Abstract
Nude athymic (nu/nu) mice on a BALB/c background and their heterozygous euthymic litter mates (nu/+) were infected with either 10(8) or 10(6) Mycobacterium lepraemurium organisms intravenously or in the left hind footpad (LHF). After LHF infection with 10(8) M. lepraemurium organisms, nu/+ mice slowly developed a response that consisted of LHF swelling and local resistance to Listeria monocytogenes. The lower inoculum induced a proportionately lower response in nu/+ mice, but the nu/nu mice developed neither LHF swelling nor resistance to L. monocytogenes in response to either dose of M. lepraemurium. Counts of M. lepraemurium in the LHF revealed no difference between the nu/+ mice and nu/nu mice. After intravenous infection the nu/+ mice developed splenomegaly, but did not otherwise differ from nu/nu mice with respect to resistance to intravenous challenge with L. monocytogenes or growth of M. lepraemurium in the spleen. In light of the poor responsiveness of nu/+ mice in this experiment, they were then compared with CB6 and B6D2 mice, which are genetically susceptible and resistant to M. lepraemurium, respectively. These mice were infected with either 10(8) or 10(6) M. lepraemurium cells or 10(6) Mycobacterium bovis BCG cells in the LHF. Once again the nu/+ mice responded poorly to M. lepraemurium, the CB6 mice responded very strongly, and the B6D2 mice gave an intermediate response with respect to LHF swelling and resistance to L. monocytogenes. However, M. lepraemurium grew to higher numbers in the LHF of nu/+ and CB6 mice than in B6D2 mice, revealing, in CB6 mice, a dissociation between resistance to L. monocytogenes and M. lepraemurium. All three mouse strains responded strongly to M. bovis BCG, but there was a suggestion that nu/+ mice might be more susceptible to this agent than the other two strains. I concluded that the failure of nu/+ mice to restrict the growth of M. lepraemurium more than nu/nu mice was due to the intrinsic genetic susceptibility of both types of mice. In effect, the nu/+ mice behaved like nu/nu mice, as if they too were deficient in T lymphocytes that were responsive to M. lepraemurium.
Full text
PDF
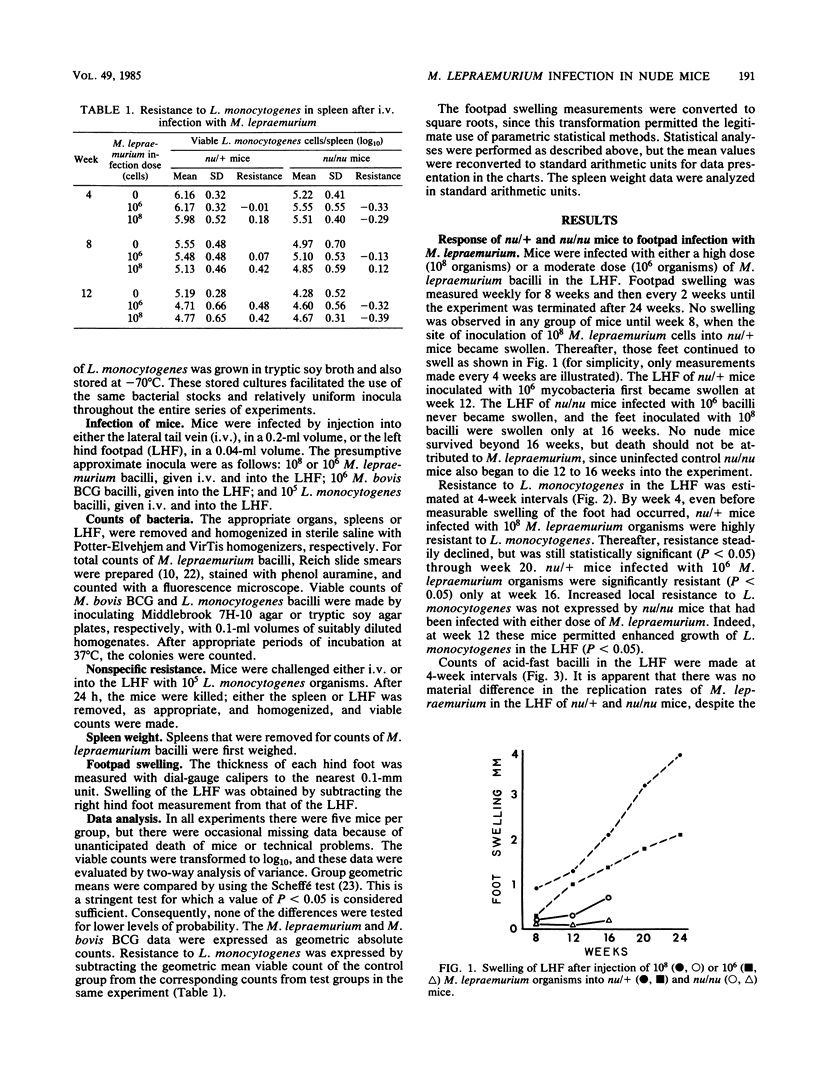
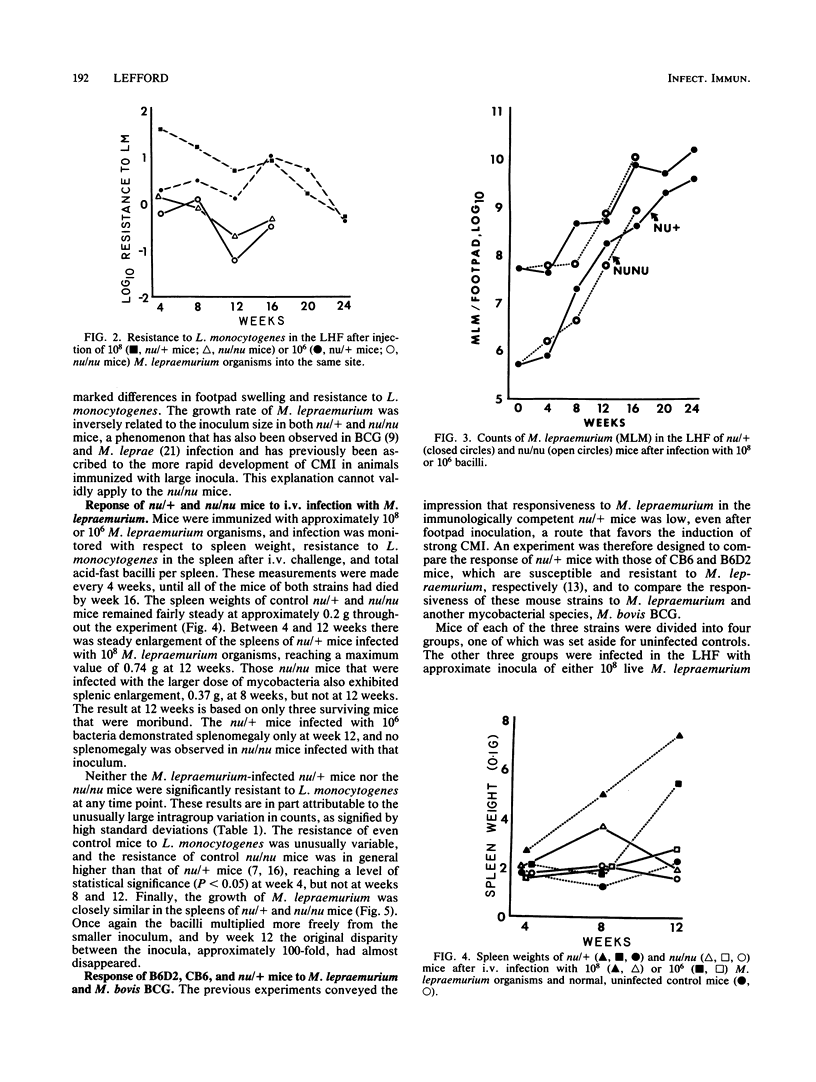
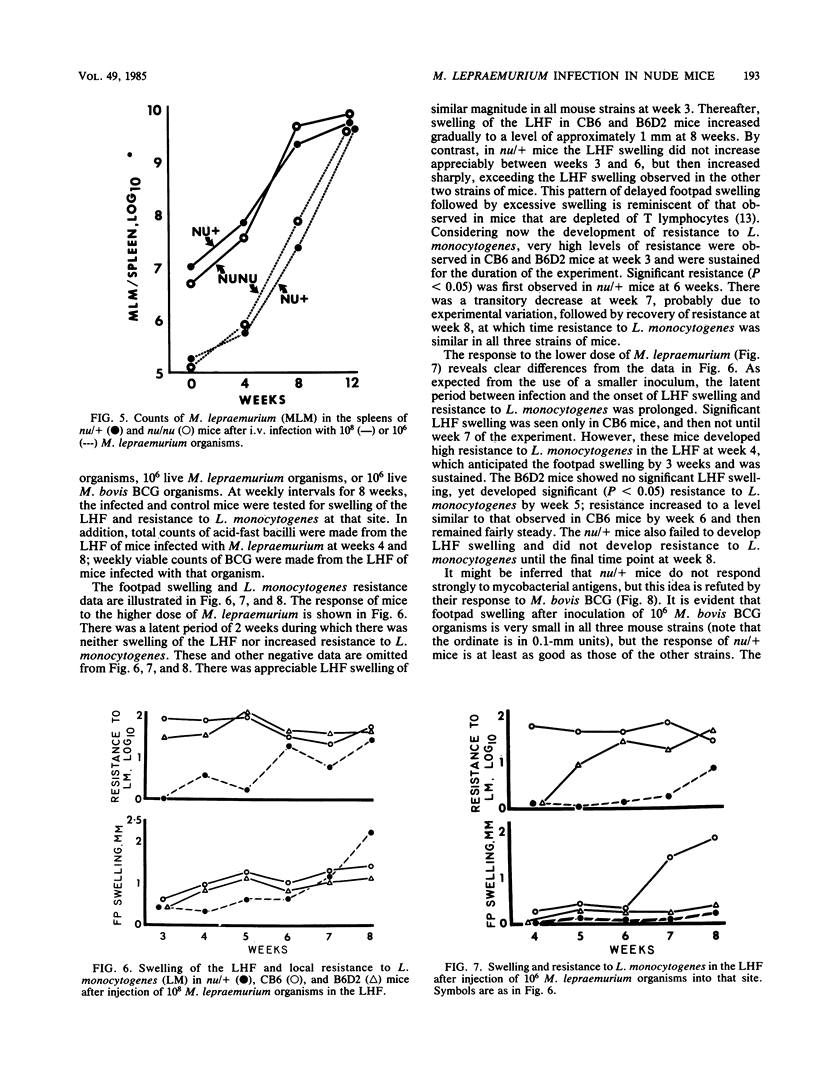
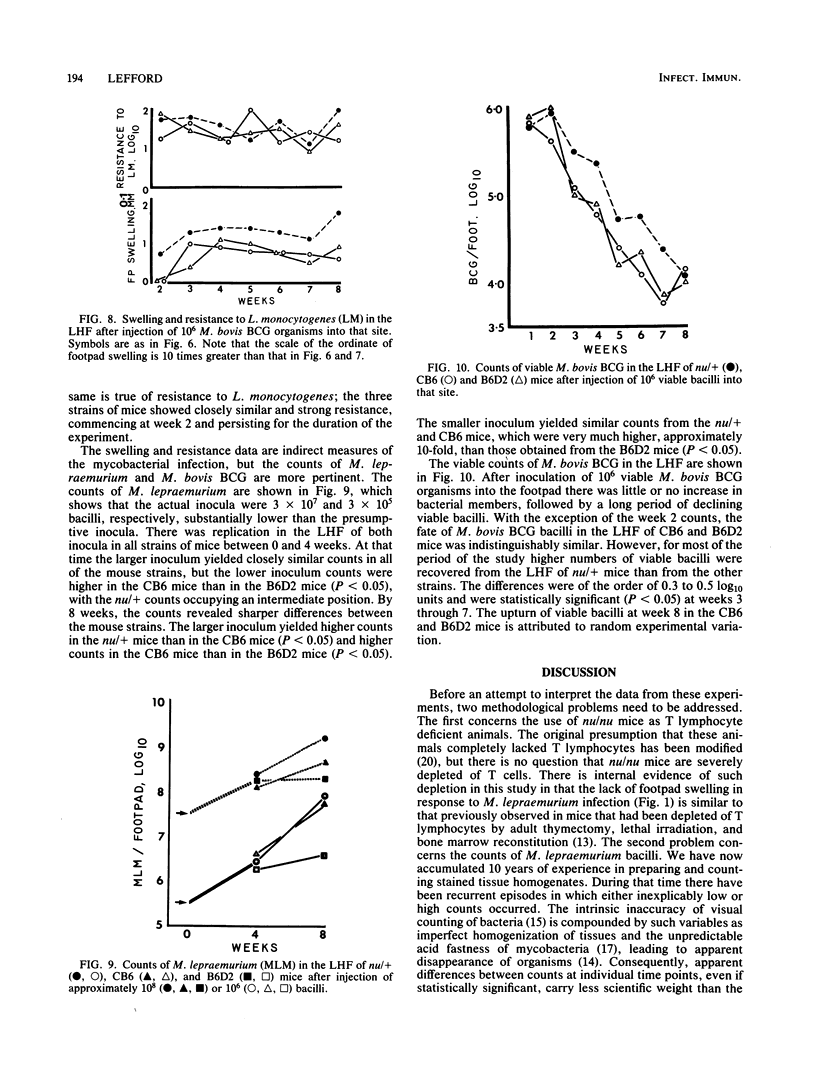


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J., Curtis J. Development of delayed hypersensitivity responses in Mycobacterium lepraemurium infections in resistant and susceptible strains of mice. Immunology. 1979 Mar;36(3):563–567. [PMC free article] [PubMed] [Google Scholar]
- Bullock W. E., Jr Perturbation of lymphocyte circulation in experimental murine leprosy. I. Description of the defect. J Immunol. 1976 Oct;117(4):1164–1170. [PubMed] [Google Scholar]
- Closs O. Experimental murine leprosy: growth of Mycobacterium lepraemurium in C3H and C57/BL mice after footpad inoculation. Infect Immun. 1975 Sep;12(3):480–489. doi: 10.1128/iai.12.3.480-489.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closs O., Haugen O. A. Experimental murine leprosy. 3. Early local reaction to mycobacterium lepraemurium in C3H and C57/BL mice. Acta Pathol Microbiol Scand A. 1975 Jan;83(1):51–58. [PubMed] [Google Scholar]
- Closs O., Haugen O. A. Experimental murine leprosy. I. Clinical histological evidence for varying susceptibility of mice to infection with Mycobacterium lepraemurium. Acta Pathol Microbiol Scand A. 1973 Jul;81(4):401–410. [PubMed] [Google Scholar]
- Curtis J., Adu H. O., Turk J. L. A lack of correlation between antigen-specific cellular reactions and resistance to Mycobacterium lepraemurium infection in mice. Immunology. 1981 Jun;43(2):293–301. [PMC free article] [PubMed] [Google Scholar]
- Emmerling P., Finger H., Bockemühl J. Listeria monocytogenes infection in nude mice. Infect Immun. 1975 Aug;12(2):437–439. doi: 10.1128/iai.12.2.437-439.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWAGUCHI Y. Classification of mouse leprosy. Jpn J Exp Med. 1959 Dec;29:651–663. [PubMed] [Google Scholar]
- Lefford M. J., Logie P. S. Induction and suppression of cross-reactive antituberculosis immunity after Mycobacterium lepraemurium infection of mice. Infect Immun. 1981 Mar;31(3):1023–1033. doi: 10.1128/iai.31.3.1023-1033.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J., Mackaness G. B. Suppression of immunity to Mycobacterium lepraemurium infection. Infect Immun. 1977 Nov;18(2):363–369. doi: 10.1128/iai.18.2.363-369.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J., Morgan R., Logie P. S. Effect of Mycobacterium bovis BCG vaccination upon Mycobacterium lepraemurium infection. Infect Immun. 1980 Jun;28(3):860–866. doi: 10.1128/iai.28.3.860-866.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J., Patel P. J., Poulter L. W., Mackaness G. B. Induction of cell-mediated immunity to Mycobacterium lepraemurium in susceptible mice. Infect Immun. 1977 Dec;18(3):654–659. doi: 10.1128/iai.18.3.654-659.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J. The effect of inoculum size on the immune response to BCG infection in mice. Immunology. 1971 Aug;21(2):369–381. [PMC free article] [PubMed] [Google Scholar]
- Levy L., Moon N., Murray L. P., O'Neill S. M., Gustafson L. E., Evans M. J. Studies of the mouse foot pad technic for cultivation of Mycobacterium leprae. 1. Fate of inoculated organisms. Int J Lepr Other Mycobact Dis. 1974 Apr-Jun;42(2):165–173. [PubMed] [Google Scholar]
- Newborg M. F., North R. J. On the mechanism of T cell-independent anti-Listeria resistance in nude mice. J Immunol. 1980 Feb;124(2):571–576. [PubMed] [Google Scholar]
- Nyka W. Method for staining both acid-fast and chromophobic tubercle bacilli with carbolfuschsin. J Bacteriol. 1967 Apr;93(4):1458–1460. doi: 10.1128/jb.93.4.1458-1460.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P. J. Antibacterial resistance in mice infected with Mycobacterium lepraemurium. Clin Exp Immunol. 1981 Sep;45(3):654–661. [PMC free article] [PubMed] [Google Scholar]
- Poulter L. W., Lefford M. J. Relationship between delayed-type hypersensitivity and the progression of Mycobacterium lepraemurium infection. Infect Immun. 1978 May;20(2):530–540. doi: 10.1128/iai.20.2.530-540.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranges G. E., Goldstein G., Boyse E. A., Schield M. P. T cell development in normal and thymopentin-treated nude mice. J Exp Med. 1982 Oct 1;156(4):1057–1064. doi: 10.1084/jem.156.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEPARD C. C. Multiplication of Mycobacterium leprae in the foot-pad of the mouse. Int J Lepr. 1962 Jul-Sep;30:291–306. [PubMed] [Google Scholar]
- Shepard C. C., McRae D. H. A method for counting acid-fast bacteria. Int J Lepr Other Mycobact Dis. 1968 Jan-Mar;36(1):78–82. [PubMed] [Google Scholar]
- Turcotte R. Influence of route of Mycobacterium lepraemurium injection on susceptibility to mouse leprosy and on lymphoblastic transformation. Infect Immun. 1980 Jun;28(3):660–668. doi: 10.1128/iai.28.3.660-668.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


