Abstract
In the marine environment, a growing body of evidence points to parasites as key players in the control of population dynamics and overall ecosystem structure. However, their prevalence and impact on marine macroalgal communities remain virtually unknown. Indeed, infectious diseases of seaweeds are largely underdocumented, partly because of the expertise required to diagnose them with a microscope. Over the last few years, however, real-time quantitative PCR (qPCR) has emerged as a rapid and reliable alternative to visual symptom scoring for monitoring pathogens. Thus, we present here a qPCR assay suitable for the detection and quantification of the intracellular oomycete pathogen Eurychasma dicksonii in its ectocarpalean and laminarialean brown algal hosts. qPCR and microscopic observations made of laboratory-controlled cultures revealed that clonal brown algal strains exhibit different levels of resistance against Eurychasma, ranging from high susceptibility to complete absence of symptoms. This observation strongly argues for the existence of a genetic determinism for disease resistance in brown algae, which would have broad implications for the dynamics and genetic structure of natural populations. We also used qPCR for the rapid detection of Eurychasma in filamentous brown algae collected in Northern Europe and South America and found that the assay is specific, robust, and widely applicable to field samples. Hence, this study opens the perspective of combining large-scale disease monitoring in the field with laboratory-controlled experiments on the genome model seaweed Ectocarpus siliculosus to improve our understanding of brown algal diseases.
Brown algae make up over 70% of the biomass on cold and temperate rocky seashores. Like other living organisms, they are plagued by diseases caused by fungi, oomycetes, bacteria, or viruses (27). Among eukaryotic pathogens of seaweeds, the oomycete Eurychasma dicksonii has been described as “the most common and widespread of the marine fungi” (see references 21, 31, and 33 and references therein). It occurs in all cold and temperate seas, although sampling biases and its microscopic size have undoubtedly contributed to this parasite remaining largely overlooked.
In culture, Eurychasma infects virtually all species of brown algae tested (>40 [25]), including representatives of all major orders of this phylum; it is therefore the marine pathogen with the widest described host range so far. In particular, Eurychasma infects the genome model seaweed Ectocarpus siliculosus, which is currently being fully sequenced. Phylogenetically, Eurychasma is the most basal member of the oomycete lineage known so far (20a, 29). Oomycetes (or water molds) are a group of fungus-like organisms which encompass many devastating pathogens such as the potato blight, grape mildew, and fish mold agents.
The role of infectious agents in the marine environment is best known for viruses, which account for an estimated 20 to 40% daily loss in prokaryotic biomass, an impact comparable to that of grazers. Their role in accelerating biogeochemical cycles and in structuring microbial communities is now widely recognized (9, 34). Circumstantial evidence suggests that eukaryotic pathogens of primary producers exert a similarly strong influence on the biology and the abundance of their hosts (e.g., references 13, 17, and 35). As brown algae are major primary producers on temperate and polar coastal rocky shores, and considering its exceptionally wide host range within this group, we infer that Eurychasma probably has a major impact on these ecosystems. Indeed, it has been found in many brown algal species in the field and was suggested to be responsible for the scarcity of Striaria attenuata on the west coast of Sweden (1). In contrast, a study by Wilce and colleagues (37) suggested that Eurychasma infection might foster fragmentation and vegetative propagation of the filamentous brown alga Pylaiella littoralis, perhaps causing so-called “brown tides.” Finally, although Eurychasma is not known to infect fully grown kelps (sporophytes of Laminariales), their microscopic gametophytic generation is subject to severe infection in laboratory culture experiments (25). Therefore, epidemics might influence the sexual reproduction and thus the recruitment of kelps, which account for most of the biomass of temperate and cold marine coastal ecosystems worldwide. Yet, despite numerous findings, most Eurychasma records are decades old and entirely based upon morphological observations. Virtually nothing is known about many fundamental aspects of its pathogenicity, biology, epidemiology, and ecology.
In the medical field, Jones et al. (20) recently reported the existence of nonrandom trends in the emergence of new infectious diseases worldwide. They showed an increase of these events over the past 6 decades, together with a significant correlation with socioeconomic, environmental, and ecological factors. Strikingly, despite the relative abundance of available data, they also concluded that current efforts to monitor emerging diseases are mostly ill targeted to the regions where they are less likely to originate. In the less well documented marine environment, the mere absence of epidemiological data or of reliable baselines for wild populations may result in major environmental shifts remaining undetected or unexplained (15). Over the last few decades, however, a few events have revealed some dramatic consequences of epidemic outbreaks, such as the iconic abrupt transition from a coral- to an alga-dominated ecosystem in Jamaica, driven by the disease-caused mass mortality of a predominant grazer, the urchin Diadema antillarum (19). From these examples, clear parallels have emerged with more comprehensive observations made of terrestrial ecosystems (5), suggesting that infectious agents may exert comparable—and so far underestimated—pressures on marine ecosystems. Overall, these lines of evidence argue in favor of an urgent need for improved methods of pathogen monitoring in order to understand their impact, in both undisturbed and disturbed environments (15).
Direct visual assessment of symptom severity is the only method that has been used to diagnose Eurychasma so far. Reliable diagnosis is time-consuming and requires a specific expertise, effectively rendering Eurychasma monitoring impracticable on a large scale. Also, visual scoring is incompatible with any elaborate statistical data analysis. Because it efficiently addresses many of these common limitations, quantitative real-time PCR (qPCR) has become a widely accepted alternative for pathogen detection (11). Compared to other conventional disease diagnostic methods, qPCR-based assays are rapid, sensitive, specific, and best suited to discriminate between slightly different levels of infection.
We present here a simple qPCR-based protocol adaptable for high-throughput analysis and suitable for quantifying the relative abundance of Eurychasma in brown algal laboratory cultures. This assay enabled us to show contrasting levels of susceptibility of various algal strains against infection, thus providing a strong and original new line of evidence in support of a genetic determinism for disease resistance in brown algae. We also demonstrate the applicability of this assay for Eurychasma detection in brown algal biomass collected in the field. This not only represents a considerable expansion of Eurychasma molecularly confirmed geographic range but also opens the possibility of large-scale disease monitoring in coastal ecosystems.
MATERIALS AND METHODS
Cultured biological material.
Unialgal clonal brown algal cultures were maintained in half-strength Provasoli medium (32), at 15°C, under illumination with daylight-type fluorescent lamps at an irradiance of 10 μE·m−2·s−1 and a 12-h photoperiod. The Eurychasma strains (CCAP 4018/1, CCAP 4018/2, and CCAP 4018/3) were maintained in the same conditions by bimensual transfer of infected material into fresh medium together with some new uninfected algal host (CCAP 1310/299, CCAP 1330/3, and CCAP 1310/300, respectively). Each of these strains originated from a single parasitic thallus transfected into a healthy algal host. CCAP strains are available from the Culture Collection for Algae and Protozoa (10) upon request.
Laboratory-controlled inoculations. (i) Simple coincubation experiments.
Inoculations were performed by coincubating a Eurychasma-infected culture with the target algal strain for 2 or 3 weeks in 50-mm-diameter, 20-mm-deep polystyrene petri dishes, as described by Müller et al. (25). The target alga was then sorted from the inoculum by visual inspection under the stereomicroscope, harvested, and frozen in liquid nitrogen.
(ii) Large-scale inoculations—separation of the inoculum and of the target alga.
In the simple coincubation experiments described above, the qPCR results might potentially be affected by contaminating inoculum that was not properly removed from the harvested culture. Thus, attempts were made to obtain spore suspensions of Eurychasma. Synchronized release of Eurychasma spores into the culture medium could be triggered by either a temperature or an osmotic shock. However, control experiments showed that the resulting spore suspensions did not retain their infectivity, and this approach was abandoned. Instead, the inoculum and the target algal strain were coincubated in six-well polystyrene plates for suspension cultures (Greiner Bio-One Ltd., United Kingdom) and physically separated using plastic inserts with a 70-μm-pore-size nylon mesh (Falcon cell strainer). The latter efficiently separates the inoculum from the target alga but allows free passage of Eurychasma spores. Since brown algal zoospores also move freely across the nylon mesh, the target alga might also become contaminated by the offspring of the inoculum. Whenever this limitation was thought to be critical, inoculations were performed using Eurychasma-infected Laminaria digitata female gametophytes (CCAP 1321/4), because this kelp life stage never releases any motile zoospores or gametes. Finally, control experiments showed that cell strainer use does not modify the outcome of the inoculation compared with a simple coincubation in a petri dish.
DNA extraction.
DNA extraction was performed either with the DNeasy plant minikit (Qiagen, United Kingdom) or by the following cetyltrimethylammonium bromide (CTAB)-based method. A few milligrams of algal biomass was transferred into a 2-ml Eppendorf tube containing a 5-mm stainless bead and frozen in liquid nitrogen. Alternatively, field samples were soaked dry on sterile filter paper and kept dehydrated at room temperature using silica gel. Seven hundred microliters of preheated CTAB buffer (65°C; 100 mM Tris-HCl [pH 8], 3% CTAB, 1.4 M NaCl, 0.02 M EDTA [pH 7.5], 2.5% polyvinylpolypyrrolidone) was added, and the samples were ground in a mechanical bead grinder twice for 1 min at 30 Hz. They were incubated at 65°C for 1 to 2 h and subjected to successive phase extractions with 1 volume phenol-CHCl3-isoamyl alcohol (25:24:1) and CHCl3-isoamyl alcohol (24:1). The aqueous phase was collected, gently mixed with 1 volume of propan-2-ol, and incubated at room temperature for 1 h. After centrifugation (15 min, 13,000 rpm), the DNA pellet was washed twice with 70% ethanol and resuspended in 100 μl mQ water. This protocol was also successfully scaled up to 96-well plate format, in a way very similar to that reported by Hoarau et al. (16).
Real-time PCR.
Twenty-microliter samples were prepared by mixing 8 μl of DNA extract with 10 μl Mesa Green Mastermix (Eurogentec, Belgium) and the appropriate primers (final concentration, 300 nM). Real-time PCRs were run in duplicate on a Quantica machine (Techne-Barloworld, United Kingdom). After a 10-min denaturation step at 95°C, samples were run for 45 cycles of 15 s at 95°C and 1 min at 60°C. After each run, a dissociation curve was acquired by heating the samples from 60 to 95°C. The primer sequences are as follows (5′ to 3′): CG60, AGTGGATGTTTCGGTCCATTTG; CG61, CAGTAAACGATGCAAGATTGATAAAC; CG64, GAATAATGAGATAGGGCCACGAC; CG65, CGTTCCTGTTAATCATTACCACAAC.
PCR and sequencing.
PCRs were run in a final volume of 50 μl, using 1 or 2 μl of template DNA and the Taq PCR Master Mix kit (Qiagen), according to the manufacturer's instructions. The PCR conditions were adjusted for each reaction and were typically as follows: denaturation at 94°C for 3 min and 35 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 2 min. The length and quality of the PCR products were checked by loading a 5-μl aliquot on an 0.8% agarose gel, and the rest was purified using the Qiaquick PCR purification kit (Qiagen). Sequencing was performed with an ABI 3730 system (Applied Biosystems) at the Edinburgh Sequencing Facility of the UK Natural Environment Research Council Molecular Genetics Facility. The primers used were CG60, CG61, and the 18S rRNA sequencing primers described by Marin et al. (22), as appropriate.
RESULTS
In this study, we have developed a qPCR-based assay to assess disease severity in algal samples infected with the oomycete Eurychasma dicksonii. It relies on the relative quantification of algal and oomycete DNA in infected samples by two qPCRs run in parallel against the oomycete and algal 18S rRNA genes.
Assay specificity, dynamic range, and robustness.
A multiple alignment of oomycete genes encoding the small rRNA subunit was generated using BioEdit (14) and manually refined. It included two Eurychasma sequences (strains CCAP 4018/1 and 4018/2, GenBank accession numbers AB368176 and AY032607, respectively [29]), representative sequences of basal oomycete groups (Haptoglossa sp. strain EU271966, Haliphthoros sp. strain AB178865, Olpidiopsis porphyrae AB287418, Halocrusticida baliensis AB284578, and Halodaphnea panulirata AB284574), saprolegnian and peronosporalean taxa (Pythium monospermum AJ238653, Saprolegnia ferax AJ238655, and Phytophthora megasperma M54938), two chytrids (Developayella elegans U37107 and Hyphochytrium catenoides X80344), and closely related stramenopile sequences of uncultured organisms from various marine environments (GenBank sequence accession numbers are in square brackets) (BOLA515 [AF372763], BOLA320 [AF372762], CCW73 [AY180031], DH144-EKD10 [AF290063], ME1-21 [AF363190]; ME1-22 [AF363191], OLI11026 [AJ402339], OLI11008 [AJ402350], Q2E10N5 [EF173010], and RA000412.91 [AY295431]). The primers CG60 and CG61 were designed to anneal in regions conserved between the two Eurychasma sequences and notably divergent in all other organisms included in the alignment. As of 1 April 2008, these two primers did not yield any significant BLAST hit in the GenBank database, except their target sequence.
DNA was extracted from Ectocarpus siliculosus CCAP 1310/56 and Laminaria digitata CCAP 1321/4 gametophyte cultures infected with the Eurychasma strains CCAP 4018/1 and CCAP 4018/2, respectively. Standard curves were obtained on DNA serial dilutions in order to check for the linearity, efficiency, and dynamic range of the quantification. Eurychasma was reliably detected and quantified for a threshold cycle (CT) value between 25 and 35 (see Fig. S1A in the supplemental material), over about 4 orders of magnitude. No aspecific amplification could be detected using these primers on equivalent quantities of DNA obtained from equivalent uninfected Ectocarpus or Laminaria cultures (see Fig. S1B in the supplemental material).
Because of the broad host spectrum of Eurychasma, we designed a primer pair suitable for as many of its potential brown algal hosts as possible, excluding any other stramenopile group. The primer pair CG64/CG65 matches perfectly with the 18S rRNA gene sequence of virtually every species of Ectocarpales and Laminariales represented in GenBank (as of April 2008), but it presents significant mismatches with most such sequences of the Fucales, Sphacelariales, and Dictyotales. Particular care was taken so that these primers would not anneal with any known diatom (diatoms often occur as epiphytes on brown algae) or oomycete sequences. Standard curves were drawn using serial dilutions of E. siliculosus (CCAP 1310/56 and CCAP 1310/300), Pylaiella littoralis (CCAP 1330/3), and L. digitata (CCAP 1321/4) DNA extracts, with a dynamic range comparable to that of the CG60/CG61 Eurychasma specific primer pair and an amplification efficiency of close to 100% (see Fig. S2 in the supplemental material).
Since Eurychasma is an obligate intracellular pathogen, pure DNA of the parasite could not be easily obtained. Therefore, we checked for the absence of interaction between algal and oomycete DNA quantification by spiking serial dilutions of Eurychasma-infected DNA samples with pure algal DNA, so that the final amount of algal DNA would be roughly the same in each sample. As shown in Fig. S3 in the supplemental material, the presence of large amounts of algal DNA in highly diluted Eurychasma samples affects neither the amplification efficiency, the quantification accuracy, nor the overall sensitivity of the assay.
DNA extraction optimization.
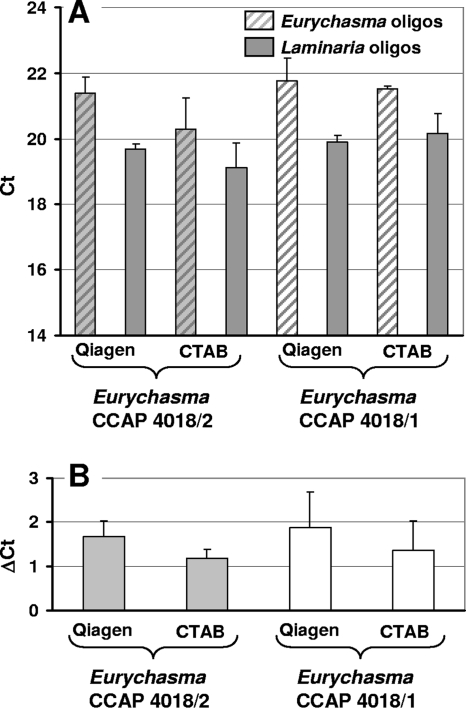
Quantitative recovery of both oomycete and algal DNA is of critical importance to guarantee the biological significance of the qPCR results. We therefore extracted replicates of Eurychasma-infected Laminaria gametophytes with a commercially available kit or a CTAB-based protocol. We then compared the overall extraction yields and oomycete/algal DNA ratios as described in detail by Gachon and Saindrenan (12). In all instances, Eurychasma and algal CTs of biological replicates extracted with these two methods were roughly identical, reflecting DNA extraction yields similar between the replicates and between the two extraction procedures (two-sided t test not significant with α = 0.05) (Fig. 1A). Furthermore, the Eurychasma/algal DNA ratios [calculated as ΔCT = (CT)Eurychasma − (CT)Laminaria] were also similar (Fig. 1B), suggesting that both protocols enable quantitative recovery of oomycete and algal DNA. The low variability between the biological replicates reflects the overall robustness of the quantification procedure.
FIG. 1.
Reproducible quantitative DNA recovery of oomycete and algal DNA from infected samples. (A) The reproducibility of DNA recovery was tested with Laminaria digitata gametophytes infected with Eurychasma strain CCAP 4018/1 or CCAP 4018/2. The DNA of two to four independent biological replicates was extracted either with a commercial kit (Qiagen) or by a CTAB-based protocol (CTAB) and used for qPCR with the primers CG60/CG61 (Eurychasma oligonucleotides) and CG64/CG65 (Laminaria oligonucleotides). (B) ΔCT values [calculated as (CT)Eurychasma − (CT)Laminaria] deduced from panel A.
Because of its much lower cost and similar throughput, the CTAB method was preferred over the commercially available kit and used for all subsequent experiments.
Evidence for differential susceptibility of cultured brown algal strains to Eurychasma infection.
The host range of the Eurychasma strains CCAP 4018/1, 4018/2, and 4018/3 was surveyed by laboratory-controlled inoculations as described by Müller et al. (25). Target algal strains were coincubated with an infected alga (referred to below as the “inoculum”), and symptom development was scored under the microscope after 2 to 3 weeks. The most frequent outcome was the development of disease symptoms, i.e., intracellularly developing parasitic thalli later differentiating into zoosporangia (29). However, some specific algal-Eurychasma strain combinations never led to the appearance of any such infection structure (Table 1), although oomycete spores were observed at the algal surface. Moreover, these algal strains were successfully infected by other Eurychasma isolates. In all instances, the infectivity of the inoculum was confirmed with a parallel coincubation with an algal strain susceptible to infection. Also, the continuous appearance of new symptoms on the inoculum (indicative of multiple reinfection cycles and thus of the release of infectious spores in the medium) was also checked. We therefore conclude that the absence of new symptoms reflects the capacity of these specific clonal Ectocarpus sp. and Pylaiella littoralis strains to resist infection by a particular Eurychasma isolate (Table 1).
TABLE 1.
Differential susceptibility of Ectocarpales to Eurychasma: microscopic observations
| Eurychasma strain | Susceptibility of algal straina:
|
|||||
|---|---|---|---|---|---|---|
| Ectocarpus siliculosus CCAP 1310/4b | Ectocarpus fasciculatus CCAP 1310/13 |
Ectocarpus sp. strain:
|
Pylaiella littoralis CCAP 1330/3 | |||
| CCAP 1310/143 | CCAP 1310/209 | CCAP 1310/300c | ||||
| CCAP 4018/1 | S | S | S | S | R | R |
| CCAP 4018/2 | S | R | S | S | S | S |
| CCAP 4018/3 | S | S | ND | ND | S | R |
S, Eurychasma-susceptible algal strain, as judged by the completion of Eurychasma infection cycle and production of mature zoosporangia; R, Eurychasma-resistant algal strain, as judged by the complete absence of infection symptoms; ND, no data available. Inoculation experiments were usually repeated twice and at least three times for resistant interactions.
Complete genome sequence under completion (26).
Healthy Ectocarpus isolate obtained from an individual originally infected in the field by Eurychasma strain CCAP 4018/3.
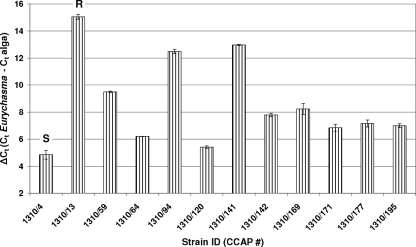
We further evaluated the performance of the qPCR assay compared to visual symptom scoring. The qPCR results were in agreement with the microscopic observations; ΔCT values clearly allowed discrimination between different levels of infection (Fig. 2). Again, replicate DNA samples extracted independently from the same culture yielded similar results, showing that this qPCR assay is useful for estimating the severity of Eurychasma infection and for rapidly identifying resistant algal candidates. Moreover, it could be used for monitoring disease progress during time course experiments.
FIG. 2.
qPCR determination of differential susceptibility of Ectocarpus strains to Eurychasma. Coincubation of Ectocarpus strains with Eurychasma strain CCAP 4018/2. CCAP 1310/4 and CCAP 1310/13 were used as susceptible and resistant controls, respectively. All other algal strains surveyed in this experiment exhibited disease symptoms. ΔCT [calculated as (CT)Eurychasma − (CT)Ectocarpus] was negatively correlated with the severity of symptoms observed microscopically, closely reflecting the variation in symptom intensity between strains. Values represent means ± standard deviations of two replicates. The small amounts of Eurychasma DNA detected in the resistant control are attributed to the inoculum.
Assay validation on field samples: detecting Eurychasma infection in geographically distant brown algal populations.
In January 2007, Eurychasma-infected brown algae were sampled on the Pacific and Atlantic coasts of South America (Table 2). Additional samples were also collected from the intertidal environment surrounding the Dunstaffnage Marine Laboratory of the Scottish Association for Marine Science (Oban, United Kingdom) in 2006 and 2007. They were microscopically inspected for the presence of Eurychasma and subjected to our qPCR assay. In all instances, the real-time PCR results were consistent with our visual assessment. Whenever possible, laboratory cultures of both Eurychasma and its original brown algal host were established from these samples. We checked that all Eurychasma isolates obtained still yielded a positive result using the CG60/CG61 primer pair, whereas all healthy algal isolates derived from this infected field material yielded negative results.
TABLE 2.
Real-time PCR detection of Eurychasma dicksonii in filamentous brown algae of various geographic originsa
| Sample source and name | Description | Microscopic diagnosis | qPCR diagnosis | EMBL accession no. (sequence length, % identityb) |
|---|---|---|---|---|
| Northern hemisphere (Dunstaffnage Bay, Oban, United Kingdom) | ||||
| Eu Oban 06-29 | Infected Ectocarpus, sampled in Oban, Scotland, May 2006 | I | + | |
| Eu Oban 06-29-7 (CCAP 4018/3) | Eurychasma isolate derived from the above; maintained in CCAP 1310/300 | I | + | FM202387 (541, 96%) |
| Ec Oban 06-29-7 (CCAP 1310/300) | Healthy Ectocarpus clonal isolate obtained from the above | NI | − | |
| Eu Oban 06-29-4 | Eurychasma isolate derived from Eu Oban 06-29; maintained in Ec Oban 06-29-5 | I | + | FM202388 (539, 99%) |
| Ec Oban 06-29-5 | Healthy Ectocarpus clonal isolate obtained from Eu Oban 06-29 | NI | − | |
| Eu Oban 06-31 | Infected Pylaiella littoralis sampled in Oban, Scotland, May 2006 | I | + | |
| Eu Oban 06-32 | Infected Pylaiella littoralis sampled in Oban, Scotland, May 2006 | I | + | |
| Eu Oban 06-33 | Infected Pylaiella littoralis sampled in Oban, Scotland, May 2006 | I | + | |
| Eu Oban 07-1 | Eurychasma isolate obtained from a field collection in June 2007; maintained in P. littoralis CCAP 1330/3 | I | + | FM202389 (530, 99%) |
| Eu Oban 07 jul | Infected Pylaiella littoralis sampled in July 2007 | I | + | |
| Eu Oban 07-2 | Eurychasma isolate obtained from the above sample, maintained in P. littoralis CCAP 1330/3 | I | + | FM202390 (1,101, 100%) |
| FS Oban 07 | Healthy Pylaiella littoralis sampled in July 2007 | NI | − | |
| Southern hemisphere | ||||
| Eu US raw | Infected Ectocarpus, sampled in Ushuaia, Argentina, January 2007 | I | + | |
| Eu US 401 | Eurychasma isolate obtained from the above | I | + | FM202392 (1,695, 98%) |
| Ec US 385 | Healthy Ectocarpus clonal isolate obtained from the above | NI | − | |
| Eu FI S63 | Mixed infected Ectocarpus and Pylaiella sampled in Stanley Harbor, Falkland Islands, United Kingdom, January 2007 | I | + | FM202391 (1,828, 99%) |
| Eu FI 64-23 | Eurychasma isolate (in Ectocarpus) derived from the above | I | + | |
| Ec FI 64-20 | Healthy Ectocarpus clonal isolate obtained from the above | NI | − | |
| Pyl FI 63-3 | Healthy Pylaiella clonal isolate obtained from the above | NI | − |
Abbreviations: Eu, Eurychasma; Ec, Ectocarpus; Pyl, Pylaiella; I, infected; NI, not infected; +, Eurychasma detected; −, Eurychasma not detected. Field samples are highlighted in bold; derived laboratory cultures are in lightface.
Finally, the identity of Eurychasma in infected samples was directly confirmed by reamplifying the DNA extracts using one Eurychasma-specific primer and a generalist eukaryotic primer (combinations CG60/ITS055R and EAF3/CG61). The corresponding PCR products were sequenced, and partial rRNA 18S sequences were obtained (Table 2). They exhibit at least 96% identity with the two previously reported Eurychasma 18S rRNA sequences from the Shetland Islands and Brittany (29), and all of them were unambiguously assigned to Eurychasma by a BLAST search against GenBank. Throughout this study, in no instance was the sequence of any other organism retrieved when either the CG60 or the CG61 primer was used, suggesting that both are highly specific for Eurychasma.
DISCUSSION
General technical considerations.
The assay reported here enables relative quantification of Eurychasma and brown alga DNA over a dynamic range sufficient for routine applications. Recently, Hoarau et al. (16) described a similar 96-well-plate-based DNA extraction protocol suitable for brown algae, which we have implemented in our lab. Combined with the possibility of performing Eurychasma inoculations in multiwell plates, this opens the opportunity of conducting large-scale inoculation experiments in order to phenotype many brown algal strains in parallel.
If needed, this dynamic range could be increased by performing the real-time PCR step on more-concentrated DNA samples. However, qPCR on such samples often fails, due to the presence of PCR inhibitors typical of brown algae. This issue can in most instances be solved by performing an additional silica-based DNA purification step prior to running the qPCR, using for example the GeneClean III kit (Q-Biogene, Illkirch, France). Also, PCR-inhibited samples can easily be identified and removed from further analysis, based on the failure of the alga-specific PCR. Thus, despite this recurrent technical issue, there is no specific risk of assigning false-negative results on Eurychasma detection due to PCR inhibition.
Applicability of qPCR to diagnose Eurychasma infections in the field.
A common limitation of qPCR for the analysis of environmental samples (like any other sequence-based approach, e.g., microarrays) is that only known sequences can be targeted, which means that unexpected genetic diversity will remain undiagnosed. On the other hand, aspecific amplification of unknown contaminants is a possible pitfall, especially in underdocumented taxa such as marine oomycetes. In other words, we cannot rule out the possibility either that some divergent Eurychasma genotypes may not be amplified using the CG60/CG61 primer pair or that the latter might successfully amplify other and as-yet-uncharacterized organisms present in some field samples. However, the consistency of our microscopic observations and real-time PCR results for samples originating from geographically distant locations makes us confident that our assay can be reliably used for large-scale disease diagnosis in brown algal populations.
Finally, qPCR leaves open the possibility of scoring the presence and relative abundance of several pathogens in parallel, in order to determine the global parasitic pressure acting on natural brown algal populations. In this respect, expanding our assay to the lesser-known hyphochytrid pathogens Anisolpidium ectocarpii and Anisolpidium rosenvingii and to the chytrid Chytridium polysiphoniae would be of high interest.
Differential susceptibility of Ectocarpus and Pylaiella clonal strains against infection: a genetic basis for disease resistance in brown algae?
Host range studies of marine seaweed pathogens have mostly been conducted at species level (25, 30). Hence, the intraspecific variation in susceptibility to pathogen infection has been very little explored, and to the best of our knowledge, the present study represents the first report of differential susceptibility to pathogen infection among brown algae. This result brings a strong new line of evidence in support of the long-standing hypothesis that disease resistance might be genetically determined in this lineage (3). This would imply that host specificity may be underpinned by an intraspecific genetic polymorphism driven by host-pathogen coevolution.
Together with the availability of an increasingly rich genomic toolbox for Ectocarpus (6), our Eurychasma-Ectocarpus model system opens exciting new opportunities to tackle this question directly and address the physiology and ecology of algal host-pathogen interactions both in the laboratory and in the field.
Implications of differential disease susceptibility for brown algal populations.
Intraspecific differential susceptibility to infection and genetic determinism of disease resistance seem to be common themes among the algal lineages for which data are available (4, 36), as is well known for terrestrial plants.
The fitness of a pathogen or that of its host is markedly affected by its capacity to infect or to resist infection, respectively. Each protagonist therefore exerts a strong selection pressure on the other, driving coevolution processes often described as an “arms race” and “trench warfare.” Such an arms race has been demonstrated or suggested in many plant-pathogen interactions (e.g., reference 2), correlating with the variations of host specificities observed at phenotypic level (18, 28). Likewise, parasitic pressure is strongly suspected to account for high genetic diversity in phytoplankton populations and, under certain circumstances, to drive the temporal succession of host genotypes (7, 23, 24). Conversely, there is also evidence in other aquatic models that rapid host evolution toward increased resistance can terminate epidemics, thereby impacting population dynamics on a short time scale (8). However, direct evidence linking host specificity and genotypic diversity is still lacking for marine models and will require laboratory-based host range studies coupled with efficient strain genotyping.
The Ectocarpus-Eurychasma system appears as a very attractive model to perform these experiments because of the genetic diversity already available in culture (>330 Ectocarpus strains from all over the world), as well as the potential to develop genomic approaches. Moreover, susceptible and resistant algal genotypes can readily be identified and directly brought into culture from infected field material (D. G. Müller, unpublished results), in contrast to other unicellular microorganisms which are either lysed when susceptible or undergo a hypersensitive response when resistant (e.g., reference 4). In this context, the capacity of monitoring Eurychasma—and potentially other pathogens—on a large scale is a first step toward shedding light on how brown algal populations are affected by epidemic outbreaks and, in fine, how this might influence their genetic structure and the overall functioning of coastal ecosystems.
Supplementary Material
Acknowledgments
C.M.M.G. expresses her gratitude to the European Commission for a Marie Curie Intra-European Fellowship (MIEF-CT-2006-022837). M.S. is the recipient of an EU ECOSUMMER Ph.D. fellowship (MEST-CT-2005-20501). F.C.K. gratefully acknowledges funding from the UK Natural Environment Research Council (NERC; grant NE/D521522/1, International Opportunities Fund of the Marine and Freshwater Microbial Biodiversity Programme, and Oceans 2025/WP 4.5) and a sequencing allocation from the NERC National Sequencing Facility (MGF 154).
Debra Brennan is acknowledged for her excellent technical assistance.
Footnotes
Published ahead of print on 14 November 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Aleem, A. 1955. Marine fungi from the west-coast of Sweden. Ark. Bot. 3:1-33. [Google Scholar]
- 2.Allen, R., P. D. Bittner-Eddy, L. J. Grenville-Briggs, J. Meitz, A. Rehmany, L. Rose, and J. L. Beynon. 2004. Host-parasite coevolutionary conflict between Arabidopsis and downy mildew. Science 306:1957-1960. [DOI] [PubMed] [Google Scholar]
- 3.Bouarab, K., B. Kloareg, P. Potin, and J. A. Correa. 2001. Ecological and biochemical aspects in algal infectious diseases. Cah. Biol. Mar. 42:91-100. [Google Scholar]
- 4.Canter, H. M., and G. Jaworski. 1979. The occurrence of a hypersensitive reaction in the planktonic diatom Asterionella formosa Hassall parasitized by the chytrid Rhizophydium planktonicum Canter emend., in culture. New Phytol. 82:187-206. [Google Scholar]
- 5.Carey, C. 2000. Infectious disease and worldwide declines of amphibian populations, with comments on emerging diseases in coral reef organisms and in humans. Environ. Health Perspect. 108:143-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charrier, B., S. M. Coelho, A. Le Bail, T. Tonon, G. Michel, P. Potin, B. Kloareg, C. Boyen, A. F. Peters, and J. M. Cock. 2008. Development and physiology of the brown alga Ectocarpus siliculosus: two centuries of research. New Phytol. 177:319-332. [DOI] [PubMed] [Google Scholar]
- 7.De Bruin, A., B. W. Ibelings, M. Rijkeboer, M. Brehm, and E. van Donk. 2004. Genetic variation in Asterionella formosa (Bacillariophyceae): is it linked to frequent epidemics of host-specific parasitic fungi? J. Phycol. 40:823-830. [Google Scholar]
- 8.Duffy, M. A., and L. Sivars-Becker. 2007. Rapid evolution and ecological host-parasite dynamics. Ecol. Lett. 10:44-53. [DOI] [PubMed] [Google Scholar]
- 9.Fuhrman, J. A. 1999. Marine viruses and their biogeochemical and ecological effects. Nature 399:541-548. [DOI] [PubMed] [Google Scholar]
- 10.Gachon, C. M. M., J. G. Day, C. N. Campbell, T. Pröschold, R. J. Saxon, and F. C. Küpper. 2007. The Culture Collection of Algae and Protozoa (CCAP): a biological resource for protistan genomics. Gene doi: 10.1016/j.gene.2007.05.018. [DOI] [PubMed]
- 11.Gachon, C. M. M., A. Mingam, and B. Charrier. 2004. Real-time PCR: what relevance to plant studies? J. Exp. Bot. 55:1445-1454. [DOI] [PubMed] [Google Scholar]
- 12.Gachon, C. M. M., and P. Saindrenan. 2004. Real-time PCR monitoring of fungal development in Arabidopsis thaliana infected by Alternaria brassicicola and Botrytis cinerea. Plant Physiol. Biochem. 42:367-371. [DOI] [PubMed] [Google Scholar]
- 13.Grahame, E. S. 1976. The occurrence of Lagenisma coscinodisci in Palmeria hardmania from Kingston harbour, Jamaica. Br. Phycol. J. 11:57-61. [Google Scholar]
- 14.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95-98. [Google Scholar]
- 15.Harvell, C. D., K. Kim, J. M. Burkholder, R. R. Colwell, P. R. Epstein, D. J. Grimes, E. E. Hofmann, E. K. Lipp, A. Osterhaus, R. M. Overstreet, J. W. Porter, G. W. Smith, and G. R. Vasta. 1999. Review: marine ecology—emerging marine diseases—climate links and anthropogenic factors. Science 285:1505-1510. [DOI] [PubMed] [Google Scholar]
- 16.Hoarau, G., J. A. Coyer, W. T. Stam, and J. L. Olsen. 2007. A fast and inexpensive DNA extraction/purification protocol for brown macroalgae. Mol. Ecol. Notes 7:191-193. [Google Scholar]
- 17.Holfeld, H. 1998. Fungal infections of the phytoplankton: seasonality, minimal host density, and specificity in a mesotrophic lake. New Phytol. 138:507-517. [Google Scholar]
- 18.Holub, E. B. 2007. Natural variation in innate immunity of a pioneer species. Curr. Opin. Plant Biol. 10:415-424. [DOI] [PubMed] [Google Scholar]
- 19.Hughes, T. P. 1994. Catastrophes, phase-shifts, and large-scale degradation of a Caribbean coral-reef. Science 265:1547-1551. [DOI] [PubMed] [Google Scholar]
- 20.Jones, K. E., N. G. Patel, M. A. Levy, A. Storeygard, D. Balk, J. L. Gittleman, and P. Daszak. 2008. Global trends in emerging infectious diseases. Nature 451:990-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a.Küpper, F. C., I. Maier, D. G. Müller, S. L.-D. Goer, and L. Guillou. 2006. Phylogenetic affinities of two eukaryotic pathogens of marine macroalgae, Eurychasma dicksonii (Wright) Magnus and Chytridium polysiphoniae Cohn. Cryptogam. Algol. 27:165-184. [Google Scholar]
- 21.Küpper, F. C., and D. G. Müller. 1999. Massive occurrence of the heterokont and fungal parasites Anisolpidium, Eurychasma and Chytridium in Pylaiella littoralis (Ectocarpales, Phaeophyceae). Nova Hedwigia 69:381-389. [Google Scholar]
- 22.Marin, B., A. Palm, M. Klingberg, and M. Melkonian. 2003. Phylogeny and taxonomic revision of plastid-containing euglenophytes based on SSU rDNA sequence comparisons and synapomorphic signatures in the SSU rRNA secondary structure. Protist 154:99-145. [DOI] [PubMed] [Google Scholar]
- 23.Martínez Martínez, J., D. C. Schroeder, A. Larsen, G. Bratbak, and W. H. Wilson. 2007. Molecular dynamics of Emiliania huxleyi and co-occurring viruses during two separate mesocosm studies. Appl. Environ. Microbiol. 73:554-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muhling, M., N. J. Fuller, A. Millard, P. J. Somerfield, D. Marie, W. H. Wilson, D. J. Scanlan, A. F. Post, I. Joint, and N. H. Mann. 2005. Genetic diversity of marine Synechococcus and co-occurring cyanophage communities: evidence for viral control of phytoplankton. Environ. Microbiol. 7:499-508. [DOI] [PubMed] [Google Scholar]
- 25.Müller, D. G., F. C. Küpper, and H. Küpper. 1999. Infection experiments reveal broad host ranges of Eurychasma dicksonii (Oomycota) and Chytridium polysiphoniae (Chytridiomycota), two eukaryotic parasites of marine brown algae (Phaeophyceae). Phycol. Res. 47:217-223. [Google Scholar]
- 26.Peters, A., D. Marie, D. Scornet, B. Kloareg, and J. M. Cock. 2004. Proposal of Ectocarpus siliculosus (Ectocarpales, Phaeophyceae) as a model organism for brown algal genetics and genomics. J. Phycol. 40:1079-1088. [Google Scholar]
- 27.Potin, P., K. Bouarab, J.-P. Salaün, G. Pohnert, and B. Kloareg. 2002. Biotic interactions of marine algae. Curr. Opin. Plant Biol. 5:308-317. [DOI] [PubMed] [Google Scholar]
- 28.Rose, L., P. D. Bittner-Eddy, C. H. Langley, E. B. Holub, R. W. Michelmore, and J. Beynon. 2004. The maintenance of extreme amino acid diversity at the disease resistance gene, RPP13, in Arabidopsis thaliana. Genetics 166:1517-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sekimoto, S., G. W. Beakes, C. M. M. Gachon, D. G. Müller, F. C. Küpper, and D. Honda. 2008. The development, ultrastructural cytology, and molecular phylogeny of the basal oomycete Eurychasma dicksonii, infecting the filamentous phaeophyte algae Ectocarpus siliculosus and Pylaiella littoralis. Protist 159:299-318. [DOI] [PubMed] [Google Scholar]
- 30.Sekimoto, S., K. Yokoo, Y. Kawamura, and D. Honda. 2008. Taxonomy, molecular phylogeny, and ultrastructural morphology of Olpidiopsis porphyrae sp. nov. (Oomycetes, straminipiles), a unicellular obligate endoparasite of Bangia and Porphyra spp. (Bangiales, Rhodophyta). Mycol. Res. 112:361-374. [DOI] [PubMed] [Google Scholar]
- 31.Sparrow, F. J. 1960. Aquatic phycomycetes, 2nd ed., vol. XV. The University of Michigan Press, Ann Arbor.
- 32.Starr, R., and J. Zeikus. 1993. UTEX—the culture collection of algae at the University of Texas at Austin 1993: list of cultures. J. Phycol. 29:S1-S106. [Google Scholar]
- 33.Strittmatter, M., C. M. M. Gachon, and F. C. Küpper. Ecology of lower oomycetes. In K. Lamour and S. Kamoun (ed.), Oomycete genetics and genomics: diversity, plant and animal interactions, and toolbox, in press. John Wiley and Sons, Hoboken, NJ.
- 34.Suttle, C. A. 2005. Viruses in the sea. Nature 437:356-361. [DOI] [PubMed] [Google Scholar]
- 35.Tillmann, U., K. J. Hesse, and A. Tillmann. 1999. Large-scale parasitic infection of diatoms in the Northfrisian Wadden Sea. J. Sea Res. 42:255-261. [Google Scholar]
- 36.West, J. A., T. A. Klochkova, G. H. Kim, and S. Loiseaux-de Goer. 2006. Olpidiopsis sp., an oomycete from Madagascar that infects Bostrychia and other red algae: host species susceptibility. Phycol. Res. 54:72-85. [Google Scholar]
- 37.Wilce, R. T., C. W. Schneider, A. V. Quinlan, and K. V. Bosch. 1982. The life history and morphology of free-living Pilayella littoralis (L) Kjellm (Ectocarpaceae, Ectocarpales) in Nahant Bay, Massachusetts. Phycologia 21:336-354. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.