Abstract
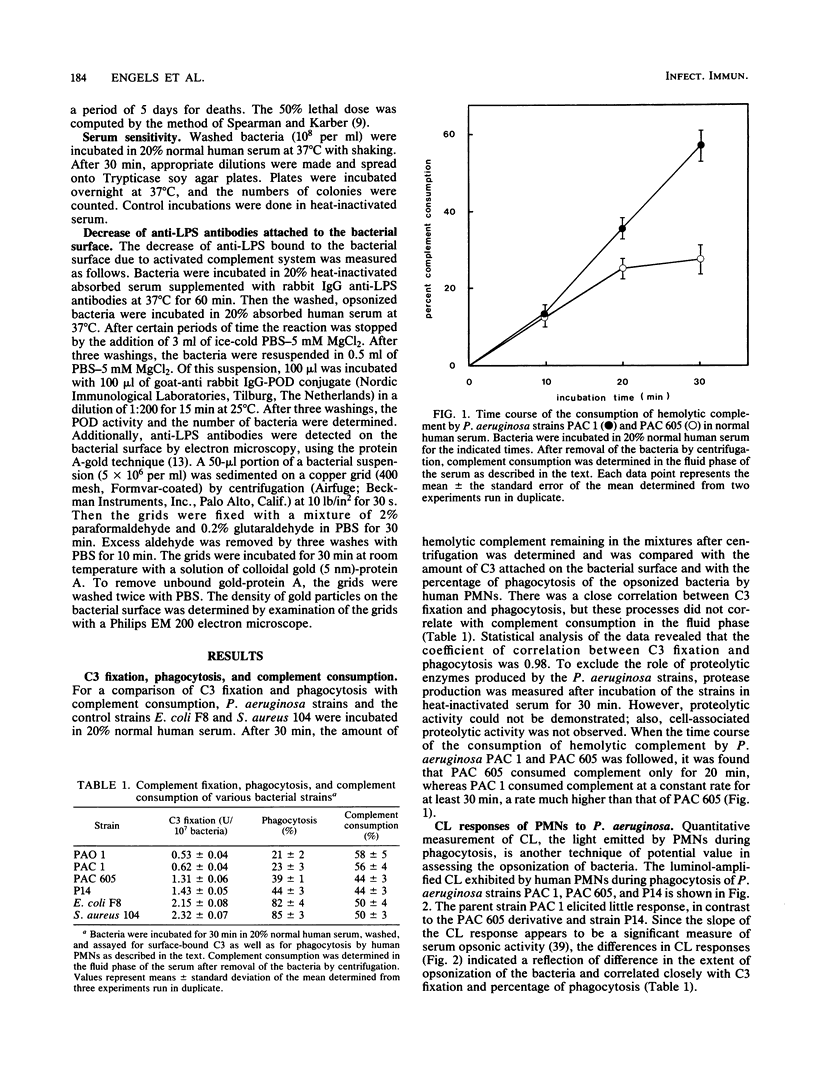
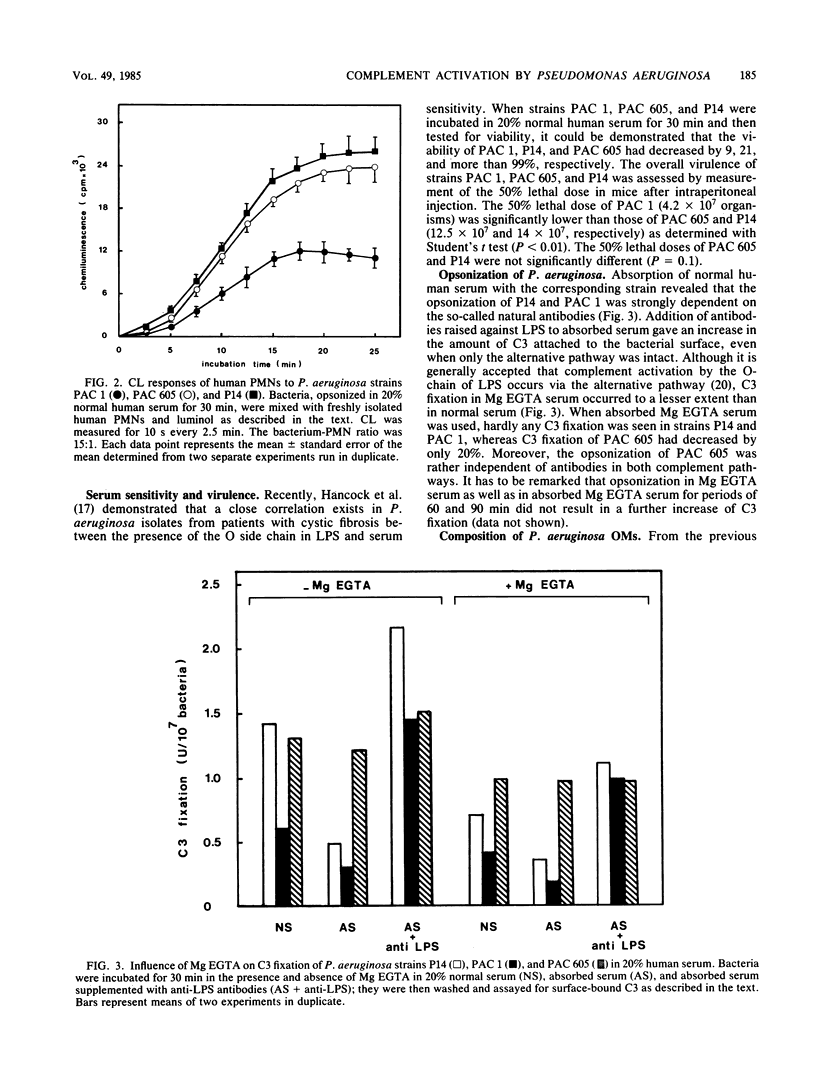
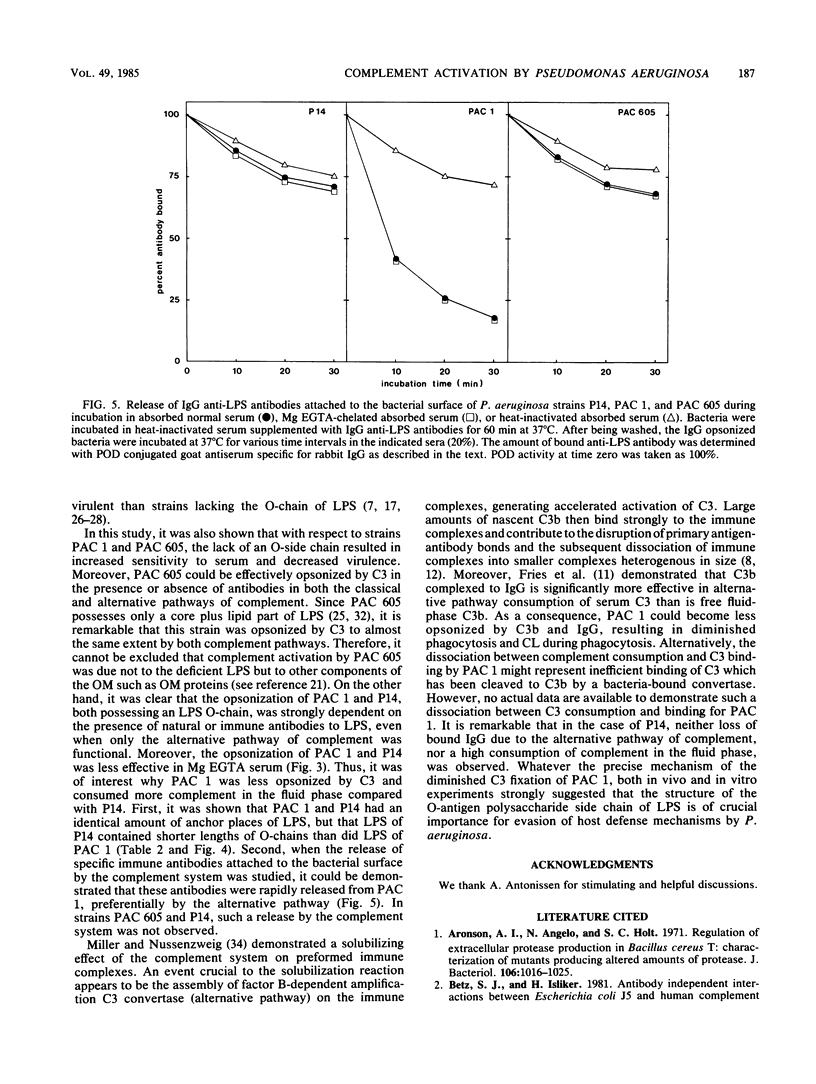
When the opsonization of various Pseudomonas aeruginosa strains--PAC 1, its O-chain-deficient mutant PAC 605, and an intermediate strain, P14--was measured either directly by determination of the amount of C3b attached to the bacterial surface or indirectly by assessing phagocytosis by human polymorphonuclear leukocytes and the responses of chemiluminescence, it was demonstrated that PAC 1 was opsonized and phagocytized to a lower extent than P14 and PAC 605. In contrast to PAC 605, PAC 1 showed an increased consumption of complement in the fluid phase and a rapid release of lipopolysaccharide antibodies bound to the bacterial surface due to the alternative pathway of the complement system. Furthermore, it was shown that with respect to PAC 1 and PAC 605, the lack of an O-chain resulted in increased sensitivity to serum and decreased virulence. From both in vivo and in vitro experiments, we concluded that the structure of the O-antigen polysaccharide chain of lipopolysaccharide is an important virulence factor of P. aeruginosa against the defense mechanisms of the host.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson A. I., Angelo N., Holt S. C. Regulation of extracellular protease production in Bacillus cereus T: characterization of mutants producing altered amounts of protease. J Bacteriol. 1971 Jun;106(3):1016–1025. doi: 10.1128/jb.106.3.1016-1025.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson A. B., Bjornson H. S. Activation of complement by opportunist pathogens and chemotypes of Salmonella minnesota. Infect Immun. 1977 Jun;16(3):748–753. doi: 10.1128/iai.16.3.748-753.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson A. B., Michael J. G. Factors in human serum promoting phagocytosis of Pseudomonas aeruginosa. I. Interaction of opsonins with the bacterium. J Infect Dis. 1974 Nov;130 (Suppl)(0):S119–S126. doi: 10.1093/infdis/130.supplement.s119. [DOI] [PubMed] [Google Scholar]
- Bjornson A. B., Michael J. G. Factors in human serum promoting phagocytosis of Pseudomonas aeruginosa. II. Interaction of opsonins with the phagocytic cell. J Infect Dis. 1974 Nov;130 (Suppl)(0):S127–S131. doi: 10.1093/infdis/130.supplement.s127. [DOI] [PubMed] [Google Scholar]
- Cooper N. R., Morrison D. C. Binding and activation of the first component of human complement by the lipid A region of lipopolysaccharides. J Immunol. 1978 Jun;120(6):1862–1868. [PubMed] [Google Scholar]
- Cryz S. J., Jr, Pitt T. L., Fürer E., Germanier R. Role of lipopolysaccharide in virulence of Pseudomonas aeruginosa. Infect Immun. 1984 May;44(2):508–513. doi: 10.1128/iai.44.2.508-513.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czop J., Nussenzweig V. Studies on the mechanism of solubilization of immune precipitates by serum. J Exp Med. 1976 Mar 1;143(3):615–630. doi: 10.1084/jem.143.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren A., Quie P. G. Influence of the alternate complement pathway in opsonization of several bacterial species. Infect Immun. 1974 Aug;10(2):402–404. doi: 10.1128/iai.10.2.402-404.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries L. F., Gaither T. A., Hammer C. H., Frank M. M. C3b covalently bound to IgG demonstrates a reduced rate of inactivation by factors H and I. J Exp Med. 1984 Dec 1;160(6):1640–1655. doi: 10.1084/jem.160.6.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Takata Y., Tamura N. Solubilization of immune precipitates by six isolated alternative pathway proteins. J Exp Med. 1981 Dec 1;154(6):1743–1751. doi: 10.1084/jem.154.6.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., van der Ley P. A., Scheffer R. C. Use of colloidal gold particles in double-labeling immunoelectron microscopy of ultrathin frozen tissue sections. J Cell Biol. 1981 Jun;89(3):653–665. doi: 10.1083/jcb.89.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonggrijp R., Volleberg M. P., Lemmens P. J., van Boven C. P. Evidence for the presence of lipopolysaccharide in a ribonuclease-sensitive ribosomal vaccine of Pseudomonas aeruginosa. Infect Immun. 1981 Mar;31(3):896–905. doi: 10.1128/iai.31.3.896-905.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman N., Leive L. Complement activation via the alternative pathway by purified Salmonella lipopolysaccharide is affected by its structure but not its O-antigen length. J Immunol. 1984 Jan;132(1):376–385. [PubMed] [Google Scholar]
- Hammer M. C., Baltch A. L., Sutphen N. T., Smith R. P., Conroy J. V. Pseudomonas aeruginosa: quantitation of maximum phagocytic and bactericidal capabilities of normal human granulocytes. J Lab Clin Med. 1981 Dec;98(6):938–948. [PubMed] [Google Scholar]
- Hancock R. E., Mutharia L. M., Chan L., Darveau R. P., Speert D. P., Pier G. B. Pseudomonas aeruginosa isolates from patients with cystic fibrosis: a class of serum-sensitive, nontypable strains deficient in lipopolysaccharide O side chains. Infect Immun. 1983 Oct;42(1):170–177. doi: 10.1128/iai.42.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Brown E. J., Frank M. M. Complement and bacteria: chemistry and biology in host defense. Annu Rev Immunol. 1984;2:461–491. doi: 10.1146/annurev.iy.02.040184.002333. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Goldman R., Schmetz M., Berger M., Hammer C. H., Frank M. M., Leive L. A quantitative analysis of C3 binding to O-antigen capsule, lipopolysaccharide, and outer membrane protein of E. coli 0111B4. J Immunol. 1984 Jan;132(1):369–375. [PubMed] [Google Scholar]
- Joiner K. A., Hammer C. H., Brown E. J., Cole R. J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. I. Terminal complement components are deposited and released from Salmonella minnesota S218 without causing bacterial death. J Exp Med. 1982 Mar 1;155(3):797–808. doi: 10.1084/jem.155.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Hammer C. H., Brown E. J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. II. C8 and C9 release C5b67 from the surface of Salmonella minnesota S218 because the terminal complex does not insert into the bacterial outer membrane. J Exp Med. 1982 Mar 1;155(3):809–819. doi: 10.1084/jem.155.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Schmetz M. A., Goldman R. C., Leive L., Frank M. M. Mechanism of bacterial resistance to complement-mediated killing: inserted C5b-9 correlates with killing for Escherichia coli O111B4 varying in O-antigen capsule and O-polysaccharide coverage of lipid A core oligosaccharide. Infect Immun. 1984 Jul;45(1):113–117. doi: 10.1128/iai.45.1.113-117.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koval S. F., Meadow P. M. The isolation and characterization of lipopolysaccharide-defective mutants of Pseudomonas aeruginosa PAC1. J Gen Microbiol. 1977 Feb;98(2):387–398. doi: 10.1099/00221287-98-2-387. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liang-Takasaki C. J., Grossman N., Leive L. Salmonellae activate complement differentially via the alternative pathway depending on the structure of their lipopolysaccharide O-antigen. J Immunol. 1983 Apr;130(4):1867–1870. [PubMed] [Google Scholar]
- Liang-Takasaki C. J., Mäkelä P. H., Leive L. Phagocytosis of bacteria by macrophages: changing the carbohydrate of lipopolysaccharide alters interaction with complement and macrophages. J Immunol. 1982 Mar;128(3):1229–1235. [PubMed] [Google Scholar]
- Liang-Takasaki C. J., Saxén H., Mäkelä P. H., Leive L. Complement activation by polysaccharide of lipopolysaccharide: an important virulence determinant of salmonellae. Infect Immun. 1983 Aug;41(2):563–569. doi: 10.1128/iai.41.2.563-569.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Meshulam T., Verbrugh H. A., Verhoef J. Opsonization and phagocytosis of mucoid and non-mucoid Pseudomonas aeruginosa strains. Eur J Clin Microbiol. 1982 Apr;1(2):112–117. doi: 10.1007/BF02014202. [DOI] [PubMed] [Google Scholar]
- Miller G. W., Nussenzweig V. A new complement function: solubilization of antigen-antibody aggregates. Proc Natl Acad Sci U S A. 1975 Feb;72(2):418–422. doi: 10.1073/pnas.72.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Kageyama M. Separation and characterization of the outer membrane of Pseudomonas aeruginosa. J Biochem. 1978 Jul;84(1):179–191. doi: 10.1093/oxfordjournals.jbchem.a132106. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Kline L. F. Activation of the classical and properdin pathways of complement by bacterial lipopolysaccharides (LPS). J Immunol. 1977 Jan;118(1):362–368. [PubMed] [Google Scholar]
- Peterson P. K., Kim Y., Schmeling D., Lindemann M., Verhoef J., Quie P. G. Complement-mediated phagocytosis of Pseudomonas aeruginosa. J Lab Clin Med. 1978 Dec;92(6):883–894. [PubMed] [Google Scholar]
- Robinson P., Wakefield D., Breit S. N., Easter J. F., Penny R. Chemiluminescent response to pathogenic organisms: normal human polymorphonuclear leukocytes. Infect Immun. 1984 Feb;43(2):744–752. doi: 10.1128/iai.43.2.744-752.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkinoja-Salonen M., Nurmiaho E. L. The effect of lipopolysaccharide composition on the ultrastructure of Pseudomonas aeruginosa. J Gen Microbiol. 1978 Mar;105(1):23–28. doi: 10.1099/00221287-105-1-23. [DOI] [PubMed] [Google Scholar]
- Takada A., Imamura Y., Takada Y. Relationships between the haemolytic activities of the human complement system and complement components. Clin Exp Immunol. 1979 Feb;35(2):324–328. [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W. Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol Rev. 1983 Mar;47(1):46–83. doi: 10.1128/mr.47.1.46-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Verbrugh H. A., Peters R., Peterson P. K., Verhoef J. Phagocytosis and killing of staphylococci by human polymorphonuclear and mononuclear leucocytes. J Clin Pathol. 1978 Jun;31(6):539–545. doi: 10.1136/jcp.31.6.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]
- Witholt B., Heerikhuizen H. V., De Leij L. How does lysozyme penetrate through the bacterial outer membrane? Biochim Biophys Acta. 1976 Sep 7;443(3):534–544. doi: 10.1016/0005-2736(76)90471-5. [DOI] [PubMed] [Google Scholar]
- Wolters G., Kuijpers L., Kacaki J., Schuurs A. Solid-phase enzyme-immunoassay for detection of hepatitis B surface antigen. J Clin Pathol. 1976 Oct;29(10):873–879. doi: 10.1136/jcp.29.10.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. S., Armstrong D. Human immunity to Pseudomonas aeruginosa. I. In-vitro interaction of bacteria, polymorphonuclear leukocytes, and serum factors. J Infect Dis. 1972 Sep;126(3):257–276. doi: 10.1093/infdis/126.3.257. [DOI] [PubMed] [Google Scholar]