Abstract
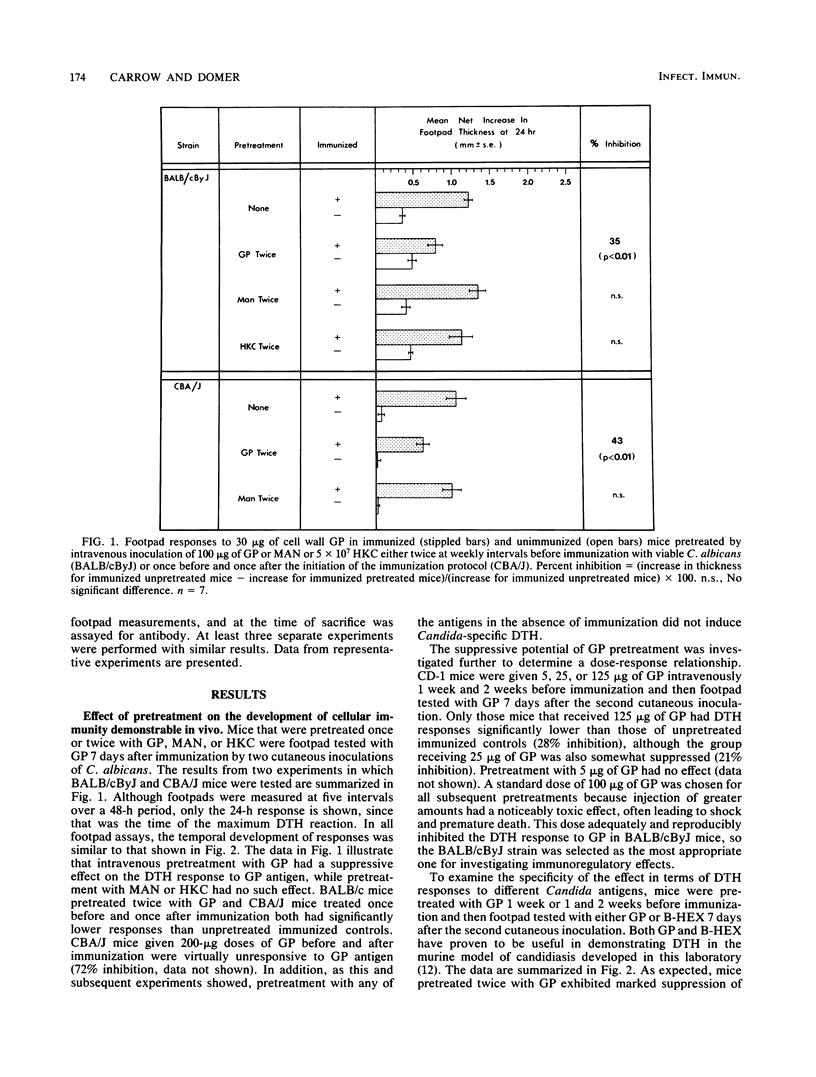
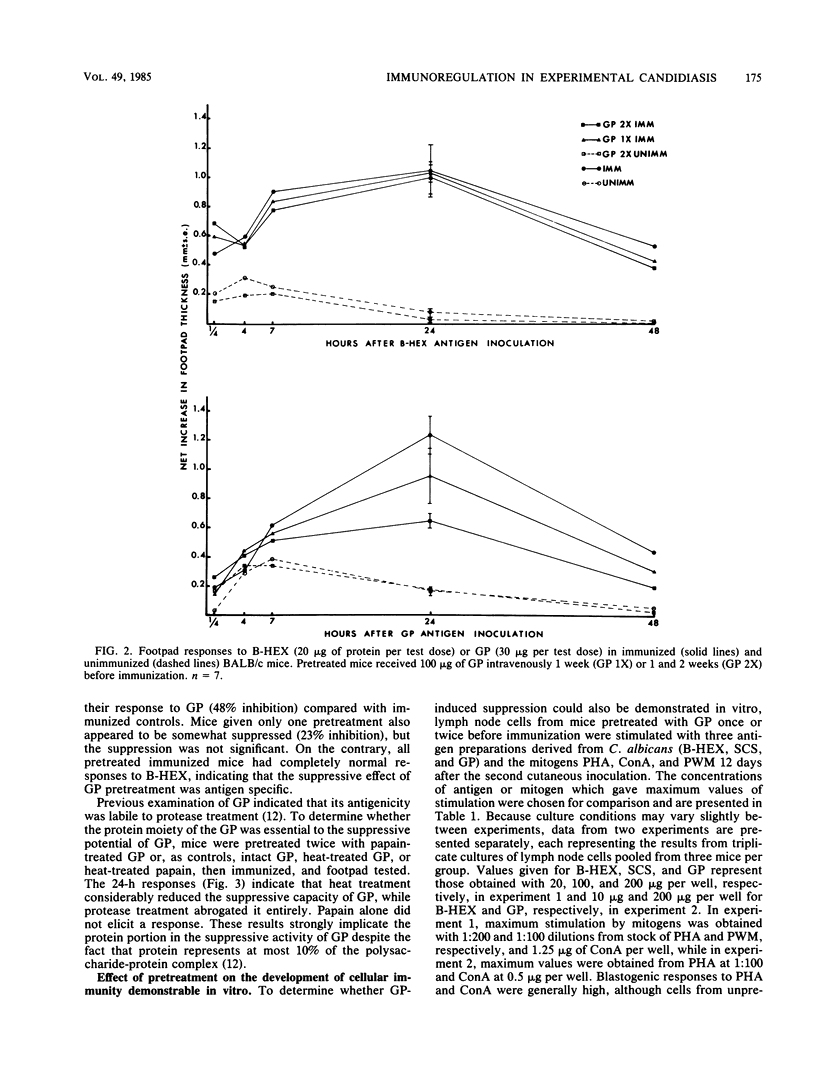
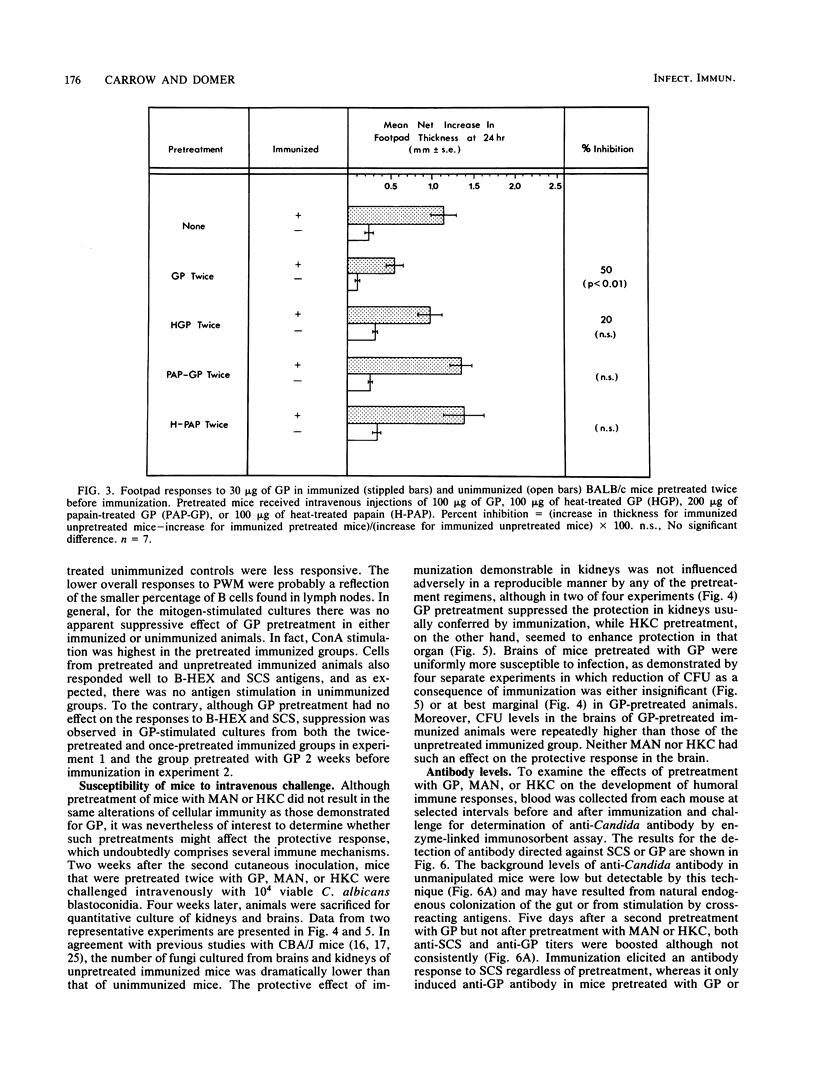
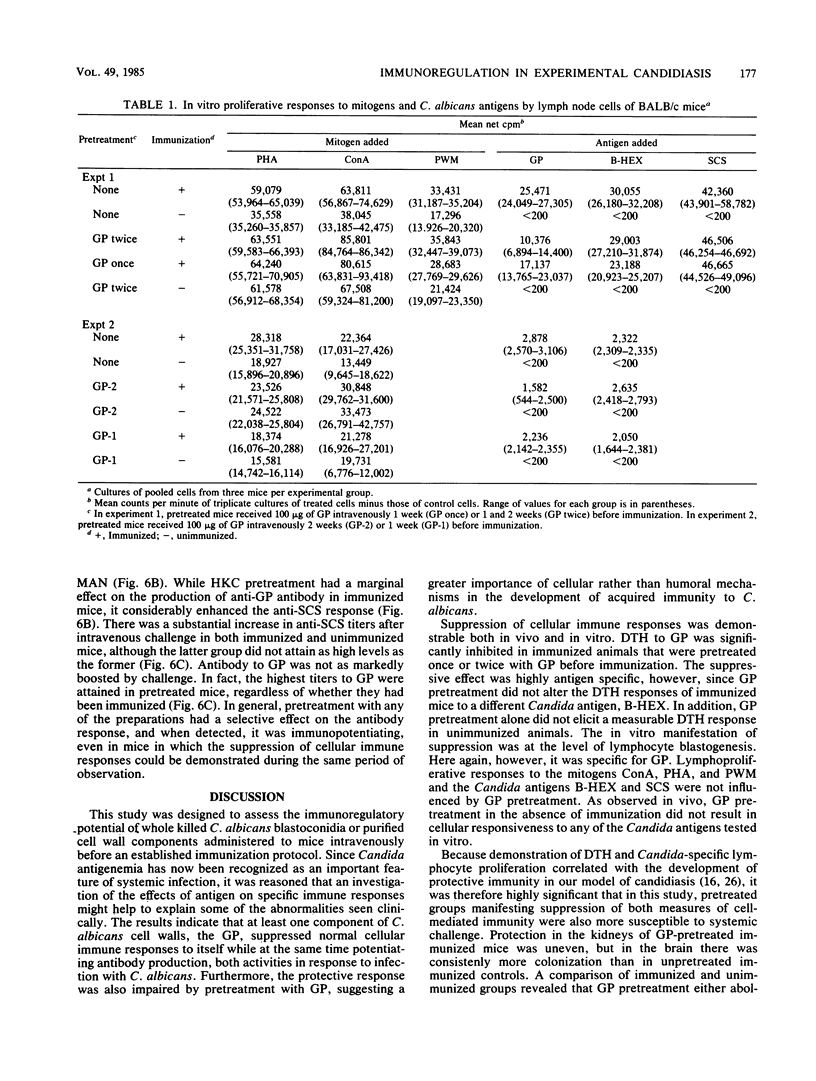
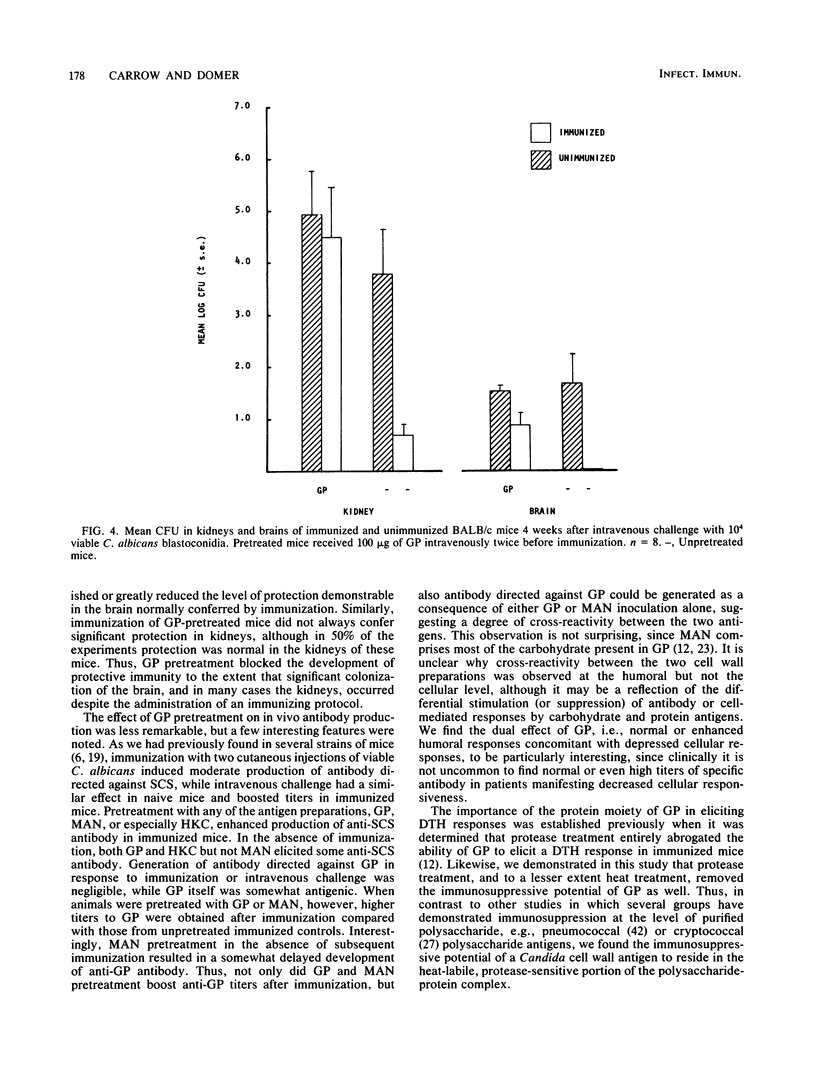
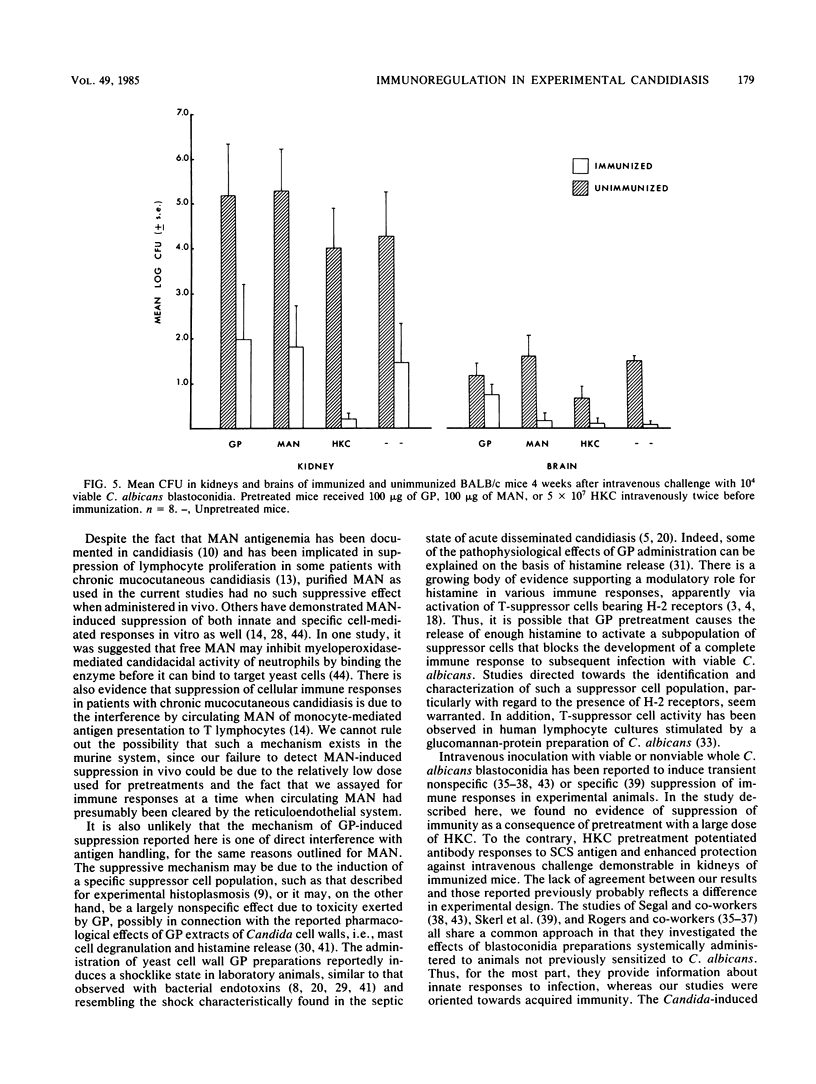
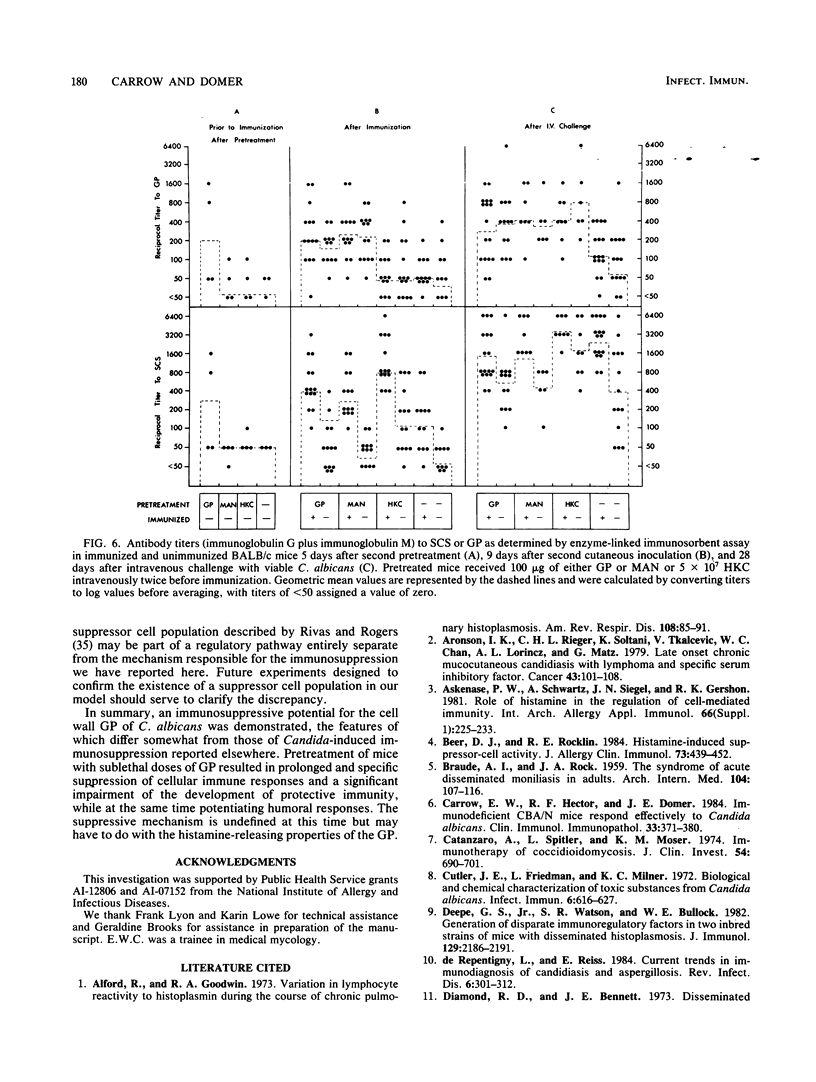
Immune regulation in candidiasis is inferred from studies of both human and animal infection, with a suppressive role suggested for cell wall polysaccharide. To study the immunosuppressive potential of Candida albicans in a murine model, whole blastoconidia or purified cell wall components of C. albicans were tested for their effects on the development of acquired immune responses by superimposing a pretreatment regimen upon an established immunization protocol. CBA/J or BALB/cByJ mice were pretreated twice intravenously with 100 micrograms of mannan (MAN), 100 or 200 micrograms of glycoprotein (GP), or 5 X 10(7) heat-killed C. albicans blastoconidia, followed 1 week later by an immunization protocol of two cutaneous inoculations of viable C. albicans blastoconidia given 2 weeks apart. Delayed hypersensitivity (DTH) to GP or to a membrane-derived antigen, B-HEX, was tested 7 days after the second inoculation, and lymphocyte stimulation was tested with mitogens and Candida antigens after 12 days. To assess protection, mice were challenged intravenously with viable C. albicans blastoconidia 14 days after the second cutaneous inoculation and sacrificed 28 days later for quantitative culture of kidneys and brains. Sera were obtained for enzyme-linked immunosorbent assays at selected intervals. Pretreatment with GP resulted in specific in vivo suppression of DTH to GP but not to B-HEX antigen and specific in vitro suppression of lymphocyte stimulation to GP but not to other Candida antigens or mitogens. MAN and heat-killed C. albicans blastoconidia had no such effects. GP pretreatment also diminished the protective effect of immunization against challenge, demonstrable in the brain, while not altering significantly the production of antibody in response to infection. Contrary to clinical evidence, MAN was not immunosuppressive in this model, and in fact, the immunosuppressive potential of GP, which is composed largely of MAN, was found to be dependent upon the presence of its heat-labile protein moiety.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alford R. H., Goodwin R. A. Variation in lymphocyte reactivity to histoplasmin during the course of chronic pulmonary histoplasmosis. Am Rev Respir Dis. 1973 Jul;108(1):85–92. doi: 10.1164/arrd.1973.108.1.85. [DOI] [PubMed] [Google Scholar]
- Aronson I. K., Rieger C. H., Soltani K., Tkalcevic V., Chan W. C., Lorincz A. L., Matz G. Late onset chronic mucocutaneous candidiasis with lymphoma and specific serum inhibitory factor. Cancer. 1979 Jan;43(1):101–108. doi: 10.1002/1097-0142(197901)43:1<101::aid-cncr2820430116>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Askenase P. W., Schwartz A., Siegel J. N., Gershon R. K. Role of histamine in the regulation of cell-mediated immunity. Int Arch Allergy Appl Immunol. 1981;66 (Suppl 1):225–233. doi: 10.1159/000232907. [DOI] [PubMed] [Google Scholar]
- Beer D. J., Rocklin R. E. Histamine-induced suppressor-cell activity. J Allergy Clin Immunol. 1984 Apr;73(4):439–452. doi: 10.1016/0091-6749(84)90353-1. [DOI] [PubMed] [Google Scholar]
- Carrow E. W., Hector R. F., Domer J. E. Immunodeficient CBA/N mice respond effectively to Candida albicans. Clin Immunol Immunopathol. 1984 Dec;33(3):371–380. doi: 10.1016/0090-1229(84)90308-8. [DOI] [PubMed] [Google Scholar]
- Catanzaro A., Spitler L., Moser K. M. Immunotherapy of coccidioidomycosis. J Clin Invest. 1974 Sep;54(3):690–701. doi: 10.1172/JCI107807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler J. E., Friedman L., Milner K. C. Biological and chemical characterization of toxic substances from Candida albicans. Infect Immun. 1972 Oct;6(4):616–627. doi: 10.1128/iai.6.4.616-627.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepe G. S., Jr, Watson S. R., Bullock W. E. Generation of disparate immunoregulatory factors in two inbred strains of mice with disseminated histoplasmosis. J Immunol. 1982 Nov;129(5):2186–2191. [PubMed] [Google Scholar]
- Domer J. E., Moser S. A. Experimental murine candidiasis: cell-mediated immunity after cutaneous challenge. Infect Immun. 1978 Apr;20(1):88–98. doi: 10.1128/iai.20.1.88-98.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A., Ballet J. J., Griscelli C. Specific inhibition of in vitro Candida-induced lymphocyte proliferation by polysaccharidic antigens present in the serum of patients with chronic mucocutaneous candidiasis. J Clin Invest. 1978 Nov;62(5):1005–1013. doi: 10.1172/JCI109204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A., Pichat L., Audinot M., Griscelli C. Defective handling of mannan by monocytes in patients with chronic mucocutaneous candidiasis resulting in a specific cellular unresponsiveness. Clin Exp Immunol. 1982 Mar;47(3):653–660. [PMC free article] [PubMed] [Google Scholar]
- Gatenby P., Basten A., Adams E. Thymoma and late onset mucocutaneous candidiasis associated with a plasma inhibitor of cell-mediated immune function. J Clin Lab Immunol. 1980 May;3(3):209–216. [PubMed] [Google Scholar]
- Giger D. K., Domer J. E., McQuitty J. T., Jr Experimental murine candidiasis: pathological and immune responses to cutaneous inoculation with Candida albicans. Infect Immun. 1978 Feb;19(2):499–509. doi: 10.1128/iai.19.2.499-509.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giger D. K., Domer J. E., Moser S. A., McQuitty J. T., Jr Experimental murine candidiasis: pathological and immune responses in T-lymphocyte-depleted mice. Infect Immun. 1978 Sep;21(3):729–737. doi: 10.1128/iai.21.3.729-737.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hector R. F., Domer J. E., Carrow E. W. Immune responses to Candida albicans in genetically distinct mice. Infect Immun. 1982 Dec;38(3):1020–1028. doi: 10.1128/iai.38.3.1020-1028.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert J., Beaudoin R., Aubin M., Fontaine M. The regulatory effect of histamine on the immune response: characterization of the cells involved. Cell Immunol. 1980 Aug 15;54(1):49–57. doi: 10.1016/0008-8749(80)90188-4. [DOI] [PubMed] [Google Scholar]
- KORN E. D., NORTHCOTE D. H. Physical and chemical properties of polysaccharides and glycoproteins of the yeast-cell wall. Biochem J. 1960 Apr;75:12–17. doi: 10.1042/bj0750012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettner M., Para R., Trnovec T. Hepatic and plasma lysosomal enzyme activity in shock-like state following administration of polysaccharide-protein complex isolated from Candida albicans. Circ Shock. 1983;10(1):31–39. [PubMed] [Google Scholar]
- Kirkpatrick C. H., Rich R. R., Bennett J. E. Chronic mucocutaneous candidiasis: model-building in cellular immunity. Ann Intern Med. 1971 Jun;74(6):955–978. doi: 10.7326/0003-4819-74-6-955. [DOI] [PubMed] [Google Scholar]
- Kocourek J., Ballou C. E. Method for fingerprinting yeast cell wall mannans. J Bacteriol. 1969 Dec;100(3):1175–1181. doi: 10.1128/jb.100.3.1175-1181.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moser S. A., Domer J. E. Effects of cyclophosphamide on murine candidiasis. Infect Immun. 1980 Feb;27(2):376–386. doi: 10.1128/iai.27.2.376-386.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser S. A., Domer J. E., Mather F. J. Experimental murine candidiasis: cell-mediated immunity after cutaneous challenge. Infect Immun. 1980 Jan;27(1):140–149. doi: 10.1128/iai.27.1.140-149.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., Moorhead J. W. Regulation of cell-mediated immunity in cryptococcosis. I. Induction of specific afferent T suppressor cells by cryptococcal antigen. J Immunol. 1982 Jan;128(1):276–283. [PubMed] [Google Scholar]
- Nelson R. D., Herron M. J., McCormack R. T., Gehrz R. C. Two mechanisms of inhibition of human lymphocyte proliferation by soluble yeast mannan polysaccharide. Infect Immun. 1984 Mar;43(3):1041–1046. doi: 10.1128/iai.43.3.1041-1046.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosál R., Nosálová V., Sikl D. Hemodynamic effects of the glycoprotein isolated from cell wall of Candida albicans. Toxicon. 1979;17(6):688–672. doi: 10.1016/0041-0101(79)90247-2. [DOI] [PubMed] [Google Scholar]
- Nosálová V., Trnovec T., Gregusková M., Nosál R. The effect of polysaccharide-protein complex isolated from Candida albicans on regional blood flow in rats. Experientia. 1979 Mar 15;35(3):341–342. doi: 10.1007/BF01964340. [DOI] [PubMed] [Google Scholar]
- Piccolella E., Lombardi G., Morelli R. Generation of suppressor cells in the response of human lymphocytes to a polysaccharide from Candida albicans. J Immunol. 1981 Jun;126(6):2151–2155. [PubMed] [Google Scholar]
- Restrepo-Moreno A., Schneidau J. D., Jr Nature of the skin-reactive principle in culture filtrates prepared from Paracoccidioides brasiliensis. J Bacteriol. 1967 Jun;93(6):1741–1748. doi: 10.1128/jb.93.6.1741-1748.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas V., Rogers T. J. Studies on the cellular nature of Candida albicans-induced suppression. J Immunol. 1983 Jan;130(1):376–379. [PubMed] [Google Scholar]
- Rogers T. J., Balish E. Effect of systemic candidiasis on blastogenesis of lymphocytes from germfree and conventional rats. Infect Immun. 1978 Apr;20(1):142–150. doi: 10.1128/iai.20.1.142-150.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. J., Balish E. Suppression of lymphocyte blastogenesis by Candida albicans. Clin Immunol Immunopathol. 1978 Jul;10(3):298–305. doi: 10.1016/0090-1229(78)90185-x. [DOI] [PubMed] [Google Scholar]
- Segal E., Sandovsky-Losica H., Vardinon N. Suppressive action of cytoplasmic and metabolite extracts of Candida albicans on the immune response in guinea pigs. Mycopathologia. 1980 Oct 31;72(2):121–128. doi: 10.1007/BF00493821. [DOI] [PubMed] [Google Scholar]
- Skerl K. G., Scheld W. M., Alliegro G. M., Calderone R. A. Lymphocyte blastogenesis during experimental endocarditis caused by Candida albicans. J Reticuloendothel Soc. 1980 Nov;28(5):495–506. [PubMed] [Google Scholar]
- Stobo J. D., Paul S., Van Scoy R. E., Hermans P. E. Suppressor thymus-derived lymphocytes in fungal infection. J Clin Invest. 1976 Feb;57(2):319–328. doi: 10.1172/JCI108283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. E., Amsbaugh D. F., Stashak P. W., Caldes G., Prescott B., Baker P. J. Cell surface antigens and other characteristics of T cells regulating the antibody response to type III pneumococcal polysaccharide. J Immunol. 1983 Jan;130(1):19–23. [PubMed] [Google Scholar]
- Vardinon N., Segal E. Suppressive action of Candida albicans on the immune response in mice. Exp Cell Biol. 1979;47(4):275–280. doi: 10.1159/000162946. [DOI] [PubMed] [Google Scholar]
- Wright C. D., Bowie J. U., Gray G. R., Nelson R. D. Candidacidal activity of myeloperoxidase: mechanisms of inhibitory influence of soluble cell wall mannan. Infect Immun. 1983 Oct;42(1):76–80. doi: 10.1128/iai.42.1.76-80.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Repentigny L., Reiss E. Current trends in immunodiagnosis of candidiasis and aspergillosis. Rev Infect Dis. 1984 May-Jun;6(3):301–312. doi: 10.1093/clinids/6.3.301. [DOI] [PubMed] [Google Scholar]


