Abstract
A new and efficient sulfide monooxygenase-producing strain, ECU0066, was isolated and identified as a Rhodococcus sp. that could transform phenylmethyl sulfide (PMS) to (S)-sulfoxide with 99% enantiomeric excess via two steps of enantioselective oxidations. Its enzyme activity could be effectively induced by adding PMS or phenylmethyl sulfoxide (PMSO) directly to a rich medium at the early log phase (6 h) of fermentation, resulting in over 10-times-higher production of the enzyme. This bacterial strain also displayed fairly good activity and enantioselectivity toward seven other sulfides, indicating a good potential for practical application in asymmetric synthesis of chiral sulfoxides.
Because of the high configurational stability of the sulfinyl group as well as their synthetic versatility, chiral sulfoxides are powerful stereodirecting groups (18), valuable asymmetric starting materials (19), and chiral auxiliaries (7). The value of chiral sulfoxide functionality is further illustrated by their diverse biological activities and pharmaceutical uses (17). In many cases, only one enantiomer of a sulfur-containing drug exhibits the desired biological activity (10); therefore, it is necessary and important to prepare enantiopure sulfoxides.
The asymmetric oxidation of a prochiral sulfide is undoubtedly a more direct and economical method for the synthesis of enantiomerically pure sulfoxides than the kinetic resolution of racemic sulfoxides. Asymmetric sulfoxidations mediated by either metal catalysts or isolated enzymes have received considerable attention over the past several years (1, 9, 14, 20, 24). However, the transformations with metal catalysts or isolated enzymes (such as peroxidases, haloperoxidases, and monooxygenases) are tedious and expensive, which are major disadvantages for preparative applications. And only very few of the enzymes used for sulfoxidation, such as the cyclohexanone monooxygenase from Acinetobacter NCBI 9871, have been isolated and characterized (8). By contrast, asymmetric sulfoxidations catalyzed by whole-cell systems (e.g., fungi and bacteria) are much cheaper and more convenient, avoiding the involvement of expensive cofactors (NADH/NADPH). Although a few microorganisms have so far been successfully used for such a biocatalytic sulfoxidation, the most frequently used cultures for this purpose were fungi (12, 21, 22). For the bacteria, there have been only a few brief reports regarding the oxidation of sulfides with whole cells (2, 15), but the results were unsatisfactory because of low enantioselectivity or poor substrate tolerance. Therefore, we decided to screen for new bacterial strains with higher enzyme activity and better enantioselectivity for asymmetric oxidation of sulfides.
In this article, we report the catalytic performance of the newly isolated bacterium Rhodococcus sp. strain ECU0066 for asymmetric oxidation of sulfides. This bacterium displayed pretty high activity and satisfactory enantioselectivity for most of the sulfides examined, indicating that this bacterium is very promising for synthetic application.
Enrichment and isolation of microbial strains for asymmetric sulfoxidation.
Bacteria with sulfide monooxygenase (SMO) activity were isolated from soil samples through two rounds of screening: the first round of screening for SMO activity and the second round of screening for enantioselective SMO activity. Soil samples used were collected from some coal gasification sites of Shanghai, Jiangsu, and Shandong Provinces, China. A tiny portion of each soil sample was suspended in a mineral salts medium containing, per liter of tap water, 1.0 g of (NH4)2SO4, 6.0 g of K2HPO4·3H2O, 3.0 g of KH2PO4, 0.5 g of NaCl, 0.5 g of MgSO4·7H2O, and 0.05 g of CaCl2. The suspension was supplemented with 40 μl of 0.5 M phenylmethyl sulfide (PMS) in methanol as the carbon source. The enrichment culture was carried out at 30°C and 160 rpm for 5 to 6 days, with one transfer into fresh medium with the same composition. After the enrichment culture, samples of the mixtures were withdrawn for a thin-layer chromatography (TLC) assay using petroleum ether-ethyl acetate (EtOAc). (2:1, vol/vol) as the elution solvent.
The soil culture samples with the obvious product phenylmethyl sulfoxide (PMSO) spot on the TLC plates were plated onto a rich medium (RM) composed of, per liter of tap water, 15 g of glucose, 5.0 g of yeast extract, 5.0 g of peptone, 0.66 g of K2HPO4·3H2O, 0.5 g of KH2PO4, 1.0 g of NaCl, 0.2 g of MgSO4, and 15 g of agar, pH 7.0. The sulfur-adapted microorganisms (800 strains) that developed on the RM agar plates were picked and inoculated individually into 2 ml of liquid RM without agar. After 24 h of incubation, 40 μl of 0.5 M PMS in methanol was added, giving a final concentration of 10 mM. After 18 h of bioconversion, the product PMSO was quickly examined by TLC. Subsequently, 100 strains of the isolates with obvious SMO activity were chosen for further screening on a larger scale. After culture in liquid RM, ca. 0.5 to 1.0 g (wet weight) washed cells of each selected strain was suspended in 9 ml of 50 mM potassium phosphate buffer (KPB; pH 7.0), and 1 ml of 100 mM PMS in KPB containing 10% (wt/vol) Tween 80 was added for bioconversion (30°C, 160 rpm, 24 h). Samples (0.5 ml each) of the reaction mixture were withdrawn for measuring the substrate conversion and product enantiomeric excess (eep) by high-pressure liquid chromatography (LC-10AT; Shimadzu, Japan) using a chiral column (Chiralcel OD-H; Daicel Co., Japan; 250 mm by 4.6 mm [inside diameter]), which was eluted with hexane-isopropanol (93:7, vol/vol; 1.0 ml/min). Detection was at 254 nm.
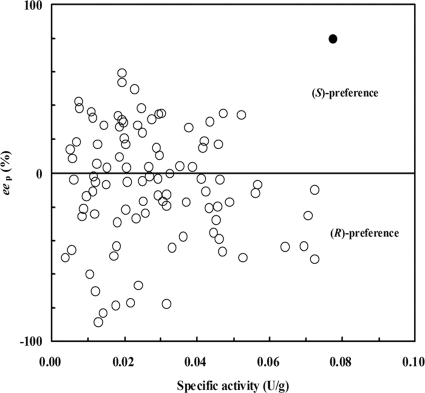
As shown in Fig. 1, 57 of the 100 strains could oxidize PMS to (R)-PMSO. Among the strains preferentially producing the R isomer, seven strains showed relatively higher enantioselectivity (eep > 60%), and the highest eep values obtained were 83.0% and 88.6%, but the specific activities of the two most selective strains were relatively low (<0.02 U/g). Among the 43 strains preferentially producing (S)-PMSO, only 2 showed satisfactory eep (60% and 80%). Fortunately, the one with 80% eep, designated ECU0066, showed the highest activity (0.08 U/g) among all 100 strains. Therefore, it was selected for further study.
FIG. 1.
Results of the secondary round of screening. The enantioselective oxidation of PMS to PMSO by 100 strains with obvious SMO activity is shown. Specific activity is in units per gram of wet cell weight. •, ECU0066; ○, other strains.
In order to obtain an effective biocatalyst from soil for this purpose, an effective two-step screening procedure was adopted in our screening system. It has greatly helped us in efficiently isolating the target strains. The fact that more than 60% of isolated strains showed over 20% conversion of PMS after 24 h of bioconversion further proves that the above-mentioned measures can greatly enhance the efficiency of obtaining active strains.
Presumed mechanism for asymmetric oxidation of PMS.
In the subsequent experiments, it was surprisingly found that the ee of (S)-PMSO could be improved to 99% when the initial PMS concentration was lowered. The reason for this phenomenon, which was revealed later, was the two steps of oxidations involved in the biotransformation process. First, PMS was sulfoxidized to sulfoxide with 80% ee for the S enantiomer, and the formed (R)-PMSO was further enantioselectively oxidized to achiral sulfone, resulting in 99% ee for the residual (S)-PMSO. Furthermore, in order to confirm this presumed mechanism, racemic PMSO (rac-PMSO) was employed as the starting material for bioconversion. It was found that the bacterium could preferentially oxidize (R)-PMSO to achiral sulfone, affording enantiopure (S)-PMSO with 99% ee. This indicates that the second oxidation step is highly enantioselective, which is different from the reaction catalyzed by a phenylacetone monooxygenase from Thermobifida fusca, which has no enantioselectivity for the second oxidation step (24). Therefore, it also provides an alternative procedure for preparing enantiopure sulfoxides from racemates for practical application. Based on the results mentioned above, a presumed pathway for the biosulfoxidation of PMS to PMSO and bio-oxidation of PMSO to phenylmethyl sulfone, which was mediated by whole cells of the strain ECU0066, was proposed (Fig. 2).
FIG. 2.
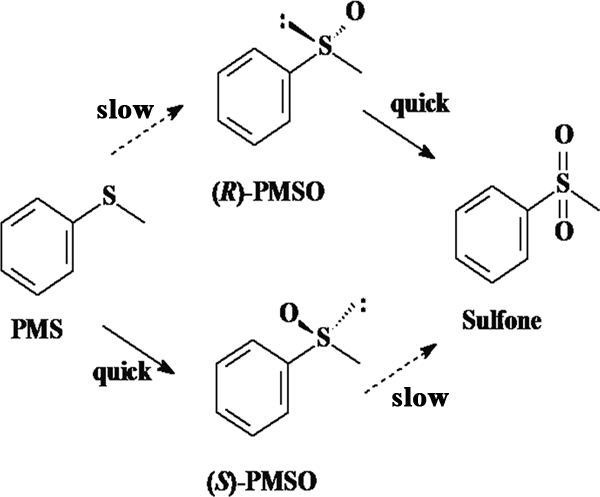
Pathway of strain ECU0066-catalyzed sulfoxidation of PMS to PMSO and phenylmethyl sulfone.
Identification of the new bacterial isolate ECU0066.
Cell morphology of the bacterial strain ECU0066 was observed using an optical microscope. The bacterial cells were sphere shaped and were gram positive. The 16S rRNA gene sequencing and taxonomic analyses revealed that this stain belongs to the genus Rhodococcus and is closely related to Rhodococcus sp. strain CHNTR42 (Fig. 3). Therefore, strain ECU0066 was marked as Rhodococcus sp. strain ECU0066. This strain was deposited in the China General Microbiological Culture Collection Center, with an accession number of CGMCC 2547. To the best of our knowledge, it is the first time that a Rhodococcus sp. was intentionally investigated for asymmetric oxidation of sulfides. In the past, more attention was focused on biodesulfurization using Rhodococcus species (11, 16), and only one report concerning the use of a reconstructed dibenzothiophene degradation bacterium, Rhodococcus erythropolis, for enantioselective oxidation of sulfides was available (13), though the results were unsatisfactory: the ee value was only 2% for the standard substrate PMS. The observed sense of the enantioselectivity of the SMO produced by Rhodococcus sp. strain ECU0066 was also opposite to that of the SMO produced by Rhodococcus erythropolis, suggesting that the SMO discovered in this work may be a novel enzyme.
FIG. 3.
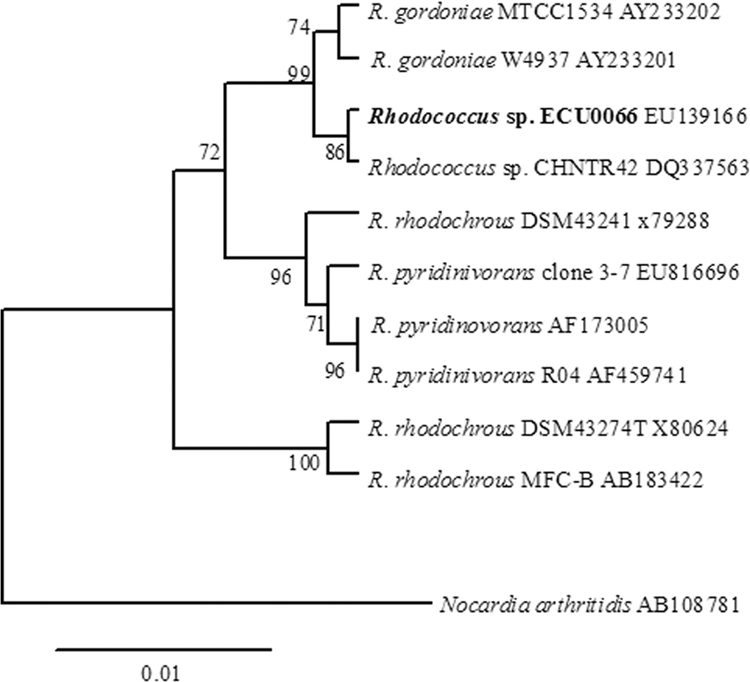
Phylogenetic dendrogram for strain ECU0066-related strains based on the 16S rRNA gene sequence. Numbers after the names of organisms are accession numbers of published sequences. Bootstrap values were based on 1,000 replicates. Nocardia arthritidis was used as the outgroup.
Fermentation and induction of SMO.
To further characterize the new bacterial isolate, the time course of SMO production was monitored by cultivating Rhodococcus sp. strain ECU0066 in 250-ml flasks with 50 ml of RM. The sterilized RM was inoculated with 5% (vol/vol) of a preculture in flasks for 12 h. Periodically, two flasks were withdrawn for determination of the enzyme activity, dry cell weight, and pH. The cells harvested from 2 ml of the culture broth by centrifuge were resuspended in 0.49 ml of 50 mM KPB (pH 8.0) and used for the assay of SMO activity on a minishaker (Thermomixer Compact; Eppendorf, Germany) at 30°C and 1,100 rpm. The reaction was started by adding 10 μl methanol solution containing 500 mM PMS (final concentration: 10 mM) and stopped after 10 min of incubation by addition of 0.3 ml EtOAc with 6 mM 4-nitroacetophenone as an internal standard. The EtOAc phase was subjected to gas chromatography analysis (GC-14C; Shimadzu, Japan) at an injector temperature of 280°C, a detector temperature of 350°C, and an oven temperature of 180°C, with a column (AT•SE-54; 30 m by 0.25 mm by 0.33 μm) and a flame ionization detector to determine the quantity of PMSO formed. One unit of SMO activity is defined as the amount of the enzyme catalyzing the formation of 1.0 μmol PMSO per minute under the above conditions.
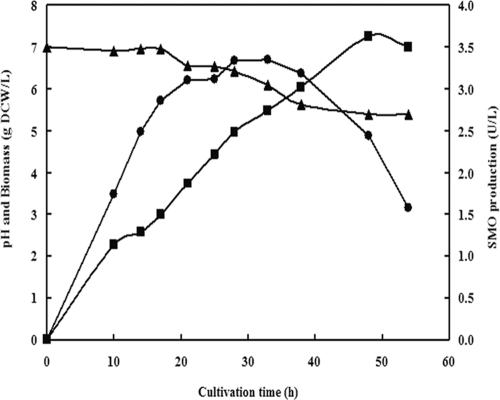
As shown in Fig. 4, the SMO activity increased in parallel with the cell growth during the first 30 h and began to decrease after 30 h, while the cell growth continued till 50 h. The maximum SMO activity (3.4 U/liter) was observed at about 30 h. The change of pH during the cultivation was small, with a trend of slight decline. However, the maximum activity observed was relatively low. Therefore, to improve the SMO production, some potential inducers were examined. PMS (0.1 mM) and rac-PMSO (0.1 mM) were tested as potential inducers in the early log phase (6 h) of fermentation. As a result, it was found that the effects of PMS and rac-PMSO as inducers on cell growth and SMO production of the bacterium were similar. The specific activities of resting cells with induction by PMS or rac-PMSO were 0.76 U/g and 0.73 U/g, respectively, which are markedly higher than that without induction (0.064 U/g), leading to more than a 10-fold enhancement of enzyme production. And the biomass obtained with induction was also slightly higher than that of the control (data not shown). Such a method of SMO induction has never been reported before. We added inducer directly to RM during the early log phase (at 6 h) of fermentation, whereas previous researchers employed camphor (2) or cyclohexanone (15) as the sole carbon source in a minimum medium for the cultivation of monooxygenase-producing bacteria. The obvious disadvantage of the latter method is the poor growth of cells, producing a much lower biomass than our method.
FIG. 4.
Profiles of cell growth and SMO production of Rhodococcus sp. strain ECU0066. •, SMO activity; ▪, biomass; ▴, pH. DCW, dry cell weight.
Based on the results above, we finally adopted the following fermentation conditions: the fermentation time was controlled at around 30 h, and PMS (0.1 mM) as an inducer was added at the 6-h point of the fermentation process.
Investigation of reaction conditions and substrate tolerance of the whole-cell transformation.
Into 0.49 ml of 50 mM KPB (pH 8.0) were added 50 mg wet cells with PMS induction and 10 μl methanol solution containing 500 mM PMS. The mixture was incubated for 10 min at different temperatures (25°C to 50°C), and the SMO activity was assayed by gas chromatography as described above. The enzyme showed the maximum activity at 35°C, and at higher temperatures the activity began to decrease significantly. The activity at 45°C was only 12% of that at 35°C. The pH optimum was determined at 30°C in buffers of various pHs (pH 4 to 6, citrate buffer; pH 7.0 to 8.5, phosphate buffer; pH 9 to 10, glycine-NaOH buffer). The optimum pH for activity of resting cells was 8.0. When the pH was over 8.0, the enzyme activity decreased dramatically, and when the pH was below pH 6.0, the activity became very low and could not be measured accurately. However, the stability of resting cell activity at 35°C was relatively poor compared with that at 30°C (data not shown). Therefore, pH 8.0 and 30°C were chosen as the optimal conditions for asymmetric oxidation of PMS with resting cells of Rhodococcus sp. strain ECU0066.
In a bioconversion process, substrate concentration is another important parameter worthy of careful investigation. One gram of resting cells with PMS induction was suspended in 9.8 ml of 50 mM KPB (pH 8.0), and the bioconversion was carried out at 30°C and 160 rpm after addition of 200 μl methanol solution containing various concentrations of PMS. As a result, the asymmetric oxidation of PMS could be successfully carried out with substrate of up to 10 mM. Figure 5 displayed the whole process of bioconversion at an initial substrate concentration of 10 mM. After 6 h, concentrations of the R and S enantiomers of sulfoxide increased from 0 to their maximum values. The ee was maintained around 80% all the time. During the whole process of bioconversion, almost no sulfone was detected. But when a lower concentration of substrate (5 mM) was used for transformation, the ee of the product could be improved to 99% and sulfone was also detected as the reaction proceeded. The reason for this phenomenon has been discussed above. Because of the toxicity and hydrophobicity of sulfide substrates, the substrate inhibition of enzyme activity appeared when the initial substrate concentration was higher than or equal to 10 mM, resulting in incomplete transformation of the substrate even if the reaction time was extended. However, to the best of our knowledge, such a concentration (10 mM) still represents the highest level that has been reported so far for whole-cell-mediated sulfoxidation (15).
FIG. 5.
Bioconversion course for enantioselective oxidation of PMS (10 mM). •, concentration of (R,S)-PMSO; ▵, concentration of (S)-PMSO; □, concentration of (R)-PMSO; ⧫, ee of (S)-PMSO.
Preparation of enantiopure sulfoxides with resting cells of the Rhodococcus sp.
In this study, the asymmetric oxidation of a broad spectrum of sulfides was examined. The general procedure for asymmetric oxidation of sulfides to sulfoxides is as follows. About 12 g of resting cells with sulfide induction was suspended in 100 ml of 50 mM KPB (pH 8.0), and then 2.5 ml of 200 mM sulfide (analytical grade; purchased from Shou & Fu Chemical Co., Ltd. [Zhejiang, China], Sigma-Aldrich [Taufkirchen, Germany], or Lancaster [Morecambe, United Kingdom]) in methanol was added, giving a final concentration of 5 mM. The reaction mixture was incubated at 30°C and 160 rpm for about 4 h. After the mixture was centrifuged, the cells were soaked with EtOAc (20 ml; twice) and the supernatant was extracted with EtOAc (50 ml; three times). The two parts of EtOAc were combined, washed twice with saturated NaCl (10 ml), dried over anhydrous Na2SO4, and finally evaporated under reduced pressure. The residue was purified by silica gel column chromatography with petroleum ether-EtOAc (2:1, vol/vol) as the elution solvent, yielding pure products of sulfoxides. The products were identified by 1H nuclear magnetic resonance analysis (for details, see the supplemental material) with Varian Mercury 300 (300 MHz). Optical rotations were measured with a Jasco P-1030 polarimeter (Japan).
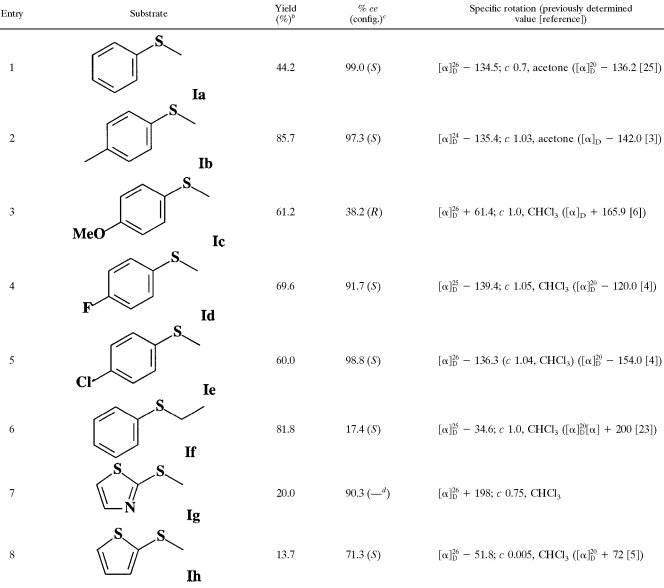
As listed in Table 1, the bacterium Rhodococcus sp. strain ECU0066 displayed pretty high activity and stereoselectivity for the majority of the sulfides examined. As a standard substrate, Ia was enzymatically sulfoxidized to (S)-IIa, affording an excellent ee (99%) and a moderate yield (44.2%), with only a trace amount of sulfone. In the case of para substitution (entries 2 to 5), essentially high yields and almost enantiopure (S)-sulfoxides were obtained except in the case of methoxy group substitution, where the R enantiomer was formed (entry 3). Interestingly, the enantioselectivity of sulfoxidization is independent of the electronic properties of the para substituents, since for either electron-withdrawing groups (F−, Cl−) or an electron-donating one (methyl−), excellent enantiomeric differentiation by the bacterium was achieved. The reason why the R enantiomer, instead of the S antipode, is formed in the case of methoxy substitution has not been clarified so far and needs further investigation. As to the effect of the alkyl side chain, when the methyl group was changed to ethyl, the highest yield (81.8%) was obtained, while the stereoselectivity decreased significantly (17.4% ee, entry 6). In contrast, the sulfides with a heterocycle produced a lower substrate acceptance of the bacterial cells. Low yields of sulfoxides were obtained and an unknown by-product also appeared for substrate Ih, though the stereoselectivity was still very excellent (entries 7 and 8).
TABLE 1.
Enantioselective oxidation of sulfides Ia to Ih to sulfoxides IIa to IIh with the resting cells of Rhodococcus sp. strain ECU0066a
I, sulfide; II, sulfoxide.
b Isolated yield of product.
c The enantioselectivity was determined by HPLC. The formula for ee is ([S] − [R]/[S] + [R]) × 100%, where [S] and [R] denote the concentrations of (S)- and (R)-PMSO, respectively. Absolute configurations (config.) were assigned by comparison of the specific rotations with the literature values.
d —, no configuration specified.
In summary, because of the encouraging results described above, our future work will focus on purification and characterization of the responsible SMO enzyme and the extensive utilization of this bacterium for large-scale application in asymmetric sulfoxidation and other oxidative biotransformations.
Nucleotide sequence accession number.
The 16S rRNA gene sequence of strain ECU0066 was deposited in the GenBank database with an accession number of EU139166.
Supplementary Material
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (grant no. 20506037 and 20672037) and the Ministry of Science and Technology, People's Republic of China (grant no. 2006AA02Z205 and 2007AA02Z225).
Footnotes
Published ahead of print on 3 October 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Adam, W., M. N. Korb, K. J. Roschmann, and C. R. Saha-Möller. 1998. Titanium-catalyzed, asymmetric sulfoxidation of alkyl aryl sulfides with optically active hydroperoxides. J. Org. Chem. 63:3423-3428. [Google Scholar]
- 2.Beer, J., P. Richardson, and A. Willetts. 1994. Baeyer-Villiger monooxygenase-dependent biotransformations: stereospecific heteroatom oxidations by camphor-grown Pseudomonas putida to produce chiral sulfoxides. Biotechnol. Lett. 16:909-912. [Google Scholar]
- 3.Bénéchie, M., and F. Khuong-Huu. 1996. Total synthesis of (-)-Maytansinol. J. Org. Chem. 61:7133-7138. [DOI] [PubMed] [Google Scholar]
- 4.Boyd, D. R., N. D. Sharma, S. A. Haughey, M. A. Kennedy, B. T. McMurray, G. N. Sheldrake, C. C. R. Allen, H. Dalton, and K. Sproule. 1998. Toluene and naphthalene dioxygenase-catalysed sulfoxidation of alkyl aryl sulfides. J. Chem. Soc. Perkin Trans. I 12:1929-1933. [Google Scholar]
- 5.Boyd, D. R., N. D. Sharma, N. Gunaratne, S. A. Haughey, M. A. Kennedy, J. F. Malone, C. C. R. Allen, and H. Dalton. 2003. Dioxygenase-catalysed oxidation of monosubstituted thiophenes: sulfoxidation versus dihydrodiol formation. Org. Biol. Chem. 1:984-994. [DOI] [PubMed] [Google Scholar]
- 6.Brunel, J. M., P. Diter, M. Duetsch, and H. B. Kagan. 1995. Highly enantioselective oxidation of sulfides mediated by a chiral titanium complex. J. Org. Chem. 60:8086-8088. [Google Scholar]
- 7.Carreno, M. C. 1995. Applications of sulfoxides to asymmetric synthesis of biologically active compounds. Chem. Rev. 95:1717-1760. [Google Scholar]
- 8.Cheesman, M. J., M. B. Kneller, E. J. Kelly, S. J. Thompson, C. K. Yeung, D. L. Eaton, and A. E. Rettie. 2001. Purification and characterization of hexahistidine-tagged cyclohexanone monooxygenase expressed in Saccharomyces cerevisiae and Escherichia coli. Protein Expr. Purif. 21:81-86. [DOI] [PubMed] [Google Scholar]
- 9.Dembitsky, V. M. 2003. Oxidation, epoxidation and sulfoxidation reactions catalysed by haloperoxidases. Tetrahedron 59:4701-4720. [Google Scholar]
- 10.Fernández, I., and N. Khiar. 2003. Recent developments in the synthesis and utilization of chiral sulfoxides. Chem. Rev. 103:3651-3705. [DOI] [PubMed] [Google Scholar]
- 11.Folsom, B. R., D. R. Schieche, P. M. Digrazia, J. Werner, and S. Palmer. 1999. Microbial desulfurization of alkylated dibenzothiophenes from a hydrodesulfurized middle distillate by Rhodococcus erythropolis I-19. Appl. Environ. Microbiol. 65:4967-4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holland, H. L., L. J. Allen, M. J. Chernishenko, M. Diez, A. Kohl, J. Ozog, and J. X. Gu. 1997. Side chain oxidation of aromatic compounds by fungi. 7. A rationale for sulfoxidation, benzylic hydroxylation, and olefin oxidation by Mortierella isabellina. J. Mol. Catal. B 3:311-324. [Google Scholar]
- 13.Holland, H. L., F. M. Brown, A. Kerridge, P. Penkos, and J. Arensdor. 2003. Biotransformation of sulfides by Rhodococcus erythropolis. J. Mol. Catal. B 22:219-223. [Google Scholar]
- 14.Kamerbeek, N. M., A. J. J. Olsthoorn, M. W. Fraaije, and D. B. Janssen. 2003. Substrate specificity and enantioselectivity of 4-hydroxyacetophenone monooxygenase. Appl. Environ. Microbiol. 69:419-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly, D. R., C. J. Knowles, J. G. Mahdi, I. N. Taylor, and M. A. Wright. 1996. The enantioselective oxidation of sulfides to sulfoxides with Acinetobacter sp. NCIMB 9871, Pseudomonas sp. NCIMB 9872, Xanthobacter autotrophicus DSM 731 (NCIMB 10811) and the Black yeast NV-2. Tetrahedron Asymmetry 7:365-368. [Google Scholar]
- 16.Kirimura, K., T. Furuya, R. Sato, Y. Ishii, K. Kino, and S. Usami. 2002. Biodesulfurization of naphthothiophene and benzothiophene through selective cleavage of carbon-sulfur bonds by Rhodococcus sp. strain WU-K2R. Appl. Environ. Microbiol. 68:3867-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Legros, J., J. R. Dehli, and C. Bolm. 2005. Applications of catalytic asymmetric sulfide oxidations to the syntheses of biologically active sulfoxides. Adv. Synth. Catal. 347:19-31. [Google Scholar]
- 18.Mata, E. G. 1996. Recent advances in the synthesis of sulfoxides from sulfides. Phosphorus 117:231-286. [Google Scholar]
- 19.Nakamura, S., Y. Watanabe, and T. Toru. 2000. Extremely efficient chiral induction in conjugate additions of p-tolyl α-lithio-β-(trimethylsilyl)ethyl sulfoxide and subsequent electrophilic trapping reactions. J. Org. Chem. 65:1758-1766. [DOI] [PubMed] [Google Scholar]
- 20.Pasta, P., G. Carrea, H. L. Holland, and S. Dallavalle. 1995. Synthesis of chiral benzyl alkyl sulfoxides by cyclohexanone monooxygenase from Acinetobacter NCIMB 9871. Tetrahedron Asymmetry 6:933-936. [Google Scholar]
- 21.Pinedo-Rivilla, C., J. Aleu, and I. G. Collado. 2007. Enantiomeric oxidation of organic sulfides by the filamentous fungi Botrytis cinerea, Eutypa lata and Trichoderma viride. J. Mol. Catal. B 49:18-23. [Google Scholar]
- 22.Ricci, L. C., J. V. Comasseto, L. H. Andrade, M. Capelari, Q. B. Cass, and A. L. M. Porto. 2005. Biotransformations of aryl alkyl sulfides by whole cells of white-rot Basidiomycetes. Enzyme Microb. Technol. 36:937-946. [Google Scholar]
- 23.Umemura, K., H. Matsuyama, N. Watanabe, M. Kobayashi, and N. Kamigata. 1989. Asymmetric alkylation of β-keto esters with optically active sulfonium salts. J. Org. Chem. 54:2374-2383. [Google Scholar]
- 24.Zambianchi, F., M. W. Fraaije, G. Carrea, G. Gonzalo, C. Rodríguez, V. Gotor, and G. Ottolina. 2007. Titration and assignment of residues that regulate the enantioselectivity of phenylacetone monooxygenase. Adv. Synth. Catal. 349:1327-1331. [Google Scholar]
- 25.Zhang, Q., and Y. K. Wu. 2007. Further explorations on bridged 1,2,4-trioxanes. Tetrahedron 63:10189-10201. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.