Abstract
Fusarium species are saprophytic molds which cause disseminated or localized infections in humans. Disseminated Fusarium infection can cause significant morbidity and mortality in immunocompromised patients. We present a case of disseminated fusariosis caused by Fusarium verticillioides in a patient with acute lymphoblastic leukemia and successfully treated using both liposomal amphotericin B and voriconazole.
CASE REPORT
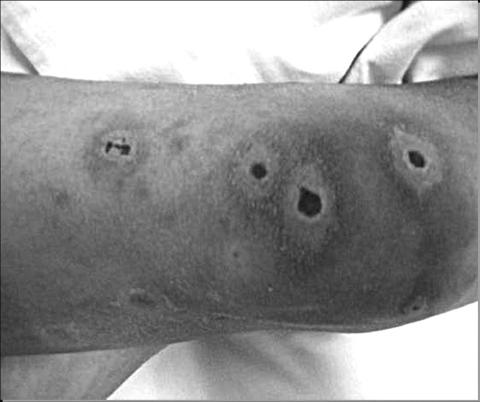
A 12-year-old boy underwent allogeneic hematopoietic stem cell transplant (allo-HSCT) from unrelated cord blood for high-risk acute lymphoblastic leukemia in its first complete remission. The conditioning regimen included busulfex (12.8 mg/kg of body weight), etoposide (40 mg/kg), and cyclophosphamide (120 mg/kg). For graft-versus-host-disease prophylaxis, cyclosporine A was added on day −1 but switched to mycophenolate mofetil due to severe allergic reaction. By the fourth day after the transplant, he was in severe neutropenia (<0.1 × 103 neutrophils/μl) and became febrile, and antibiotic treatment (meropenem) was initiated for febrile neutropenia. Although the fever disappeared within few days of antibiotic onset, on day 23 posttransplant, he again became febrile, and treatment with liposomal amphotericin B (LAmB) was then started, with a dosage of 3 mg/kg/day. Cultures of separate blood samples obtained percutaneously and from a central venous catheter yielded coagulase-negative Staphylococcus epidermidis, and teicoplanin was added. On day 59 posttransplant, the patient developed multiple skin lesions, starting from the extremities and spreading to the face and trunk. The lesions had necrotic centers surrounded by spreading erythema (Fig. 1). A biopsy of the skin lesion showed the presence of histopathological symptoms consistent with a septate pathogenic mold, and blood cultures taken on the same day were positive for a Fusarium species.
FIG. 1.
Multiple necrotic skin lesions on the patient's leg.
The diagnosis of disseminated fusariosis was established, and LAmB was raised to a dose of 5 mg/kg/day. During the days following, the lesions worsened and treatment with voriconazole (loading dose, 6 mg/kg/day, followed by 4 mg/kg/day intravenously every 12 h) was initiated. A chest X-ray and a high-resolution computed tomography scan of the lungs were normal. Since profound neutropenia still continued, it was accepted that the patient had graft failure and new, unrelated donor search was started. Since a fully HLA-matched donor was not available, the patient underwent peripheral blood stem cell transplantation from a 9/10-matched, unrelated donor on day 82 posttransplant. The conditioning regimen included cyclophosphamide (120 mg/kg), and for graft-versus-host-disease prophylaxis, antithymocyte globulin (60 mg/kg), mycophenolate mofetil, and methotrexate were used. Neutrophil engraftment occurred on day 13 posttransplant. Combined-antifungal therapy and antifungal therapy with only voriconazole were continued until the ends of first and third months after peripheral blood stem cell transplantation, respectively. Two weeks after discontinuation of voriconazole therapy, the patient developed swelling and increased heat in the right knee joint and tenderness on movement, consistent with the diagnosis of arthritis. The patient was not neutropenic. On the days following, other joints also became affected. Examination of synovial fluid obtained from the right knee disclosed two or three granulocytes, and it was transudative. Culture yielded Fusarium spp., and combined-antifungal therapy with LAmB and voriconazole was restarted, and although the severity of the symptoms reduced, they did not disappear completely. Successive blood cultures within 15 days were found sterile. LAmB therapy was continued for 1 month, and at the end of 1 month, he was discharged home on oral voriconazole alone.
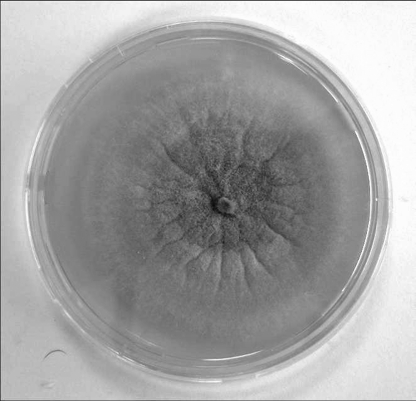
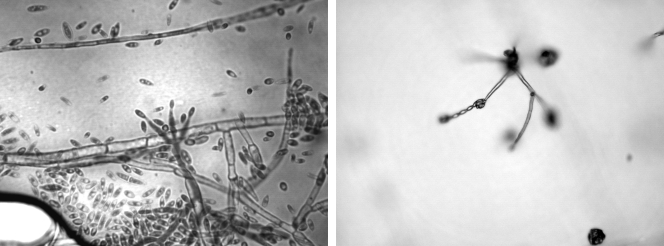
The macroscopic and microscopic morphologies of isolated Fusarium sp. were examined, and it was identified as Fusarium verticillioides on the basis of these characteristics. In short, it has a cottony colony with white aerial mycelium tinged with purple with a colorless reverse macroscopically (Fig. 2). In microscopic examination, it was found to have septate and hyaline hyphae; conidiophores arising laterally from hyphae in the aerial mycelium, sparsely branched; and abundant microconidia, in chains, ovoidal to clavate. Conidiogenous cells are monophialidic (Fig. 3).
FIG. 2.
Macroscopic colonies on Sabouraud dextrose agar with purple aerial mycelium.
FIG. 3.
(Left panel) Microscopy showing septate and hyaline hyphae, abundant microconidia, and monophialidic conidiogenous cells. (Right panel) Microscopic morphology of microconidia in chains in potassium chloride agar.
Molecular identification was performed in order to confirm the identification to the species level. The Fusarium isolate was cultured in GYEP medium (0.3% yeast extract, 1% peptone; Difco, Soria Melguizo S.A., Madrid, Spain) with 2% glucose (Sigma Aldrich Química, Madrid, Spain) for 24 to 48 h at 30°C. Genomic DNA was extracted by using a previously described procedure (10). DNA segments comprising a region of elongation factor alpha (EFα) and the internal transcribed spacers (ITS) were amplified with primers EF1 (5′-ATGGGTAAGARGACAAGAC-3′), EF2 (5′-GGARGTACCAGTSATCATGTT-3′), ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′), and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) in a GeneAmp 9700 PCR system (Applied Biosystems, Madrid, Spain) (11, 21). The reaction mixtures contained 0.5 μM of each primer, 0.2 μM of each deoxynucleoside triphosphate, 5 μl of 10× PCR buffer (Applied Biosystems), 2.5 U Taq DNA polymerase (Amplitaq; Applied Biosystems), and 25 ng of DNA in a final volume of 50 μl. The samples were amplified in a GeneAmp 9700 PCR system (Applied Biosystems) by using the following cycling conditions: 1 initial cycle of 5 min at 94°C, followed by 35 cycles of 30 s at 94°C, 45 s at 47°C (EFα) or 56°C (ITS), and 2 min at 72°C, with 1 final cycle of 5 min at 72°C. The reaction products were analyzed with a 0.8% agarose gel.
Sequencing reactions were done with 2 μl of a mixture from a sequencing kit (BigDye Terminator cycle sequencing ready reaction; Applied Biosystems), 1 μl of the primers (EF1, EF2, ITS1, and ITS4), and 3 μl of the PCR product in a final volume of 10 μl.
Sequences were assembled and edited using the SeqMan II and EditSeq software packages (Lasergene; DNAstar, Inc., Madison, WI). Sequence analysis was performed by comparison of the DNA sequences with EFα sequences of Fusarium strains (with accession numbers DQ246834, DQ247188, AY337433, AY337436, AF008480, AY337437, AJ543560, AJ543570, DQ295140, DQ295141, DQ295142, and DQ246834) obtained from the GenBank database (http://www.ncbi.nih.gov/Genbank/).
All phylogenetic analyses were conducted with InfoQuest FP software, version 4.50 (Bio-Rad Laboratories, Madrid, Spain). Blood and synovial fluid isolates were identical according to DNA sequence analysis.
The in vitro susceptibilities of F. verticillioides to antifungal drugs were determined using the broth dilution method, following the European Committee for Antimicrobial Susceptibility Testing procedures (19). This standard is similar to the one published by the CLSI (formerly NCCLS) reference method for broth dilution antifungal susceptibility testing of filamentous fungi, but with the following minor modifications: (i) RPMI 1640 was supplemented with glucose to reach a 2% concentration and (ii) the inoculum sizes were between 1.0 × 105 and 5.0 × 105 CFU/ml (1, 3, 16, 18). Inoculum preparations were performed by counting spores in a hematocytometer. Aspergillus fumigatus ATCC 2004305 and Aspergillus flavus ATCC 2004304 were used as quality control strains.
The antifungal agents used in the study were amphotericin B (range, 16 to 0.03 μg/ml) (Sigma Aldrich Química), itraconazole (range, 8 to 0.015 μg/ml) (Janssen Pharmaceutica S.A., Madrid, Spain), voriconazole (range, 8 to 0.015 μg/ml) (Pfizer S.A., Madrid, Spain), posaconazole (range, 8 to 0.015 μg/ml) (Schering-Plough Research Institute, Kenilworth, NJ), and terbinafine (range, 16 to 0.03 μg/ml) (Novartis, Basel, Switzerland). The endpoint was the antifungal concentration that produced a complete inhibition of visual growth at 48 h.
The antifungal susceptibility results for the blood isolate were as follows: amphotericin B, 1 μg/ml; itraconazole, >8 μg/ml; voriconazole, 4 μg/ml; posaconazole, >8 μg/ml; and terbinafine, 2 μg/ml.
The antifungal susceptibility results for the synovial fluid isolate were as follows: amphotericin B, 2 μg/ml; itraconazole, >8 μg/ml; voriconazole, 8 μg/ml; posaconazole, >8 μg/ml; and terbinafine, 4 μg/ml.
Fusarium species are saprophytic molds that are prevalent in the soil and air. They can cause local cutaneous infections and infections of surgical and burn wounds. However, this fungus has caught particular attention as an emerging pathogen of immunocompromised patients with involvement of multiple organs and numerous skin lesions (5, 13).
The Fusarium spp. most frequently involved in human infections are Fusarium solani, Fusarium oxysporum, and Fusarium verticillioides (6, 12). Of note, in tissue samples Fusarium spp. are often confused with Aspergillus spp., as both pathogens have similar histopathologic appearances, with septate, dichotomously branching hyphae (8). Yet, therapeutic options are scarce, and mortality reaches 80 to 90% in patients subjected to allo-HSCT (14, 20). We describe a pediatric allo-HSCT patient with disseminated fusariosis who responded to early combination of voriconazole and LAmB.
The epidemiology of fungal infections in hemopoietic stem cell transplant recipients has changed in the last 20 years. Candida species were still the most frequent agents of invasive mycoses (15). In recent years, invasive fungal infections caused by Aspergillus spp. and other emerging molds, such as Fusarium spp., have increased in frequency in hemopoietic stem cell transplant recipients (14). Invasive fusariosis can be life-threatening, and treatment options are limited. The increasing incidence, rapid progression, and high mortality rates of invasive fusariosis have necessitated early aggressive management of patients. There is no definitive effective treatment of invasive fusariosis. Currently, high-dose amphotericin B and its lipid formulations are the primary treatment for invasive fusariosis. Expanded-spectrum triazoles, particularly voriconazole and posaconazole, have also been used, and voriconazole is approved as front-line treatment for fusariosis (2, 15).
Fusarium spp. are very resistant to antifungal agents. Different species may exhibit variable susceptibility patterns. In the case of isolation of the organism, it is recommended that antifungal susceptibility testing be performed and that the active agent be administered at the highest tolerable dose (15). In the presented case, the MICs of amphotericin B and voriconazole were 1 μg/ml and 4 μg/ml, respectively. Because of the high mortality rate of invasive fusariosis under monotherapy, new treatment strategies, such as combination therapy, can be considered life-saving (9). At present, no randomized prospective studies evaluating combination therapy for invasive fusariosis are available. There are isolated case reports of successful treatment of invasive fusariosis with an amphotericin B and voriconazole combination. All of these case reports showed that the efficacy of combination therapy is better than that of monotherapy for fungal infections (4, 7, 17, 20). Our patient was successfully treated with combination therapy of amphotericin B and voriconazole, suggesting that the combination therapy is a potential alternative for patients with invasive fusariosis.
Acknowledgments
This study was supported by the Akdeniz University Scientific Research Unit.
Footnotes
Published ahead of print on 12 November 2008.
REFERENCES
- 1.Aberkane, A., M. Cuenca-Estrella, A. Gomez-Lopez, E. Petrikkou, E. Mellado, A. Monzon, J. L. Rodriguez-Tudela, and the Eurofung Network. 2002. Comparative evaluation of two different methods of inoculum preparation for antifungal susceptibility testing of filamentous fungi. J. Antimicrob. Chemother. 50719-722. [DOI] [PubMed] [Google Scholar]
- 2.Bhatti, Z., A. Shaukat, N. G. Almyroudis, and B. H. Segal. 2006. Review of epidemiology, diagnosis, and treatment of invasive mould infections in allogeneic hematopoietic stem cell transplant recipients. Mycopathologia 1621-15. [DOI] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. 2005. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi: approved standard M38-A. CLSI, Wayne, PA.
- 4.Durand-Joly, I., S. Alfandari, Z. Benchikh, M. Rodrigue, A. Espinel-Ingroff, B. Catteau, C. Cordevant, D. Camus, E. Dei-Cas, F. Bauters, L. Delhaes, and S. Botton. 2003. Successful Outcome of Disseminated Fusarium Infection with Skin Localization Treated with Voriconazole and Amphotericin B-Lipid Complex in a Patient with Acute Leukemia. J. Clin. Microbiol. 414898-4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleming, R. V., T. J. Walsh, and E. J. Anaissie. 2002. Emerging and less common fungal pathogens. Infect. Dis. Clin. North. Am. 16915-933. [DOI] [PubMed] [Google Scholar]
- 6.Gupta, A. K., R. Baran, and R. C. Summerbell. 2000. Fusarium infections of the skin. Curr. Opin. Infect. Dis. 13121-128. [DOI] [PubMed] [Google Scholar]
- 7.Guzman-Cottrill, J. A., X. Zheng, and E. G. Chadwick. 2004. Fusarium solani endocarditis successfully treated with liposomal amphotericin B and voriconazole. Pediatr. Infect. Dis. J. 231059-1061. [DOI] [PubMed] [Google Scholar]
- 8.Hayden, R. T., P. A. Isotalo, T. Parrett, X. Qian, G. D. Roberts, and R. V. Lloyd. 2003. In situ hybridization for the differentiation of Aspergillus, Fusarium and Pseudoallescheria species in tissue section. Diagn. Mol. Pathol. 1221-26. [DOI] [PubMed] [Google Scholar]
- 9.Ho., D. Y., J. D. Lee, F. Rosso, and J. G. Montoya. 2007. Treating disseminated fusariosis: amphotericin B, voriconazole or both? Mycoses 50227-231. [DOI] [PubMed] [Google Scholar]
- 10.Holden, D. W. 1994. DNA mini prep method for Aspergillus fumigatus (and other filamentous fungi), p. 3-4. In B. Maresca and G. S. Kobayashi (ed.), Molecular biology of pathogenic fungi, a laboratory manual. Telos Press, New York, NY.
- 11.Kristensen, R., M. Torp, B. Kosiak, and A. Holst-Jensen. 2005. Phylogeny and toxigenic potential is correlated in Fusarium species as revealed by partial translation elongation factor 1 alpha gene sequences. Mycol. Res. 109173-186. [DOI] [PubMed] [Google Scholar]
- 12.Nelson, P. E., M. C. Dignani, and E. J. Anaissie. 1994. Taxonomy, biology, and clinical aspects of Fusarium species. Clin. Microbiol. Rev. 7479-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nucci, M., and E. Anaissie. 2002. Cutaneous infection by Fusarium species in healthy and immunocompromised hosts: implications for diagnosis and management. Clin. Infect. Dis. 35909-920. [DOI] [PubMed] [Google Scholar]
- 14.Nucci, M., K. A. Marr, F. Queiroz-Telles, C. A. Martins, P. Trabasso, S. Costa, J. C. Voltarelli, A. L. Colombo, A. Imhof, R. Pasquini, A. Maiolino, C. A. Souza, and E. Anaissie. 2004. Fusarium infection in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 381237-1242. [DOI] [PubMed] [Google Scholar]
- 15.Nucci, M., and E. Anaissie. 2006. Emerging fungi. Infect. Dis. Clin. North Am. 20563-579. [DOI] [PubMed] [Google Scholar]
- 16.Petrikkou, E., J. L. Rodriguez-Tudela, M. Cuenca-Estrella, A. Gomez, A. Molleja, and E. Mellado. 2001. Inoculum standardization for antifungal susceptibility testing of filamentous fungi pathogenic for humans. J. Clin. Microbiol. 391345-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez, C. A., J. Luján-Zilbermann, P. Woodard, M. Andreansky, and E. E. Adderson. 2003. Successful treatment of disseminated fusariosis. Bone marrow Transplant. 31411-412. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Tudela, J. L., E. Chryssanthou, E. Petrikkou, J. Mosquera, D. W. Denning, and M. Cuenca-Estrella. 2003. Interlaboratory evaluation of hematocytometer method of inoculums preparation for testing antifungal susceptibilities of filamentous fungi. J. Clin. Microbiol. 415236-5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriquez Tudela, J. L., J. P. Donnelly, M. C. Arendrup, S. Arikan, F. Barchiesi, J. Bille, E. Chryssanthou, M. Cuenca-Estrella, E. Dannaoui, D. Denning, W. Fegeler, P. Gaustad, C. Lass-Flörl, C. Moore, M. Richardson, A. Schmalreck, J. A. Velegraki, P. Verweij, et al. 2008. EUCAST Technical Note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. Clin. Microbiol. Infect. 14982-984. [DOI] [PubMed] [Google Scholar]
- 20.Stanzani, M., N. Vianelli, G. Bandini, S. Paolini, M. Arpinati, F. Bonifazi, B. Giannini, C. Agostinelli, M. Baccarani, and P. Ricci. 2006. Successful treatment of disseminated fusariosis after allogeneic hematopoietic stem cell transplantation with the combination of voriconazole and liposomal amphotericin B. J. Infect. 53e243-e246. [DOI] [PubMed] [Google Scholar]
- 21.White, T. J., T. Bruns, S. Lee, and J. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315-322. In M. Innis (ed.), PCR protocols: a guide to methods and applications. Academic Press, San Diego, CA.