Abstract
Seventy-six children ≤2 years old were prospectively followed for 1 year in a peri-urban community of Mexico City to determine asymptomatic infection and acute diarrhea associated with diarrheagenic Escherichia coli pathotypes (DEPs). By use of a pathogen-specific multiplex PCR, DEPs were sought in 795 stool samples, of which 125 (16%) were positive for DEP; of these, 4 represented shedding episodes and 4 parasite coinfections. Most single-DEP infections (85/117) were asymptomatic (P < 0.001), and of the 32 DEP diarrhea episodes, 41% were associated with atypical enteropathogenic E. coli (aEPEC), 37.5% with enterotoxigenic E. coli, 9% with typical EPEC, 9% with enteroinvasive E. coli, and 3% with Shiga toxin-producing E. coli strains. Among the 76 children, 54 had at least one stool positive for DEP, of which 23 experienced a DEP-associated diarrhea episode. In the last group of children, DEP infection was significantly associated with a diarrhea episode (relative risk [RR] = 2.5; 95% confidence interval [CI], 1.79 to 3.57; P < 0.001), with ETEC (RR = 2.30; 95% CI, 1.49 to 3.54; P = 0.003) and aEPEC (RR = 1.92; 95% CI, 1.23 to 3.0; P = 0.019) being the pathotypes associated with diarrhea. aEPEC-associated diarrhea episodes were frequently in the <12-month age group (RR = 2.57; 95% CI, 1.05 to 6.27; P = 0.04). aEPEC infections were distributed all year round, but associated diarrheal episodes were identified from April to October, with a May-June peak (rainy season). Most ETEC infections and diarrhea episodes characteristically occurred during the summer (rainy season), with a diarrhea peak in August. Of all DEPs, only aEPEC was associated with acute diarrhea episodes lasting 7 to 12 days (P = 0.019). DEPs are important causes of community-acquired enteric infection and diarrhea in Mexican children.
Diarrheal diseases continue to be a health problem worldwide (4, 16), especially in developing countries, where they are estimated to be responsible for 2.5 million infant deaths per year, with an annual mortality rate of 4.9 per 1,000 children and an incidence of 3.2 episodes per child per year among children under 5 years of age (16). Diarrheagenic Escherichia coli pathotypes (DEPs) represent a leading bacterial cause of pediatric diarrhea in developing regions (21), with some responsible for traveler's diarrhea (21, 27), and are also an emerging cause of diarrhea in industrialized countries (6, 29). The DEPs that cause diarrhea include enteropathogenic E. coli (EPEC), enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC), Shiga toxin-producing E. coli (STEC), diffusely adherent E. coli, and enteroaggregative E. coli (15, 21). These pathotypes are defined by the presence or absence of one or more definable E. coli virulence factors. However, a less well-characterized DEP is atypical EPEC (aEPEC), which carries the attaching and effacing intimin (eaeA) gene of typical EPEC (tEPEC) strains but lacks the plasmid-mediated bundle-forming pilus (bfp) of tEPEC and Shiga toxins 1 and 2 (21, 31).
Although DEPs are of public health relevance, they are not routinely sought as enteric pathogens in clinical laboratories worldwide; thus, their incidence in children ≤2 years of age and their importance in community-acquired diarrhea are generally unknown, particularly in areas of endemicity. In order to assess the prevalence of DEPs and their association with diarrhea, stools collected from a cohort of children recruited and followed prospectively for 1 year in peri-urban communities of Mexico City were screened for the presence of ETEC, EIEC, tEPEC, aEPEC, and STEC by using a pathogen-specific multiplex PCR (19). In order to assess DEP frequency and association with diarrhea, a cohort of children from a peri-urban area of Mexico City were analyzed for the presence of ETEC, EIEC, tEPEC, aEPEC, and STEC by a pathogen-specific multiplex PCR (19); however, enteroaggregative E. coli and diffusely adherent E. coli were not sought. The study was the placebo arm of a larger study looking at the effect of vitamin A supplementation on the occurrence of diarrhea and enteric infection (18).
MATERIALS AND METHODS
Subjects and microbiological analysis.
A cohort of children <2 years old, from La Magdalena Atlicpac, a peri-urban community of Mexico City, Mexico, were enrolled between 1 January and 31 December 1998 and prospectively followed for up to 15 months. The study was a randomized clinical trial of the impact of vitamin A supplementation on asymptomatic enteric infection and acute diarrhea. At the beginning of the study, mothers were interviewed regarding their educational statuses, access to piped water, and possession of a bathroom in the house or on the property. Children were assigned to receive vitamin A or a placebo every 2 months, with study households being visited twice a week. During visits, mothers were interviewed regarding the presence of diarrhea in household children, the number and consistency of evacuations, the presence of fever, and the passage of stools containing gross blood and/or mucus. A stool sample was collected twice a month from healthy children, and two samples were collected within 1 week of the episode onset. Each stool sample was processed within 4 hours of collection at the laboratory of the Ministry of Health hospital located in the respective zone. Stool samples were analyzed for the presence of parasites (20) and enterobacteria (13). Of note, although sought, Salmonella and Shigella species were not identified in the stools of these children.
DEP characteristics and identification.
Three lactose-fermenting colonies with E. coli morphology were selected from MacConkey agar plates and biochemically identified (13). DEPs were characterized by a single multiplex PCR technique, as previously described (19). The pathotypes identified by this PCR technique, their characteristics, and their target loci are given in Table 1. STEC bacteria isolated from patients were further characterized by the expression of the O157 lipopolysaccharide antigen and the enterohemolysine gene (hlyA), using a latex particle agglutination kit (Oxoid Limited, Basingstoke, United Kingdom) and PCR, respectively (25). We used the following working definitions: (i) a diarrhea episode was defined as a change in the child's stool patterns as reported by the mother and confirmed by the passage of three or more liquid stools in 1 day; (ii) a single-DEP infection involved the presence of only one DEP in a stool; (iii) a DEP-associated diarrhea episode involved the presence of a DEP in a stool sample collected during, within 7 days before, or after a diarrhea episode; and (iv) DEP shedding involved confirmation of the same DEP during the 2-week time period after a DEP diarrhea episode.
TABLE 1.
Phenotypic and genotypic characteristics of DEPs
| DEP | Defining characteristic(s)a | Target(s) |
|---|---|---|
| tEPEC | Presence of both intimin (as a marker of the LEE) and the BFP contained in the EAF plasmid | eaeA, bfpA |
| aEPEC | Presence of intimin (as a marker of LEE); absence of the EAF plasmid and Shiga toxins 1 and 2 | eaeA |
| ETEC | Presence of ST or/and LT | lt, st |
| EIEC | Presence of the invasion-associated locus of the invasion plasmid | ial |
| STEC | Presence of Shiga toxin 1 and/or Shiga toxin 2; in addition, some strains also have intimin (as marker of LEE) | stx1, stx2, eaeA |
See reference 15. Abbreviations: LEE, locus of enterocyte effacement; BFP, fundle-forming pilus; EAF, EPEC adherence factor.
Statistical analysis.
the cases and controls were compared using Mantel and Haenszel's x2 test and Fisher's two-tailed exact test. Relative risk (RR) values were calculated by standard methods and were adjusted for age, sex, and seasonality. When possible, the subjects were used as their own controls. Differences between groups were considered significant when P was <0.05.
RESULTS
Seventy-six children (39 boys and 37 girls) <2 years old assigned to the placebo arm in the randomized clinical trial were included in the study. Table 2 illustrates that 46% of households of study children had no access to piped water whereas almost all households had a bathroom in the house or on the property. Additionally, close to 50% of the mothers of the enrolled children had completed a secondary education or higher. A total of 173 diarrhea episodes were reported, with an overall rate of 3.96 (250 episodes/63.1 child years).
TABLE 2.
Baseline characteristics of study children and households
| Characteristic | % of patients |
|---|---|
| Child age (mo) | |
| 6-12 | 81.3 |
| 12-18 | 17.5 |
| Male sex | 51.3 |
| Mother's education | |
| Less than 6 yr | 14.4 |
| Completion of elementary school | 36.5 |
| Completion of secondary school | 40.4 |
| Beyond secondary school | 8 |
| Water source | |
| Indoor faucet | 13.1 |
| Outdoor faucet | 38.3 |
| No access to piped water | 46.7 |
| Bathroom location | |
| Inside house | 33.6 |
| Outside house | 63.5 |
| No bathroom | 2.8 |
Among the 76 children, 54 (71%) had at least one stool positive for DEP, while 32 (59%) had multiple positive stool samples, including 2 children who had DEPs isolated from six stools each. Of the 54 children positive for DEPs, 23 (46%) had a minimum of one DEP-associated diarrhea episode. In Table 3, we list the general characteristics of these 23 children, including the total numbers of diarrhea episodes and stool samples, the occurrences of asymptomatic DEP infections and associated diarrhea episodes (in chronological order), the durations of the diarrhea episodes (in days), and the occurrences of shedding events (see definitions in Materials and Methods). Sixteen children had one DEP-associated diarrhea episode (seven had ETEC, four aEPEC, three EIEC, and two tEPEC), six children had two (four had ETEC and aEPEC and two aEPEC and aEPEC), and one had four (aEPEC, ETEC, tEPEC, and STEC). Most children (16; 70%) had another asymptomatic DEP infection during the year. From the dates of the asymptomatic infections, we identified four shedding events (in patients 8, 9, 10, and 19). Additionally, patient 2, who had an aEPEC-associated diarrhea episode, was found to have two stools positive for the same DEP in the previous month. For these children, there was a significant association between the presences of a DEP infection and a diarrhea episode (RR = 2.5; 95% confidence interval [CI], 1.79 to 3.57; P < 0.001). ETEC and aEPEC were the pathotypes significantly associated with diarrhea, with RR values of 2.30 (95% CI, 1.49 to 3.54; P = 0.003) and 1.92 (95% CI, 1.23 to 3.0; P = 0.019), respectively. For EIEC, tEPEC, and STEC, no significant associations were observed, which may relate to the reduced prevalences of these DEPs. Of all DEP-associated diarrhea episodes, only aEPEC episodes (30%) were significantly associated with durations of >6 days (P = 0.019), given that all other DEP-associated diarrhea episodes lasted ≤6 days.
TABLE 3.
Characteristics of patients with acute DEP diarrhea episodes
| Patient no. | No. of diarrhea episodes/no. of stools | DEP(s) (date[s]) associated with:
|
Duration of diarrhea episode (days) or presence of sheddingc | |
|---|---|---|---|---|
| Asymptomatic infection | Diarrheaa,b | |||
| 14 | 4/19 | tEPEC (January 22), aEPEC (March 13), ETEC (September 2) | aEPEC (October 5) | 8 |
| 24 | 3/17 | aEPEC (April 2), aEPEC (July 2) | ETEC (July 30) | 1 |
| aEPEC (October 8), aEPEC (October 22) | aEPEC (October 29) | 1 | ||
| 28 | 5/24 | aEPEC (March 10), tEPEC (April 1), aEPEC (September 2), aEPEC (October 13) | EIEC (May 25) | 1 |
| 79 | 6/25 | Mixedd (July 1), aEPEC (October 7) | EIEC (May 24) | 2 |
| 93 | 5/14 | aEPEC (May 16) | 1 | |
| 95 | 4/13 | ETEC (September 25) | ETEC (July 8) | 3 |
| 109 | 4/24 | aEPEC (January 28), aEPEC (September 1) | aEPEC (July 20) | 11 |
| ETEC (September 23), tEPEC (October 14) | ETEC (August 8) | 3 | ||
| 110 | 4/20 | aEPEC (May 20) | 3 | |
| aEPEC (June 4) | + | |||
| ETEC (August 31) | 1 | |||
| 125 | 4/17 | aEPEC (May 6), tEPEC (July 31) | ETEC (August 9) | 4 |
| ETEC (August 25) | + | |||
| 150 | 3/10 | ETEC (February 25) | ETEC (August 12) | 1 |
| ETEC (August 26) | + | |||
| 160 | 2/16 | aEPEC (September 28), ETEC (October 7) | ETEC (August 23) | 2 |
| 167 | 4/17 | aEPEC (September 1) | aEPEC (March 31) | 1 |
| aEPEC (June 8) | 2 | |||
| 174 | 5/13 | EIEC (August 11) | aEPEC (June 18) | 3 |
| 201 | 4/15 | ETEC (October 28) | aEPEC (May 24) | 2 |
| 237 | 3/9 | aEPEC (June 24) | 3 | |
| aEPEC (September 16) | 2 | |||
| 241 | 1/5 | ETEC (August 7 | 2 | |
| 264 | 1/10 | ETEC (July 22), aEPEC (October 7) | tEPEC (July 29) | 3 |
| 268 | 5/7 | EIEC (July 31) | 1 | |
| 291 | 3/12 | tEPEC (June 2) | aEPEC (June 23) | 7 |
| aEPEC (July 3) | + | |||
| ETEC (October 10 | 2 | |||
| 309 | 2/10 | ETEC (July 30), ETEC (August 7) | ETEC (October 1) | 1 |
| 319 | 7/14 | tEPEC (July 23) | 1 | |
| aEPEC (August 1) | 12 | |||
| ETEC (October 16) | 6 | |||
| STEC (November 28) | 2 | |||
| 335 | 3/6 | ETEC (July 27) | 3 | |
| 345 | 1/5 | tEPEC (September 3) | 1 | |
P < 0.001; RR = 2.5; 95% CI, 1.79 to 3.57.
ETEC (RR = 2.30; 95% CI, 1.49 to 3.54; P = 0.003) and aEPEC (RR = 1.92; 95% CI, 1.23 to 3.0; P = 0.019) were the pathotypes significantly associated with diarrhea.
P = 0.019. “+” indicates a shedding episode.
“Mixed” indicates a mixed infection of ETEC and STEC strains. (A mixed DEP is defined as two or more DEPs identified in the same stool sample.)
Of the 795 stools collected during the study, 125 (16%) were positive for diarrheagenic E. coli, of which 4 were associated with a shedding event (2 with ETEC and 2 with aEPEC) and 4 were coinfections between a DEP and a parasite: 1 ETEC-Giardia lamblia, 1 aEPEC-Giardia lamblia, 1 aEPEC-Entamoeba histolytica, and 1 aEPEC-Ascaris lumbricoides coinfection. The remaining 117 single-DEP infections were analyzed according to age distribution, presence of asymptomatic infections, and association with diarrhea for each DEP, which is illustrated in Table 4. More DEP infections were significantly associated (P < 0.001) with asymptomatic events (73%) than with diarrhea episodes (27%). aEPEC was the most frequent DEP identified, accounting for 44.5% of all infections, followed by ETEC (36%), tEPEC (10%), EIEC (5%), and STEC (2.5%), and 2% were mixed-DEP infections. Seventy-seven percent of asymptomatic infections were in the ≥12-month age group, whereas 41% (12/29) of DEP-associated diarrhea episodes were in the <12-month age group, but there was not a significant association. However, aEPEC-associated diarrhea episodes (46%; 6/13) were significantly associated with children in the <12-month age group, with an RR value of 2.57 (95% CI, 1.05 to 6.27; P = 0.04), while ETEC diarrhea episodes were similarly distributed between the two age groups: 33% (2/6) for <12 months and 28% (10/36) for ≥12 months. Of the 32 associated diarrhea episodes, 41% were due to aEPEC, 37.5% to ETEC, 9% to tEPEC, 9% to EIEC, and 3% to STEC. Mixed-DEP infections were not associated with diarrhea. Of the total 173 diarrhea episodes reported in this group, 32 (18%) were found to be associated with a single-DEP infection. The incidence of diarrhea for all DEP infections was 0.84 episodes per child per year (32 episodes/38.1 child years).
TABLE 4.
Diarrheagenic E. coli asymptomatic infections and diarrhea episodes over a 1-year time period (1 January to 31 December 1998) among children ≤2 years of age living in a peri-urban community of Mexico City
| DEP(s) | No. (%) during the year for indicated age group
|
||||||
|---|---|---|---|---|---|---|---|
| Total infections | Asymptomatic infectionsa
|
Diarrhea episodes
|
|||||
| Total | <12 mo | ≥12 mo | Total | <12 mo | ≥12 mo | ||
| aEPEC | 52 (44.5) | 39 (75) | 7 (18) | 32 (82) | 13 (25) | 6 (46)b | 7 (53) |
| ETEC | 42 (36) | 30 (71) | 4 (13) | 26 (87) | 12 (29) | 2 (17) | 10 (83) |
| tEPEC | 12 (10) | 9 (75) | 4 (44) | 5 (56) | 3 (25) | 2 (75) | 1 (25) |
| EIEC | 6 (5) | 3 (50) | 0 | 3 (100) | 3 (50) | 1 (25) | 2 (75) |
| STEC | 3 (2.5) | 2 (67) | 1 (50) | 1 (50) | 1 (33) | 1 (100) | 0 |
| Mixed | 2 (2) | 2 (100) | 1 (50) | 1 (50) | 0 | 0 | 0 |
| Total | 117 | 85 (73) | 17 (20) | 68 (80) | 32 (27) | 12 (38) | 20 (62) |
P < 0.001.
P = 0.04; RR = 2.57; 95% CI, 1.05 to 6.27.
Most ETEC isolates (39; 92%) were lt positive, only 2 were lt-st positive, and only 1 was st positive. All ETEC-associated diarrhea episodes were due to lt-positive strains. The 3 STEC strains identified were one stx1-eaeA, one stx1-stx2, and one stx1 strain, the last of which was the only strain associated with a diarrhea episode. None of the STEC strains expressed the O157 lipopolysaccharide antigen or contained the hlyA gene.
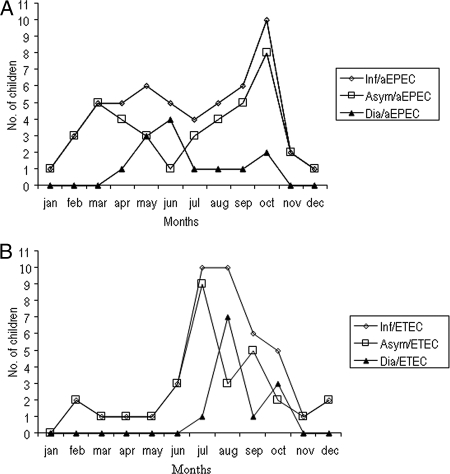
aEPEC infections were reported throughout the year (Fig. 1A) among the children, with the highest frequency of associated diarrhea episodes (92%) occurred during May to October; Mexico City rainy season (Fig. 1A). ETEC infections were found from February to December; nevertheless, most infections and diarrhea episodes were seen during summer months of July to October (rainy season) with a clear diarrhea peak in August (Fig. 1B). There was no apparent seasonality for infection by tEPEC, EIEC and STEC, which may relate to their reduced prevalence (Table 4). Interestingly the 3 STEC cases were identified during the winter.
FIG. 1.
Seasonal occurrence of asymptomatic (Asym) infection (Inf) cases and episodes of diarrhea (Dia) due to aEPEC (A) or ETEC (B) over a 1-year time period (1 January to 31 December 1998) among children ≤2 years of age living in a peri-urban community of Mexico City.
DISCUSSION
In the present study we describe in a DEP endemic country like Mexico, the prevalence of 4 of these pathotypes in a cohort of children ≤2 years old from a Mexico City peri-urban community. In these children that were enrolled during a year and followed up for the same period most DEPs infections were significantly associated (P < 0.001) with asymptomatic events. Similar results have been reported at least for ETEC in domicile carrier studies from developing countries (33) and in some studies of EPEC and aEPEC around the world (23).
We are reporting here for the first time a significant association of aEPEC with community-acquired acute diarrhea lasting 7 to 12 days (P = 0.019). None of the other pathotypes were associated with illness of this duration, suggesting that aEPEC is more associated with protracted diarrhea than the other DEP. In addition, aEPEC-associated diarrhea episodes were significantly associated with children in the <12-month age group (RR = 2.57; 95% CI, 1.05 to 6.27; P = 0.04), in contrast to the tendency previously described for Norwegian children (1). Until now, the strongest association between aEPEC and diarrhea has been for persistent diarrhea (≥14 days) in children in industrialized countries (1, 22); however, in the present study, persistent diarrhea was not investigated, due to its low prevalence. We recently identified aEPEC strains in children hospitalized with acute diarrhea in Mexico (7). Although aEPEC had the highest prevalence among DEPs, most isolates produced asymptomatic infection. The worldwide importance of aEPEC as a cause of diarrhea pathogenesis is uncertain (2, 31). It is likely that aEPEC strains differ in their pathogenic potentials. DNA microarray analysis of aEPEC strains isolated from Norwegian children with and without diarrhea revealed that indeed there are certain genes statistically associated with diarrhea (2). Of note, there was a child (patient 2) (Table 4) who harbored aEPEC on five occasions during the year, suggesting a propensity for aEPEC to persist longer in the intestine than other DEPs. Further studies of the importance of aEPEC strains as causes of diarrhea are needed for other countries.
The aEPEC pathotypes were distributed throughout the year, with a clear peak in October, when most infections were asymptomatic. Diarrhea episodes associated with aEPEC were observed only from April to October, with a peak in June (early summer). A number of studies in both developed and developing countries have reported seasonal peaks in tEPEC and aEPEC infection (1, 10). A study in Norway found that aEPEC was isolated from children throughout the year but was found most frequently in the late summer/early autumn period (1). Studies in both Brazil and Bangladesh have found clear seasonal peaks in tEPEC as well, but these peaks can occur at different times during the calendar year, possibly as a result of local meteorological patterns (3). From the present study, it appears that aEPEC is an emerging diarrheagenic pathogen, not only in industrialized countries, as previously described (1, 6, 22, 29), but also in developing countries, like Mexico (7), Brazil (31), and India (32). aEPEC strains identified in Mexico were previously shown to possess lower levels of antibiotic resistance than other diarrhea-producing E. coli pathotypes in Mexico (7), suggesting that aEPEC strains may have recently been acquired in this country.
The high prevalence of ETEC is consistent with the rates of ETEC isolation reported previously for indigenous populations of and travelers to Mexico (5). The low proportion of symptomatic ETEC infections (33%) may be due to the fact that most isolated strains found (95%) were heat-labile enterotoxin (LT) positive only. Previous studies have shown that heat-stable enterotoxin (ST)-positive ETEC strains are more commonly associated with outbreaks and diarrhea episodes (27). Nevertheless, in recent years, studies in Mexico have shown a higher prevalence of LT strains associated with diarrhea episodes than ST strains (7, 8). In our study, ETEC infections were distributed almost all year round (February to December), with a majority of infections occurring from June to October, similar to seasonal patterns found for LT strains in Egypt (28). However, ETEC-associated diarrhea episodes were identified only from July to October, with a clear peak in August, during the summer rainy season in Mexico City, rather than the warm season (spring in Mexico City), as has been reported for other regions of the world (10, 11, 27). In addition, a smaller, second peak was observed in October, similar to the autumn peaks reported for Bangladesh (27). Overall, these results suggest that humidity is an important factor for ETEC spread and disease in Mexico City.
In the present study, tEPEC infections were not commonly associated with diarrhea, which may relate to the older ages of the children included in the study. tEPEC strains have been reported to most commonly infect infants ≤6 months old (21, 23). It is possible that in Mexico, tEPEC prevalence is diminishing as aEPEC infection rates are increasing, a pattern already described for industrialized regions (29, 31) and more recently for several developing countries (22, 23, 31, 32). EIEC was the DEP with the highest degree of association with diarrhea, found in half of the subjects, revealing once more its virulence for infants in Mexico (24). Since EIEC strains are the only invasive DEP (15), their identification may have therapeutic implications. Non-O157 STEC was the least prevalent pathotype in this study, with only one of the three isolates being associated with illness. Similar to what was found for other settings in Mexico (7) and other developing countries, non-O157 STEC prevalence has been shown to be low (12, 26) or not even present (32). Despite its low prevalence in developing countries, non-O157 STEC can cause diarrhea (7, 12).
Our observations that most DEP infections were significantly associated with asymptomatic events and that only 1 of the 54 children who had one or several DEP infections had at least one DEP-associated diarrhea episode strongly suggest that only some children are susceptible to DEP diarrhea. In line with this, recent studies have shown that both genetic predisposition (9, 14) and nutritional status (17, 18, 30) increase the probability of a DEP diarrhea episode.
Finally, our results reveal that locus pathogen-specific multiplex PCR is an excellent tool for the identification of both well-established and emerging pathotypes, allowing the study of the incidence of pathogen-specific infection and diarrhea. The present study demonstrates the importance of diarrhea-causing E. coli in children living in a peri-urban area of Mexico City, which is responsible for at least one-third of all diarrhea episodes and the emergence of aEPEC as an important pathogen of acute protracted diarrhea.
Acknowledgments
This work was supported by CONACyT grant 46068-Q to T.E.-G. and by a National Institutes of Health grant (K01 DK06142-02) to K.Z.L. R.T.-B. (169483) and D.L.-H. (200494) were supported by CONACyT scholarships.
Footnotes
Published ahead of print on 19 November 2008.
REFERENCES
- 1.Afset, J. E., L. Bevanger, P. Romundstand, and K. Bergh. 2004. Association of atypical enteropathogenic Escherichia coli (EPEC) with prolonged diarrhea. J. Med. Microbiol. 531137-1144. [DOI] [PubMed] [Google Scholar]
- 2.Afset, J. E., G. Bruant, R. Brousseau, J. Harel, E. Anderssen, L. Bevanger, and K. Bergh. 2006. Identification of virulence genes linked with diarrhea due to atypical enteropathogenic Escherichia coli by DNA microarray analysis and PCR. J. Clin. Microbiol. 443703-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albert, M. J., S. M. Faruque, A. S. G. Faruque, P. K. B. Neogi, M. Ansaruzzaman, N. A. Bhuiyan, K. Alam, and M. S. Akbar. 1995. Controlled study of Escherichia coli diarrheal infections in Bangladeshi children. J. Clin. Microbiol. 33973-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avendaño, P., D. O. Matson, J. Long, S. Whitney, C. C. Matson, and L. K. Pickering. 1993. Costs associated with office visits for diarrhea in infants and toddlers. Pediatr. Infect. Dis. J. 12897-902. [DOI] [PubMed] [Google Scholar]
- 5.Bouckenooghe, A. R., Z. D. Jiang, F. J. De la Cabada, C. D. Ericsson, and H. L. DuPont. 2002. Enterotoxigenic Escherichia coli as cause of diarrhea among Mexican adults and US travelers in Mexico. J. Travel Med. 9137-140. [DOI] [PubMed] [Google Scholar]
- 6.Cohen, M. B., J. P. Nataro, D. I. Bernstein, J. Hawkins, N. Roberts, and M. A. Staat. 2005. Prevalence of diarrheagenic Escherichia coli in acute childhood enteritis: a prospective controlled study. J. Pediatr. 14654-61. [DOI] [PubMed] [Google Scholar]
- 7.Estrada-Garcia, T., J. F. Cerna, L. Paheco-Gil, R. F. Velázquez, T. J. Ochoa, J. Torres, and H. L. DuPont. 2005. Drug-resistant diarrheogenic Escherichia coli, Mexico. Emerg. Infect. Dis. 111306-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flores, J., H. L. DuPont, Z. D. Jiang, J. Belkind-Gerson, J. A. Mohamed, L. G. Carlin, R. Padda, M. Paredes, J. F. Martinez-Sandoval, N. A. Villa, and P. C. Okhuysen. 2008. Enterotoxigenic Escherichia coli heat-labile toxin seroconversion in US travelers to Mexico. J. Travel Med. 15156-161. [DOI] [PubMed] [Google Scholar]
- 9.Flores, J., H. L. DuPont, S. A. Lee, J. Belkind-Gerson, M. Paredes. J. A. Mohamed, L. Y. Armitige, D.-C. Guo, and P. C. Okhuysen. 2008. Influence of host interleukin-10 polymorphisms on development of traveler's diarrhea due to heat-labile enterotoxin-producing Escherichia coli in travelers from the United States who are visiting Mexico. Clin. Vaccine Immunol. 151194-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomes, T. A., V. Rassi, K. L. MacDonald, R. T. S. Ramos, L. R. Trabulsi, M. A. Vieira, B. E. Guth, J. A. Candeias, C. Ivey, M. R. Toledo, and P. A. Blake. 1991. Enteropathogens associated with acute diarrheal disease in urban infants in Sao Paulo, Brazil. J. Infect. Dis. 164331-337. [DOI] [PubMed] [Google Scholar]
- 11.Guerrant, R. L., L. V. Kirchhoff, D. S. Shields, M. K. Nations, J. Leslie, M. A. de Sousa, J. G. Araujo, L. L Correia, K. T. Sauer, K. E. McClelland, F. L. Trowbridge, and J. M. Hughes. 1983. Prospective study of diarrheal illness in northeastern Brazil: patterns of disease, nutritional impact, etiologies, and risk factors. J. Infect. Dis. 148986-997. [DOI] [PubMed] [Google Scholar]
- 12.Guth, B. E., S. R. Ramos, A. M. Cerqueira, J. R. Andrade, and T. A. Gomes. 2002. Phenotypic and genotypic characteristics of Shiga toxin-producing Escherichia coli strains isolated from children in São Paulo, Brazil. Mem. Inst. Oswaldo Cruz 971085-1089. [DOI] [PubMed] [Google Scholar]
- 13.Isenberg, H. D. 1992. Clinical microbiology procedures handbook. American Society for Microbiology, Washington, DC.
- 14.Jiang, Z.-D., P. C. Okhuysen, D.-C. Guo, R. He, T. M. King, H. L. DuPont, and D. M. Milewicz. 2003. Genetic susceptibility to enteroaggregative Escherichia coli diarrhea: polymorphism in the interleukin-8 promotor region. J. Infect. Dis. 188506-511. [DOI] [PubMed] [Google Scholar]
- 15.Kaper, J. B., J. P. Nataro, and H. L. Mobley. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2123-140. [DOI] [PubMed] [Google Scholar]
- 16.Kosek, M., C. Bern, and R. L. Guerrant. 2003. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull. W. H. O. 81197-204. [PMC free article] [PubMed] [Google Scholar]
- 17.Lima, A. A., S. R. Moore, M. S. Barboza, Jr., A. M. Soares, M. A. Schleupner, R. D. Newman, C. L. Sears, J. P. Nataro, D. P. Fedorko, T. Wuhib, J. B. Schorling, and R. L. Guerrant. 2000. Persistent diarrhea signals a critical period of increased diarrhea burdens and nutritional shortfalls: a prospective cohort study among children in northeastern Brazil. J. Infect. Dis. 1811643-1651. [DOI] [PubMed] [Google Scholar]
- 18.Long, K. Z., J. I. Santos, J. L. Rosado, C. Lopez-Saucedo, R. Thompson-Bonilla, M. Abonce, H. L. DuPont, E. Hertzmark, and T. Estrada-Garcia. 2006. Impact of vitamin A on selected gastrointestinal pathogen infections and associated diarrheal episodes among children in Mexico City, Mexico. J. Infect. Dis. 1941217-1225. [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Saucedo, C., J. F. Cerna, N. Villegas-Sepulveda, R. Thompson, F. R. Velazquez, J. Torres, P. I. Tarr, and T. Estrada-García. 2003. Single multiplex polymerase chain reaction to detect diverse loci associated with diarrheagenic Escherichia coli. Emerg. Infect. Dis. 9127-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin, L. K., and P. C. Beaver. 1968. Evaluation of Kato thick-smear technique for quantitative diagnosis of helminth infections. Am. J. Trop. Med. Hyg. 17382-391. [DOI] [PubMed] [Google Scholar]
- 21.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen, R. N., L. S. Taylor, M. Tauschek, and R. M. Robins-Browne. 2006. Atypical enteropathogenic Escherichia coli infection and prolonged diarrhea in children. Emerg. Infect. Dis. 12597-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ochoa, T. J., F. Barletta, C. Contreras, and E. Mercado. 2008. New insights into the epidemiology of enteropathogenic Escherichia coli infection. Trans. R. Soc. Trop. Med. Hyg. 102852-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pacheco-Gil, L., T. J. Ochoa, L. Flores-Romo, H. L. DuPont, and T. Estrada-Garcia. 2006. Enteroinvasive Escherichia coli severe dysentery complicated by rotavirus gastroenteritis. J. Infect. 53e211-e213. [DOI] [PubMed] [Google Scholar]
- 25.Paton, A. W., and J. C. Paton. 1998. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J. Clin. Microbiol. 36598-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phantouamath, B., N. Sithivong, S. Insisiengmay, N. Higa, C. Toma, N. Nakasone, and M. Iwanaga. 2003. The incidence of Escherichia coli having pathogenic genes for diarrhea: a study in the People's Democratic Republic of Lao. Jpn. J. Infect. Dis. 56103-106. [PubMed] [Google Scholar]
- 27.Qadri, F., A. M. Svennerholm, A. S. Faruque, and R. B. Sack. 2005. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin. Microbiol. Rev. 18465-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao, M. R., R. Abu-Elyazeed, S. J. Savarino, A. B. Naficy, T. F. Wierzba, I. Abdel-Messih, H. Shaheen, R. W. Frenck, Jr., A. M. Svennerholm, and J. D. Clemens. 2003. High disease burden of diarrhea due to enterotoxigenic Escherichia coli among rural Egyptian infants and young children. J. Clin. Microbiol. 414862-4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robins-Browne, R. M., A.-M. Bordun, M. Tauschek, V. R. Bennett-Wood, J. Russell, F. Oppedisano, N. A. Lister, K. A. Bettelheim, C. K. Fairley, M. I. Sinclair, and M. E. Hellard. 2004. Escherichia coli and community acquired gastroenteritis, Melbourne, Australia. Emerg. Infect. Dis. 101797-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steiner, T. S., A. A. Lima, J. P. Nataro, and R. L. Guerrant. 1998. Enteroaggregative Escherichia coli produce intestinal inflammation and growth impairment and cause interleukin-8 release from intestinal epithelial cells. J. Infect. Dis. 17788-96. [DOI] [PubMed] [Google Scholar]
- 31.Trabulsi, L. R., R. Keller, and T. A. Tardelli Gomes. 2002. Typical and atypical enteropathogenic Escherichia coli. Emerg. Infect. Dis. 8508-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wani, S. A., A. Nabi, I. Fayaz, I. Ahmad, Y. Nishikawa, K. Qureshi, M. A. Khan, and J. Chowdhary. 2006. Investigation of diarrhoeic faecal samples for enterotoxigenic, Shiga toxin-producing and typical or atypical enteropathogenic Escherichia coli in Kashmir, India. FEMS Microbiol. Lett. 261238-244. [DOI] [PubMed] [Google Scholar]
- 33.Wenneras, C., and V. Erling. 2004. Prevalence of enterotoxigenic Escherichia coli-associated diarrhoea and carrier state in the developing world. J. Health Popul. Nutr. 22370-382. [PubMed] [Google Scholar]