Abstract
NusA, a modulator of RNA polymerase, interacts with the DNA polymerase DinB. An increased level of expression of dinB or umuDC suppresses the temperature sensitivity of the nusA11 strain, requiring the catalytic activities of these proteins. We propose that NusA recruits translesion DNA synthesis (TLS) polymerases to RNA polymerases stalled at gaps, coupling TLS to transcription.
DNA is damaged from a variety of endogenous and exogenous sources, which can result in a variety of cellular problems including cell death (12). All organisms have mechanisms of DNA repair and DNA damage tolerance to help them to survive DNA damage (12). One important mechanism of DNA damage tolerance is translesion DNA synthesis (TLS), a process in which a specialized DNA polymerase copies past DNA lesions that block the highly accurate, stringent replicative DNA polymerases. Although certain TLS polymerases can catalyze proficient DNA synthesis across from cognate lesions, they have reduced fidelity on undamaged templates (11, 16). TLS polymerases are conserved throughout all domains of life, with the majority being members of the Y family of DNA polymerases (37). Escherichia coli has two Y-family DNA polymerases, DinB (polymerase IV [Pol IV]) and UmuD′2C (Pol V).
DinB (termed DNA Pol kappa in eukaryotes) is the only Y-family polymerase found in all domains of life, yet despite its striking conservation, the role of DinB in vivo is still incompletely understood. E. coli DinB is known to be involved in the phenomenon of λ untargeted mutagenesis (4) and adaptive mutagenesis (19), and when expressed at increased levels, it causes an increase in −1 frameshift mutations (22). It was recently discovered that ΔdinB strains are sensitive to the DNA-damaging agents nitrofurazone and 4-nitroquinolone-1-oxide and that DinB preferentially and accurately bypasses certain N2-dG adducts (21, 24, 32, 51). This ability to preferentially bypass these N2-dG adducts is conserved evolutionarily (21), suggesting a possible reason for the conservation across all domains of life. Additionally, DinB has been shown to incorporate oxidized nucleotides (50) and possess lyase activity (42) and was suggested to be involved in replication-arrest-stimulated recombination (29). Intriguingly, mammalian Pol kappa has been implicated in nucleotide excision repair and was proposed to function in the patching or gap-filling step (36). Given that improper access to DNA or misregulation by increased levels of expression of TLS polymerases can be mutagenic under normal conditions, it is extremely important that TLS polymerases are properly regulated. In E. coli, dinB and umuDC are both transcriptionally induced as part of the SOS response to DNA damage (12). In addition, the activity of UmuC is controlled by an elaborate posttranscriptional regulatory process that includes the RecA-mediated cleavage of its partner UmuD to UmuD′ and interactions with the β processivity clamp and RecA (12, 31, 46). DinB also interacts with the β clamp, and its activity has recently been shown to be controlled by the umuD gene products and RecA (14). Both DinB and UmuC also interact with the molecular chaperone GroEL (8, 14, 48).
A DinB affinity column assay used to search for potential DinB-interacting proteins within lysates of cells that constitutively express the SOS response found that UmuD, UmuD′, and RecA physically associate with DinB (14). An extension of this study (14), by binding purified recombinant His6-HMK (heart muscle kinase)-DinB to an Ni2+-charged affinity resin to generate a DinB affinity column, identified NusA as being a potential interactor, as determined by N-terminal sequencing (Fig. 1A). However, identification of protein interaction partners by affinity methods can lead to a high frequency of false-positive interactions (9, 38). Furthermore, confirmation of an interaction by other methods does not necessarily imply any relevance in vivo. Here, we report that NusA, long known to be an RNA polymerase-associated factor, physically interacts with DinB and that nusA genetically interacts with both dinB and umuDC. These unexpected findings suggest additional biological roles for NusA besides modulating RNA polymerase function.
FIG. 1.
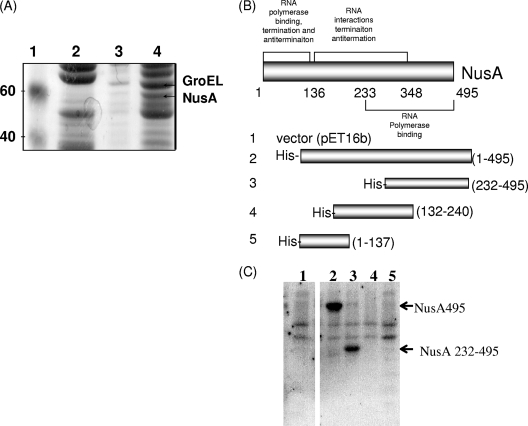
DinB physically interacts with NusA. (A) Coomassie-stained sodium dodecyl sulfate (SDS)-polyacrylamide gel showing several steps of traditional immobilized metal ion chromatography where purified recombinant His6-HMK-DinB was bound to an Ni2+-charged affinity resin using conditions and reagents recommended by Qiagen. Recombinant His6-HMK-DinB was purified as previously described (3). Lane 1, molecular weight markers (in thousands); lane 2, nonspecific binding to resin; lane 3, washes with 20 mM imidazole; lane 4, DinB affinity column eluate eluted with 300 mM imidazole. The interacting proteins NusA and GroEL were identified by Edman degradation (MIT CCR Core Facility). (B) Schematic of NusA constructs used for far-Western experiments. (Adapted from reference 30 with permission of the publisher.) (C) Far-Western blot demonstrates that the interaction between DinB and NusA is direct. BL21(DE3) cell lysates expressing NusA and NusA derivatives (30) (Table 1) were separated by SDS-polyacrylamide gel electrophoresis, transferred onto a polyvinylidene difluoride membrane, and probed with 32P-labeled His6-HMK-DinB as previously described (14, 45). Lane 1, vector (pET16b); lane 2, pNusA(1-495); lane 3, pNusA(232-495); lane 4, pNusA(132-240); lane 5, pNusA(1-137).
NusA is an essential protein that functions in both the termination and antitermination of transcription and is thought to be associated with the RNA polymerase throughout the elongation and termination steps of transcription (6, 10, 17, 25, 26, 28, 40). Originally reported in 1974, NusA forms an antitermination complex with the λN protein that is required for successful λ phage infection (13) and was recently found to form a shield with the λQ protein to protect the emerging transcript from termination mechanisms (41). NusA is highly conserved throughout bacterial and archaeal domains of life; however, to date, no eukaryotic sequence or functional homolog has been identified.
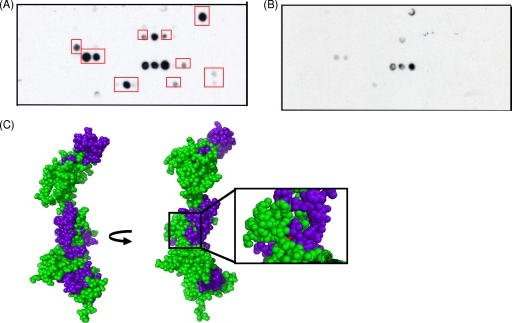
To test whether the interaction between DinB and NusA detected by affinity chromatography is direct or indirect, we performed a far-Western experiment using cell lysates expressing NusA and NusA derivatives (30) (Fig. 1B and Table 1). As shown in Fig. 1C, our observations indicate that DinB and NusA do indeed interact and that they do so directly. Interestingly, the C-terminal 263 amino acids of NusA, which seem to be especially important for the interaction with DinB, are also implicated in binding to RNA polymerase (30), implying that this region is involved in the binding of both RNA polymerase and DinB. We also used cellulose filter peptide arrays to search for peptides of NusA that might potentially interact with DinB. Filter peptide arrays containing 12-mer peptides of NusA each overlapped by two residues were probed with purified recombinant DinB (Fig. 2A) or were performed without a DinB incubation step (Fig. 2B). Interacting peptides were then mapped to a homology model of NusA based on the Thermotoga maritima crystal structure (43) (Fig. 2C). Interestingly, we found that one potential DinB binding region of NusA encompasses several surface residues around the site of the temperature-sensitive mutation of the nusA11 allele (33). While some peptides found to potentially bind to DinB are located within the C-terminal 263 amino acids of NusA, consistent with the far-Western results, others are found outside of this region. Further study will be required to define the exact details of how DinB and NusA interact, but it is possible that there are multiple contact sites since neither the far-Western approach nor the peptide array approach takes into account the full tertiary structures of the proteins.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Genotype | Reference or source |
|---|---|---|
| Strains | ||
| P90C | Δ(lac-pro)XIIara gal | 5 |
| IQ419 | zha-132::Tn10 arg rpsL257 | CGSC |
| YN2351 | metBtrpE9829(Am) tyr(Am) sup-126 nusA11 | 33 |
| SEC26 | Same as YN2351 except for zha-132::Tn10, constructed by P1vir transduction (IQ419 × YN2351) | This work |
| SEC29 | Same as P90C except for nusA11zha0132::Tn10 | This work |
| BL21(DE3) | Novagen | |
| AB1157 | thr-1 leuB6 proA2 hisG4 thi1 argE3 lacY1 galK2 ara-14 xyl-5 mtl-1 tsx-33 rpsL31 supE44 | Laboratory stock |
| Plasmids | ||
| pWSK29 | Vector; pSC101-like replicon; Ampr | 21 |
| pYG782(pdinB+) | dinB gene cloned into plasmid pWSK30 (Ampr), which is the same as pWSK29 but with the multiple-cloning site cloned in the opposite orientation under the lac promoter | 22 |
| pdinB003 | Same as pYG782 except for the dinB gene encoding the D103N mutation | 49 |
| pdinBΔβ | Same as pYG782 except for the dinB gene encoding a truncation mutant to delete C-terminal residues, deleting the β binding motif; premature stop codon inserted using a site-directed mutagenesis kit from Stratagene | This work |
| pdinB(F13V) | Same as pYG782 except for the dinB gene encoding the F13V mutation | 21 |
| pBR322 | Vector; Ampr | New England Biolabs |
| pDCoc | umuDC cloned into pBR322 under its operator constitutive promoter o1c | 2 |
| pDC(101) | Same as pDCoc except that it encodes catalytically inactive UmuC(D101N); constructed using a site-directed mutagenesis kit from Stratagene | This work |
| pDC(Δβ) | Same as pDCoc except that the UmuCβ1 motif, residues 357-361, is mutated to alanine | 2 |
| pD′Coc | UmuD′C cloned into pBR322 under the operator constitutive promoter o1c; constructed by subcloning from pGY9738a | This work |
| pDC(122) | Same as pDCoc expect that stop codons in all three reading frames using a site-directed mutagenesis kit from Stratagene were inserted to correspond to the UmuC122 truncation, lacking the last 102 residues | This work |
| pD(S60A)C | Same as pDCoc except for the umuD gene encoding the S60A mutation; constructed using a site-directed mutagenesis kit from Stratagene | This work |
| pGW2020(pUmuD) | umuD cloned into pBR322 under the operator constitutive promoter o1c | 35 |
| pGW2122(pUmuD′) | UmuD′ cloned into pBR322 under the operator constitutive promoter o1c | 35 |
| pET16b | Vector; pBR322 origin of replication; Ampr; similar to pET11d except that it contains His6 | Novagen |
| pNusA(1-495) | N-terminal His6-tagged full-length NusA cloned into pET11d | 30 |
| pNusA(232-495) | N-terminal His6-tagged amino acids 232-495 of NusA cloned into pET11d | 30 |
| pNusA(132-240) | N-terminal His6-tagged amino acids 132-240 of NusA cloned into pET11d | 30 |
| pNusA(1-137) | N-terminal His6-tagged amino acids 1-137 of NusA cloned into pET11d | 30 |
See reference 44.
FIG. 2.
Peptides of NusA which bind to DinB encompass the site of the nusA11 temperature-sensitive mutation. (A) One-hour exposure of a cellulose filter peptide array consisting of 12-mer peptides scanning the primary sequence of NusA, with each peptide being offset by two residues from the previous sequence (MIT CCR Core Facility), probed with 150 nM purified recombinant DinB, and developed with an anti-DinB antibody as described previously (34). Recombinant DinB was purified as previously described (3). Red boxes highlight peptides that interacted with DinB and were mapped onto the homology model described below (C). (B) One-hour and 15-min exposures of a control peptide array, which was performed as described above (A) except without a DinB incubation step. (C) Mapping of interacting peptides onto a homology model of NusA. NusA residues are shown in green, interacting peptides are shown in blue, and the temperature-sensitive mutation of the nusA11 allele is shown in red. The NusA homology model, constructed with SWISS-MODEL, is based on the crystal structure of full-length NusA from Thermotoga maritima, with the N terminus of NusA at the top.
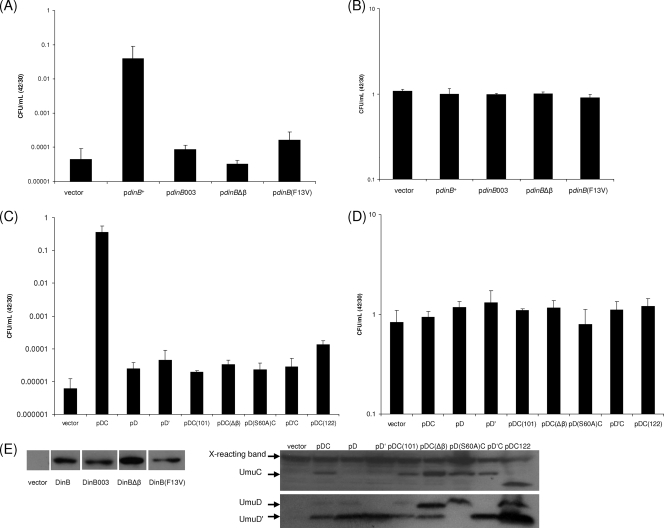
Nevertheless, the peptide array data led us to consider the possibility that elevated levels of DinB might stabilize the NusA11 protein, resulting in dinB+ serving as a multicopy suppressor of the temperature sensitivity of a nusA11 strain. We found that an increased level of expression of DinB, from a low-copy-number plasmid under the lac promoter, indeed suppresses the temperature sensitivity of the nusA11 strain and does so by approximately 3 orders of magnitude (Fig. 3A).
FIG. 3.
DinB or umuDC acts as a multicopy suppressor of the nusA11 temperature sensitivity. (A) Survival of nusA11 strains harboring plasmids at 30°C versus 42°C. nusA11 cells (SEC29) harboring plasmids containing dinB+ and dinB derivates (Table 1) were allowed to reach saturation in LB plus ampicillin (100 μg/ml) at 30°C, diluted (1:100) into LB supplemented with ampicillin and IPTG (isopropyl-β-d-thiogalactopyranoside) (1 mM), and again allowed to reach saturation. The cultures were then diluted in M9 salts and plated onto LB agar plates supplemented with ampicillin and IPTG, preheated to either 30°C or 42°C, and incubated at the respective temperature; CFU/ml on plates growing at 30°C and 42°C were then scored. Shown are pWSK29, the empty vector; pdinB+; pdinB003, which encodes a catalytically inactive DinB; pdinBΔβ, which encodes a truncation mutation of DinB eliminating its β processivity motif; and pdinB(F13V), which encodes a variant of DinB that can catalyze DNA synthesis but not across N2-adducted dG residues (in vitro). (B) Survival of nusA+ strains (P90C) harboring the same set of plasmids as described above (A) at 30°C versus 42°C, showing that these plasmids do not confer any growth phenotypes in a wild-type background. (C) Survival of nusA11 strains harboring plasmids at 30°C versus 42°C. umuDC suppression assays were performed as described above for the dinB suppression assays except that IPTG was omitted. Shown are pBR322 (empty vector); pDCoc (pumuD+C+, operator constitutive promoter); pD, which carries only umuD; pD′, which expresses only UmuD′; pDC(101), which encodes a catalytically inactive UmuC; pDC(Δβ), which encodes a variant of UmuC with the β binding motif mutated to alanines; pD(S60A)C, which encodes a noncleavable UmuD variant; pD′Coc, which contains UmuD′C under the operator constitutive promoter; and pDC(122), which encodes a truncated variant of UmuC. (D) Survival of nusA+ strains harboring the same set of plasmids described above (C) at 30°C versus 42°C, showing that these plasmids do not confer any growth phenotypes in a wild-type background. (E) Western blots demonstrate that dinB and umuDC derivatives are stably expressed as well as dinB+ or umuD+C+. nusA11 cells harboring plasmids described above (A) were grown as they would be grown for multicopy suppression assays except that cells were harvested, lysed with lysozyme, and treated with DNase I (Sigma). Fifteen micrograms of total protein of each lysate was loaded onto a 4 to 20% SDS-polyacrylamide gel. After electrophoresis, proteins were transferred onto a polyvinylidene difluoride membrane and probed with an anti-DinB antibody, under Western conditions described previously (2) except with the addition of a high-salt (0.5 M NaCl) wash after incubation with the secondary antibody. Antibodies against UmuD and UmuC can detect plasmid-borne protein levels only after SOS induction. Thus, the expression of umuDC under the conditions used for nusA11 multicopy suppression cannot be detected by Western blotting. In order to check that the different mutants used were stably expressed compared to the wild type, AB1157 cells harboring plasmids described above (C) were irradiated with UV to induce the SOS response, and Western blot assays were then performed as described previously (2).
Although this multicopy suppression could have resulted from DinB stabilization of the NusA11 protein, it could also have resulted from DinB functioning as a TLS DNA polymerase. To distinguish between these possibilities, we tested the abilities of various dinB derivatives to serve as a multicopy suppressor of nusA11. Strikingly, we found that DinB requires its catalytic activity to act as a multicopy suppressor of the nusA11 temperature sensitivity, as shown by the failure of a nusA11 strain to grow at 42°C when harboring pdinB003, which encodes catalytically inactive DinB(D103N). Furthermore pdinBΔβ, which encodes a truncation mutation of DinB eliminating its β processivity motif (7), and pdinB(F13V), which encodes a variant of DinB that can catalyze DNA synthesis but not across N2-adducted dG residues in vitro (21), also fail to support growth of the nusA11 strain at 42°C (Fig. 3A). These observations indicate that the suppression of the temperature sensitivity of the nusA11 strain by DinB requires not only DinB's ability to catalyze DNA synthesis and to interact with the processivity clamp but also its ability to perform TLS. Western blotting experiments showed that the various dinB mutants were expressed as well as dinB+ (Fig. 3E), and none of the above-mentioned plasmids affected the growth of a nusA+ strain (Fig. 3B). It is possible that the interaction with DinB is also stabilizing the NusA11 protein, but if so, this stabilization is not sufficient to account for the multicopy suppression that we have observed.
Our discovery that the TLS function of DinB is necessary for the multicopy suppression of the nusA11 temperature sensitivity prompted us to test whether umuD+C+ could also function as a multicopy suppressor. Using a plasmid with umuD+C+ under its native promoter with an operator constitutive mutation (o1c) on a medium-copy-number plasmid, pDC, we found that elevated levels of the umuDC gene products indeed increase the level of survival of the nusA11 strain at 42°C by approximately 4 orders of magnitude (Fig. 3C). The difference in suppression between dinB and umuDC may reflect a qualitative difference, but it may also be explained at least in part by the differences in the vector and promoter used for expression. Neither of the umuD gene products alone, pD or pD′, under the operator constitutive promoter (o1c), can support growth of the nusA11 strain at 42°C, indicating that UmuC function is required for the multicopy suppression of the nusA11 strain. Similar to the suppression by DinB, the suppression of nusA11 strains at 42°C requires UmuC's catalytic activity. This is shown by the fact that the temperature sensitivity of a nusA11 strain cannot be suppressed by pDC(101), which encodes catalytically inactive UmuC(D101N), or by pDC(Δβ), which encodes a variant of UmuC that is deficient for binding to the β clamp. It also requires the autoproteolytic activities of UmuD, shown by pD(S60A)C, which encodes a noncleavable UmuD variant. However, to our surprise, pD′C, which directly expresses UmuD′C from the plasmid, does not support growth of the nusA11 strain at 42°C, suggesting that both UmuD and UmuD′ must be present along with UmuC for the multicopy suppression to occur. Interestingly, UmuD and UmuD′ form a heterodimer that is considerably more stable than the UmuD2 or UmuD′2 homodimer (1), raising the possibility that the UmuD·UmuD′ heterodimer may be required for the multicopy suppression by umuDC. Alternatively, the additional methionine used to initiate the translation of UmuD′ in our pD′C construct may alter the ability for multicopy suppression. pDC(122), which encodes a truncation of the UmuC protein that results in hydroxyurea resistance (15), also does not support growth at 42°C, implying that the requirements for hydroxyurea resistance are not the same for multicopy suppression (Fig. 3C). Additionally, Western blotting experiments show that the various mutant umuDC gene products are expressed at least as well as umuD+C+ (Fig. 3E) and that they do not alter the growth of nusA+ strains (Fig. 3D).
In summary, we find that NusA, an essential E. coli protein, physically interacts with the DNA polymerase DinB in addition to its well known RNA polymerase contacts. We have shown that peptides implicated in DinB binding form distinct patches on the NusA surface, including residues around the site of mutation of the nusA11(Ts) allele. Furthermore, elevated levels of expression of dinB+ or umuD+C+ result in a multicopy suppression of the temperature sensitivity of the nusA11(Ts) strain. For both of these translesion DNA polymerases, this multicopy suppression requires their catalytic activities as well as their ability to bind to the β clamp.
Taken together, our results suggest the existence of a previously unsuspected cellular process involving physical and genetic interactions between an important RNA polymerase modulator and translesion DNA polymerases. Furthermore, the fact that the lethality of a nusA11 mutant at 42°C can be suppressed by elevating the level of expression of either of two translesion DNA polymerases implies a hitherto unrecognized role for NusA in DNA repair and/or DNA damage tolerance. Such a role for NusA might be an additional reason that the nusA gene is present in all bacteria and archaea.
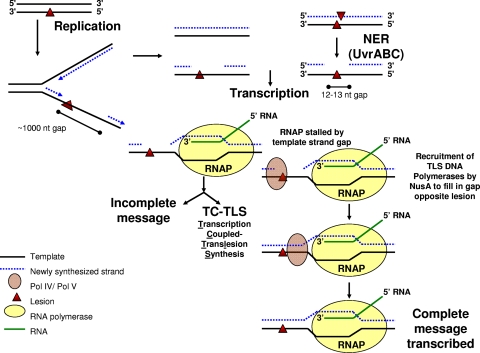
What type of molecular mechanism could account for these unanticipated results? We propose that by binding to DinB or some complex involving UmuC and the umuD gene products, NusA can couple the process of transcription to the process of TLS to enable transcription-coupled TLS (TC-TLS) in a manner analogous to the coupling of transcription to nucleotide excision repair during transcription-coupled repair (TCR). In principle, a process of TC-TLS could be helpful when transcription becomes stalled by gaps in the transcribed strand that are opposite lesions in the nontranscribed strand. Such gaps can be caused by lesions that cannot be bypassed by the replicative DNA polymerase. On the lagging strand, gaps are generated by replication resuming at the site of the next Okasaki fragment, while a replication restart can generate similar gaps on the leading strand; gaps formed in this manner are estimated to average about 1,000 nucleotides in length (18, 20, 23, 39). Alternatively, gaps opposite lesions could also be formed by UvrABC-dependent nucleotide excision repair if two lesions are very close together but on opposing strands or by UvrABC-dependent incisions during the repair of an intrastrand cross-link (12). In the latter two cases, the gaps would be smaller, 12 to 13 nucleotides (12). DinB has recently been shown to be capable of accurately filling the gaps that could be generated during the repair of N2,N2-guanine intrastrand cross-links (24), an observation that might help rationalize the involvement of DNA Pol kappa in mammalian nucleotide excision repair (36).
We hypothesize that if an RNA polymerase encounters one of these gaps in the transcribed strand opposite a lesion, it would stall. In this case, NusA, which is associated with the RNA polymerase throughout the elongation phase of transcription, might then recruit a TLS polymerase to fill in the gap in the template strand (Fig. 4). Repairing the gap would permit the transcription of the gene by subsequent RNA polymerases, possibly even by the original RNA polymerase if it is retained during the process as in TCR. TC-TLS would provide a way of prioritizing the use of the cell's TLS resources to maximally benefit transcription in the same way that TCR prioritizes nucleotide excision repair resources to maximally benefit transcription.
FIG. 4.
Proposed model of TC-TLS. This model of TC-TLS is described in the text. Briefly, we propose that an RNA polymerase (RNAP) stalled by a gap in the template strand opposite a DNA lesion on the nontranscribed strand could recruit TLS polymerases through NusA to fill in the gap opposite the lesion to allow for the continuation of transcription. NER, nucleotide excision repair; nt, nucleotide.
The mechanism of TC-TLS that we are proposing is unrelated to the phenomenon of template strand gap bypass that was characterized previously (27, 52). It has been shown that two phage RNA polymerases and E. coli RNA polymerase are capable of bypassing a small gap in the transcribed strand, thereby generating faithfully transcribed, but internally deleted, mRNAs that would be nonfunctional in most cases. Although T7 RNA polymerase can bypass a template strand gap of up to 24 nucleotides, E. coli RNA polymerase only inefficiently bypasses a 1-nucleotide gap and generates the equivalent of a −1 frameshift mutation in the process (27, 52). Many of the gaps that we are considering would be too large to bypass in this fashion, and the mechanism which we are proposing would usually result in the production of biologically functional mRNAs.
If a process of transcription-coupled TLS does exists, and if it is universal, as most DNA repair and DNA damage tolerance processes tend to be, it might be of particular importance for mammals, where some mRNAs can take many hours to transcribe (47), so the consequences of encountering a template gap late in the transcriptional process would be severe. Alternatively, NusA may be important for the recruitment of DinB to serve as the polymerase in the patching step of nucleotide excision repair that takes place during transcription-coupled repair, as has been implicated for DNA Pol kappa in eukaryotes (36).
Acknowledgments
We thank members of the Walker laboratory for thoughtful comments on the manuscript. We thank Penny J. Beuning (Northeastern University) for construction of plasmid pDC(C101) and Daniel F. Jarosz for construction of plasmid pdinBΔβ. G.C.W. is an American Cancer Society Research Professor.
This work was supported by NIH grant CA21615 to G.C.W. as well as NIEHS grant P30 ES002109 to the MIT Center for Environmental Health Sciences.
Footnotes
Published ahead of print on 7 November 2008.
REFERENCES
- 1.Battista, J. R., T. Ohta, T. Nohmi, W. Sun, and G. C. Walker. 1990. Dominant negative umuD mutations decreasing RecA-mediated cleavage suggest roles for intact UmuD in modulation of SOS mutagenesis. Proc. Natl. Acad. Sci. USA 877190-7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beuning, P. J., D. Sawicka, D. Barsky, and G. C. Walker. 2006. Two processivity clamp interactions differentially alter the dual activities of UmuC. Mol. Microbiol. 59460-474. [DOI] [PubMed] [Google Scholar]
- 3.Beuning, P. J., S. M. Simon, V. G. Godoy, D. F. Jarosz, and G. C. Walker. 2006. Characterization of Escherichia coli translesion synthesis polymerases and their accessory factors. Methods Enzymol. 408318-340. [DOI] [PubMed] [Google Scholar]
- 4.Brotcorne-Lannoye, A., and G. Maenhaut-Michel. 1986. Role of RecA protein in untargeted UV mutagenesis of bacteriophage λ: evidence for the requirement for the dinB gene. Proc. Natl. Acad. Sci. USA 833904-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cairns, J., and P. L. Foster. 1991. Adaptive reversion of a frameshift mutation in Escherichia coli. Genetics 128695-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan, C. L., and R. Landick. 1993. Dissection of the his leader pause site by base substitution reveals a multipartite signal that includes a pause RNA hairpin. J. Mol. Biol. 23325-42. [DOI] [PubMed] [Google Scholar]
- 7.Dalrymple, B. P., K. Kongsuwan, G. Wijffels, N. E. Dixon, and P. A. Jennings. 2001. A universal protein-protein interaction motif in the eubacterial DNA replication and repair systems. Proc. Natl. Acad. Sci. USA 9811627-11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnelly, C. E., and G. C. Walker. 1992. Coexpression of UmuD′ with UmuC suppresses the UV mutagenesis deficiency of groE mutants. J. Bacteriol. 1743133-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dziembowski, A., and B. Seraphin. 2004. Recent developments in the analysis of protein complexes. FEBS Lett. 5561-6. [DOI] [PubMed] [Google Scholar]
- 10.Farnham, P. J., J. Greenblatt, and T. Platt. 1982. Effects of NusA protein on transcription termination in the tryptophan operon of Escherichia coli. Cell 29945-951. [DOI] [PubMed] [Google Scholar]
- 11.Friedberg, E. C., R. Wagner, and M. Radman. 2002. Specialized DNA polymerases, cellular survival, and the genesis of mutations. Science 2961627-1630. [DOI] [PubMed] [Google Scholar]
- 12.Friedberg, E. C., G. C. Walker, W. Siede, R. D. Wood, R. A. Schultz, and T. Ellenberger. 2005. DNA repair and mutagenesis, 2nd ed. ASM Press, Washington, DC.
- 13.Friedman, D. I., and L. S. Baron. 1974. Genetic characterization of a bacterial locus involved in the activity of the N function of phage lambda. Virology 58141-148. [DOI] [PubMed] [Google Scholar]
- 14.Godoy, V. G., D. F. Jarosz, S. M. Simon, A. Abyzov, V. Ilyin, and G. C. Walker. 2007. UmuD and RecA directly modulate the mutagenic potential of the Y family DNA polymerase DinB. Mol. Cell 281058-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godoy, V. G., D. F. Jarosz, F. L. Walker, L. A. Simmons, and G. C. Walker. 2006. Y-family DNA polymerases respond to DNA damage-independent inhibition of replication fork progression. EMBO J. 25868-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodman, M. F. 2002. Error-prone repair DNA polymerases in prokaryotes and eukaryotes. Annu. Rev. Biochem. 7117-50. [DOI] [PubMed] [Google Scholar]
- 17.Greenblatt, J., and J. Li. 1981. Interaction of the sigma factor and the nusA gene protein of E. coli with RNA polymerase in the initiation-termination cycle of transcription. Cell 24421-428. [DOI] [PubMed] [Google Scholar]
- 18.Heller, R. C., and K. J. Marians. 2006. Replication fork reactivation downstream of a blocked nascent leading strand. Nature 439557-562. [DOI] [PubMed] [Google Scholar]
- 19.Hersh, M. N., R. G. Ponder, P. J. Hastings, and S. M. Rosenberg. 2004. Adaptive mutation and amplification in Escherichia coli: two pathways of genome adaptation under stress. Res. Microbiol. 155352-359. [DOI] [PubMed] [Google Scholar]
- 20.Iyer, V. N., and W. D. Rupp. 1971. Usefulness of benzoylated naphthoylated DEAE-cellulose to distinguish and fractionate double-stranded DNA bearing different extents of single-stranded regions. Biochim. Biophys. Acta 228117. [DOI] [PubMed] [Google Scholar]
- 21.Jarosz, D. F., V. G. Godoy, J. C. Delaney, J. M. Essigmann, and G. C. Walker. 2006. A single amino acid governs enhanced activity of DinB DNA polymerases on damaged templates. Nature 439225-228. [DOI] [PubMed] [Google Scholar]
- 22.Kim, S.-R., G. Maenhaut-Michel, M. Yamada, Y. Yamamoto, K. Matsui, T. Sofuni, T. Nohmi, and H. Ohmori. 1997. Multiple pathways for SOS-mutagenesis in Escherichia coli: an overexpression of dinB/dinP results in strongly enhancing mutagenesis in the absence of any exogenous treatment to damage DNA. Proc. Natl. Acad. Sci. USA 9413792-13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kornberg, A., and T. A. Baker. 1992. DNA replication, 2nd ed. W. H. Freeman and Company, New York, NY.
- 24.Kumari, A., I. G. Minko, M. B. Harbut, S. E. Finkel, M. F. Goodman, and R. S. Lloyd. 2008. Replication bypass of interstrand cross-link intermediates by Escherichia coli DNA polymerase IV. J. Biol. Chem. 28327433-27437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landick, R., and C. Yanofsky. 1987. Isolation and structural analysis of the Escherichia coli trp leader paused transcription complex. J. Mol. Biol. 196363-377. [DOI] [PubMed] [Google Scholar]
- 26.Linn, T., and J. Greenblatt. 1992. The NusA and NusG proteins of Escherichia coli increase the in vitro readthrough frequency of a transcriptional attenuator preceding the gene for the beta subunit of RNA polymerase. J. Biol. Chem. 2671449-1454. [PubMed] [Google Scholar]
- 27.Liu, J., and P. W. Doetsch. 1996. Template strand gap bypass is a general property of prokaryotic RNA polymerases: implications for elongation mechanisms. Biochemistry 3514999-15008. [DOI] [PubMed] [Google Scholar]
- 28.Liu, K., Y. Zhang, K. Severinov, A. Das, and M. M. Hanna. 1996. Role of Escherichia coli RNA polymerase alpha subunit in modulation of pausing, termination and anti-termination by the transcription elongation factor NusA. EMBO J. 15150-161. [PMC free article] [PubMed] [Google Scholar]
- 29.Lovett, S. T. 2006. Replication arrest-stimulated recombination: dependence on the RecA paralog, RadA/Sms and translesion polymerase, DinB. DNA Repair (Amsterdam) 51421-1427. [DOI] [PubMed] [Google Scholar]
- 30.Mah, T. F., J. Li, A. R. Davidson, and J. Greenblatt. 1999. Functional importance of regions in Escherichia coli elongation factor NusA that interact with RNA polymerase, the bacteriophage lambda N protein and RNA. Mol. Microbiol. 34523-537. [DOI] [PubMed] [Google Scholar]
- 31.Maor-Shoshani, A., and Z. Livneh. 2002. Analysis of the stimulation of DNA polymerase V of Escherichia coli by processivity proteins. Biochemistry 4114438-14446. [DOI] [PubMed] [Google Scholar]
- 32.Minko, I. G., K. Yamanaka, I. D. Kozekov, A. Kozekova, C. Indiani, M. E. O'Donnell, Q. Jiang, M. F. Goodman, C. J. Rizzo, and R. S. Lloyd. 2008. Replication bypass of the acrolein-mediated deoxyguanine DNA-peptide cross-links by DNA polymerases of the DinB family. Chem. Res. Toxicol. 211983-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura, Y., S. Mizusawa, D. L. Court, and A. Tsugawa. 1986. Regulatory defects of a conditionally lethal nusAts mutant of Escherichia coli. Positive and negative modulator roles of NusA protein in vivo. J. Mol. Biol. 189103-111. [DOI] [PubMed] [Google Scholar]
- 34.Niehbuhr, K., and J. Wehland. 1997. Screening of antibody epitopes and regions of protein-protein interaction sites using SPOT peptides. Academic Press, San Diego, CA.
- 35.Nohmi, T., J. R. Battista, L. A. Dodson, and G. C. Walker. 1988. RecA-mediated cleavage activates UmuD for mutagenesis: mechanistic relationship between transcriptional derepression and posttranslational activation. Proc. Natl. Acad. Sci. USA 851816-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogi, T., and A. R. Lehmann. 2006. The Y-family DNA polymerase kappa (pol kappa) functions in mammalian nucleotide-excision repair. Nat. Cell Biol. 8640-642. [DOI] [PubMed] [Google Scholar]
- 37.Ohmori, H., E. C. Friedberg, R. P. Fuchs, M. F. Goodman, F. Hanaoka, D. Hinkle, T. A. Kunkel, C. W. Lawrence, Z. Livneh, T. Nohmi, L. Prakash, S. Prakash, T. Todo, G. C. Walker, Z. Wang, and R. Woodgate. 2001. The Y-family of DNA polymerases. Mol. Cell 87-8. [DOI] [PubMed] [Google Scholar]
- 38.Reiner, A., D. Yekutieli, and Y. Benjamini. 2003. Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics 19368-375. [DOI] [PubMed] [Google Scholar]
- 39.Rupp, W. D., and P. Howard-Flanders. 1968. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J. Mol. Biol. 31291-304. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt, M. C., and M. J. Chamberlin. 1987. nusA protein of Escherichia coli is an efficient transcription termination factor for certain terminator sites. J. Mol. Biol. 195809-818. [DOI] [PubMed] [Google Scholar]
- 41.Shankar, S., A. Hatoum, and J. W. Roberts. 2007. A transcription antiterminator constructs a NusA-dependent shield to the emerging transcript. Mol. Cell 27914-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen, X., R. Woodgate, and M. F. Goodman. 2005. Lyase activities intrinsic to Escherichia coli polymerases IV and V. DNA Repair (Amsterdam) 41368-1373. [DOI] [PubMed] [Google Scholar]
- 43.Shin, D. H., H. H. Nguyen, J. Jancarik, H. Yokota, R. Kim, and S. H. Kim. 2003. Crystal structure of NusA from Thermotoga maritima and functional implication of the N-terminal domain. Biochemistry 4213429-13437. [DOI] [PubMed] [Google Scholar]
- 44.Sommer, S., F. Boudsocq, R. Devoret, and A. Bailone. 1998. Specific RecA amino acid changes affect RecA-UmuD′C interaction. Mol. Microbiol. 28281-291. [DOI] [PubMed] [Google Scholar]
- 45.Sutton, M. D., T. Opperman, and G. C. Walker. 1999. The Escherichia coli SOS mutagenesis proteins UmuD and UmuD′ interact physically with the replicative DNA polymerase. Proc. Natl. Acad. Sci. USA 9612373-12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang, M., X. Shen, E. G. Frank, M. O'Donnell, R. Woodgate, and M. F. Goodman. 1999. UmuD′2C is an error-prone DNA polymerase, Escherichia coli pol V. Proc. Natl. Acad. Sci. USA 968919-8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tennyson, C. N., H. J. Kalmut, and R. G. Worton. 1995. The human dystrophin gene requires 16 hours to be transcribed and is cotranscriptionally spliced. Nat. Genet. 9184-190. [DOI] [PubMed] [Google Scholar]
- 48.Wagner, J., S. Fujii, P. Gruz, T. Nohmi, and R. P. Fuchs. 2000. The beta clamp targets DNA polymerase IV to DNA and strongly increases its processivity. EMBO Rep. 1484-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagner, J., P. Gruz, S.-R. Kim, M. Yamada, K. Matsui, R. P. P. Fuchs, and T. Nohmi. 1999. The dinB gene encodes a novel E. coli DNA polymerase, DNA Pol IV, involved in mutagenesis. Mol. Cell 4281-286. [DOI] [PubMed] [Google Scholar]
- 50.Yamada, M., T. Nunoshiba, M. Shimizu, P. Gruz, H. Kamiya, H. Harashima, and T. Nohmi. 2006. Involvement of Y-family DNA polymerases in mutagenesis caused by oxidized nucleotides in Escherichia coli. J. Bacteriol. 1884992-4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuan, B., H. Cao, Y. Jiang, H. Hong, and Y. Wang. 2008. Efficient and accurate bypass of N2-(1-carboxyethyl)-2′-deoxyguanosine by DinB DNA polymerase in vitro and in vivo. Proc. Natl. Acad. Sci. USA 1058679-8684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou, W., and P. W. Doetsch. 1993. Effects of abasic sites and DNA single-strand breaks on prokaryotic RNA polymerases. Proc. Natl. Acad. Sci. USA 906601-6605. [DOI] [PMC free article] [PubMed] [Google Scholar]