Abstract
Infection with Toxoplasma gondii is often asymptomatic and, when acquired during pregnancy, may lead to connatal toxoplasmosis in the offspring. The newly introduced Vitros anti-Toxoplasma immunoglobulin G (IgG) and IgM assays, designed for the Vitros ECiQ immunodiagnostic system, a fully automated system based on chemiluminescence, were evaluated as a screening method for the serological detection of acute and chronic Toxoplasma infections in the sera of 719 pregnant women. The combination of the Vitros IgG and IgM assays demonstrated a sensitivity and a specificity of 100% for the successful detection of all acute T. gondii infections by comparison with the Sabin-Feldman dye test as the reference test. The Vitros IgG assay parameter revealed a sensitivity of 95.0%, a specificity of 100.0%, a positive predictive value of 100.0%, a negative predictive value of 86.2%, and an overall agreement of 96.2% by comparison with the dye test. Comparison of the Vitros Toxoplasma IgM assay with the immunosorbent agglutination assay yielded values of 77.1%, 99.0%, 97.7%, 88.5%, and 91.1%, respectively. Subsequent receiver operating characteristic curve analysis for the accurate detection of Toxoplasma IgM in acute (n = 90) and chronic (n = 461) infections demonstrated high sensitivity (92.2%) and specificity (81.6%). The combination of a Toxoplasma-specific IgG assay with specific IgM antibody detection has improved the diagnosis of T. gondii infection by decreasing follow-up testing. Nonetheless, positive Toxoplasma IgM test results during pregnancy necessitate confirmatory testing by a reference laboratory to ensure fast and, above all, accurate test results.
Infection with the protozoan Toxoplasma gondii is mostly asymptomatic for immunocompetent individuals (11). The incidence of gestational Toxoplasma infection in European countries ranges from 0.2 to 1.0% (7). Maternal infection during pregnancy may cause placental and fetal infections. Connatal toxoplasmosis is associated with a wide spectrum of clinical symptoms, such as retinochoroiditis, intracerebral calcifications, and hydrocephalus. These symptoms may be present at birth or may develop later in life, leading finally to blindness, psychomotor retardation, and hearing difficulties (13, 21).
Austria and France are the only countries that have implemented nationwide obligatory serological screening programs for the detection of gestational Toxoplasma infections. These systems provide systematic serological assessment early in pregnancy and periodic follow-up of pregnant women at risk (7). Serological diagnosis of infection with T. gondii is performed indirectly by enzyme immunoassays, an indirect immunofluorescence test, and, more precisely, by the Sabin-Feldman dye test (18). The dye test is considered the reference test for the detection of Toxoplasma infection (16).
Any serological test system has to meet several criteria of adequacy, such as high sensitivity and specificity, easy handling, and reproducible results under routine laboratory conditions. The present study investigated the newly introduced Vitros ECiQ Toxoplasma immunoglobulin G (IgG) and IgM assays (Ortho-Clinical Diagnostics, NJ) as a screening method for the diagnosis of acute and chronic Toxoplasma infections in the sera of pregnant women. The Vitros test results were compared with those of the Sabin-Feldman dye test and the immunosorbent agglutination assay (ISAGA) for the determination of anti-T. gondii-specific IgM (10). Diagnosis of maternal infection status was provided via routine serology by the toxoplasmosis reference laboratory at the Department of Pediatrics and Adolescent Medicine, Medical University of Vienna, Vienna, Austria. In addition, the technical precision of both the Vitros Toxoplasma IgG and IgM assays was evaluated by serial specimen measurements.
MATERIALS AND METHODS
Samples and patients.
Serum samples were collected from 719 healthy pregnant women according to the recommendations of the Austrian toxoplasmosis screening program and were submitted to the laboratory for routine analysis. The Sabin-Feldman dye test and the IgM ISAGA were performed within 24 to 48 h from the time when the samples were received. Sera were stored at −20°C. For the evaluation of the Vitros Toxoplasma IgG and IgM assays, aliquots of sera were thawed and retrospectively analyzed in this study. The results were compared with in-house serology using the dye test and with the determination of anti-T. gondii-specific IgM by the ISAGA (bioMérieux, France).
Vitros ECiQ system.
The automated Vitros ECiQ system is based on an immunometric technique.
(i) Vitros Toxoplasma IgG assay.
The IgG assay involves the reaction of anti-Toxoplasma IgG present in the sample with a Toxoplasma antigen applied to the reaction wells. After a wash step, a horseradish peroxidase (HRP)-conjugated antibody (mouse monoclonal anti-human IgG), which complexes with bound anti-Toxoplasma IgG, is added.
(ii) Vitros Toxoplasma IgM assay.
An antibody class capture technique is used for the IgM assay. This involves an automatic dilution of the sample and the simultaneous reaction of human IgM in the diluted sample with a biotinylated antibody (mouse monoclonal anti-human IgM). The immune complex is captured by streptavidin on the wells, and unbound materials are removed by washing. An HRP-labeled mouse monoclonal anti-Toxoplasma antibody [F(ab)2 fragment], which complexes with inactive Toxoplasma antigen (conjugate), is captured by anti-Toxoplasma-specific IgM bound to the wells.
(iii) Final step.
Unbound material is removed by washing. The bound HRP conjugate is measured by a luminescent reaction (20). A reagent containing luminogenic substrates (a luminol derivative and a peracid salt) and an electron transfer agent is added to the wells. The HRP in the bound conjugate catalyzes the oxidation of the luminol derivative, thus producing light. The electron transfer agent (a substituted acetanilide) increases the level of light produced and prolongs its emission. The light signals are read by the Vitros immunodiagnostic system. The amount of HRP conjugate bound is directly proportional to the concentration of anti-Toxoplasma IgG/IgM present in the sample.
Results are expressed in international units per milliliter in the IgG assay and as a ratio in the IgM assay. This ratio is calculated by dividing the signal for the test sample by the signal at the cutoff (cutoff value). Interpretation of Vitros results was based on the manufacturer's criteria, as follows: ≤3.99 IU/ml, negative for IgG antibodies; 4.00 to 7.99 IU/ml, borderline; ≥8.00 IU/ml, positive. For IgM antibodies the ratio was classified as follows: <0.80, negative; ≥0.80 to <1.20, borderline; ≥1.20, positive.
The Vitros test kits were used according to the manufacturer's protocol. Sera with IgG levels higher than >500 IU/ml were automatically diluted by the ECiQ system and reanalyzed.
Serological tests.
The Sabin-Feldman dye test and the IgM ISAGA were performed as previously described (4). The final determination of acute and chronic infection status was performed according to the criteria of Lebech et al. (8). For a definite diagnosis, the patients were further investigated by subsequent serum sample analysis (by the Sabin-Feldman dye test and IgM ISAGA) and/or IgG avidity. The IgM ISAGA has been reported to be more sensitive than a double sandwich enzyme-linked immunosorbent assay (5, 10). The IgM ISAGA is suitable for the diagnosis and screening of acute Toxoplasma infection in pregnant women, as well as for the detection of IgM in the blood samples of children to identify connatal infection (2, 12).
Technical performance.
The technical precision of the Vitros system was evaluated by intra- and interassay testing of sera. Intra-assay precision was determined by processing eight consecutive runs of two sera for IgG and six consecutive runs of four sera for IgM antibodies. Interassay precision was determined by testing negative and positive controls, each in triplicate, over a period of 15 days (Liquichek ToRCH Plus positive control for Toxoplasma IgG and Liquichek ToRCH Plus IgM control positive and negative for Toxoplasma IgM [both from Bio-Rad]).
Statistics.
Calculations were performed using Excel 2007 (Microsoft Inc.), SPSS 16.0 (SPSS Science), and MedCalc 9.0 (MedCalc Sofware, Belgium) for receiver operating characteristic curve (ROC) analysis. Spearman's rank correlation coefficient (r) and Cohen's kappa were used to determine the statistical agreement between the comparison assays (22). The coefficient of variation was used to determine intra- and interassay precision.
RESULTS
Performance of Vitros IgG and IgM assays.
The analysis of 719 serum samples by the Vitros IgG assay resulted in a sensitivity of 95.0%, a specificity of 100.0%, a positive predictive value of 100.0%, a negative predictive value of 86.2%, and an overall agreement of 96.2% (r = 0.905; kappa = 0.900) by comparison with the Sabin-Feldman dye test. Table 1 shows the comparison of the IgM ISAGA with the Vitros IgM test. Of the 719 serum samples tested, 259 (36.0%) were positive by the IgM ISAGA. Of the latter sera, 172 (23.9%) were positive, 36 (5.0%) borderline, and 51 (7.1%) negative by the Vitros IgM assay. Of the 407 (56.6%) serum samples that were negative by the IgM ISAGA, 391 (54.4%) were negative, 12 (1.7%) borderline, and 4 (0.6%) positive by the Vitros IgM assay. The results of the IgM ISAGA were equivocal for 53 (7.4%) of the serum samples. The results of the Vitros IgM assay were equivocal for 61 (8.5%) serum samples. Thirteen (1.8%) serum samples gave equivocal results in both tests. Sera with equivocal results were excluded from final analysis. Compared with the IgM ISAGA, the Vitros IgM assay had a sensitivity of 77.1%, a specificity of 99.0%, a positive predictive value of 97.7%, a negative predictive value of 88.5%, and an overall agreement of 91.1% (r = 0.768; kappa = 0.626).
TABLE 1.
Comparison of the IgM ISAGA with the Vitros Toxoplasma-specific IgM assay
| IgM ISAGA result | No. (%) with the following Vitros IgM test result:
|
Total no. (%) | ||
|---|---|---|---|---|
| Negative | Borderline | Positive | ||
| Negative | 391 (54.4) | 12 (1.7) | 4 (0.6) | 407 (56.6) |
| Borderline | 27 (3.8) | 13 (1.8) | 13 (1.8) | 53 (7.4) |
| Positive | 51 (7.1) | 36 (5.0) | 172 (23.9) | 259 (36.0) |
| Total | 469 (65.2) | 61 (8.5) | 189 (26.3) | 719 (100.0) |
The Sabin-Feldman dye test is capable of detecting Toxoplasma-specific immunoglobulins of all classes (IgM, IgA, and IgG antibodies). Therefore, dye test results should not be compared with the results of isolated IgG or IgM assays but only with combined IgG and IgM results (14). Table 2 shows a comparison of the results of the Sabin-Feldman dye test and the combined IgG and IgM results obtained by the Vitros system. All 34 samples found to be negative within the Vitros system represented chronic T. gondii infections (positive Sabin-Feldman dye test results). Based on the dye test as a reference standard, the Vitros system demonstrated 100.0% sensitivity, 100.0% specificity, and positive and negative predictive values of 100.0% for the detection of acute Toxoplasma infections.
TABLE 2.
Comparison of the Sabin-Feldman dye test with the combination of the Vitros anti-Toxoplasma-specific IgG and IgM assays
| Sabin-Feldman dye test result | No. (%) with the following Vitros IgG and IgM assay result:
|
Total | |
|---|---|---|---|
| Negative | Positive | ||
| Negative | 168 (23.4) | 0 | 168 (23.4) |
| Positive | 34a (4.7) | 517 (71.9) | 551 (76.6) |
| Total | 202 (28.1) | 517 (71.9) | 719 (100.0) |
All 34 samples that were negative by the Vitros Toxoplasma IgG and/or IgM assay but positive by the Sabin-Feldman dye test represented chronic infections.
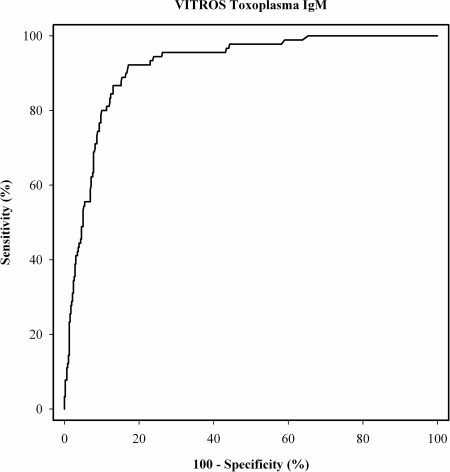
ROC curve analysis of Vitros IgM assay values for potential discrimination of acute (n = 90) and chronic (n = 461) infections was performed (Fig. 1). Table 3 displays the sensitivity and specificity for the cutoff value of ≥1.2 (92.2% and 77.0%, respectively; cutoff value according to the manufacturer's recommendation), and for the optimal cutoff value of ≥1.47 (92.2% and 81.6%) calculated in this sample setup. The calculated positive and negative predictive values for the ≥1.2 cutoff value were 43.9% and 98.0%, respectively, and those for the ≥1.47 cutoff value were 50.9% and 98.0%, respectively. An increase in the cutoff value resulted in enhanced specificity for the discrimination of acute and chronic T. gondii infections in this study.
FIG. 1.
ROC curve analysis of the Vitros IgM assay values for the discrimination of acute (n = 90) and chronic (n = 461) Toxoplasma infections.
TABLE 3.
Criterion values and characteristics of the ROC curve analysisa
| Criterion value | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | Likelihood ratio
|
|
|---|---|---|---|---|
| Positive | Negative | |||
| ≥1.2a | 92.2 (84.6-96.8) | 77.0 (73.1-81.0) | 4.05 | 0.10 |
| ≥1.47b | 92.2 (84.6-96.8) | 81.6 (79.1-86.2) | 5.38 | 0.09 |
Cutoff value according to the manufacturer's recommendation.
Optimal cutoff value calculated in this study.
Technical precision.
The intra- and interassay coefficients of variation for the Vitros IgG and IgM assays for the positive controls and sera were below 9.6% and 4.6% for the IgG assay and below 1.9% and 6.6% for the IgM assay, respectively. The serial measurements of the Liquichek ToRCH Plus IgM negative control resulted in a maximum IgM ratio of 0.12 (a ratio of <0.80 indicates a negative test result).
DISCUSSION
The Vitros ECiQ immunodiagnostic system is a simple-to-use, fully automated laboratory test system. This is the first study to evaluate the Vitros system in comparison with the Sabin-Feldman dye test. Analysis of anti-Toxoplasma-specific IgG and IgM antibodies with the Vitros system demonstrated a good correlation with the results obtained by the dye test. The Sabin-Feldman dye test is considered the “gold standard” and detects all classes of Toxoplasma-specific immunoglobulins, including IgG, IgM, and IgA (15). However, the Sabin-Feldman dye test is expensive, time-consuming and difficult to standardize. Due to its high sensitivity, the dye test still serves as the recommended reference test for the confirmation of acute infection in pregnant women and for the validation of commercial kits (16).
When the Vitros IgG assay results were compared with those of the Sabin-Feldman dye test, high sensitivity and specificity, 95% and 100%, respectively, were achieved. A comparison of the Vitros IgM assay to the IgM ISAGA yielded a high specificity (99%) but a sensitivity of 77%, indicating that the IgM ISAGA is more sensitive than the Vitros IgM assay. It is well known that the accurate detection of anti-Toxoplasma-specific IgM antibodies by an automated test system is a common problem in the diagnosis of T. gondii infection (17).
The combination of anti-Toxoplasma-specific IgG assays with assays for specific IgM antibodies has improved the diagnosis of T. gondii infection (17). The good correlation between the dye test and the combination of the Vitros IgG and IgM assays indicates that the Vitros system is valuable for the detection of recently acquired T. gondii infection. In acute infection there is an excellent correlation between the combination of the Vitros IgG and IgM assays, on the one hand, and the Sabin-Feldman dye test, on the other, expressed by a sensitivity and specificity of 100%. Consequently, the Vitros system can be used as a screening test for seronegative women.
Subsequent ROC curve analysis for the detection of Toxoplasma IgM in acute and chronic infections demonstrated high sensitivity (92.2%) and specificity (82.9%). However, chronic infection with T. gondii can lead to a long persistence of IgM antibodies (1, 3). IgM antibodies are detectable for a median of a duration of 12.8 months (interquartile range, 6.9 to 24.9 months); moreover, in a substantial minority of women, IgM-positive test results can persist beyond 2 years (6). Since IgM can persist years after infection, Toxoplasma IgM positivity alone is unable to distinguish an acute from a chronic infection (19).
Diagnosis of the infection status is achieved by interpretation of the immunoglobulin concentrations in follow-up serology. Decreasing or stable IgG levels (depending on the gestational age and test interval) denote chronic infection in immunocompetent pregnant women, while a significant IgG increase within two samples proves acute infection (9). Additional tests (e.g., IgG avidity, differential agglutination [AC/HS]) and collection of serial serum samples at an interval of 2 to 4 weeks are mandatory for the detection of acute infection with T. gondii. It is recommended that sera with positive IgM test results obtained at nonreference laboratories be sent to a Toxoplasma reference laboratory. The serological diagnosis must be verified by confirmatory testing before treatment is considered.
The Vitros ECiQ system is a practicable, easy-to-use test system that produces screening results in <40 min (with automated dilution of samples in <2 h). Nonetheless, our results revealed 34 (6.2%) false-negative results by the Vitros system, even though the Sabin-Feldman dye test and IgM ISAGA demonstrated a chronic infection status of these study patients. When the serological findings of the Vitros system are negative, retesting in a subsequent pregnancy is recommended in order to rule out an acute Toxoplasma infection.
Acknowledgments
We are grateful for the work being done by Birgit Panzenböck, Andrea Konstantin, and Sabina Kropfreiter.
Footnotes
Published ahead of print on 5 November 2008.
REFERENCES
- 1.Bobić, B., D. Sibalić, and O. Djurković-Djaković. 1991. High levels of IgM antibodies specific for Toxoplasma gondii in pregnancy 12 years after primary toxoplasma infection. Case report. Gynecol. Obstet. Investig. 31182-184. [DOI] [PubMed] [Google Scholar]
- 2.Carlier, Y., D. Bout, J. P. Dessaint, A. Capron, F. Van Knapen, E. J. Ruitenberg, R. Bergquist, and G. Huldt. 1980. Evaluation of the enzyme-linked immunosorbent assay (ELISA) and other serological tests for the diagnosis of toxoplasmosis. Bull. W. H. O. 5899-105. [PMC free article] [PubMed] [Google Scholar]
- 3.Del Bono, V., A. Canessa, P. Bruzzi, M. A. Fiorelli, and A. Terragna. 1989. Significance of specific immunoglobulin M in the chronological diagnosis of 38 cases of toxoplasmic lymphadenopathy. J. Clin. Microbiol. 272133-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desmonts, G., Y. Naot, and J. S. Remington. 1981. Immunoglobulin M-immunosorbent agglutination assay for diagnosis of infectious diseases: diagnosis of acute congenital and acquired Toxoplasma infections. J. Clin. Microbiol. 14486-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duffy, K. T., P. J. Wharton, J. D. Johnson, L. New, and R. E. Holliman. 1989. Assessment of immunoglobulin-M immunosorbent agglutination assay (ISAGA) for detecting toxoplasma specific IgM. J. Clin. Pathol. 421291-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gras, L., R. E. Gilbert, M. Wallon, F. Peyron, and M. Cortina-Borja. 2004. Duration of the IgM response in women acquiring Toxoplasma gondii during pregnancy: implications for clinical practice and cross-sectional incidence studies. Epidemiol. Infect. 132541-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayde, M., H. R. Salzer, G. Gittler, H. Aspock, and A. Pollak. 1995. Microparticle enzyme immunoassay (MEIA) for toxoplasma specific immunoglobulin G in comparison to the Sabin-Feldman dye test. A pilot study. Wien Klin. Wochenschr. 107133-136. [PubMed] [Google Scholar]
- 8.Lebech, M., D. H. Joynson, H. M. Seitz, P. Thulliez, R. E. Gilbert, G. N. Dutton, B. Ovlisen, E. Petersen, et al. 1996. Classification system and case definitions of Toxoplasma gondii infection in immunocompetent pregnant women and their congenitally infected offspring. Eur. J. Clin. Microbiol. Infect. Dis. 15799-805. [DOI] [PubMed] [Google Scholar]
- 9.Liesenfeld, O., C. Press, J. G. Montoya, R. Gill, J. L. Isaac-Renton, K. Hedman, and J. S. Remington. 1997. False-positive results in immunoglobulin M (IgM) toxoplasma antibody tests and importance of confirmatory testing: the Platelia Toxo IgM test. J. Clin. Microbiol. 35174-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meek, B., T. van Gool, H. Gilis, and R. Peek. 2001. Dissecting the IgM antibody response during the acute and latent phase of toxoplasmosis. Diagn. Microbiol. Infect. Dis. 41131-137. [DOI] [PubMed] [Google Scholar]
- 11.Montoya, J. G., and O. Liesenfeld. 2004. Toxoplasmosis. Lancet 3631965-1976. [DOI] [PubMed] [Google Scholar]
- 12.Naot, Y., and J. S. Remington. 1980. An enzyme-linked immunosorbent assay for detection of IgM antibodies to Toxoplasma gondii: use for diagnosis of acute acquired toxoplasmosis. J. Infect. Dis. 142757-766. [DOI] [PubMed] [Google Scholar]
- 13.Petersen, E. 2007. Toxoplasmosis. Semin. Fetal Neonatal Med. 12214-223. [DOI] [PubMed] [Google Scholar]
- 14.Petersen, E., M. V. Borobio, E. Guy, O. Liesenfeld, V. Meroni, A. Naessens, E. Spranzi, and P. Thulliez. 2005. European multicenter study of the LIAISON automated diagnostic system for determination of Toxoplasma gondii-specific immunoglobulin G (IgG) and IgM and the IgG avidity index. J. Clin. Microbiol. 431570-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Press, C., J. G. Montoya, and J. S. Remington. 2005. Use of a single serum sample for diagnosis of acute toxoplasmosis in pregnant women and other adults. J. Clin. Microbiol. 433481-3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reiter-Owona, I., E. Petersen, D. Joynson, H. Aspock, M. L. Darde, R. Disko, O. Dreazen, H. Dumon, R. Grillo, U. Gross, M. Hayde, R. Holliman, D. O. Ho-Yen, K. Janitschke, P. A. Jenum, K. Naser, M. Olszewski, P. Thulliez, and H. M. Seitz. 1999. The past and present role of the Sabin-Feldman dye test in the serodiagnosis of toxoplasmosis. Bull. W. H. O. 77929-935. [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts, A., K. Hedman, V. Luyasu, J. Zufferey, M. H. Bessieres, R. M. Blatz, E. Candolfi, A. Decoster, G. Enders, U. Gross, E. Guy, M. Hayde, D. Ho-Yen, J. Johnson, B. Lecolier, A. Naessens, H. Pelloux, P. Thulliez, and E. Petersen. 2001. Multicenter evaluation of strategies for serodiagnosis of primary infection with Toxoplasma gondii. Eur. J. Clin. Microbiol. Infect. Dis. 20467-474. [DOI] [PubMed] [Google Scholar]
- 18.Sabin, A. B., and H. A. Feldman. 1948. Dyes as microchemical indicators of a new immunity phenomenon affecting a protozoan parasite (Toxoplasma). Science 108660-663. [DOI] [PubMed] [Google Scholar]
- 19.Sensini, A. 2006. Toxoplasma gondii infection in pregnancy: opportunities and pitfalls of serological diagnosis. Clin. Microbiol. Infect. 12504-512. [DOI] [PubMed] [Google Scholar]
- 20.Summers, M., T. Booth, T. Brockas, H. Edgar, J. Edwards, C. Nunnerley, S. Peterson, A. Friedman, T. Kissel, and S. Groulx. 1995. Luminogenic reagent using 3-chloro 4-hydroxy acetanilide to enhance peroxidase/luminol chemiluminescence. Clin. Chem. 4173. (Abstract.)7813084 [Google Scholar]
- 21.Wilson, C. B., J. S. Remington, S. Stagno, and D. W. Reynolds. 1980. Development of adverse sequelae in children born with subclinical congenital Toxoplasma infection. Pediatrics 66767-774. [PubMed] [Google Scholar]
- 22.Zar, J. H. 1999. Biostatistical analysis, 4th ed. Prentice Hall, Upper Saddle River, NJ.