Abstract
Live cell imaging of human malaria parasites Plasmodium falciparum during gametocytogenesis revealed that the apicoplast does not grow, whereas the mitochondrion undergoes remarkable morphological development. A close connection of the two organelles is consistently maintained. The apicoplast and mitochondrion are not components of the male gametes, suggesting maternal inheritance.
The human malaria parasite Plasmodium falciparum is responsible for severe disease and persists as a major global health problem. P. falciparum belongs to the phylum Apicomplexa and harbors a relic plastid (apicoplast) acquired via secondary endosymbiosis (10, 13). Although the apicoplast has lost its photosynthetic ability (10), it still retains critical metabolic pathways such as type II fatty acid synthesis, isoprenoid synthesis, and part of heme synthesis (11, 20, 29, 39, 41). Because these pathways are fundamentally bacterial and distinct from equivalent human pathways, the apicoplast is a promising drug target (28).
Like all plastids, apicoplasts cannot be created de novo and must be vertically inherited. We previously demonstrated that both the apicoplast and the mitochondrion begin as small globular structures in the initial erythrocyte phase and then grow and branch before segmenting into multiple daughter organelles that are segregated into the daughter parasites (3, 15, 16, 20, 37, 39).
Despite detailed investigation of organelle inheritance in the asexual erythrocyte stages, little is known about other life cycle stages. The falciparum malaria parasite's life cycle is divided between the human and the mosquito. Transition from the human to the mosquito stages involves gametocytogenesis, which commences in erythrocytes and then proceeds in the mosquito gut, where the macrogametocyte (female) produces a single macrogamete and the microgametocyte (male) produces eight microgametes after three serial nuclear divisions. Gametes fuse to produce a zygote, which eventually develops into motile forms ready for injection into a new human host.
We wanted to explore the behavior of the apicoplast and the mitochondrion in gametocytes (20, 27, 37, 39). We therefore created a new, tagged organelle line in the gametocyte-competent line 3D7 (2, 25, 30) with a novel fluorophore, mOrange (32), in the apicoplast for improved labeling. The 3D7 ACP(L):mOrange-CS(L):GFPmut2 line has red apicoplasts and green mitochondria, and examination of the asexual erythrocyte stages confirmed previous observations of organelle development and partitioning (37).
Five morphological developmental stages of gametocytes (14) are recognized (Fig. 1 and 2). In contrast to the apicoplast in asexual erythrocyte phases, we observed little to no apicoplast change during gametocytogenesis; the apicoplast stayed small and rounded or was only slightly elongated. Despite its morphological inertia in gametocytes, the apicoplast is functionally active. Sullivan et al. (35) demonstrated that inhibition of the apicoplast translation in the P. berghii gametocyte by thiostrepton blocks parasite transmission (12, 23). Furthermore, gametocyte transcriptomics shows upregulation of genes for apicoplast type II fatty acid biosynthesis (FASII), lipoate synthase and the pyruvate dehydrogenase E1 alpha subunit (42), and at least two FASII enzymes are detected in gametocyte proteomes (18).
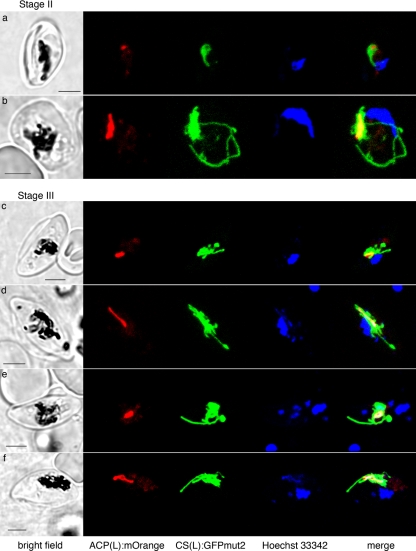
FIG. 1.
Live cell images of 3D7 ACP(L):mOrange-CS(L):GFPmut2 gametocytes at stage II (a and b) and stage III (c to f), showing a small apicoplast (red) that is always encased by the mitochondrion (green). At stage III, the apicoplast is round (c and e) or slightly elongated (d and f). In contrast, the mitochondrion forms branches from the cluster that encase the apicoplast. Sometimes the GFP labeling for the mitochondrion is heterologous, but continuous (c and d). Scale bars, 3 μm.
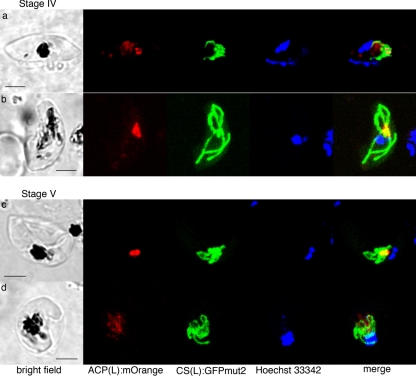
FIG. 2.
Live cell images of 3D7 ACP(L):mOrange-CS(L):GFPmut2, at stage IV (a and b) and stage V (c and d), showing the apicoplast (red) and mitochondrion (green) association in macrogametocytes (a and c) and microgametocytes (b and d). The apicoplast (red) does not elongate or branch but stays small. Often, the mOrange signal for the apicoplast is weak in the mature microgametocyte (d). Scale bars, 3 μm.
In contrast to the morphologically static apicoplast, the mitochondrion undergoes spectacular morphological development as gametocytes mature. In stage II gametocytes, the mitochondrion elongates and branches in a manner somewhat reminiscent of that in the asexual schizont cell, except that it forms a cluster around the small apicoplast (Fig. 1a and b; see also the movies in the supplemental material). The mitochondrion keeps this unusual association with the apicoplast throughout the whole gametocyte developmental process, typically appearing like a thicket of elongate mitochondrial profiles virtually encasing the apicoplast.
As the gametocyte elongates at Stage III, the mitochondrion also elongates longitudinally (Fig. 1c to f; see also the movies in the supplemental material). In these cells, part of the mitochondrion is often folded a few times along the elongated apicoplast. As the gametocytes enlarge and mature (stage IV and V; Fig. 2), the mitochondrion in some cells forms a denser cluster with short, round branches that emerge from a center and emcompass the apicoplast (Fig. 2a, c, and d; see also the movies in the supplemental material). However, the morphology is diverse and does not correspond to gametocyte sex or maturity.
Our observation of an expanding mitochondrion in the gametocyte is consistent with the activation of mitochondrial metabolism in gametocytes. Parasites in the asexual erythrocyte phase rely exclusively on cytosolic glycolysis and eschew the mitochondrial tricarboxylic acid (TCA) cycle. Indeed, Painter et al. suggest that mitochondrial electron transport in erythrocytes is only necessary for the reduction of dihydroorotate dehydrogenase (4, 8, 27, 38). However, transcriptomics reveals that 15 of the 16 mitochondrial TCA cycle enzymes are upregulated in gametocytes (42), and nine of these enzymes are detected proteomically in females and four are detected in males (18). Gametocyte mitochondria also develop the tubular mitochondrial cristae that are largely absent in the asexual stages (21) but are required for full mitochondrial function. The elaboration of the mitochondria during gametocytogenesis reflects this development of mitochondrial activity.
The proximity of the mitochondrion and the apicoplast is intriguing. In the asexual phase, there is a constant physical association of the apicoplast and the mitochondrion (1, 15, 37), which even persists when the organelles are isolated (19, 26). This contact is proposed to facilitate metabolite exchange (20, 39). Available evidence suggests that synthesis of heme by malaria parasites is a cooperative activity between the apicoplast and mitochondrion and that protoporphyrin intermediates are shuttled between the organelles (31, 39, 40). The gametocyte proteome includes at least one apicoplast and one mitochondrial enzyme for the putatively conjoined heme synthesis pathway (42), and the connection reported here may facilitate substrate shuttling. It has also been suggested that the two organelles may share tRNAs (16).
In the present study, we observed a single mitochondrion with the different segments visibly connected by thin strands. This is similar to rhodamine-123 staining of live gametocytes (17). The highly lobed mitochondrial structure may be responsible for the reports of multiple (four to eight) mitochondria based solely on electron microscopy of gametocytes of P. falciparum (33) and other Plasmodium species (21).
We also wanted to explore how the organelles might be inherited during sexual reproduction. In plants, both the plastid and the mitochondria are typically maternally inherited (22), but there are examples of biparental or paternal inheritance (24). We attempted to induce microgametogenesis (exflagellation) in our 3D7 ACP(L):mOrange-CS(L):GFPmut2 line without success. Instead, we used immunofluorescent markers for the mitochondrion and apicoplast to examine fixed parasites of the exflagellation-competent strain NF54 (25, 30).
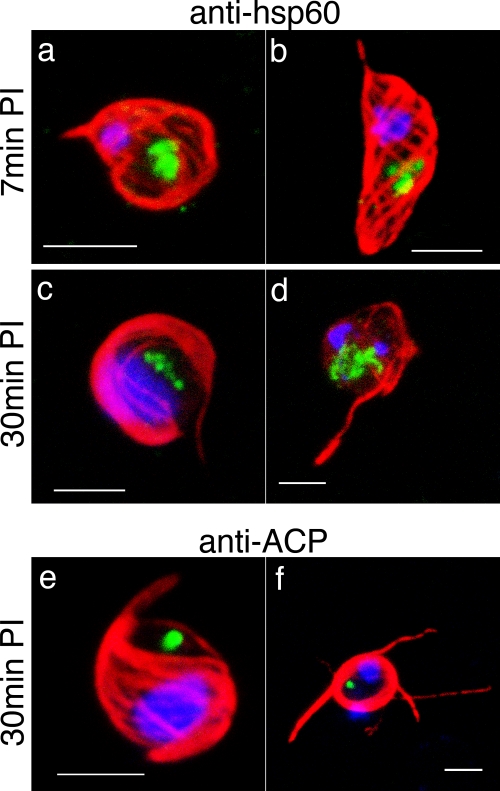
We focused on the microgametogenesis, or exflagellation, since the obvious metamorphosis allows us to unambiguously distinguish mature gametes. NF54 parasites developed into gametocytes and the males could be induced to exflagellate as previously described (9). Seven minutes after the exflagellation induction, the microgametocyte begins to develop axonemes (Fig. 3a and b), retaining a single apicoplast and a single mitochondrion. At 30 min after the induction, when microgametes were being released, the single apicoplast and the single mitochondrion remain lodged in the original gametocyte cytoplasm and hence are not partitioned into the microgamete (Fig. 3c to f). The absence of mitochondria and apicoplasts in male gametes is consistent with genetic evidence for the maternal inheritance of the mitochondrial and apicoplast genomes (5), as well as electron microscopy (33, 34). The absence of apicoplasts and mitochondria in microgametes of other apicomplexan parasites (6, 7) suggests that this may be a common feature of the phylum (33).
FIG. 3.
Exflagellated microgametes. Immunofluorescence images of the mitochondrion in NF54 parasites, fixed 7 min (a and b) or 30 min (c to f) after exflagellation induction. (a to d) The mitochondrion (labeled with the anti-Hsp60 antibody [green]) stays single even after each microgamete (whose axoneme is labeled with the anti-alpha tubulin antibody [red]) is formed (a and b) and exflagellated (c and d). (e and f) The apicoplast (labeled with the anti-ACP antibody [green]) stays single and does not enter the exflagellated microgametes (whose axoneme is labeled with the anti-alpha tubulin antibody [red]). Scale bars, 3 μm.
Conclusions and future prospects.
Gametocytogenesis in P. falciparum is the first step of the sexual reproduction, which is an essential bridging stage from humans into the mosquito host. In the present study, we made thorough morphological observations of the different gametocyte stages to demonstrate that the gametocyte has a single unelaborated apicoplast that undergoes minimal expansion through gametocytogenesis. In contrast, the single mitochondrion expands greatly to form a cluster around/along the apicoplast during gametocyte development. The morphologies are congruent with metabolic activity in the two organelles at this stage.
Recent transcriptome and proteome analyses of the hepatocyte phase of the rodent malaria P. yoelii revealed high activity of the apicoplast and the mitochondrion (36), so it will be interesting to visualize the organelles in these stages, as well as sexual reproduction process in the mosquito host. A complete picture of what the organelles are doing at various life cycle phases will inform development of intervention strategies, particularly when and how to use drugs targeting apicoplast or mitochondrial metabolisms.
Supplementary Material
Acknowledgments
We thank to Alan F. Cowman (Walter and Eliza Hall Institute of Medical Research, Australia) for the NF54 strain and heat inactivated human serum. We also thank for Nirbhay Kumar (Johns Hopkins Malaria Research Institute) for advice on exflagellation. We thank the Australian Red Cross for human blood samples.
N.O. is funded by the Japan Society for Promotion of Sciences. G.M. is an ARC Federation Fellow and a Howard Hughes Medical Institute International Scholar. The project is supported by a Program Grant from the NH&MRC.
Footnotes
Published ahead of print on 7 November 2008.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Aikawa, M. 1966. The fine structure of the erythrocytic stages of three avian malarial parasites, Plasmodium fallax, P. lophurae, and P. cathemerium. Am. J. Trop. Med. Hyg. 15449-471. [DOI] [PubMed] [Google Scholar]
- 2.Alano, P. 2007. Plasmodium falciparum gametocytes: still many secrets of a hidden life. Mol. Microbiol. 66291-302. [DOI] [PubMed] [Google Scholar]
- 3.Bannister, L. H., J. M. Hopkins, R. E. Fowler, S. Krishna, and G. H. Mitchell. 2000. A brief illustrated guide to the ultrastructure of Plasmodium falciparum asexual blood stages. Parasitol. Today 16427-433. [DOI] [PubMed] [Google Scholar]
- 4.Biagini, G. A., N. Fisher, N. Berry, P. A. Stocks, B. Meunier, D. P. Williams, R. Bonar-Law, P. G. Bray, A. Owen, P. M. O'Neill, and S. A. Ward. 2008. Acridinediones: selective and potent inhibitors of the malaria parasite mitochondrial bc1 complex. Mol. Pharmacol. 731347-1355. [DOI] [PubMed] [Google Scholar]
- 5.Creasey, A., K. Mendis, J. Carlton, D. Williamson, I. Wilson, and R. Carter. 1994. Maternal inheritance of extrachromosomal DNA in malaria parasites. Mol. Biochem. Parasitol. 6595-98. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson, D. J. P., S. A. Campbell, F. L. Henriquez, L. Phan, E. Mui, T. A. Richards, S. P. Muench, M. Allary, J. Z. Lu, S. T. Prigge, F. Tomley, M. W. Shirley, D. W. Rice, R. McLeod, and C. W. Roberts. 2007. Enzymes of type II fatty acid synthesis and apicoplast differentiation and division in Eimeria tenella. Int. J. Parasitol. 3733-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson, D. J. P., F. L. Henriquez, M. J. Kirisits, S. P. Muench, S. T. Prigge, D. W. Rice, C. W. Roberts, and R. L. McLeod. 2005. Maternal inheritance and stage-specific variation of the apicoplast in Toxoplasma gondii during development in the intermediate and definitive host. Eukaryot. Cell 4814-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher, N., P. G. Bray, S. A. Ward, and G. A. Biagini. 2008. Malaria-parasite mitochondrial dehydrogenases as drug targets: too early to write the obituary. Trends Parasitol. 249-10. [DOI] [PubMed] [Google Scholar]
- 9.Fivelman, Q. L., L. McRobert, S. Sharp, C. J. Taylor, M. Saeed, C. A. Swales, C. J. Sutherland, and D. A. Baker. 2007. Improved synchronous production of Plasmodium falciparum gametocytes in vitro. Mol. Biochem. Parasitol. 154119-123. [DOI] [PubMed] [Google Scholar]
- 10.Foth, B. J., and G. I. McFadden. 2003. The apicoplast: a plastid in Plasmodium falciparum and other apicomplexan parasites. Int. Rev. Cytol. 22457-110. [DOI] [PubMed] [Google Scholar]
- 11.Goodman, C. D., and G. I. McFadden. 2007. Fatty acid biosynthesis as a drug target in apicomplexan parasites. Curr. Drug Targets 815-30. [DOI] [PubMed] [Google Scholar]
- 12.Goodman, C. D., V. Su, and G. I. McFadden. 2007. The effects of antibacterials on the malaria parasite Plasmodium falciparum. Mol. Biochem. Parasitol. 152181-191. [DOI] [PubMed] [Google Scholar]
- 13.Gould, S. B., R. F. Waller, and G. I. McFadden. 2008. Plastid evolution. Annu. Rev. Plant Biol. 59491-517. [DOI] [PubMed] [Google Scholar]
- 14.Hawking, F., M. E. Wilson, and K. Gammage. 1971. Evidence for cyclic development and short-lived maturity in gametocytes of Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 65549-559. [DOI] [PubMed] [Google Scholar]
- 15.Hopkins, J., R. Fowler, S. Krishna, I. Wilson, G. Mitchell, and L. Bannister. 1999. The plastid in Plasmodium falciparum asexual blood stages: a three-dimensional ultrastructural analysis. Protist 150283-295. [DOI] [PubMed] [Google Scholar]
- 16.Howe, C. J., and S. Purton. 2007. The little genome of apicomplexan plastids: its raison d'etre and a possible explanation for the ‘delayed death’ phenomenon. Protist 158121-133. [DOI] [PubMed] [Google Scholar]
- 17.Kato, M., K. Tanabe, A. Miki, K. Ichimori, and S. Waki. 1990. Membrane potential of Plasmodium falciparum gametocytes monitored with rhodamine 123. FEMS Microbiol. Lett. 69283-288. [DOI] [PubMed] [Google Scholar]
- 18.Khan, S. M., B. Franke-Fayard, G. R. Mair, E. Lasonder, C. J. Janse, M. Mann, and A. P. Waters. 2005. Proteome analysis of separated male and female gametocytes reveals novel sex-specific Plasmodium biology. Cell 121675-687. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi, T., S. Sato, S. Takamiya, K. Komaki-Yasuda, K. Yano, A. Hirata, I. Onitsuka, M. Hata, F. Mi-ichi, T. Tanaka, T. Hase, A. Miyajima, S. Kawazu, Y. Watanabe, and K. Kita. 2007. Mitochondria and apicoplast of Plasmodium falciparum: behaviour on subcellular fractionation and the implication. Mitochondrion 7125-132. [DOI] [PubMed] [Google Scholar]
- 20.Krungkrai, J. 2004. The multiple roles of the mitochondrion of the malarial parasite. Parasitology 129511-524. [DOI] [PubMed] [Google Scholar]
- 21.Krungkrai, J., P. Prapunwattana, and S. R. Krungkrai. 2000. Ultrastructure and function of mitochondria in gametocytic stage of Plasmodium falciparum. Parasite 719-26. [DOI] [PubMed] [Google Scholar]
- 22.Kuroiwa, T., S. Kawano, S. Nishibayashi, and C. Sato. 1982. Epifluorescent microscopic evidence for maternal inheritance of chloroplast DNA. Nature 298481-483. [DOI] [PubMed] [Google Scholar]
- 23.McConkey, G. A., M. J. Rogers, and T. F. McCutchan. 1997. Inhibition of Plasmodium falciparum protein synthesis: targeting the plastid-like organelle with thiostrepton. J. Biol. Chem. 2722046-2049. [DOI] [PubMed] [Google Scholar]
- 24.Mogensen, H. L. 1996. The hows and whys of cytoplasmic inheritance in seed plants. Am. J. Bot. 83383-404. [Google Scholar]
- 25.Moore, J. M., N. Kumar, L. D. Shultz, and T. V. Rajan. 1995. Maintenance of the human malarial parasite, Plasmodium falciparum, in scid mice and transmission of gametocytes to mosquitos. J. Exp. Med. 1812265-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mullin, K. A., L. Lim, S. A. Ralph, T. P. Spurck, E. Handman, and G. I. McFadden. 2006. Membrane transporters in the relict plastid of malaria parasites. Proc. Natl. Acad. Sci. USA 1039572-9577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Painter, H. J., J. M. Morrisey, M. W. Mather, and A. B. Vaidya. 2007. Specific role of mitochondrial electron transport in blood-stage Plasmodium falciparum. Nature 44688-91. [DOI] [PubMed] [Google Scholar]
- 28.Ralph, S. A., M. C. D'Ombrain, and G. I. McFadden. 2001. The apicoplast as an antimalarial drug target. Drug Resist. Update 4145-151. [DOI] [PubMed] [Google Scholar]
- 29.Ralph, S. A., G. G. van Dooren, R. F. Waller, M. J. Crawford, M. J. Fraunholz, B. J. Foth, C. J. Tonkin, D. S. Roos, and G. I. McFadden. 2004. Metabolic maps and functions of the Plasmodium falciparum apicoplast. Nat. Rev. Microbiol. 2203-216. [DOI] [PubMed] [Google Scholar]
- 30.Rossario, V. 1981. Cloning of naturally occurring mixed infections of malaria parasites. Science 2121037-1038. [DOI] [PubMed] [Google Scholar]
- 31.Sato, S., B. Clough, L. Coates, and R. J. M. (I.) Wilson. 2004. Enzymes for heme biosynthesis are found in both the mitochondrion and plastid of the malaria parasite Plasmodium falciparum. Protist 155117-125. [DOI] [PubMed] [Google Scholar]
- 32.Shaner, N. C., R. E. Campbell, P. A. Steinbach, B. N. G. Giepmans, A. E. Palmer, and R. Y. Tsien. 2004. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 221567-1572. [DOI] [PubMed] [Google Scholar]
- 33.Sinden, R. E., E. U. Canning, R. S. Bray, and M. E. Smalley. 1978. Gametocyte and gamete development in Plasmodium falciparum. Proc. R. Soc. Lond. B 201375-380. [DOI] [PubMed] [Google Scholar]
- 34.Sinden, R. E., E. U. Canning, and B. Spain. 1976. Gametogenesis and fertilization in Plasmodium yeolii nigeriensis: transmission electron microscope study. Proc. R. Soc. Lond. B 19355-76. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan, M., J. Li, S. Kumar, M. J. Rogers, and T. F. McCutchan. 2000. Effects of interruption of apicoplast function on malaria infection, development, and transmission. Mol. Biochem. Parasitol. 10917-23. [DOI] [PubMed] [Google Scholar]
- 36.Tarun, A. S., X. Peng, R. F. Dumpit, Y. Ogata, H. Silva-Rivera, N. Camargo, T. M. Daly, L. W. Bergman, and S. H. I. Kappe. 2008. A combined transcriptome and proteome survey of malaria parasite liver stages. Proc. Natl. Acad. Sci. USA 105305-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Dooren, G. G., M. Marti, C. J. Tonkin, L. M. Stimmler, A. F. Cowman, and G. I. McFadden. 2005. Development of the endoplasmic reticulum, mitochondrion and apicoplast during the asexual life cycle of Plasmodium falciparum. Mol. Microbiol. 57405-419. [DOI] [PubMed] [Google Scholar]
- 38.van Dooren, G. G., and G. I. McFadden. 2007. Malaria: differential parasite drive. Nature 450955-956. [DOI] [PubMed] [Google Scholar]
- 39.van Dooren, G. G., L. M. Stimmler, and G. I. McFadden. 2006. Metabolic maps and functions of the Plasmodium mitochondrion. FEMS Microbiol. Rev. 30596-630. [DOI] [PubMed] [Google Scholar]
- 40.Varadharajan, S., B. K. C. Sagar, P. N. Rangarajan, and G. Padmanaban. 2004. Localization of ferrochelatase in Plasmodium falciparum. Biochem. J. 384429-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiesner, J., and H. Jomaa. 2007. Isoprenoid biosynthesis of the apicoplast as drug target. Curr. Drug Targets 83-13. [DOI] [PubMed] [Google Scholar]
- 42.Young, J. A., Q. L. Fivelman, P. L. Blair, P. de la Vega, K. G. Le Roch, Y. Zhou, D. J. Carucci, D. A. Baker, and E. A. Winzeler. 2005. The Plasmodium falciparum sexual development transcriptome: a microarray analysis using ontology-based pattern identification. Mol. Biochem. Parasitol. 14367-79. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.