Abstract
To characterize the mode of action of lacticin Q (LnqQ), its membrane-permeabilizing activity was compared with that of nisin A because of the similar antimicrobial features of these compounds. Lipid II, the receptor for nisin A, was not required for LnqQ activity. LnqQ induced high-level membrane permeability in the absence of specific receptors.
Membrane-permeabilizing antimicrobial peptides are promising candidates for new antibiotics for various applications (16). Small cationic peptides (20 to 40 residues) from multicellular organisms have been studied, and in most cases their antimicrobial mechanism is thought to be membrane permeabilization (3). Cationic peptides first recognize anionic bacterial membranes without specific receptors and then cause membrane permeabilization. Magainin 2, a well-characterized cationic antimicrobial peptide, forms a toroidal pore that results in leakage of cellular components via its α-helical structure on the bacterial membrane (12). Magainin 2 inhibits the growth of bacteria at concentrations in the micromolar range (1 to 20 μM), whereas no lysis of blood cells occurs at concentrations in this range (14).
Many lactic acid bacteria (LAB) secrete ribosomally synthesized antimicrobial peptides termed bacteriocins. The antimicrobial mechanisms of many LAB bacteriocins, including nisin A and pediocin PA-1, require specific receptors (so-called docking molecules) for activity, in addition to the peptide itself and the phospholipid bilayer (7, 9, 10, 15). Docking molecules enhance LAB bacteriocin activity and provide target cell specificity. Thus, docking molecules not only increase the antimicrobial activity of bacteriocins but also result in selective toxicity of bacteriocins.
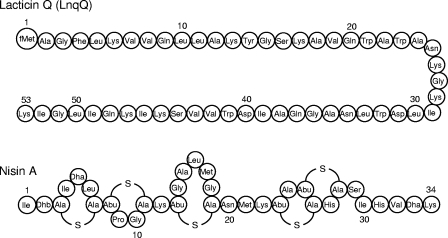
We previously reported that Lactococcus lactis QU 5 produces a bacteriocin termed lacticin Q (LnqQ) (8). Both LnqQ and nisin A have been reported to have widespread antimicrobial activity against gram-positive bacteria at concentrations in the nanomolar range. Figure 1 shows that the structures of LnqQ, which consists of 53 unmodified amino acids, and nisin A, which consists of 34 amino acids with posttranslational modifications, are clearly different (7, 14). LnqQ causes efflux of ATP from cells, indicating that its bactericidal activity is due to membrane permeabilization (7). Pediocin PA-1 is a well-known nonlantibiotic that has activity at concentrations in the nanomolar range, but strong activity has been observed only with Listeria and limited species; pediocin AP-1 forms small pores that cannot cause leakage of ATP from cells (4). Here, we characterized the membrane-permeabilizing activity of LnqQ using live cells and artificial membranes and compared this activity with that of nisin A.
FIG. 1.
Primary structures of LnqQ and nisin A. fMet, formylmethionine; Dha, dehydroalanine; Dhb, dehydrobutyrine; Ala-S-Ala lanthionine; Abu-S-Ala, 3-methyllanthionine; S, sulfur atom of the thioether bridge.
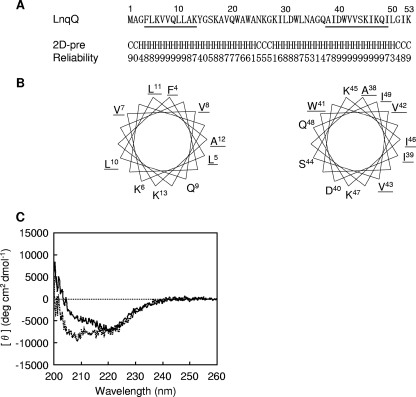
Initially, the secondary structure of LnqQ was examined, because amphiphilic secondary structures have been reported for antimicrobial peptides but not for nisin A (3, 6). The LnqQ and nisin A used in this study were purified by a method described previously (7). Using PSIPRED (http://bioinf.cs.ucl.ac.uk/psipred/), LnqQ was predicted to contain two α-helixes with high reliability at positions 4 to 13 and 38 to 49 (Fig. 2A). Amphiphilic α-helices in these regions were revealed by helical wheel analysis (Fig. 2B). Circular dichroism (CD) spectra of LnqQ were recorded in the presence and absence of negatively charged small unilamella vesicles (SUVs) using previously described methods (13) (Fig. 2C). Negative peaks at wavelengths around 207 and 222 nm, indicating α-helical structures, were observed in the presence of SUVs. The helix contents calculated from the CD spectrum ellipticities at 222 nm were similar under the two conditions (31.4% for buffer and 35.1% for SUVs). However, the spectrum in buffer at wavelengths around 207 nm was different from the spectrum obtained with SUVs. We concluded that LnqQ forms an amphiphilic α-helical structure in the presence of SUVs.
FIG. 2.
Analysis of the secondary structure of LnqQ. (A) The LnqQ line shows the amino acid sequence of LnqQ. The 2D-pre and Reliability lines show the predicted secondary structure (C, random coil; H, helix) and the reliability (9, highest; 0, lowest), respectively. (B) Helical wheel analysis for positions 4 to 13 and 38 to 49 of LnqQ (underlined regions in panel A). The underlined amino acids are hydrophobic residues. (C) CD spectra of LnqQ. The solid and dotted lines show data for LnqQ in buffer A (10 mM Tris-HCl, 75 mM NaCl, 1 mM EDTA; pH 7.4) and in the presence of SUVs (PC/PG, 1:1), respectively. The final peptide and lipid concentrations were 4 and 400 μM, respectively.
The MICs of LnqQ and nisin A for gram-positive bacteria were determined by using a turbidimetric assay (11). Both of these bacteriocins exhibited antimicrobial activity at concentrations in the nanomolar range (Table 1). However, the patterns were different, as were the structural features mentioned above.
TABLE 1.
Antimicrobial spectra of LnqQ and nisin A
| Organisma | MIC (nM)
|
|
|---|---|---|
| LnqQ | Nisin A | |
| Bacillus coagulans JCM 2257T | 75 | 250 |
| Lactococcus lactis subsp. lactis ATCC 19435T | 5 | 500 |
| Listeria innocua ATCC 33090T | 750 | 1,000 |
| Pediococcus pentosaceus JCM 5890T | 500 | 100 |
Abbreviations: ATCC, American Type Culture Collection, Manassas, VA; JCM, Japan Collection of Microorganisms, Wako, Japan.
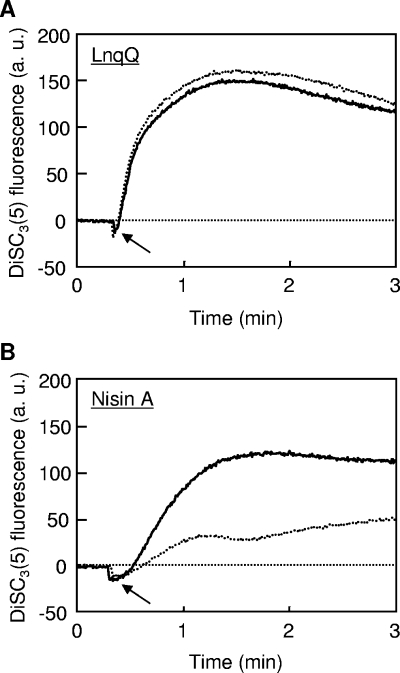
Nisin A requires lipid II as a docking molecule to exhibit high antimicrobial activity at concentrations in the nanomolar range (2, 10). Thus, we speculated that LnqQ could also require lipid II for membrane-permeabilizing activity. Using the method of Breukink et al. (2), we monitored bacteriocin-caused disruption of the membrane potential when the cells were treated with vancomycin (Fig. 3). The fluorescence of 3,3′-dipropylthiadicarbocyanine iodide [DiSC3(5)] (Invitrogen, Carlsbad, CA) was used as an indicator of membrane potential. LnqQ disrupted the membrane potentials of intact and vancomycin-treated cells equally (Fig. 3A). As previously reported (2), the membrane-permeabilizing activity of nisin A was blocked by vancomycin (Fig. 3B). Thus, the modes of action of LnqQ and nisin A were different, especially with respect to use of lipid II as a docking module.
FIG. 3.
Effects of vancomycin on disruption of the membrane potential of Bacillus coagulans JCM 2257T caused by LnqQ (A) and nisin A (B). The solid and dotted lines show the bacteriocin-caused disruption of the membrane potential of intact and vancomycin-treated cells, respectively. The arrows indicate when bacteriocins (20 nM) were added. All experiments were performed in a buffer (250 mM sucrose, 5 mM MgSO4, 10 mM potassium phosphate; pH 7.0) containing 1 μM DiSC3(5) at 30°C. a.u., arbitrary units.
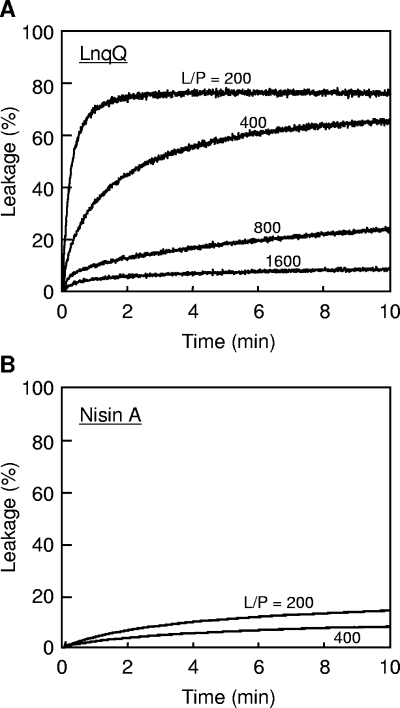
We then investigated whether LnqQ requires a docking molecule for its membrane-permeabilizing activity. We used liposomes, which are large unilamellar vesicles (LUVs), because it was reported previously that high concentrations of nisin A or pediocin PA-1 were required for membrane-permeabilizing activity against membranes lacking docking molecules (lipid/peptide [L/P] molar ratio, 2 to 10) (1, 5). We constructed negatively charged LUVs entrapping the fluorescent dye calcein (Dojindo, Kumamoto, Japan) using egg yolk l-α-phosphatidylcholine (PC) (Sigma, St. Louis, MO) and l-α-phosphatidyl-dl-glycerol (PG) (Sigma), as described previously (13). Without a docking molecule, treatment with LnqQ induced rapid and significantly high levels (75.7%) of leakage of calcein at an L/P ratio of 200 (Fig. 4A). The amount and rate of calcein leakage depended on the LnqQ concentration, and treatment with LnqQ caused leakage of 8.4% of the calcein at a low peptide concentration (31 nM) at an L/P ratio of 1,600. On the other hand, treatment with nisin A induced leakage of only 14.2% of the calcein, even at a high concentration (250 nM) with an L/P ratio of 200 (Fig. 4B). The calcein leakage results obtained with LnqQ and nisin A shown in Fig. 4 were similar to the results obtained for vancomycin-treated cells (Fig. 3). In addition, the high membrane-permeabilizing activity of LnqQ with LUVs was similar to the activity reported for nisin Z (a natural nisin variant) with lipid II-containing LUVs (∼60% leakage at an L/P ratio of 500) (15). These results suggest that LnqQ does not require a docking molecule for membrane-permeabilizing activity, in contrast to other LAB bacteriocins, including nisin A and pediocin PA-1.
FIG. 4.
Leakage of calcein entrapped in LUVs (PC/PG, 1:1) caused by LnqQ (A) and nisin A (B). The lines show the peptide-induced calcein leakage for different L/P ratios (mol/mol). The final lipid concentration was 50 μM. All experiments were performed in buffer A at 30°C.
In conclusion, we propose the following LnqQ killing mechanism: (i) binding to a negatively charged bacterial membrane with a cationic α-helical structure, (ii) membrane permeation without the aid of docking molecules, and (iii) cell death due to the loss of intracellular components. The features of LnqQ reported in this study are more similar to the features of antimicrobial peptides from multicellular eukaryotes than to the features of nisin A. However, many antimicrobial peptides from multicellular eukaryotes have antimicrobial activity at concentrations in the micromolar range. Further research is required to determine how LnqQ exerts antimicrobial activity at concentrations in the nanomolar range and to characterize the mode of action of LnqQ on bacterial membranes in detail.
Acknowledgments
We thank N. Igura and S. M. Asaduzzaman of Kyusyu University, Fukuoka, Japan, for their assistance in this study.
This work was supported in part by a grant-in-aid for scientific research from the Japan Society for the Promotion of Science and as a research project for utilizing advanced technologies in agriculture, forestry, and fisheries by the Ministry of Agriculture, Forestry and Fisheries of Japan.
Footnotes
Published ahead of print on 14 November 2008.
REFERENCES
- 1.Breukink, E., C. van Kraaij, R. A. Demel, R. J. Siezen, O. P. Kuipers, and B. de Kruijff. 1997. The C-terminal region of nisin is responsible for the initial interaction of nisin with the target membrane. Biochemistry 36:6968-6976. [DOI] [PubMed] [Google Scholar]
- 2.Breukink, E., I. Wiedemann, C. van Kraaij, O. P. Kuipers, H.-G. Sahl, and B. de Kruijff. 1999. Use of the cell wall precursor lipid II by a pore-forming peptide antibiotic. Science 286:2361-2364. [DOI] [PubMed] [Google Scholar]
- 3.Brogden, K. A. 2005. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3:238-250. [DOI] [PubMed] [Google Scholar]
- 4.Chen, Y., and T. J. Montville. 1995. Efflux of ions and ATP depletion induced by pediocin PA-1 are concomitant with cell death in Listeria monocytogenes Scott A. J. Appl. Bacteriol. 79:684-690. [Google Scholar]
- 5.Chen, Y., R. Shapira, M. Eisenstein, and T. J. Montville. 1997. Functional characterization of pediocin PA-1 binding to liposomes in the absence of a protein receptor and its relationship to a predicted tertiary structure. Appl. Environ. Microbiol. 63:524-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotter, P. D., C. Hill, and R. P. Ross. 2005. Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3:777-788. [DOI] [PubMed] [Google Scholar]
- 7.Dalet, K., Y. Cenatiempo, P. Cossart, and Y. Hechard. 2001. A σ54-dependent PTS permease of the mannose family is responsible for sensitivity of Listeria monocytogenes to mesentericin Y105. Microbiology 147:3263-3269. [DOI] [PubMed] [Google Scholar]
- 8.Fujita, K., S. Ichimasa, T. Zendo, S. Koga, F. Yoneyama, J. Nakayama, and K. Sonomoto. 2007. Structural analysis and characterization of lacticin Q, a novel bacteriocin belonging to a new family of unmodified bacteriocins of gram-positive bacteria. Appl. Environ. Microbiol. 73:2871-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gravesen, A., P. Warthoe, S. Knochel, and K. Thirstrup. 2000. Restriction fragment differential display of pediocin-resistant Listeria monocytogenes 412 mutants shows consistent overexpression of a putative β-glucoside-specific PTS system. Microbiology 146:1381-1389. [DOI] [PubMed] [Google Scholar]
- 10.Hasper, H. E., N. E. Kramer, J. L. Smith, J. D. Hillman, C. Zachariah, O. P. Kuipers, B. de Kruijff, and E. Breukink. 2006. An alternative bactericidal mechanism of action for lantibiotic peptides that target lipid II. Science 313:1636-1637. [DOI] [PubMed] [Google Scholar]
- 11.Kuipers, O. P., H. S. Rollema, W. M. Yap, H. J. Boot, R. J. Siezen, and W. M. de Vos. 1992. Engineering dehydrated amino acid residues in the antimicrobial peptide nisin. J. Biol. Chem. 267:24340-24346. [PubMed] [Google Scholar]
- 12.Matsuzaki, K., O. Murase, N. Fujii, and K. Miyajima. 1996. An antimicrobial peptide, magainin 2, induced rapid flip-flop of phospholipids coupled with pore formation and peptide translocation. Biochemistry 35:11361-11368. [DOI] [PubMed] [Google Scholar]
- 13.Matsuzaki, K., O. Murase, H. Tokuda, S. Funakoshi, N. Fujii, and K. Miyajima. 1994. Orientational and aggregational states of magainin 2 in phospholipid bilayers. Biochemistry 33:3342-3349. [DOI] [PubMed] [Google Scholar]
- 14.Matsuzaki, K., K. Sugishita, M. Harada, N. Fujii, and K. Miyajima. 1997. Interactions of an antimicrobial peptide, magainin 2, with outer and inner membranes of Gram-negative bacteria. Biochim. Biophys. Acta 1327:119-130. [DOI] [PubMed] [Google Scholar]
- 15.Wiedemann, I., E. Breukink, C. van Kraaij, O. P. Kuipers, G. Bierbaum, B. de Kruijff, and H.-G. Sahl. 2001. Specific binding of nisin to the peptidoglycan precursor lipid II combines pore formation and inhibition of cell wall biosynthesis for potent antibiotic activity. J. Biol. Chem. 276:1772-1779. [DOI] [PubMed] [Google Scholar]
- 16.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389-395. [DOI] [PubMed] [Google Scholar]