Abstract
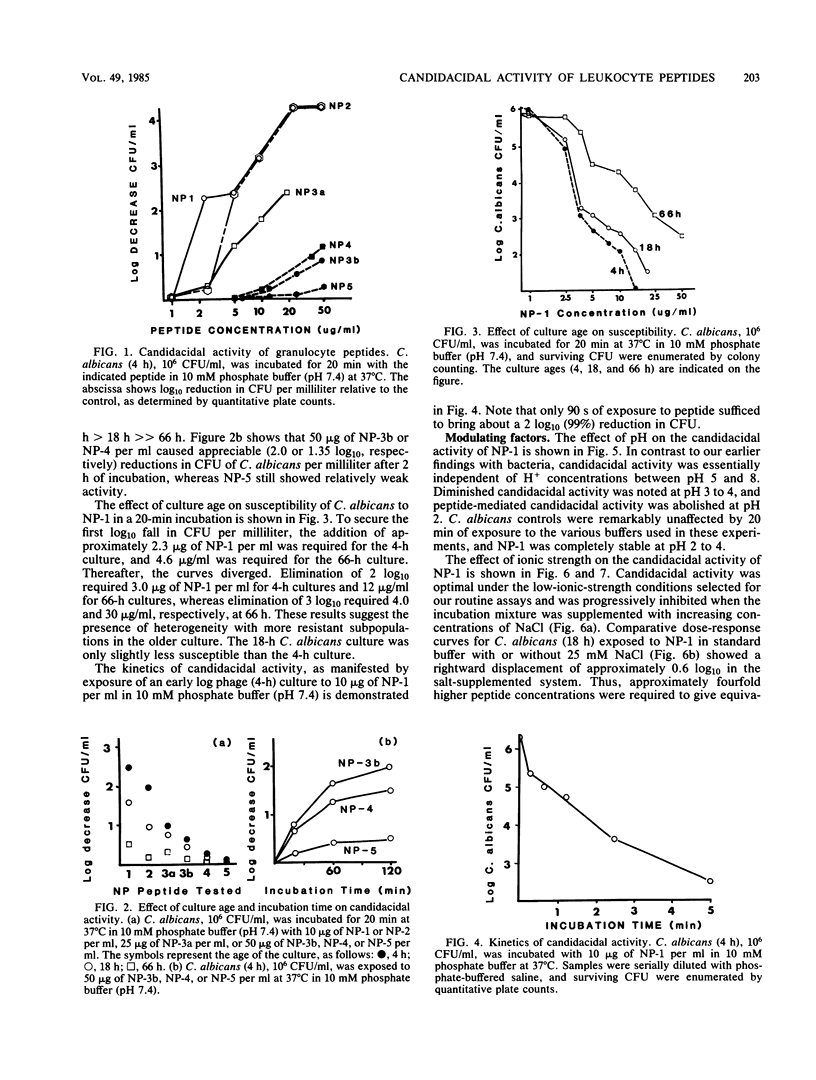
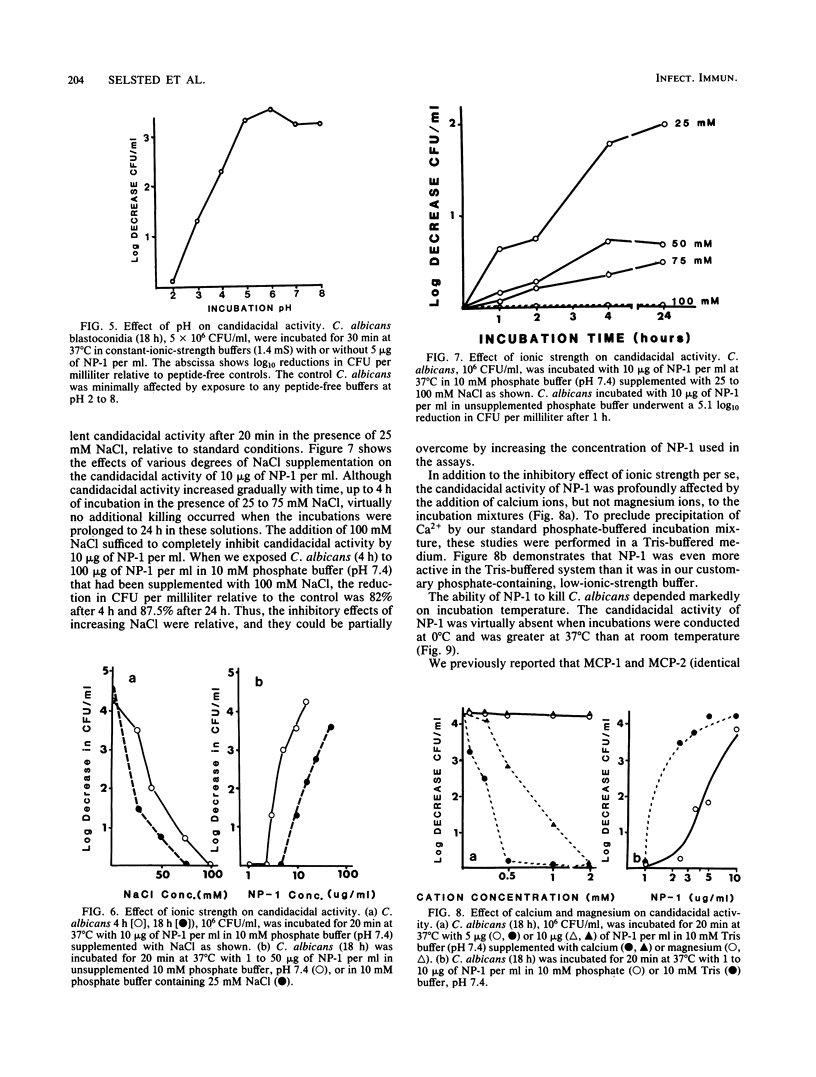
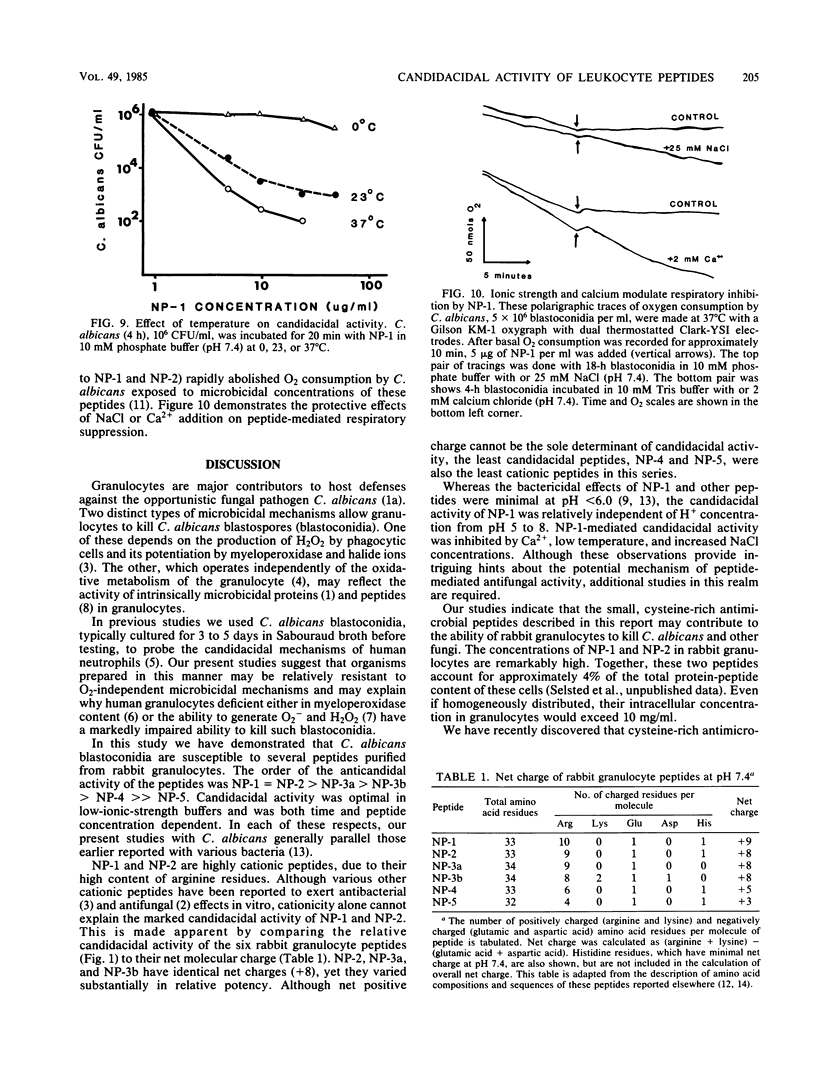
Six related cysteine-rich, low-molecular-weight peptides were purified from rabbit peritoneal granulocytes and tested in vitro for fungicidal activity against Candida albicans. Two peptides (NP-1 and NP-2) were highly effective, one (NP-3a) was moderately active, and three (NP-3b greater than NP-4 much greater than NP-5) had substantially less potency. There was a general, but imperfect, correlation between the candidacidal potency of each peptide and its net cationic charge. Candidacidal activity by NP-1 was concentration and time dependent and occurred rapidly under optimal low-ionic-strength conditions. It was inhibited by increasing either the ionic strength or Ca2+ concentration of the incubation mixtures, but was relatively unaffected by Mg2+. Candidacidal activity was independent of H+ concentrations between pH 5 and 8, but decreased below pH 5. Candidacidal activity was temperature sensitive and was virtually abolished when NP-1 was incubated with C. albicans at 0 degrees C. Cysteine-rich antimicrobial peptides such as NP-1 and NP-2 may equip leukocytes to deal with infections caused by C. albicans and other fungi that are susceptible to their microbicidal effects.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Drazin R. E., Lehrer R. I. Fungicidal properties of a chymotrypsin-like cationic protein from human neutrophils: adsorption to Candida parapsilosis. Infect Immun. 1977 Aug;17(2):382–388. doi: 10.1128/iai.17.2.382-388.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadebusch H. H., Johnson A. G. Natural host resistance to infection with Cryptococcus neoformans. IV. The effect of some cationic proteins on the experimental disease. J Infect Dis. 1966 Dec;116(5):551–565. doi: 10.1093/infdis/116.5.551. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Interaction of Candida albicans with human leukocytes and serum. J Bacteriol. 1969 Jun;98(3):996–1004. doi: 10.1128/jb.98.3.996-1004.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969 Aug;48(8):1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. Functional aspects of a second mechanism of candidacidal activity by human neutrophils. J Clin Invest. 1972 Oct;51(10):2566–2572. doi: 10.1172/JCI107073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Ladra K. M., Hake R. B. Nonoxidative fungicidal mechanisms of mammalian granulocytes: demonstration of components with candidacidal activity in human, rabbit, and guinea pig leukocytes. Infect Immun. 1975 Jun;11(6):1226–1234. doi: 10.1128/iai.11.6.1226-1234.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Selsted M. E., Szklarek D., Fleischmann J. Antibacterial activity of microbicidal cationic proteins 1 and 2, natural peptide antibiotics of rabbit lung macrophages. Infect Immun. 1983 Oct;42(1):10–14. doi: 10.1128/iai.42.1.10-14.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson-Delafield J., Martinez R. J., Lehrer R. I. Microbicidal cationic proteins in rabbit alveolar macrophages: a potential host defense mechanism. Infect Immun. 1980 Oct;30(1):180–192. doi: 10.1128/iai.30.1.180-192.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson-Delafield J., Szklarek D., Martinez R. J., Lehrer R. I. Microbicidal cationic proteins of rabbit alveolar macrophages: amino acid composition and functional attributes. Infect Immun. 1981 Feb;31(2):723–731. doi: 10.1128/iai.31.2.723-731.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted M. E., Brown D. M., DeLange R. J., Harwig S. S., Lehrer R. I. Primary structures of six antimicrobial peptides of rabbit peritoneal neutrophils. J Biol Chem. 1985 Apr 25;260(8):4579–4584. [PubMed] [Google Scholar]
- Selsted M. E., Brown D. M., DeLange R. J., Lehrer R. I. Primary structures of MCP-1 and MCP-2, natural peptide antibiotics of rabbit lung macrophages. J Biol Chem. 1983 Dec 10;258(23):14485–14489. [PubMed] [Google Scholar]
- Selsted M. E., Szklarek D., Lehrer R. I. Purification and antibacterial activity of antimicrobial peptides of rabbit granulocytes. Infect Immun. 1984 Jul;45(1):150–154. doi: 10.1128/iai.45.1.150-154.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Arginine-rich proteins of polymorphonuclear leukocyte lysosomes. Antimicrobial specificity and biochemical heterogeneity. J Exp Med. 1968 May 1;127(5):927–941. doi: 10.1084/jem.127.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Cationic proteins of polymorphonuclear leukocyte lysosomes. II. Composition, properties, and mechanism of antibacterial action. J Bacteriol. 1966 Feb;91(2):755–762. doi: 10.1128/jb.91.2.755-762.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


