% lactic acid solutions, while a longer dipping time was needed to inhibit L. monocytogenes growth on frankfurters than that for bologna (Fig. 3). This finding may be due to the cut surface of bologna slices, which may allow more absorption of lactic acid than the frankfurters’ intact surface, resulting in lower pH values of bologna than those of frankfurters (bologna, 5.27 to 5.66 [1%], 4.71 to 5.28 [2%], 4.27 to 4.88 [3%], and 4.19 to 4.73 [4%]; frankfurters, 5.67 to 5.75 [1%], 5.52 to 5.62 [2%], 5.45 to 5.62 [3%], and 5.30 to 5.51 [4%]). The probability of growth curves shown in Fig. 3 may be useful in choosing suitable lactic acid concentrations and dipping times to obtain a desired level of control of L. monocytogenes growth on bologna and frankfurters. The predicted boundaries between growth and no growth of L. monocytogenes were produced as a function of lactic acid concentration, dipping time, and storage temperature at the probability level of 0.5, using the estimates listed in Table 1 (Fig. 4). This may help in the prediction of combinations of minimal levels of the treatments needed to limit L. monocytogenes growth at desired probabilities.
FIG. 4.
Boundary surfaces between growth and no growth of Listeria monocytogenes on bologna (A) and on frankfurters (B) as a function of lactic acid concentration, dipping time, and storage temperature predicted by the developed model using equation 1 at a probability of 0.5.
For measures of goodness of fit of the models developed, the concordance indices (i.e., the degree of agreement between the predicted probabilities and the observed responses) were used (11, 12). As determined by the concordance indices of logistic regression analysis, the degree of agreement between the predicted probabilities and the observations was 96.5% for bologna and 95.4% for frankfurters, while corresponding discordance values were 0.2% and 4.2%, respectively. In addition to the concordance indices, predicted probabilities of growth were compared to the observed growth response data on which the model was based for further evaluation of the goodness of fit (11, 12). The results showed that observed growth responses of one combination from bologna (1.3%) and five combinations from frankfurters (6.7%) disagreed with the model prediction; in the model prediction, growth or no growth of the combinations were determined at a probability of 0.5. Moreover, deviance statistics were 16.46 (P = 1.000) and 39.67 (P = 0.9987) for bologna and frankfurters, respectively. The model developed was validated with the limited available data from RTE meat products, and the results indicated that the model displayed agreement with the majority of actual growth responses of L. monocytogenes in RTE meat products except for those in a study by Samelis et al. (23), which showed significant growth of L. monocytogenes on bologna within 20 to 30 days at 4°C (Table 2).
TABLE 2.
Comparison between observed responses of Listeria monocytogenes in various RTE meat products from published studies, including a final research report, and probabilities of growth predicted by the model developed in this studya
| Productc | Temp (°C) | LA (%) | DT (min) | Observed responseb | Ppred | Reference |
|---|---|---|---|---|---|---|
| Bologna | 10 | 2.5 | 2 | NG | 0.03 | 5 |
| 7 | 2 | 0.5 | G | 0.99 | 24 | |
| 4 | 2.5 | 1 | G | 0.13 | 23 | |
| Frankfurters | 7 | 2 | 0.5 | G | 0.95 | 24 |
| Frankfurters (TSBYE) | 10 | 2.5 | 2 | G | 0.76 | 6 |
| Frankfurters (SSH) | 10 | 2.5 | 2 | G | 0.76 | 6 |
| Frankfurters (BIO) | 10 | 2.5 | 2 | G | 0.76 | 6 |
| Frankfurters | 10 | 2.5 | 2 | G | 0.76 | 1 |
LA, lactic acid concentration; DT, dipping time; Ppred, predicted probability of growth.
G, growth; NG, no growth.
TSBYE, samples inoculated with cells grown planktonically in tryptic soy broth plus 0.6% yeast extract; SSH, samples inoculated with cells grown planktonically in a smoked sausage homogenate; BIO, samples inoculated with detached cells from biofilms formed on stainless steel coupons.
In conclusion, the minimum concentrations of lactic acid solutions needed to inhibit L. monocytogenes growth on bologna and frankfurters decreased with increasing dipping times, and lower storage temperatures would enhance antimicrobial effects of lactic acid. In addition, longer dipping time was needed to inhibit L. monocytogenes growth on frankfurters compared to that for bologna. Models developed in this study may be useful in selecting lactic acid concentrations, dipping times, and storage temperatures to control L. monocytogenes growth on bologna and frankfurters and in determining the probabilities of growth under the selected conditions, while the modeling procedures presented may be useful for application in various foods, pathogens, and antimicrobial factors.
Acknowledgments
This study was funded by the National Integrated Food Safety Institute of the Cooperative State Research, Education and Extension Service of the United State Department of Agriculture (agreements 2004-51110-02160 and 2005-51110-03278) and by the Colorado Agriculture Experiment Station.
We express our gratitude to Konstantinos P. Koutsoumanis for his friendly and critical advice.
Published ahead of print on 14 November 2008.
REFERENCES
- 1.Barmpalia, I. M., I. Geornaras, K. E. Belk, J. A. Scanga, P. A. Kendall, G. C. Smith, and J. N. Sofos. 2004. Control of Listeria monocytogenes on frankfurters with antimicrobials in the formulation and by dipping in organic acid solutions. J. Food Prot. 67:2456-2464. [DOI] [PubMed] [Google Scholar]
- 2.Bedie, G. K., J. Samelis, J. N. Sofos, K. E. Belk, J. A. Scanga, and G. C. Smith. 2001. Antimicrobials in the formulation to control Listeria monocytogenes postprocessing contamination on frankfurters stored at 4°C in vacuum packages. J. Food Prot. 64:1949-1955. [DOI] [PubMed] [Google Scholar]
- 3.European Food Safety Authority. 2007. Request for updating the former SCVPH opinion on Listeria monocytogenes risk related to ready-to-eat foods and scientific advice on different levels of Listeria monocytogenes in ready-to-eat foods and the related risk for human illness. EFSA J. 599:1-42. [Google Scholar]
- 4.Genigeorgis, C., S. Martin, C. E. Franti, and H. Reimann. 1971. Initiation of staphylococcal growth in laboratory media. Appl. Microbiol. 21:934-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geornaras, I., K. E. Belk, J. A. Scanga, P. A. Kendall, G. C. Smith, and J. N. Sofos. 2005. Postprocessing antimicrobial treatments to control Listeria monocytogenes in commercial vacuum-packaged bologna and ham stored at 10°C. J. Food Prot. 68:991-998. [DOI] [PubMed] [Google Scholar]
- 6.Geornaras, I., P. N. Skandamis, K. E. Belk, J. A. Scanga, P. A. Kendall, G. C. Smith, and J. N. Sofos. 2006. Postprocess control of Listeria monocytogenes on commercial frankfurters formulated with and without antimicrobials and stored at 10°C. J. Food Prot. 69:53-61. [DOI] [PubMed] [Google Scholar]
- 7.Glass, K. A., and M. P. Doyle. 1989. Fate of Listeria monocytogenes in processed meat products during refrigerated storage. Appl. Environ. Microbiol. 55:1565-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glass, K. A., D. A. Granberg, A. L. Smith, A. M. McNamara, M. Hardin, J. Mattias, K. Ladwig, and E. A. Johnson. 2002. Inhibition of Listeria monocytogenes by sodium diacetate and sodium lactate on wieners and cooked bratwurst. J. Food. Prot. 65:116-123. [DOI] [PubMed] [Google Scholar]
- 9.Islam, M., J. Chen, M. P. Doyle, and M. Chinnan. 2002. Effect of selected generally recognized as safe preservative sprays on growth of Listeria monocytogenes on chicken luncheon meat. J. Food Prot. 65:794-798. [DOI] [PubMed] [Google Scholar]
- 10.Islam, M., J. Chen, M. P. Doyle, and M. Chinnan. 2002. Control of Listeria monocytogenes on turkey frankfurters by generally-recognized-as-safe preservatives. J. Food Prot. 65:1411-1416. [DOI] [PubMed] [Google Scholar]
- 11.Koutsoumanis, K. P., P. A. Kendall, and J. N. Sofos. 2004. Modeling the boundaries of growth of Salmonella Typhimurium in broth as a function of temperature, water activity and pH. J. Food Prot. 67:53-59. [DOI] [PubMed] [Google Scholar]
- 12.Koutsoumanis, K. P., P. A. Kendall, and J. N. Sofos. 2004. A comparative study on growth limits of Listeria monocytogenes as affected by temperature, pH and aw when grown in suspension or on a solid surface. Food Microbiol. 21:415-422. [Google Scholar]
- 13.Mattick, K. L., R. A. Bailey, F. Jorgensen, and T. J. Humphrey. 2002. The prevalence and number of Salmonella in sausages and their destruction by frying, grilling or barbecuing. J. Appl. Microbiol. 93:541-547. [DOI] [PubMed] [Google Scholar]
- 14.Mbandi, E., and L. A. Shelef. 2002. Enhanced antimicrobial effects of combination of lactate and diacetate on Listeria monocytogenes and Salmonella spp. in beef bologna. Int. J. Food Microbiol. 76:191-198. [DOI] [PubMed] [Google Scholar]
- 15.McKellar, R. C., and X. Lu. 2001. A probability of growth model for Escherichia coli O157:H7 as a function of temperature, pH, acetic acid, and salt. J. Food Prot. 64:1922-1928. [DOI] [PubMed] [Google Scholar]
- 16.McMeekin, T. A., J. Olley, D. A. Ratkowsky, and T. Ross. 2002. Predictive microbiology: toward the interface and beyond. Int. J. Food Microbiol. 73:395-407. [DOI] [PubMed] [Google Scholar]
- 17.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nuñez de Gonzalez, M. T., J. T. Keeton, G. R. Acuff, L. J. Ringer, and L. M. Lucia. 2004. Effectiveness of acidic calcium sulfate with propionic and lactic acid and lactates as postprocessing dipping solutions to control Listeria monocytogenes on frankfurters with or without potassium lactate and stored vacuum packaged at 4.5°C. J. Food Prot. 67:915-921. [DOI] [PubMed] [Google Scholar]
- 19.Palumbo, S. A., and A. C. Williams. 1994. Control of Listeria monocytogenes on the surface of frankfurters by acid treatments. Food Microbiol. 11:293-300. [Google Scholar]
- 20.Pin, C., J. P. Sutherland, and J. Baranyi. 1999. Validating predictive models of food spoilage organisms. J. Appl. Microbiol. 87:491-499. [DOI] [PubMed] [Google Scholar]
- 21.Ratkowsky, D. A., and T. Ross. 1995. Modeling the bacterial growth/no growth interface. Lett. Appl. Microbiol. 20:29-33. [Google Scholar]
- 22.Samelis, J., G. K. Bedie, J. N. Sofos, K. E. Belk, J. A. Scanga, and G. C. Smith. 2002. Control of Listeria monocytogenes with combined antimicrobials after postprocess contamination and extended storage of frankfurters at 4°C in vacuum packages. J. Food Prot. 65:299-307. [DOI] [PubMed] [Google Scholar]
- 23.Samelis, J., J. N. Sofos, M. L. Kain, J. A. Scanga, K. E. Belk, and G. C. Smith. 2001. Organic acids and their salts as dipping solutions to control Listeria monocytogenes inoculated following processing of sliced pork bologna stored at 4°C in vacuum packages. J. Food Prot. 64:1722-1729. [DOI] [PubMed] [Google Scholar]
- 24.Sofos, J. N., I. M. Barmpalia, I. Geornaras, Y. Yoon, P. A. Kendall, K. E. Belk, J. A. Scanga, and G. C. Smith. 2005. Comparison of use of activated lactoferrin with use of a “gold standard” combination/concentration of antimicrobials for post-processing control of Listeria monocytogenes in ready-to-eat meat products. American Meat Institute Foundation final report. American Meat Institute Foundation, Washington, DC.
- 25.Tienungoon, S., D. A. Ratkowsky, T. A. McMeekin, and T. Ross. 2000. Growth limits of Listeria monocytogenes as a function of temperature, pH, NaCl, and lactic acid. Appl. Environ. Microbiol. 66:4979-4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.U.S. Department of Agriculture Food Safety and Inspection Service. 2003. Control of Listeria monocytogenes in ready-to-eat meat and poultry products; final rule. Fed. Regist. 68:34208-34254. [Google Scholar]
- 27.U.S. Department of Agriculture Food Safety and Inspection Service. 2006. Compliance guidelines to control Listeria monocytogenes in post-lethality exposed ready-to-eat meat and poultry products. http://www.fsis.usda.gov/oppde/rdad/FRPubs/97-013F/LM_Rule_Compliance_Guidelines_May_2006.pdf.
- 28.U.S. Department of Health and Human Services Food and Drug Administration. 2008. Draft compliance policy guide sec. 555.320—Listeria monocytogenes. Fed. Regist. 73:7293-7298. [Google Scholar]
- 29.U.S. Department of Health and Human Services Food and Drug Administration's Center for Food Safety and Applied Nutrition/U.S. Department of Agriculture Food Safety and Inspection Service. 2003. Quantitative assessment of the relative risk to public health from foodborne Listeria monocytogenes among selected categories of ready-to-eat foods. http://www.cfsan.fda.gov/∼dms/lmr2-toc.html.
- 30.Wilson, P. D. G., T. F. Brocklehurst, S. Arino, D. Thuault, M. Jakobsen, M. Lange, J. Farkas, J. W. T. Wimpenny, and J. F. Van Impe. 2002. Modeling microbial growth in structured foods: towards a unified approach. Int. J. Food Microbiol. 73:275-289. [DOI] [PubMed] [Google Scholar]