Abstract
The Yersinia enterocolitica YtxR protein is a LysR-type transcriptional regulator that induces expression of the ytxAB locus, which encodes a putative ADP-ribosylating toxin. The ytxR and ytxAB genes are not closely linked in the Y. enterocolitica chromosome, and whereas ytxR is present in all sequenced Yersinia spp., the ytxAB locus is not. These observations suggested that there might be other YtxR-regulon members besides ytxAB and prompted us to investigate coregulated genes and gene products by using transcriptional and proteomic approaches. Microarray and reverse transcription-PCR analysis showed that YtxR strongly activates expression of the yts2 locus, which encodes a putative type 2 secretion system, as well as several uncharacterized genes predicted to encode extracytoplasmic proteins. Strikingly, we also discovered that under Ysc-Yop type 3 secretion system-inducing conditions, YtxR prevented the appearance of Yop proteins in the culture supernatant. Microarray and lacZ operon fusion analysis showed that this was due to specific repression of ysc-yop gene expression. YtxR was also able to repress VirF-dependent Φ(yopE-lacZ) and Φ(yopH-lacZ) expression in a strain lacking the virulence plasmid, which suggested a direct repression mechanism. This was supported by DNase I footprinting, which showed that YtxR interacted with the yopE and yopH control regions. Therefore, YtxR is a newly identified regulator of the ysc-yop genes that can act as an overriding off switch for this critical virulence system.
The genus Yersinia consists of several species, of which three are widely recognized as significant human pathogens. Yersinia pestis causes bubonic and pneumonic plague, whereas Yersinia pseudotuberculosis and Yersinia enterocolitica primarily cause self-limiting intestinal disease. Y. enterocolitica, the species most frequently isolated from humans, is usually acquired from contaminated food or water (4, 8, 9). Following acquisition, the bacteria transit to the terminal ileum and gain access to M cells overlaying the Peyer's patches. Here the bacteria multiply and have the potential to move into the mesenteric lymph nodes (25). On very rare occasions Y. enterocolitica disease can also progress to life-threatening systemic infections (9). The intestinal diseases caused by Y. enterocolitica and Y. pseudotuberculosis contrast sharply with the severe outcomes resulting from Y. pestis infection. Nevertheless, all three species share similarities in their pathogenesis and virulence mechanisms. In particular, all use an essentially identical type 3 secretion system (T3SS) to counteract the mammalian host's innate immune response (11).
The Ysc-Yop T3SS is encoded by an approximately 70-kb virulence plasmid, named pYV, which is common to all three pathogenic yersiniae. It allows yersiniae to survive and multiply in lymphoid tissues and is essential for their virulence in murine models of infection (reviewed in reference 11). The system exports a group of proteins, collectively known as the Yops, directly into host immune cells. The Yops interfere with various intracellular signaling processes, causing a number of outcomes, including the inhibition of phagocytosis (31).
Ysc-Yop is the prototype T3SS, other examples of which have now been described for many gram-negative pathogenic and symbiotic bacteria (18). They are complex protein export and delivery systems used by bacteria occupying diverse niches and interacting with varied hosts, including both animals and plants. With the complexity of these systems, and the energetic cost of their production to the cell, it is perhaps not surprising that both their production and activity are tightly regulated (reviewed in reference 15). Environmental cues such as temperature, divalent cations, pH, and quorum sensing are some of the stimuli used to regulate transcription of T3SS-encoding genes. Commonly employed regulatory mechanisms include AraC-like activators, small histone-like proteins, and changes in DNA topology. T3SSs are often encoded by accessory genetic elements such as plasmid and pathogenicity islands. Critical transcriptional regulatory proteins are frequently encoded within these elements, but in some cases T3SS genes have also been integrated into other regulatory circuits encoded elsewhere in the genome (15). In addition to environmental control, many T3SSs are also subject to another level of control known as feedback inhibition. This mechanism is triggered when protein export is inhibited, and it leads to reduced T3SS gene expression. It is mediated by the intracellular accumulation of inhibitory proteins, which are themselves substrates of the T3SS (reviewed in reference 5).
The Ysc-Yop T3SS is also tightly regulated. In the laboratory, expression of the genes encoding the Ysc-Yop system is induced at 37°C when the growth medium is depleted of calcium ions. There are a number of mechanisms underlying this transcriptional regulation. Temperature regulation is mediated by the AraC-like regulator VirF, which positively regulates ysc-yop gene expression by binding to several promoter regions (21, 33). The activity of VirF is controlled by a combination of the histone-like protein YmoA and temperature-induced changes in the supercoiling of pYV DNA (10, 28). There is also feedback inhibition of ysc-yop gene expression that is triggered when Yop secretion is prevented, such as by a millimolar calcium ion concentration in the laboratory culture medium. Feedback inhibition is itself dependent on pYV-encoded components, and the LcrQ/YscM protein has been specifically implicated (27). The Y. enterocolitica virulence plasmid encodes two LcrQ/YscM homologues, YscM1 and YscM2, which are both involved in mediating feedback inhibition of ysc-yop gene expression (6, 7, 29). In addition, a YopD-SycD complex binds to the 5′ untranslated region of yop mRNAs and represses their translation (1). There is also a functional relationship between the YscM1/2 and YopD-SycD mechanisms that is not yet completely understood (6, 7).
Our own interest in Y. enterocolitica gene regulation led to the previously reported identification of YtxR, a LysR-type transcriptional regulator (LTTR) that directly induces expression of the ytxAB genes (2). It was speculated that YtxR might have multiple regulatory targets because ytxR and ytxAB are not closely linked in Y. enterocolitica, and ytxR is conserved in all yersiniae whereas ytxAB is not (2). Therefore, in this study we have investigated the possibility of a broader YtxR regulon. We report that YtxR is both a positive and a negative global regulator. Remarkably, we show that YtxR negatively regulates expression of the ysc-yop regulon. Our studies suggest that the mechanism involves direct interference with VirF-dependent transcriptional activation, mediated by YtxR binding to ysc-yop promoter regions. Therefore, YtxR is a global regulator that can act as an overriding transcriptional off switch for the Ysc-Yop T3SS.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth media.
Bacterial strains and plasmids used in this study are listed in Table 1. Y. enterocolitica strains were routinely propagated in Luria-Bertani-Miller broth (LB broth) or on LB agar at 26°C. For experiments involving induction of the Ysc-Yop system, strains were grown in brain heart infusion supplemented with 20 mM MgCl2 and 20 mM sodium oxalate (BHI-MOX). The phospholipase indicator agar was MacConkey agar base (Difco) supplemented with 1% (vol/vol) Tween 80 and 1 mM CaCl2 (36). When appropriate, the following antibiotics were used at the concentrations indicated in parentheses: nalidixic acid (20 μg/ml), kanamycin (100 μg/ml), and chloramphenicol (12.5 μg/ml).
TABLE 1.
Strains and plasmids
| Strain or plasmid | Genotype/features | Reference or source |
|---|---|---|
| Yersinia enterocolitica strainsa | ||
| JB580v | ΔyenR (r− m+) | 20 |
| AJD239 | ΔyenR (r− m+) ΔytxR | 2 |
| AJD1022 | ΔyenR (r− m+) ΔaraGFB::[Φ(yopH-lacZY)] | 23 |
| AJD1685 | ΔyenR (r− m+) ΔaraGFB::[Φ(ytxA-lacZY)] | This study |
| AJD1950 | ΔyenR (r− m+) ΔaraGFB::[Φ(yscA-lacZY)] | This study |
| AJD1951 | ΔyenR (r− m+) ΔaraGFB::[Φ(lcrG-lacZY)] | This study |
| AJD2351 | ΔyenR (r− m+) ΔaraGFB::[Φ(rscB-lacZY)] | This study |
| AJD2407 | ΔyenR (r− m+) ΔaraGFB::[Φ(yopE-lacZY)] | This study |
| GY460 | ΔyenR (r− m+) ΔflhDC | 37 |
| YVM925 | ΔyenR (r− m+) Φ(ysaE-lacZYA) | 32 |
| YVM987 | ΔyenR (r− m+) Φ(sycB-lacZYA) | 32 |
| Plasmids | ||
| pBAD18-kan | KmraraBp expression vector, ColE1 ori | 16 |
| pBAD33 | CmraraBp expression vector, p15A ori | 16 |
| pVLT33 | Kmrtacp expression vector, RSF1010 ori | 14 |
| pAJD594 | tacp-ytxR in pVLT33 | This study |
| pAJD654 | araBp-ytxR in pBAD33 | 2 |
| pAJD679 | araBp-His6-ytxR in pBAD18-Km | 2 |
| pAJD905 | Cmr R6K ori mob+ (RP4) sacB1+, promoterless lacZY | 23 |
| pAJD952 | yopHp fragment in pAJD905 | 23 |
| pAJD1062 | ytxAp fragment in pAJD905 | 2 |
| pAJD1284 | yscAp fragment in pAJD905 | This study |
| pAJD1285 | lcrGp fragment in pAJD905 | This study |
| pAJD1435 | araBp-virF in pBAD33 | This study |
| pAJD1437 | rscBp fragment in pAJD905 | This study |
| pAJD1439 | tacp-rscR in pVLT33 | This study |
| pAJD1473 | yopEp fragment in pAJD905 | This study |
| pGY20 | tacp-flhDC in pVLT33 | 37 |
All strains listed have the virulence plasmid (pYV). In some cases pYV− derivatives were made, as described previously (13), but they are not listed in the table.
Strain and plasmid construction.
Primer sequences used in this study will be supplied upon request (please contact the corresponding author). DNA sequences of all fragments generated by PCR were determined to ensure that there were no spurious mutations. To construct Φ(yopE-lacZ), Φ(yscA-lacZ), Φ(lcrG-lacZ), and Φ(rscB-lacZ) single-copy operon fusion strains, control region fragments were amplified from strain JB580v chromosomal DNA by PCR and cloned into plasmid pAJD905. A ytxAp-containing pAJD905 derivative was constructed previously (2). All of these pAJD905-encoded fusions were integrated into the ara locus exactly as described previously (23).
A tacp-ytxR+ expression plasmid was made by cloning the ytxR+ fragment from plasmid pAJD654 into pVLT33. To construct a tacp-rscR+ plasmid, the rscR gene was amplified from strain JB580v chromosomal DNA by PCR and cloned into pVLT33. The araBp-virF+ plasmid was made by amplifying the virF+ gene from virulence plasmid DNA and cloning it into pBAD33.
RNA extraction and microarray analysis.
To identify YtxR-regulated genes during growth at 26°C in LB broth, total RNA was isolated from three independent cultures of strain AJD239 containing either pBAD33 (YtxR negative) or pAJD654 (YtxR positive). Saturated cultures were diluted into 5 ml LB broth in 18-mm-diameter test tubes so that the optical density (600 nm) was approximately 0.1. The cultures were grown on a roller drum at 26°C until the optical density (600 nm) was approximately 0.5, at which time arabinose was added (0.2% final concentration). Incubation was then continued for an additional 2.5 h. The equivalent of 1 ml of culture with an optical density (600 nm) of 1.0 was mixed with RNAprotect (Qiagen) to preserve its integrity. Total RNA was isolated with the RNeasy minikit (Qiagen) and treated with DNase I. To identify YtxR-regulated genes during growth under Ysc-Yop-inducing conditions, total RNA was isolated from three independent cultures of strain AJD239 containing either pVLT33 (YtxR negative) or pAJD594 (YtxR positive). Saturated cultures were diluted into 5 ml BHI-MOX in 18-mm-diameter test tubes so that the optical density (600 nm) was approximately 0.1. The cultures were grown on a roller drum at 26°C for 2 h at which time IPTG (isopropyl-β-d-thiogalactopyranoside) was added (5 μM final concentration). Cultures were then transferred to a roller drum at 37°C for an additional 3 h. Cultures were harvested, and RNA was prepared as described above.
Yersinia enterocolitica PCR-based microarrays included all 4,215 predicted coding sequences from the sequenced genome of strain 8081. They were constructed at the Bacterial Microarray facility at St. George's Hospital Medical School essentially as described previously (17). Ten micrograms of RNA was denatured at 95°C in the presence of 3 μg random primers (Invitrogen) and snap-cooled on ice. The RNA was then reverse transcribed to generate Cy-dye-labeled cDNA using 500 U Superscript II reverse transcriptase (Invitrogen) in the presence of 1× first-strand buffer, 10 mM dithiothreitol, 460 μM dATP, 460 μM dGTP, 460 μM dTTP, 184 μM dCTP, and 850 pM of either Cy5-dCTP or Cy3-dCTP (Amersham Pharmacia). After incubation at 42°C in the dark for 90 min, labeled cDNAs from comparative samples were mixed and purified together using a single Qiagen minielution column. Microarray slides were prehybridized in 3.5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 0.1% sodium dodecyl sulfate (SDS), 10 mg/ml bovine serum albumin at 65°C for 20 min before being washed for 1 min in H2O followed by 1 min in 100% isopropanol. The purified cDNA mixture was denatured at 95°C before being applied to the microarray slide in hybridization solution (4× SSC, 0.3% SDS). Hybridization was performed for 18 h at 65°C, before the slides were washed once in 1× SSC, 0.05% SDS at 65°C and twice in 0.06× SSC for 2 min. Dye swaps were performed with the three independent RNA isolations being hybridized to the arrays on two separate occasions. With duplicate spots on each array, this resulted in a total of 12 replicates for each of the represented reporter elements.
Microarray slides were scanned using a Genetic Microsystems GMS 418 scanner (MWG Biotech), and images were analyzed using Imagene (BioDiscovery) and Genespring (Silicon Genetics) software. Forty percent of the data was used to calculate the Lowess curve, which was fitted to the log ratio plot. This curve was used to adjust the control value for each measurement. Only data points determined to be “present or marginal” by the Imagene software were used in the final analysis. Statistical significance was determined using a P value of <0.05 when analyzed by t test with the Benjamini and Hochberg false-discovery rate as a cutoff.
Reverse transcription-PCR.
Total RNA was isolated as described for the microarray experiments conducted at 26°C in LB broth. cDNA synthesis was performed with the SuperScript III first-strand synthesis system (Invitrogen) according to the manufacturer's instructions using 0.5 μg of RNA as the template. After completion these reaction mixtures were diluted 200-fold and 1 μl was used as the template in PCRs with Taq DNA polymerase. Each PCR mixture also contained 2 μM of each of two gene-specific primers and 200 mM deoxynucleoside triphosphates. The PCR primers were designed to amplify ∼200- to 400-bp coding region fragments from each target gene. Four microliters of each PCR mixture was analyzed on a 1.5% agarose gel.
Analysis of culture supernatant proteins.
For Ysc-Yop-inducing conditions, saturated cultures were used to inoculate 5 ml of BHI-MOX in an 18-mm-diameter test tube so that the optical density (600 nm) was 0.1. The cultures were incubated at 26°C on a roller drum for 2 h, at which time IPTG was added (5 μM final concentration). Cultures were then transferred to a roller drum at 37°C for an additional 3 h. Bacterial cells from the 5-ml culture were removed by centrifugation in a microcentrifuge at 13,000 rpm for 5 min. The upper two-thirds of the supernatant was passed through a 0.2-μm-pore-size low-protein-binding filter (Millipore). Proteins were precipitated by adding trichloroacetic acid to a final concentration of 10% (vol/vol) and incubating the mixture on ice for 12 to 16 h. The proteins were collected by centrifugation in a microcentrifuge at 13,000 rpm for 30 min at 4°C. The pellets were washed with 1 ml of ice-cold acetone, followed by incubation on ice for 15 min. Proteins were then collected by centrifugation in a microcentrifuge at 13,000 rpm for 30 min at 4°C. The pellets were resuspended in SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer containing β-mercaptoethanol. The resuspension volumes were adjusted according to the final optical density (600 nm) of the cultures so that equivalent amounts were analyzed. Proteins were separated by SDS-PAGE (12.5% polyacrylamide) and visualized by staining with BioSafe Coomassie blue (Bio-Rad).
For Ysa-Ysp-inducing conditions, saturated cultures were used to inoculate 5 ml of LB broth containing 290 mM NaCl and 5 μM IPTG in an 18-mm-diameter test tube so that the optical density (600 nm) was 0.1. The cultures were incubated at 26°C on a roller drum for 6 h. Bacterial cells from the 5-ml culture were removed by centrifugation at 8,000 × g for 5 min. The upper two-thirds of the supernatant was passed through a 0.2-μm-pore-size low-protein-binding filter (Millipore). Proteins were precipitated with trichloroacetic acid exactly as described above. The pellets were resuspended in SDS-PAGE sample buffer containing β-mercaptoethanol, separated by SDS-PAGE (12.5% polyacrylamide), and visualized by staining with silver (3).
Swimming motility assays.
For each strain 10 μl of a saturated culture was spotted onto the surface of 1% (wt/vol) tryptone, 0.5% (wt/vol) NaCl, 0.25% (wt/vol) agar, 5 μM IPTG, and 100 μg/ml kanamycin in a petri dish. Plates were incubated at 26°C for 21 h, and the radius of the ring of bacteria was measured. Values reported are the averages from triplicate assays ± the standard deviations.
β-Galactosidase assays.
To determine the effects of tacp-ytxR+ and tacp-rscR+ plasmids on Φ(yopE-lacZ), Φ(yopH-lacZ), Φ(yscA-lacZ), Φ(lcrG-lacZ), Φ(rscB-lacZ), and Φ(ytxA-lacZ) expression before and after exposure to Ysc-Yop-inducing conditions, saturated cultures were diluted into 5 ml BHI-MOX medium in an 18-mm-diameter test tube so that the optical density (600 nm) was 0.1. The cultures were incubated at 26°C on a roller drum for 2 h, at which time a 1-ml sample was harvested (preinduction). IPTG was then added (5 μM final concentration), and the cultures were transferred to a roller drum at 37°C for an additional 3 h, at which time another 1-ml sample was harvested (postinduction).
To determine the effect of the tacp-ytxR+ plasmid on Φ(sycB-lacZ), Φ(ysaE-lacZ), and Φ(ytxA-lacZ) expression in Ysa system-inducing conditions, saturated cultures were diluted into 5 ml of Luria-Bertani broth containing 5 μM IPTG and a total NaCl concentration of either 5 mM (low) or 290 mM (high) in an 18-mm-diameter test tube so that the optical density (600 nm) was 0.1. The cultures were incubated for 6 h on a roller drum at 26°C prior to harvesting.
To determine the effect of tacp-ytxR+ and araBp-virF+ plasmids on Φ(yopE-lacZ) and Φ(yopH-lacZ) expression, saturated cultures were diluted into 5 ml of LB broth in an 18-mm-diameter test tube so that the optical density (600 nm) was 0.1. Cultures were incubated for 2 h at 37°C on a roller drum, and then arabinose and IPTG were added at the indicated concentrations. Incubation continued for an additional 3 h prior to harvesting.
β-Galactosidase activity was determined at room temperature (approximately 22°C) using permeabilized cells (22). Activities were expressed in arbitrary units determined according to the formula described by Miller (24). Each individual culture was assayed in duplicate, and the activities reported are averages of two to four independent cultures.
Purification of His6-YtxR.
The pAJD679 expression plasmid was used to purify His6-YtxR protein essentially as described previously (2). In addition, a mock purification was done in which the empty vector for plasmid pAJD679 (pBAD18-kan) was used in its place. One modification to the previous purification protocol (2) was that after binding, the nickel-nitrilotriacetic acid agarose was washed with 10 ml each of 50 mM NaH2PO4, 300 mM NaCl, 5 mM β-mercaptoethanol, 0.1% Tween 20, and 5 mM MgCl2 (pH 7.5), containing either 25 mM, 50 mM, 75 mM, or 100 mM imidazole (in that order). The His6-YtxR protein was then eluted with 10 ml of 50 mM NaH2PO4, 300 mM NaCl, 5 mM β-mercaptoethanol, 0.1% Tween 20, 5 mM MgCl2, 250 mM imidazole (pH 7.5). One-milliliter fractions were collected and used directly in DNase I footprinting reactions. The purified protein precipitated upon storage. Therefore, it was always used for DNase I footprinting assays on the same day on which it was purified and not stored. Protein concentrations were estimated using a NanoDrop ND-1000 spectrophotometer to measure absorbance at 280 nm in comparison to a bovine serum albumin standard.
Preparation of probes for DNase I footprinting.
The yopE control region fragment was generated by PCR amplification from virulence plasmid DNA. The forward primer annealed approximately ∼190 bp upstream of the yopE position +1 and incorporated an XbaI site. The reverse primer annealed approximately 30 bp downstream of the yopE start codon and incorporated a BamHI site. The product was digested with BamHI and dephosphorylated with calf intestinal alkaline phosphatase (Promega). The bottom (template) strand was labeled at the 5′ end with [γ-32P]ATP by T4 polynucleotide kinase (Promega). Unincorporated [γ-32P]ATP was removed with the Promega Wizard SV gel and PCR cleanup system. To eliminate any label from the other end of the DNA fragment, the product was digested with XbaI and purified again with the Promega Wizard SV gel and PCR cleanup system.
yopH control region fragments were generated by PCR amplification from plasmid pAJD952. The forward primer annealed 380 bp upstream of the yopH position +1 and incorporated an XbaI site. The reverse primers annealed either 130 bp upstream or 40 bp downstream of the yopH +1 position and incorporated a BamHI site. The products were digested with BamHI and dephosphorylated, and the bottom (template) strand was labeled as described above.
DNase I footprinting assays.
Labeled yopH or yopE control region fragments (approximately 2 nM) were mixed with His6-YtxR protein at the indicated concentrations (or an equivalent amount of the His6-YtxR elution buffer for the no-protein control reaction) in a buffer containing 400 μg/ml salmon sperm DNA (Sigma-Aldrich), 100 mM HEPES (pH 7.6), 50 mM (NH4)2SO4, 5 mM dithiothreitol, 1% (vol/vol) Tween 20, and 150 mM KCl in a total reaction volume of 50 μl. The reaction mixtures were incubated at 32°C for 15 min, and then 53 μl of a solution containing 5 mM CaCl2, 10 mM MgCl2, and 0.005 U/μl of DNase I was added. The mixtures were incubated for 2 min, and digestion was stopped by adding 25 μl of a solution of 2 M ammonium acetate, 250 mM EDTA, 100 μg/ml salmon sperm DNA, and 1 mg/ml glycogen. The DNA was precipitated with ethanol and resuspended in formamide loading dye. To generate size markers, the unlabeled, undigested yopE control region PCR fragment (yopEp) or plasmid pAJD952 (yopHp) was used in DNA sequencing reactions with the fmol DNA cycle sequencing system (Promega). The sequencing primers had 5′ ends that corresponded exactly to the labeled ends of the fragments used in the footprint reactions. Samples were resolved by denaturing 8% polyacrylamide-urea electrophoresis and visualized by autoradiography.
Microarray data accession numbers.
Fully annotated microarray data have been deposited in BμG@Sbase (accession number E-BUGS-53; http://bugs.sgul.ac.uk/E-BUGS-53) and also ArrayExpress (accession number E-BUGS-53).
RESULTS
Microarray analysis of the transcriptional response to increased ytxR expression at 26°C.
YtxR was identified in a search for a regulator of the ytxAB locus (2). However, ytxR and ytxAB are separated on the Y. enterocolitica chromosome, and whereas ytxR is conserved in all yersiniae, ytxAB is not. This led to speculation that YtxR might have additional regulatory targets. Therefore, we used whole-genome microarrays to compare the transcription profiles of a Y. enterocolitica ΔytxR strain containing either an araBp-ytxR+ plasmid or the corresponding empty vector control, during routine laboratory growth conditions (LB broth at 26°C).
YtxR increased the expression of almost 300 genes (≥2-fold, P ≤ 0.05; see Table S1 in the supplemental material). However, most of this large number of induced genes probably reflects indirect effects of ytxR overexpression rather than direct regulation by YtxR. Direct targets are more likely to be among the most highly induced genes. Indeed, this is true for the known direct target ytxA, which was induced by 188-fold (see Table 2, which lists the most highly induced loci). None of these highly induced genes has been characterized in any yersiniae, which suggests that YtxR activates a novel regulon. Strikingly, almost all the loci are predicted to encode at least one protein with an extracytoplasmic location (e.g., with an N-terminal sec-dependent signal sequence; Table 2). This suggests that YtxR induces a family of cell envelope or secreted proteins, which includes a putative type 2 secretion system, previously named Yts2 (19). YtxR also reduced the expression of approximately 250 genes (≥2-fold, P ≤ 0.05; see Table S2 in the supplemental material). Many of these were metabolic genes with nothing obvious in common, and they were not studied further.
TABLE 2.
Locia induced ≥50-fold in response to ytxR expression at 26°C
| Geneb | Inductionc (fold) | P valued | Comment(s) |
|---|---|---|---|
| YE4154 | 341 | 1.34 × 10−6 | Putative exported protein |
| YE4155 | 200 | 9.73 × 10−8 | Putative exported protein |
| YE4156 | 62 | 1.47 × 10−6 | Putative outer membrane protein |
| YE1068 | 325 | 1.49 × 10−6 | Putative exported protein |
| YE2423 | 258 | 8.70 × 10−8 | Putative exported protein |
| YE4086 | 234 | 2.45 × 10−9 | Signal sequence, unknown function |
| YE4085 | 42 | 2.17 × 10−8 | Putative hemolysin activator protein |
| YE4084 | 27 | 2.16 × 10−6 | Putative exported protein |
| YE2124 (ytxA) | 188 | 1.77 × 10−7 | Putative ADP-ribosyltransferase |
| YE2123 (ytxB) | 35 | 7.88 × 10−5 | Putative subunit of YtxAB toxin |
| YE3351 | 79 | 1.27 × 10−6 | Yts2 T2SSe locus |
| YE3350 | 53 | 6.93 × 10−5 | Yts2 T2SS locus |
| YE3349 | 37 | 6.47 × 10−6 | Yts2 T2SS locus |
| YE3348 | 163 | 1.86 × 10−6 | Yts2 T2SS locus |
| YE3347 | 177 | 3.31 × 10−6 | Yts2 T2SS locus |
| YE3346 | 111 | 2.83 × 10−7 | Yts2 T2SS locus |
| YE3345 | 131 | 7.93 × 10−6 | Yts2 T2SS locus |
| YE3344 | 136 | 6.41 × 10−6 | Yts2 T2SS locus |
| YE3343 | 156 | 3.59 × 10−6 | Yts2 T2SS locus |
| YE3342 | 65 | 1.64 × 10−5 | Yts2 T2SS locus |
| YE3341 | 59 | 1.60 × 10−9 | Yts2 T2SS locus |
| YE0607 | 156 | 2.11 × 10−6 | Signal sequence, unknown function |
| YE1083 | 119 | 1.94 × 10−6 | Unknown function |
| YE0396 | 76 | 5.87 × 10−10 | Signal sequence, unknown function |
| YE1033 | 67 | 7.27 × 10−6 | Signal sequence, unknown function |
| YE0749 | 54 | 3.74 × 10−6 | Unknown function |
| YE0750 | 13 | 6.91 × 10−7 | Unknown function |
All genes induced 50-fold or more are listed. Genes that might be in the same operon as those induced 50-fold or more are grouped together and listed regardless of their induction.
Y. enterocolitica numerical annotation is from reference 30.
Rounded to the nearest whole number.
The values are multiple testing corrected using the Benjamini and Hochberg false-discovery rate.
T2SS, type 2 secretion system.
To test the validity of the microarray data, genes from six of the most highly induced loci were selected for semiquantitative reverse transcription-PCR analysis. The same results were obtained when the source of RNA was the same as that used in the microarray experiments (data not shown) or when RNA was prepared from biological replicates not used in the arrays (Fig. 1). In all cases this analysis confirmed that the transcript levels of these genes were significantly elevated upon increased ytxR expression.
FIG. 1.
Semiquantitative reverse transcription-PCR validation of selected genes identified by microarray analysis as highly induced by YtxR. Total RNA was isolated from strain AJD239 containing either plasmid pBAD33 (− YtxR) or pAJD654 (+ YtxR) grown at 26°C in LB broth with 0.2% arabinose. cDNA synthesis reactions were done either with (+) or without (−) the presence of reverse transcriptase (RT) prior to the use of cDNAs as templates in PCRs as described in Materials and Methods. The identity of the genes targeted in each set of PCRs is indicated at the top of the figure. PCR products were separated on 1.5% agarose gels, which were photographed under UV illumination after ethidium bromide staining (the negative images are shown). Lanes M, DNA size markers ranging from 100 bp (bottom) to 500 bp (top) in 100-bp increments.
Increased ytxR expression prevents the appearance of Yop proteins in the culture supernatant.
The genes most highly induced by YtxR are predicted to encode components of a type 2 secretion system as well as other putative cell envelope or secreted proteins (Table 2). Therefore, we next investigated whether increased ytxR expression changed the culture supernatant protein profile. However, we could not detect any YtxR-dependent proteins in the supernatant when strains were grown under various environmental conditions, including those used to generate RNA for the microarray (data not shown).
The best-studied secretion conditions for pathogenic yersiniae are those that induce Yop export by the Ysc T3SS (37°C, high Mg2+, and low Ca2+), and we were also interested to test the effect of YtxR under these conditions. We have found Yop export to be more efficient in BHI-based media than in LB (e.g., reference 12). However, this medium contains glucose, which represses the araB promoter. Therefore, we used a tacp-ytxR+ expression plasmid for these and all subsequent experiments. Wild-type Y. enterocolitica containing either a tacp-ytxR+ plasmid or the corresponding empty vector control was grown in BHI-MOX containing 5 μM IPTG to induce the tac promoter (see Materials and Methods). Culture supernatant proteins were analyzed by SDS-PAGE, which revealed that increased ytxR expression completely prevented the appearance of the pYV-dependent Yop proteins (Fig. 2A).
FIG. 2.
Effects of increased ytxR expression on protein export. (A) Analysis of culture supernatant proteins in Ysc-Yop-inducing conditions. Strain JB580 either with (pYV+) or without (pYV−) the virulence plasmid had either tacp-ytxR expression plasmid pAJD594 (+ YtxR) or the empty vector control pVLT33 (− YtxR). Proteins were isolated from culture supernatants following growth in Ysc-Yop-inducing conditions and analyzed by SDS-PAGE and Coomassie blue staining as described in Materials and Methods. (B) Analysis of culture supernatant proteins in Ysa-Ysp-inducing conditions. Strain JB580v had either tacp-ytxR expression plasmid pAJD594 (+ YtxR) or the empty vector control pVLT33 (− YtxR). Proteins were isolated from culture supernatants following growth in Ysa-Ysp-inducing conditions and analyzed by SDS-PAGE and silver staining as described in Materials and Methods. Each lane contains culture supernatant derived from the equivalent of a 1-ml culture at an optical density (600 nm) of 1.0. (C) Phenotypes on phospholipase indicator agar. Strains with the indicated genotypes and plasmids were grown on phospholipase indicator agar at 26°C for 48 h as described in Materials and Methods. The dark precipitate, which was red on the original plates, indicates phospholipase activity (only a black-and-white image is shown). The ΔflhDC strain containing either pVLT33 or pVLT33-flhDC+ (pGY20) serves as a negative and positive control, respectively (36).
We were concerned that this negative effect on Yop export might reflect a nonspecific artifact resulting from overexpression of ytxR. However, when a similar experiment was done under conditions that induce Ysp export by the chromosomal Ysa T3SS (35), increased ytxR expression did not alter the protein profile in the culture supernatant (Fig. 2B). This suggests that the Ysa-Ysp system is unaffected by YtxR, a notion which was supported by subsequent transcriptional studies described below. In addition, increased ytxR expression did not affect the phenotype on phospholipase activity indicator plates (Fig. 2C), which suggested that the flagellar type 3 export system was unaffected (36). This was supported by the observation that increased ytxR expression did not affect swimming motility, with the wild-type strain containing either a tacp-ytxR+ plasmid or the corresponding empty vector control forming motility rings of 2.2 ± 0.3 cm and 2.4 ± 0.2 cm, respectively. Together, all of these observations suggest that increased ytxR expression specifically prevents Yop production or export.
YtxR represses ysc-yop regulon gene expression.
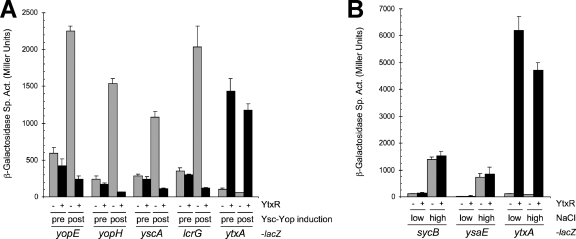
YtxR is a DNA binding transcriptional regulator (2). A simple hypothesis to explain its negative effect on the Yop proteins is that YtxR represses ysc-yop regulon transcription. To test this, we constructed strains with lacZ operon fusions to four promoters, yopE, yopH, yscA, and lcrG, which express different functional components of the system. These strains had the native ytxR gene on their chromosomes, but it is not expressed under the conditions used here and its presence or absence has no effect on ysc-yop expression (data not shown). The strains were grown as described for the Yop secretion assays, and β-galactosidase activities were determined after an initial 2-h growth period at 26°C (pre-Ysc-Yop induction) and a subsequent 3-h growth period at 37°C (post-Ysc-Yop induction). As expected, without increased ytxR expression, all four ysc-yop regulon fusions were significantly induced after the growth period at 37°C (Fig. 3A). However, increased ytxR expression completely prevented this induction, with YtxR-mediated repression ranging from 10- to 23-fold. In contrast, Φ(ytxA-lacZ) fusion expression was induced 21-fold by YtxR, consistent with previous results (2). These data show that increased ytxR expression interferes with the Ysc-Yop system by repressing the expression of its genes.
FIG. 3.
Repression of the Ysc-Yop regulon by YtxR is specific. (A) Effect of YtxR on Φ(yopE-lacZ), Φ(yopH-lacZ), Φ(yscA-lacZ), Φ(lcrG-lacZ), and Φ(ytxA-lacZ) operon fusion expression. Strains contained either tacp-ytxR expression plasmid pAJD594 (+ YtxR; black bars) or the empty vector control pVLT33 (− YtxR; gray bars). Cultures were grown in BHI supplemented with 5 μM IPTG, 20 mM sodium oxalate, and 20 mM MgCl2. Samples were isolated either before (pre) or after (post) transfer from 26°C to 37°C incubation for 3 h as described in Materials and Methods. (B) Effect of YtxR on Φ(sycB-lacZ), Φ(ysaE-lacZ), and Φ(ytxA-lacZ) operon fusion expression. Strains contained either tacp-ytxR expression plasmid pAJD594 (+ YtxR; black bars) or the empty vector control pVLT33 (− YtxR; gray bars). Cultures were grown in LB broth containing 5 μM IPTG and either 5 mM (low) or 290 mM (high) NaCl as described in Materials and Methods. For both panels error bars indicate the positive standard deviations from the means.
Once again our use of a ytxR expression plasmid made it important to investigate the specificity of this repressive effect on ysc-yop regulon expression. First, we tested the effect of YtxR on the expression of two promoters from the chromosomal Ysa-Ysp T3SS. Φ(sycB-lacZ) and Φ(ysaE-lacZ) operon fusion strains (32) were grown at 26°C in LB broth containing either 5 mM NaCl (non-Ysa-Ysp inducing) or 290 mM NaCl (Ysa-Ysp inducing), and β-galactosidase assays were performed (Fig. 3B). As expected, the expression of both fusions was induced by high salt levels. However, increased ytxR expression did not have any effect. A Φ(ytxA-lacZ) fusion was induced approximately 50-fold by YtxR, which confirmed YtxR activity under these growth conditions (Fig. 3B).
To further probe specificity, we tested the effect of overproducing a different LTTR on Φ(yopH-lacZ) expression. RscR is an LTTR that induces expression of the rscB locus when it is overexpressed (26). We compared the effect of tacp-ytxR+ and tacp-rscR+ plasmids on Φ(yopH-lacZ) expression under Ysc-Yop-inducing conditions. The results showed that while YtxR strongly repressed Φ(yopH-lacZ) expression, RscR did not (data not shown). As a control, increased rscR expression did induce Φ(rscB-lacZ) expression by 17-fold (data not shown), which confirmed RscR activity under these growth conditions. We have also found that the overexpression of two other putative LTTR-encoding genes, YE1892 and YE4033, has no effect on ysc-yop expression (data not shown).
Taken together, all of these data indicate that YtxR represses ysc-yop regulon expression and that this is not indicative of nonspecific repression of T3SSs or something that occurs when any LTTR is overproduced. We expanded upon these conclusions in the next series of experiments.
Microarray analysis confirms global repression of the ysc-yop regulon.
The original microarray experiments, described above, were done using growth conditions in which the ysc-yop regulon is not well expressed (LB broth at 26°C). However, our subsequent finding that YtxR represses the Ysc-Yop system motivated us to investigate the global effect of YtxR under Ysc-Yop-inducing conditions. Therefore, we used whole-genome microarrays to compare the transcription profiles of Y. enterocolitica strains containing either a tacp-ytxR+ plasmid or the corresponding empty vector control during growth in BHI-MOX at 37°C.
Once again, YtxR increased the expression of almost 300 genes (≥2-fold, P ≤ 0.05; see Table S3 in the supplemental material), and the most highly induced genes were similar to those identified in the original microarray experiment. Similarly, many of the genes repressed by YtxR at 26°C were also repressed in BHI-MOX at 37°C (see Table S4 in the supplemental material). However, following growth in BHI-MOX at 37°C, approximately half of the 50 most highly repressed genes were encoded on the virulence plasmid. All together, 43 predicted coding sequences on the virulence plasmid were identified as being downregulated by YtxR (Table 3; ≥2-fold, P ≤ 0.05; note that yscA is not listed because the array reported downregulation of slightly less than twofold). Therefore, increased ytxR expression causes global downregulation of the ysc-yop regulon. Furthermore, it appears that the Ysc-Yop system is the major target for downregulation by YtxR, at least under the growth conditions used here. Next, we turned our attention to investigating the mechanism by which YtxR represses ysc-yop regulon expression.
TABLE 3.
Virulence plasmid genes repressed ≥2-fold by ytxR expression under low-calcium, 37°C conditions
| Genea | Normalized expressionb | P valuec | Comment(s) |
|---|---|---|---|
| yopE | 0.04 | 7.97 × 10−8 | YopE effector |
| lcrG | 0.04 | 3.97 × 10−6 | LcrG protein |
| sycD | 0.05 | 1.47 × 10−6 | YopB/D chaperone SycB |
| yopM | 0.06 | 2.51 × 10−10 | YopM effector |
| lcrD | 0.07 | 1.79 × 10−8 | LcrD protein |
| yopH | 0.09 | 8.85 × 10−9 | YopH effector |
| yopB | 0.09 | 1.54 × 10−11 | YopB translocator |
| lcrV | 0.11 | 4.95 × 10−9 | LcrV protein |
| tyeA | 0.12 | 4.76 × 10−15 | TyeA protein |
| yopQ | 0.12 | 1.69 × 10−11 | YopQ effector |
| yopD | 0.13 | 4.29 × 10−11 | YopD translocator |
| yscX | 0.14 | 4.20 × 10−12 | YscX protein |
| lcrR | 0.14 | 2.00 × 10−8 | LcrR protein |
| yscF | 0.15 | 6.68 × 10−7 | YscF T3SS apparatus protein |
| yscM2 | 0.15 | 3.38 × 10−5 | YscM2 regulatory protein |
| yscI | 0.18 | 9.32 × 10−6 | YscI T3SS apparatus protein |
| sycE | 0.19 | 4.03 × 10−7 | YopE chaperone SycE |
| orf91B | 0.21 | 3.43 × 10−4 | Unknown function |
| yopT | 0.21 | 2.13 × 10−10 | YopT effector |
| sycN | 0.22 | 2.20 × 10−9 | YopN chaperone SycN |
| yscH | 0.22 | 8.59 × 10−9 | YscH T3SS apparatus protein |
| yopN | 0.22 | 1.41 × 10−10 | YopN regulatory protein |
| yopP | 0.24 | 3.72 × 10−5 | YopP effector |
| yscJ | 0.25 | 2.64 × 10−6 | YscJ T3SS apparatus protein |
| yscQ | 0.26 | 7.27 × 10−6 | YscQ T3SS apparatus protein |
| yopO | 0.26 | 1.52 × 10−5 | YopO effector |
| yadA | 0.28 | 3.19 × 10−3 | YadA adhesin |
| sycT | 0.32 | 1.59 × 10−5 | YopT chaperone SycT |
| yscS | 0.33 | 1.57 × 10−6 | YscS T3SS apparatus protein |
| yscE | 0.33 | 2.97 × 10−4 | YscE T3SS apparatus protein |
| yscD | 0.33 | 1.56 × 10−6 | YscD T3SS apparatus protein |
| yscC | 0.34 | 2.96 × 10−7 | YscC T3SS apparatus protein |
| yscP | 0.35 | 5.95 × 10−10 | YscP T3SS apparatus protein |
| yscG | 0.35 | 2.38 × 10−4 | YscG T3SS apparatus protein |
| yscR | 0.36 | 3.27 × 10−7 | YscR T3SS apparatus protein |
| yscK | 0.38 | 1.92 × 10−8 | YscK T3SS apparatus protein |
| yscM1 | 0.38 | 4.44 × 10−9 | YscM2 regulatory protein |
| yscL | 0.38 | 6.34 × 10−4 | YscL T3SS apparatus protein |
| yscB | 0.38 | 4.15 × 10−5 | YscB T3SS apparatus protein |
| yscT | 0.39 | 2.48 × 10−6 | YscT T3SS apparatus protein |
| yscN | 0.43 | 1.37 × 10−4 | YscN T3SS apparatus protein |
| yscO | 0.44 | 3.77 × 10−4 | YscO T3SS apparatus protein |
| yscU | 0.49 | 4.45 × 10−3 | YscU T3SS apparatus protein |
Y. enterocolitica numerical annotation is from reference 30.
Expression with YtxR normalized to expression without YtxR, rounded to two decimal places (e.g., 0.04 corresponds to 25-fold repression by YtxR).
The values are multiple testing corrected using the Benjamini and Hochberg false-discovery rate.
YtxR interferes with VirF-dependent transcriptional activation in the absence of the virulence plasmid.
It is possible that the effect of YtxR on the Ysc-Yop system could simply be an indirect consequence of increased ytxR expression negatively affecting the stability and/or copy number of the virulence plasmid. It is also possible that YtxR does not directly affect ysc-yop gene expression but instead works through the poorly understood feedback inhibition mechanism. For example, like ytxR, the yscM1 and yscM2 genes were found to repress ysc-yop expression when they were overexpressed (29). However, overexpression of the yscM genes repressed VirF-dependent expression of a Φ(yopH-cat) operon fusion in a pYV+ strain but not in a pYV− strain. This allowed the authors to conclude that YscM1 and YscM2 do not act directly as transcriptional repressors or as anti-VirF factors. Rather, they act indirectly through other pYV-encoded components. Therefore, they are probably part of the feedback inhibition mechanism that represses ysc-yop expression when secretion is blocked. We designed a similar experiment to test whether YtxR repression of ysc-yop transcript levels might simply be due to an effect on pYV stability and/or require the presence of other pYV-encoded components. For these, and subsequent experiments, we chose the yopE and yopH promoters as model representatives.
The virulence plasmid was cured from strains with single-copy Φ(yopE-lacZ) and Φ(yopH-lacZ) fusions on their chromosomes. Then, araBp-virF+ and tacp-ytxR+ plasmids were introduced, or the corresponding empty-vector controls were used. β-Galactosidase activities were determined after growth at 37°C in LB broth. The medium contained 0.002% arabinose and 5 μM IPTG to induce virF and ytxR expression, respectively. In the absence of ytxR expression, the araBp-virF+ plasmid significantly induced Φ(yopE-lacZ) and Φ(yopH-lacZ) expression, as expected (Fig. 4). However, when the tacp-ytxR+ plasmid was also included, the expression of both fusions was reduced close to the basal level. These results indicate that YtxR-mediated repression of ysc-yop genes is mediated either by direct binding to their promoters or by somehow inactivating VirF. It does not require other pYV-encoded components, a finding which shows that feedback inhibition is not involved.
FIG. 4.
YtxR represses VirF-dependent yop gene expression in the absence of the virulence plasmid. The presence of araBp-virF expression plasmid pAJD1435 or tacp-ytxR expression plasmid pAJD594 is indicated by a plus sign in the appropriate row. A minus sign indicates the presence of the appropriate empty vector control (pBAD33 for virF and pVLT33 for ytxR). Cultures were grown at 37°C in LB broth containing 0.002% arabinose and 5 μM IPTG as described in Materials and Methods. Error bars indicate the positive standard deviations from the means.
YtxR interacts with the yopE and yopH control regions.
The simplest hypothesis to explain how YtxR interferes with ysc-yop expression is that it binds to their control regions. Unfortunately, the YtxR protein has proved difficult to work with in vitro. For example, an overproduced maltose binding protein-YtxR fusion protein was completely insoluble (data not shown). However, we previously showed that a His6-YtxR fusion protein was active in vivo and at least partially soluble when overproduced (2). Even so, the purified His6-YtxR protein remains in solution for only a few hours, and upon storage at any temperature it precipitates. We speculate that the purified protein is prone to aggregation. One consequence of this is that we have so far been unable to identify conditions that allow us to perform DNA mobility shift assays. Protein-DNA mixtures do not enter a polyacrylamide gel, which may be due to the tendency of the His6-YtxR protein to aggregate. However, we have been successful with DNase I footprinting experiments. Purified His6-YtxR protected nucleotides within both the ytxA and ytxR control regions from DNase I cleavage in vitro (2). Furthermore, the in vivo relevance of these protected sites was confirmed, which supports the use of the DNase I footprinting approach. Therefore, we used the same approach to investigate the possibility of YtxR binding to the yopE and yopH control regions in vitro.
His6-YtxR protected the yopE control region from approximately the transcription start site (+1) to 70 bp upstream (Fig. 5). This region protected by YtxR overlaps significantly with that previously shown to be protected by VirF (−29 to −112 [33]), which is consistent with YtxR interfering with VirF-mediated activation of transcription in vivo (Fig. 4).
FIG. 5.
DNase I footprint analysis of the yopE and yopH control regions. Labeled yopE or yopH control region fragments were incubated with different concentrations of His6-YtxR protein as shown and then treated with DNase I. G, A, T, and C are sequencing reactions of each control region fragment calibrated with respect to the number of base pairs from the transcription start site (shown at left of each panel). Brackets show the approximate regions of protection from DNase I. Asterisks denote sites hypersensitive to DNase I cleavage in the presence of His6-YtxR. For the central panel the order of the DNase I footprint reactions has been inverted from the original gel with respect to the sequencing reactions. However, all samples were originally run on the same gel. The sequencing reaction failed for the reactions shown in the right panel. In this case calibration was derived from similar footprinting and sequencing reactions run on different gels (not shown). Lane Mk, incubation with a sample from a mock His6-YtxR purification as described in Materials and Methods (the volume of the mock purification fraction used was equivalent to the volume of the His6-YtxR fraction used in the 45 μM lane).
The yopH gene has an almost-700-bp noncoding region upstream. We used two overlapping fragments for DNase I footprinting assays of the region closest to the yopH gene. Like yopE, His6-YtxR also protected specific areas of the yopH control region from DNase I cleavage. One was well upstream of the gene, centered at approximately position −260. The other extended from approximately position −50 to −120 (Fig. 5). Protection of this downstream region was much weaker than that of the upstream region, suggesting a lower binding affinity. However, in addition to protected nucleotides, the downstream footprint also contained sites hypersensitive to DNase I cleavage, as did all of the other His6-YtxR footprints here and in a previous study (2). As an additional control, there was no effect on the DNase I digestion pattern when a sample from a mock purification was used (i.e., purification from an Escherichia coli strain containing the empty vector control for the His6-YtxR expression plasmid; see Materials and Methods). This mock protein sample was from the corresponding fraction and was equal in volume to the sample used in the 45 μM His6-YtxR reaction. This demonstrates that the footprint was due to His6-YtxR rather than any minor contaminating proteins. The downstream region protected by YtxR (−50 to −120) partially overlaps with two separate regions previously shown to be protected from DNase I by VirF (−134 to −94 and −63 to −19 [21, 33]). Once again, this is fully consistent with the observation that YtxR interferes with VirF-mediated activation of yopH expression in vivo (Fig. 4). The possibility of other VirF binding sites, significantly upstream of the −134 position, was not investigated in the previous analyses of VirF binding sites (21, 33).
These data suggest that His6-YtxR interacts with defined regions upstream of yopE and yopH in vitro. Furthermore, the apparent overlap between YtxR and VirF-protected regions suggests that YtxR may inhibit yopE and yopH transcription in vivo by antagonizing VirF-mediated activation. We investigated this hypothesis further in the final set of experiments.
Evidence supporting antagonism between YtxR and VirF in vivo.
The preceding in vitro experiments suggested an antagonistic relationship between YtxR and VirF in controlling yopE and yopH expression. This predicts that increasing the VirF level should at least partially overcome the YtxR-mediated repression of yopE and yopH expression in vivo. To test this, we used the pYV-free Φ(yopE-lacZ) or Φ(yopH-lacZ) system (Fig. 4).
β-Galactosidase activities of Φ(yopE-lacZ) and Φ(yopH-lacZ) strains containing araBp-virF+ and tacp-ytxR+ plasmids were determined after growth in LB broth containing 5 μM IPTG to induce ytxR expression. This concentration of IPTG led to efficient YtxR-mediated repression in the previous experiment (Fig. 4). The medium also contained various concentrations of arabinose to induce virF expression. For the conditions used in Fig. 4 (0.002% arabinose), the relative β-galactosidase activity was assigned a value of 1. As the arabinose concentration was increased, the relative β-galactosidase activity also increased (Fig. 6A). Therefore, increasing the VirF level is able to overcome YtxR-mediated repression.
FIG. 6.
In vivo evidence for antagonism between YtxR and VirF. (A) Increased virF expression overcomes YtxR-mediated repression of yopE and yopH expression. Each strain contained araBp-virF expression plasmid pAJD1435 and tacp-ytxR expression plasmid pAJD594. Cultures were grown at 37°C in LB broth containing 5 μM IPTG and various concentrations of arabinose as indicated. For each strain the relative β-galactosidase activity is shown with respect to the culture containing 0.002% arabinose, which was the concentration used for Fig. 4. (B) Increasing YtxR levels repress yopE and yopH expression even in the presence of a higher VirF level. Each strain contained araBp-virF expression plasmid pAJD1435 and tacp-ytxR expression plasmid pAJD594. Cultures were grown at 37°C in LB broth containing 0.2% arabinose and various concentrations of IPTG as indicated. For each strain the relative β-galactosidase activity is shown with respect to the culture containing 5 μM IPTG, which was the concentration used for Fig. 4. For both panels error bars indicate the positive standard deviations from the means.
We next tested whether elevating the YtxR level could reduce yopE and yopH expression even in the presence of a higher VirF concentration. β-Galactosidase activities of Φ(yopE-lacZ) and Φ(yopH-lacZ) strains containing araBp-virF+ and tacp-ytxR+ plasmids were determined after growth in LB broth containing 0.2% arabinose to induce a high level of virF expression. The medium also contained various concentrations of IPTG to induce ytxR expression. For the conditions used in the preceding experiment (5 μM IPTG) (Fig. 6A), the relative β-galactosidase activity was assigned a value of 1. As the IPTG concentration was increased, the relative β-galactosidase activity decreased (Fig. 6B). Therefore, increasing the YtxR level reduces yopE and yopH expression even in the presence of an elevated VirF concentration.
These experiments support the prediction from the in vitro experiments that YtxR might reduce yop gene expression by antagonizing VirF-mediated activation.
DISCUSSION
YtxR is a Y. enterocolitica LTTR that activates expression of the ytxAB locus and is also positively autoregulated (2). Based on amino acid sequence similarity (>50% identity) and on its having the same relative chromosomal location, ytxR is conserved in all Yersinia spp. with a sequenced genome as well as Photorhabdus luminescens, Photorhabdus asymbiotica, Proteus mirabilis, and Providencia stuartii (data not shown). However, the role of YtxR is not known in any of these organisms. We previously speculated that YtxR was likely to regulate other Y. enterocolitica genes besides ytxAB (2). This was based on the fact that ytxR and ytxAB are separated on the chromosome and that the ytxAB target is not conserved in all species that have ytxR. Therefore, to provide more insight into the role of YtxR, we have begun to identify and characterize other regulatory targets in Y. enterocolitica. Here we report that YtxR induces the expression of a novel family of genes that are significantly enriched for the potential to encode proteins with an extracytoplasmic location (Table 2). Most significantly, we also found that YtxR specifically represses the ysc-yop regulon, which encodes a T3SS, and its exported effectors, which together are the most important virulence factor of the three pathogenic Yersinia species.
Microarray analysis revealed a number of loci that were highly induced by YtxR, which included the already-known target ytxAB. Like YtxAB, many of the proteins encoded by these highly induced genes are predicted to have N-terminal sec-dependent signal sequences. It also included the members of the yts2 locus, which encodes a putative type 2 secretion system (19). It is tempting to speculate that YtxAB and other YtxR-induced proteins could be substrates for the Yts2 system. However, we cannot make this conclusion for Y. enterocolitica. When ytxR was overexpressed, in a variety of different growth conditions, we could not detect the appearance of any YtxR-dependent proteins in the culture supernatant. We also saw no accumulation of proteins in periplasmic or whole-cell extracts under these conditions (G. L. Axler-DiPerte and A. J. Darwin, unpublished data). We do not yet understand the reason(s) for this. Perhaps ytxR overexpression leads to increased transcription of its target genes but they are not sufficiently abundant for detection by protein staining of SDS-polyacrylamide gels. In fact many of the YtxR-induced genes have a much higher A+T content than the average for the Y. enterocolitica genome (reference 2 and data not shown). This high A+T content might contribute to relatively inefficient translation. It is also possible that YtxR regulon members might be rapidly degraded when synthesized under what are presumably unnatural production conditions or that only very low protein levels are needed for their normal functions. Regardless, several of the genes induced by YtxR are conserved in the different Yersinia spp., and we speculate that they may represent a conserved YtxR-dependent regulon.
During our investigations into the potential for YtxR-dependent extracellular proteins, we discovered that increased ytxR expression prevented the appearance of Yops in the culture supernatant. We have also confirmed that this leads to inhibition of Y. enterocolitica-mediated cytotoxicity toward cultured RAW 264.7 mouse macrophage cells in vitro (Axler-DiPerte and Darwin, unpublished). Investigating this surprising phenomenon became the focus of this study. The relationship between YtxR and the Ysc-Yop T3SS is a specific one. Increased ytxR expression did not affect the chromosomally encoded Ysa T3SS or the flagellar export system. In addition, increased expression of three other LTTR-encoding genes, including rscR, did not affect the Ysc-Yop system.
Microarray analysis revealed that YtxR represses the expression of many pYV-encoded genes. Using the yopE and yopH promoters as models, we found that His6-YtxR binds to them in locations consistent with antagonizing VirF-dependent activation (Fig. 5). Previously, we noticed some sequence similarity in the regions of the ytxA and ytxR promoters bound by His6-YtxR (2). These regions include the two similar sequences TTTAAATGATAATGA for ytxA and GTTAACTGATTTTGT for ytxR. The region furthest upstream of yopH protected by His6-YtxR contains the sequence TCTAAATGATAATGA, which differs in only one position from that upstream of ytxA. Therefore, it is possible that these motifs form at least part of the sequence specifically recognized by YtxR. There are also sequences with some similarity to this in the other His6-YtxR-protected regions upstream of yopE and yopH, but we could not unequivocally identify corresponding motifs. This is probably because there is significant sequence divergence among distinct binding sites as evidenced by the comparison of the ytxA and ytxR control regions. In addition, the yopE and yopH control regions are very A-T rich, as is the sequence motif, which makes its unequivocal identification difficult. VirF may also recognize an A-T-rich sequence, and distinct VirF recognition sites were similarly difficult to clearly identify upstream of ysc-yop promoters (33).
There is overlap between some of the regions protected from DNase I by His6-YtxR and VirF. Therefore, a simple hypothesis would be that two transcription factors recognize the same binding sequences. However, this is unlikely, because we have found that an araBp-virF+ plasmid does not prevent induction of Φ(ytxR-lacZ) by tacp-ytxR+, suggesting that unlike YtxR, VirF cannot bind to the ytxR control region (Axler-DiPerte and Darwin, unpublished).
In this study we used increased ytxR expression to activate the regulon, which is somewhat artificial. However, in defense of this approach, the native ytxR promoter is strongly upregulated by YtxR, which means that increased ytxR expression is a normal feature of YtxR regulon induction (2). Of course, the question that remains is under what circumstances the YtxR regulon is naturally activated. More specifically, why have an overriding off switch for the Ysc-Yop system? Perhaps there is an environment where Y. enterocolitica might encounter the necessary stimuli for ysc-yop activation but where the production of the T3SS would be detrimental. One intriguing possibility would be during a commensal relationship in a mammalian reservoir. Alternatively, it might occur in an environment where the organism is free living and production of the Ysc-Yop system would simply interfere with the production and/or function of the putative extracellular proteins induced by YtxR. To date we have been unable to identify a laboratory growth medium that activates the YtxR regulon. However, the conservation of intact ytxR genes in all yersiniae, and some other genera, strongly suggests that it serves an important function in these organisms. Identifying the conditions that activate the regulon remains an important goal for the future.
All of the genes highly induced by YtxR have not been studied before in any of the yersiniae. This indicates the surprising existence of a completely novel regulon that is highly enriched for genes encoding extracellular proteins. It shows the value of attempting to characterize the numerous genes present in bacterial genomes for which natural expression conditions are yet to be defined. What the YtxR regulon does for the yersiniae and when it does it are difficult questions to answer, but for which there is now ample motivation to pursue. However, our finding that the Ysc-Yop system, the prototype T3SS, has been integrated into a newly discovered global regulon is especially significant. This is somewhat reminiscent of other T3SSs. For example, the T3SS encoded by the LEE region in enteropathogenic E. coli and the Psc T3SS of Pseudomonas aeruginosa have both been integrated into more global regulons (reviewed in references 15 and 34).
In summary, a new level of complexity has been added to those mechanisms already known to control ysc-yop expression. This further underlines the obvious importance to the organism of the fine-tuned regulation of this system to ensure that it is made only when absolutely required.
Supplementary Material
Acknowledgments
This work was supported by institutional startup funds from the NYU School of Medicine. G.L.A.-D. was supported in part by grant T32 AI007180 from the NIH.
We thank Kim Walker and Virginia Miller for strains YVM925 and YVM987 and Glenn Young for strain GY460 and plasmid pGY20. We are grateful to Heran Darwin for comments on a draft version of the manuscript and the Bacterial Pathogen Microarray Facility at St. George's (BUGS) for provision of microarrays.
Footnotes
Published ahead of print on 14 November 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Anderson, D. M., K. S. Ramamurthi, C. Tam, and O. Schneewind. 2002. YopD and LcrH regulate expression of Yersinia enterocolitica YopQ by a posttranscriptional mechanism and bind to yopQ RNA. J. Bacteriol. 1841287-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Axler-Diperte, G. L., V. L. Miller, and A. J. Darwin. 2006. YtxR, a conserved LysR-like regulator that induces expression of genes encoding a putative ADP-ribosyltransferase toxin homologue in Yersinia enterocolitica. J. Bacteriol. 1888033-8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blum, H., H. Beier, and H. J. Gross. 1987. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 893-99. [Google Scholar]
- 4.Bottone, E. J. 1999. Yersinia enterocolitica: overview and epidemiologic correlates. Microbes Infect. 1323-333. [DOI] [PubMed] [Google Scholar]
- 5.Brutinel, E. D., and T. L. Yahr. 2008. Control of gene expression by type III secretory activity. Curr. Opin. Microbiol. 11128-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cambronne, E. D., and O. Schneewind. 2002. Yersinia enterocolitica type III secretion: yscM1 and yscM2 regulate yop gene expression by a posttranscriptional mechanism that targets the 5′ untranslated region of yop mRNA. J. Bacteriol. 1845880-5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cambronne, E. D., J. A. Sorg, and O. Schneewind. 2004. Binding of SycH chaperone to YscM1 and YscM2 activates effector yop expression in Yersinia enterocolitica. J. Bacteriol. 186829-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carniel, E., and H. H. Mollaret. 1990. Yersiniosis. Comp. Immunol. Microbiol. Infect. Dis. 1351-58. [DOI] [PubMed] [Google Scholar]
- 9.Cornelis, G., Y. Laroche, G. Balligand, M.-P. Sory, and G. Wauters. 1987. Y. enterocolitica, a primary model for bacterial invasiveness. Rev. Infect. Dis. 964-87. [DOI] [PubMed] [Google Scholar]
- 10.Cornelis, G., C. Sluiters, I. Delor, D. Geib, K. Kaniga, C. Lambert de Rouvroit, M.-P. Sory, J.-C. Vanooteghem, and T. Michiels. 1991. ymoA, a Yersinia enterocolitica chromosomal gene modulating the expression of virulence functions. Mol. Microbiol. 51023-1034. [DOI] [PubMed] [Google Scholar]
- 11.Cornelis, G. R. 2002. The Yersinia Ysc-Yop “type III” weaponry. Nat. Rev. Mol. Cell Biol. 3742-754. [DOI] [PubMed] [Google Scholar]
- 12.Darwin, A. J., and V. L. Miller. 1999. Identification of Yersinia enterocolitica genes affecting survival in an animal host using signature-tagged transposon mutagenesis. Mol. Microbiol. 3251-62. [DOI] [PubMed] [Google Scholar]
- 13.Darwin, A. J., and V. L. Miller. 2001. The psp locus of Yersinia enterocolitica is required for virulence and for growth in vitro when the Ysc type III secretion system is produced. Mol. Microbiol. 39429-444. [DOI] [PubMed] [Google Scholar]
- 14.de Lorenzo, V., L. Eltis, B. Kessler, and K. N. Timmis. 1993. Analysis of Pseudomonas gene products using lacIq/Ptrp-lac plasmids and transposons that confer conditional phenotypes. Gene 12317-24. [DOI] [PubMed] [Google Scholar]
- 15.Francis, M. S., H. Wolf-Watz, and A. Forsberg. 2002. Regulation of type III secretion systems. Curr. Opin. Microbiol. 5166-172. [DOI] [PubMed] [Google Scholar]
- 16.Guzman, L., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 1774121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinchliffe, S. J., K. E. Isherwood, R. A. Stabler, M. B. Prentice, A. Rakin, R. A. Nichols, P. C. Oyston, J. Hinds, R. W. Titball, and B. W. Wren. 2003. Application of DNA microarrays to study the evolutionary genomics of Yersinia pestis and Yersinia pseudotuberculosis. Genome Res. 132018-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwobi, A., J. Heesemann, E. Garcia, E. Igwe, C. Noelting, and A. Rakin. 2003. Novel virulence-associated type II secretion system unique to high-pathogenicity Yersinia enterocolitica. Infect. Immun. 711872-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinder, S. A., J. L. Badger, G. O. Bryant, J. C. Pepe, and V. L. Miller. 1993. Cloning of the YenI restriction endonuclease and methyltransferase from Yersinia enterocolitica serotype O8 and construction of a transformable R-M+ mutant. Gene 136271-275. [DOI] [PubMed] [Google Scholar]
- 21.Lambert de Rouvroit, C., C. Sluiters, and G. R. Cornelis. 1992. Role of the transcriptional activator, VirF, and temperature in the expression of the pYV plasmid genes of Yersinia enterocolitica. Mol. Microbiol. 6395-409. [PubMed] [Google Scholar]
- 22.Maloy, S. R., V. J. Stewart, and R. K. Taylor. 1996. Genetic analysis of pathogenic bacteria. Cold Spring Harbor Laboratory Press, Plainview, NY.
- 23.Maxson, M. E., and A. J. Darwin. 2005. Improved system for construction and analysis of single-copy β-galactosidase operon fusions in Yersinia enterocolitica. Appl. Environ. Microbiol. 715614-5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 25.Naktin, J., and K. G. Beavis. 1999. Yersinia enterocolitica and Yersinia pseudotuberculosis. Clin. Lab. Med. 19523-536. [PubMed] [Google Scholar]
- 26.Nelson, K. M., G. M. Young, and V. L. Miller. 2001. Identification of a locus involved in systemic dissemination of Yersinia enterocolitica. Infect. Immun. 696201-6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rimpilainen, M., A. Forsberg, and H. Wolf-Watz. 1992. A novel protein, LcrQ, involved in the low-calcium response of Yersinia pseudotuberculosis shows extensive homology to YopH. J. Bacteriol. 1743355-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rohde, J. R., J. M. Fox, and S. A. Minnich. 1994. Thermoregulation in Yersinia enterocolitica is coincident with changes in DNA supercoiling. Mol. Microbiol. 12187-199. [DOI] [PubMed] [Google Scholar]
- 29.Stainier, I., M. Iriarte, and G. R. Cornelis. 1997. YscM1 and YscM2, two Yersinia enterocolitica proteins causing downregulation of yop transcription. Mol. Microbiol. 26833-843. [DOI] [PubMed] [Google Scholar]
- 30.Thomson, N. R., S. Howard, B. W. Wren, M. T. Holden, L. Crossman, G. L. Challis, C. Churcher, K. Mungall, K. Brooks, T. Chillingworth, T. Feltwell, Z. Abdellah, H. Hauser, K. Jagels, M. Maddison, S. Moule, M. Sanders, S. Whitehead, M. A. Quail, G. Dougan, J. Parkhill, and M. B. Prentice. 2006. The complete genome sequence and comparative genome analysis of the high pathogenicity Yersinia enterocolitica strain 8081. PLoS Genet. 2e206. doi: 10.1371/journal.pgen.0020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trosky, J. E., A. D. Liverman, and K. Orth. 2008. Yersinia outer proteins: Yops. Cell. Microbiol. 10557-565. [DOI] [PubMed] [Google Scholar]
- 32.Walker, K. A., and V. L. Miller. 2004. Regulation of the Ysa type III secretion system of Yersinia enterocolitica by YsaE/SycB and YsrS/YsrR. J. Bacteriol. 1864056-4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wattiau, P., and G. R. Cornelis. 1994. Identification of DNA sequences recognized by VirF, the transcriptional activator of the Yersinia yop regulon. J. Bacteriol. 1763878-3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yahr, T. L., and M. C. Wolfgang. 2006. Transcriptional regulation of the Pseudomonas aeruginosa type III secretion system. Mol. Microbiol. 62631-640. [DOI] [PubMed] [Google Scholar]
- 35.Young, B. M., and G. M. Young. 2002. YplA is exported by the Ysc, Ysa, and flagellar type III secretion systems of Yersinia enterocolitica. J. Bacteriol. 1841324-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young, G. M., D. H. Schmiel, and V. L. Miller. 1999. A new pathway for the secretion of virulence factors by bacteria: the flagellar export apparatus functions as a protein-secretion system. Proc. Natl. Acad. Sci. USA 966456-6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young, G. M., M. J. Smith, S. A. Minnich, and V. L. Miller. 1999. The Yersinia enterocolitica motility master regulatory operon, flhDC, is required for flagellin production, swimming motility, and swarming motility. J. Bacteriol. 1812823-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.