Abstract
Transcription factor σ28 in Chlamydia trachomatis (σ28Ct) plays a role in the regulation of genes that are important for late-stage morphological differentiation. In vitro mutational and genetic screening in Salmonella enterica serovar Typhimurium was performed in order to identify mutants with mutations in region 4 of σ28Ct that were defective in σ28-specific transcription. Specially, the previously undefined but important interactions between σ28Ct region 4 and the flap domain of the RNA polymerase β subunit (β-flap) or the −35 element of the chlamydial hctB promoter were examined. Our results indicate that amino acid residues E206, Y214, and E222 of σ28Ct contribute to an interaction with the β-flap when σ28Ct associates with the core RNA polymerase. These residues function in contacts with the β-flap similarly to their counterpart residues in Escherichia coli σ70. Conversely, residue Q236 of σ28Ct directly binds the chlamydial hctB −35 element. The conserved counterpart residue in E. coli σ70 has not been reported to interact with the −35 element of the σ70 promoter. Observed functional disparity between σ28Ct and σ70 region 4 is consistent with their divergent properties in promoter recognition. This work provides new insight into understanding the molecular basis of gene regulation controlled by σ28Ct in C. trachomatis.
Chlamydia trachomatis is a leading causative pathogen of bacterial sexually transmitted diseases and ocular infections (trachoma) in humans (36). The obligate intracellular parasitic feature of Chlamydia has hindered genetic and biochemical studies. As a result, little is known about how gene expression is regulated in Chlamydia. Recent studies indicate that gene expression takes place coordinately at the level of transcription throughout the developmental cycle of Chlamydia (2, 33, 37). Bacterial RNA polymerase (RNAP) is a key enzyme that controls transcription (15). Chlamydial RNAP is identical to the well-studied Escherichia coli RNAP in containing subunits α2ββ′ and a σ factor, despite the lack of a recognizable ω subunit (43). The σ factors associate with the RNAP core to form an RNAP holoenzyme that initiates promoter-specific transcription (15, 19, 46). Three known σ factors, σ66, σ54, and σ28, and some transcription regulators have been revealed in the C. trachomatis genome (43). Both chlamydial σ66 and C. trachomatis σ28 (σ28Ct), but not σ54, belong to members of the E. coli σ70 family that share up to four conserved amino acid regions (regions 1 to 4) (26). Chlamydial σ66 serves as the primary σ factor that directs transcription of housekeeping genes or most constitutively expressed genes (2, 7, 23, 27, 33), whereas σ28Ct directs transcription of several late-stage genes that are required for differentiation from the noninfectious but metabolically active reticulate bodies to the infectious but metabolically inactive elementary bodies (3, 39, 48). σ28Ct also performs its task of transcription by responding to stressful conditions (39, 50). It is not known how the σ28Ct function is regulated in Chlamydia. C. trachomatis does not appear to encode an identifiable anti-σ28 homologue, which is known as FlgM in Salmonella (18, 22). A putative chlamydial RsbW, encoded by the rsbW gene, was shown to bind σ28Ct in a glutathione S-transferase pull-down assay (A. L. Douglas and T. P. Hatch, personnel communication). However, there is no direct evidence to show an inhibitory effect of RsbW on chlamydial σ28Ct-specific transcription (17, 22). Even less is known about the function of chlamydial σ54, and only two putative σ54-like promoters have been reported (28). Defining the molecular basis of σ factor action is central for understanding the mechanism by which Chlamydia completes its unique intracellular developmental cycle (16) and adapts to environmental cues.
Several lines of evidence demonstrate that the DNA-σ and σ-core interactions are essential for the process of transcription initiation and elongation (5, 11, 30, 31, 35). Such molecular interactions are well characterized in E. coli σ70-mediated transcription regulation (5, 11, 25). Regions 2 and 4 of E. coli σ70 recognize the promoter −10 and −35 elements, respectively (4, 11, 34). Region 2 of σ70 also interacts with the β′ coiled coil in core RNAP. This interplay is essential for holoenzyme formation (1, 5, 12, 34). Also, the σ70 region 4 interacts with the flexible flap structure in the β subunit (β-flap). This interaction is required to properly position σ70 regions 2 and 4, allowing for simultaneous contact with the −35/−10 promoter elements. Furthermore, the σ70 region 4/β-flap interaction can be a target of transcription factors, such as the anti-σ70 factor T4 AsiA (13, 21). By sequence analogy with E. coli σ70, conserved region 4 in an alternative σ factor is likely to interact with the β-flap for recognition of the −35/−10 promoters. Indeed, direct interactions of region 4 of Helicobacter pylori σ28 with the β-flap (6), as well as those of region 4 of E. coli σ38 with the β-flap (27, 36) have been identified. An apparent difference in the strengths of the E. coli σ38/β-flap and E. coli σ70/β-flap interactions was found, although E. coli σ38 and σ70 recognize similar promoter consensus sequences (19). The question is raised of whether the variability of σ region 4 and the β-flap interaction contributes to promoter recognition in a σ-specific manner.
Previously, we have characterized the range of promoter elements that are recognized by chlamydial σ28 (39, 40). This study was performed in order to explore the details of σ28Ct action in the process of transcription. Specifically, we report that certain σ28Ct determinants are required for contact with the chlamydial β-flap of RNAP or the −35 promoter element. Because σ28Ct plays a role in regulation of genes important for late-stage morphological differentiation and the stress response, our data can shed light on molecular mechanisms underlying these processes. Importantly, given the uncertainty about whether a transcription regulator would affect the σ28Ct activity, our ability to define the molecular interactions of the transcription machinery provides us with a useful genetic tool and information to facilitate the discovery and characterization of potential regulatory factors for σ28Ct in Chlamydia, a genetically intractable pathogen.
MATERIALS AND METHODS
Bacterial strains and growth.
The bacterial strains used are listed in Table 1. C. trachomatis was propagated and purified as previously described (38). DH5α or XL1-Blue was used as the host for cloning. Salmonella enterica serovar Typhimurium fliA, encoding Salmonella σ28, mutant strains TH5504 and TH7034 were kindly provided by Kelly T. Hughes (University of Utah). Reporter strains BN317 (34) and KS1 (8) were kindly provided by Ann Hochschild (Harvard University). Reporter strains ZY101 containing the test promoter Plac_hctB-35 linked to lacZ on an F′ episome were constructed as described previously (45) (see Fig. 6). Briefly, the EcoRI-HindIII-digested DNA fragment carrying the synthetic promoter Plac_hctB- 35 was cloned into pFW11 via EcoRI and HindIII sites. The resultant plasmid was introduced into strain CSH100, allowing homologous recombination of Plac_hctB- 35 and lacZ onto an F′ episome. Further mating with strain FW102 was performed in order to finalize construction of strain ZY101. In this test promoter, an ectopic chlamydial hctB promoter −35 element with the sequence TAAAGTTT was centered at bp −45.5 relative to the transcription initiation site of lac. Using a similar strategy, the reporter strain ZY102 was constructed. ZY102 is identical to ZY101, except that it contains a mutated ectopic chlamydial hctB promoter −35 element (gAAAGTTT). E. coli and S. enterica serovar Typhimurium strains were grown in Luria-Bertani (LB) medium at 37°C. MacConkey agar supplemented with 1% lactose (Difco) plus 0.02% arabinose (MacConkey-lactose-arabinose) was used in indicator plates for β-galactosidase activity. When required, medium was supplemented with 25 μg/ml chloramphenicol, 50 μg/ml kanamycin, 10 μg/ml tetracycline, and/or 100 μg/ml carbencillin.
TABLE 1.
Strains and plasmids used in this study
| Species and strain or plasmid | Description | Reference or source |
|---|---|---|
| C. trachomatis ATCC VR-346 | Serovar F/IC-Cal-13 | ATCC |
| E. coli | ||
| KS1 | MC1000 F′ lacIq integrated with a single copy of promoter plac OR2-62 controlled by lacZ in the chromosome | 8 |
| ZY101 | MC1000 F′ lacIq integrated with a single copy of Plac_hctB-35(TAAAGTTT)::lacZ on an F′episome | This study |
| ZY102 | MC1000 F′ lacIq integrated with a single copy of Plac_hctB-35(gAAAGTTT)::lacZ on an F′ episome | This study |
| BN317 | MC1000 F′ lacIq integrated with a single copy of placCons-35C::lacZ on an F′ episome | 34 |
| S. enterica serovar Typhimurium | ||
| TH7034 | Isogenic with wild-type strain LT2; fliA5886 [R91C L207P] fliC5050::MudJ | K. T. Hughes |
| TH5504 | Isogenic with wild-type strain LT2; fliA5647::FRT | K. T. Hughes |
| Plasmids | ||
| pFW11 | 45 | |
| pS28H | ParaB-His6 chlamydial fliA | 39 |
| pES28H | ParaB-His6E. coli fliA | 39 |
| pHσ28Ct-Ec4 | ParaB-His6 hybrid fliA; encoding σ28Ct residues 1-178 fused to region 4 of σ28Ec residues 166 to 239 | This study |
| pHσ28Ec-Ct4 | ParaB-His6 hybrid fliA; encoding σ28Ec (residues 1-165) fused to region 4 of σ28Ct (residues 179-253) | This study |
| pRV5 | Promoter probe vector containing less lacZ; ligation of the XmnI-SphI fragment of pQF50KgroE and the MfeI (blunted)-SphI fragment of pTara; origin of replication of p15A; Cmr | This study |
| pRVPhctB | pRV5 with promoter of chlamydial hctB (−125 to +45) | This study |
| pQF50KgroE | Promoter reporter vector containing PgroE::lacZ | 24 |
| pTara | Expression vector carrying ParaB-T7RNAP | 47 |
| pACλcI | PlacUV5-directed synthesis of the λCI protein | 8 |
| pACλcI-β-flap | PlacUV5-directed synthesis of the λCI protein fused via three alanines to residues 858-946 of the β subunit of E. coli RNAP | 25 |
| pACλcI-β-flapCt | PlacUV5-directed synthesis of the λCI protein fused via three alanines to residues 798-887 of the β subunit of C. trachomatis RNAP | This study |
| pBRα | PlacUV5/Plpp-directed synthesis of the α subunit of E. coli RNAP | 8 |
| pBRαLN | PlacUV5/Plpp-directed synthesis of N terminus (residues 1-248) of the α subunit of E. coli RNAP | 8, 10 |
| pBRα-σ66 | PlacUV5/Plpp-directed synthesis of the αNTD fused to C. trachomatis σ66 region 4 (residues 468-571 of σ66) | This work |
| pBRα-σ70 | PlacUV5/Plpp-directed synthesis of the αNTD fused to E. coli σ70 region 4 (residues 528-613 of σ70) | 10 |
| pBRα-σ70D581G | PlacUV5/Plpp-directed synthesis of the αNTD fused to E. coli σ70 region 4 carrying the D581G substitution. | 25 |
| pBRα-σ28 | PlacUV5/Plpp-directed synthesis of the αNTD fused to chlamydial σ28Ct region 4 (residues 148-253 of σ28) | This study |
| pBRα-σ28E206G | pBRα-σ28 encoding the E206G substitution in the β moiety of the fusion | This study |
| pBRα-σ28Y214C | pBRα-σ28 encoding the Y214C substitution in the β moiety of the fusion | This study |
| pBRα-σ28E222G | pBRα-σ28 encoding the E222G substitution in the β moiety of the fusion | This study |
| pBRα-σ28Q236L | pBRα-σ28 encoding the Q236L substitution in the β moiety of the fusion | This study |
| pBRα-σ28L243K | pBRα-σ28 encoding the L243K substitution in the β moiety of the fusion | This study |
| pGPhctB | Contains a chlamydial-hctB-controlled guanine-less cassette and an E. coli fliC promoter-controlled guanine-less cassette; Apr | 40 |
| pGPhctBΔup | Derivative of pGPhctB with the AT-rich sequence upstream of the promoter −35 element deleted | 40 |
| pGS9 | Derivative of pGPhctB containing a 9-bp spacer between the −35/−10 elements | 40 |
| pGS14 | Derivative of pGPhctB containing a 14-bp spacer between the −35/−10 elements | 40 |
| pGPtar | Contains an E. coli tar-controlled guanine-less cassette and an E. coli fliC promoter-controlled guanine-less cassette; Apr | 40 |
| pGPtar+ | Derivative of pGPtar fusing a DNA sequence upstream of PhctB to the core promoter of Ptar | 40 |
| pGHR | Contains a PhctB-controlled guanine-less cassette and a chlamydial rRNA P1 promoter-controlled guanine-less cassette | This study |
| pLF28 | Low-copy-number expression vector carrying chlamydial fliA; Tcr | 39 |
| pLF28a | Derivative of pLF28 with a silent mutation at nucleotide position 723 to remove the natural HindIII site | This study |
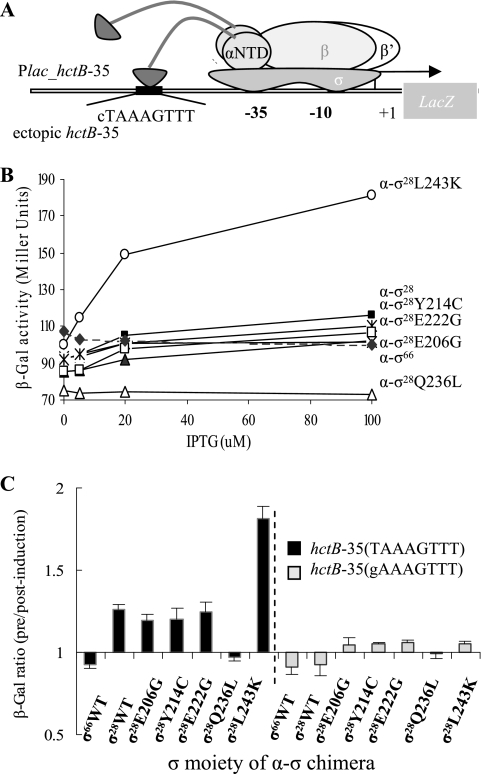
FIG. 6.
Bacterial one-hybrid assay: characterization of the σ28Ct substitutions that affect interaction with the hctB −35 DNA element. (A) Cartoon illustrating the test promoter with wild-type or mutant ectopic hctB −35. The ectopic hctB −35 element serves as a binding site for the tethered σ28Ct region 4 moiety. When they interact with each other, transcription from the test promoter is activated, resulting in an increase of reporter lacZ (β-galactosidase). (B) Effects of substitution in the σ moiety of the α-σ28Ct chimera on transcription from the test promoter. Strain ZY101 harboring plasmids directing the synthesis of the α-σ28Ct or derivatives was grown in the presence of different concentrations of IPTG as indicated. β-Galactosidase activity was measured in duplicate on at least three independent occasions. Values shown are the averages from one experiment. (C) Change (n-fold) in the β-galactosidase activity before and after IPTG (100 μM) induction. Shown are changes of transcription from the test promoter with wild-type hctB −35 (TAAAGTTT) (in ZY101, black bars) or mutant hctB −35 (gAAAGTTT) (in ZY102, light gray bars). ZY101 and ZY102 harboring plasmids that directed the synthesis of α-σ28Ct or derivatives were grown in the absence or presence of IPTG and assayed for β-galactosidase activity. β-Galactosidase activity was measured in duplicate on at least three independent occasions.
Plasmid construction.
The plasmids used in this study are listed in Table 1. The hybrid σ28 genes (Fig. 1) were generated by overlapping PCR amplification and recloned into expression vectors pS28H and pES28H (39), creating pHσ28Ct-Ec4 and pHσ28Ec-Ct4. The hybrid σ28 genes are under the control of an arabinose-inducible Para promoter. Plasmids pACλcI, pBRα, pBRαLN and pACλcI-β-flap (8-10, 25) were kindly provided by Ann Hochschild (Harvard University). pACλcI-β-flapCt directs synthesis of λcI fused to chlamydial β-flap under the control of a lacUV5 promoter. pBRα-σ28 encodes the E. coli N-terminal domain of the α subunit of RNAP (αNTD) fused to the chlamydial σ28 region 4 under the control of tandem lpp and IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible lacUV5 promoters. pBRα-σ66 encodes αNTD fused to region 4 of chlamydial σ66. Plasmid pGHR, carrying promoters of chlamydial hctB and the rRNA, was created by inserting the hctB promoter region into XbaI-EcoRV-digested pMT504 (44). pGHR together with pGS9, pGPhctB, pGS14, pGPhctBΔup, pGPtar, or pGPtar+ (40) was used as a supercoiled template in the in vitro transcription assay. Inserts in all constructs were confirmed by restriction mapping and DNA sequencing. Details of primers and procedures for these constructs are available upon request.
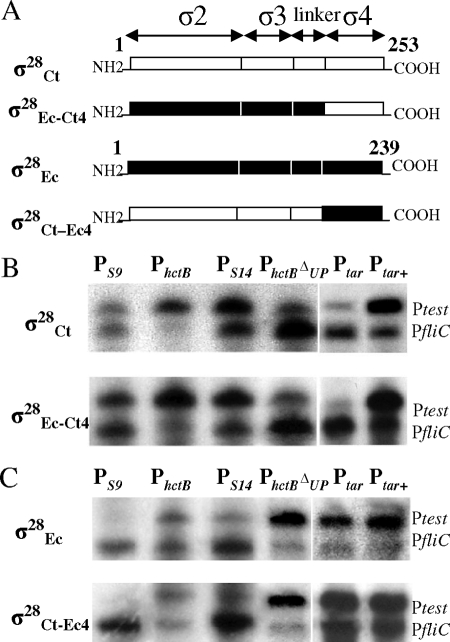
FIG. 1.
In vitro promoter selectivity of holoenzymes containing hybrid σ28 proteins. (A) Construction of hybrid σ28, showing the organization of σ28Ct, σ28Ec, σ28Ec-Ct4 (σ28Ec residues 1 to 165 fused to region 4 of the σ28Ct [residues 179-253]), and σ28Ct-Ec4 (σ28Ct residues 1 to 178 fused to region 4 of σ28Ec [residues 166 to 239]). (B) Gel analysis of in vitro transcription products using the RNAP holoenzyme containing σ28Ct or hybrid σ28Ec-Ct4 protein. (C) Gel analysis of in vitro transcription products using the RNAP holoenzyme containing σ28Ec or hybrid σ28Ct-Ec4 protein. RNAP is designated by the σ28 proteins as indicated on the left. Above each lane is shown the test promoter used in the transcription assay. On the right side of each panel are shown transcripts from different test promoters. PfliC serves as an internal control in the same reaction.
Mutant σ28Ct library and genetic screening.
For convenience in cloning, a silent mutation (T to A) was introduced at position 723 of the σ28Ct coding region using PCR-based site-directed mutagenesis in order to remove a natural HindIII site in pLF28 (40). The resultant plasmid was named pLF28a. Error-prone PCR was performed with Taq DNA polymerase (New England Biolabs) for introducing random mutations of the σ28Ct gene into appropriate fragments of pLF28a. The mutagenized PCR fragments were digested with HindIII-SphI, and a 187-bp DNA fragment containing region 4 of σ28Ct was ligated with the HindIII-SphI-digested fragment of pLF28a. DH5α was transformed with the ligation mixture in order to generate a mutant library of σ28Ct region 4. This mutant library was then subjected to a genetic screen in S. enterica serovar Typhimurium fliA mutant strain TH7034. The selection strains formed white or pink colonies on MacConkey-lactose-arabinose indicator plates when transformed with mutant σ28Ct (the wild type was red). The plasmids carrying potential mutations were analyzed by restriction enzyme digestion, and the full-length σ28Ct gene was sequenced in order to confirm the presence of mutations.
Expression and purification of wild-type σ28Ct and derivatives.
For the production of N-terminally His6-tagged σ28Ct and derivatives, pS28H and derived plasmids were introduced into S. enterica serovar Typhimurium strain TH7034. Cells were grown in LB containing carbenicillin. The fusion proteins were induced by treatment with 0.02% arabinose for 2 h. Proteins were purified under denaturing conditions on an Ni-nitrilotriacetic column (Qiagen), followed by a HiTrap HP column (GE Healthcare), as described previously (42). Purified proteins were renatured by serial dialysis. The final dialysis buffer contained 50% glycerol, 50 mM Tris-HCl (pH 7.9), 0.01% (vol/vol) Triton X-100, 0.1 mM EDTA, 150 mM NaCl, and 0.1 mM dithiothreitol. Protein was stored at −20°C for future use. Protein concentrations were determined using a protein assay kit (USB Corporation) and confirmed by Western blot analysis.
In vivo assay of chlamydial hctB promoter activity in Salmonella: relative β-galactosidase activity.
To examine the effect of mutant σ28Ct proteins on transcription from the chlamydial hctB promoter, pS28H or derived plasmids were introduced into Salmonella fliA mutant strain TH5504 cells harboring pRVhctB, which carries the reporter hctB::lacZ. Cells were grown in LB containing carbencillin and chloramphenicol to ensure selection of both plasmids. The protein was induced by the addition of 0.02% (wt/vol) arabinose starting from an optical density at 600 nm of ≈0.3 for 1 hour. Aliquots of cells were collected. Cell lysates in sodium dodecyl sulfate (SDS) loading buffer were electrophoresed on a 10% glycine-SDS-polyacrylamide gel, followed by Western blot analysis. Levels of cellular σ28Ct and RNAP β subunit were measured by Western blot analysis with polyclonal anti-σ28Ct antibody (a gift from Thomas P. Hatch, University of Tennessee) and monoclonal antibody 8RB13 against the β subunit of RNAP (NeoClone) as probes, respectively. The β-subunit band was used as an internal standard for correcting the amount of protein loading. The activity of β-galactosidase was measured as described previously (29) and then normalized to the abundance of cellular σ28Ct protein. The resultant relative β-galactosidase activity was used to evaluate σ28Ct function.
Core-binding assays: coimmunoprecipitation.
To test the in vivo core-σ binding, cellular lysates of Salmonella fliA mutant TH7034 expressing σ28Ct (from pLF28 or its derivatives) in buffer (10 mM Tris [pH 7.5], 150 mM NaCl) were used for coimmunoprecipitation. To test core-σ binding in vitro, a reaction mixture (15 μl) containing purified σ28 proteins (4 pmol) and E. coli core RNAP (1 pmol) (Epicenter) in buffer A (10 mM Tris-Cl [pH 7.5], 1 mM β-mercaptoethanol, 0.3 M NaCl, 10 mM MgCl2, 0.1 mM EDTA, 0.01% Triton 100, 250 μg/ml bovine serum albumin) was used. Monoclonal antibody B8R13 (1 μl) against the β subunit was incubated with cell lysate or a mixture of σ28 and core at 4°C overnight, and then complexes of core and σ were purified using protein A-agarose (Sigma). After three time washes with buffer A to remove unbound σ factor, the bound proteins were separated on a 10% SDS-polyacrylamide gel, followed by Western blot analysis. Bands of the bound σ28Ct and β subunit were probed with polyclonal σ28Ct antibody and β antibody (8RB13), respectively. Protein bands were quantified with Quantity One software (Bio-Rad).
In vitro transcription assays.
The σ28RNAP holoenzyme was formed by incubation of a threefold excess of purified His6-tagged σ28 protein or derivatives with E. coli core RNAP enzyme (Epicenter) on ice for 15 min. In some cases, the ratio of mutated σ28 to core enzyme was increased in order to make σ28-saturated RNAP. σ70RNAP holoenzyme was purchased from Epicenter. The in vitro transcription system contained RNAP holoenzyme, supercoiled plasmids (1 μg), 10 mM Tris-HCl (pH 8.0), 200 mM NaCl, and 5 mM dithiothreitol, and transcription was initiated by adding 400 μM ATP, 400 μM UTP, 1.2 μM CTP, 0.20 μM [α-32P]CTP (3,000 μCi/mmol), and 100 μM 3′-O-methylguanosine 5′-triphosphate (GE Healthcare). The reaction was performed as described previously (39). mRNA made by the RNAP holoenzyme was separated and detected on a 6% (wt/vol) polyacrylamide-8 M urea polyacrylamide gel electrophoresis. Signal intensities from autoradiographs were determined with Quantity One software (Bio-Rad).
RESULTS
Replacement of σ28Ct region 4 with σ28Ec region 4 changes the behavior of RNAP holoenzyme in vitro.
Previously, we found that σ28CtRNAP is permissive in recognizing promoters with altered length between the promoter −35 and −10 elements, and preferentially activates promoters with upstream AT-rich sequences; in contrast the σ28RNAP of E. coli (σ28EcRNAP) does not have such a preference (40). In order to test whether differences in region 4 of σ28 were related to the observed disparity, we assessed the complementary functionality of σ28Ct region 4 by making a reciprocal pair of hybrid σ28 proteins. Specifically, region 4 of σ28Ec was exchanged with the relevant region 4 of σ28Ct (Fig. 1A). S. enterica serovar Typhimurium fliA mutant strain TH7034 carrying the constructs directing the synthesis of the hybrid σ28 (σ28Ec-Ct4 and σ28Ct-Ec4) appeared as red colonies on MacConkey-lactose-arabinose indicator plates, suggesting that both σ28Ec-Ct4 and σ28Ct-Ec4 transcribe from the same fliC promoter that was recognized by wild-type σ28Ct (23, 40).
We next tested the in vitro activities of purified σ28Ec-Ct4 or σ28Ct-Ec4 in the context of a holoenzyme with several defined σ28 promoters. We chose the chlamydial hctB promoter (PhctB) (native AT-rich upstream sequence plus core promoter of hctB), chlamydial hctB promoters with spacer lengths of 9 or 14 bp between the −35 and −10 element (PS9 and PS14), the core promoter of hctB (PhctBΔup), and the E. coli tar promoter (Ptar) (native GC-rich upstream sequence plus core promoter of tar) and its derivative Ptar+ (AT-rich upstream sequence of hctB plus core promoter of tar) (40). In the reaction mixture containing plasmid templates and reconstituted RNAP holoenzymes, the resultant transcripts were dependent on the addition of RNAP holoenzyme. RNAP holoenzyme made from hybrid σ28Ec-Ct4, which contains σ28Ct region 4, exhibited higher activities with PS9, PhctB, PS14, and Ptar+ (Ptest/PfliC transcript ratio of ≥1) but was weakly active with PhctBΔup and Ptar (Ptest/PfliC ration of <1) (Fig. 1B). Such behaviors of σ28Ec-Ct4 RNAP are comparable to those of wild-type σ28CtRNAP. In contrast, reconstituted σ28Ct-Ec4RNAP, which contains σ28Ec region 4, conferred strong activities for PhctB, PhctBΔup, Ptar, and Ptar+, but it was weakly active with PS9 and PS14 (Fig. 1C). These actions are in agreement with results from using wild-type σ28EcRNAP. Taken together, the results obtained with the hybrid σ28 proteins indicate that the observed differences in promoter selectivity between σ28Ct and σ28Ec are, at least in part, specified by region 4.
Identification of mutants defective in σ28Ct-dependent transcription in Salmonella.
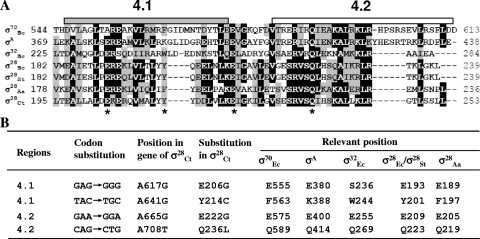
We next sought to identify residues in region 4 of σ28Ct that are important for σ28Ct-dependent transcription. A plasmid library carrying mutagenized σ28Ct genes driven from an arabinose-inducible ParaB promoter was transformed into Salmonella fliA mutant TH7034, which allowed us to screen mutants defective in transcription from σ28-dependent fliC::lacZ. In this background, only σ28Ct-dependent transcription can be detected, because no functional Salmonella σ28 exists. Cells were selected for a lower level of σ28-dependent transcription of lacZ (white or pink colonies) than for the wild type (red colony) by plating on MacConkey-lactose-arabinose agar. The candidates were further confirmed by measurement of β-galactosidase activity. Approximately 7,000 clones were screened, and four with single-residue substitutions in σ28Ct (E206G, Y214C, E222G, and Q236L) were isolated (Fig. 2). These mutants were further characterized as described below.
FIG. 2.
Mutagenesis of σ28Ct region 4. (A) Alignment of the amino acid sequences of region 4 from E. coli σ70, Thermus aquaticus σA, E. coli σ32 (σ32Ec), Salmonella σ28 (σ28St), σ28Ec, Aquifex aeolicus σ28 (σ28Aa), and σ28Ct using the CLUSTAL W program. The amino acid sequence of σ28Sa is identical to that of σ28Ec. Regions were defined based on previous studies (26, 41). The number for each amino acid position is relative to that from the start of each protein sequence. Identical or similar residues are shown in black or gray shadow, respectively. Asterisks at the bottom indicate residues undergoing mutation. (B) Isolation of σ28Ct mutants. Mutated nucleotides and the deduced amino acid residues are indicated based on the position of σ28Ct and corresponding residue positions in different σ factors.
Mutations in region 4 of σ28Ct decrease transcription from the chlamydial hctB promoter.
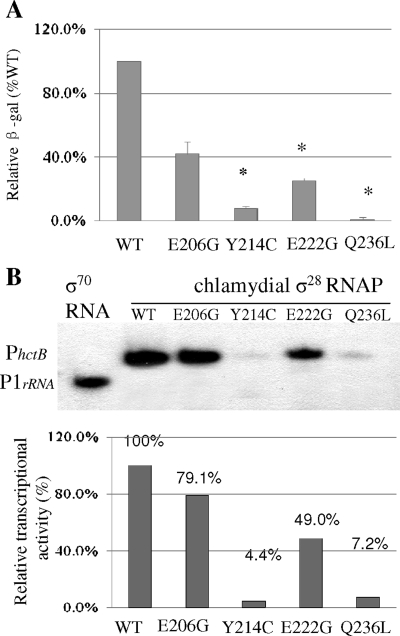
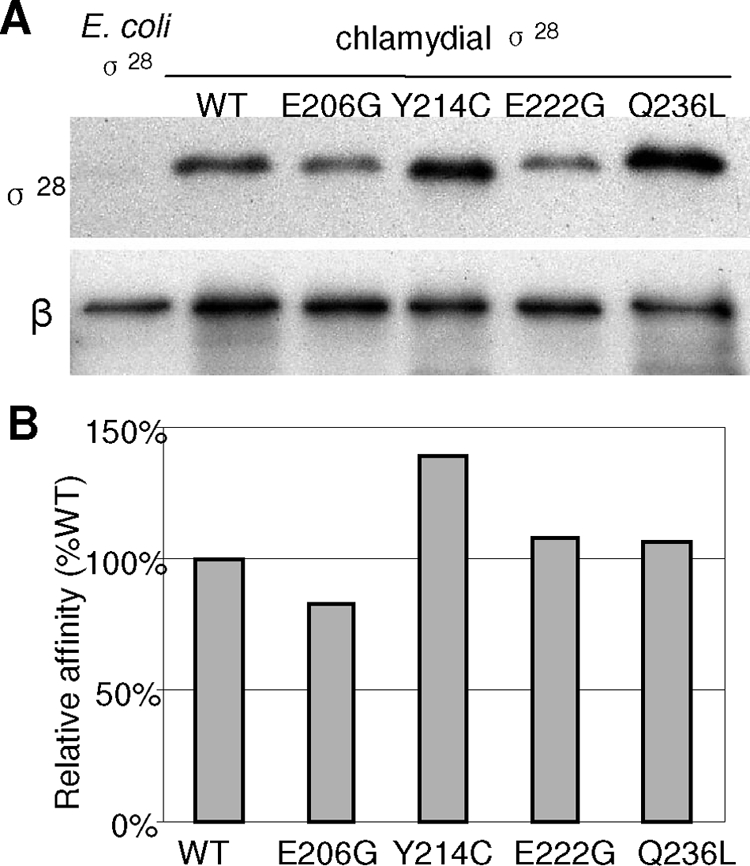
We compared the effects of σ28Ct mutants on transcription from the σ28Ct-dependent hctB promoter in Salmonella. Plasmid-encoded wild-type σ28Ct or derivatives were expressed in Salmonella fliA mutant strain TH5504 carrying pRVhctB, and σ28Ct-driven transcription from PhctB::lacZ was assessed by measurement of β-galactosidase activity as described in Materials and Methods. Relative β-galactosidase activities from strains carrying σ28Ct substitutions Y214C, E222G, and Q236L were significantly lower than that of wild-type σ28Ct (i.e., 7.9%, 25.1%, and 1.0%, respectively) (Fig. 3A). σ28Ct E206G also decreased levels of β-galactosidase activity, to about 41.9% relative to wild-type σ28Ct.
FIG. 3.
Effect of single-residue substitution on σ28-dependent transcription from chlamydial hctB promoter. (A) Activity of mutated σ28Ct in vivo using a lacZ reporter assay. Cellular β-galactosidase activity was normalized to levels of σ28Ct determined by Western blot analysis using specific anti-σ28 antibody. We previously found that mutation in region 4 of σ28Ct did not impair its reactivity with the antibody to σ28Ct. The β-galactosidase activity from each strain containing the indicated mutated proteins is represented as a percentage relative to the β-galactosidase activity of the strain with wild-type σ28Ct. Asterisks above the bars indicated significantly reduced activities compared with that of the wild type (P < 0.05). (B) Transcripts from single-round transcription assay in vitro. Plasmid pGHR containing two chlamydial promoters (PhctB and P1CtrRNA) was used as a template in the presence of σ70RNAP or σ28CtRNAP as indicated on the top. The transcripts generated from promoters are indicated on the left. The intensity of each transcript band was quantified using Quantity One. The amount of transcript produced by each mutant RNAP is reported as a percentage of the transcription of wild-type σ28CtRNAP.
Next, the direct effect of mutant σ28CtRNAP holoenzyme on transcription from PhctB was examined in vitro. In the presence of both σ28CtRNAP and σ70CtRNAP holoenzymes, plasmid template pGHR produced two divergent transcripts: a 152-bp transcript from the σ28-dependent PhctB and a 130-bp transcript from the σ66-dependent rRNA P1 (P1CtrRNA) (data not shown). The RNAP containing σ28Ct Y214C or Q236L was severely defective in generating transcripts from hctB, showing a yield of less than 10% of the wild-type level (Fig. 3B). The RNAP containing σ28Ct E206G or E222G reduced transcription activity to 79.1% and 49.0% of that of wild-type σ28CtRANP, respectively (Fig. 3B). None of the mutant σ28CtRNAPs was able to transcribe from the P1CtrRNA. We also noted that the RNAP saturated with mutated σ28Ct did not mount transcription activity in vitro. Moreover, mutated σ28CtRNAPs did not change their behavior for transcription from PS9, PhctB, PS14, PhctBΔUP, Ptar, and Ptar+ in vitro (data not shown).
Taken together, these transcription studies indicate that σ28Ct substitutions Y214C and Q236L severely decreased transcription from hctB, whereas substitutions E206G and E222G moderately affected hctB transcription.
Effect of σ28Ct substitutions on core binding.
The abilities of wild-type and mutant σ28Ct to bind core RNAP were compared using coimmunoprecipitations. The levels of mutant proteins that bound to Salmonella core RNAP were similar to or slightly higher than the wild-type level. (Fig. 4). As validation, we also examined potential core-binding differences among these proteins using the E. coli core and purified recombinant σ28Ct or derivatives in vitro. We found that each of the σ28Ct mutants effectively bound E. coli core RNAP by coimmunopreciptation relative to wild-type σ28Ct (data not shown). This confirms that none of the σ28Ct substitutions severely reduced σ affinity for the core RNAP under our test conditions.
FIG. 4.
Binding of σ28 and derivatives to core RNAP in Salmonella. (A) Immunoblot showing the results of a coimmunoprecipitation assay from Salmonella fliA mutant TH7034, which expresses σ28 or derivatives as indicated above each lane. A strain expressing E. coli σ28, which is identical to Salmonella σ28, was the negative control. Cell lysates were subjected to immunoprecipitation using anti-β monoclonal antibody as described in Materials and Methods. Precipitated proteins were separated by PAGE and immunoblotted with anti-σ28 antibody or anti-β monoclonal antibody. (B) Relative binding affinity of σ28 or derivatives for core. The amount of precipitated σ28 protein is reported as a percentage of the level of β in core RNAP.
Influence of σ28Ct substitutions on the interaction of σ28Ct region 4 with the β-flap.
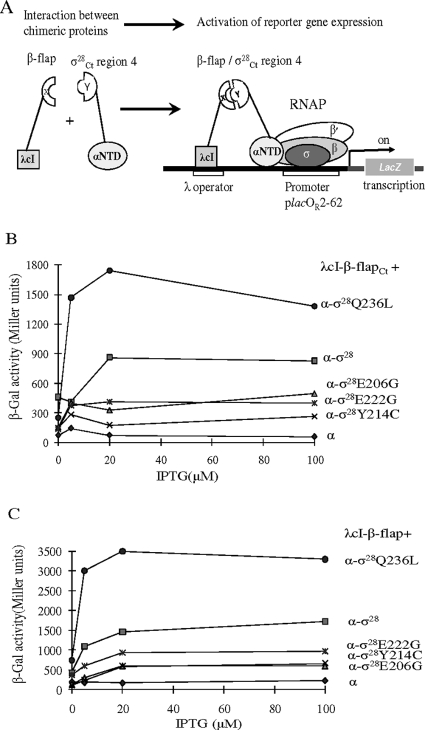
We examined whether substitutions in the σ28Ct region 4 would disrupt protein-protein interaction of σ28Ct region 4 with the β-flap in a bacterial two-hybrid assay (8, 10). This assay involves the use of (i) a reporter strain, KS1, which carries a chromosomal copy of the test promoter plac OR2-62 linked to lacZ; (ii) a plasmid encoding the λcI fused to the β-flap from Chlamydia (λcI-β-flapCt) or E. coli (λcI-β-flap); and (iii) a second plasmid encoding the N-terminal domain of αNTD fused to σ28Ct region 4 or its derivatives. Interaction of the β-flap with σ28Ct region 4 stabilizes RNAP binding to the test promoter, thus, mediating transcription activation from the test promoter plac OR2-62::lacZ. This can be monitored by measuring β-galactosidase activity (Fig. 5A).
FIG. 5.
Bacterial two-hybrid assay: characterization of σ28Ct substitution that affects its interaction with the β-flap. (A) Diagram showing how interaction of the σ28Ct region 4 (in pBRα-σ28Ct) with the β-flap (in pACλcI-β-flapCt or pACλcI-β-flap) activates transcription from the test promoter plac OR2-62 located on the chromosome in KS1. (B) Effects of substitution in the σ moiety of the α-σ28Ct chimera on transcription from plac OR2-62 in the presence of the chimera λcI-β-flapCt. (C) Effects of substitutions in the σ moiety of the α-σ28Ct chimera on transcription from plac OR2-62 in the presence of the chimera λcI-β-flap. KS1 cells harboring two compatible plasmids, which direct the synthesis of α-σ28Ct or derivatives and λcI-β-flapCt or pACλcI-β-flap as indicated, were grown in the presence of carbencillin, chloramphenicol, and kanamycin. Protein expression was induced by treatment with different concentrations of IPTG for 1 hour when cultures reached an optical density at 600 nm of 0.3. β-Galactosidase activity was measured in duplicate on at least three independent occasions. Values shown are the averages from one experiment.
As shown in Fig. 5B, in the presence of the chlamydial β-flap fusion (cI-β-flapCt), the chimera α-σ28Ct activates transcription from plac OR2-62 up to ∼14.0-fold relative to that of the negative control α expressed from pBR-α. Such an increased magnitude compared to no obvious increase in the paired α-σ70/cI-β-flap did not surprise us, because σ28Ct contains a residue at G228 corresponding to σ70D581 (25, 34). Substitution at σ70D581G stabilizes the folded structure of the tethered σ70 region 4 moiety and enhances interaction of σ70 region 4 with the DNA −35 element (25, 34). In strains coexpressing chimera λcI-β-flapCt and chimera α-σ28E206G, α-σ28Y214C, or α-σ28E222G, the magnitude of transcription activation from plac OR2-62 decreased about ∼8.3-, ∼4.5-, and ∼6.9-fold, respectively. The decrease of transcription activation observed is not due to instability of chimera proteins, as the chimeras were able to interact with the hctB −35 element in a one-hybrid assay (Fig. 6). In contrast, chimera α-σ28Q236L strengthened the interaction between the β-flap and σ28 in the presence of λcI-β-flapCt; transcription from plac OR2-62 was increased up to ∼23.4-fold (Fig. 5B) compared to the wild-type σ28Ct (∼14.0-fold increase).
In the presence of an E. coli β-flap fusion (cI-β-flap), the α-σ28Ct chimera increased transcription from plac OR2-62 up to ∼8.5-fold, compared to ∼14-fold in the presence of the cI-β-flapCt chimera. Substitutions E206G, Y214C, and E222G in σ28Ct reduced transcription from plac OR2-62 to ∼3.4-, ∼3.4-, and ∼5.4-fold, respectively. However, the α-σ28Ct Q236L chimera stimulates transcription from plac OR2-62 ∼14.4-fold, compared to a change of ∼23.4-fold in the presence of the cI-β-flapCt chimera (Fig. 5C).
These results indicate that substitution E206G, Y214C, or E222G in σ28Ct weakens interactions between σ28Ct and the β-flap from both E. coli and Chlamydia. Thus, these residues are involved in interaction of the cI-β-flap with σ28 region 4, whereas σ28Ct residue Q236 seems not to be important for β-flap binding. E. coli and chlamydial β-flap share 62.2% amino acid identity and 73.8% similarity. The finding that σ28Ct can bind the E. coli β-flap could be relevant to its ability to transcribe from both σ28Ec- and σ28Ct-dependent promoters when it associates with the E. coli core enzyme in a heterologous genetic system or in vitro.
Effect of σ28Ct mutants on binding of the −35 element from the hctB promoter.
We next examined whether residue substitutions in σ28Ct region 4 would affect interaction of σ region 4 and the −35 element of the promoter, using a one-hybrid assay (34). This in vivo DNA-binding assay is designed to use a test promoter, which contains an ectopic hctB −35 element upstream of the lac promoter in strain ZY101, and a plasmid-encoded α-σ28 fusion. The direct interaction of σ28 region 4 with the ectopic hctB −35 element recruits and stabilizes RNAP to the test promoter, causing an increase of reporter gene (lacZ) expression. This can be monitored by measuring β-galactosidase activity (Fig. 6A).
We introduced a plasmid encoding chimera α-σ28 or its derivatives into the reporter strain ZY101. We chose chimera α-σ66 as a negative control, as previous report showed that chlamydial σ66 did not recognize the σ28-dependent promoter (48). The σ28L243K mutant bears enhanced transcriptional activity from the hctB promoter (Z. Hua et al., unpublished data) and was the positive control. In the presence of α-σ28, transcription activation from the test promoter was observed, and the increase of stimulation occurred in an IPTG dose-dependent fashion (Fig. 6B). Similarly, expression of α-σ28E206G, α-σ28E222G, or α-σ28Y214C was able to activate transcription from the test promoter. Although the stimulations are small (<1.5-fold), these increases are reproducible in all experiments. Chimera α-σ28L243K increased transcription up to 1.8-fold, indicating that this system functioned. In contrast, chimera α-σ28Q236L failed to stimulate transcription from the test promoter (Fig. 6B). Because α-σ28Q236L interacts with chimera λcI-β-flap (Fig. 5), the inability of α-σ28Q236L to interact with the ectopic promoter is unlikely to be related to the poor protein expression. The substitution σ28CtQ236L disrupted α-σ28/hctB −35 interactions, which may be an explanation for our findings. As expected, chimera α-σ66 was unable to activate transcription from test promoter in ZY101 (Fig. 6B and C); however, α-σ66 stimulated transcription from placCons-35C in BN317, which contains a σ70-specific ectopic −35 element (data not shown).
In ZY102, which bears a mutant ectopic hctB −35 element (gAAAGTTT), none of the wild-type and mutant α-σ28 chimeras stimulated transcription from the test promoter (Fig. 6C). The first base pair T of the hctB −35 element has been shown to be the major determinant for σ28Ct recognition (49). This result confirmed that the observed stimulatory effects of α-σ28Ct, α-σ28E206G, α-σ28Y214C, and α-σ28E222G in ZY101 are a result of the specific interaction of the chimeras with the ectopic hctB −35 element. Substitution σ28CtQ236L interrupts interaction between σ28 and the hctB −35 element, indicating that σ28Ct Q236 is essential to be in contact with the −35 element.
DISCUSSION
We focused on characterization of several σ28Ct mutants with mutations in region 4 that are defective in σ28-dependent transcription. Since there is no successful genetic system available to manipulate genes in Chlamydia, our strategy has been to study σ28-dependent transcription using complementary approaches. By switching σ28Ct region 4 with σ28Ec region 4, we found that amino acids in σ28Ct region 4 might specify the function of the σ28CtRNAP holoenzyme for use of an altered spacer length between promoter −35 and −10 elements, as well as preference for the UP-like sequences in vitro (Fig. 1). We also screened for σ28Ct mutants defective in transcription from the σ28-dependent fliC promoter in Salmonella (Fig. 2). We then examined the effects of four single-residue substitutions in σ28Ct region 4 on transcription from the chlamydial hctB promoter both in vitro and in a fliA mutant Salmonella strain. We found that substitutions σ28Ct, Y214C and Q236L, significantly decreased transcription from the hctB promoter, while σ28CtE206G and -E222G moderately reduced hctB transcription (Fig. 3); the promoter specificity was unchanged, and the effect was stronger in vivo than in vitro. A possible explanation for this is that the existence of additional host cell regulatory factors in vivo might lead to a bias for a diminished in vitro effect seen with σ28Ct mutations. It is unlikely that the difference observed is due to the inhibition of FlgM encoded by the surrogate strain of S. enterica serovar Typhimurium, because FlgM specifically negatively regulates Salmonella σ28 but not σ28Ct (22).
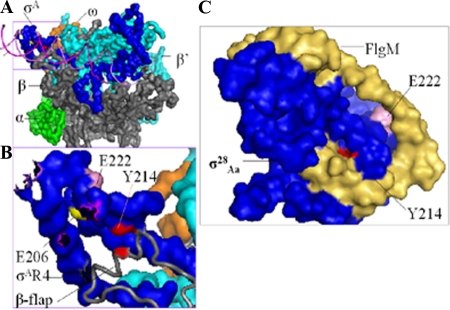
The apparent transcriptional deficiency observed might result from σ28Ct mutations that have a reduced affinity of σ with the core RNAP, a disrupted σ/β-flap interaction, and/or an occluded σ/promoter interaction. Our data do not support a direct strong effect of mutant σ28Ct on core affinity, since RNAP saturated by mutant σ28 did not increase transcription. Moreover, the mutant σ28Ct effectively bound core RNAP in a coimmunoprecipitation experiment (Fig. 4). However, modest indirect effects cannot be excluded. By taking advantage of the bacterial two-hybrid system, we found that the three substitutions in σ28Ct, E206G, Y214C, and E222G, impaired interaction of σ28Ct with the β-flap from Chlamydia or from E. coli (Fig. 5). Of these residues, only the corresponding residue σ28CtY214 has been mapped and directly contacts the β-flap in the X-ray structure of the Thermus aquaticus σA holoenzyme-DNA complex (34) (Fig. 7A and B). The corresponding residues of σ28CtE206 and -E222 may indirectly involve such σ/β-flap interactions. The X-ray structure of Aquifex aeolicus σ28, free or in complex with FlgM, the anti-σ28 factor, does not explain our observations, as both core and DNA-binding domains are buried in the folded compact structure (Fig. 7C) (41, 42). We speculate that σ28Ct is mostly in an active conformation when it associates with the core RNAP in Salmonella or in vitro, consistent with their transcription activities. Perhaps substitution E206G, Y214C, or E222G in σ28Ct caused a deficiency of σ28Ct in binding the β-flap, resulting in an unstable or mispositioned region 4 in the σ28CtRNAP holoenzyme and an inability to simultaneously bind promoter −10 and −35 elements, leading to poor transcription activity. Our results further add support to the idea that RNAP remodeling of σ28 region 4 is required to expose the recognition domains of both the −35 binding site and β-flap on σ28 (41, 42). Previous studies indicated that the σ70 residues E555, F563, and E575 (corresponding to σ28Ct E206, Y214, and E222, respectively) (Fig. 3) have been implicated in interaction with the E. coli β-flap (12, 13).
FIG. 7.
Positions of residues in substitutions that affect the interaction of σ28Ct region 4 with the β-flap. (A) Locations of residues based on the crystal structures of Thermus aquaticus σA holoenzyme and DNA (PDB ID no. 1I9Z) (32). Shown are the surfaces of α (green), β-flap (gray), β′ (cyan), ω (orange), and σ factor (blue). The DNA promoter (magenta) is shown as a cartoon. (B) Enlarged illustration of region 4 of T. aquaticus σA (labeled with corresponding amino acid numbers in σ28Ct) (see amino acid alignments in Fig. 2) and the β-flap contact. The β-flap (gray) is shown as the cartoon. The DNA promoter is hidden. (C) Locations of residues based on the crystal structures of the σ28Aa/FlgM complex (labeled with corresponding amino acid numbers in chlamydial σ28Ct) (PDB ID no. 1SC5 and 1RP3) (41). Blue, σ28Aa; yellow-brown, FlgM. Residues corresponding to the T aquaticus σA or σ28Aa were identified and mutated to the naturally occurring residues in chlamydial σ28Ct as indicated. This figure was generated using Pymol 0.99rc6 (http://pymol.sourceforge.net).
Our data suggest a role of residue Q236 of σ28Ct in binding the −35 element of the hctB promoter because (i) substitution Q236L in σ28Ct disrupted the interaction of σ28Ct with the hctB −35 sequence as determined by the one-hybrid assay (Fig. 6), and the same protein interacted with the β-flap as determined by the two-hybrid assay (Fig. 5); (ii) Q236 is located in the DNA-binding helix-turn-helix motif in of σ28Ct; and (iii) in T. aquaticus σA, the corresponding residue Q414 is structurally positioned on the DNA-binding interface (4). Consistent with our results, Kourennaia et al. (24) reported that the substitution Q269A in E. coli σ32 (corresponding to σ28Ct Q236) impeded recognition of the −35 element of the E. coli groE promoter but did not reduce its ability to bind to the core enzyme. The corresponding residue in σ70 (Q589) has not been implicated in contact with a specific DNA base. Instead, the adjacent residue R588 of σ70 can help position residue 585 for direct contact with bacteriophage PRM DNA and mediate binding to the DNA activator (20). Presumably, the σ28Ct substitution Q236L, which changes a polar residue to an aliphatic hydrophobic residue, interrupts the side chain contacting the −35 element sequence and/or indirectly affects its ability to bind DNA by distorting or destabilizing the structure of σ28Ct region 4.
Given the favorable effect of the presence of AT-rich sequences upstream of the promoter on σ28Ct-dependent transcription (40), we wondered whether a possible positive influence is induced by the C-terminal domain of the α subunit (αCTD). The interaction of σ region 4 with the αCTD can stimulate transcription from a subset of UP element-dependent promoters by tightening RNAP-promoter associations (14). We failed to detect direct interaction of wild-type or mutant σ28Ct with the αCTD in a two-hybrid assay (Hua et al., unpublished data). We still cannot rule out the possibility that this association, if any, might be transient and/or weak, thus making it difficult to detect in vivo.
These studies have allowed us to begin defining the determinants in σ28Ct that are essential for contact with the β-flap and with the −35 element; both are required for recognition of the −35/−10 promoter. Because there is no overlapping promoter recognition specificity between σ28Ct and the primary σ66 (σ70) (39, 40), it is fair to assume that there might be some differences regarding the σ/core interaction as well as σ/promoter interaction. Supporting this, σ28Ct Q236 has been shown to be important for the σ28Ct-specific −35 element binding. This function has not been reported for the counterpart residue of E. coli σ70. While this study does not directly address whether σ28 activity is inhibited by a regulator, observations with several σ28Ct region 4 mutants defective in contact with the β-flap indicates that inactivation may play a role in chlamydial σ28 activity control. In the future, we will use a stabilized or deficient protein-binding mutant of chlamydial σ28 in order to facilitate identification and characterization of potential σ28 regulators in a two-hybrid assay.
Acknowledgments
We gratefully acknowledge Ann Hochschild and Padraig G. Deighan for their suggestions and help with design of the one-hybrid and two-hybrid assays used in this study, as well as for comments on the manuscript. We thank You-xun Zhang and Sean J. Garrity for helpful discussions, Kelly T. Hughes for S. enterica serovar Typhimurium strains, Tom P. Hatch for chlamydial σ28 antibody, Pieter L. deHaseth and Kathleen Mathews for plasmids, and Ronald Luftig and Timothy P. Foster for critical reading of the manuscript.
This work was supported by grants from the National Institutes of Health (AI055869) and the Louisiana State University Health Sciences Center Fund to L.S.
Footnotes
Published ahead of print on 31 October 2008.
REFERENCES
- 1.Arthur, T. M., and R. R. Burgess. 1998. Localization of a sigma70 binding site on the N terminus of the Escherichia coli RNA polymerase beta′ subunit. J. Biol. Chem. 27331381-31387. [DOI] [PubMed] [Google Scholar]
- 2.Belland, R. J., G. Zhong, D. D. Crane, D. Hogan, D. Sturdevant, J. Sharma, W. L. Beatty, and H. D. Caldwell. 2003. Genomic transcriptional profiling of the developmental cycle of Chlamydia trachomatis. Proc. Natl. Acad. Sci. USA 1008478-8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brickman, T. J., C. E. Barry III, and T. Hackstadt. 1993. Molecular cloning and expression of hctB encoding a strain-variant chlamydial histone-like protein with DNA-binding activity. J. Bacteriol. 1754274-4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell, E. A., O. Muzzin, M. Chlenov, J. L. Sun, C. A. Olson, O. Weinman, M. L. Trester-Zedlitz, and S. A. Darst. 2002. Structure of the bacterial RNA polymerase promoter specificity sigma subunit. Mol. Cell 9527-539. [DOI] [PubMed] [Google Scholar]
- 5.Campbell, E. A., L. F. Westblade, and S. A. Darst. 2008. Regulation of bacterial RNA polymerase sigma factor activity: a structural perspective. Curr. Opin. Microbiol. 11121-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colland, F., J. C. Rain, P. Gounon, A. Labigne, P. Legrain, and H. De Reuse. 2001. Identification of the Helicobacter pylori anti-sigma28 factor. Mol. Microbiol. 41477-487. [DOI] [PubMed] [Google Scholar]
- 7.Douglas, A. L., and T. P. Hatch. 1995. Functional analysis of the major outer membrane protein gene promoters of Chlamydia trachomatis. J. Bacteriol. 1776286-6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dove, S. L., J. K. Joung, and A. Hochschild. 1997. Activation of prokaryotic transcription through arbitrary protein-protein contacts. Nature 386627-630. [DOI] [PubMed] [Google Scholar]
- 9.Dove, S. L., F. W. Huang, and A. Hochschild. 2000. Mechanism for a transcriptional activator that works at the isomerization step. Proc. Natl. Acad. Sci. USA 9713215-13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dove, S. L., and A. Hochschild. 2001. Bacterial two-hybrid analysis of interactions between region 4 of the sigma(70) subunit of RNA polymerase and the transcriptional regulators Rsd from Escherichia coli and AlgQ from Pseudomonas aeruginosa. J. Bacteriol. 1836413-6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dove, S. L., S. A. Darst, and A. Hochschild. 2003. Region 4 of sigma as a target for transcription regulation. Mol. Microbiol. 48863-874. [DOI] [PubMed] [Google Scholar]
- 12.Geszvain, K., T. M. Gruber, R. A. Mooney, C. A. Gross, and R. Landick. 2004. A hydrophobic patch on the flap-tip helix of E. coli RNA polymerase mediates sigma (70) region 4 function. J. Mol. Biol. 343569-587. [DOI] [PubMed] [Google Scholar]
- 13.Gregory, B. D., B. E. Nickels, S. J. Garrity, E. Severinova, L. Minakhin, R. J. Urbauer, T. Heyduk, K. Severinov, and A. Hochschild. 2004. A regulator that inhibits transcription by targeting an intersubunit interaction of the RNA polymerase holoenzyme. Proc. Natl. Acad. Sci. USA 1014554-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gourse, R. L., W. Ross, and T. Gaal. 2000. UPs and downs in bacterial transcription initiation: the role of the alpha subunit of RNA polymerase in promoter recognition. Mol. Microbiol. 37687-695. [DOI] [PubMed] [Google Scholar]
- 15.Gross, C. A., C. Chan, A. Dombroski, T. Gruber, M. Sharp, J. Tupy, and B. Young. 1998. The functional and regulatory roles of sigma factors in transcription. Cold Spring Harbor Symp. Quant. Biol. 63141-155. [DOI] [PubMed] [Google Scholar]
- 16.Hatch, T. P. 1999. Developmental biology, p 29-67. In R. S. Stephens (ed.), Chlamydia. Intracellular biology, pathogenesis, and immunity. American Society for Microbiology, Washington, DC.
- 17.Hua, L., P. S. Hefty, Y. J. Lee, Y. M. Lee, R. S. Stephens, and C. W. Price. 2006. Core of the partner switching signalling mechanism is conserved in the obligate intracellular pathogen Chlamydia trachomatis. Mol. Microbiol. 59623-636. [DOI] [PubMed] [Google Scholar]
- 18.Hughes, K. T., and K. Mathee. 1998. The anti-sigma factors. Annu. Rev. Microbiol. 52231-286. [DOI] [PubMed] [Google Scholar]
- 19.Ishihama, A. 2000. Functional modulation of Escherichia coli RNA polymerase. Annu. Rev. Microbiol. 54499-518. [DOI] [PubMed] [Google Scholar]
- 20.Jain, D., B. E. Nickels, L. Sun, A. Hochschild, and S. A. Darst. 2004. Structure of a ternary transcription activation complex. Mol. Cell 1345-53. [DOI] [PubMed] [Google Scholar]
- 21.Jishage, M., and A. Ishihama. 1998. A stationary phase protein in Escherichia coli with binding activity to the major sigma subunit of RNA polymerase. Proc. Natl. Acad. Sci. USA 954953-4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karlinsey, J. E., and K. T. Hughes. 2006. Genetic transplantation: Salmonella enterica serovar Typhimurium as a host to study sigma factor and anti-sigma factor interactions in genetically intractable systems. J. Bacteriol. 188103-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koehler, J. E., R. R. Burgess, N. E. Thompson, and R. S. Stephens. 1990. Chlamydia trachomatis RNA polymerase major sigma subunit. Sequence and structural comparison of conserved and unique regions with Escherichia coli sigma 70 and Bacillus subtilis sigma 43. J. Biol. Chem. 26513206-13214. [PubMed] [Google Scholar]
- 24.Kourennaia, O. V., L. Tsujikawa, and P. L. Dehaseth. 2005. Mutational analysis of Escherichia coli heat shock transcription factor sigma 32 reveals similarities with sigma 70 in recognition of the −35 promoter element and differences in promoter DNA melting and −10 recognition. J. Bacteriol. 1876762-6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuznedelov, K., L. Minakhin, A. Niedziela-Majka, S. L. Dove, D. Rogulja, B. E. Nickels, A. Hochschild, T. Heyduk, and K. Severinov. 2002. A role for interaction of the RNA polymerase flap domain with the sigma subunit in promoter recognition. Science 295855-857. [DOI] [PubMed] [Google Scholar]
- 26.Lonetto, M., M. Gribskov, and C. A. Gross. 1992. The sigma 70 family: sequence conservation and evolutionary relationships. J. Bacteriol. 1743843-3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathews, S. A., and R. S. Stephens. 1999. DNA structure and novel amino and carboxyl termini of the Chlamydia sigma 70 analogue modulate promoter recognition. Microbiology 1451671-8181. [DOI] [PubMed] [Google Scholar]
- 28.Mathews, S. A., and P. Timms. 2000. Identification and mapping of sigma-54 promoters in Chlamydia trachomatis. J. Bacteriol. 1826239-6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 30.Mooney, R. A., S. A. Darst, and R. Landick. 2005. Sigma and RNA polymerase: an on-again, off-again relationship? Mol. Cell 20335-345. [DOI] [PubMed] [Google Scholar]
- 31.Murakami, K. S., and S. A. Darst. 2003. Bacterial RNA polymerases: the whole story. Curr. Opin. Struct. Biol. 1331-39. [DOI] [PubMed] [Google Scholar]
- 32.Murakami, K. S., S. Masuda, E. A. Campbell, O. Muzzin, and S. A. Darst. 2002. Structural basis of transcription initiation: an RNA polymerase holoenzyme-DNA complex. Science 2961285-1290. [DOI] [PubMed] [Google Scholar]
- 33.Nicholson, T. L., L. Olinger, K. Chong, G. Schoolnik, and R. S. Stephens. 2003. Global stage-specific gene regulation during the developmental cycle of Chlamydia trachomatis. J. Bacteriol. 1853179-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nickels, B. E., S. L. Dove, K. S. Murakami, S. A. Darst, and A. Hochschild. 2002. Protein-protein and protein-DNA interactions of sigma70 region 4 involved in transcription activation by λcI. J. Mol. Biol. 32417-34. [DOI] [PubMed] [Google Scholar]
- 35.Nickels, B. E., S. J. Garrity, V. Mekler, L. Minakhin, K. Severinov, R. H. Ebright, and A. Hochschild. 2005. The interaction between sigma70 and the beta-flap of Escherichia coli RNA polymerase inhibits extension of nascent RNA during early elongation. Proc. Natl. Acad. Sci. USA 1024488-4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schachter, J. 1999. Infection and diseases epidemiology, p. 139-169. In R. S. Stephens (ed.), Chlamydia. Intracellular biology, pathogenesis, and immunity. American Society for Microbiology, Washington, DC.
- 37.Shaw, E. I., C. A. Dooley, E. R. Fischer, M. A. Scidmore, K. A. Fields, and T. Hackstadt. 2000. Three temporal classes of gene expression during the Chlamydia trachomatis developmental cycle. Mol. Microbiol. 37913-925. [DOI] [PubMed] [Google Scholar]
- 38.Shen, L., Y. Shi, A. L. Douglas, T. P. Hatch, C. M. O'Connell, J. M. Chen, and Y. X. Zhang. 2000. Identification and characterization of promoters regulating tuf expression in Chlamydia trachomatis serovar F. Arch. Biochem. Biophys. 37946-56. [DOI] [PubMed] [Google Scholar]
- 39.Shen, L., M. Li, and Y. X. Zhang. 2004. Chlamydia trachomatis sigma28 recognizes the fliC promoter of Escherichia coli and responds to heat shock in chlamydiae. Microbiology 150205-215. [DOI] [PubMed] [Google Scholar]
- 40.Shen, L., X. Feng, Y. Yuan, X. Luo, T. P. Hatch, K. T. Hughes, J. S. Liu, and Y. X. Zhang. 2006. Selective promoter recognition by chlamydial σ28 holoenzyme. J. Bacteriol. 1887364-7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sorenson, M. K., S. S. Ray, and S. A. Darst. 2004. Crystal structure of the flagellar sigma/anti-sigma complex sigma(28)/FlgM reveals an intact sigma factor in an inactive conformation. Mol. Cell 14127-138. [DOI] [PubMed] [Google Scholar]
- 42.Sorenson, M. K., and S. A. Darst. 2006. Disulfide cross-linking indicates that FlgM-bound and free sigma28 adopt similar conformations. Proc. Natl. Acad. Sci. USA 10316722-16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stephens, R. S., S. Kalman, C. Lammel, J. Fan, R. Marathe, L. Aravind, W. Mitchell, L. Olinger, R. L. Tatusov, Q. Zhao, E. V. Koonin, and R. W. Davis. 1998. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science 282754-759. [DOI] [PubMed] [Google Scholar]
- 44.Tan, M., and J. N. Engel. 1996. Identification of sequences necessary for transcription in vitro from the Chlamydia trachomatis rRNA P1 promoter. J. Bacteriol. 1786975-6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whipple, F. W. 1998. Genetic analysis of prokaryotic and eukaryotic DNA-binding proteins in Escherichia coli. Nucleic Acids Res. 263700-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wosten, M. M. 1998. Eubacterial sigma-factors. FEMS Microbiol. Rev. 22127-150. [DOI] [PubMed] [Google Scholar]
- 47.Wycuff, D. R., and K. S. Matthews. 2000. Generation of an AraC-araBAD promoter-regulated T7 expression system. Anal. Biochem. 27767-73. [DOI] [PubMed] [Google Scholar]
- 48.Yu, H. H., and M. Tan. 2003. Sigma28 RNA polymerase regulates hctB, a late developmental gene in Chlamydia. Mol. Microbiol. 50577-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu, H. H., E. G. Di Russo, M. A. Rounds, and M. Tan. 2006. Mutational analysis of the promoter recognized by Chlamydia and Escherichia coli sigma(28) RNA polymerase. J. Bacteriol. 1885524-5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu, H. H., D. Kibler, and M. Tan. 2006. In silico prediction and functional validation of sigma28-regulated genes in Chlamydia and Escherichia coli. J. Bacteriol. 1888206-8212. [DOI] [PMC free article] [PubMed] [Google Scholar]