Abstract
Human parvovirus B19 has been linked to a variety of cardiac diseases, as well as to erythema infectiosum, acute arthropathy, and fetal hydrops. A causal association between viral infection and cardiac disease was frequently postulated following the detection of B19 DNA by PCR in endomyocardial biopsy specimens. Since the lifelong persistence of B19 DNA in bone marrow, skin, synovia, tonsils, and liver was previously reported, the aim of our study was to investigate the possibility of asymptomatic B19 DNA persistence in heart tissue. Myocardial autopsy and postmortem blood samples were prospectively collected from 69 bodies sent to the Department of Forensic Medicine, Freiburg University Medical Center, for inquests. All study subjects were screened for B19-specific antibodies using a commercial enzyme immunoassay. Tissue samples were analyzed by real-time PCR for the presence of viral DNA. Since the presence of B19 genotype 2, known to have been circulating before 1960, would prove long-lasting persistence, the presence of the B19 genotype was retrospectively determined in seven of the study subjects by melting temperature analysis and sequencing of the PCR product. B19 DNA was found in myocardial samples from 46 of 48 seropositive and in none of 21 seronegative individuals. B19 genotype 1 was found in three patients born between 1950 and 1969. Genotype 2 was found in four patients born between 1927 and 1957. Our findings suggest lifelong persistence of B19 DNA in heart tissue. Thus, the detection of B19 DNA in myocardial biopsy specimens alone is not sufficient to postulate a relationship between B19 infection and cardiac disease.
Parvovirus B19, a member of the genus Erythrovirus, is a nonenveloped single-stranded DNA virus which replicates in erythroid progenitor cells of bone marrow. Its role as a human pathogen causing erythema infectiosum (fifth disease) (2), fetal hydrops after infection in utero (1), and transient aplastic crisis in patients with preexisting erythropathy is well established (23).
After the development of PCR technology and its application for the sensitive detection of viral DNA, B19 infection has been linked to a variety of acute and chronic disease processes. Based on the amplification of B19 genome sequences from tissue samples, an association with chronic arthropathy (6, 12) and acute fulminant liver failure in children (13), as well as with several entities of cardiac disease, i.e., acute myocarditis (5, 27), dilative cardiomyopathy or idiopathic left ventricular dysfunction (14, 16, 19), and peripartum cardiomyopathy (3), was suggested.
In the case of chronic arthropathy, the causal relationship of B19 infection has been questioned, since a case-control study showed a higher percentage of B19 DNA-positive synovial tissue samples in the control group than in the cases (28% in children with chronic arthropathy versus 48% in patients without it) (28). Likewise, the role of B19 in inducing acute hepatic failure was doubted when the persistence of viral DNA in histologically normal livers of adults was demonstrated (8). The persistence of parvoviral DNA has also been shown for the bone marrow and skin of healthy adults (4, 29). Recently, B19 DNA was detected in 17 to 64% of skin, synovia, tonsil, and liver tissue samples from heterogeneous sources, including both patients with a variety of symptoms and healthy donors (22). Norja et al. also showed that B19 genotype 2 (Gt-2), which was circulating before 1960, was present in tissue samples of subjects born before 1973, thus proving the long-term persistence of B19 DNA (22). The aim of our study was to investigate whether there is a lifelong asymptomatic persistence of B19 DNA in the heart after primary infection, as was previously shown for other tissues. This finding would be instrumental in the interpretation of PCR results from cardiac biopsy samples.
MATERIALS AND METHODS
Samples.
Sixty-nine myocardial tissue and blood samples were randomly collected in three periods between November 2002 and July 2004. Corpses with putrefactive changes were excluded. The cause of death was unknown except in those cases showing massive signs of traumatization. In no case was acute viral infection suspected on the basis of information available. Heart tissue samples of approximately 1 by 1 by 0.5 cm in size from left and right ventricular myocardia were transferred into sterile empty containers for DNA analysis. Heart or femoral vein blood was drawn for serological analysis. All samples were immediately frozen at −20°C and tested in a blind manner in the virology laboratories (Freiburg University Medical Center and Labor Enders und Partner) as well as in the histology laboratory of the Freiburg University Medical Center.
Serology.
B19 immunoglobulin M (IgM) and IgG antibody titers were measured by commercial enzyme immunoassay (Biotrin, Dublin, Ireland) in an automated BEP device (Dade Behring, Marburg, Germany). In addition, seropositive samples were subsequently tested by a parvovirus recomBlot assay (Mikrogen, Munich, Germany) to identify recent primary infections. These are characterized by a prominent IgG immune response against the viral protein (VP) C-terminal linear epitopes within VP2, usually detectable only within the first months after acute infection.
DNA analyses.
For DNA analysis, myocardial tissue fragments of approximately 2 mm3 were digested overnight with 20 μl (≥0.6 U/μl) proteinase K (Roche Applied Science, Mannheim, Germany) in 180 μl ATL lysis buffer (Qiagen, Hilden, Germany) at 56°C. Automated DNA extraction was performed with a total nucleic acid isolation kit in a MagNA Pure liquid chromatography (LC) instrument (Roche Applied Science, Mannheim, Germany). Five microliters of these nucleic acid preparations was used for amplification with the Fast Start DNA master hybridization probe kit in a LightCycler 1.0 instrument (Roche Applied Science, Mannheim, Germany), with a universal reaction profile of 10 min at 40°C, 10 min at 95°C, and 45 cycles of 15 s at 95°C and 30 s at 60°C, followed by a final cooling step of 1 min at 40°C. PCR primers and fluorescence-labeled TaqMan probes were specific for the detection of B19 (PCR-1) (PAR-UP, 5′-TGT GGC CCA TTT TCA AGG AA-3′; PAR-DP, 5′-CTG AAG TCA TGC TTG GGT ATT TTT CT-3′; and PAR-P, 5′-FAM-TTT GCC GGA AGT TCC CGC TTA CAA C-TAMRA-3′, where FAM is 6-carboxyfluorescein and TAMRA is 6-carboxytetramethylrhodamine). Serial dilutions of a recombinant plasmid construct were used as a quantification standard. The lower limit of detection proved to be 10 copies of target DNA per reaction. UNG amperase (Fermentas, St. Leon-Rot, Germany) was added to the reaction mixture to exclude false-positive results due to PCR product contamination, and each sample was tested for the presence of inhibitory substances in a parallel reaction.
To demonstrate the long-term persistence of B19 Gt-2 DNA in heart tissue, the seven available samples from individuals born before 1973 were retrospectively subjected to a LightCycler consensus PCR (PCR-2). PCR-2 was performed as described previously with primers that bind within the VP1 coding sequence, resulting in an amplicon size of 212 bp (26). Immediately after PCR-2, an additional analytical cycle for melting temperature (Tm) analysis was included. Melting curves were automatically converted by LC software version 3.5 (Roche) into melting peaks. Isolates with a Tm value differing from that determined for B19 Gt-1 are considered to belong to Gt-2 or -3. The 212-bp amplicons were purified by preparative agarose gel electrophoresis with a QIAquick gel extraction kit (Qiagen, Hilden, Germany). Bidirectional sequencing of the 212-bp amplicons was performed with a Dual CyDye terminator sequencing kit (Amersham Biosciences Europe GmbH, Freiburg, Germany). Unincorporated dye terminators were directly removed from the sequencing reaction mixtures using a DyeEx 2.0 spin kit (Qiagen, Hilden, Germany). Sequence analysis was performed by the Trugene genotyping system (Siemens Medical Solutions Diagnostics GmbH, Bad Nauheim, Germany). Recombinant plasmid constructs carrying B19 DNA of the different genotypes (Gt-1, B19-NAN; Gt-2, B19-LaLi; Gt-3, B19-V9) were used as controls. Gt-1- and Gt-2-containing B19 plasmid constructs (11) were kindly provided by Maria Söderlund-Venermo and Klaus Hedman, Haartman Institute, University of Helsinki, Finland. The cloned genome of the V9 prototype (Gt-3) was obtained from the Collection Nationale de Cultures de Microorganisms (identification reference number PCD.V9.C22, accession number CNCM I-2066).
Histology.
For histological evaluation of the myocardium, samples from the right and left ventricles were taken at standardized locations of each heart. Neutral phosphate-buffered formaldehyde (pH 7.0) was used as a fixative for up to 48 h. Sections (5 μm) of the paraffin-embedded tissue were stained with hematoxylin-eosin and investigated for myocarditis according to the Dallas criteria.
RESULTS
Study population and histopathology.
Sixty-nine subjects—29 (42%) females and 40 (58%) males—with a mean age of 51.4 years (range, 15 to 85 years; median, 53 years) were investigated. In 35 cases (51%), death was caused by multiple traumas, craniocerebral injury, exsanguination, or suffocation in consequence of an accident, suicide, or homicide. Cardiovascular disease, i.e., thrombosis of coronary arteries, cardiac decompensation, and pulmonary embolism, were established in 18 cases (26%). Causes of death are summarized in Table 1. In a 19-year-old man, histological analysis of heart tissue revealed diffuse, prominent lympho-monocytic infiltrations of the myocardium, with scattered destruction of myocytes, and myocarditis of unknown origin was diagnosed. All other myocardial samples did not show any signs of acute or chronic inflammatory disease and did not fulfill the strict criteria of dilative cardiomyopathy.
TABLE 1.
Age distribution and seropositivity rates grouped by different causes of death
| Cause(s) of death | No. of subjects | Mean age (yr) (range) | % PVB19 seropositivity | % B19 PCR positivity |
|---|---|---|---|---|
| Craniocerebral injury | 6 | 43.2 (22-77) | 100 | 100 |
| Multiple traumas with a blunt instrument | 14 | 47.1 (25-83) | 78.6 | 71.4 |
| Suffocation, drowning, stabbing, or gunshot injury | 15 | 48.3 (15-77) | 60.0 | 60 |
| Intoxication | 10 | 44.0 (23-65) | 50.0 | 50 |
| Natural death other than cardiovascular disease | 6 | 63.7 (29-82) | 66.7 | 50 |
| Cardiovascular disease | 18 | 59.2 (19-83) | 72.2 | 72.2 |
Serology.
The overall rate of B19 seropositivity was 69.6%. B19 IgG antibodies were detected in 72% of cases with a cardiovascular cause of death versus 69% of cases without (Table 1). None of the serum samples was positive for B19 IgM. Subsequent testing of IgG-seropositive samples using the parvovirus recomBlot test (Mikrogen, Munich, Germany) suggested one possible recent primary infection on the basis of a prominent band for the VP C-terminal linear epitopes in a 29-year-old man, who had died of progressive Becker's muscular dystrophy. In all other seropositive cases, the immunoblot results showed antibody patterns consistent with past infection.
DNA analyses.
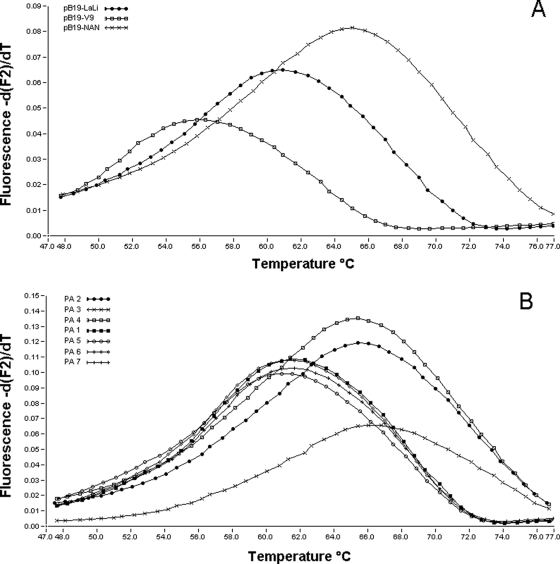
B19 DNA was detected by PCR-1 in the myocardial tissue samples of 46 (96%) of 48 seropositive and in 0 of 21 seronegative study subjects. There was no significant difference in the prevalences of B19 DNA in tissue samples obtained from subjects with and without cardiac disease (Table 1). Estimated viral loads ranged between 2 × 101 and 5 × 104 genome equivalents (GE)/μg extracted DNA from heart tissue. The patient diagnosed with acute myocarditis was also positive for B19 DNA, with a viral load of 3 × 103 GE/μg myocardial DNA. Results of the Tm analysis obtained with PCR-2 products of seven myocardial tissue samples and different recombinant B19 plasmid constructs are demonstrated in Fig. 1. DNA of Gt-1 was found in three patients born between 1950 and 1969. DNA of Gt-2 was found in four patients born between 1927 and 1957.
FIG. 1.
Melting peaks generated by LC PCR-2. (A) Recombinant B19 plasmid constructs pB19-V9 (Gt-3), pB19-LaLi (Gt-2), and pB19-NAN (Gt-1); (B) heart DNA from seven autopsy samples. While the Gt-1-specific probe/target hybrid melts at 65 to 66°C, the Gt-2-specific hybrid melts at 61 to 62°C and the Gt-3-specific hybrid melts at 56 to 57°C. The fluorescence values are given on the y axis, and the increasing temperature is noted on the x axis.
DISCUSSION
B19 infection has been linked to a variety of human diseases, based on the detection of viral DNA by PCR in affected tissue (6, 12, 13). In chronic arthropathy and juvenile hepatic failure, the causative role of viral infection was questioned when healthy controls showed the frequent persistence of B19 DNA in synovial membranes and liver tissue (8, 28). In addition, viral DNA was detected in the bone marrow (4), skin (29), and tonsils of unaffected adults (22).
Several authors have postulated a causative role of B19 in cardiac disease, such as acute myocarditis (5, 17, 27), dilative cardiomyopathy or idiopathic left ventricular dysfunction (7, 14, 16, 19), and peripartum cardiomyopathy (3). A search of the Internet on “parvovirus, human, cardiac disease” yields more than 100 hits in 29 publications since 2005. Most of these studies suggest the causal link between B19 infection and cardiac disease, based on the detection by PCR or in situ hybridization of B19 DNA, while data on serostatus were scarce and sufficient control populations were rarely included. Moreover, demonstration of parvoviral protein expression in situ was missing.
Donoso Mantke et al. (7) showed the presence of B19 DNA in tissue samples in 7% (4 of 56) of explanted hearts and no viral DNA in healthy control samples. Interestingly, in the same study, this was contradicted by the detection of B19 DNA in 9% (5 of 54) of the healthy donor samples. Kühl et al. examined heart tissue samples from 172 patients with suspected myocarditis and found B19 DNA in 37% (17). However, DNA of other viruses was also detected frequently, whereas the patient's serostatus was not evaluated, samples showed no histological signs of inflammation, and control subjects were not tested. Klingel et al. compared the viral loads of cases with acute myocarditis and controls. They found up to 4 × 105 GE in patients, whereas viral titers of controls were below 103 GE per μg myocardial nuclei acid (15). Viral loads of subjects investigated in our study ranged between 2 × 101 GE and 5 × 104 GE per μg DNA, with the majority of samples containing between 2 × 102 and 5 × 103 GE/μg. There was no difference in the viral loads of subjects with different causes of death. The subject with the highest concentration (5 × 104 GE/μg) was a 26-year-old suicide victim. Thus, differences in viral load alone are not sufficient to postulate a pathogenic role of B19 in cardiac disease. Bultmann et al. demonstrated equal rates of B19 PCR positivity in endomyocardial biopsy samples taken from women with peripartum cardiomyopathy (4 of 26 patients [15%]) and controls with nonviral cardiomyopathies (5 of 33 [15%]), indicating viral persistence in the absence of inflammatory disease (3). Those authors also found higher viral loads in cases than in controls and postulated viral reactivation followed by an autoimmune process to explain a pathogenic role of B19 in cardiac inflammatory disease. However, although the persistence of B19 DNA in peripheral blood after acute primary infection has been shown in human immunodeficiency virus-infected patients as well as organ or stem cell transplant patients, the reactivation of B19 was never demonstrated in patients under immunosuppression (9, 10, 25). PCR-1 is frequently performed in cases of aplastic anemia or engraftment failure in stem cell transplant patients, but we never found PCR positivity in the peripheral blood of patients who were seropositive before transplantation (unpublished data). Also, a recent study showed no differences in B19 T-cell immunity when patients assigned to a group with “B19 DNA-positive myocarditis” were compared to seropositive healthy controls (18).
Analyses of B19 genotypes in tissue samples from Finland, Scotland, and Germany revealed the presence of B19 Gt-1 in all examined age groups, whereas the detection of Gt-2 was predominantly confined to those born before 1973 (16, 20, 22). Those authors suggested that Gt-2 DNA originated from primary infection at times of Gt-2 predominance and supported the concept of lifelong asymptomatic persistence of B19 DNA in various tissues. Furthermore, Norja and colleagues recently postulated Gt-2 being the ancestor of Gt-1 in the late 1950s (21). Consistent with these reports, we found four B19 Gt-2 infections in patients born between 1927 and 1957, while two of three Gt-1 infections were from patients born after 1960 (Table 2). These results confirm the lifelong persistence of B19 DNA in myocardial tissue. Since various normal tissues from B19-seropositive individuals were shown to contain B19 DNA, it is hard to prove a causal role of the virus in diseased organs.
TABLE 2.
Characteristics of patients with B19-positive myocardial tissue samples and a known B19 genotype
| Patient's yr of birth | Patient code | Yr of death | Cause(s) of death | B19 genotype | DNA load (GE/μg) |
|---|---|---|---|---|---|
| 1927 | PA1 | 2004 | Suicide | 2 | 7.5 × 102 |
| 1928 | PA3 | 2004 | Chronic heart failure | 2 | 1 × 103 |
| 1940 | PA5 | 2004 | Head injury | 2 | 7.5 × 102 |
| 1950 | PA4 | 2004 | Myocardial infarction | 1 | 2 × 103 |
| 1957 | PA7 | 2004 | Multiple traumas | 2 | 5.5 × 103 |
| 1964 | PA3 | 2004 | Myocardial infarction | 1 | 7.5 × 102 |
| 1969 | PA2 | 2004 | Multiple traumas | 1 | 1 × 103 |
To our knowledge, this is the first study to examine B19 DNA positivity in myocardial samples from an unselected population. In none of the cases did the recent medical history suggest viral infection. The rate of seropositivity matched published data on IgG positivity in the local healthy population (24). In only one of 48 seropositive samples could recent infection not be excluded by the Western blot band pattern. However, B19 DNA was detected by PCR in more than 96% (46 of 48) of the myocardial samples taken from seropositive donors. The high rate of DNA positivity in our study might in part be explained by efficient DNA extraction methods and the use of sensitive real-time PCR for detection. We could show differences of up to 2 logs in the levels of recovery of viral DNA by different extraction methods (data not shown). Furthermore, autolytic processes in cadavers might contribute to more-efficient lysis of viral particles. In addition, we were able to use large postmortem tissue samples for our study, whereas most published data depend on the use of smaller biopsy samples taken in vivo. Cross contamination can be ruled out, as samples were analyzed in a blind manner without knowledge of serostatus and PCR was negative with all seronegative samples.
In conclusion, our data strongly suggest the lifelong asymptomatic persistence of B19 DNA in myocardial tissue after primary infection. The detection of B19 DNA by a sensitive PCR technology has to be interpreted carefully and is not a proof of a causal relationship between B19 infection and cardiac disease.
Footnotes
Published ahead of print on 12 November 2008.
REFERENCES
- 1.Anand, A., E. S. Gray, T. Brown, J. P. Clewley, and B. J. Cohen. 1987. Human parvovirus infection in pregnancy and hydrops fetalis. N. Engl. J. Med. 316183-186. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, M. J., S. E. Jones, S. P. Fisher-Hoch, E. Lewis, S. M. Hall, C. L. Bartlett, B. J. Cohen, P. P. Mortimer, and M. S. Pereira. 1983. Human parvovirus, the cause of erythema infectiosum (fifth disease)? Lancet 11378. [DOI] [PubMed] [Google Scholar]
- 3.Bultmann, B. D., K. Klingel, M. Nabauer, D. Wallwiener, and R. Kandolf. 2005. High prevalence of viral genomes and inflammation in peripartum cardiomyopathy. Am. J. Obstet. Gynecol. 193363-365. [DOI] [PubMed] [Google Scholar]
- 4.Cassinotti, P., G. Burtonboy, M. Fopp, and G. Siegl. 1997. Evidence for persistence of human parvovirus B19 DNA in bone marrow. J. Med. Virol. 53229-232. [PubMed] [Google Scholar]
- 5.Dettmeyer, R., R. Kandolf, A. Baasner, S. Banaschak, A. M. Eis-Hubinger, and B. Madea. 2003. Fatal parvovirus B19 myocarditis in an 8-year-old boy. J. Forensic Sci. 48183-186. [PubMed] [Google Scholar]
- 6.Dijkmans, B. A., A. M. van Elsacker-Niele, M. M. Salimans, G. A. van Albada-Kuipers, E. de Vries, and H. T. Weiland. 1988. Human parvovirus B19 DNA in synovial fluid. Arthritis Rheum. 31279-281. [DOI] [PubMed] [Google Scholar]
- 7.Donoso Mantke, O., A. Nitsche, R. Meyer, K. Klingel, and M. Niedrig. 2004. Analysing myocardial tissue from explanted hearts of heart transplant recipients and multi-organ donors for the presence of parvovirus B19 DNA. J. Clin. Virol. 3132-39. [DOI] [PubMed] [Google Scholar]
- 8.Eis-Hubinger, A. M., U. Reber, T. Abdul-Nour, U. Glatzel, H. Lauschke, and U. Putz. 2001. Evidence for persistence of parvovirus B19 DNA in livers of adults. J. Med. Virol. 65395-401. [DOI] [PubMed] [Google Scholar]
- 9.Geetha, D., J. B. Zachary, H. M. Baldado, J. D. Kronz, and E. S. Kraus. 2000. Pure red cell aplasia caused by Parvovirus B19 infection in solid organ transplant recipients: a case report and review of literature. Clin. Transplant. 14586-591. [DOI] [PubMed] [Google Scholar]
- 10.Hayes-Lattin, B., T. J. Seipel, K. Gatter, M. C. Heinrich, and R. T. Maziarz. 2004. Pure red cell aplasia associated with parvovirus B19 infection occurring late after allogeneic bone marrow transplantation. Am. J. Hematol. 75142-145. [DOI] [PubMed] [Google Scholar]
- 11.Hokynar, K., P. Norja, H. Laitinen, P. Palomaki, A. Garbarg-Chenon, A. Ranki, K. Hedman, and M. Soderlund-Venermo. 2004. Detection and differentiation of human parvovirus variants by commercial quantitative real-time PCR tests. J. Clin. Microbiol. 422013-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kandolf, R., P. Kirschner, P. H. Hofschneider, and T. L. Vischer. 1989. Detection of parvovirus in a patient with “reactive arthritis” by in situ hybridization. Clin. Rheumatol. 8398-401. [DOI] [PubMed] [Google Scholar]
- 13.Karetnyi, Y. V., P. R. Beck, R. S. Markin, A. N. Langnas, and S. J. Naides. 1999. Human parvovirus B19 infection in acute fulminant liver failure. Arch. Virol. 1441713-1724. [DOI] [PubMed] [Google Scholar]
- 14.Klein, R. M., H. Jiang, D. Niederacher, O. Adams, M. Du, M. Horlitz, P. Schley, R. Marx, M. R. Lankisch, M. U. Brehm, B. E. Strauer, H. E. Gabbert, T. Scheffold, and H. Gulker. 2004. Frequency and quantity of the parvovirus B19 genome in endomyocardial biopsies from patients with suspected myocarditis or idiopathic left ventricular dysfunction. Z. Kardiol. 93300-309. [DOI] [PubMed] [Google Scholar]
- 15.Klingel, K., M. Sauter, C. T. Bock, G. Szalay, J. J. Schnorr, and R. Kandolf. 2004. Molecular pathology of inflammatory cardiomyopathy. Med. Microbiol. Immunol. 193101-107. [DOI] [PubMed] [Google Scholar]
- 16.Kühl, U., D. Lassner, M. Pauschinger, U. M. Gross, B. Seeberg, M. Noutsias, W. Poller, and H. P. Schultheiss. 2008. Prevalence of erythrovirus genotypes in the myocardium of patients with dilated cardiomyopathy. J. Med. Virol. 801243-1251. [DOI] [PubMed] [Google Scholar]
- 17.Kühl, U., M. Pauschinger, B. Seeberg, D. Lassner, M. Noutsias, W. Poller, and H. P. Schultheiss. 2005. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation 1121965-1970. [DOI] [PubMed] [Google Scholar]
- 18.Lindner, J., S. Zehentmeier, R. Franssila, S. Barabas, J. Schroeder, L. Deml, and S. Modrow. 2008. CD4+ T helper cell responses against human bocavirus viral protein 2 viruslike particles in healthy adults. J. Infect. Dis. 1981677-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lotze, U., R. Egerer, C. Tresselt, B. Gluck, G. Dannberg, A. Stelzner, and H. R. Figulla. 2004. Frequent detection of parvovirus B19 genome in the myocardium of adult patients with idiopathic dilated cardiomyopathy. Med. Microbiol. Immunol. 19375-82. [DOI] [PubMed] [Google Scholar]
- 20.Manning, A., S. J. Willey, J. E. Bell, and P. Simmonds. 2007. Comparison of tissue distribution, persistence, and molecular epidemiology of parvovirus B19 and novel human parvoviruses PARV4 and human bocavirus. J. Infect. Dis. 1951345-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norja, P., A. M. Eis-Hubinger, M. Soderlund-Venermo, K. Hedman, and P. Simmonds. 2008. Rapid sequence change and geographical spread of human parvovirus B19: comparison of B19 virus evolution in acute and persistent infections. J. Virol. 826427-6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norja, P., K. Hokynar, L. M. Aaltonen, R. Chen, A. Ranki, E. K. Partio, O. Kiviluoto, I. Davidkin, T. Leivo, A. M. Eis-Hubinger, B. Schneider, H. P. Fischer, R. Tolba, O. Vapalahti, A. Vaheri, M. Soderlund-Venermo, and K. Hedman. 2006. Bioportfolio: lifelong persistence of variant and prototypic erythrovirus DNA genomes in human tissue. Proc. Natl. Acad. Sci. USA 1037450-7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pattison, J. R., S. E. Jones, J. Hodgson, L. R. Davis, J. M. White, C. E. Stroud, and L. Murtaza. 1981. Parvovirus infections and hypoplastic crisis in sickle-cell anaemia. Lancet i664-665. [DOI] [PubMed] [Google Scholar]
- 24.Röhrer, C., B. Gärtner, A. Sauerbrei, S. Böhm, B. Hottenträger, U. Raab, W. Thierfelder, P. Wutzler, and S. Modrow. 2008. Seroprevalence of parvovirus B19 in the German population. Epidemiol. Infect. 1361564-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanphasitvong, W., K. Poovorawan, P. Boonsuk, T. Assanasen, T. Na Nakorn, and Y. Poovorawan. 2005. Parvovirus b19 infection in HIV patient with pure red cell aplasia. Southeast Asian J. Trop. Med. Public Health 361216-1220. [PubMed] [Google Scholar]
- 26.Schalasta, G., M. Schmid, T. Lachmund, and G. Enders. 2004. LightCycler consensus PCR for rapid and differential detection of human erythrovirus B19 and V9 isolates. J. Med. Virol. 7354-59. [DOI] [PubMed] [Google Scholar]
- 27.Schowengerdt, K. O., J. Ni, S. W. Denfield, R. J. Gajarski, N. E. Bowles, G. Rosenthal, D. L. Kearney, J. K. Price, B. B. Rogers, G. M. Schauer, R. E. Chinnock, and J. A. Towbin. 1997. Association of parvovirus B19 genome in children with myocarditis and cardiac allograft rejection: diagnosis using the polymerase chain reaction. Circulation 963549-3554. [DOI] [PubMed] [Google Scholar]
- 28.Soderlund, M., R. von Essen, J. Haapasaari, U. Kiistala, O. Kiviluoto, and K. Hedman. 1997. Persistence of parvovirus B19 DNA in synovial membranes of young patients with and without chronic arthropathy. Lancet 3491063-1065. [DOI] [PubMed] [Google Scholar]
- 29.Soderlund-Venermo, M., K. Hokynar, J. Nieminen, H. Rautakorpi, and K. Hedman. 2002. Persistence of human parvovirus B19 in human tissues. Pathol. Biol. (Paris) 50307-316. [DOI] [PubMed] [Google Scholar]