Abstract
In this work we investigated the role of the tyrosine decarboxylation pathway in the response of Enterococcus faecium E17 cells to an acid challenge. It was found that 91% of the cells were able to remain viable in the presence of tyrosine when they were incubated for 3 h in a complex medium at pH 2.5. This effect was shown to be related to the tyrosine decarboxylation pathway. Therefore, the role of tyrosine decarboxylation in pH homeostasis was studied. The membrane potential and pH gradient, the parameters that compose the proton motive force (PMF), were measured at different pHs (pH 4.5 to 7). We obtained evidence showing that the tyrosine decarboxylation pathway generates a PMF composed of a pH gradient formed due to proton consumption in the decarboxylation reaction and by a membrane potential which results from electrogenic transport of tyrosine in exchange for the corresponding biogenic amine tyramine. The properties of the tyrosine transporter were also studied in this work by using whole cells and right-side-out vesicles. The results showed that the transporter catalyzes homologous tyrosine/tyrosine antiport, as well as electrogenic heterologous tyrosine-tyramine exchange. The tyrosine transporter had properties of a typical precursor-product exchanger operating in a proton motive decarboxylation pathway. Therefore, the tyrosine decarboxylation pathway contributes to an acid response mechanism in E. faecium E17. This decarboxylation pathway gives the strain a competitive advantage in nutrient-depleted conditions, as well as in harsh acidic environments, and a better chance of survival, which contributes to higher cell counts in food fermentation products.
Tyramine is one of the toxicologically important biogenic amines and is formed in foods by the enzymatic action of tyrosine decarboxylase (TDC) produced by food-related bacteria that are often present in fermented meat products (10, 26). Since it is a potent vasoconstrictor, tyramine can induce hypertension, migraines, brain hemorrhage, and heart failure when high concentrations are present in an organism (17, 26). Tyramine is broken down in mammals by monoamine oxidase (MAO)-catalyzed oxidative deimination (18). However, the detoxifying mechanisms in humans are not sufficient when the intake in a diet is too high, when individuals are allergic, and when patients consume drugs that act on MAO inhibitors (anti-Parkinson disease drugs and antidepressants that inhibit MAO) (24, 26). For this reason it is understandable that from the viewpoint of food safety it is important that only a fraction of the strains in a given microbial genus or species are able to decarboxylate tyrosine. In fact, the tyrosine decarboxylation pathway is present only in some species of lactic acid bacteria (LAB) and is considered strain specific rather than species specific (28). Due to the high sequence identity of the genes coding for the decarboxylases, horizontal gene transfer has been proposed as the mechanism for dissemination of these genes between LAB (8, 28).
Recently, genes encoding bacterial TDCs were identified in various lactic acid bacteria, including Enterococcus faecalis, Lactobacillus brevis, and Lactococcus lactis. It was also reported previously (5) that in L. brevis and L. lactis the decarboxylase gene was located in an operon containing four genes; the tdc gene was preceded by a gene homologous to genes coding for tyrosyl-tRNA synthetases and was followed by two genes coding for secondary transporters, a putative tyrosine transporter and a putative Na+:H+ antiporter.
Because decarboxylase enzymes are induced at acidic pHs (1, 2, 6, 14), it is commonly accepted that the decarboxylation pathways are activated to increase the acid resistance of cells by maintaining the cellular pH homeostasis when cells are subjected to acid stress (9, 21, 28). Recently, an acid resistance locus was reported for L. brevis, and it was shown to contain genes that form two distinct operons encoding amino acid decarboxylation pathways (14) that are putatively involved in acid resistance mechanisms.
Besides the role that amino acid decarboxylation pathways play as a response to acid stress, these pathways can also be responsible for generation of a proton motive force (PMF). The electrochemical gradient of protons across the cytoplasmic membrane is a major store of free energy in the bacterial cell. Usually, a PMF is generated by translocation of protons against the gradient across the cell membrane, which results in the two components of the PMF, a membrane potential formed by the charge gradient and a pH gradient (ΔpH) due to proton gradients (25).
In some LAB there is a mechanism for PMF generation that involves the action of secondary transporters rather than primary pumps, and therefore this PMF generation is called secondary PMF generation (6, 8, 27). Mechanisms for generation of a PMF involving amino acid decarboxylation pathways have been described for several amino acid-biogenic amine pairs, such as histidine-histamine in Lactobacillus buchneri (19) and tyrosine-tyramine in L. brevis (28).
The simplest type of amino acid decarboxylation pathway involves two proteins, a decarboxylase and a transporter protein. The former protein converts the amino acid in the cytoplasm into the corresponding biogenic amine and carbon dioxide, whereas the latter protein is responsible for the uptake of the amino acid from the medium and excretion of the corresponding biogenic amine in the exchange mode (5, 12, 13, 14, 28). Since a proton is consumed in the amino acid decarboxylation reaction, the decarboxylation contributes to the intracellular pH homeostasis triggered when cells are exposed to acidic environments (4, 6, 7, 19). Moreover, the amino acid/biogenic amine antiporter is able to increase the membrane potential provided the transporter is an electrogenic precursor-product exchanger.
A similar function for the tyrosine decarboxylation pathway in Enterococcus faecium E17 is proposed here. A role for tyrosine decarboxylation in pH homeostasis has been proposed for other genera of LAB, but this pathway has never been evaluated for Enterococcus spp.
In this study we evaluated the role of the tyrosine transporter and showed that in E. faecium E17 transport of tyrosine in exchange for the corresponding biogenic amine tyramine generates a PMF. We also demonstrated that this tyrosine transporter translocates a net positive charge across the membrane during exchange.
As far as we know, this is first time that such a study has been performed to demonstrate the role of the tyrosine decarboxylation pathway in the generation of a PMF and as a mechanism for an acid challenge response in an Enterococcus sp. strain isolated from a fermented meat product.
MATERIALS AND METHODS
Materials.
l-[U-14C]tyrosine (483 mCi/mmol) was purchased from Amersham Biosciences (Piscataway, NJ). The 2′,7′-bis-(2-carboxyethyl)-5(and-6)-carboxyfluorescein (BCECF) and 3,3′-dipropylthiocarbocyanine iodine [DiSC3(5)] probes were obtained from Molecular Probes (Eugene, OR). The ATPase inhibitor N,N′-dicyclohexylcarbodiimide (DCCD), l-tyrosine, tyramine, valinomycin, and nigericin were purchased from Sigma (Sigma Aldrich, Germany). Brain heart infusion (BHI) broth was obtained from Oxoid (Hampshire, United Kingdom). A BCA protein assay kit was purchased from Pierce (Rockford, IL). All other chemicals were reagent grade and were obtained from commercial sources.
Bacterial strain and growth conditions.
The tyramine producer E. faecium strain E17 used in this work was isolated from a fermented sausage from Portugal and has been deposited in the IBET/ITQB culture collection. This strain was routinely grown at 37°C without agitation in BHI broth prepared according to the manufacturer's instructions. Where indicated, tyrosine and pyridoxal phosphate were added to the growth medium to final concentrations of 5 and 1 mM, respectively.
Frozen stocks of the strain were prepared from cultures grown overnight under the conditions described above and were stored at −20°C with 50% (vol/vol) glycerol until they were needed. A preculture was prepared by diluting 200 μl of a frozen stock in 5 ml of fresh medium.
Acid challenge assays.
Acid challenge assays were performed as described by Iyer et al. (9), with some modifications. Briefly, cells were grown overnight in BHI medium. Stationary-phase cultures were diluted 1:1,000 into prewarmed BHI medium at pH 2.5 that was not supplemented with tyrosine (control) or was supplemented with 5 mM tyrosine and then incubated at 37°C. At time zero and after 3 h, 100-μl aliquots were serially diluted, and 100 μl of each dilution was plated on BHI agar plates in triplicate. The numbers of CFU were determined, and the percent survival was calculated by comparison with the time-zero data. The results presented below are the means of three experiments.
To check the capacity of strain E17 to increase the pH by tyrosine decarboxylation, cells were grown overnight as described above, pelleted by centrifugation (10,000 × g at 4°C for 10 min), washed with 10 mM potassium phosphate buffer (pH 5.5), and resuspended in the same buffer (without tyrosine or supplemented with 5 mM tyrosine) to obtain an optical density at 600 nm (OD600) of 0.6 to 0.8. At time intervals 1-ml samples were removed and centrifuged, and the supernatant buffer pH was determined.
Measurement of the internal pH and membrane potential.
Cells were grown until OD600 was 0.6 to 0.8. Then 20 ml of the culture was centrifuged (10,000 × g at 4°C for 10 min), and the cells were washed twice with buffer and then resuspended in 15 μl of the same buffer. The buffers used in the assays were 50 mM potassium phosphate buffer and 50 mM sodium phosphate buffer (pH 4.5, 5.5, and 7). Glucose, l-tyrosine, and tyramine were added at final concentrations of 150, 0.75, and 1.5 mM, respectively, as indicated below. Valinomycin was added at a final concentration of 75 μM. To inhibit the activity of FoF1 ATPase, cells were preincubated for 30 min at 37°C with 100 μM (final concentration) DCCD.
The intracellular pH was determined by loading the cells with the pH-sensitive fluorescent probe BCECF as follows. Briefly, 5 μl of 10 mM BCECF was added to 15 μl of a cell suspension, prepared as described above, and then 2.5 μl of 0.5 M HCl was added to shock the probe into the cells. The suspension was left for 5 min at room temperature in the dark, after which 500 μl of the appropriate ice-cold buffer (50 mM) was added. The cells were pelleted by short spin centrifugation (13,200 × g, 1 min), resuspended in 100 μl of buffer, and kept on ice. In each experiment, 30 μl of the BCECF-containing cells was diluted in 2 ml of buffer in a 3-ml cuvette equilibrated at 30°C. The cells in the cuvette were stirred constantly during acquisition of fluorescence data. The fluorescence signal was determined every second.
The intracellular pH was correlated with the fluorescence signal using BCECF-containing cells in 2 ml of 10 mM phosphate buffer (pH 5.5) to which 40 μl of Triton X-100 (Sigma), 75 μM valinomycin, and 75 μM nigericin were added. Successive additions of 4 μl of a 0.1 M NaOH solution were made, and the pH inside the cuvette was determined with a microelectrode (Crison). The fluorescence signal was recorded, and a calibration plot of fluorescence versus pH was drawn. Fluorescence emission at 529 nm (with excitation at 503 nm) was determined with an SLM-AMINCO spectrofluorometer.
The membrane potential was measured qualitatively using the fluorescent probe DiSC3(5). This probe partitions in the membrane, and its fluorescence is quenched by a difference in the electrical potential across the membrane (15). The intensity of the probe fluorescence is sensitive to changes in the membrane potential. Changes in the membrane potential, as determined by DiSC3(5) fluorescence, were recorded as a function of time. An increase in the electrical potential across the membrane correlated with a decrease in fluorescence intensity. For each experiment, 30 μl of the cell suspension was added to 2 ml of buffer, as described above, and 5 μl of a 3 mM solution of DiSC3(5) prepared in dimethyl sulfoxide was added. The fluorescence signal was sampled every second (emission at 680 nm and excitation at 653 nm).
Preparation of RSO membrane vesicles.
Right-side-out (RSO) membrane vesicles of E. faecium strain E17 were prepared by using osmotic shock as previously described (20), with some modifications. Briefly, 20 ml of a culture grown in BHI medium supplemented with 5 mM tyrosine and 1 mM pyridoxal phosphate was diluted into 2 liters of fresh medium. After 16 to 18 h of growth, cells were harvested, washed twice with 100 mM potassium phosphate buffer (pH 7.0), and resuspended in 17.5 ml of the same buffer containing 10 mM MgSO4 and 20 mg/ml lysozyme. The cell suspension was incubated for 1 h at 30°C. With continuous stirring, 16 ml of K2SO4 was then added slowly until the final concentration was 0.36 M. Next, the suspension was slowly diluted into 120 ml of buffer containing 50 μg/ml of RNase and 50 μg/ml of DNase. The suspension was incubated for 20 min at 30°C with continuous stirring, after which K-EDTA (pH 7.5) was added to a final concentration of 20 mM. After 10 min of incubation at 30°C, enough MgSO4 was added to obtain a final concentration of 25 mM. The suspension was centrifuged for 30 min at 750 × g at 4°C, and the supernatant was centrifuged for 30 min at 45,000 × g at 4°C. The pellet was resuspended in 30 ml of 50 mM potassium phosphate buffer (pH 6) and then centrifuged for 60 min at 750 × g at 4°C. The RSO vesicles were collected from the supernatant by centrifugation for 30 min at 45,000 × g at 4°C, and the pellet obtained was resuspended in 2 ml of 50 mM potassium phosphate buffer (pH 6.0). Aliquots (100 μl) were rapidly frozen in liquid nitrogen and stored at −80°C until they were used.
Protein quantification.
To quantify the total protein in the vesicles prepared from cells, a 100-μl aliquot of vesicles was diluted into 1 ml of 50 mM potassium phosphate buffer (pH 7). For whole cells, cells from a 5-ml culture were harvested by centrifugation (13,200 × g, 8 min,), washed with 50 mM potassium phosphate buffer, and then diluted with 1 ml of the same buffer. In both cases (cells and vesicles) sonication was then performed on ice at 50% potency for 30 s followed by a 30-s pause for a total of 3 min. The total protein was then quantified using the BCA protein assay kit from Pierce with bovine serum albumin as the protein standard.
Transport assays with whole cells.
E. faecium E17 was grown in BHI medium containing 1 mM pyridoxal phosphate with (tyr+ cells) or without (tyr− cells) 5 mM tyrosine. Cells were harvested and washed once with ice-cold 50 mM potassium phosphate buffer (pH 6.0) and resuspended to an OD600 of approximately 20. Following addition of 0.2% (wt/vol) glucose, a 100-μl aliquot of suspended resting cells was incubated for 5 min at room temperature with constant stirring. At time zero, enough l-[U-14C]tyrosine was added to obtain a final concentration of 2 μM. Uptake was stopped at the times indicated below by addition of 2 ml of an ice-cold 0.1 M LiCl solution, which was followed immediately by filtration through a 0.45-μm-pore-size nitrocellulose filter (BA85; Schleicher & Schuell GmbH). The filter was washed once with 2 ml of ice-cold 0.1 M LiCl and submerged in scintillation liquid (Scint Liquid; Perkin Elmer), and the retained radioactivity was counted with a Tri-Carb 2000CA liquid scintillation counter (model LS-6500; Beckman Coulter). The background was estimated by adding 2 μl of the radiolabeled substrate to the cell suspension immediately after addition of 2 ml of ice-cold LiCl, followed by filtering. In the chase experiments, tyramine and/or unlabeled (cold) tyrosine was added after 1 min to a final concentration of 0.5 mM. The results described below are the means of three independent assays.
Transport assays with RSO membranes.
Concentrated RSO membranes were loaded with the appropriate buffer by 1 h of incubation at room temperature. Then 2-μl aliquots of concentrated membranes were diluted 100-fold into 50 mM phosphate buffer containing labeled or unlabeled substrates. The final protein concentration in the samples was approximately 140 μg/ml. Transport was quenched and samples were processed as described above.
In PMF-driven uptake assays, membrane vesicles were preloaded with 50 mM potassium phosphate buffer (pH 6.0) containing 100 mM potassium acetate in the presence of 150 μM valinomycin. Membranes were then diluted into sodium phosphate buffer (pH 6.0) containing 50 mM Na2SO4 and 3 μM l-[U-14C]tyrosine. In efflux assays, vesicles were preloaded with 50 mM potassium phosphate buffer (pH 6.0) containing 250 μM l-[U-14C]tyrosine in the presence of 150 μM valinomycin and 75 μM nigericin. For exchange or counterflow experiments, membrane vesicles were loaded with the same buffer containing 250 μM labeled tyrosine or tyramine. In the exchange assays, vesicles were diluted into buffer containing 250 μM l-tyrosine or tyramine. In the counterflow assays the membranes were diluted into buffer containing 100 μM tyramine.
In the exchange experiments the results were analyzed by plotting the release of radiolabel from the membranes versus time.
RESULTS
Tyrosine decarboxylation in the acid tolerance response of E. faecium E17.
E. faecium E17 cells were grown in BHI medium, and then survival in the same medium at pH 2.5 was tested. After 3 h of incubation at 37°C, the number of CFU of control cells (in medium without supplemental tyrosine) was 44% less than the number at time zero (data not shown). However, when cells were incubated in the same medium but the medium was supplemented with 5 mM tyrosine, the level of survival was 91%. These results clearly indicate that the presence of tyrosine had a positive effect on the acid tolerance response.
In another experiment, exponentially grown cells were incubated in 10 mM potassium phosphate buffer at pH 5.5 for 3 days (Fig. 1). When no tyrosine was added, the extracellular pH decreased to less than 5.4 within the first 3 h. This acidification indicated that there was production of lactic acid by metabolizing cells. With increasing time the external pH increased slowly, and it reached a value close to pH 5.45, which was maintained. However, when cells were incubated in the same conditions but the medium was supplemented with 5 mM tyrosine, no significant decrease in the external pH was observed. On the contrary, the external pH increased to almost 5.8. Alkalinization of the external medium (almost 0.3 pH unit in buffer) in the presence of tyrosine is a indication of tyrosine decarboxylation, and the extracellular pH alkalinization surely contributed to the increase in the survival of strain E. faecium E17 when it was exposed to an acid environment.
FIG. 1.
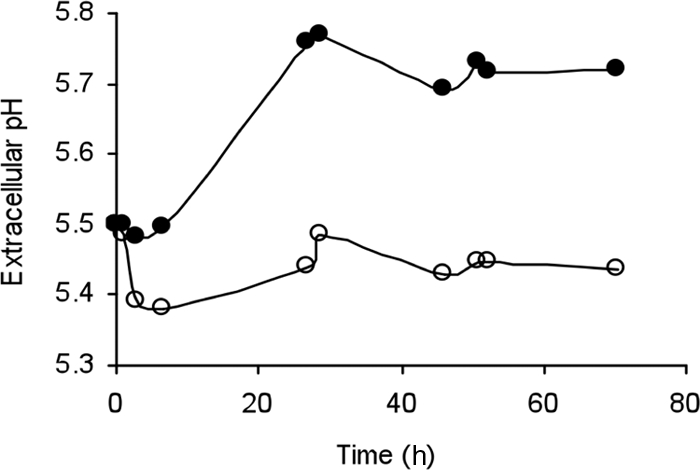
Cells of E. faecium E17 were resuspended and incubated in phosphate buffer (pH 5.5) that was not supplemented with tyrosine (○) or was supplemented with 5 mM tyrosine (•).
Role of tyrosine in intracellular pH homeostasis.
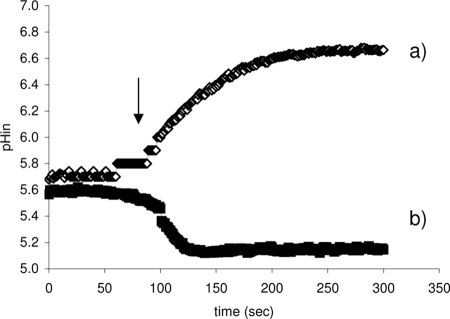
When exponentially growing cells of E. faecium E17 were resuspended in 50 mM potassium phosphate buffer at pH 4.5, they were able to maintain an intracellular pH between 5.6 and 5.7. When these cells were energized with glucose, an increase in the intracellular pH was observed (Fig. 2, line a). The fermentative glucose metabolism in Enterococcus spp. produces lactic acid, which leads to acidification of the cytoplasm, but the activity of FoF1 ATPase regulates the intracellular concentration of protons by extruding them to the extracellular medium. This results in a continuous increase in the intracellular pH. The role of FoF1 ATPase in the increase in the intracellular pH when there was glucose fermentation was confirmed when cells were pretreated with DCCD, a strong inhibitor of FoF1 ATPase activity. In this situation (Fig. 2, line b) the intracellular concentration of H+ increased continuously, which decreased the cytoplasmic pH.
FIG. 2.
Variation of the intracellular pH (pHin) of resting cells of E. faecium E17 when glucose was added. Cells were suspended in 50 mM potassium phosphate buffer (pH 4.5). At the time indicated by the arrow glucose was added. Control cells were preincubated for 30 min at 37°C with only phosphate buffer (line a). Cells whose FoF1ATPase activity was inhibited were preincubated for 30 min at 37°C with phosphate buffer containing 30 mM DCCD (line b).
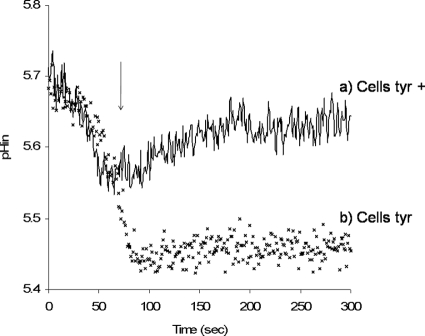
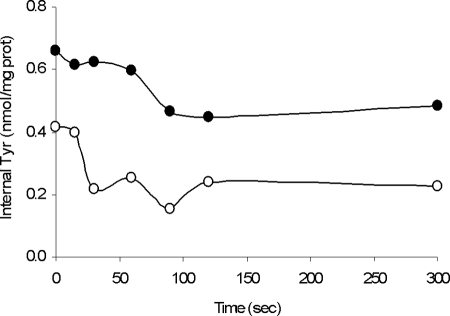
To examine the role of tyrosine decarboxylation in maintenance of the intracellular pH of resting cells of strain E17, two preparations of cells were used: cells grown in the presence of 5 mM tyrosine (Tyr+ cells) and cells grown in medium without amino acid supplementation (Tyr− cells) (Fig. 3). Addition of tyrosine to E17 cells resuspended in potassium phosphate buffer at pH 4.5 enabled them to stabilize their internal pH at pH 5.45 (for Tyr− cells) (Fig. 3). In the case of Tyr+ cells, the cells were able to increase their internal pH to pH 5.6 to 5.7, creating a ΔpH of 1.2 pH units (Fig. 3). In this case the role of tyrosine decarboxylation in the maintenance of intracellular homeostasis was proven to be independent of the FoF1ATPase activity. In fact, the assay was repeated with cells pretreated with DCCD. When the FoF1ATPase activity of cells was inhibited, there was no difference between these cells and cells having an active FoF1ATPase (data not shown), showing that the effect of tyrosine was the same whether FoF1ATPase was active or not. These results indicate that the decrease in the intracellular concentration of H+ is not dependent on the extrusion of H+ by FoF1ATPase activity but is due to proton consumption in the tyrosine decarboxylation reaction.
FIG. 3.
Effect of tyrosine addition (at ∼100 s) to resting cells of E. faecium E17 at pH 4.5 when cells were grown in the presence of tyrosine (Cells tyr+) (line a) and in the absence of tyrosine (Cells tyr−) (×) in the growth medium. At the time indicated by the arrow, tyrosine was added to the reaction mixture to a final concentration of 0.75 μM. pHin, intracellular pH.
When assays were performed at external pHs of 5.5 and 7, the effect of addition of tyrosine was always an increase in the intracellular pH (Table 1). However, the values reported for the ΔpH in Table 1 show that the increase in the internal pH was always dependent on the external pH; i.e., there was a decrease in the ΔpH when the external pH increased. Also, at an external pH of 7 the ΔpH that formed upon tyrosine addition was very small (0.04 pH unit) and even inverted (pH lower inside) compared to the data obtained with an external pH of 4.5 or 5.5 (pH higher inside).
TABLE 1.
Effect of addition of tyrosine on the intracellular pH of resting cells of E. faecium E17 at different external pH values
| Cells | ΔpHa
|
||
|---|---|---|---|
| External pH 4.5 | External pH 5.5 | External pH 7 | |
| Tyr− | −0.95 | −0.15 | 0.04 |
| Tyr+ | −1.20 | −0.30 | 0.04 |
ΔpH = pHout − pHin.
A comparison of the results obtained for Tyr− cells and Tyr+ cells showed that the ΔpH values obtained after tyrosine addition were (for pH 4.5 and 5.5) one-half the ΔpH values obtained for Tyr− cells. As expected, the presence of tyrosine in the growth medium seemed to have a positive effect on the activity of the enzymes of the tyrosine decarboxylation pathway, and this was reflected in the rate of medium alkalinization.
Role of tyrosine decarboxylation in membrane potential generation.
The results obtained for the membrane potential parallel those obtained for the transmembrane ΔpH. In a control assay, addition of glucose to resting cells in buffer at pH 4.5 resulted in an increase in the membrane potential. Glucose metabolism induces greater accumulation of positive charges outside the cells, as a result of proton extrusion from the cell, causing an increase in the membrane potential. However, this effect is transient since the membrane potential is subsequently converted into a ΔpH. When cells preincubated with DCCD were used, an increase in the membrane potential was observed. The inhibition of FoF1ATPase caused an increase in the positive charges that accumulated inside the cell, and as a consequence the membrane potential increased. As expected, this increase was less in more acidic environments. In fact, when tyrosine was added to resting cells of E. faecium E17, a small but significant increase in the membrane potential was observed.
Role of Na+ ions in the tyrosine decarboxylation pathway of E. faecium E17.
A gene coding for a putative Na+:H+ antiporter was reported to be present in the operon containing the tyrosine decarboxylase gene in L. brevis (5, 14). This observation suggests that Na+ ions have a role in the tyrosine decarboxylation pathway (11). To clarify this, both the ΔpH and membrane potential were measured in cells resuspended in sodium phosphate buffer instead of potassium phosphate buffer.
The first results (Fig. 4) indicated that cells in sodium phosphate buffer at pH 4.5 were able to maintain a higher intracellular pH (pH 5.7) than cells resuspended in potassium phosphate buffer (pH 5.5). The presence of an Na+:H+ antiporter could, therefore, catalyze uptake of Na+ in exchange for H+ extrusion. Although electroneutral, this antiport activity would increase the capacity to extrude protons even when FoF1ATPase was inhibited (data not shown).
FIG. 4.
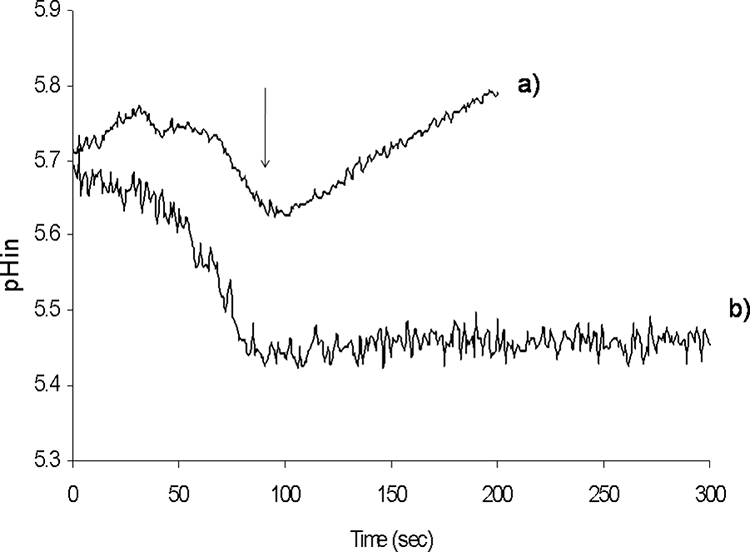
Effect of tyrosine addition on the intracellular pH in sodium phosphate buffer (line a) and in potassium phosphate buffer (line b). pHin, intracellular pH.
Tyrosine uptake by resting cells of E. faecium E17.
Tyrosine uptake was demonstrated by measuring the uptake of l-[U-14C]tyrosine by resting cells of E. faecium E17. Cells were energized with 20 mM glucose, and the uptake was monitored over time in Tyr+ cells and Tyr− cells.
At an initial l-[U-14C]tyrosine concentration of 2 μM, the Tyr+ cells took up the labeled tyrosine at a lower initial rate than the Tyr− cells (1.5 pmol min−1 · mg−1 of cell protein for Tyr+ cells compared to 1.8 pmol min−1 · mg−1 of cell protein for Tyr− cells). However, because the amount of total cell protein was larger in the Tyr+ cells, the actual rate of tyrosine uptake by Tyr+ cells could have been underestimated. However, the intracellular concentrations of labeled tyrosine accumulated by Tyr+ cells were higher than the intracellular concentrations of labeled tyrosine accumulated by Tyr− cells (data not shown).
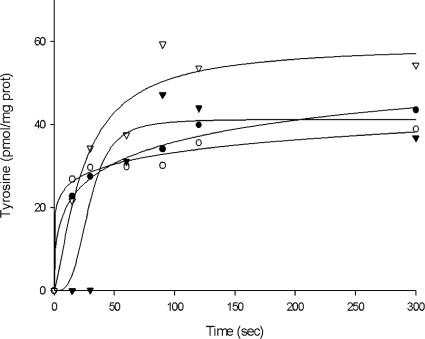
Tyrosine/tyramine antiport was also demonstrated using cells previously incubated with 0.5 mM tyramine (Fig. 5). Valinomycin and nigericin were added in order to completely eliminate the membrane potential. After 1 h of incubation at 37°C, both Tyr+ and Tyr− cells were diluted in buffer containing 2 μM l-[U-14C]tyrosine, and the uptake of labeled tyrosine was monitored over time. Again, the results showed that cells grown in the presence of tyrosine had a higher rate of tyrosine uptake (1.4 pmol min−1 · mg−1 of cell protein for Tyr+ cells, compared to 0.9 pmol min−1 · mg−1 of cell protein for Tyr− cells). In addition, it was observed that when cells were preloaded with tyramine, the uptake of tyrosine was much greater. This strongly suggests that there is a tyrosine/tyramine antiport mechanism.
FIG. 5.
Uptake of l-[U-14C]tyrosine by resting cells of E. faecium E17 (circles) or cells preincubated with 5 mM tyramine (triangles). The cells used were grown in the absence of tyrosine (open symbols) or in the presence of 5 mM tyrosine (filled symbols) in the growth medium.
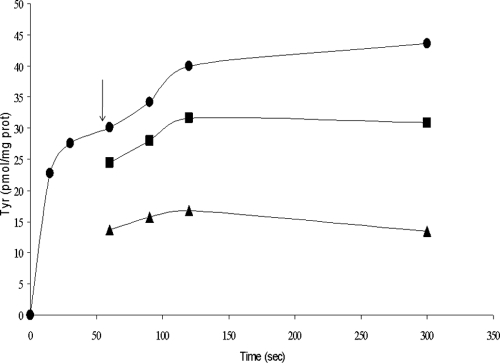
Subsequent chase experiments showed that the tyrosine transporter catalyzed the tyrosine-tyrosine exchange as well as the tyrosine-tyramine exchange (Fig. 6). Cells were allowed to take up l-[U-14C]tyrosine for 1 min, after which 0.5 μM unlabeled tyrosine or tyramine was added to the cell suspension. In a control assay cells preloaded with l-[U-14C]tyrosine were diluted in buffer alone. Addition of tyrosine and tyramine to cells resulted in immediate release of previously accumulated l-[U-14C]tyrosine.
FIG. 6.
Chase experiments in which resting cells were allowed to take up labeled tyrosine (•). After 1 min (indicated by the arrow) cells were diluted in buffer with 5 mM unlabeled tyrosine (▴) or in buffer with 5 mM unlabeled tyramine (▪). prot, protein.
Tyrosine transport in RSO vesicles of E. faecium E17.
RSO vesicles are well-defined experimental systems used to study transport activities of secondary transporters. The effect of an artificially imposed PMF on tyrosine transport was investigated. An electrical potential across the membrane with the inside negative can be created by diluting K+-loaded membranes into a K+-free (i.e., Na+) buffer in the presence of the K+ ionophore valinomycin. Dilution into an Na+ buffer results in generation of both a chemical gradient of Na+ across the membrane and a membrane potential. A ΔpH across the membrane with the inside alkaline can be created by diluting acetate-loaded membranes into a solution containing a less permeable ion, such as sulfate. Acetate is membrane permeable only in its protonated state; i.e., acetic acid results in quantitative removal of protons from the intravesicular space. Simultaneous imposition of both a K+ diffusion gradient and an acetate diffusion gradient resulted in transient generation of a PMF. Under these conditions it was observed that RSO vesicles of E. faecium E17 were able to take up tyrosine at low but significant levels (Fig. 7).
FIG. 7.
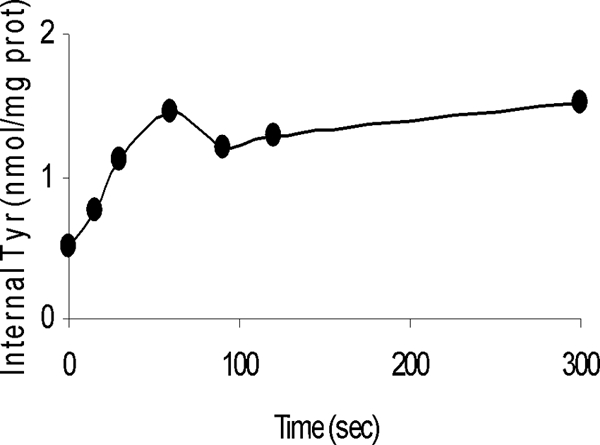
Uptake of labeled tyrosine by vesicles of E. faecium E17 in the presence of an imposed PMF. prot, protein.
The exchange capacity of this tyrosine transporter was also studied using RSO vesicles preequilibrated with a known concentration of labeled tyrosine and labeled tyrosine plus tyramine. Moreover, the ionophores valinomycin and nigericin were added in combination to completely collapse the PMF (Fig. 8). When vesicles were diluted in buffer alone, efflux of tyrosine was observed. However, when vesicles previously loaded with tyrosine and tyramine were diluted in buffer with the same concentration of labeled tyrosine, an immediate increase in the internal pool of labeled tyrosine was observed, which corresponded to the exchange between internal tyramine and labeled external tyrosine. Soon the internal and external pools of tyrosine reached equilibrium.
FIG. 8.
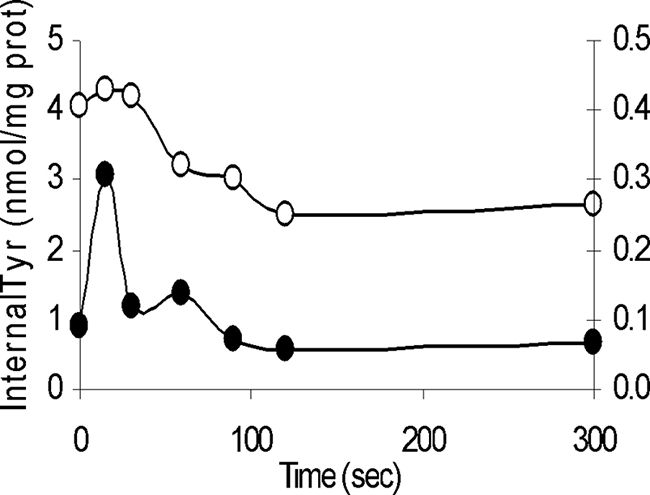
Tyrosine efflux assays. Vesicles were preequilibrated with labeled tyrosine (○) and with labeled tyrosine plus tyramine (•) (y axis on the right). prot, protein.
Dilution of RSO vesicles preloaded with l-[U-14C]tyrosine in buffer containing the same concentration of unlabeled tyrosine and tyramine (Fig. 9) confirmed the observations obtained in the chase experiments performed with whole cells.
FIG. 9.
Tyrosine exchange in vesicles preloaded with l-[U-14C]tyrosine and diluted in buffer with unlabeled tyrosine (•) and in buffer with tyramine (○). prot, protein.
Both external tyrosine and external tyramine caused the release of internal l-[U-14C]tyrosine from RSO membranes. In fact, in the presence of external tyrosine essentially all labeled tyrosine was released from the vesicles within 30 s, while addition of external tyramine did not result in significant release during the first 100 s. The release of internal labeled tyrosine is driven by an exchange between internal labeled substrate and external unlabeled substrate.
The heterologous exchange hypothesis was also confirmed (Fig. 10) when vesicles that were preloaded with 25 μM labeled tyrosine were diluted in buffer containing tyramine. Efflux of the internal labeled tyrosine pool was observed in exchange with tyramine.
FIG. 10.
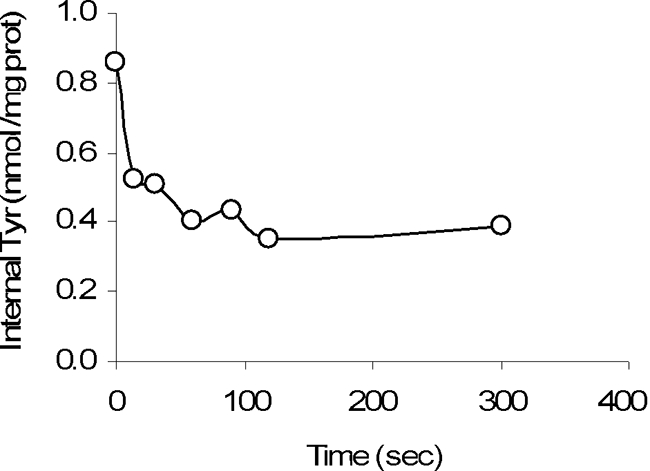
Counterflow experiment. Vesicles were incubated with labeled tyrosine and then diluted in buffer containing tyramine. prot, protein.
DISCUSSION
Tyramine, which results from decarboxylation of the amino acid tyrosine, is the biogenic amine most commonly found in fermented meat products (24, 26). Tyrosine decarboxylation is catalyzed by a specific enzyme, TDC, and the sequence of this enzyme in E. faecium strain E17 has been determined (C. I. Pereira, I. Manarte, F. Gaspar, D. Matos, M. V. San Romão, and M. T. B. Crespo, submitted for publication). The tdc gene coding for TDC was previously reported to be in an operon with other genes of the pathway, including one gene coding for a tyrosine-tyramine exchanger in L. lactis (28).
It has been suggested that decarboxylation of amino acids by LAB might protect cells against intracellular acidification and be an acid tolerance response mechanism. pH regulation by a histidine/histamine antiporter has also been suggested for L. buchneri (19). It is commonly accepted that amino acid decarboxylation activity is strain dependent rather than related to species or even genera (28), which enables a strain with this activity to compete with other strains during the food fermentation process. Based on our results, we propose a putative role in acid resistance for the tyrosine decarboxylase system in E. faecium E17.
The acid tolerance response involves induction of acid shock genes and depends on several regulatory systems (1). In this study we elucidated a mechanism underlying the ability of E. faecium to survive in low-pH environments, an important and desirable characteristic in food strains. We showed that this mechanism is related to tyrosine decarboxylation.
The fact that alkalinization of the cytoplasm due to the tyrosine decarboxylation reaction was a key factor for the cellular pH homeostasis of E. faecium E17 when it was subjected to acid stress was investigated further. Measurement of the intracellular pH after addition of tyrosine to resting cells of E. faecium E17 showed that tyrosine decarboxylation consumes intracellular protons. ATP formed in the glycolysis pathway may have many functions in the cell, including driving proton extrusion from cytoplasm and playing a role in cellular pH homeostasis. LAB are therefore able to regulate the intracellular pH at the expense of energy produced by substrate level phosphorylation. The role of the tyrosine decarboxylation pathway in the acid response of E. faecium E17 seems to be different and independent of FoF1ATPase activity. Our results showed that TDC was more active at acidic pHs, confirming previous reports for other LAB that amino acid decarboxylases are induced at low pHs (16). Tyrosine decarboxylation increases the ΔpH, a component of the PMF.
Also, Tyr+ cells showed a higher rate of pH increase upon addition of tyrosine than Tyr− cells. This result was anticipated, since other authors (13, 14, 16, 28) have reported that the rate of tyrosine decarboxylation is dependent on the TDC enzyme activity, which is induced by the presence of external tyrosine. A continuous cycle of proton consumption through amino acid decarboxylation raises the internal pH of cells exposed to acid. This increase in the intracellular pH is thought to be required for survival in extreme acid stress conditions. It has been proposed that the siphoning of intracellular protons enhances pH homeostasis and allows the cell to maintain an internal pH compatible with viability in E. faecium E17.
In the decarboxylation reaction tyrosine is converted into tyramine. At physiological pH tyramine has a positive charge, whereas tyrosine has no net charge. Under the assay conditions used, tyrosine/tyramine antiport is active, and tyramine is exported from the cytoplasm, increasing the concentration of positive charges outside the membrane. Consequently, the membrane potential increases. Moreover, during the decarboxylation reaction protons are consumed in the cytoplasm, and this should increase the membrane potential even more. However, one explanation for the fact that the increase in the membrane potential was not as significant as initially expected could be that although the intracellular consumption of protons due to tyrosine decarboxylation and tyramine (positively charged) extrusion from the cytoplasm would increase the positive charges outside, this effect would be simultaneously compensated for by the uptake of other cations.
Based on the results obtained in this study, the role of Na+ ions in the tyrosine decarboxylation pathway appears to be more important. These ions could regulate acid resistance and play important roles in housekeeping pH homeostasis systems. The presence of a gene coding for a putative Na+:H+ antiporter in an operon involved in the acid response of L. brevis (5, 14) suggests that Na+ ions also have an important role in the tyrosine decarboxylation pathway. Moreover, the roles of Na+ and K+ ions in pH homeostasis during growth at different pHs have also been reported (8). We demonstrated in this study that when tyrosine was added, cells incubated in sodium buffer (i.e., potassium-depleted conditions) exhibited a greater increase in the intracellular pH than cells suspended in potassium buffer. We believe that Na+ ions can function as counterions in an antiport mechanism that directly removes H+ from the cytoplasm. Alternatively, Na+ could act at the gene level by influencing the induction of key system components, as it does in other bacteria (23). Surprisingly, the Na+:H+ antiport system, which is thought to be important for pH homeostasis under pH conditions more suitable for growth, appeared to be unimportant in the tyrosine decarboxylation pathway experiments performed with E. faecium E17.
In the amino acid decarboxylation pathway, in addition to TDC, an antiporter that facilitates the coupled transport of amino acids and their decarboxylation products across the membrane is also important in PMF formation in E. faecium E17. The tyrosine transporter studied here was shown to catalyze tyrosine-tyramine exchange with high efficiency. In this exchange a membrane potential is generated during turnover due to the charge difference between the two transported substrates.
In summary, the results reported here support the hypothesis that there is a PMF pathway, as follows: (i) both tyrosine and its decarboxylation product, tyramine, are substrates of the transporter; (ii) the tyrosine transporter catalyzes exchange with tyramine more efficiently than unidirectional tyrosine transport; and (iii) tyrosine-tyramine exchange is electrogenic.
One physiological function of the tyrosine decarboxylation pathway in E. faecium E17 was demonstrated to be generation of a PMF at the expense of the free energy released in the decarboxylation reaction. The two components of the PMF, membrane potential and ΔpH, are generated separately in the two steps of the pathway, since at the physiological pH range studied tyramine is positively charged, while tyrosine has no net charge. Therefore, exchange of the two substrates in the absence of any cotransported ions is electrogenic. Turnover of the tyrosine transporter results in a membrane potential with physiological polarity (outside positive), since the monovalent, positively charged compound tyramine is exchanged for uncharged tyrosine. The decarboxylation of tyrosine catalyzed by TDC consumes a proton that alkalinizes the cytoplasm compared to the external medium. For each cycle of the pathway in which one external tyrosine molecule is converted to one external tyramine molecule, one positive charge is translocated across the membrane and one proton is removed from the cytoplasm, which is equivalent to pumping one proton across the membrane. Hence, the pathway functions as an indirect proton pump, as reported in other cases (17, 19, 22, 28).
Tyrosine accumulation in E. faecium E17 in response to a PMF was also demonstrated in this study. Transport down a tyrosine concentration gradient was evident, indicating that there was also uniport of tyrosine. However, tyrosine uptake is faster in the presence of tyramine. We cannot exclude the possibility that there are other transporters that are involved in the transport of tyrosine in the absence of tyramine. Here, we assumed that the antiporter catalyzes at least part of this transport. In fact, most secondary transport systems catalyze exchange much faster than uniport or symport systems (3, 22), which is the case for the tyrosine transporter in E. faecium strain E17.
These results are consistent with a tyrosine/tyramine antiport mechanism in which a net positive charge is transported with tyramine. It is not clear whether this transport system mediates only a strictly coupled antiport reaction or can also catalyze uniport or proton symport of these substrates. Exchange of internal and external substrates is a partial reaction of a symporter or uniporter, and it is believed that the bacterial precursor-product exchangers are unidirectional transporters that have been optimized to catalyze exchange. Precursor-product exchangers are members of transporter families that, in addition to exchangers, contain symporters, uniporters, and antiporters, indicating that they are structurally and mechanistically similar (17, 22).
Depending on the organism and the conditions, the primary goal of an amino acid decarboxylation pathway may be to prolong the period during which a sufficiently high intracellular pH is present or to be a source of metabolic energy. In the case of E. faecium E17 this is accomplished by the tyrosine decarboxylation pathway. Protons entering the cell are consumed by the decarboxylase reaction (17, 22). The pathway, which couples the decarboxylation reaction and exchange by the antiporter, promotes the uptake of extracellular tyrosine, its conversion into tyramine, the release of tyramine from the cell, and, importantly, the consumption of cytoplasmic protons, thereby generating a ΔpH across the membrane (6). In this way the tyrosine decarboxylase pathway in E. faecium E17 helps the organism regulate its cytoplasmic pH in acid environments.
Although such a model for the role of an amino acid decarboxylation pathway has been proposed by other authors for other genera of LAB, to the best of our knowledge, no model has been experimentally proven previously, particularly for a meat strain like an Enterococcus sp. strain. The ability of microorganisms to survive in acidic environment is important for both in vivo functions and fermentation stability. Therefore, mechanisms that contribute to the capacity of a microorganism to tolerate acidic pH are essential for the production and functionality of a microbial culture. Thus, it is timely to consider the mechanisms used by gram-positive organisms to protect themselves from the challenges posed by low-pH environments, such as food fermentation and exposure to gastric juice, and comment on the strategies by which these mechanisms can be aided or impeded. Acid tolerance is one of the desirable properties used to select potentially probiotic strains (2). Ultimately, because biogenic amines in general and tyramine in particular are such a health threat when they are present at high concentrations in fermented meat products, insight into the tyramine production pathway in LAB would help prevent accumulation of these products in fermented foods.
Acknowledgments
We acknowledge Fundação para a Ciência e Tecnologia (FCT) for providing Ph.D. grant BD/19135/2004 to Cristina Pereira and also for providing grant POCTI/AGR/39613/2001.
Footnotes
Published ahead of print on 14 November 2008.
REFERENCES
- 1.Azcarate-Peril, A., E. Altermman, R. L. Hoover-Fitzule, R. J. Cano, and T. R. Klaenhammer. 2004. Identification and inactivation of genetic loci involved with Lactobacillus acidophilus acid tolerance. Appl. Environ. Microbiol. 70:5315-5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker-Austin, C., and M. Dopson. 2007. Life at acid: pH homeostasis on acidophiles. Trends Microbiol. 15:165-171. [DOI] [PubMed] [Google Scholar]
- 3.Bandell, M., M. E. Lhote, C. Marty-Teysset, A. Veyrat, H. Prevost, V. Dartois, C. Divies, W. N. Konings, and J. S. Lolkema. 1998. Mechanism of citrate transporters in carbohydrate and citrate cometabolism in Lactococcus and Leuconostoc species. Appl. Environ. Microbiol. 64:1594-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bearson, S., B. Bearson, and J. W. Foster. 1997. Acid stress responses in enterobacteria. FEMS Microbiol. Lett. 147:173-180. [DOI] [PubMed] [Google Scholar]
- 5.Connil, N., Y. Le Breton, X. Dousset, Y. Auffray, A. Rince, and H. Prevust. 2002. Identification of the Enterococcus faecalis tyrosine decrboxylase operon involved in tyramine production. Appl. Environ. Microbiol. 68:3537-3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotter, P. D., and C. Hill. 2003. Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol. Mol. Biol. Rev. 67:429-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dimroth, P., and B. Schink. 1998. Energy conservation in the decarboxylation of dicarboxylic acids by fermenting bacteria. Arch. Microbiol. 170:69-77. [DOI] [PubMed] [Google Scholar]
- 8.Fernández, M., D. M. Linares, and M. A. Alvarez. 2004. Sequencing of the tyrosine decarboxylase cluster of Leuconostoc lactis IPLA 655 and the development of a PCR method for detecting tyrosine decarboxylating lactic acid bacteria. J. Food Prot. 67:2521-2529. [DOI] [PubMed] [Google Scholar]
- 9.Iyer, R., C. Williams, and C. Miller. 2003. Arginine-agmatine antiporter in extreme acid resistance in Escherichia coli. J. Bacteriol. 185:6556-6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komprda, T., R. Burdychová, V. Dohnal, O. Cwiková, P. Sládková, and H. Dvoracková. 2008. Tyramine production in Dutch-type semi-hard cheese from two different producers. Food Microbiol. 25:219-227. [DOI] [PubMed] [Google Scholar]
- 11.Kosono, S., S. Morotomi, M. Kitada, and T. Kudo. 1999. Analysis of a Bacillus subtilis homologue of the Na+/H+ antiporter gene which is important for pH homeostasis of alkaliphilic Bacillus sp. C-125. Biochim. Biophys. Acta Bioenerg. 1409:171-175. [DOI] [PubMed] [Google Scholar]
- 12.Lucas, P., and A. Lonvaud-Funel. 2002. Purification and partial gene sequence of the tyrosine decarboxylase of Lactobacillus brevis IOEB 9809. FEMS Microbiol. Lett. 211:85-89. [DOI] [PubMed] [Google Scholar]
- 13.Lucas, P., J. Landete, M. Cotton, E. Cotton, and A. Lonvaud-Funel. 2003. The tyrosine decarboxylase operon of Lactobacillus brevis IOEB 9809: characterization and conservation in tyramine-producing bacteria. FEMS Microbiol. Lett. 229:65-71. [DOI] [PubMed] [Google Scholar]
- 14.Lucas, P. M., V. S. Blancato, O. Claisse, C. Magni, J. S. Lolkema, and A. Lonvaud-Funel. 2007. Agmatine deiminase pathway genes in Lactobacillus brevis are linked to the tyrosine decarboxylation operon in a putative acid resistance locus. Microbiology 153:2221-2230. [DOI] [PubMed] [Google Scholar]
- 15.Magni, C., D. Mendoza, W. N. Konings, and J. S. Lolkema. 1999. Mechanism of citrate metabolism in Lactococcus lactis: resistance against lactate toxicity at low pH. J. Bacteriol. 181:1451-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marques, A. P., M. C. Leitão, and M. V. San Romão. 2008. Biogenic amines in wines: influence of oenological factors. Food Chem. 107:853-860. [Google Scholar]
- 17.Marty-Teysset, C., J. S. Lolkema, P. Scmitt, C. Divies, and W. N. Konings. 1995. Membrane potential generating transport of citrate and malate catalyzed by the citP gene product of Leuconostoc mesenteroides. J. Biol. Chem. 270:25370-25376. [DOI] [PubMed] [Google Scholar]
- 18.McCabe-Sellers, B. J., C. G. Staggs, and M. L Bogle. 2006. Tyramine in foods and monoamine oxidase inhibitor drugs: a crossroad where medicine, nutrition, pharmacy, and food industry converge. J. Food Compos. Anal. 19:S58-S65. [Google Scholar]
- 19.Molenaar, D., J. S. Bosscher, B. Ten Brink, A. J. Driessen, and W. N. Konings. 1993. Generation of a proton motive force by histidine decarboxylation and electrogenic histidine/histamine antiport in Lactobacillus buchneri. J. Bacteriol. 175:2864-2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otto, R., R. G. Lageveen, H. Veldkamp, and W. N. Konings. 1982. Lactate efflux-induced electrical potential in membrane vesicles of Streptococcus cremoris. J. Bacteriol. 149:733-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park, Y.-K., B. Bearson, S. H. Bang, I. S. Bang, and J. W. Foster. 1996. Internal pH crisis, lysine decarboxylase and the acid tolerance response of Salmonella typhimurium. Mol. Microbiol. 20:605-611. [DOI] [PubMed] [Google Scholar]
- 22.Poolman, B., K. J. Hellingwerf, and W. N. Konings. 1987. Regulation of glutamate-glutamine transport system by intracellular pH in Streptococcus lactis. J. Bacteriol. 169:2272-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richard, H., and J. W. Foster. 2007. Sodium regulates Escherichia coli acid resistance, and influences GadX- and GadW-dependent activation of gadE. Microbiology 153:3154-3161. [DOI] [PubMed] [Google Scholar]
- 24.Ruiz-Capillas, C., and F. Jiménez-Colmenero. 2004. Biogenic amines in meat and meat products. Crit. Rev. Food Sci. Nutr. 44:489-499. [DOI] [PubMed] [Google Scholar]
- 25.Salema, S., J. S. Lolkema, M. V. San Romão, and M. C. L. Dias. 1996. The proton motive force generated in Leuconostoc oenos by l-malate fermentation. J. Bacteriol. 178:3127-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silla Santos, M. H. 1996. Biogenic amines: their importance in foods. Int. J. Food Microbiol. 29:213-231. [DOI] [PubMed] [Google Scholar]
- 27.Sobczak, I., and J. S. Lolkema. 2005. The hydrocarboxylate transporter family. Microbiol. Mol. Biol. Rev. 69:665-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolken, W. A. M., P. Lucas, A. Lonvaud-Funel, and J. S. Lolkema. 2006. The mechanism of the tyrosine transporter TyrP supports a proton motive tyrosine decarboxylation pathway in Lactobacillus brevis. J. Bacteriol. 188:2198-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]