Abstract
Q fever is a zoonosis caused by Coxiella burnetii, a bacterium largely carried by ruminants and shed into milk, vaginal mucus, and feces. The main potential hazard to humans and animals is due to shedding of bacteria that can then persist in the environment and be aerosolized. The purpose of this study was to evaluate shedding after an outbreak of Q fever abortion in goat herds and to assess the relationship with the occurrence of abortions and antibody responses. Aborting and nonaborting goats were monitored by PCR for C. burnetii shedding 15 and 30 days after the abortion episodes. PCR analysis of all samples showed that 70% (n = 50) of the aborting and 53% (n = 70) of the nonaborting goats were positive. C. burnetii was shed into vaginal mucus, feces, and milk of 44%, 21%, and 38%, respectively, of goats that aborted and 27%, 20%, and 31%, respectively, of goats that delivered normally. Statistical comparison of these shedding results did not reveal any difference between these two groups. PCR results obtained for the vaginal and fecal routes were concordant in 81% of cases, whereas those for milk correlated with only 49% of cases with either vaginal or fecal shedding status. Serological analysis, using enzyme-linked immunosorbent assay (ELISA), indirect immunofluorescence assay (IFA), and complement fixation tests, showed that at least 24% of the seronegative goats shed bacteria. Positive vaginal and fecal shedding, unlike positive milk shedding, was observed more often in animals that were weakly positive or negative by ELISA or IFA. Two opposite shedding trends were thus apparent for the milk and vaginal-fecal routes. Moreover, this study showed that a nonnegligible proportion of seronegative animals that delivered normally could excrete C. burnetii.
Q fever is a public health concern throughout the world (28, 29). The agent of this disease, Coxiella burnetii, is a highly infectious bacterium which is strictly intracellular and possesses an adaptive pathway differentiating into dormant survival forms during starvation (13, 25, 26). Humans contract infection mainly through inhalation of the infectious aerosols, which can resist various conditions and be spread. Primary sources of C. burnetii include birth products, vaginal secretions, milk, and feces of infected domestic ruminants. Evidence that C. burnetii is a food-borne pathogen was obtained in experiments where contaminated milk was fed to volunteers, causing seroconversion but any clinical disease (5, 12, 22). In fact, vaginal and fecal bacterial discharges seem to have a major impact on environmental contamination as a result of practices at kidding and effluent management. The well-known clinical manifestations are abortion, stillbirth, and premature delivery in ruminants. Although most wild animals and domestic species have persistent infections, high rates of abortion and stillbirth have been observed in goat herds (2, 9, 10, 24, 27, 38). Numerous studies have suggested that epizootics of Q fever in goats are related to cases of this disease in humans (19, 20, 35-37).
Our understanding of C. burnetii shedding modalities in ruminants requires improvement to allow the implementation of rational prophylactic measures (2, 23, 33). Studies are currently limited due to a lack of simple and sensitive detection tools. Initial investigations were carried out on Q fever abortions by identifying the causal agent, by isolation in laboratory animals and presumptive bacterial staining on smears, and/or by demonstration of an antibody response, using complement fixation tests (CFTs) or agglutination tests (23). Advances in PCR detection and enzyme-linked immunosorbent assay (ELISA) serological tests later helped to better describe the characteristics of bacterial shedding routes and the antibody response during both experimental and natural infections (2-4, 11, 16). Experimental reproduction of the disease in goats is recent (3, 4, 34). C. burnetii inoculation led to abortions in almost all pregnant females, particularly during the end of gestation, as in naturally infected animals. Shedding of C. burnetii in vaginal mucus, feces, and milk lasted 1 to 5 weeks, 2 to 5 weeks, and 1 day to 6 weeks, respectively (3). In addition, goats that had aborted or delivered normally in naturally infected herds shed the bacteria (9, 10, 18). However, each of these C. burnetii shedding studies conducted under field conditions was carried out with a single herd of goats. Moreover, the interpretation of the serological test results can be questioned because of the seronegative response of several aborting goats experimentally infected with C. burnetii (3, 4). Recently, diagnostic test performances were compared and monitored for eight clinically infected dairy goat herds (32). One CFT exhibited poor sensitivity, whereas results obtained using an ELISA and an indirect immunofluorescence assay (IFA) were significantly associated with abortion above the cutoffs of 80% optical density (OD) and a titer of 80, respectively. Good agreement was obtained between the ELISA and IFA serological results. However, the tests at the individual level were poorly indicative of Q fever abortion because a relevant proportion of nonaborting goats presented high antibody levels and close to 20% of aborting goats did not (32). Also, the occurrence of C. burnetii shedding in some seronegative animals, even using experimentally infected goats and PCR and ELISA tests, means that the serological screening of infected animals is problematic (1, 4, 8, 11, 14, 16, 17). Actually, among results derived from postabortion investigations of naturally infected ruminants, the relationships between abortion events, bacterial shedding, and antibody responses have never been assessed statistically, apart from recent studies with dairy cows (15, 16).
The present study aimed at providing epidemiological information, using available diagnostic tools, to appreciate the C. burnetii shedding prevalence in eight herds of goats with cases of Q fever abortions. A high prevalence of strong antibody responses suggested extensive bacterial circulation within these herds (32). In this study, the objective was first to describe the proportions of animal shedders among those having aborted or not, considering the three shedding routes. Secondly, potential relationships were investigated between shedding routes and serological results in order to contribute to testing strategies for identification of shedding animals in this type of herd. The shedding of C. burnetii was tested using PCR detection applied to vaginal, fecal, and milk samples collected from goats 15 and 30 days (D15 and D30, respectively) after abortion or according to the expected dates of parturition. The present data were obtained from the same 120 monitored animals, for which blood samples were collected at D15, D30, and D60 and serological results were recently reported (32).
MATERIALS AND METHODS
Herds and goats investigated.
Eight herds of dairy goats were recruited and 120 goats were selected for examination as previously presented (32). Briefly, the sizes of herds ranged between 90 and 390 goats, except for one herd with 39 goats. A herd was included in the protocol if at least five goats had aborted due to Q fever and if neither a Q fever event nor vaccination against Q fever had been reported during the three previous years. In each herd, targeted animals were 5 aborting goats and 10 nonaborting goats. The latter were chosen by taking into account the parity status (multiparous or primiparous) of the selected aborting goats within the herd. The targeted nonaborting animals were five goats at full-term gestation and five goats during the final month of gestation, regardless of the date of parturition calculated from that of covering. A total of 50 females that aborted and 70 that delivered normally were monitored, as 10 of the selected pregnant goats eventually aborted.
Sampling.
Samples of blood, vaginal mucus, feces, and milk were concomitantly collected from each selected goat at D15 and D30, with D0 being the abortion peak for the aborting animals and either parturition or the last gestation month for the nonaborting animals. Vaginal mucus samples were taken from inside the vagina with a dry, sterile cotton wool swab (10 cm). At least 1 g of feces was taken directly from the rectum into a sterile container. At least 5 ml of milk was sampled aseptically into a sterile container. The samples were transported to the laboratory in a biosafety container at 4°C. The samples were then frozen at −20°C for subsequent analysis.
Serological tests.
Sera were tested for specific anti-C. burnetii antibodies by use of three serological tests as previously described (32), including an ELISA Chekit Q fever test (Idexx Laboratories, Broomfield, CO), an IFA (Coxiella burnetii Spot IF; bioMerieux SA, Marcy-l'Etoile, France), and a CFT using the C. burnetii antigen provided by Symbiotics Europ (Lyon, France). Analytical results were interpreted using the cutoffs recommended by the manufacturers (%OD of >50 for ELISA, titer of >80 for IFA, and titer of >10 for CFT). The D15 and D30 results were combined for each test and for each goat to compute an individual serological profile, which was either seronegative (repeated negative results) or seropositive (positive results for at least one of the paired samples, showing either a seronegative or seropositive conversion).
PCR assay.
Samples were processed for PCR assay by established protocols (6, 7). Briefly, each vaginal swab was resuspended in 1 ml of physiological saline solution. Total DNA was extracted from 200 μl of vaginal suspension, 20 mg of feces, or 100 μl of milk by use of a QIAamp tissue kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. The PCR was carried out with 2.5 μl of purified DNA in a total volume of 25 μl in an automated DNA thermal cycler (UNO Thermobloc; Biometra, Gottingen, Germany). PCR products (687 bp; 10 μl) were analyzed by electrophoresis on a 1.5% agarose gel, stained with ethidium bromide, visualized under a UV transilluminator, and photographed. For a given shedding route, a goat was considered to be a positive shedder if at least one sample was PCR positive, whatever the day (D15 or D30), and to be a negative shedder if neither of the two samples was PCR positive. For a given day, a goat was defined as a C. burnetii positive shedder if at least one of the available samples was PCR positive, whatever the shedding route (vaginal mucus, feces, or milk), and otherwise was considered a negative shedder.
Statistical analyses.
For the eight monitored herds, PCR results for bacterial shedding in vaginal mucus, feces, and milk were available at both D15 and D30 for 120, 60, and 93 goats, respectively. PCR results for bacterial shedding in feces and milk were available for only one of the two sampling times for 15 and 25 goats, respectively. In other words, shedding data at D15 and/or D30 for vaginal mucus, feces, and milk were obtained for 120, 75, and 118 goats, respectively. Serological data for three different tests were achieved at both D15 and D30 for all 120 selected goats.
Statistical analyses were done using R (30). The results were computed for aborting and nonaborting animals. The associations between D15 and D30 PCR results, as well as the shedder proportions, were tested using Fisher's exact test. The coincidence (or the mutual exclusion) of the three routes was assessed by paired comparisons, with the association being tested using Fisher's exact test. The shedding status for each route and for each individual was compared with the respective serological profile. The strength of the association was quantified by computing the odds ratios (OR) and 95% confidence interval (CI). Finally, the association between serological and PCR results was studied quantitatively from the variations in the proportions of PCR-positive results according to titer (IFA and CFT) or %OD (ELISA). Four groups of goats were defined for each serological test, using the 25th, 50th, and 75th percentiles of the titers or %OD distribution. The proportions of PCR-positive results by group were then computed and tested using a χ2 test for trends in proportions.
RESULTS
Coxiella burnetii shedding in vaginal, fecal, and milk samples, detected by PCR.
Seventy percent (n = 50) of the aborting goats and over one-half (53%; n = 70) of the nonaborting goats yielded at least one PCR-positive sample during the study and were thus classified as positive shedders (Table 1). These two percentages were not significantly different. C. burnetii was shed into 21%, 38%, and 44% of the fecal, milk, and vaginal samples, respectively, from the aborting goats. Among goats that delivered normally, each of the three routes presented a percentage of PCR-positive results that was close to 25%. The percentages of PCR-positive results for each route did not differ significantly between aborting and nonaborting goats.
TABLE 1.
PCR results for samples taken from aborting and nonaborting goats and tested for the presence of Coxiella burnetii DNA
| Animal category | Shedding route | No. of positive samples/total no. of samples tested (%)
|
||
|---|---|---|---|---|
| D15 | D30 | Any daya | ||
| Aborting goats | Vaginal mucus | 20/50 (40) | 7/50 (14) | 22/50 (44) |
| Feces | 5/34 (15) | 3/29 (10) | 7/34 (21) | |
| Milk | 11/43 (26) | 9/49 (18) | 19/50 (38) | |
| Any routeb | 25/50 (50) | 16/50 (32) | 35/50 (70) | |
| Nonaborting goats | Vaginal mucus | 14/70 (20) | 8/70 (11) | 19/70 (27) |
| Feces | 5/41 (12) | 3/31 (10) | 8/41 (20) | |
| Milk | 6/53 (11) | 17/66 (26) | 21/68 (31) | |
| Any routeb | 21/70 (30) | 22/70 (31) | 37/70 (53) | |
Goats for which at least one sample tested PCR positive throughout the study.
Goats that tested positive for at least one shedding route.
Among the aborting goats, two positive PCR results were obtained for 23% (n = 22), 30% (n = 7), and 21% (n = 19) of the vaginal mucus, feces, and milk samples, respectively. Among the goats that delivered normally, 16% (n = 19) of the vaginal mucus samples and 38% (n = 21) of the milk samples were PCR positive at both sampling times. None of the animals (n = 8) presented positive paired fecal samples. Regarding the shedding route, no significant association was found between D15 and D30 PCR-positive results for aborting or nonaborting goats for which both PCR results were available.
Comparison of the three shedding routes.
A significant association was found between vaginal and fecal shedding (Table 2) for both the aborting (Fisher's exact test; P = 0.007) and nonaborting goats (Fisher's exact test; P < 0.0001). For the 75 animals tested for both vaginal and fecal shedding during the study, two positive PCR results were obtained for 14 goats and two negative PCR results were obtained for 47 goats, showing that 81% of the goats exhibited a concordant individual shedding status. Moreover, 13 of the 14 goats with a discordant status were PCR positive for the vaginal swab and PCR negative for the fecal sample, while one gave a PCR-negative vaginal swab and a PCR-positive fecal sample. Thus, except for one goat, bacterial shedding occurred systematically in the vaginal tract when positive fecal shedding was observed (but not the contrary).
TABLE 2.
Comparison of Coxiella burnetii shedding routes in aborting and nonaborting goats assessed by PCR
| Shedding route paira | No. of goats
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Aborting
|
Nonaborting
|
|||||||
| − − | + + | + − | − + | − − | + + | + − | − + | |
| Vaginal mucus-feces | 17 | 7 | 10 | 0 | 30 | 7 | 3 | 1 |
| Vaginal mucus-milk | 15 | 6 | 16 | 13 | 33 | 4 | 15 | 17 |
| Feces-milk | 13 | 2 | 5 | 14 | 19 | 3 | 5 | 14 |
+, goats from which at least one sample tested PCR positive throughout the study; −, goats from which all of the samples tested PCR negative throughout the study. According to the order of the names in the considered sample pair, the first sign of the shedding route pattern corresponds to the first sample type and the second sign corresponds to the second one.
No significant association was found between bacterial shedding in milk and that in the vaginal mucus or feces (Fisher's exact test; P > 0.05). The status of half of the animals was concordant and that of the other half was discordant in both populations (Table 2). Furthermore, when negative shedders for both routes were discarded from the analysis, most goats were of discordant status, including 61 of 71 animals (86%) for the vaginal-milk shedding comparison and 38 of 43 animals (88%) for the feces-milk shedding comparison. Thus, few animals simultaneously excreted C. burnetii via both the milk route and the vaginal or fecal route.
Both categories of goats most frequently exhibited concomitant vaginal and fecal shedding status, whereas the milk shedding status was rarely associated with either of the other two routes.
Relationships between shedding routes and serological responses.
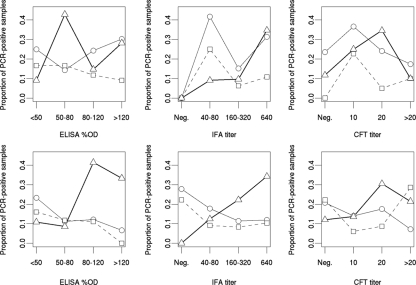
Among the 72 goats with positive shedding status (at least one positive PCR sample), 25, 24, and 39% were seronegative according to the ELISA, IFA, and CFT analyses, respectively (Table 3). For nonaborting animals, positive shedding in the milk was positively linked with the IFA serological profile (Fisher's exact test; P = 0.02), as the shedding status of 60% of the animals (n = 68) was concordant with the IFA profile, i.e., 17 were seropositive and positive shedders and 24 were seronegative and negative shedders. The corresponding OR was significantly more than 1 (OR = 4.3; 95% CI, 1.2 to 20.4), indicating a larger proportion of positive shedders among animals with a positive serological response by IFA. Moreover, a significant association was found between positive vaginal shedding and serological profiles with both ELISA (Fisher's exact test; P = 0.01) and IFA (Fisher's exact test; P = 0.03). The vaginal shedding status, unlike the milk shedding-IFA profile association, of most of the nonaborting goats did not concord with the respective serological profile, i.e., there was 70% (n = 70) and 66% (n = 70) concordance with ELISA and IFA, respectively. Consequently, the corresponding ORs were significantly less than 1 for both ELISA (OR = 0.23; 95% CI, 0.06 to 0.77) and IFA (OR = 0.30; 95% CI, 0.08 to 0.99), indicating a negative association. In fact, positive vaginal shedding seemed to be more frequent for nonaborting animals when the individual ELISA or IFA serological profile was negative, as 12 of 19 (63%) of these positive shedders were seronegative. No association was observed between the individual shedding status and the serological profile for aborting goats. Nevertheless, the proportion of milk PCR-positive results increased with increasing IFA titer (χ2 = 7.3; P = 0.007) for aborting animals (Fig. 1). For nonaborting animals, the proportion of milk positive shedders increased significantly with both the IFA titer (χ2 = 10.4; P = 0.001) and the ELISA %OD (χ2 = 9.5; P = 0.002). The proportions of positive shedders for the two other routes decreased with increasing ELISA %OD and with IFA titer. However, these trends were less marked and nonsignificant.
TABLE 3.
Comparison of Coxiella burnetii shedding routes assessed by PCR with ELISA, IFA, and CFT serological profiles for aborting and nonaborting goats
| Serological profilea | Shedding routeb | No. of goatsc
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Aborting
|
Nonaborting
|
||||||||
| − − | + + | + − | − + | − − | + + | + − | − + | ||
| ELISA | Vaginal mucus | 3 | 20 | 25 | 2 | 14 | 7 | 37 | 12 |
| Feces | 1 | 6 | 26 | 1 | 9 | 4 | 24 | 4 | |
| Milk | 5 | 19 | 26 | 0 | 20 | 16 | 27 | 5 | |
| Any route | 3 | 33 | 12 | 2 | 10 | 21 | 23 | 16 | |
| IFA | Vaginal mucus | 4 | 20 | 24 | 2 | 17 | 7 | 34 | 12 |
| Feces | 2 | 6 | 25 | 1 | 12 | 4 | 21 | 4 | |
| Milk | 6 | 19 | 25 | 0 | 24 | 17 | 23 | 4 | |
| Any route | 4 | 33 | 11 | 2 | 14 | 22 | 19 | 15 | |
| CFT | Vaginal mucus | 8 | 14 | 20 | 8 | 25 | 10 | 26 | 9 |
| Feces | 9 | 4 | 18 | 3 | 15 | 4 | 18 | 4 | |
| Milk | 8 | 11 | 23 | 8 | 25 | 14 | 22 | 7 | |
| Any route | 4 | 23 | 11 | 12 | 18 | 21 | 15 | 16 | |
−, seronegative at D15 and D30; +, other profiles (both positive results; seropositive or seronegative conversion).
−, samples tested PCR negative at D15 and D30; +, at least one PCR-positive sample was present.
The first sign of the patterns “− −”, “+ +”, “+ −,” and “− +” corresponds to the serological profile obtained with the considered test, and the second sign corresponds to bacterial shedding through the considered route.
FIG. 1.
Results obtained for samples taken concomitantly from goats tested for the presence of Coxiella burnetii DNA by PCR and for antibodies against Coxiella burnetii by three different serological tests (ELISA, IFA, and CFT). Circles and thin line, vaginal mucus samples; squares and dashed line, fecal samples; triangles and thick line, milk samples. Aborting animals (top) were sampled at D15 and D30 after the abortion peak. Samples from nonaborting animals (bottom) were taken at D15 and D30 after parturition or during the last gestation month, with the date of parturition being calculated from that of covering.
No particular trend according to CFT titer was observed, whatever the shedding route, for either nonaborting or aborting goats. Thus, the milk and vaginal-fecal shedding routes in nonaborting animals appeared to be differently linked with IFA and ELISA serological status.
DISCUSSION
The shedding of C. burnetii by ruminants is an important public health threat. However, possible control measures are difficult to apply and evaluate because of a lack of epidemiological information and simple tools to identify shedders. To our knowledge, this is the first study aimed at describing the global proportion of shedders and assessing the relationships between C. burnetii shedding routes and serological responses among herds of goats with cases of abortion.
This investigation showed the identification of 60% (72/120 goats) of goats shedding C. burnetii into vaginal mucus, feces, and/or milk taken from animals distributed in eight herds in different locations in France (Table 1). This estimated prevalence of shedders confirmed that after an abortion episode, goats constitute an important risk of direct or indirect exposure within and between herds and to the human population (19, 20, 35-37). Furthermore, a study showed that bacteria were shed by some goats for almost 4 months after an outbreak and also at the two successive parturitions (9). Adding to the problem of long-term shedders is that of environmental contamination persistence.
No significant differences in the proportions of C. burnetii shedders were found between aborting and nonaborting goats (Table 1). These findings are consistent with another study performed on cows sampled less than 2 months after Q fever abortion or calving (15). Therefore, after a Q fever outbreak, herds may contain more than one-half goats without characteristic clinical signs but excreting C. burnetii. Such information shows that it is important to pay attention to both goats that abort and those that deliver normally. The latter might transmit the bacteria to neonates in the first month of life during suckling, but little is known about infection by the oral route. Nevertheless, the observation of apparently healthy goats shedding bacteria in the vaginal mucus and feces could be a potential source of environmental contamination. In France, abortions in ruminants are notifiable. C. burnetii is diagnosed as a cause of abortion, but not systematically. A Q fever abortion report is usually recommended for an area when an increase in human cases has occurred. The findings in the present study suggest the use of sanitary precautions for diseased animals for limitation of C. burnetii transmission within the herd, but also, animals from a herd with abortions due to C. burnetii should not be transferred to other herds. However, the period of application is difficult to estimate and is dependent on control measure efficacy, which still remains to be defined. Recently, in The Netherlands, a ban on spreading manure during the 3 months following the detection of Q fever at a farm was imposed (37).
Our study showed that no particular route of bacterial shedding was dominant in either aborting or nonaborting goats, which is in good agreement with data recently obtained for bovine herds (15). Thus, all three routes need to be investigated before the C. burnetii shedding status of an animal can be classified. Our study also showed a preponderance of shedding negativity or positivity after an interval of 15 days, ranging from 62 to 100% of goats, depending on the route tested. These observations are consistent with discontinuous C. burnetii shedding, which has already been noted in goats (3, 4, 9, 10). Thus, false-negative results may be obtained if only a single sampling time is chosen for shedder identification.
Analysis of the relationships between the PCR results for the three routes indicated that different pairs of shedding routes gave different results. A statistically significant concordant shedding status was obtained more frequently for vaginal and fecal routes (81%) than for vaginal and milk routes (49%) or fecal and milk routes (49%) in both aborting and nonaborting goats (Table 2). In a recent study, it was observed that goats from one clinically affected herd shed bacteria in vaginal mucus and feces, whereas goats from two asymptomatic herds did not (31). Taken together, these findings suggest that shedding route modalities are more likely to be associated with the clinical or latent form of Q fever at the herd level than at the individual goat level. Future testing programs should be carried out on herds without a history of Q fever abortions.
Several studies suggested that animals may shed C. burnetii without infectious antibodies being detectable (1, 4, 8, 11, 14, 16, 17). In this study, a large proportion of discordant PCR and serological results was observed during the study period. Among the 72 positive shedders, 18, 17, and 28 presented a negative serological profile with the ELISA, IFA, and CF tests used, respectively (Table 3). According to ELISA, IFA, and CFT, the seronegative shedders were distributed in five, four, and seven of the eight herds, respectively. A possible link could not be observed between the occurrence of these animals and an episode directed mainly at primiparous goats. Thus, the capacity and therefore potential interest of these serological tools to determine currently shedding animals were inadequate. Previous serological data showed strong relationships between all ELISA and IFA results and abortion, but these tests cannot provide evidence of Q fever abortion at an individual level (32). Serological testing seems rather useful for carrying out preliminary surveys of infection.
In this investigation, there were no significant trends in the serological profiles of aborting goats with regard to the shedding route, with the proportion of strong responses being high, as mentioned above. Among nonaborting goats, the proportion of C. burnetii shedders into milk was apparently linked to a strong antibody response, whereas a nonnegligible proportion of seronegative goats excreted bacteria in the vaginal mucus or feces. These milk and vaginal-fecal shedding observations could imply that in accordance with the tropism of C. burnetii for the mammary glands and for the genital and digestive tracts, very different types of antibodies could be involved. It was reported that the detection or lack of detection of various anti-C. burnetii antibodies depended essentially on the antigen used (8, 21, 32). We believed that some types would not be revealed by the tests used in our study. The relationships between routes and serological responses need to be studied thoroughly but did not appear of interest for screening of shedders. PCR is thus the method of choice to trace shedders.
In conclusion, positive shedding and a negative serological response were observed for at least one-quarter of the tested animals. The results revealed that PCR testing should be done at different times and with different types of samples in order to not miss shedding goats. Nevertheless, the statically supported results presented here might be useful to elaborate a surveillance program. The sample size could be calculated according to the shedding goat proportions obtained. Also, the results provided an estimation of the false-negative result proportions if testing is based on only one type of biological sample. For cost and practical reasons, a screen might be performed on vaginal swabs upon a Q fever outbreak and successive parturitions. In the veterinary communities, a national project has been initiated for implementation of medical and sanitary control measures and their evaluation. Clinically affected livestock are firstly targeted. The obtained findings could also facilitate future experiments with goats to study the efficacy of inactivated phase 1 vaccines (2), antibiotic treatment, sanitary precautions, and disinfection measures against Coxiella in the environment.
Acknowledgments
This work was supported by grant S98/34 from the DGAl (National Direction of Alimentation).
We are grateful to Annick Mazeau from FNGDSB for involving the following organizations: CNIEL (French National Interprofessional Center for Dairy Economy), Institut de l'Elevage (Institute of Breeding), and SNGTV (French National Company of Veterinary Technical Groups). All stockbreeders, departmental laboratory agents, and veterinarians are also kindly acknowledged. We thank Christophe Lacz for his excellent technical assistance in PCR analysis.
Footnotes
Published ahead of print on 14 November 2008.
REFERENCES
- 1.Adesiyun, A. A., A. G. Jagun, J. K. Kwaga, and L. B. Tekdek. 1985. Shedding of Coxiella burnetii in milk by Nigerian dairy and dual purposes cows. Int. J. Zoonoses 12:1-5. [PubMed] [Google Scholar]
- 2.Arricau-Bouvery, N., and A. Rodolakis. 2005. Is Q fever an emerging or re-emerging zoonosis? Vet. Res. 36:327-349. [DOI] [PubMed] [Google Scholar]
- 3.Arricau-Bouvery, N., A. Souriau, C. Bodier, P. Dufour, E. Rousset, and A. Rodolakis. 2005. Effect of vaccination with phase I and phase II Coxiella burnetii vaccines in pregnant goats. Vaccine 23:4392-4402. [DOI] [PubMed] [Google Scholar]
- 4.Arricau-Bouvery, N., A. Souriau, P. Lechopier, and A. Rodolakis. 2003. Experimental Coxiella burnetii infection in pregnant goats: excretion routes. Vet. Res. 34:423-433. [DOI] [PubMed] [Google Scholar]
- 5.Benson, W. W., D. W. Brock, and J. Mather. 1963. Serologic analysis of a penitentiary group using raw milk from a Q fever infected herd. Public Health Rep. 78:707-710. [PMC free article] [PubMed] [Google Scholar]
- 6.Berri, M., N. Arricau-Bouvery, and A. Rodolakis. 2003. PCR-based detection of Coxiella burnetii from clinical samples. Methods Mol. Biol. 216:153-161. [DOI] [PubMed] [Google Scholar]
- 7.Berri, M., K. Laroucau, and A. Rodolakis. 2000. The detection of Coxiella burnetii from ovine genital swabs, milk and fecal samples by the use of a single touchdown polymerase chain reaction. Vet. Microbiol. 72:285-293. [DOI] [PubMed] [Google Scholar]
- 8.Berri, M., E. Rousset, J. L. Champion, N. Arricau-Bouvery, P. Russo, M. Pepin, and A. Rodolakis. 2003. Ovine manure used as garden fertiliser as a suspected source of human Q fever. Vet. Rec. 153:269-270. [DOI] [PubMed] [Google Scholar]
- 9.Berri, M., E. Rousset, J. L. Champion, P. Russo, and A. Rodolakis. 2007. Goats may experience reproductive failures and shed Coxiella burnetii at two successive parturitions after a Q fever infection. Res. Vet. Sci. 83:47-52. [DOI] [PubMed] [Google Scholar]
- 10.Berri, M., E. Rousset, C. Héchard, J. L. Champion, P. Dufour, and A. Rodolakis. 2005. Progression of Q fever and Coxiella burnetii shedding in milk after an outbreak of enzootic abortion in a goat herd. Vet. Rec. 150:548-549. [DOI] [PubMed] [Google Scholar]
- 11.Berri, M., A. Souriau, M. Crosby, D. Crochet, P. Lechopier, and A. Rodolakis. 2001. Relationships between the shedding of Coxiella burnetii, clinical signs and serological responses of 34 sheep. Vet. Rec. 148:502-505. [DOI] [PubMed] [Google Scholar]
- 12.Cerf, O., and R. Condron. 2006. Coxiella burnetii and milk pasteurization: an early application of the precautionary principle? Epidemiol. Infect. 134:946-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coleman, S. A., E. R. Fischer, D. Howe, D. J. Mead, and R. A. Heinzen. 2004. Temporal analysis of Coxiella burnetii morphological differentiation. J. Bacteriol. 186:7344-7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enright, J. B., W. M. Longhurst, C. E. Franti, D. E. Behymer, V. J. Dutson, and M. E. Wright. 1971. Coxiella burnetii in a wildlife-livestock environment. Isolations of rickettsiae from sheep and cattle. Am. J. Epidemiol. 94:72-78. [DOI] [PubMed] [Google Scholar]
- 15.Guatteo, R., F. Beaudeau, M. Berri, A. Rodolakis, A. Joly, and H. Seegers. 2006. Shedding routes of Coxiella burnetii in dairy cows: implications for detection and control. Vet. Res. 37:827-833. [DOI] [PubMed] [Google Scholar]
- 16.Guatteo, R., F. Beaudeau, A. Joly, and H. Seegers. 2007. Coxiella burnetii shedding by dairy cows. Vet. Res. 38:849-860. [DOI] [PubMed] [Google Scholar]
- 17.Hassig, M., and J. Lubsen. 1998. Relationship between abortions and seroprevalences to selected infectious agents in dairy cows. Zentralbl. Vetmed. B 45:435-441. [DOI] [PubMed] [Google Scholar]
- 18.Hatchette, T., N. Campbell, R. Hudson, D. Raoult, and T. J. Marrie. 2003. Natural history of Q fever in goats. Vector Borne Zoonotic Dis. 3:11-15. [DOI] [PubMed] [Google Scholar]
- 19.Hatchette, T. F., R. C. Hudson, W. F. Schlech, N. A. Campbell, J. E. Hatchette, S. Ratnam, D. Raoult, C. Donovan, and T. J. Marrie. 2001. Goat-associated Q fever: a new disease in Newfoundland. Emerg. Infect. Dis. 7:413-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kovacova, E., J. Kazar, and A. Simkova. 1998. Clinical and serological analysis of a Q fever outbreak in western Slovakia with four-year follow-up. Eur. J. Clin. Microbiol. Infect. Dis. 17:867-869. [DOI] [PubMed] [Google Scholar]
- 21.Kovacova, E., J. Kazar, and D. Spanelova. 1998. Suitability of various Coxiella burnetii antigen preparations for detection of serum antibodies by various tests. Acta Virol. 42:365-368. [PubMed] [Google Scholar]
- 22.Krumbiegel, E. R., and H. J. Wisniewski. 1970. Q fever in the Milwaukee area. II. Consumption of infected raw milk by human volunteers. Arch. Environ. Health 21:63-65. [DOI] [PubMed] [Google Scholar]
- 23.Lang, G. H. 1990. Coxiellosis (Q fever) in animals, p. 23-48. In T. J. Marrie (ed.), Q fever, the disease, vol. 1. CRC Press, Boca Raton, FL. [Google Scholar]
- 24.Masala, G., R. Porcu, C. Daga, S. Denti, G. Canu, C. Patta, and S. Tola. 2007. Detection of pathogens in ovine and caprine abortion samples from Sardinia, Italy, by PCR. J. Vet. Diagn. Investig. 19:96-98. [DOI] [PubMed] [Google Scholar]
- 25.McCaul, T. F., and J. C. Williams. 1981. Developmental cycle of Coxiella burnetii: structure and morphogenesis of vegetative and sporogenic differentiations. J. Bacteriol. 147:1063-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ormsbee, R. A., M. G. Peacock, R. Gerloff, G. Tallent, and D. Wike. 1978. Limits of rickettsial infectivity. Infect. Immun. 19:239-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer, N. C., M. Kierstead, D. W. Key, J. C. Williams, M. G. Peacock, and H. Vellend. 1983. Placentitis and abortion in sheep and goats in Ontario caused by Coxiella burnetii. Can. Vet. J. 24:60-61. [PMC free article] [PubMed] [Google Scholar]
- 28.Parker, N. R., J. H. Barralet, and A. M. Bell. 2006. Q fever. Lancet 367:679-688. [DOI] [PubMed] [Google Scholar]
- 29.Raoult, D., T. Marrie, and J. Mege. 2005. Natural history and pathophysiology of Q fever. Lancet Infect. Dis. 5:219-226. [DOI] [PubMed] [Google Scholar]
- 30.R Development Core Team, R Foundation for Statistical Computing. 2006. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
- 31.Rodolakis, A., M. Berri, C. Hechard, C. Caudron, A. Souriau, C. C. Bodier, B. Blanchard, P. Camuset, P. Devillechaise, J. C. Natorp, J. P. Vadet, and N. Arricau-Bouvery. 2007. Comparison of Coxiella burnetii shedding in milk of dairy bovine, caprine, and ovine herds. J. Dairy Sci. 90:5352-5360. [DOI] [PubMed] [Google Scholar]
- 32.Rousset, E., B. Durand, M. Berri, P. Dufour, M. Prigent, P. Russo, T. Delcroix, A. Touratier, A. Rodolakis, and M. Aubert. 2007. Comparative diagnostic potential of three serological tests for abortive Q fever in goat herds. Vet. Microbiol. 124:286-297. [DOI] [PubMed] [Google Scholar]
- 33.Rousset, E., V. Duquesne, P. Russo, and M. Aubert. 2008. Chapter 2.1.12. Q fever, p. 292-303. In B. Vallat and S. Edwards (ed.), OIE manual of diagnostic tests and vaccines for terrestrial animals (mammals, birds and bees), 6th ed., vol. 1. World Organization for Animal Health, Paris, France. [Google Scholar]
- 34.Sanchez, J., A. Souriau, A. J. Buendia, N. Arricau-Bouvery, C. M. Martinez, J. Salinas, A. Rodolakis, and J. A. Navarro. 2006. Experimental Coxiella burnetii infection in pregnant goats: a histopathological and immunohistochemical study. J. Comp. Pathol. 135:108-115. [DOI] [PubMed] [Google Scholar]
- 35.Sanford, S. E., G. K. Josephson, and A. MacDonald. 1994. Coxiella burnetii (Q fever) abortion storms in goat herds after attendance at an annual fair. Can. Vet. J. 35:376-378. [PMC free article] [PubMed] [Google Scholar]
- 36.Serbezov, V. S., J. Kazar, V. Novkirishki, N. Gatcheva, E. Kovacova, and V. Voynova. 1999. Q fever in Bulgaria and Slovakia. Emerg. Infect. Dis. 5:388-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schimmer, B., G. Morroy, F. Dijkstra, P. M. Schneeberger, G. Weers-Pothoff, A. Timen, C. Wijkmans, and W. van der Hoek. 2008. Large ongoing Q fever outbreak in the south of The Netherlands, 2008. Eurosurveillance 13:1-3. [PubMed] [Google Scholar]
- 38.Woldehiwet, Z. 2004. Q fever (coxiellosis): epidemiology and pathogenesis. Res. Vet. Sci. 77:93-100. [DOI] [PubMed] [Google Scholar]