Abstract
It has been proposed that the relative scarcity of Staphylococcus aureus and Streptococcus pneumoniae cocolonization in the nasopharynxes of humans can be attributed to hydrogen peroxide-mediated interference competition. Previously it has been shown in vitro that H2O2 produced by S. pneumoniae is bactericidal to S. aureus. To ascertain whether H2O2 has this inhibitory effect in the nasal passages of neonatal rats, colonization experiments were performed with S. aureus and S. pneumoniae. The results of these experiments with neonatal rats are inconsistent with the hypothesis that hydrogen peroxide-mediated killing of S. aureus by S. pneumoniae is responsible for the relative scarcity of cocolonization by these bacteria. In mixed-inoculum colonization experiments and experiments where S. aureus invaded the nasopharynxes of rats with established S. pneumoniae populations, the density of S. aureus did not differ whether the S. pneumoniae strain was H2O2 secreting or non-H2O2 secreting (SpxB). Moreover, the advantage of catalase production by S. aureus in competition with a non-catalase-producing strain (KatA) during nasal colonization was no greater in the presence of H2O2-producing S. pneumoniae than in the presence of non-H2O2-producing S. pneumoniae.
Recent epidemiological investigations of the carriage of Staphylococcus aureus and Streptococcus pneumoniae suggest that cocolonization by these two commensal (and occasionally invasive) bacteria is negatively correlated (1, 7, 11, 16, 24). One corollary of this observation is that a reduction in the frequency of colonization by one of these species would lead to a corresponding increase in the frequency of colonization by the other. In fact, it has been proposed that the reduction of S. pneumoniae colonization due to the pneumococcal conjugate vaccine has played a role in the increase in S. aureus acute otitis media and bacteremia (5, 23). The mechanism proposed to account for this cocolonization pattern is interference competition, or allelopathy, mediated by the killing of S. aureus by S. pneumoniae-produced hydrogen peroxide (H2O2) (19). In broth, high densities of S. pneumoniae mixed with S. aureus result in the demise of S. aureus, and killing does not occur if the pneumococci genetically lack pyruvate oxidase (SpxB) or if catalase is present to neutralize the H2O2 (19). While much attention has been given to the appealing hypothesis that loss of H2O2-mediated interference competition by S. pneumoniae is responsible for the increase in invasive S. aureus infections, there has been only indirect confirmation (14) that this allelopathic mechanism operates during nasal colonization.
In this study, I tested the effect of H2O2 production by S. pneumoniae on nasal colonization of S. aureus in two scenarios where interspecies interference may occur in a neonatal rat model: the invasion of an established population of S. pneumoniae by S. aureus and the use of mixed inocula of both species. By using isogenic strains of S. pneumoniae that either produce or do not produce H2O2 (SpxB), I demonstrated that H2O2 production by S. pneumoniae has no effect on S. aureus colonization. Furthermore, the advantage of catalase-producing S. aureus in the presence of S. pneumoniae is manifest whether S. pneumoniae produces or does not produce H2O2. I discuss some potential reasons why S. pneumoniae-mediated H2O2 allelopathy is effective against S. aureus in vitro but not in vivo.
MATERIALS AND METHODS
Bacterial strains, media, and inoculum preparation.
S. pneumoniae TIGR4 (21) and an SpxB-negative variant of TIGR4 (designated SxpB) (19) were provided by Marc Lipsitch. S. aureus PS80 (serotype 8, ATTC 27700) and Newman (NCTC 8178) were obtained from the American Type Culture Collection. A catalase-deficient, KatA-negative variant of Newman (designated KatA) (14) was provided by George Liu. S. aureus strains were cultivated in Luria-Bertani (LB) broth cultures and agar plates incubated at 37°C. S. pneumoniae strains were grown in Todd-Hewitt broth supplemented with 5 g of yeast extract (THY), and plates were supplemented with 40 ml of sheep blood (BBL). Broth cultures and agar plates of S. pneumoniae were incubated at 37°C with 5% CO2.
Inocula for all the infant rat experiments were prepared by initially growing strains to late logarithmic phase (optical density of 0.35 to 0.8). These were stored at −80°C and then thawed on the day of the experiment before being suspended in 2 ml of either LB or THY. Cultures that reached mid-exponential phase were centrifuged (5,000 × g, 3 min) and resuspended in phosphate-buffered saline with 0.1% gelatin (PBSG). Inocula and animal specimen densities were estimated by plating dilutions on LB agar plates for Newman or PS80, on LB plates supplemented with spectinomycin (100 mg/liter) for KatA, and on THY blood plates supplemented with streptomycin (40 mg/liter) for TIGR4 or kanamycin (75 mg/liter) for SpxB.
Infant rat model.
All in vivo experiments were preformed under the guidelines approved by the Emory University Institutional Animal Care and Use Committee. Three-day-old pups, born of timed-pregnant Sprague-Dawley rats (Charles River Laboratories), were pooled, randomly reassigned to dams, and maintained in microisolator cages in a biocontainment facility. At 3 or 5 days of age, rats were intranasally inoculated by touching a drop of 106 to 107 bacteria of either a mixture of S. aureus and S. pneumoniae strains or one strain alone suspended in 5 μl PBSG to the right and nares then another such drop to the left external nares (9, 12, 13).
Two days after the inoculation, nasal wash was collected from 200 μl of PBSG instilled into a 5-cm intramedic polyetylene tubing (PE50; Clay Adams) placed into the trachea, and nasal epithelium was scraped from the nasal passages after a second wash with 200 μl of PBSG and removal of the frontal bones. The nasal epithelium, which has been suggested to represent a distinct population (2), was homogenized in 1 ml of PBSG.
In all experiments, 100 μl of the nasal wash and nasal epithelium samples were plated directly and serially diluted onto selective plates. Plates were incubated overnight at 37°C, and all colonies were counted. The limit for detection at any site was 20 CFU/ml.
Experimental design.
In the mixed-inoculum experiments, 5-day-old rats were intranasally inoculated with 106 to 107 bacteria of a mixture of an S. aureus strain(s) (Newman, PS80, KatA, or a mixture of Newman and KatA) and an S. pneumoniae strain (TIGR4 or SpxB) at a ratio of 1:5 (S. aureus to S. pneumoniae). For each pairing between an S. aureus strain and an S. pneumoniae strain, this experiment was replicated in three different groups of 6 to 12 rats; results from a single replicate are shown (all others are available at www.eclf.net).
For the experiments testing whether S. aureus can invade when S. pneumoniae is established, groups of six 3-day-old rats were inoculated in both nostrils with 106 S. pneumoniae bacteria (TIGR4 or SpxB) or with PBS. All of these rats were then inoculated 48 h later with 106 to 107 S. aureus bacteria (Newman, PS80, KatA, or a mixture of Newman and KatA). For each pairing between an S. aureus strain and an S. pneumoniae strain, this experiment was replicated in two different groups of 6 to 12 rats; results from a single replicate are shown (all others are available on www.eclf.net).
Statistical analysis.
Welch's t test was used to evaluate the statistical significance of H2O2 production by S. pneumoniae for the bacterial density of S. aureus following growth on agar surfaces or during nasal colonization. To ascertain whether catalase-producing S. aureus was being selected during nasal colonization, the selection rate constant (rnk) was calculated. The selection rate is a measure of the relative recoveries of the catalase-producing S. aureus strain (Newman) and the catalase-deficient S. aureus strain (KatA) inoculated into the nasal passages over the 48 h and is given by rnk = [Nn(48)/Nn(0)] − [Nk(48)/Nk(0)], where Nn(0) and Nk(0) are the initial densities in the inoculum of Newman and KatA, respectively, and Nn(48) and Nk(48) are the densities detected at the specific site after 48 h in the rat (22). A selection rate of 0 indicates that there is no selection for catalase production, and a positive rate indicates an advantage for the catalase-producing strain. The P values given for the selection rates were determined using the two-tailed probability (from the t distribution with n − 1 degrees of freedom) of rejecting by chance the null hypothesis that the selection rate constant equals zero, indicating equal fitness for the catalase-producing (Newman) and catalase-deficient (KatA) strains.
RESULTS
Previously it has been shown that in vitro H2O2 production by S. pneumoniae limits the growth of S. aureus in liquid culture (19). To determine whether this occurs in vivo, neonatal rats were used, because both S. aureus and S. pneumoniae readily colonize the nasal passages of neonatal rats in single-clone and mixed cultures (with low inoculum densities). Within 48 h after a single species is inoculated alone, both S. aureus and S. pneumoniae reach a population of 103 CFU/ml in the nasal wash and epithelium, and this population is maintained for at least 5 days (data not shown). Despite large individual variation in bacterial densities with either species, the average bacterial densities in neonatal rats are similar to the bacterial loads reported for humans (20). To determine whether H2O2 plays a role in competition during colonization when both S. pneumoniae and S. aureus are introduced in a mixed inoculum, 5-day-old neonatal rats were challenged with a mixture of S. pneumoniae and S. aureus. The three strains of S. aureus were separately mixed with either an H2O2-producing S. pneumoniae strain (TIGR4) or a non-H2O2-producing S. pneumoniae strain (SpxB). If H2O2 produced by S. pneumoniae killed the S. aureus, one would expect that S. aureus would reach a statistically significantly lower density in the presence of the H2O2-producing strain (TIGR4) than when inoculation was with SpxB. This was not observed (Fig. 1).
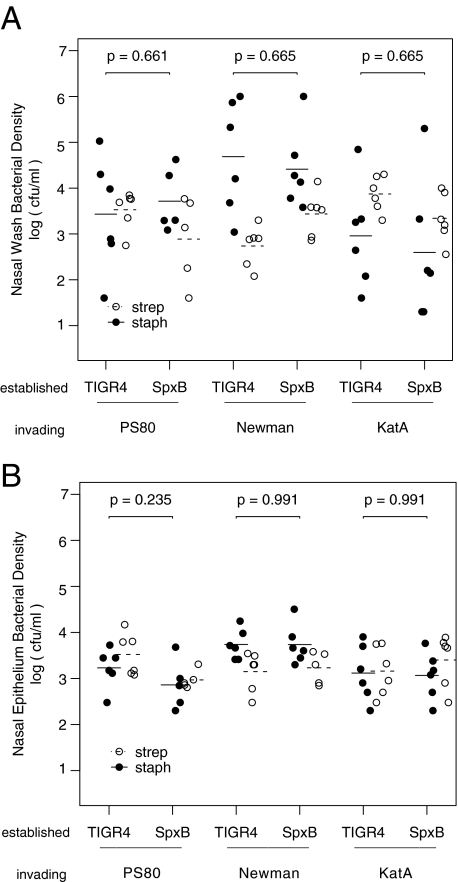
FIG. 1.
Forty-eight-hour densities of S. aureus and S. pneumoniae from nasal washes (a) and epithelia (b) of colonized rats. Five-day-old neonatal rats were colonized with 5 × 106 CFU of an S. pneumoniae strain which produces H2O2 (TIGR4) or one that does not (SpxB) in the left nostril and with 1 × 106 CFU of either the S. aureus PS80, Newman, or catalase-deficient Newman (KatA) strain.
Since the amount of H2O2 produced is proportional to the number of bacteria, the maximum amount of H2O2 would be present when the population of the producing strain was at a high density. Consequently, one would expect that H2O2 production would be most effective in preventing invasion in habitats that are already colonized by the H2O2-producing population. To ascertain whether this is the case for S. pneumoniae H2O2 production, S. aureus was inoculated intranasally into neonatal rats with established populations (inoculated 48 h earlier) of either H2O2-producing (TIGR4) or non-H2O2-producing (SpxB) S. pneumoniae. At 48 h, the nasal wash and epithelium were sampled and the densities of both S. pneumoniae and S. aureus estimated. The results of this experiment suggest that S. aureus is equally able to invade populations of S. pneumoniae in the nasal passages of rats whether the S. pneumoniae strain is capable of producing H2O2 or not and whether the S. aureus produces catalase or not (Fig. 2).
FIG. 2.
Forty-eight-hour densities of S. aureus and S. pneumoniae from nasal washes (a) and epithelia (b) of colonized rats. Three-day-old neonatal rats were colonized with 107 CFU of an S. pneumoniae strain which produces H2O2 (TIGR4) or one that does not (SpxB). Five-day-old neonatal rats were colonized with 106 CFU of either the S. aureus PS80, Newman, or catalase-deficient Newman (KatA) strain.
One explanation for why S. aureus is uninfected by S. pneumoniae-produced H2O2 during cocolonization and is able to invade established populations of H2O2-producing S. pneumoniae is that wild-type S. aureus produces a sufficient amount of catalase to neutralize the H2O2. Recent results by Park and colleagues (14) suggest that this may be the case. In their experiments with a mouse cocolonization model, S. pneumoniae producing H2O2 selected for catalase-producing S. aureus in mixtures with otherwise isogenic KatA mutants that do not produce catalase.
To discern whether H2O2 production by S. pneumoniae selects for catalase-producing S. aureus in the neonatal rat model, mixtures of catalase-producing (Newman) and non-catalase-producing (KatA) S. aureus were inoculated either in coinoculation with or invading on an established population of either H2O2-producing (TIGR4) or non-H2O2-producing (SpxB) S. pneumoniae or a PBS buffer control. The selection rate, which compares the relative recoveries of the two strains in the nasal epithelium, measured the competitive performance of the catalase-producing S. aureus strain and the non-catalase-producing S. aureus strain. If the selection rate constant is 0, there is no advantage for the catalase producer, while a value in excess of 0 would mean that catalase production is favored. The results of this experiment for the nasal epithelium are presented in Table 1.
TABLE 1.
Selection for catalase production in S. aureus in the nasal epithelium
| Established population or control | Mixed inoculum
|
Established S. pneumoniae inoculum
|
||
|---|---|---|---|---|
| Selection rate (mean ± SEM)a | P valueb | Selection rate (mean ± SEM) | P value | |
| PBS | 0.48 ± 0.83 | 0.583 | ||
| TIGR4 | 0.48 ± 0.42 | 0.296 | 5.52 ± 0.59 | 0.006 |
| SpxB | 1.63 ± 0.39 | 0.006 | 3.40 ± 0.64 | 0.002 |
KatA+ relative to KatA−.
Two-tailed probability from the t distribution of rejecting by chance the null hypothesis that the selection rate constant equals 0, indicating equal fitness for the catalase-producing (Newman) and catalase-deficient (KatA) strains.
The catalase-producing strain had a marked advantage over the nonproducer when coinoculated with a non-H2O2-producing strain (SpxB); however, there was no detectable selection for catalase production without S. pneumoniae present (PBS control) or with coinoculation with the H2O2-producing strain (TIGR4). The selective advantage for catalase production was even more pronounced when the mixture of S. aureus strains was invading neonatal rats with established populations of S. pneumoniae. In this case, the fitness advantage of the catalase-producing strain was significant regardless of whether the established S. pneumoniae strain produced H2O2. Similar results were obtained when fitness was estimated from densities determined from the nasal wash (results not shown).
DISCUSSION
The hypothesis that hydrogen peroxide-mediated killing of S. aureus by S. pneumoniae is responsible for the relative scarcity of cocolonization of these two species of bacteria is appealing. It provides an explanation for why the increase in invasive S. aureus infections could be due to the vaccine-associated decrease in pneumococcal colonization. This hypothesis is supported not only by epidemiological studies (1, 7, 11, 16, 24) but also by in vitro experiments (19) that show that H2O2 produced by S. pneumoniae is bactericidal to S. aureus and limits the density which S. aureus reaches in liquid culture. The neonatal rat nasal colonization results of this study are inconsistent with this hypothesis. Hydrogen peroxide production provided no advantage to S. pneumoniae in competing with S. aureus either in colonizing the nasal mucosa of these rats or preventing established populations of S. pneumoniae from being colonized by S. aureus. Although the results of our experiments indicated that the production of catalase (which neutralizes H2O2) provides a competitive advantage to S. aureus in the presence of S. pneumoniae, that advantage occurred whether S. pneumoniae was H2O2 producing or not.
I propose three classes of explanations for why S. pneumoniae-mediated H2O2 killing of S. aureus is effective in vitro but fails to prevent colonization of S. aureus in the nasal passages of neonatal rats: (i) S. pneumoniae does not produce H2O2 in sufficient quantities in vivo, (ii) H2O2 produced in the nasal passages is inactivated either by the host or by other members of the nasal flora, or (iii) the rate of replication of S. aureus more than makes up for killing by S. pneumoniae-produced H2O2. In support of the first explanation is the observation that in broth when S. pneumoniae is at low densities relative to S. aureus, there are insufficient amounts of H2O2 to inhibit the growth of S. aureus (16). The same would be expected on surfaces (plates or nasal epithelium) if the S. aureus and S. pneumoniae colonies are too far apart for S. aureus to come into contact with the zone of inhibition formed by S. pneumoniae (3). This may have been the case in the nasal passages of neonatal rats if S. aureus and S. pneumoniae were not colocalized in the nasal passages or if their densities were too low. It should be noted that in the neonatal rat, at least the recovered density of S. aureus or S. pneumoniae was never greater than 105 CFU/nose. It would be of interest to ascertain whether the densities of S. pneumoniae in the nasal passages of humans are greater than that observed in these rats. In support of the second explanation is the observation that the nasal epithelium produces both catalase and glutathione peroxidase (a scavenger of H2O2) (4). As for the third explanation, I am unaware of estimates of the exponential growth rates of S. aureus in the nasal passage, much less the extent to which that growth rate is reduced by H2O2 killing.
Although the results of these experiments are consistent with earlier observations that catalase production provides a fitness advantage to S. aureus when it is coinoculated with S. pneumoniae in a mouse model (14), they suggest that this advantage is not due to the production of H2O2 by S. pneumoniae. In my experiments, this advantage of catalase-producing S. aureus over otherwise isogenic strains that did not produce this enzyme occurred whether the S. pneumoniae in the nasal epithelia of the rats can produce H2O2 or not. Why, then, would catalase production provide a fitness advantage to S. aureus only when S. pneumoniae is present? Perhaps in this habitat catalase production by S. aureus increases the survival rate in neutrophils by reducing oxidative stress (6, 8), or S. pneumoniae may indirectly select for catalase production in S. aureus, as the presence of both species could synergistically elicit a stronger innate immune response (particularly neutrophil infiltration) as has recently been observed for Haemophilus influenzae and S. pneumoniae (10, 15).
The results of the study can be seen as a cautionary tale, with the moral being that what occurs in a flask may not predict what occurs in a bacterium's natural habitat. They also support the recent epidemiological evidence reported by Regev-Yochay and colleagues (17) that H2O2 is not the major determinant to explain the pattern of cocolonization. Why, then, is S. pneumoniae-S. aureus cocolonization rarer than expected? One can speculate that the scarcity of cocolonization may be due either to different host preferences by these bacterial species or to competitive interactions other than H2O2 allelopathy, perhaps resource or immune-mediated competition (18). In support of the possibility of immune-mediated competition, there was a negative association between S. pneumoniae and S. aureus colonization only in human immunodeficiency virus-negative children and not in human immunodeficiency virus-positive carriers (11). Distinguishing between bacterial interactions and host preferences is especially important for vaccination efforts; reducing the incidence of one species with a vaccine may have the undesired consequence of increasing the incidence of a competing commensal or pathogenic species.
Acknowledgments
I am particularly grateful to George Liu, Marc Lipsitch, and Bill Shafer for generously providing strains. I would like to thank my advisor, Bruce Levin, for his constant support and his persistent kibitzing. Thanks go to both Bruce and Rebecca Sanders for critically reading an earlier version of the manuscript and making many insightful comments and suggestions.
This research was supported by NIH grant AI40662 (to Bruce Levin) and NIH grant T32GM08169 (Emory Medical Scientist Training Program).
Footnotes
Published ahead of print on 14 November 2008.
REFERENCES
- 1.Bogaert, D., A. van Belkum, M. Sluijter, A. Luijendijk, R. de Groot, H. C. Rmke, H. A. Verbrugh, and P. W. M. Hermans. 2004. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet 3631871-1872. [DOI] [PubMed] [Google Scholar]
- 2.Briles, D. E., L. Novak, M. Hotomi, F. W. van Ginkel, and J. King. 2005. Nasal colonization with streptococcus pneumoniae includes subpopulations of surface and invasive pneumococci. Infect. Immun. 736945-6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chao, L., and B. R. Levin. 1981. Structured habitats and the evolution of anticompetitor toxins in bacteria. Proc. Natl. Acad. Sci. USA 786324-6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coursin, D. B., H. P. Cihla, T. D. Oberley, and L. W. Oberley. 1992. Immunolocalization of antioxidant enzymes and isozymes of glutathione stransferase in normal rat lung. Am. J. Physiol. 263L679-L691. [DOI] [PubMed] [Google Scholar]
- 5.Herz, A. M., T. L. Greenhow, J. Alcantara, J. Hansen, R. P. Baxter, S. B. Black, and H. R. Shinefield. 2006. Changing epidemiology of outpatient bacteremia in 3- to 36-month-old children after the introduction of the heptavalent-conjugated pneumococcal vaccine. Pediatr. Infect. Dis. J. 25293-300. [DOI] [PubMed] [Google Scholar]
- 6.Kanafani, H., and S. E. Martin. 1985. Catalase and superoxide dismutase activities in virulent and nonvirulent Staphylococcus aureus isolates. J. Clin. Microbiol. 21607-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madhi, S. A., P. Adrian, L. Kuwanda, C. Cutland, W. C. Albrich, and K. P. Klugman. 2007. Long-term effect of pneumococcal conjugate vaccine on nasopharyngeal colonization by Streptococcus pneumoniae—and associated interactions with Staphylococcus aureus and Haemophilus influenzae colonization—in HIV-infected and HIV-uninfected children. J. Infect. Dis. 1961662-1666. [DOI] [PubMed] [Google Scholar]
- 8.Mandell, G. L. 1975. Catalase, superoxide dismutase, and virulence of staphylococcus aureus. In vitro and in vivo studies with emphasis on staphylococcal-leukocyte interaction. J. Clin. Investig. 55561-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Margolis, E., and B. R. Levin. 2007. Within-host evolution for the invasiveness of commensal bacteria: an experimental study of bacteremias resulting from Haemophilus influenzae nasal carriage. J. Infect. Dis. 1961068-1075. [DOI] [PubMed] [Google Scholar]
- 10.Matthias, K. A., A. M. Roche, A. J. Standish, M. Shchepetov, and J. N. Weiser. 2008. Neutrophil-toxin interactions promote antigen delivery and mucosal clearance of Streptococcus pneumoniae. J. Immunol. 1806246-6254. [DOI] [PubMed] [Google Scholar]
- 11.McNally, L. M., P. M. Jeena, K. Gajee, A. W. Sturm, A. M. Tomkins, H. M. Coovadia, and D. Goldblatt. 2006. Lack of association between the nasopharyngeal carriage of Streptococcus pneumoniae and Staphylococcus aureus in HIV-1-infected South African children. J. Infect. Dis. 194385-390. [DOI] [PubMed] [Google Scholar]
- 12.Moxon, E. R., A. L. Smith, D. R. Averill, and D. H. Smith. 1974. Haemophilus influenzae meningitis in infant rats after intranasal inoculation. J. Infect. Dis. 129154-162. [DOI] [PubMed] [Google Scholar]
- 13.Moxon, E. R., and K. A. Vaughn. 1981. The type b capsular polysaccharide as a virulence determinant of Haemophilus influenzae: studies using clinical isolates and laboratory transformants. J. Infect. Dis. 143517-524. [DOI] [PubMed] [Google Scholar]
- 14.Park, B., V. Nizet, and G. Y. Liu. 2008. Role of Staphylococcus aureus catalase in niche competition against Streptococcus pneumoniae. J. Bacteriol. 1902275-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ratner, A. J., E. S. Lysenko, M. N. Paul, and J. N. Weiser. 2005. Synergistic proinflammatory responses induced by polymicrobial colonization of epithelial surfaces. Proc. Natl. Acad. Sci. USA 1023429-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regev-Yochay, G., R. Dagan, M. Raz, Y. Carmeli, B. Shainberg, E. Derazne, G. Rahav, and E. Rubinstein. 2004. Association between carriage of Streptococcus pneumoniae and Staphylococcus aureus in children. JAMA 292716-720. [DOI] [PubMed] [Google Scholar]
- 17.Regev-Yochay, G., R. Malley, E. Rubinstein, M. Raz, R. Dagan, and M. Lipsitch. 2008. In vitro bactericidal activity of Streptococcus pneumoniae and bactericidal susceptibility of Staphylococcus aureus strains isolated from cocolonized versus noncocolonized children. J. Clin. Microbiol. 46747-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Regev-Yochay, G., K. Trzcinski, C. M. Thompson, M. Lipsitch, and R. Malley. 2007. spxB is a suicide gene of Streptococcus pneumoniae and confers a selective advantage in an in vivo competitive colonization model. J. Bacteriol. 1896532-6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Regev-Yochay, G., K. Trzcinski, C. M. Thompson, R. Malley, and M. Lipsitch. 2006. Interference between Streptococcus pneumoniae and Staphylococcus aureus: In vitro hydrogen peroxide-mediated killing by Streptococcus pneumoniae. J. Bacteriol. 1884996-5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solberg, C. O. 1965. A study of carriers of Staphylococcus aureus with special regard to quantitative bacterial estimations. Acta Med. Scand. Suppl. 4361-96. [PubMed] [Google Scholar]
- 21.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293498-506. [DOI] [PubMed] [Google Scholar]
- 22.Travisano, M., and R. E. Lenski. 1996. Long-term experimental evolution in Escherichia coli. iv. Targets of selection and the specificity of adaptation. Genetics 14315-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veenhoven, R., D. Bogaert, C. Uiterwaal, C. Brouwer, H. Kiezebrink, J. Bruin, E. Izerman, P. Hermans, R. de Groot, B. Zegers, W. Kuis, G. Rijkers, A. Schilder, and E. Sanders. 2003. Effect of conjugate pneumococcal vaccine followed by polysaccharide pneumococcal vaccine on recurrent acute otitis media: a randomised study. Lancet 3612189-2195. [DOI] [PubMed] [Google Scholar]
- 24.Watson, K., K. Carville, J. Bowman, P. Jacoby, T. V. Riley, A. J. Leach, D. Lehmann, and the Kalgoorlie Otitis Media Research Project Team. 2006. Upper respiratory tract bacterial carriage in aboriginal and non-aboriginal children in a semi-arid area of western Australia. Pediatr. Infect. Dis. J. 25782-790. [DOI] [PubMed] [Google Scholar]