Abstract
Helix α4 of Bacillus thuringiensis Cry toxins is thought to play a critical role in the toxins' mode of action. Accordingly, single-site substitutions of many Cry1Aa helix α4 amino acid residues have previously been shown to cause substantial reductions in the protein's pore-forming activity. Changes in protein structure and formation of intermolecular disulfide bonds were investigated as possible factors responsible for the inactivity of these mutants. Incubation of each mutant with trypsin and chymotrypsin for 12 h did not reveal overt structural differences with Cry1Aa, although circular dichroism was slightly decreased in the 190- to 210-nm region for the I132C, S139C, and V150C mutants. The addition of dithiothreitol stimulated pore formation by the E128C, I132C, S139C, T142C, I145C, P146C, and V150C mutants. However, in the presence of these mutants, the membrane permeability never reached that measured for Cry1Aa, indicating that the formation of disulfide bridges could only partially explain their loss of activity. The ability of a number of inactive mutants to compete with wild-type Cry1Aa for pore formation in brush border membrane vesicles isolated from Manduca sexta was also investigated with an osmotic swelling assay. With the exception of the L147C mutant, all mutants tested could inhibit the formation of pores by Cry1Aa, indicating that they retained receptor binding ability. These results strongly suggest that helix α4 is involved mainly in the postbinding steps of pore formation.
During the last few decades, the insecticidal toxins produced by Bacillus thuringiensis have been used increasingly in the forms of formulated sprays and transgenic plants for the highly focused biological control of insect pests (29). At the same time, the mechanism by which these proteins form pores in the apical membrane of midgut epithelial cells of targeted insects has been studied extensively (7, 29). In the case of the three-domain Cry toxins, specificity is mostly attributable to their capacity to bind to certain proteins located on the surface of the intestinal membrane through specific segments of domains II and III, composed mainly of β sheets (16, 27). On the other hand, membrane insertion and pore formation are thought to occur through elements of domain I, composed of a bundle of six amphipathic α-helices surrounding the highly hydrophobic helix α5 (17, 20).
Several lines of evidence indicate that helices α4 and α5 play a particularly important role in these processes (3). Spectroscopic studies with synthetic peptides corresponding to domain I helices revealed that α4 and α5 have the greatest propensity for insertion into artificial membranes, although insertion and pore formation were most efficient when α4 and α5 were connected by a segment corresponding to the α4-α5 loop of the toxin (13, 14). A particularly large number of single-site mutations with altered amino acids from these helices, which lead to a strong reduction in the toxicity and pore-forming ability of the toxin, have been characterized (2, 9, 10, 15, 18, 23, 25, 30, 31, 33). Finally, a site-directed chemical modification study has provided strong evidence that α4 lines the lumens of the pores formed by the toxin (23).
Recent studies have established that toxin activity is especially sensitive to modifications not only in the charged residues of α4 (31) but in most of its hydrophilic residues (15). Furthermore, the loss of activity of most of these mutants did not result from an altered selectivity or size of the pores but from a reduced pore-forming capacity of the toxin (15, 31). In order to better understand the role of α4 in the mechanism of pore formation, the present study was carried out with a series of previously characterized Cry1Aa mutants in which most of the residues from this helix were replaced by cysteines (15). By subjecting these mutants to circular dichroism (CD), protease sensitivity, pore formation inhibition, and electrophoretic mobility analyses, our data suggest that the mutations in α4 which alter the pore-forming ability of Cry1Aa do so mainly by preventing the proper oligomerization or membrane insertion of the toxin.
MATERIALS AND METHODS
Toxin preparation.
Several amino acids located in helix α4 and V150, located in the loop linking helices α4 and α5, were individually replaced by a cysteine by using oligonucleotide-directed in vitro mutagenesis in Escherichia coli, using the double oligonucleotide method (11). These mutations were created in the pMP39 plasmid (22), except for E128C and N135C, which were prepared in the E. coli/B. thuringiensis shuttle vector pBA1 (4).
Toxins were produced in E. coli or B. thuringiensis and activated by incubating 50 mg of protoxin in 40 ml of 50 mM carbonate buffer (pH 10.5) containing 50 mM NaCl and 5 mg trypsin (Gibco BRL, Burlington, Ontario, Canada) for 3 h at 37°C. The toxins were then purified by fast protein liquid chromatography using a Mono-Q anion-exchange column as described previously (21, 22).
CD spectroscopy.
The secondary structures of Cry1Aa mutants were analyzed by CD spectroscopy. Data were obtained from 190 nm to 280 nm with a Chirascan CD spectrometer (Applied Photophysics, Leatherhead, United Kingdom) at room temperature, using a 0.2-mm optical path length and a toxin concentration of 0.5 mg/ml. CD was measured every 0.5 nm with an integration time of 0.5 s, and each experiment was repeated 10 times. Values measured for the solubilization buffer containing 150 mM KCl and 10 mM 3-(cyclohexylamino)-1-propanesulfonic acid-KOH (pH 10.5) were subtracted from the toxin spectra.
Protein digestion and electrophoresis.
Proteolysis was analyzed by incubating 3 μg of each of the inactive mutants (trypsin activated for 3 h) with 3 μg of trypsin or chymotrypsin at 37°C. Each sample was brought to a total volume of 15 μl by the addition of a small amount of water. The reaction was stopped immediately after the addition of the protease or after 12 h by boiling the samples for 5 min. The samples were diluted with loading buffer (final concentration of 100 mM dithiothreitol, 50 mM Tris-HCl, 2% sodium dodecyl sulfate, 0.1% bromophenol blue, 10% glycerol) and resolved by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (19). The gels were stained with GelCode blue stain reagent (Pierce, Rockford, IL) by following the manufacturer's instructions.
To identify the possible formation of homodimers through a disulfide bridge, all of the helix α4 mutants were analyzed by polyacrylamide gel electrophoresis as described above, except that 3 μg of each toxin was mixed with loading buffer containing dithiothreitol or no reducing agent.
Preparation of brush border membrane vesicles.
Brush border membrane vesicles were prepared from Manduca sexta midgut homogenates by using a procedure involving magnesium precipitation and differential centrifugation (32).
Osmotic swelling assay.
The toxin-induced permeability of brush border membrane vesicles was analyzed with a light-scattering assay (8). Toxins were first incubated in the presence or absence of 2 mM EDTA and 5 mM dithiothreitol at a concentration of 0.5 mg/ml for at least 1 h. They were then incubated with the vesicles (0.4 mg membrane protein/ml, 10 mM HEPES-KOH, and 1 mg/ml bovine serum albumin [pH 7.5]) for 1 h. An osmotic shock was initiated by rapidly mixing the vesicles with an equal volume of a hypertonic salt solution (150 mM KCl, 10 mM HEPES-KOH, 1 mg/ml bovine serum albumin [pH 7.5]), using a stopped-flow apparatus (Hi-Tech Scientific, Salisbury, United Kingdom). The vesicle volume was monitored by measuring the scattered-light intensity at a wavelength of 450 nm with a photomultiplier tube at an angle of 90° relative to the incident light beam in a Photon Technology International (South Brunswick, NJ) spectrofluorometer. Data were recorded at 23°C every 0.1 s for 1 min. The percent volume recovery is defined as 100(1 − It), where It is the relative scattered-light intensity at a given time, t.
This technique was also used to study the ability of inactive mutants to compete with wild-type Cry1Aa for pore formation (9). In this competition assay, 500 pmol of each of the inactive mutants per mg of brush border membrane protein was present in the hypertonic solution with or without 50 pmol of Cry1Aa per mg of membrane protein. Vesicles were not preincubated with the toxins but were exposed to them as they were subjected to the osmotic shock. Scattered-light intensity was monitored as described above for 6 min. The percent volume recovery was calculated at every experimental point. Values obtained for control vesicles in the absence of toxin were subtracted from those measured in the presence of toxin. Data are the means ± standard errors of the means from three experiments carried out with a different vesicle preparation. Each replicate consists of the average of five traces obtained using the same vesicle preparation.
RESULTS
As changes in toxin structure are likely to affect its activity, several Cry1Aa mutants previously shown to be substantially inactive in osmotic swelling experiments using brush border membrane vesicles from M. sexta (15) were examined for structural alterations, using different approaches.
Protease digestion.
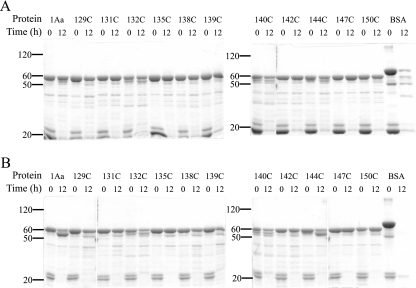
Changes in protein conformation could expose proteolysis sites that are normally hidden. Therefore, the mutants that were previously activated with trypsin for 3 h were further incubated with either trypsin (Fig. 1A) or chymotrypsin (Fig. 1B) for 12 h. The activated form of Cry1Aa had an apparent molecular mass of 60 kDa, as was the case for all mutants (Fig. 1A and B) at 0 h, at which point the activated toxin samples were boiled immediately after the addition of trypsin or chymotrypsin. The lower-molecular-weight bands present at 0 h correspond to trypsin (Fig. 1A) and chymotrypsin (Fig. 1B). Some mutants appear to be slightly susceptible to trypsin during the activation of the protoxin, since small amounts of protein were observed just below the 60-kDa protein band at 0 h. Some of this, however, could reflect small differences in the purities of the different toxin preparations. No subsequent digestion was obvious after 12 h of incubation with trypsin for either Cry1Aa or any of its mutants (Fig. 1A). However, chymotrypsin was able to digest a fraction of the activated Cry1Aa to a protein fragment of 55 kDa after 12 h (Fig. 1B). All mutants were also partially degraded to a 55-kDa fragment, albeit to various extents, but no additional digestion was observed. Both proteases efficiently degraded bovine serum albumin after 12 h (Fig. 1A and B).
FIG. 1.
Protease sensitivity of Cry1Aa and its inactive mutants. Samples (3 μg) of each of the indicated toxins were incubated with 3 μg of trypsin (A) or chymotrypsin (B) at 37°C. The reaction was stopped at the beginning of the experiment or after 12 h by boiling the samples for 5 min. The samples were then analyzed by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis. Numbers at left are molecular weight markers (in thousands).
CD spectra.
Cry1Aa and most of its inactive mutants, such as the T142C mutant, had similar CD spectra. However, the spectra of the I132C, S139C, and V150C mutants deviated slightly from that of Cry1Aa between 190 and 210 nm, a region which is strongly influenced by the α-helical content of the protein (Fig. 2).
FIG. 2.
CD spectra of wild-type and mutant toxins. Data were obtained with a Chirascan CD spectrometer (Applied Photophysics, Leatherhead, United Kingdom) at room temperature, using a 0.2-mm optical path length and a toxin concentration of 0.5 mg/ml. The traces shown correspond to the averages of 10 replicate scans.
Homodimer formation.
The introduction of a cysteine residue, an amino acid which is normally absent from Cry1Aa, could favor the formation of homodimers through a disulfide bridge and significantly impair the activity of the toxin. To investigate this possibility, cysteine mutants, both active and inactive, were analyzed by electrophoresis in the presence and absence of dithiothreitol. Cry1Aa and most mutants (the L126C, I132C, N138C, T142C, A144C, I145C, A149C, and V150C mutants) did not dimerize or did so to only a barely detectable level (the E129C, R131C, N135C, A140C, P146C, and L147C mutants) (Fig. 3A). However, substantial amounts of dimers were observed for the R127C, E128C, M130C, Q133C, M137C, and S139C mutants or, to a lesser extent, for the F134C and L141C mutants. In all cases, the dimers dissociated when reducing agent was added to the loading buffer (Fig. 3B).
FIG. 3.
Polyacrylamide gel electrophoresis (10% polyacrylamide) of Cry1Aa and its mutants in the absence (A) and presence (B) of dithiothreitol. Each well contained 3 μg of the indicated toxin. Numbers at left are molecular weight markers (in thousands).
In order to investigate the effect of dimer formation on the activity of the mutant toxins, toxins were incubated for a least 1 h in the presence of dithiothreitol prior to a 1-h incubation period with the vesicles, in preparation for osmotic swelling experiments. Toxin concentrations for these experiments were chosen based on the activities previously observed for these mutants: 150 (Fig. 4A) and 50 (Fig. 4B) pmol of toxin/mg of membrane protein were used for those mutants that had reduced activity and for those that retained a near wild-type level of activity, respectively (15, 31). As expected, the reducing agent had no significant effect on the light-scattering experiments performed in the presence of Cry1Aa or in the absence of toxin (Fig. 4). However, significant increases in volume recovery were observed in the presence of dithiothreitol for the E128C, I132C, S139C T142C, I145C, P146C, and V150C mutants (Fig. 4A). For all of these mutants, the mutated residue is located either on the hydrophilic face of α4 or within the α4-α5 loop, locations which are well exposed to the solvent, according to the crystal structure (15, 17). The cysteines introduced at these positions are therefore likely to form disulfide bridges in solution, in contrast to those introduced on the hydrophobic face of the helix, which is buried within the protein structure.
FIG. 4.
Effect of dithiothreitol on pore formation by Cry1Aa and its mutants. Each toxin was first incubated in the presence (+DTT) and absence (−DTT) of 2 mM EDTA and 5 mM dithiothreitol at a concentration of 0.5 mg/ml for a least 1 h. Vesicles (0.4 mg membrane protein/ml) isolated from fifth-instar larvae were equilibrated overnight in 10 mM HEPES-KOH (pH 7.5). Before the experiments, bovine serum albumin was added to a final concentration of 1 mg/ml. The toxin was then incubated for 1 h with the vesicles prior to the experiment, at a concentration of 150 pmol of toxin/mg of membrane protein for mutants with reduced activity (A) or 50 pmol of toxin/mg of membrane protein for those with activity near that of the wild type (B). The vesicles were then rapidly mixed with an equal volume of a solution containing 150 mM KCl, 1 mg/ml bovine serum albumin, and 10 mM HEPES-KOH (pH 7.5) directly in a cuvette by using a stopped-flow apparatus. Osmotic swelling of the vesicles was monitored by measuring scattered-light intensity at an angle of 90°. Significant differences using Student's t test were found between values obtained in the presence versus the absence of dithiothreitol (*, P < 0.05; **, P < 0.01).
Competition assay.
Binding of the inactive mutants was also investigated by using a competition assay. For this assay, the vesicles were not preincubated with the toxins for 1 h as in the previous experiments, and the rate of pore formation was monitored for 6 min following exposure to the toxins. Addition of an excess amount of an inactive mutant that retains the ability to bind to its receptors should inhibit Cry1Aa-induced vesicle swelling. Concentrations of 50 pmol of Cry1Aa per mg of membrane protein (Fig. 5A) and 500 pmol of each of the inactive mutants per mg of membrane protein were used in these experiments. In the absence of Cry1Aa, all mutants tested were nearly completely inactive (Fig. 5), with the exception of the I132C (Fig. 5D) and N138C (Fig. 5F) mutants, for which slight pore-forming activities were detected. Competition experiments were performed with a wild-type-to-mutant ratio of 50:500 pmol of toxin/mg of membrane protein. The E129C (Fig. 5B) and N135C (Fig. 5E) mutants completely inhibited pore formation by Cry1Aa. A strong inhibition was observed for the R131C (Fig. 5C), I132C (Fig. 5D), N138C (Fig. 5F), S139C (Fig. 5G), A140C (Fig. 5H), T142C (Fig. 5I), A144 (Fig. 5J), and V150C (Fig. 5L) mutants. Only the L147C mutant was unable to inhibit pore formation (Fig. 5K).
FIG. 5.
Competitive binding assay between Cry1Aa and its inactive mutants. Permeability of the vesicles to KCl was assayed as described in the legend to Fig. 4, except that the toxins were not preincubated with the vesicles but were mixed with the KCl solution prior to the osmotic shock. The concentrations (Cry1Aa-to-mutant ratios) are in pmol of toxin/mg of membrane protein. The percent volume recovery was calculated for every experimental point, and values obtained for control vesicles, assayed without added toxin, were subtracted from those obtained in the presence of toxin. For clarity, error bars are shown for every 50th experimental point.
DISCUSSION
Trypsin had no detectable effect on the activated form of Cry1Aa or its mutants (Fig. 1A). However, chymotrypsin was able to cleave part of Cry1Aa to a stable fragment of approximately 55 kDa (Fig. 1B and Table 1). These results suggest that chymotrypsin has access to a portion of the protein that could not be cleaved by trypsin. Similar findings were obtained with the mutant toxins when they were exposed to chymotrypsin, although some mutants appeared somewhat less susceptible to this digestion than Cry1Aa (Fig. 1B). However, there was no correlation between the extent of cleavage (Fig. 1B) and the pore-forming activity of the mutants (Fig. 4).
TABLE 1.
Summary of results
| Toxin | Chymotrypsin digestiona | CD spectrumb | Effect of DTT on toxin activity
|
Competitione | |
|---|---|---|---|---|---|
| SDS-PAGEc | Pore formationd | ||||
| Cry1Aa | Partial | Wild type | − | − | NAf |
| L126C mutant | NDg | ND | − | − | ND |
| R127C mutant | ND | ND | ++ | − | ND |
| E128C mutant | ND | ND | ++ | + | ND |
| E129C mutant | Minor | Wild type | Trace | − | Complete |
| M130C mutant | ND | ND | ++ | − | ND |
| R131C mutant | Minor | Wild type | Trace | − | Strong |
| I132C mutant | Partial | Lower | − | + | Strong |
| Q133C mutant | ND | ND | ++ | − | ND |
| F134C mutant | ND | ND | + | − | ND |
| N135C mutant | Minor | Wild type | Trace | − | Complete |
| M137C mutant | ND | ND | ++ | − | ND |
| N138C mutant | Minor | Wild type | − | − | Strong |
| S139C mutant | Minor | Lower | ++ | + | Strong |
| A140C mutant | Partial | Wild type | Trace | − | ND |
| L141C mutant | ND | ND | + | − | ND |
| T142C mutant | Minor | Wild type | − | + | Strong |
| A144C mutant | Partial | Wild type | − | − | Strong |
| I145C mutant | ND | ND | Trace | + | ND |
| P146C mutant | ND | ND | Trace | + | ND |
| L147C mutant | Minor | Wild type | Trace | − | None |
| A149C mutant | ND | ND | − | − | ND |
| V150C mutant | Minor | Lower | − | + | Strong |
Apparent amounts indicate minor or partial digestion of the toxin to a fragment of about 55 kDa.
Compared with the Cry1Aa spectrum. “Wild type”, the spectrum of the mutant was similar to that of Cry1Aa; “Lower,” the α-helix content of the protein was decreased.
The presence of bands corresponding to a 120-kDa protein, characteristic of homodimers, was revealed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in the absence of dithiothreitol (DTT) in amounts that were substantial (++), moderate (+), or trace. The absence of homodimer bands is indicated by a minus.
The presence of disulfide bridges as revealed by the effect of dithiothreitol on pore formation. The addition of dithiothreitol had either no effect (−) or produced increased (+) toxin activity.
Ability of each mutant to inhibit the formation of pores by Cry1Aa.
NA, not applicable.
ND, not determined.
Cry1Aa (17) shares a three-dimensional structure that is remarkably similar to that of the other Cry toxins (5, 6, 12, 20, 24). As expected, the CD spectrum of Cry1Aa (Fig. 2) was also similar to that of other Cry toxins (1, 26, 28). Slight differences observed between the spectra of the I132C, S139C, and V150C mutants and those of Cry1Aa and most of its mutants (Table 1) may help to explain their reduced ability to form pores (Fig. 4). As discussed below, these three mutants were among those that displayed a higher pore-forming ability in the presence of dithiothreitol. Therefore, their altered spectra may be a result of extraneous intermolecular disulfide bridging to a polypeptide fragment, possibly during activation.
The effect of dithiothreitol on both the electrophoretic mobility (Fig. 3) and pore-forming ability (Fig. 4) of each mutant was tested. This compound had no readily apparent effect on the L126C, E129C, R131C, N135C, N138C, A140C, A144C, L147C, and A149C mutants, although trace amounts of a band corresponding to toxin dimers were detected for some of these mutants and disappeared after the addition of the reducing agent (Table 1). On the other hand, the E128C and S139C mutants displayed both disulfide-linked homodimer formation and dithiothreitol-stimulated pore-forming activity (Table 1). Increased volume recovery was especially notable for the S139C mutant, which was almost completely inactive in the absence of reducing agent (Fig. 4A). Nevertheless, although homodimer formation may contribute to a reduction in the activity of both mutants, the proportion of dimers detected, at least in the case of the S139C mutant (Fig. 3A), appears insufficient to account fully for the low activity measured for these mutants in the absence of dithiothreitol. Homodimer formation was readily detectable in polyacrylamide gels for the R127C, M130C, Q133C, and M137C mutants and to a lesser extent for the F134C and L141C mutants (Fig. 3 and Table 1). However, the addition of dithiothreitol had no significant effect on volume recovery measured in the presence of these mutants (Fig. 4 and Table 1). For each of these mutants, the modified residue is located on or near the hydrophobic side of helix α4 and, according to the published structure of Cry1Aa, most of them face the core of the protein and are therefore not accessible to the solvent (17). The denaturing conditions used in these experiments probably exposed this region of the protein and allowed the formation of disulfide bridges, which were absent under the nondenaturing conditions used in the osmotic swelling assay. The remaining mutants (the I132C, T142C, I145C, P146C, and V150C mutants) did not display detectable homodimer formation but did have significant dithiothreitol-enhanced pore-forming activity (Table 1). This group of mutants appears to have formed disulfide bridges with other polypeptides. These could be molecules that are insufficiently large to significantly alter the electrophoretic mobility of the heterodimers. Alternatively, the mutant toxins could have reacted with large proteins during an early step in their preparation. This protein moiety would then have been largely degraded during the activation step in the presence of trypsin. Formation of such heterodimers, in addition to the homodimers discussed above, could also contribute to the alteration of the activity of the E128C and S139C mutants.
Previously published toxicity results for the E128C, S139C, and I145C mutants did not correlate well with their activity measured with the osmotic swelling assay (15, 31). For all three of these mutants, toxicity approached that of the wild-type toxin, but pore formation in brush border membrane vesicles was significantly reduced. These experiments, however, were conducted in the absence of a reducing agent. As stated above, the addition of dithiothreitol strongly increased the activity of these mutants (Table 1). The reducing conditions present in the insect midgut therefore probably contribute to the toxins' higher in vivo activity. On the other hand, despite a significant stimulation of pore formation by the I132C, T142C, P146C, and V150C mutants under reducing conditions, the activities of these mutants remain significantly lower than that of Cry1Aa, at least qualitatively, in agreement with their reduced toxicities.
A number of mutants were completely inactive or, in the case of the I132C (Fig. 5D) and N138C (Fig. 5F) mutants, very slightly active, when they were tested alone at 500 pmol of toxin/mg of membrane protein (Fig. 5). These results are in agreement with those of previous experiments using 150 pmol of toxin/mg of membrane protein (15, 31) and imply that any volume recovery observed in the competition experiments illustrated in Fig. 5 is due mainly to the permeability induced by Cry1Aa. Results also imply that activity that is reduced relative to that of Cry1Aa in these competition experiments is due to the ability of the mutant to compete with Cry1Aa for its binding site on the membrane receptors (9). These experiments thus demonstrate that with the exception of the L147C mutant, all mutants tested retain the ability to bind to vesicles despite their poor pore-forming ability (Fig. 5 and Table 1). In agreement with these results and with the fact that helix α4 is identical in Cry1Aa, Cry1Ab, and Cry1Ac, except for the amino acid at position 148, the N135Q mutant from Cry1Ab and Cry1Ac (9, 30) and the R131L, I132S, I132L, I132V, I132N, and Q133R mutants from Cry1Ac (18) have also been shown to bind to M. sexta brush border membrane vesicles, despite their inability to increase membrane permeability.
To date, the only mutation of a helix α4 residue in a Cry1 toxin to show an effect on receptor binding is L147C, presented in this study. The reason for the mutant's inability to bind to the midgut membrane will, nevertheless, require further investigation, since it does not appear to result from an altered secondary or tertiary structure or from the formation of a disulfide bond.
In summary, although disulfide bridge formation can account for some loss of activity for a few mutants (Table 1), it remains clear that other factors are also involved. For instance, the activity of the E128C mutant, in the absence of reducing agent, has previously been shown to be significantly increased by raising the pH from 7.5 to 10.5, a procedure which restores the negative charge of the mutated residue (31). Even for those mutants that were strongly stimulated by dithiothreitol, volume recovery values always remained lower than those measured for Cry1Aa. With one exception, all mutations tested herein that caused a loss of pore-forming ability did not prevent the toxin from binding to its receptors. In addition, no obvious structural alteration could explain the loss of activity of the resulting mutants. This implies that these mutations primarily affect later steps in pore formation, including either toxin oligomerization or insertion into the membrane or both. In any case, the results of the present study underscore the importance of helix α4 in the mechanism of pore formation and, in particular, in its postbinding steps.
Acknowledgments
We thank Andreea Schmitzer, Université de Montréal, for allowing us to perform the CD experiments and for valuable advice.
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada, the Fonds Québécois de la Recherche sur la Nature et les Technologies, and Valorisation-Recherche Québec.
Footnotes
Published ahead of print on 14 November 2008.
REFERENCES
- 1.Arnold, S., A. Curtiss, D. H. Dean, and O. Alzate. 2001. The role of a proline-induced broken-helix motif in α-helix 2 of Bacillus thuringiensis δ-endotoxins. FEBS Lett. 490:70-74. [DOI] [PubMed] [Google Scholar]
- 2.Aronson, A. I., C. Geng, and L. Wu. 1999. Aggregation of Bacillus thuringiensis Cry1A toxins upon binding to target insect larval midgut vesicles. Appl. Environ. Microbiol. 65:2503-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aronson, A. I., and Y. Shai. 2001. Why Bacillus thuringiensis insecticidal toxins are so effective: unique features of their mode of action. FEMS Microbiol. Lett. 195:1-8. [DOI] [PubMed] [Google Scholar]
- 4.Bah, A., K. van Frankenhuyzen, R. Brousseau, and L. Masson. 2004. The Bacillus thuringiensis Cry1Aa toxin: effects of trypsin and chymotrypsin site mutations on toxicity and stability. J. Invertebr. Pathol. 85:120-127. [DOI] [PubMed] [Google Scholar]
- 5.Boonserm, P., P. Davis, D. J. Ellar, and J. Li. 2005. Crystal structure of the mosquito-larvicidal toxin Cry4Ba and its biological implications. J. Mol. Biol. 348:363-382. [DOI] [PubMed] [Google Scholar]
- 6.Boonserm, P., M. Mo, C. Angsuthanasombat, and J. Lescar. 2006. Structure of the functional form of the mosquito larvicidal Cry4Aa toxin from Bacillus thuringiensis at a 2.8-angstrom resolution. J. Bacteriol. 188:3391-3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bravo, A., S. S. Gill, and M. Soberón. 2007. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 49:423-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carroll, J., and D. J. Ellar. 1993. An analysis of Bacillus thuringiensis δ-endotoxin action on insect-midgut-membrane permeability using a light-scattering assay. Eur. J. Biochem. 214:771-778. [DOI] [PubMed] [Google Scholar]
- 9.Cooper, M. A., J. Carroll, E. R. Travis, D. H. Williams, and D. J. Ellar. 1998. Bacillus thuringiensis Cry1Ac toxin interaction with Manduca sexta aminopeptidase N in a model membrane environment. Biochem. J. 333:677-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dean, D. H., F. Rajamohan, M. K. Lee, S.-J. Wu, X. J. Chen, E. Alcantara, and S. R. Hussain. 1996. Probing the mechanism of action of Bacillus thuringiensis insecticidal proteins by site-directed mutagenesis: a minireview. Gene 179:111-117. [DOI] [PubMed] [Google Scholar]
- 11.Deng, W. P., and J. A. Nickoloff. 1992. Site-directed mutagenesis of virtually any plasmid by eliminating a unique site. Anal. Biochem. 200:81-87. [DOI] [PubMed] [Google Scholar]
- 12.Galitsky, N., V. Cody, A. Wojtczak, D. Ghosh, J. R. Luft, W. Pangborn, and L. English. 2001. Structure of the insecticidal bacterial δ-endotoxin Cry3Bb1 of Bacillus thuringiensis. Acta Crystallogr. Sect. D 57:1101-1109. [DOI] [PubMed] [Google Scholar]
- 13.Gazit, E., P. La Rocca, M. S. P. Sansom, and Y. Shai. 1998. The structure and organization within the membrane of the helices composing the pore-forming domain of Bacillus thuringiensis δ-endotoxin are consistent with an “umbrella-like” structure of the pore. Proc. Natl. Acad. Sci. USA 95:12289-12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerber, D., and Y. Shai. 2000. Insertion and organization within membranes of the δ-endotoxin pore-forming domain, helix 4-loop-helix 5, and inhibition of its activity by a mutant helix 4 peptide. J. Biol. Chem. 275:23602-23607. [DOI] [PubMed] [Google Scholar]
- 15.Girard, F., V. Vachon, G. Préfontaine, L. Marceau, Y. Su, G. Larouche, C. Vincent, J.-L. Schwartz, L. Masson, and R. Laprade. 2008. Cysteine scanning mutagenesis of α4, a putative pore-lining helix of the Bacillus thuringiensis insecticidal toxin Cry1Aa. Appl. Environ. Microbiol. 74:2565-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gómez, I., L. Pardo-López, C. Muñoz-Garay, L. E. Fernandez, C. Pérez, J. Sánchez, M. Soberón, and A. Bravo. 2007. Role of receptor interaction in the mode of action of insecticidal Cry and Cyt toxins produced by Bacillus thuringiensis. Peptides 28:169-173. [DOI] [PubMed] [Google Scholar]
- 17.Grochulski, P., L. Masson, S. Borisova, M. Pusztai-Carey, J.-L. Schwartz, R. Brousseau, and M. Cygler. 1995. Bacillus thuringiensis CryIA(a) insecticidal toxin: crystal structure and channel formation. J. Mol. Biol. 254:447-464. [DOI] [PubMed] [Google Scholar]
- 18.Kumar, A. S. M., and A. I. Aronson. 1999. Analysis of mutations in the pore-forming region essential for insecticidal activity of a Bacillus thuringiensis δ-endotoxin. J. Bacteriol. 181:6103-6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227:680-685. [DOI] [PubMed] [Google Scholar]
- 20.Li, J., J. Carroll, and D. J. Ellar. 1991. Crystal structure of insecticidal δ-endotoxin from Bacillus thuringiensis at 2.5 Å resolution. Nature 353:815-821. [DOI] [PubMed] [Google Scholar]
- 21.Masson, L., A. Mazza, L. Gringorten, D. Baines, V. Aneliunas, and R. Brousseau. 1994. Specificity domain localization of Bacillus thuringiensis insecticidal toxins is highly dependent on the bioassay system. Mol. Microbiol. 14:851-860. [DOI] [PubMed] [Google Scholar]
- 22.Masson, L., G. Préfontaine, L. Péloquin, P. C. K. Lau, and R. Brousseau. 1990. Comparative analysis of the individual protoxin components in P1 crystals of Bacillus thuringiensis subsp. kurstaki isolates NRD-12 and HD-1. Biochem. J. 269:507-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masson, L., B. E. Tabashnik, Y.-B. Liu, R. Brousseau, and J.-L. Schwartz. 1999. Helix 4 of the Bacillus thuringiensis Cry1Aa toxin lines the lumen of the ion channel. J. Biol. Chem. 274:31996-32000. [DOI] [PubMed] [Google Scholar]
- 24.Morse, R. J., T. Yamamoto, and R. M. Stroud. 2001. Structure of Cry2Aa suggests an unexpected receptor binding epitope. Structure 9:409-417. [DOI] [PubMed] [Google Scholar]
- 25.Nuñez-Valdez, M., J. Sánchez, L. Lina, L. Güereca, and A. Bravo. 2001. Structural and functional studies of α-helix 5 region from Bacillus thuringiensis Cry1Ab δ-endotoxin. Biochim. Biophys. Acta 1546:122-131. [DOI] [PubMed] [Google Scholar]
- 26.Park, H.-W., and B. A. Federici. 2004. Effect of specific mutations in helix α7 of domain I on the stability and crystallization of Cry3A in Bacillus thuringiensis. Mol. Biotechnol. 27:89-100. [DOI] [PubMed] [Google Scholar]
- 27.Pigott, C. R., and D. J. Ellar. 2007. Role of receptors in Bacillus thuringiensis crystal toxin activity. Microbiol. Mol. Biol. Rev. 71:255-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puntheeranurak, T., P. Uawithya, L. Potvin, C. Angsuthanasombat, and J.-L. Schwartz. 2004. Ion channels formed in planar lipid bilayers by the dipteran-specific Cry4B Bacillus thuringiensis toxin and its α1-α5 fragment. Mol. Membr. Biol. 21:67-74. [DOI] [PubMed] [Google Scholar]
- 29.Schnepf, E., N. Crickmore, J. Van Rie, D. Lereclus, J. Baum, J. Feitelson, D. R. Zeigler, and D. H. Dean. 1998. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62:775-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tigue, N. J., J. Jacoby, and D. J. Ellar. 2001. The α-helix 4 residue, Asn135, is involved in the oligomerization of Cry1Ac1 and Cry1Ab5 Bacillus thuringiensis toxins. Appl. Environ. Microbiol. 67:5715-5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vachon, V., G. Préfontaine, C. Rang, F. Coux, M. Juteau, J.-L. Schwartz, R. Brousseau, R. Frutos, R. Laprade, and L. Masson. 2004. Helix 4 mutants of the Bacillus thuringiensis insecticidal toxin Cry1Aa display altered pore-forming abilities. Appl. Environ. Microbiol. 70:6123-6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolfersberger, M., P. Luethy, A. Maurer, P. Parenti, V. F. Sacchi, B. Giordana, and G. M. Hanozet. 1987. Preparation and partial characterization of amino acid transporting brush border membrane vesicles from the larval midgut of the cabbage butterfly (Pieris brassicae). Comp. Biochem. Physiol. A 86:301-308. [Google Scholar]
- 33.Wu, D., and A. I. Aronson. 1992. Localized mutagenesis defines regions of the Bacillus thuringiensis δ-endotoxin involved in toxicity and specificity. J. Biol. Chem. 267:2311-2317. [PubMed] [Google Scholar]