Abstract
Shewanella livingstonensis Ac10, a psychrotrophic gram-negative bacterium isolated from Antarctic seawater, produces eicosapentaenoic acid (EPA) as a component of phospholipids at low temperatures. EPA constitutes about 5% of the total fatty acids of cells grown at 4°C. We found that five genes, termed orf2, orf5, orf6, orf7, and orf8, are specifically required for the synthesis of EPA by targeted disruption of the respective genes. The mutants lacking EPA showed significant growth retardation at 4°C but not at 18°C. Supplementation of a synthetic phosphatidylethanolamine that contained EPA at the sn-2 position complemented the growth defect. The EPA-less mutant became filamentous, and multiple nucleoids were observed in a single cell at 4°C, indicating that the mutant has a defect in cell division. Electron microscopy of the cells by high-pressure freezing and freeze-substitution revealed abnormal intracellular membranes in the EPA-less mutant at 4°C. We also found that the amounts of several membrane proteins were affected by the depletion of EPA. While polyunsaturated fatty acids are often considered to increase the fluidity of the hydrophobic membrane core, diffusion of a small hydrophobic molecule, pyrene, in the cell membranes and large unilamellar vesicles prepared from the lipid extracts was very similar between the EPA-less mutant and the parental strain. These results suggest that EPA in S. livingstonensis Ac10 is not required for bulk bilayer fluidity but plays a beneficial role in membrane organization and cell division at low temperatures, possibly through specific interaction between EPA and proteins involved in these cellular processes.
Long-chain ω-3 polyunsaturated fatty acids (PUFAs) such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) occur in organisms from bacteria to humans as the acyl group of phospholipids in the membrane. These fatty acids have been attracting a great deal of attention, mainly because they have beneficial effects on human health (1, 5, 9). Many biophysical studies have been conducted with model membranes and have revealed that PUFAs significantly alter the basic properties of lipid bilayers such as fluidity, acyl chain order, phase behavior, elastic compressibility, and permeability (31). However, despite accumulating information on the properties of PUFA-containing bilayers, information on the physiological role of PUFAs and their molecular mode of action in living cells is very limited.
Various marine gram-negative bacteria that inhabit cold and high-pressure environments such as the polar regions and the deep sea produce EPA and DHA as components of their membrane phospholipids (18). These bacteria, which belong to the genera of Shewanella, Photobacterium, Moritella, Colwellia, and Psychromonas, have a gene cluster solely dedicated to the synthesis of PUFA (3, 18, 21, 26). The gene cluster involved in the synthesis of PUFA was first cloned from Shewanella pneumatophori SCRC-2738 (formerly Shewanella sp. strain SCRC-2738) (36). Five genes in the cluster, showing sequence similarity to the polyketide synthase genes, are essential for the synthesis of EPA in a recombinant Escherichia coli strain. The bacterial EPA synthesis system is distinct from the PUFA synthesis system of fungi and nematodes in that the latter system involves the elongation and desaturation of preexisting fatty acids, whereas the bacterial system does not (22). Thus, in marine bacteria, PUFAs are produced independently of the conventional fatty acid synthesis system. The occurrence of the gene cluster specifically required for the synthesis of PUFAs suggests that PUFAs are physiologically important for the survival of these bacteria at low temperatures and high pressures. It is generally believed that PUFAs contribute to the maintenance of membrane fluidity under these conditions. However, direct in vivo evidence for this hypothesis is lacking (34). It was recently reported that EPA provides marine bacteria with resistance to oxidative stress (25, 27). Because low temperature increases the solubility of oxygen and other reactive oxygen species, this function is supposed to be important for cell growth at low temperatures. In addition to the antioxidative function, PUFAs may have other physiologically important roles.
In this study, we explored the physiological roles of EPA in a psychrotrophic bacterium, Shewanella livingstonensis Ac10. This bacterium, which was isolated from Antarctic seawater, produces EPA at low temperatures (13). Analysis of the draft genome sequence of this bacterium (unpublished results) revealed the presence of a gene cluster similar to the EPA biosynthesis gene cluster of S. pneumatophori SCRC-2738. Here we constructed EPA-less mutants of S. livingstonensis Ac10 by targeted gene disruption and analyzed the effects of EPA depletion on the growth, membrane fluidity, membrane proteins, and morphology of this bacterium. As a result, we found novel physiological functions of EPA.
MATERIALS AND METHODS
Materials.
1-Oleoyl-2-eicosapentaenoyl-sn-glycero-3-phosphoethanolamine (OEPE) was synthesized as described previously (16). 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) was purchased from Avanti Polar Lipids, Inc. (Alabaster, AL).
Bacterial strains, plasmid, and growth conditions.
The bacterial strains and plasmid used in this study are described in Table 1. A rifampin-resistant mutant of S. livingstonensis Ac10 was obtained by mutation of the rpoB gene, which codes for the RNA polymerase β subunit. Sequence analysis of the gene revealed that His526 of the wild-type enzyme was replaced with Asp in the mutant. The rifampin-resistant mutant was used as the parental strain for gene disruption. S. livingstonensis Ac10 and its derivatives were grown in Luria-Bertani (LB) medium at various temperatures. E. coli MG1655 was grown in LB medium at 37°C. When required, antibiotics were added to the medium at the following concentrations: kanamycin, 40 μg ml−1 for E. coli and 30 μg ml−1 for S. livingstonensis Ac10; rifampin, 50 μg ml−1 for S. livingstonensis Ac10. The EPA-less mutants of S. livingstonensis Ac10 (Δorf2, Δorf5, Δorf6, Δorf7, and Δorf8) were also grown in LB medium containing OEPE or DOPE. Phospholipid solutions containing chloroform were dried in sterilized tubes with nitrogen gas and hydrated with 5 ml LB medium by vortexing at room temperature. Growth was monitored by measuring turbidity at 600 nm. Bio-photorecorder (compact rocking incubator) TVS062CA (ADVANTEC Toyo, Japan) was used to monitor the growth of the EPA-less strain in the medium containing phospholipids.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Reference |
|---|---|---|
| Escherichia coli | ||
| S17-1/λpir | S17-1 derivative, host for pir-dependent plasmids | 7 |
| MG1655 | Control strain to determine lateral diffusion of pyrene | |
| Shewanella livingstonensis | ||
| Ac10 | Wild type | |
| Ac10-Rifr | Parent strain, rifampin-resistant mutant of Ac10 | This work |
| Δorf2 | Rifrorf2::pKNOCK-Kmr | This work |
| Δorf5 | Rifrorf5::pKNOCK-Kmr | This work |
| Δorf6 | Rifrorf6::pKNOCK-Kmr | This work |
| Δorf7 | Rifrorf7::pKNOCK-Kmr | This work |
| Δorf8 | Rifrorf8::pKNOCK-Kmr | This work |
| pKNOCK-Kmr | RP4 oriT and R6K γ-ori; Kmr | 2 |
Construction of EPA-less mutants.
Putative EPA synthesis genes of S. livingstonensis Ac10 were disrupted by the integration of suicide plasmid pKNOCK-Kmr as previously reported (2, 7, 33). An internal fragment of each gene was amplified by PCR with the primers listed in Table 2. The purified PCR products were cloned into pCR-Blunt II-TOPO (Invitrogen Corp., Carlsbad, CA) and subcloned into the PstI and SpeI sites of pKNOCK-Kmr. The plasmids obtained were introduced into competent E. coli S17-1/λpir cells (7). The plasmids were transferred from the recombinant E. coli S17-1/λpir cells into the rifampin-resistant mutant of S. livingstonensis Ac10 by conjugation at 18°C on LB plates. Following overnight incubation at 18°C, the cells were collected from the plates, suspended in medium, and plated onto LB plates containing kanamycin (30 μg ml−1) and rifampin (50 μg ml−1). Transformants were obtained by incubation at 18°C. Integration of the plasmid at the appropriate locus was confirmed by PCR with a combination of a plasmid-specific primer, pKR3, and primers that anneal to the flanking regions of the target genes (Table 2).
TABLE 2.
Primers used in this study
| Primer | Sequence | Target gene |
|---|---|---|
| Used for gene disruptiona | ||
| orf2F | GCTTTGGTGTCGACATTGAA | orf2 |
| orf2R | GCTGAGCTAGCCAACCCCAAAGT | |
| orf5F | TATGATGCGGGTGCTCGAGT | orf5 |
| orf5R | GTGTTACCGTTTGCCCTTGC | |
| orf6F | TCCCCAATGTGTATGCCGAA | orf6 |
| orf6R | CATTAACAGCAACGGCCATC | |
| orf7F | TGCCAAATGCTGTGCTACC | orf7 |
| orf7R | TACAGCGGCAATAATGATGG | |
| orf8F | TCAGCAAGCCCAAGTCGTAA | orf8 |
| orf8R | GCACCAGCATAATAAGCGAA | |
| Used to verify gene disruptionb | ||
| orf2F′ | CGATCAACAGGGCTTTCTCA | orf2 |
| orf5F′ | CTATATGCAAACCAAATTACCAGCGGCCAA | orf5 |
| orf6F′ | TCAGTCAAATGGGCTTAGT | orf6 |
| orf7F′ | GTCAGTGGCATTGGCTTATC | orf7 |
| orf8F′ | TGTAGGCATGGATAAGAAG | orf8 |
| pKR3 | GTCTAGCTATCGCCATGTAA | pKNOCK-Kmr |
Internal fragments of the target genes were amplified with these primers and used for gene disruption as described in Materials and Methods.
Gene disruption was verified by PCR with pKR3 and the primer specific to the target gene as described in Materials and Methods.
Analysis of fatty acid composition by GC-MS.
Cells were grown to the early stationary phase (optical density at 600 nm was about 1.0) as described above. Fatty acid methyl esters were prepared by previously described methods (10) and analyzed with a gas chromatography-mass spectrometry (GC-MS) system (AutoSystem XL Gas Chromatograph, TurboMass Mass Spectrometer; Perkin-Elmer, Wellesley, MA) equipped with an ULBON HR-1 capillary column (Shinwa Chemical Industries, Ltd., Kyoto, Japan). Individual compounds were identified by comparing their mass spectra and retention times with those of the authentic compounds.
Analysis of phospholipid composition by ESI-MS.
Phospholipids were extracted from cells grown to the early stationary phase at 4°C with chloroform-methanol by the method of Bligh and Dyer (6). Total phospholipids were analyzed by electrospray ionization-mass spectrometry (ESI-MS) with a triple-quadrupole Sciex API 3000 liquid chromatography-tandem mass spectrometry system (Applied Biosystems, Foster City, CA) equipped with an IonSpray ion source in the negative mode. The fatty acyl residues in each molecular species were analyzed in the precursor ion scan mode.
Fluorescence microscopy.
The cells were analyzed by fluorescence microscopy by a method previously reported, with modifications (12). 4′,6′-Diamidino-2-phenylindole (DAPI; Sigma, St. Louis, MO) was added to a final concentration of 0.5 μg ml−1. After 5 min on ice, FM4-64 (Molecular Probes; Invitrogen Corp., Carlsbad, CA) was added to a final concentration of 10 μM. For video-enhanced microscopy, a BX51 fluorescence microscope (Olympus, Tokyo, Japan) equipped with an OrcaII cooled charge-coupled device camera (Hamamatsu Photonics, Hamamatsu, Japan) controlled by imaging software (Metaview; Nippon Roper, Tokyo, Japan) was used.
Transmission electron microscopic analysis.
Bacterial samples were sucked into copper tubes (inner diameter, 350 μm) mounted in the freezing holder. The specimens were inserted into a high-pressure freezing instrument (EM PACT2; Leica, Solms, Germany) at a pressure of approximately 2,000 atm and at the temperature of liquid nitrogen. The samples were then placed in liquid nitrogen, and the top of the copper tube was peeled away for further processing. The specimens were freeze-substituted in acetone containing 2% OsO4 at −80°C for 72 h. The samples were kept at −30°C for 2 h and then at 4°C for 2 h. They were incubated in acetone at 4°C for 10 min, rinsed three times in room temperature acetone (for 10 min each time), and embedded in Epon at 60°C for 48 h. Ultrathin sections were prepared with Ultracut S (Leica), double stained with uranium acetate and lead nitrate, and observed with a JEM-1010 transmission electron microscope (JEOL, Tokyo, Japan) at 80 kV.
Measurement of pyrene fluorescence.
Bacterial spheroplasts were prepared according to the method of Osborn et al. (28). Cells were harvested by centrifugation at 2,000 × g for 10 min at 4°C. The cell pellet was rapidly resuspended in cold buffer containing 0.75 M sucrose and 10 mM Tris-HCl (pH 7.8). Lysozyme (Wako Pure Chemical Industries, Ltd., Osaka, Japan) was immediately added to a final concentration of 100 μg ml−1, and the mixture was incubated for 2 min at 4°C. The suspension was then slowly diluted with 2 volumes of cold 1.5 mM EDTA (pH 7.5).
To prepare large unilamellar liposomes, phospholipid extracts from the cells in chloroform were transferred into a glass tube and dried with an argon stream. Dried phospholipids were then hydrated with 10 mM Tris-HCl (pH 7.8) and 1.5 mM EDTA (pH 7.5) at a lipid concentration of 2 mM. The lipid suspension prepared by vortex mixing was extruded with a Mini-Extruder equipped with a Nuclepore membrane (400-nm pore diameter) (Avanti Polar Lipids Inc.) at 20°C.
After pyrene in N,N-dimethylformamide was added to the mixture containing spheroplasts (optical density at 420 nm = 0.2) or liposomes (0.2 mM phospholipids), the fluorescence spectra were measured with an 850 fluorescence spectrometer (Hitachi, Tokyo, Japan) at 6, 18, and 37°C by excitation at 340 nm. The concentration of N,N-dimethylformamide did not exceed 0.1%.
Membrane proteome analysis of the EPA-less mutant.
Membrane proteins were extracted with the ReadyPrep Membrane Protein Extraction Kit (Membrane I) (Bio-Rad Laboratories, Inc., Hercules, CA). The protein concentration was determined with the DC Protein Assay Kit (Bio-Rad). The membrane proteins (300 μg) were purified with the 2-D Cleanup Kit (Bio-Rad), diluted with isoelectric focusing buffer as reported by Mineki et al. (23), and subjected to two-dimensional gel electrophoresis.
For isoelectric focusing of the samples, Immobiline DryStrips, pH 4 to 7 (18 cm; GE Healthcare UK Ltd., Buckinghamshire, United Kingdom), were used. Isoelectric focusing was performed with a Protean IEF cell (Bio-Rad) at a voltage of 7,000 V for 10 h. For second-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis, we used a sodium dodecyl sulfate gradient gel (10 to 20%, 200 by 200 by 1 mm; Daiichi Pure Chemicals Co., Ltd., Tokyo, Japan). The gels were stained with SYPRO Ruby (Invitrogen Corp.) and scanned with a Typhoon 9400 (GE Healthcare UK Ltd.) to analyze the spot intensities. Image analysis was performed with the ImageQuant software (Bio-Rad). Proteins whose relative abundance in the EPA-less mutant was more than twofold higher or less than twofold lower than in the parental strain were excised. The proteins in the gels were identified by peptide mass fingerprinting as described previously (19).
RESULTS
Fatty acid and phospholipid composition of S. livingstonensis Ac10.
The fatty acid composition of S. livingstonensis Ac10 grown at 4 and 18°C was analyzed by GC-MS. The major fatty acids of this bacterium were palmitoleic acid (16:1) and isopentadecanoic acid (i15:0) at both temperatures (see Table S1 in the supplemental material). The contents of these fatty acids were 43.0 and 26.9%, respectively, at 4°C and 40.4 and 23.7%, respectively, at 18°C. The content of EPA was 5.1% at 4°C and 0.7% at 18°C, indicating that EPA is inducibly synthesized at low temperatures. ESI-MS analysis of the phospholipids indicated that EPA is present as an acyl group of phosphatidylethanolamine and phosphatidylglycerol (see Table S2 in the supplemental material).
Construction of EPA-less mutants and their fatty acid and phospholipid composition.
To investigate the physiological role of EPA, EPA-less mutants of S. livingstonensis Ac10 were constructed. We disrupted the putative EPA synthesis genes orf2, orf5, orf6, orf7, and orf8 (GenBank accession no. AB284096, AB284098, AB373983, AB373984, and AB373985, respectively), which showed sequence similarity to the EPA synthesis genes (GenBank accession no. U73935) of S. pneumatophori SCRC-2738, and found that each of these genes is essential for the synthesis of EPA (see Table S1 and S2 in the supplemental material). The requirement of each of these genes from S. pneumatophori SCRC-2738 for EPA synthesis in recombinant E. coli cells was previously demonstrated (22, 36), which supports the view that the lack of EPA in the disruptants of S. livingstonensis Ac10 was not due to polar effects of the gene disruption but all of these genes are necessary for EPA synthesis in this strain.
The fatty acid composition of the EPA-less mutants was similar to that of the parental strain, except that the contents of isopentadecanoic acid (i15:0), isoheptadecanoic acid (i17:0), and oleic acid (18:1) were slightly higher in the EPA-less mutants than in the parental strain at 4°C (see Table S1 in the supplemental material). For a summary of the phospholipid compositions of the EPA-less mutant (Δorf5) and the parental strain at 4°C, see Table S2 in the supplemental material. The contents of phospholipids such as 16:0-16:1PG, 16:0-17:1PG, 13:0-16:1PE, and 15:0-16:1PE were higher in the EPA-less mutant, whereas the contents of phospholipids such as 16:1-17:1PG and 13:0-15:0PE were lower in the EPA-less mutant. The physiological significance and the molecular basis of these changes caused by the loss of EPA should be addressed in future studies.
Growth retardation of the EPA-less mutants at low temperatures.
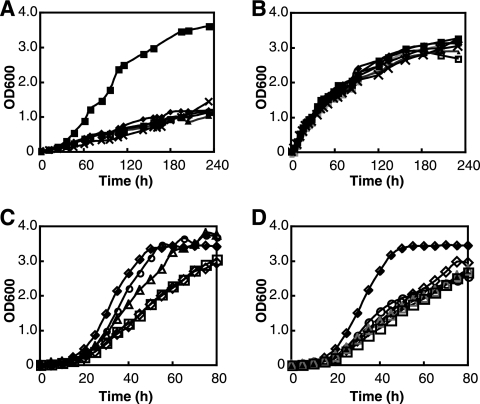
The EPA-less mutants (Δorf2, Δorf5, Δorf6, Δorf7, and Δorf8) were cultured at 4 and 18°C. Figure 1B shows that the lack of EPA did not affect the growth of S. livingstonensis Ac10 at 18°C. In contrast, the growth rates of the EPA-less mutants were significantly lower than that of the parental strain at 4°C (Fig. 1A). The doubling times of the optical density were 7.5 and 13.5 h for the parental and EPA-less strains, respectively, at 4°C. To confirm that this retardation was caused by the lack of EPA, the medium for the EPA-less mutant (Δorf5 was used throughout in the following experiments) was supplemented with OEPE as a phospholipid containing EPA and DOPE as a phospholipid not containing EPA. We found that the addition of OEPE restored the growth of the EPA-less mutant to a normal level (Fig. 1C). The growth rate of the EPA-less strain was increased as the concentration of OEPE added to the medium increased. In contrast, supplementation with DOPE did not increase the growth of the EPA-less mutant (Fig. 1D). Supplementation with the free form of EPA (13 μM) did not increase the growth rate, and supplementation with the methyl ester of EPA (13 μM) decreased the growth rate (data not shown).
FIG. 1.
Lack of EPA caused growth retardation of S. livingstonensis Ac10 at low temperatures. The growth of S. livingstonensis Ac10 (▪) and the EPA-less mutants (Δorf2 [□], Δorf5 [▵], Δorf6 [×], Δorf7 [⋄], and Δorf8 [○]) at 4°C (A) and 18°C (B) in LB medium was monitored. The growth of the EPA-less mutant (Δorf5) in LB medium supplemented with OEPE (C) and DOPE (D) at 6°C was also monitored. The concentrations of the phospholipids in the medium were 0 μM (⋄), 1.3 μM (□), 13 μM (▵), and 130 μM (○). The growth of the parental strain in LB medium (⧫) is shown as a control. OD600, optical density at 600 nm.
Defect of the EPA-less mutant in cell division at low temperatures.
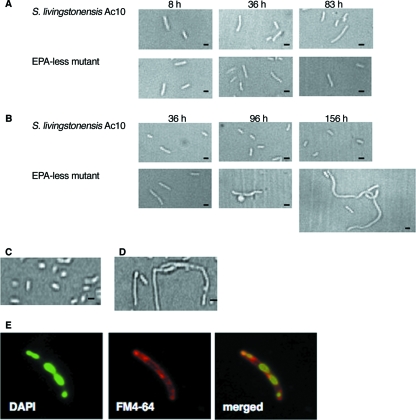
S. livingstonensis Ac10 changed its morphology, depending on the cultivation temperature: the length of the cells was 1 to 2 μm at 4°C (Fig. 2B) and 3 to 5 μm at 18°C (Fig. 2A). We found that the morphology of the EPA-less mutant was significantly different from that of the parental strain at 4°C: the mutant cells became filamentous as cultivation proceeded (Fig. 2B). In contrast, the morphology of the EPA-less mutant was indistinguishable from that of the parental strain at 18°C (Fig. 2A). The size of the EPA-less strain at 4°C became normal when the medium was supplemented with OEPE, whereas the cells remained filamentous when the medium was supplemented with DOPE (Fig. 2C and D).
FIG. 2.
Lack of EPA caused a defect in cell division at low temperatures. Morphological changes in S. livingstonensis Ac10 (parental strain) and the EPA-less strain (Δorf5) at 18°C (A) and 4°C (B) were seen. Effects of OEPE (C) and DOPE (D) supplementation on the morphology of the EPA-less strain grown at 6°C are shown. Fluorescence microscopic images of an EPA-less mutant cell grown at 4°C stained with DAPI and FM4-64 and the merged image are also shown (E). The bars indicate 1 μm.
In order to see whether the filamentation of the EPA-less mutant at 4°C was caused by a defect in cell division, the nucleoids and membranes of the cells were stained with DAPI and a membrane probe, FM4-64, respectively, and analyzed by fluorescence microscopy (Fig. 2E). Irregularly spaced multiple nucleoids were observed in a single cell, suggesting that EPA depletion caused a defect in cell division in a step after DNA replication.
Development of intracellular membranes in the EPA-less mutant grown at low temperatures.
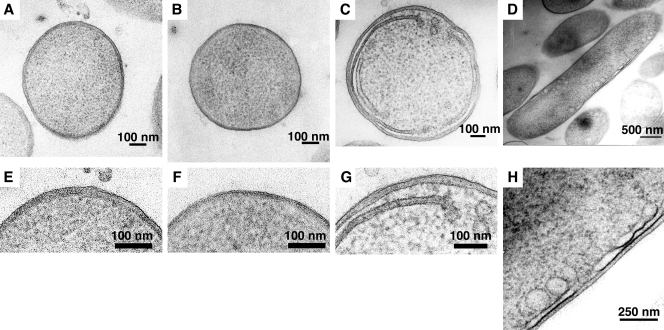
The effects of EPA depletion on cell morphology were studied by electron microscopy. In order to preserve membrane morphology, the specimens were prepared by high-pressure freezing and freeze-substitution. As a result, the ultrafine structure of the cells was clearly observed by transmission electron microscopy (Fig. 3). The parental strain grown at 4 and 18°C showed the typical morphology of a gram-negative bacterium: the outer membrane, periplasm, and inner membrane (Fig. 3A, B, E, and F). The periplasm of parental cells grown at 4°C was much thicker than that of cells grown at 18°C (22.0 ± 2.6 nm [Fig. 3E] and 6.9 ± 1.7 nm [Fig. 3F], respectively [n = 16]). The EPA-less mutant (Δorf5) had a greater cell diameter than the parental strain at 4°C (939 ± 51 nm [Fig. 3C] and 765 ± 45 nm [Fig. 3A], respectively [n = 5]).
FIG. 3.
Lack of EPA caused the development of intracellular membranes. Ultrathin sections of the parental strain of S. livingstonensis Ac10 grown at 4°C (A) and 18°C (B) and the EPA-less mutant (Δorf5) (C and D) grown at 4°C were analyzed by transmission electron microscopy. Images of cross sections (A, B, and C) and longitudinal sections (D) are shown. The boxed areas in panels A to D are shown magnified in panels E to H, respectively.
The striking feature of the EPA-less mutant grown at 4°C was the development of intracellular membranes (Fig. 3C, D, G, and H). When the cross section along the short axis of the cell was observed (Fig. 3C and G), the intracellular membranes appeared to form two-layer structures with their ends connected by a membrane with low electron density (Fig. 3C and G). The boundary between the ends of the intracellular membranes and the cytoplasm was not clear in some sections. The intracellular membranes were observed in a peripheral region of the cytoplasm in many cases and formed a large arc. In the cytoplasm near the ends of the intracellular membranes, vesicle-like structures were occasionally observed. The electron density of the region surrounded by the intracellular membranes was similar to that of the periplasm. Some of the cells had several sets of these structures. When the longitudinal section of the cell was observed (Fig. 3D and H), many vesicle-like structures were observed in a peripheral region of the cytoplasm. Some of them were attached to the inner membrane. When the cells were grown at 18°C, no significant difference was observed between the parental strain and the EPA-less mutant regarding the size of the cells, the thickness and electron density of the periplasm, or the appearance of the outer and inner membranes (data not shown). Intracellular membranes were not observed in the EPA-less mutant grown at 18°C.
Membrane fluidity of the EPA-less mutant assessed by pyrene excimer formation.
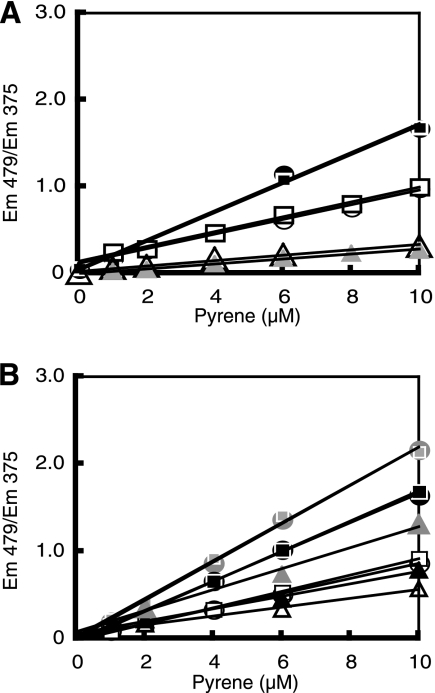
The membrane fluidity of the EPA-less mutant and the parental strain was examined by measuring the diffusion of pyrene in the membrane bilayer. Monomeric pyrene emits fluorescence at 375 nm when excited at 340 nm. When the excited pyrene forms an excimer by collision with an unexcited pyrene, it emits fluorescence at 479 nm. We prepared spheroplasts and liposomes from S. livingstonensis Ac10 cells grown at 4°C and measured the pyrene fluorescence at different temperatures (Fig. 4). The excimer-to-monomer ratio (Em479/Em375) increased linearly with the pyrene concentration. The slope of the line, which is in proportion to the rate constant for the lateral diffusion of pyrene (14), increased as the assay temperature increased, verifying that membrane fluidity can be monitored by this method. We next compared the excimerization of pyrene in the membranes of the EPA-less mutant and the parental strain. Unexpectedly, we detected no significant difference in the slope of the line between these strains, although EPA is generally believed to contribute to the maintenance of membrane fluidity. Irrespective of the presence or absence of EPA, the membrane of S. livingstonensis Ac10 was more fluid than that of E. coli MG1655 (Fig. 4). The apparent membrane fluidity of the spheroplasts from S. livingstonensis Ac10 at 6°C was about 2.8-fold higher than that of the spheroplasts from E. coli MG1655 at 37°C.
FIG. 4.
Lack of EPA did not affect the lateral diffusion of pyrene in the membrane. Fluorescence spectra of pyrene in spheroplasts (A) and liposomes (B) were measured at 6°C (open symbols), 18°C (closed symbols), and 37°C (gray symbols) by excitation at 340 nm. Spheroplasts and liposomes were prepared from the parental strain of S. livingstonensis Ac10 grown at 4°C (squares), the EPA-less mutant grown at 4°C (circles), and E. coli MG1655 grown at 37°C (triangles).
Effects of EPA depletion on the membrane proteins.
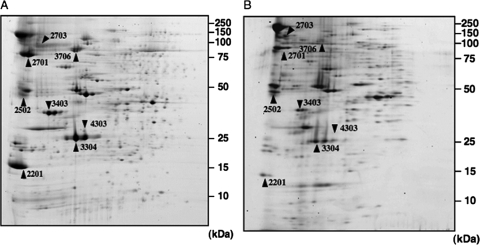
The membrane proteins from the EPA-less mutant and the parental strain grown at 4°C were compared by two-dimensional polyacrylamide gel electrophoresis (Fig. 5). Five spots were decreased and three spots were increased in the EPA-less mutant. These spots were analyzed by peptide mass fingerprinting, and four decreased spots and one increased spot were identified (Table 3). The former were a TonB-dependent receptor encoded by cirA(C496_25) (CirA_C496), two outer membrane porins encoded by omp(C176_150) and omp(C456_1) (Omp_C176 and Omp_C456, respectively), and an efflux pump protein encoded by tolC(C334_18) (TolC). The latter was another TonB-dependent receptor encoded by cirA(C313_13) (CirA_C313). TonB-dependent receptors are putative outer membrane receptor proteins for relatively large molecules, such as siderophores and vitamin B12 (24). In the EPA-less mutant, the amount of CirA_C496 was 8% of the amount produced in the parental strain, whereas the amount of CirA_C313 was 7.9-fold higher in the EPA-less mutant.
FIG. 5.
Lack of EPA affected the composition of membrane proteins. Two-dimensional gel electrophoresis of the membrane proteins of the parental strain (A) and the EPA-less strain (B) grown at 4°C. The arrowheads indicate spots whose relative intensity in the EPA-less mutant was reproducibly more than twofold higher or less than twofold lower than in the parental strain.
TABLE 3.
Membrane proteins affected by EPA depletion
| Identification no. | Spot intensity
|
Relative abundance (Δorf5/Ac10-Rifr) | Gene | Product | Predicted pI | Predicted mol wt | Accession no. | |
|---|---|---|---|---|---|---|---|---|
| Ac10-Rifr | Δorf5 | |||||||
| Decreased by EPA depletion | ||||||||
| 2701 | 43,038 | 3,596 | 0.08 | cirA(C496_25) | TonB-dependent receptor | 4.24 | 71,895.6 | AB373987 |
| 3403 | 20,669 | 5,633 | 0.27 | omp(C176_150) | Outer membrane porin | 4.46 | 37,735.6 | AB284090 |
| 3304 | 48,407 | 20,620 | 0.43 | tolC(C334_18) | TolC family protein | 4.86 | 47,706.1 | AB373986 |
| 4303 | 1,788 | 30 | 0.02 | omp(C456_1) | Hypothetical porin | 5.16 | 41,406.3 | AB373988 |
| Increased by EPA depletion | ||||||||
| 2703 | 3,194 | 25,386 | 7.9 | cirA(C313_13) | TonB-dependent receptor | 4.07 | 79,342.6 | AB373989 |
DISCUSSION
Effects of EPA depletion on the growth of S. livingstonensis Ac10 at low temperatures.
Various bacteria adapted to low temperatures and high pressures produce PUFAs, such as EPA, as a component of phospholipids in the membrane, leading to the hypothesis that PUFAs are important for the bacteria to adapt to these extreme environments (18). In the present study, we found that the lack of EPA causes significant growth retardation of S. livingstonensis Ac10 at low temperatures (Fig. 1). The lack of EPA was complemented by the addition of OEPE but not by that of DOPE, which confirmed that S. livingstonensis Ac10 requires EPA for its adaptation to cold. In contrast, it was previously reported that an EPA-producing piezophilic bacterium, Photobacterium profundum SS9, requires monounsaturated fatty acids, such as oleic acid, but not EPA, for growth at low temperatures and high pressures (3). EPA production appears to be the major, but not the sole, mechanism of cold adaptation.
Effects of EPA depletion on membrane fluidity.
Whereas EPA is often speculated to regulate membrane fluidity (34), we detected no significant difference between the EPA-less mutant and the parental strain, as assessed by the excimer formation of pyrene (Fig. 4). Irrespective of the presence or absence of EPA, the bilayer of S. livingstonensis Ac10 was more fluid than that of E. coli MG1655 (Fig. 4). The high efficiency of the excimer formation of pyrene in the membrane of S. livingstonensis Ac10 was not due to the formation of inverted micelles because the liposomes were stably formed from the lipid extracts. The cell membrane of E. coli contains a high concentration of palmitic acid (16:0, 30%) and palmitoleic acid (16:1, 30%) at 37°C (38), whereas S. livingstonensis Ac10 produces palmitoleic acid (16:1, 43.0% at 4°C and 40.4% at 18°C) and isopentadecanoic acid (iso-15:0, 26.9% at 4°C and 23.7% at 18°C) as the major components of the cell membrane (see Table S1 in the supplemental material). Palmitoleic acid and isopentadecanoic acid were the major fatty acids also in the EPA-less mutants at 4°C (39.4 to 47.6% and 28.2 to 33.1%, respectively). The high membrane fluidity of S. livingstonensis Ac10 is probably ensured by the large amount of palmitoleic acid and isopentadecanoic acid but not by the small amount of EPA. The results suggest that EPA has a more specific physiological role than that of contributing to membrane fluidity in S. livingstonensis Ac10.
It was recently demonstrated that EPA has an antioxidative function in a marine bacterium, Shewanella marinintestina IK-1 (27, 29). In this bacterium, EPA probably plays a role in the protection of cells by shielding them from the entry of exogenous oxidants (25, 27). Since S. livingstonensis Ac10 is a close relative of this strain, EPA may play a similar antioxidative function. If this is the case, the loss of EPA might induce oxidative stress and thereby cause a growth defect in S. livingstonensis Ac10. Besides the antioxidative function, EPA may have a beneficial role for the proper function of a certain set of membrane proteins through specific interaction with those proteins. This possibility is further discussed in the following part.
Effects of EPA depletion on membrane proteins.
The membrane proteome analysis clearly indicated that EPA deficiency specifically affected the expression of several membrane proteins (Fig. 5 and Table 3). We revealed that four putative outer membrane proteins (Omp_C176, Omp_C456, CirA_C496, and TolC) decreased and one putative outer membrane protein (CirA_C313) increased in the EPA-less mutant at 4°C. We previously reported that a large amount of Omp_C176, which probably serves as a membrane passage for hydrophilic nutrients, is inducibly produced in the parental S. livingstonensis Ac10 strain at low temperatures (19). The present study showed that low-temperature-induced expression of Omp_C176 requires EPA. We recently found that the growth of the omp(C176_150) disruption-containing mutant of S. livingstonensis Ac10 is significantly inhibited at low temperatures (unpublished results). Thus, the growth retardation of the EPA-less mutant may be partially due to a decrease in the rate of permeation through Omp_C176.
As a possible background of reduced amounts of Omp_C176 and other membrane proteins, we suggest that their stability at low temperatures depends on EPA-containing phospholipids. Folding of some membrane proteins, including bacterial outer membrane proteins, depends on membrane lipid composition or the presence of certain specific phospholipids (30). For example, OmpA isolated from E. coli in an unfolded form is differently refolded in large unilamellar phosphatidylcholine vesicles in a manner dependent on the acyl chains (15, 20). It is also interesting that PUFA is implicated in the stability of membrane microdomains in some mammalian cells (35). In S. livingstonensis Ac10, EPA-containing phospholipids might also affect the lateral distribution and/or oligomerization of these and other membrane proteins. We are therefore undertaking a systematic approach, including the evaluation of the specific association of EPA-containing phospholipids with individual membrane proteins.
Effects of EPA depletion on cell division and membrane organization.
Fluorescence microscopy of the cells revealed that the EPA-less mutant had a defect in cell division in a step after DNA replication and became longer than the parental strain (Fig. 2). Although in this respect the EPA-less mutant resembles the csdA deletion-containing E. coli strain, which forms filamentous cells at low temperatures, the molecular mode of action of EPA should be different from that of CsdA, which functions as an RNA helicase (4, 17). Bacterial cell division begins with chromosome segregation and Z-ring assembly (37). EPA deficiency appears to affect the latter. In E. coli, assembly of the Z ring, which involves a dozen proteins, including membrane proteins such as FtsK, FtsW, ZipA, and FtsX, is restricted to the mid-cell by the MinCDE system. This process requires phospholipids, as the MinD ATPase activity is stimulated by MinE in the presence of the lipids (32). The targeting of these proteins likely involves the association of one or some of these proteins with a specific class of lipids in the cytoplasmic leaflet of the inner membrane. In the EPA-less S. livingstonensis Ac10 mutant, one possible cause of the defect in cell division is the defect in this targeting/assembly of the cell division apparatus. Notably, the cells lacking EPA formed abnormal intracellular membranes at 4°C (Fig. 3). These membranes were formed in the vicinity of the cell membrane and appeared to be connected to the apparently normal inner membrane and, possibly, to the outer membrane as well. This suggests that the EPA deficiency and the low temperature affected the organization of the cell membrane and the regulation of the surface area. With these defects, it is likely that multiple mechanisms for organizing outer and inner membranes were dysfunctional. The membranes at the mid-cell regions in dividing cells form a highly curved structure. Although the bending rigidity of PUFA-containing bilayers is generally low, the presence of highly curved intracellular vesicles suggests that deficiency in EPA did not impair the membrane curvature in general (8). Our results, rather, suggest that EPA was required for the precise localization of a curvature-generating mechanism at low temperatures. This might be mediated by direct interaction between proteins and EPA-containing phospholipids.
We found that EPA is required for normal membrane organization and cell division of S. livingstonensis Ac10 at low temperatures of around 4°C but not at temperatures of around 18°C. This result can be explained by assuming that interaction between EPA and proteins involved in these cellular processes is essential only at low temperatures. It was recently reported that DHA, which is similar to EPA in that it has unconjugated multiple cis double bonds, has much greater diversity of energetically accessible conformational states than saturated fatty acids (11). This allows DHA to solvate rough membrane protein surface with little energetic penalty. Solvation of membrane proteins by lipid molecules is supposed to be important for the stability and function of membrane proteins, and little energetic penalty for solvation should be more important at lower temperatures than at higher temperatures. A certain set of membrane proteins of S. livingstonensis Ac10 may require EPA for their energetically feasible solvation at low temperatures.
Although much remains to be studied, our results suggest that EPA in S. livingstonensis Ac10 is not required for bulk bilayer fluidity but plays a beneficial role in membrane organization and cell division at low temperatures. This role possibly relies on specific interaction between EPA-containing phospholipids and proteins involved in these cellular processes. An approach employing the extracellular supplementation of the EPA-less mutant with chemically defined EPA-containing or other PUFA-containing phospholipids would advance our understanding of the structural and genetic basis of PUFA-dependent protein functions.
Supplementary Material
Acknowledgments
We thank Yoshinori Fujiyoshi and Koki Nishikawa of the Graduate School of Science, Kyoto University, Japan, for their help in preparing the samples for electron microscopy.
This work was supported in part by the Global COE program Integrated Materials Science (B-09) (to N.E.), Grants-in-Aid for Scientific Research (B) 17404021 and 19404020 from JSPS (to T.K.), and a grant for Research for Promoting Technological Seeds from JST (to T.K.).
Footnotes
Published ahead of print on 14 November 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Albert, C. M., C. H. Hennekens, C. J. O'Donnell, U. A. Ajani, V. J. Carey, W. C. Willett, J. N. Ruskin, and J. E. Manson. 1998. Fish consumption and risk of sudden cardiac death. JAMA 27923-28. [DOI] [PubMed] [Google Scholar]
- 2.Alexeyev, M. F. 1999. The pKNOCK series of broad-host-range mobilizable suicide vectors for gene knockout and targeted DNA insertion into the chromosome of gram-negative bacteria. BioTechniques 26824-826, 828. [DOI] [PubMed] [Google Scholar]
- 3.Allen, E. E., D. Facciotti, and D. H. Bartlett. 1999. Monounsaturated but not polyunsaturated fatty acids are required for growth of the deep-sea bacterium Photobacterium profundum SS9 at high pressure and low temperature. Appl. Environ. Microbiol. 651710-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Awano, N., C. Xu, H. Ke, K. Inoue, M. Inouye, and S. Phadtare. 2007. Complementation analysis of the cold-sensitive phenotype of the Escherichia coli csdA deletion strain. J. Bacteriol. 1895808-5815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birch, E. E., S. Garfield, D. R. Hoffman, R. Uauy, and D. G. Birch. 2000. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev. Med. Child Neurol. 42174-181. [DOI] [PubMed] [Google Scholar]
- 6.Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37911-917. [DOI] [PubMed] [Google Scholar]
- 7.Brown, S. D., M. Martin, S. Deshpande, S. Seal, K. Huang, E. Alm, Y. Yang, L. Wu, T. Yan, X. Liu, A. Arkin, K. Chourey, J. Zhou, and D. K. Thompson. 2006. Cellular response of Shewanella oneidensis to strontium stress. Appl. Environ. Microbiol. 72890-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruno, M. J., R. E. Koeppe II, and O. S. Andersen. 2007. Docosahexaenoic acid alters bilayer elastic properties. Proc. Natl. Acad. Sci. USA 1049638-9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dewey, A., C. Baughan, T. Dean, B. Higgins, and I. Johnson. 2007. Eicosapentaenoic acid (EPA, an omega-3 fatty acid from fish oils) for the treatment of cancer cachexia. Cochrane Database Syst. Rev. doi: 10.1002/14651858.CD004597.pub2. [DOI] [PMC free article] [PubMed]
- 10.Fang, J., C. Kato, T. Sato, O. Chan, and D. McKay. 2004. Biosynthesis and dietary uptake of polyunsaturated fatty acids by piezophilic bacteria. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 137455-461. [DOI] [PubMed] [Google Scholar]
- 11.Feller, S. E. 2008. Acyl chain conformations in phospholipid bilayers: a comparative study of docosahexaenoic acid and saturated fatty acids. Chem. Phys Lipids 15376-80. [DOI] [PubMed] [Google Scholar]
- 12.Fishov, I., and C. L. Woldringh. 1999. Visualization of membrane domains in Escherichia coli. Mol. Microbiol. 321166-1172. [DOI] [PubMed] [Google Scholar]
- 13.Galkin, A., L. Kulakova, H. Ashida, Y. Sawa, and N. Esaki. 1999. Cold-adapted alanine dehydrogenases from two Antarctic bacterial strains: gene cloning, protein characterization, and comparison with mesophilic and thermophilic counterparts. Appl. Environ. Microbiol. 654014-4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galla, H. J., and E. Sackmann. 1974. Lateral diffusion in the hydrophobic region of membranes: use of pyrene excimers as optical probes. Biochim. Biophys. Acta 339103-115. [DOI] [PubMed] [Google Scholar]
- 15.Hong, H., and L. K. Tamm. 2004. Elastic coupling of integral membrane protein stability to lipid bilayer forces. Proc. Natl. Acad. Sci. USA 1014065-4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosokawa, M., K. Takahashi, H. M., and M. Egi. 1995. Phospholipase A2-mediated synthesis of phosphatidylethanolamine containing highly unsaturated fatty acids. Int. J. Food Sci. Technol. 29721-725. [Google Scholar]
- 17.Jones, P. G., M. Mitta, Y. Kim, W. Jiang, and M. Inouye. 1996. Cold shock induces a major ribosomal-associated protein that unwinds double-stranded RNA in Escherichia coli. Proc. Natl. Acad. Sci. USA 9376-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato, C., and Y. Nogi. 2001. Correlation between phylogenetic structure and function: examples from deep-sea Shewanella. FEMS Microbiol. Ecol. 35223-230. [DOI] [PubMed] [Google Scholar]
- 19.Kawamoto, J., T. Kurihara, M. Kitagawa, I. Kato, and N. Esaki. 2007. Proteomic studies of an Antarctic cold-adapted bacterium, Shewanella livingstonensis Ac10, for global identification of cold-inducible proteins. Extremophiles 11819-826. [DOI] [PubMed] [Google Scholar]
- 20.Kleinschmidt, J. H., and L. K. Tamm. 2002. Secondary and tertiary structure formation of the beta-barrel membrane protein OmpA is synchronized and depends on membrane thickness. J. Mol. Biol. 324319-330. [DOI] [PubMed] [Google Scholar]
- 21.Methé, B. A., K. E. Nelson, J. W. Deming, B. Momen, E. Melamud, X. Zhang, J. Moult, R. Madupu, W. C. Nelson, R. J. Dodson, L. M. Brinkac, S. C. Daugherty, A. S. Durkin, R. T. DeBoy, J. F. Kolonay, S. A. Sullivan, L. Zhou, T. M. Davidsen, M. Wu, A. L. Huston, M. Lewis, B. Weaver, J. F. Weidman, H. Khouri, T. R. Utterback, T. V. Feldblyum, and C. M. Fraser. 2005. The psychrophilic lifestyle as revealed by the genome sequence of Colwellia psychrerythraea 34H through genomic and proteomic analyses. Proc. Natl. Acad. Sci. USA 10210913-10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Metz, J. G., P. Roessler, D. Facciotti, C. Levering, F. Dittrich, M. Lassner, R. Valentine, K. Lardizabal, F. Domergue, A. Yamada, K. Yazawa, V. Knauf, and J. Browse. 2001. Production of polyunsaturated fatty acids by polyketide synthases in both prokaryotes and eukaryotes. Science 293290-293. [DOI] [PubMed] [Google Scholar]
- 23.Mineki, R., H. Taka, T. Fujimura, M. Kikkawa, N. Shindo, and K. Murayama. 2002. In situ alkylation with acrylamide for identification of cysteinyl residues in proteins during one- and two-dimensional sodium dodecyl sulphate-polyacrylamide gel electrophoresis. Proteomics 21672-1681. [DOI] [PubMed] [Google Scholar]
- 24.Moeck, G. S., and J. W. Coulton. 1998. TonB-dependent iron acquisition: mechanisms of siderophore-mediated active transport. Mol. Microbiol. 28675-681. [DOI] [PubMed] [Google Scholar]
- 25.Nishida, T., Y. Orikasa, Y. Ito, R. Yu, A. Yamada, K. Watanabe, and H. Okuyama. 2006. Escherichia coli engineered to produce eicosapentaenoic acid becomes resistant against oxidative damages. FEBS Lett. 5802731-2735. [DOI] [PubMed] [Google Scholar]
- 26.Nogi, Y., C. Kato, and K. Horikoshi. 2002. Psychromonas kaikoae sp. nov., a novel piezophilic bacterium from the deepest cold-seep sediments in the Japan Trench. Int. J. Syst. Evol. Microbiol. 521527-1532. [DOI] [PubMed] [Google Scholar]
- 27.Okuyama, H., Y. Orikasa, T. Nishida, K. Watanabe, and N. Morita. 2007. Bacterial genes responsible for the biosynthesis of eicosapentaenoic and docosahexaenoic acids and their heterologous expression. Appl. Environ. Microbiol. 73665-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osborn, M. J., J. E. Gander, and E. Parisi. 1972. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Site of synthesis of lipopolysaccharide. J. Biol. Chem. 2473973-3986. [PubMed] [Google Scholar]
- 29.Satomi, M., H. Oikawa, and Y. Yano. 2003. Shewanella marinintestina sp. nov., Shewanella schlegeliana sp. nov. and Shewanella sairae sp. nov., novel eicosapentaenoic-acid-producing marine bacteria isolated from sea-animal intestines. Int. J. Syst. Evol. Microbiol. 53491-499. [DOI] [PubMed] [Google Scholar]
- 30.Schulz, G. E. 2003. Transmembrane beta-barrel proteins. Adv. Protein Chem. 6347-70. [DOI] [PubMed] [Google Scholar]
- 31.Stillwell, W., and S. R. Wassall. 2003. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem. Phys. Lipids 1261-27. [DOI] [PubMed] [Google Scholar]
- 32.Suefuji, K., R. Valluzzi, and D. RayChaudhuri. 2002. Dynamic assembly of MinD into filament bundles modulated by ATP, phospholipids, and MinE. Proc. Natl. Acad. Sci. USA 9916776-16781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson, D. K., A. S. Beliaev, C. S. Giometti, S. L. Tollaksen, T. Khare, D. P. Lies, K. H. Nealson, H. Lim, J. Yates III, C. C. Brandt, J. M. Tiedje, and J. Zhou. 2002. Transcriptional and proteomic analysis of a ferric uptake regulator (fur) mutant of Shewanella oneidensis: possible involvement of fur in energy metabolism, transcriptional regulation, and oxidative stress. Appl. Environ. Microbiol. 68881-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valentine, R. C., and D. L. Valentine. 2004. Omega-3 fatty acids in cellular membranes: a unified concept. Prog. Lipid Res. 43383-402. [DOI] [PubMed] [Google Scholar]
- 35.van Meer, G., D. R. Voelker, and G. W. Feigenson. 2008. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9112-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watanabe, K., K. Yazawa, K. Kondo, and A. Kawaguchi. 1997. Fatty acid synthesis of an eicosapentaenoic acid-producing bacterium: de novo synthesis, chain elongation, and desaturation systems. J. Biochem. (Tokyo) 122467-473. [DOI] [PubMed] [Google Scholar]
- 37.Weiss, D. S. 2004. Bacterial cell division and the septal ring. Mol. Microbiol. 54588-597. [DOI] [PubMed] [Google Scholar]
- 38.Yamanaka, K. 1999. Cold shock response in Escherichia coli. J. Mol. Microbiol. Biotechnol. 1193-202. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.