Abstract
Hepatitis B virus (HBV) expresses two structural forms of the nucleoprotein, the intracellular nucleocapsid (hepatitis core antigen [HBcAg]) and the secreted nonparticulate form (hepatitis e antigen [HBeAg]). The aim of this study was to evaluate the ability of HBcAg- and HBeAg-specific genetic immunogens to induce HBc/HBeAg-specific CD4+/CD8+ T-cell immune responses and the potential to induce liver injury in HBV-transgenic (Tg) mice. Both the HBcAg- and HBeAg-specific plasmids primed comparable immune responses. Both CD4+ and CD8+ T cells were important for priming/effector functions of HBc/HBeAg-specific cytotoxic T-lymphocyte (CTL) responses. However, a unique two-step immunization protocol was necessary to elicit maximal CTL priming. Genetic vaccination did not prime CTLs in HBe- or HBc/HBeAg-dbl-Tg mice but elicited a weak CTL response in HBcAg-Tg mice. When HBc/HBeAg-specific CTLs were adoptively transferred into HBc-, HBe-, and HBc/HBeAg-dbl-Tg mice, the durations of the liver injury and inflammation were significantly greater in HBeAg-Tg recipient mice than in HBcAg-Tg mice. Importantly, liver injury in HBc/HBeAg-dbl-Tg mice was similar to the injury observed in HBeAg-Tg mice. Loss of HBeAg synthesis commonly occurs during chronic HBV infection; however, the mechanism of selection of HBeAg-negative variants is unknown. The finding that hepatocytes expressing wild-type HBV (containing both HBcAg and HBeAg) are more susceptible to CTL-mediated clearance than hepatocytes expressing only HBcAg suggest that the HBeAg-negative variant may have a selective advantage over wild-type HBV within the livers of patients with chronic infection during an immune response and may represent a CTL escape mutant.
Hepatitis B virus (HBV) is an enveloped virus with a partially double-stranded circular DNA genome of approximately 3.2 kb encoding structural and nonstructural proteins. Control and clearance of acute and chronic HBV infections are thought to be dependent on multispecific T-cell responses directed to several HBV-encoded antigens (6, 31, 38, 42, 43). HBV expresses two forms of the nucleoprotein: the 21-kDa intracellular nucleocapsid (hepatitis core antigen [HBcAg]), which self-assembles into particles and encapsidates the viral genome and polymerase, and the secreted nonparticulate form (hepatitis e antigen [HBeAg]). HBeAg and HBcAg are translated from two distinct RNA species that have different 5′ initiation sites (19). The HBeAg or precore mRNA encodes a hydrophobic signal sequence that directs the HBeAg to the endoplasmic reticulum, where it undergoes N- and C-terminal cleavage within the secretory pathway and is secreted as an 18-kDa monomeric protein (32, 41, 44, 56). Because of the structural differences between the HBcAg and HBeAg (referred to below as the HBc/HBeAgs), they are distinctly recognized by antibodies (24), but due to extensive amino acid homology, they are highly cross-reactive at the CD4+ and CD8+ T-cell levels (6, 28, 37, 55). In contrast to the well-established structural and replicative functions of HBcAg, the function of the secreted HBeAg in the viral life cycle is less clear because it is not required for assembly, infection, or replication (10, 11, 46). However, studies in a number of murine transgenic (Tg) systems indicate that secreted HBeAg functions as an immunoregulatory protein that downregulates the immune response to HBcAg via a variety of mechanisms, including deletional, nondeletional, central, and peripheral immune tolerance (12, 13, 33-36).
The cytotoxic T-lymphocyte (CTL) response is believed to be involved in both viral clearance and liver disease during HBV infection (14). CTL responses directed against HBcAg have been suggested to be of major importance in the clearance of HBV infections in humans (6). Several reports have indicated that both HBcAg and HBeAg expressed as endogenous proteins can prime and be the targets of CTL effector cells (27, 28, 52, 55). The ability of the HBeAg, as well as the intracellular HBcAg, to prime and be recognized as a target of CTL effector cells indicates that intracellular HBeAg and/or its precursors are processed and presented in the context of major histocompatibility complex (MHC) class I molecules for recognition by CTL effector cells. Furthermore, previous studies (27, 28, 52, 55) and the experiments reported here indicate that the HBc/HBeAgs appear to be indistinguishable in terms of priming CTLs and CTL target recognition in vitro.
In the current study the comparative abilities of HBc/HBeAg-based genetic vaccines and/or HBc/HBeAg-expressing tumor cell lines to induce CTL responses in wild-type and HBc/HBeAg-Tg mice and to induce liver injury were examined. These studies indicated that a unique two-step immunization protocol was necessary to elicit maximal CTL priming in vivo and that endogenously expressed HBc/HBeAgs can function as tolerogens at the CTL level. Most importantly, although the HBc/HBeAgs were indistinguishable in terms of priming CTLs and as targets for CTL recognition in vitro, CTL recognition of the HBc/HBeAgs expressed in hepatocytes in vivo was significantly different and resulted in different phenotypes of liver injury.
MATERIALS AND METHODS
Plasmid DNA, recombinant proteins, and synthetic peptides.
An HBcAg gene fragment (552 nucleotides) of the ayw subtype was amplified and cloned into the eukaryotic expression vector pVAX1 (Invitrogen, San Diego, CA) as previously described (29). The HBcAg expression plasmid was designated HBcAg-pVAX1. An HBeAg gene fragment (639 nucleotides) was amplified by PCR from tail DNA extracted from an HBeAg-Tg mouse (23). The amplified gene fragment was ligated into a HindIII- and ApaI-digested pVAX1 vector. The HBeAg expression plasmid was designated HBeAg-pVAX1. Sequencing of the HBcAg-pVAX1 and HBeAg-pVAX1 expression plasmids showed that the inserted genes had the correct reading frame. Plasmid DNA was grown and purified as described previously (18). The purified plasmid DNA was dissolved and diluted in sterile phosphate-buffered saline (PBS) to a concentration of 1 mg/ml. Recombinant HBcAg (rHBcAg) of the ayw subtype was produced in Escherichia coli and purified as described previously (48). Yeast-derived rHBcAg of the ayw subtype was purchased from Meridian Life (Saco, ME). An rHBeAg with a sequence corresponding to serum-derived HBeAg encompassing the 10 precore amino acids that prevent particle assembly and that is recognized efficiently by HBeAg-specific monoclonal antibodies but displays little HBc antigenicity was produced in E. coli as described previously (49). The 8-mer peptide (sequence MGLKFRQL, designated HBcAg93-100) corresponds to an HBcAg MHC class I-restricted (H-2Kb) epitope in mice (27). The HBe/HBcAg-derived 21-mer synthetic peptide representing two T-helper (Th) cell recognition sites was used and designated, by amino acid position from the N terminus of HBcAg ayw (120 to 140), p120-140 (VSFGVWIRTPPAYRPPNAPIL) (37). The peptides were synthesized by Michael Levi (Tripep AB, Stockholm, Sweden) using an automated peptide synthesizer as described previously (45).
In vitro transcription and translation assay.
To ensure that the HBcAg and HBeAg genes were intact and could be translated, an in vitro transcription and translation assay using the prokaryotic T7 coupled reticulocyte lysate system (TNT; Promega, Madison, WI) was performed as previously described (18).
Mice.
Inbred C57BL/6 (H-2b), C57BL/10 (H-2b), B-cell−/−, CD4−/− (H-2b), CD8−/− (H-2b), and nude (Foxn1nu/Foxn1nu) mice were obtained from The Jackson Laboratory. C57BL/10 Tg mice with intrahepatic expression of the HBcAg protein (HBc-Tg, 0.2 to 2 μg/mg liver protein) (13, 22) or the HBeAg protein (HBe-Tg, 4 to 10 μg/ml serum) (13, 23) were obtained from the breeding colony of the Vaccine Research Institute of San Diego. Female mice, at least 6 to 12 weeks old, were used in the experiments described here. All animal care was performed according to National Institutes of Health standards (38a).
Cell lines.
The Rauscher virus-induced T-cell lymphoma (RBL-5) (20) cell line (H-2b) was maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (GIBCO-BRL, Gaithersburg, MD). RBL-5 cells with stable expression of HBcAg (RBL-5/C) and HBeAg (RBL-5/E) (27, 28) (provided by Francis V. Chisari, The Scripps Research Institute, La Jolla, CA) were maintained in 1,000 μg Geneticin (G418)/ml in complete RPMI 1640 medium. RBL-5/C predominantly expressed HBcAg intracellularly at 1 μg HBcAg per 10 × 106 RBL-5/C tumor cells as determined by enzyme-linked immunosorbent assay (ELISA) performed on cell lysates. RBL-5/E expressed HBeAg as a secreted protein in the supernatant (11.3 ng HBeAg per ml of RBL-5/E cell culture supernatant) as determined by ELISA. The parental RBL-5 tumor cell line was negative for HBc/HBeAg in both cell lysates and cell culture supernatants. All RBL-5 cell lines tested negative in the infectious-microbe PCR amplification test, using the profile III panel, performed at the Research Animal Diagnostic Laboratory, University of Missouri.
RMA-S cells (kindly provided by Klas Kärre, Karolinska Institutet, Sweden) (26) were maintained in RPMI 1640 medium supplemented with 5% FBS, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. All cells were grown in a 5% CO2 humidified 37°C incubator.
Analysis of expression of HBcAg and HBeAg protein from transfected RBL-5 cell lines.
Detection of intracellular HBcAg or secreted HBeAg expressed from the RBL-5 cell lines was analyzed by ELISA. In brief, RBL-5 cells were counted, and equal amounts of cells were lysed in distilled water using the freeze-thaw method. Cells were rapidly frozen in liquid nitrogen and subsequently thawed in a 37°C water bath. The procedure was repeated three times prior to centrifugation (12,000 × g for 5 min) of cell lysates. After centrifugation, supernatants containing cytosolic proteins were collected and frozen at −80°C until use. Supernatants from RBL-5 cells were collected 3 days after cell seeding. HBcAg was measured in either RBL-5 cell lysates or cell culture supernatants by using a previously described modified commercial anti-HBc assay (ETI-AB-COREK Plus; Diasorin, Stillwater, MN) (13), and rHBcAg was used as a standard. HBeAg was measured in either RBL-5 cell lysates or cell culture supernatants by a commercial ELISA (ETI-EBK Plus kit; DiaSorin, Stillwater, MN), and rHBeAg was used as a standard. Nontransfected RBL-5 cells were used as a negative control in each assay.
Analysis of surface markers on transfected RBL-5 cell lines by flow cytometry.
RBL-5 cell lines was analyzed for expression of Thy1.2 (CD90.2), MHC class I (clone 28-14-8), MHC class II (clone M5/114.15.2), CD4, and CD8 on the cell surface by flow cytometry. In brief, cells were washed and resuspended in PBS-3% fetal calf serum (fluorescence-activated cell sorter buffer) and incubated with anti-mouse CD16/32 antibodies (to block Fc binding). Cells were then washed and incubated with fluorescein isothiocyanate-conjugated anti-mouse CD90.2, phycoerythrin-conjugated anti-mouse MHC class I and II antibody, fluorescein isothiocyanate-conjugated anti-mouse CD8 antibody, and Cy-Chrome anti-mouse CD4 antibody. After being washed, the cells were diluted in fluorescence-activated cell sorter buffer containing 4′,6-diamino-2-phenylindol (DAPI). A total of approximately 100,000 events from each sample were counted on an LSR flow cytometer (Becton Dickinson Biosciences, San Jose, CA), and dead cells (DAPI-positive cells) were excluded in the analysis. All antibodies were purchased from eBioscience (San Diego, CA). The RBL-5/C, RBL-5/E, and parental RBL-5 cell lines were positive for the T-cell marker Thy1.2 and for MHC class I molecules. All cell lines were negative for expression of the surface markers MHC class II, CD4, and CD8 (data not shown).
Immunization protocols.
Groups (4 to 10 mice/group) of female mice (H-2b), at least 6 to 12 weeks old, were immunized by needle injections with 100 μg plasmid DNA encoding HBV proteins. Plasmid DNA in PBS was given intramuscularly (i.m.) in the tibialis anterior (TA) muscles. Where indicated in the text, mice were injected i.m. with 50 μl/TA of 0.01 mM Cardiotoxin (Latoxan, Rosans, France) in 0.9% sterile physiological sodium chloride (NaCl) 5 days prior to DNA immunization. The mice were boosted at 4-week intervals.
Recombinant protein immunization was performed by intraperitoneal (i.p.) immunization with 10 μg protein mixed 1:1 in incomplete Freund adjuvant. The mice were boosted i.p. at week 4 using 1 μg protein mixed 1:1 in incomplete Freund adjuvant. RBL-5 tumor cell immunization was performed by subcutaneous (s.c.) inoculation of 5 × 106 tumor cells (diluted in 200 μl PBS) in the right flank. A two-step protocol of first one or two i.m. immunizations using 100 μg plasmid DNA (with 4-week intervals between DNA immunizations) and then an s.c. boost using 5 × 106 RBL-5 tumor cells in the right flank was used when indicated below.
ELISA for detection of murine anti-HBc or anti-HBe antibodies.
Sera were collected by retro-orbital bleeding of isofluorane-anesthetized mice. Antibodies were detected by an indirect solid-phase ELISA with rHBcAg or rHBeAg as the solid-phase ligands as described previously (13, 37).
Detection of HBcAg- and HBeAg-specific lytic CTLs.
Spleen cells from C57BL/10 mice immunized with DNA, tumor cells, or DNA plus tumor cells were resuspended in complete RPMI 1640 medium supplemented with 10% FBS, 2 mM l-glutamine, 10 mM HEPES, 100 U/ml penicillin, 100 μg/ml streptomycin, 1 mM nonessential amino acids, 50 μM β-mercaptoethanol, and 1 mM sodium pyruvate. Detection of HBcAg- and HBeAg-specific lytic CTLs was analyzed by an in vitro stimulation followed by a standard 51Cr release assay performed as described by Lazdina et al. (29), except that a total of 25 μl supernatant was harvested after the 51Cr release assay and the radioactivity was measured using a gamma counter (Packard Top Count NXT microplate scintillation and luminescence counter).
Results were expressed according to the following formula: percent specific lysis = (experimental release − spontaneous release)/(maximum release − spontaneous release). Experimental release is the mean counts per minute released by the target cells in the presence of effector cells. Maximum release is the radioactivity released after lysis of target cells with 10% Triton X-100. Spontaneous release is the leakage of radioactivity into the medium of target cells.
In vivo challenge with the HBcAg- or HBeAg-expressing RBL-5 lymphoma.
In vivo challenge of naïve or immunized mice with HBcAg- and HBeAg-expressing RBL-5 lymphoma cell lines (designated RBL-5/C and RBL-5/E) was performed according to a previously described method (17). In brief, groups of C57BL/10 wild-type, Tg, knockout, or nude Foxn1nu/Foxn1nu mice were left untreated or immunized using different immunogens at weeks 0 and 4 as described in “Immunization protocols” above. At 2 weeks after the last immunization, a total of 5 × 106 RBL-5/C or RBL-5/E tumor cells were injected s.c. in the right flank. The kinetics of tumor growth was determined by measuring the tumor size using a sliding caliper through the skin at days 0 to 30. Tumors were measured every 2 to 3 days. The kinetics of tumor development in two groups of mice were compared using the area under the curve. The mean tumor sizes were compared using the analysis of variance (ANOVA) test. Mice were sacrificed at the end of the experiment.
Determination of CD4+ cytokine production in response to HBcAg and HBeAg.
Spleen cells from either unprimed or primed wild-type, Tg, or knockout mice were cultured (5 × 106 cells/ml) with various concentrations of a series of antigens. Culture supernatants were harvested at 48 h for interleukun-2 (IL-2) determination and at 96 h for gamma interferon (IFN-γ) determination. Cell culture supernatants from RBL-5 tumor cell lines were tested for the presence of IL-2, IL-4, IL-10, and IFN-γ cytokines. Cytokines were measured using commercial ELISA kits according to the manufacturer's protocol (eBiosciences).
Adoptive transfer experiments with primed HBcAg- or HBeAg-specific CTLs.
Wild-type C57BL/10 mice were immunized once or twice with 100 μg HBcAg- or HBeAg-pVAX1 plasmid DNA and boosted with 5 × 106 RBL-5/C or RBL-5/E tumor cells. At 2 to 4 weeks after the booster tumor cell injection, spleens were harvested and stimulated in vitro with the HBcAg93-100 MHC class I peptide. Five days after in vitro stimulation, activated CTLs were washed, counted, suspended in PBS, and injected intravenously (i.v.) into Tg and non-Tg recipients in various numbers (2 × 106 to 20 × 106 per mouse in 200 μl). Liver injury was monitored biochemically by measuring serum alanine aminotransferase (sALT) activity. sALT levels were measured at 0, 12, 24, 48, 72, 96, 120, and 168 h and 14 days after adoptive transfer. Results were expressed as mean sALT activity (U/liter) ± standard error (SE). Values of below 100 U/liter were considered normal ALT levels.
In vivo depletion of CD4+ T cells.
CD4+ T cells were depleted in vivo by i.p. injection of purified anti-CD4 (clone GK1.5, rat immunoglobulin G2b [IgG2b]) or a nonspecific rat IgG2b isotype control (clone LTF-2). A total of 0.2 mg of depletion antibody per mouse was injected every 3 to 4 days, starting 2 weeks prior to immunization (−2 weeks) and continuing throughout the whole experiment until the mice were sacrificed (+4 weeks). Flow cytometric analyses of peripheral blood mononuclear cell populations and splenic cell populations at week 0 demonstrated that more than 99% of the CD4+ T cells were depleted. GK1.5 and LTF-2 were purchased from BioXCell (West Lebanon, NH).
Biochemical analysis.
Serum samples were tested for ALT activity using the DTSC II Module (Ortho-Clinical Diagnostics) and the Kodak Ektachem DT60 Analyzer with ALT DT slides according to the manufacturer's protocol (Ortho-Clinical Diagnostics).
Gene array analysis of HBcAg-Tg and HBeAg-Tg livers at day 0 and day 3 after CTL transfer.
Mouse whole-genome array analysis was performed to compare mRNA profiles in HBcAg-Tg and HBeAg-Tg mice at day 0 and day 3 after CTL transfer. Livers were harvested from representative mice, and total RNA was isolated from each liver using the TRIzol extraction method. The RNA was then used to generate Cy3- and Cy5-labeled cRNA probes which were then applied to Whole Mouse Genome Oligo microarrays to analyze gene expression. Gene expression intensities were quantified using the Agilent Feature Extraction software (Agilent Technologies, Palo Alto, CA), and Rosetta Biosoftware (Kirkland, WA) was used to determine differential expression. Of the genes differentially expressed in HBcAg-Tg and HBeAg-Tg livers at day 0 and day 3 after CTL transfer, several were readily identified based on their association with inflammation and apoptosis. Additionally, several antistress and antiapoptotic genes were identified.
Statistical analysis.
Statistical comparisons were performed using the Statview 5.0 and Excel:mac software packages for Macintosh. Kinetics of tumor growth in groups of mice were compared using the area under the curve, and the values were compared using ANOVA (Statview).
RESULTS
Characterization of the HBcAg and HBeAg gene expression vectors.
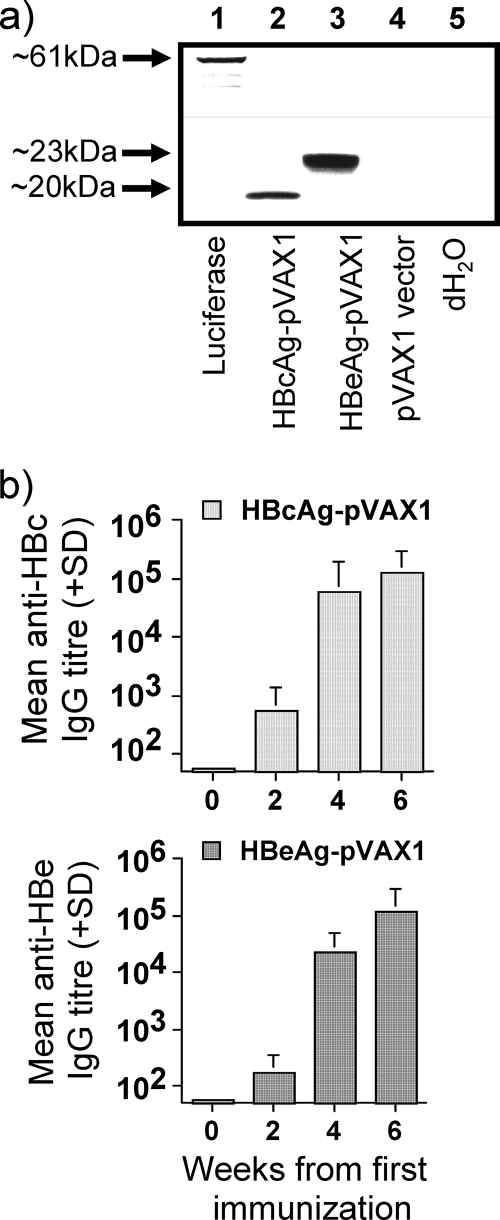
The expression constructs HBcAg- and HBeAg-pVAX1 were analyzed by an in vitro transcription and translation assay to confirm that the proteins were expressed from the respective plasmids (Fig. 1a). To characterize the immunogenicity of the HBcAg and HBeAg genes, groups of C57BL/10 mice were immunized twice i.m. using 100 μg plasmid DNA, followed by monitoring of the anti-HBc and anti-HBe IgG responses. Escalating-dose immunization studies comparing the HBc/HBeAg proteins have revealed that HBcAg is approximately 1,000-fold more efficient in terms of antibody production (37), yet HBc/HBeAg-pVAX1-immunized mice developed similar IgG antibody titers (>105) after two i.m. immunizations (Fig. 1b). Thus, the HBcAg and HBeAg expression plasmids were equally efficient in priming humoral immune responses in wild-type C57BL/10 mice. The equivalence of anti-HBc and anti-HBe antibody production after HBc/HBeAg-pVAX1 immunization can be explained by the fact that HBeAg is efficiently secreted, whereas HBcAg is an intracellular protein and only the small amount that “leaks” from HBcAg-pVAX1-transfected cells or cross-presenting antigen-presenting cells is available for B-cell recognition and subsequent anti-HBc production. Next, the Th cell phenotype primed by HBcAg and HBeAg expression plasmids was analyzed. To compare directly the Th1 and Th2 skewing of the T-cell response primed by HBc/HBeAg-pVAX1 immunization, the levels of HBc/HBeAg-specific IgG1 (Th2) and IgG2a (Th1) antibodies were analyzed. The HBcAg and HBeAg expression plasmids predominantly primed HBc/HBe-specific IgG2a and IgG2b isotype antibodies, indicating a typical Th1 phenotype (see Fig. 3b). This differs from immunization with protein antigens in that HBeAg elicits primarily IgG1 anti-HBe and HBcAg elicits IgG2a/IgG2b anti-HBc antibodies (37). The predominant IgG2a/2b anti-HBe response after HBeAg-pVAX1 immunization is likely due to Toll-like receptor activation mediated by the plasmid vector.
FIG. 1.
(a) Analysis of the translation products from the HBcAg-pVAX1 and HBeAg-pVAX1 plasmids by an in vitro transcription and translation assay in the presence of [35S]methionine by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Lane 1, luciferase plasmid control (∼61 kDa); lane 2, HBcAg-pVAX1; lane 3, HBeAg-pVAX1; lane 4, pVAX1 (empty vector); lane 5, negative control (distilled water). (b) Antibody responses primed by two doses of 100 μg HBcAg-pVAX1 or HBeAg-pVAX1 in groups of four H-2b mice. Mice were immunized i.m. in the TA muscles at week 0 and boosted at week 4. IgG antibody titers were measured at weeks 0, 2, 4, and 6. Values are given as mean end point IgG antibody titers ± standard deviation (SD).
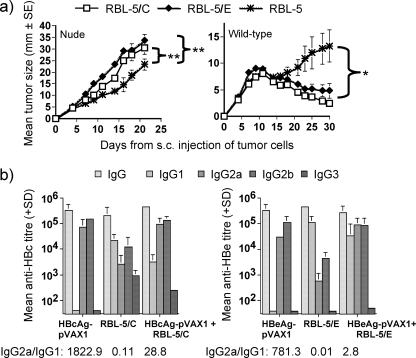
FIG. 3.
(a) In vivo growth potential of RBL-5 tumor cells in naïve nude Foxn1nu/Foxn1nu and wild-type H-2b mice. Groups of five naïve Foxn1nu/Foxn1nu or H-2b mice were injected s.c. with 5 × 106 RBL-5/C, RBL-5/E, or parental RBL-5 cells. Tumor sizes were measured through the skin every 2 to 3 days after the tumor cell injection. Values are given as the mean tumor size ± SE. **, P < 0.01; *, P < 0.05 (areas under the curve compared by ANOVA). (b) Anti-HBc/HBe IgG isotype distribution in groups of five or six H-2b mice immunized twice with 100 μg HBcAg-pVAX1 or HBeAg-pVAX1 given i.m., immunized once with 5 × 106 RBL-5/C or RBL-5/E tumor cells given s.c., or a immunized in a two-step procedure with two monthly doses of 100 μg HBcAg-pVAX1 or HBeAg-pVAX1 given i.m. and boosted with a 5 × 106 RBL-5/C or RBL-5/E tumor cells given s.c. Values are given as mean end point IgG isotype antibody titers (±SD). The titer ratio was obtained by dividing the mean end point titer of IgG2a antibodies to HBc or HBe by the mean end point titer of IgG1 antibodies to HBc or HBe. A high ratio (>3) indicates a Th1-like response, a low ratio (<0.3) indicates a Th2-like response, and an intermediate value (0.3 to 3) indicates a mixed Th1-Th2 response.
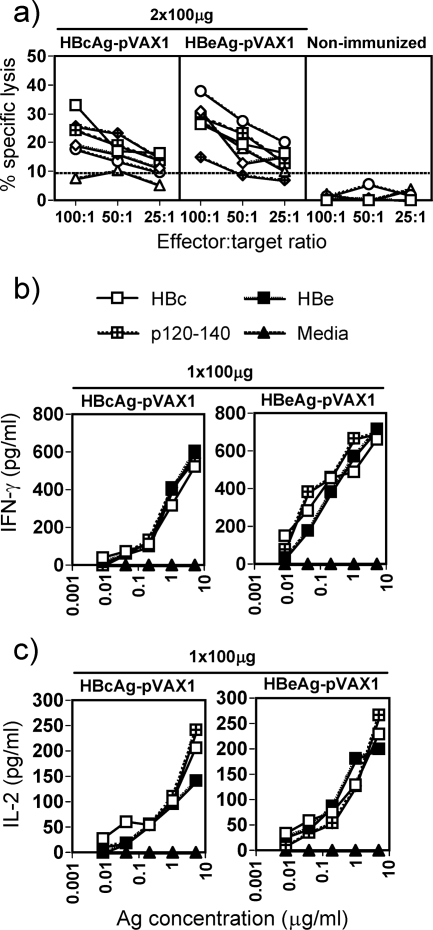
To compare directly the in vitro lytic activities of HBc/HBeAg-specific CTLs, a standard 51Cr release assay was performed after two i.m. immunizations using the HBcAg and HBeAg expression plasmids. The lytic activity of the in vivo primed CTLs was assayed on HBcAg93-100 peptide-loaded RMA-S cells. After two doses, both plasmids induced similar CTL responses (Fig. 2a). Neither of the expression plasmids was a potent inducer of HBc/HBe-specific CTL responses even after two doses. Splenic cytokine responses were also determined following single HBcAg- or HBeAg-pVAX1 i.m. immunizations and cardiotoxin pretreatment. Splenic IFN-γ and IL-2 cytokine levels were determined after culture with a panel of recall antigens (i.e., HBcAg, HBeAg, and HBc/HBe120-140), and similar levels of IFN-γ and IL-2 were detected in HBcAg- and HBeAg-immunized mice (Fig. 2b and c). The antibody isotype and the cytokine profiles indicated that a dominant Th1 phenotype was primed by both the HBcAg- and HBeAg-expression plasmids.
FIG. 2.
(a) Priming of in vitro-detectable CTLs in H-2b mice. Groups of six H-2b mice were immunized i.m. twice with 100 μg HBcAg-pVAX1 or HBeAg-pVAX1 (4 weeks between immunizations) or left unimmunized. The percent specific lysis corresponds to the percent lysis obtained with HBcAg93-100 peptide-loaded RMA-S cells minus the percent lysis obtained with unloaded RMA-S cells. Values are given for effector-to-target cell ratios of 100:1, 50:1, and 25:1. Each line indicates an individual mouse. (b and c) Cytokine responses to HBcAg-pVAX1 and HBeAg-pVAX1 in spleens of immunized H-2b mice. All mice were pretreated with Cardiotoxin. Spleen cells from three mice were pooled and cultured for 48 h (IL-2) or for 96 h (IFN-γ) with various concentrations of rHBcAg (HBc), rHBeAg (HBe), p120-140, or medium, and T-cell activation was determined by measuring IL-2 and IFN-γ production in culture supernatants.
Immune response to the RBL-5/C and RBL-5/E tumor cell lines.
To fully characterize the RBL-5 cell lines, we analyzed their in vivo growth potentials. First, RBL-5 tumor cell lines were inoculated into T-cell-deficient nude Foxn1nu/Foxn1nu mice to test their growth potential without any effect from inherent T-cell antitumor immunity. The RBL-5/C and RBL-5/E cell lines generated significantly larger tumors than the parental RBL-5 cell line in nude Foxn1nu/Foxn1nu mice, in which tumor growth was not inhibited (Fig. 3) (P < 0.01 for RBL-5/C versus RBL-5 and P < 0,01 for RBL-5/E versus RBL-5). Next, RBL-5 tumor growth was tested in naïve wild-type C57BL/10 mice with an intact immune system. Wild-type mice inoculated with RBL-5/C or RBL-5/E tumor cells spontaneously cleared the tumors within 4 to 6 weeks after an approximately 2-week period of tumor growth, whereas mice inoculated with the parental RBL-5 tumor cells did not (Fig. 3) (P < 0.05 for RBL-5/C and RBL-5/E versus RBL-5). These results indicate that immunity to the RBL-5 tumor cell lines is specific for the HBc/HBeAgs. Therefore, HBc/HBeAg-expressing RBL-5 tumor cells are an appropriate means of examining cell-mediated immune responses to the HBc/HBeAgs and CTL responses in particular. It is interesting to note that immunization with the HBc/HBeAg-expressing RBL-5 tumor cell lines elicited primarily Th2-like IgG isotype patterns of anti-HBc and anti-HBe antibodies, as opposed to the Th1-like pattern induced by the DNA vectors (Fig. 3b). Unstimulated RBL-5 tumor cell lines did not secrete any detectable levels of IL-2, IL-4, IL-10, or IFN-γ when grown in culture as determined by cytokine ELISA. Thus, the RBL-5 tumor cell lines do not themselves affect the phenotype of the T-cell immune response by secretion of endogenous cytokines (data not shown).
A two-step immunization protocol is necessary to achieve efficient CTL responses to the HBc/HBeAgs.
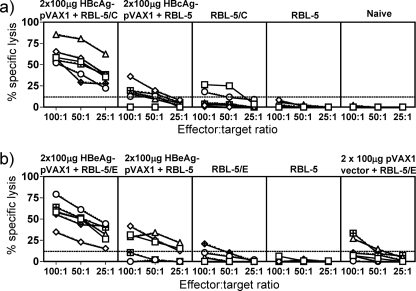
As stated above, two injections with the HBcAg-pVAX1 or HBeAg-pVAX1 expression plasmid did not elicit very efficient CTL responses (Fig. 2 and 4a and b). Similarly, injection with the RBL-5/C and RBL-5/E tumor lines elicited only inefficient HBc/HBeAg-specific CTL responses (Fig. 4a and b). However, the combination of both immunogens (i.e., two doses of the pVAX1 expression plasmid followed by injection of the RBL-5/C or RBL-5/E tumor line) primed very efficient HBc/HBeAg-specific CTL responses (Fig. 4a and b). These results suggest that the two disparate vectors act synergistically for maximal CTL induction. Because the DNA vectors elicit a Th1-like response and the RBL-5/C and RBL-5/E tumor lines elicit primarily a Th2-like response, it is possible that a diversified HBc/HBeAg-specific Th1/Th2 CD4+ response is superior to either a Th1- or Th2-like response singly. The two-step approach of immunizing with plasmid DNA and boosting with tumor cells elicited a Th1/Th2-like phenotype skewed toward a Th1-like response, especially in HBcAg-specific T cells, as evidenced by high levels of IgG1, IgG2a, and IgG2b antibodies (Fig. 3b). Also, note that the HBcAg and HBeAg expression vectors elicited comparable levels of CTL responses. Furthermore, the DNA vectors encoding either HBcAg or HBeAg used in combination with either HBcAg- or HBeAg-expressing RBL-5 tumor lines elicited comparable CTL responses that can be assayed on either HBcAg- or HBeAg-expressing RMA-S target cells (data not shown). This is consistent with previous studies that have reported no differences in the efficiency of CTL priming by plasmid DNA encoding secreted HBeAg or cytosolic HBcAg as well as no differences in the efficiency of class I MHC-restricted processing and presentation of the HBc/HBeAg epitopes by transfected cell lines (27, 28).
FIG. 4.
Effect of using a two-step immunization protocol on priming of in vitro-detectable CTLs in H-2b mice. (a) HBcAg-specific cellular immune responses; (b) HBeAg-specific cellular immune responses. Groups of six H-2b mice were either immunized twice with 100 μg plasmid DNA and boosted with 5 × 106 RBL-5/C, RBL-5/E, or RBL-5 tumor cells given s.c. or immunized with RBL-5/C(E) only or RBL-5 only. At day 42 after the tumor cell booster immunization, spleens were harvested, restimulated for 5 days in the presence of the HBcAg93-100 peptide, and thereafter used in a standard 4-hour 51Cr release assay. The percent specific lysis corresponds to the percent lysis obtained with HBcAg93-100 peptide-loaded RMA-S cells minus the percent lysis obtained with unloaded RMA-S cells. Values are given for effector-to-target cell ratios of 100:1, 50:1, and 25:1. Each line indicates an individual mouse.
Development of an HBc/HBeAg-specific CTL in vivo tumor model.
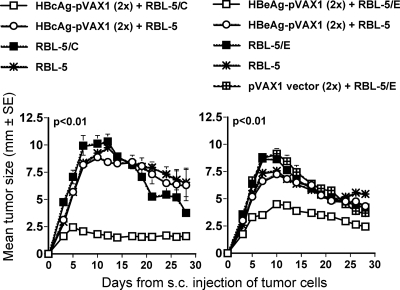
To test the efficiency of HBc/HBeAg-specific CTL responses in vivo following i.m. DNA immunization, we challenged mice with HBcAg- or HBeAg-expressing RBL-5 tumor cell lines. Groups of 6 to 10 C57BL/10 mice were either unimmunized or immunized twice with 100 μg HBcAg-pVAX1, HBeAg-pVAX1, or the pVAX1 empty vector. At 2 weeks after the last immunization, mice were challenged using a s.c. injection of 5 × 106 parental RBL-5 or HBc/HBeAg-expressing RBL-5 tumor cells. As shown in Fig. 5, mice primed with HBcAg-pVAX1 or HBeAg-pVAX1 were protected from RBL-5/C(E)-expressing tumor cell growth. Moreover, groups of C57BL/10 mice immunized with rHBcAg protein and having anti-HBc IgG titers ranging between 3 × 106 and 17 × 106 prior to tumor challenge or mice immunized with the HBcAg93-100 MHC class I peptide were not protected against tumor growth when challenged with RBL-5/C tumor cells (data not shown). Thus, an endogenous production of HBc/HBeAg appears to be required to prime in vivo protective CTLs.
FIG. 5.
Inhibition of in vivo tumor growth after immunization. Groups of six H-2b mice were either left unimmunized or given two monthly immunizations with 100 μg HBcAg-pVAX1, HBeAg-pVAX1, or pVAX1 (empty vector) plasmid. Two weeks after the last DNA plasmid immunization, mice were inoculated with 5 × 106 RBL-5/C, RBL-5/E, or RBL-5 tumor cells given s.c. Tumor sizes were measured through the skin every 2 to 3 days after the tumor cell injection. Values are given as the mean tumor size (±SE). The area under the curve for the HBc(e)Ag-pVAX1-immunized mice challenged with RBL-5/C(E) tumor cells was statistically significantly different from the curves for the other groups (ANOVA, P < 0.01).
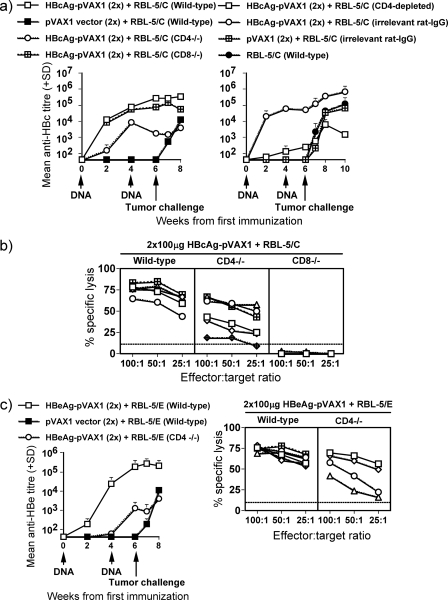
Next, we investigated the importance of B cells, CD4+ T cells, and CD8+ T cells for in vivo priming of HBc/HBeAg-specific immune responses. To define the effect of each cell population on priming and effector function, groups (six mice per group) of wild-type, B−/−, CD4−/−, and CD8−/− mice were used. Moreover, in selected experiments we depleted the CD4+ T cells in wild-type mice prior to and during immunization and tumor challenge. All groups of mice were immunized twice using 100 μg HBcAg-pVAX1 (Fig. 6a and b), HBeAg-pVAX1 (Fig. 6c), or the pVAX1 empty vector, and at 2 weeks after the last immunization, they all received an s.c. inoculation of 5 × 106 RBL-5/C or RBL-5/E tumor cells. These experiments revealed that HBcAg-specific B cells were necessary for anti-HBc/HBe antibody production but were not of importance for induction of CTLs (data not shown). In wild-type and CD8−/− mice, equally high levels of anti-HBc specific IgG antibody titers (>105) were produced, whereas in CD4−/− and CD4+ T-cell-depleted wild-type mice, the ability to produce anti-HBc antibodies was significantly reduced (∼103 to 104) (Fig. 6a). Hence, the humoral immune response toward HBcAg is at least partially CD4+ T-cell dependent. The lytic activity primed by two HBcAg-pVAX1 plasmid DNA immunizations and an RBL-5/C tumor cell boost in wild-type, CD4−/−, and CD8−/− mice was completely CD8+ T-cell dependent, whereas the lytic activity in CD4−/− mice was only partially reduced compared to that in wild-type mice (Fig. 6b). Similar results were obtained when wild-type and CD4−/− mice were immunized twice with 100 μg HBeAg-pVAX1 plasmid DNA and boosted with RBL-5/E tumor cells. The magnitude of the anti-HBe-specific IgG antibody response was lower in CD4−/− mice (∼103 to 104) than in wild-type mice (>105) (Fig. 6c). In addition, the lytic activity primed in CD4−/− mice was also weaker than the activity in wild-type mice (Fig. 6c). Therefore, HBc/HBeAg-specific CD8+ T-cell CTL function is not strictly dependent on CD4+ T cells; however, the presence of HBc/HBeAg-specific primed CD4+ T cells enhances the efficiency of CD8+ CTL function.
FIG. 6.
The CD4+/CD8+ T-cell dependence of antibody and CTL responses primed by two monthly i.m. immunizations with 100 μg HBc(e)Ag-pVAX1 or pVAX1 vector (empty vector, negative control) and a booster immunization with 5 × 106 RBL-5/C(E) tumor cells. Groups of six or seven H-2b (wild-type, CD4−/−, CD8−/−, and CD4+ T cell-depleted wild-type) mice were immunized with the indicated antigens. Arrows indicate time points for DNA plasmid immunizations (4-week intervals) and tumor cell challenge. IgG antibody titers were measured at weeks 0, 2, 4, 6, and 8. Values are given as mean end point IgG antibody titers (±SD). For CTL analysis, 22 days after the tumor cell booster immunization, spleens were harvested and restimulated for 5 days in the presence of the HBcAg93-100 peptide and thereafter used in a standard 4-hour 51Cr release assay. The percent specific lysis corresponds to the percent lysis obtained with HBcAg93-100 peptide-loaded RMA-S cells minus the percent lysis obtained with unloaded RMA-S cells. Values are given for effector-to-target cell ratios of 100:1, 50:1, and 25:1. Each line indicates an individual mouse.
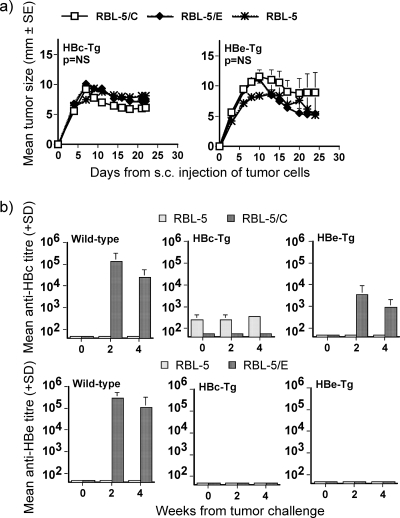
CD8+ CTL immune tolerance in HBc/HBeAg-Tg mice.
Previous studies have demonstrated that liver-derived HBc/HBeAgs expressed in Tg murine models function as tolerogens at the level of CD4+ T cells and that the HBeAg is a more effective tolerogen due to the fact that it is a secreted protein (12, 13, 35). To examine the tolerogenic potential of the HBc/HBeAgs at the level of CD8+ CTLs, RBL-5 parental tumor cells or tumor cells expressing either HBcAg or HBeAg were inoculated into naïve wild-type or HBcAg- or HBeAg-expressing C57BL/10 mice. As shown in Fig. 7a, neither HBcAg-Tg nor HBeAg-Tg mice eradicated the inoculated tumor cells, whereas wild-type mice rejected both HBc- and HBeAg-expressing tumor cells by day 30 (see Fig. 3a). Similarly, wild-type mice produced high-titer anti-HBc or anti-HBe antibodies after tumor inoculation, whereas HBcAg-Tg mice produced only low-level anti-HBc and HBeAg-Tg mice produced no anti-HBe antibodies (Fig. 7b). These results indicate that the HBc/HBeAg-Tg animals are tolerant to the HBc/HBeAgs presented by RBL-5 tumor cells. The fact that HBc/HBeAg-specific tumor regression is CD8+ T cell-dependent indicates that HBc/HBeAg-specific CD8+ T cells are tolerant, similar to the tolerance of CD4+ T cells, as indicated by the low-level to absent anti-HBc(HBe) antibody production after RBL-5/C(E) inoculation.
FIG. 7.
Immune response of HBcAg-Tg and HBeAg-Tg mice to RBL-5/C(E) tumor cells. (a) Groups of five naïve HBcAg- and HBeAg-Tg mice were injected s.c. with 5 × 106 RBL-5/C, RBL-5/E, or parental RBL-5 cells. Tumor sizes were measured through the skin every 2 to 3 days after the tumor cell injection. Values are given as the mean tumor size (±SE). NS, no statistical difference (area under the curve values compared by ANOVA). (b) Antibody responses primed by a single immunization of RBL-5/C, RBL-5/E, or parental RBL-5 tumor cells in groups of five H-2b (wild-type, HBcAg-Tg, and HBeAg-Tg) mice. Mice were immunized s.c. with 5 × 106 tumor cells at week 0, and anti-HBc/anti-HBe IgG antibodies were measured at weeks 0, 2, and 4. Values are given as mean end point IgG antibody titers (±SD).
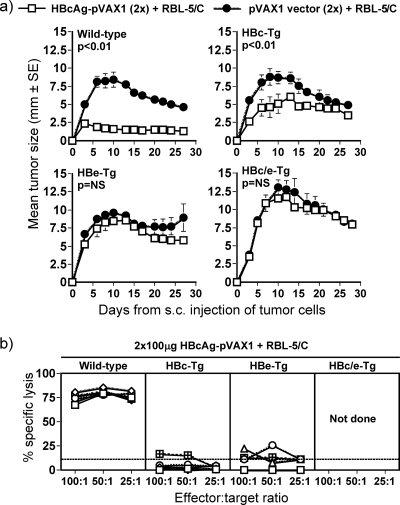
As a more stringent test of CD8+ T-cell tolerance, groups of wild-type, HBcAg- or HBeAg-expressing, or HBc/HBe-dbl-Tg mice were immunized twice with 100 μg HBcAg-pVAX1 plasmid DNA, and 2 weeks after the last immunization they were challenged with RBL-5/C tumor cells and monitored for tumor growth in vivo. Immunized wild-type C57BL/10 mice were completely protected against tumor growth (P < 0.01), whereas HBeAg and HBc/HBeAg-dbl-Tg mice were completely unable to clear the tumors (Fig. 8a) (P = not significant)). Interestingly, the immunized HBcAg-Tg mice were partially protected against tumor growth compared to the pVAX1-immunized control group, and the HBcAg-Tg mice had statistically significantly smaller tumors (P < 0.01). Therefore, mice expressing HBcAg are not totally tolerized at the CD8+ T-cell level. However, mice expressing HBcAg in the presence of HBeAg (i.e., HBc/HBe-dbl-Tg mice) are completely tolerant at the CD8+ level (Fig. 8a). Direct comparison of in vitro lytic activities after DNA and tumor cell immunization in wild-type and Tg mice revealed high lytic activity in wild-type mice and low or absent activity in HBc/HBeAg-expressing Tg animals (Fig. 8b). Furthermore, no elevations in sALT levels in HBc-, HBe-, or HBc/e-Tg mice were observed after DNA and RBL-5/C(E) immunizations. sALT levels were measured at days 3 and 7 after the DNA and RBL-5/C(E) immunizations (data not shown). Therefore, no liver injury was induced by the DNA and tumor immunizations owing to the level of T-cell tolerance apparent in HBc/HBeAg-expressing Tg mice.
FIG. 8.
Inhibition of in vivo tumor growth after immunization in HBcAg-, HBeAg-, and HBc/HBeAg-dbl-Tg mice. (a) Groups of six to eight H-2b (wild-type, HBcAg-Tg, HBeAg-Tg, and HBc/eAg-Tg) mice were given two monthly immunizations with 100 μg HBcAg-pVAX1 or pVAX1 (empty vector) plasmid. Two weeks after the last DNA plasmid immunization, mice were inoculated with 5 × 106 RBL-5/C tumor cells given s.c. Tumor sizes were measured through the skin every 2 to 3 days after the tumor cell injection. Values are given as the mean tumor size (±SE). The areas under the curves were compared for statistically significantly difference by ANOVA. (b) The indicated mice were immunized as described for panel a, and spleen cells were harvested for in vitro CTL analysis as described for Fig. 4.
Induction of transient liver injury mediated by the adoptive transfer of HBcAg-specific CD8+ CTLs.
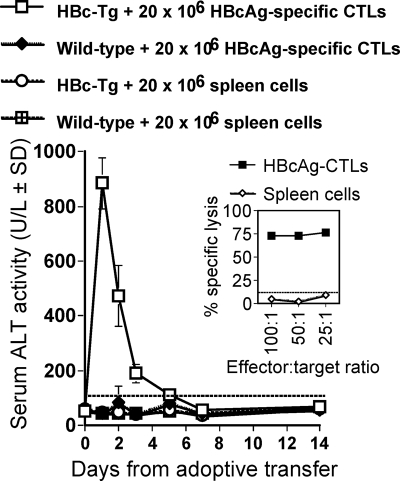
Because direct immunization with HBcAg-pVAX1 and RBL-5/C tumor cells into HBc/HBeAg-Tg mice did not induce liver injury due to CD4+ and CD8+ CTL tolerance, an adoptive transfer model was developed to determine if HBc/HBeAg-specific CTL could mediate liver injury in HBc/HBeAg-Tg recipients. As shown above, it was necessary to immunize wild-type mice with both HBcAg-pVAX1 (twice) and RBL-5/C tumor cells and to restimulate the spleen cells in vitro with HBc/HBeAg93-100 in order to elicit a vigorous CTL response (Fig. 4). These highly activated (Fig. 9, inset), polyclonal HBcAg-specific CTLs (20 × 106) were transferred into nonirradiated HBcAg-Tg recipients, and liver injury was monitored by measuring sALT levels for 2 weeks after CTL transfer (Fig. 9). The transferred HBcAg-specific CTLs elicited liver injury of a very transient nature in HBcAg-Tg recipients. sALT became elevated within the first 12 h after CTL transfer and peaked at 24 h. sALT levels were only slightly elevated at day 3 and normalized by day 4 to 5. No liver injury occurred in wild-type mice injected with HBcAg-specific CTLs or normal spleen cells or in HBcAg-Tg mice injected with normal spleen cells (Fig. 9).
FIG. 9.
Adoptive transfer of HBcAg-specific CTLs elicits transient liver injury in HBcAg-Tg recipients. A total of 20 × 106 HBcAg-specific CTLs or spleen cells were adoptively transferred i.v. into groups of three to six HBcAg-Tg or wild-type mice at day 0. sALT activity was measured at days 1, 2, 3, 4, 5, 7, and 14 after adoptive transfer and is expressed as units/liter ± SD. Values of below 100 U/liter were considered normal ALT levels. The inset shows the in vitro lytic activity of the primed HBcAg-specific CTLs prior to adoptive transfer. The percent specific lysis corresponds to the percent lysis obtained with HBcAg93-100 peptide-loaded RMA-S cells minus the percent lysis obtained with unloaded RMA-S cells. Values are given for effector-to-target cell ratios of 100:1, 50:1, and 25:1. Also shown is the lytic activity of control spleen cells.
Differential CTL-mediated liver injury in HBeAg-Tg versus HBcAg-Tg recipients.
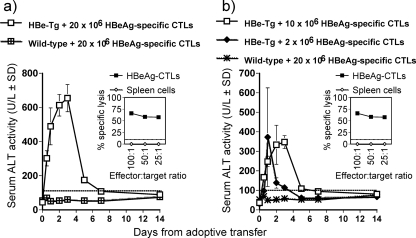
In a separate set of adoptive transfer experiments, HBeAg-specific CTLs were transferred into HBeAg-Tg recipients. Donor polyclonal, activated HBeAg-specific CTLs were generated by immunizing wild-type mice with HBeAg-pVAX1 (twice) and RBL-5/E tumor cells, and spleen cells were restimulated with HBc/HBeAg93-100 in vitro. The HBeAg-specific CTLs were highly functional as determined by in vitro specific lysis of target cells (Fig. 10a, inset). Unexpectedly, upon adoptive transfer of HBeAg-specific CTLs, the nature of the liver injury observed in the HBeAg-Tg recipients was quite different from that observed in HBcAg-Tg recipients receiving HBcAg-specific CTLs. The duration of the liver injury was significantly longer in HBeAg-Tg recipients than in HBcAg-recipients (Fig. 10a). The initiation of liver injury within 12 h after CTL transfer was similar in HBeAg-Tg and HBcAg-Tg recipients of CTLs. However, sALT levels did not peak at 24 h, as observed in HBcAg-Tg recipients, but rather at 72 h after CTL transfer in HBeAg-Tg recipients, and they remained slightly elevated until day 5 and normalized by day 7 (Fig. 10a). Liver injury was dependent on the number of HBeAg-specific CTLs transferred. Transfer of 10 × 106 HBeAg-specific CTLs elicited lower levels of sALT than transfer of 20 × 106 CTLs, although the extended kinetics of liver injury (i.e., peak at day 3) was not altered. However, adoptive transfer of 2 × 106 HBeAg-specific CTLs into HBeAg-Tg recipients recapitulated the transient liver injury observed in HBcAg-Tg recipients of 20 × 106 HBcAg-specific CTLs (Fig. 10b).
FIG. 10.
Adoptive transfer of HBeAg-specific CTLs elicits prolonged liver injury in HBeAg-Tg recipients. A total of 20 × 106, 10 × 106, or 2 × 106 HBeAg-specific CTLs, as indicated, were adoptively transferred i.v. into groups of three to six HBeAg-Tg or wild-type mice at day 0. sALT activity was measured at days 0.5, 1, 2, 3, 4, 5, 7, and 14 after adoptive transfer and is expressed as units/liter ± SD. Values of below 100 U/liter were considered normal ALT levels. The insets show the in vitro lytic activity of the primed HBeAg-specific CTLs prior to adoptive transfer.
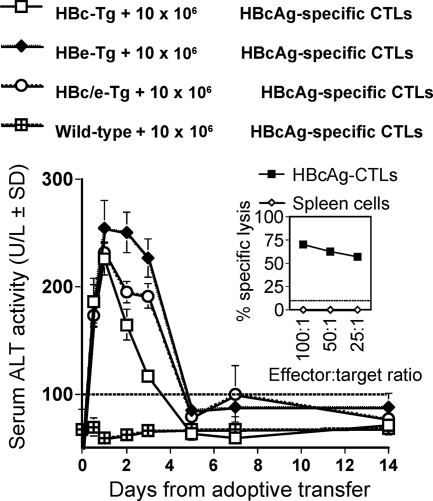
In order to determine if the differential kinetics of liver injury observed was due to the specificity of the CTLs (i.e., HBcAg specific versus HBeAg specific) or to the specificity of the target antigen expressed in the liver of the recipient, the same population of HBcAg-specific CTLs (10 × 106) was adoptively transferred into three different Tg recipients that expressed either HBcAg, HBeAg, or both HBc/HBeAgs (Fig. 11). Adoptive transfer of HBcAg-specific CTLs into HBcAg-Tg recipients resulted in a transient elevation of sALT, which peaked at day 1, whereas the transfer of HBcAg-specific CTLs into HBeAg-Tg recipients resulted in liver injury of longer duration. Interestingly, transfer of HBcAg-specific CTLs into HBc/HBeAg-dbl-Tg recipients resulted in the longer-duration phenotype of liver injury (Fig. 11). Therefore, the target antigen expressed in the liver determined the phenotype of liver injury that occurs after adoptive transfer of HBc/HBeAg-specific CTLs, and the specificity of the CTLs for HBcAg or HBeAg appears to be irrelevant. This is consistent with the highly cross-reactive nature of the HBc/HBeAgs at the CTL level observed in vitro. The difference between the HBc/HBeAgs becomes apparent only at the level of CTL recognition of intracellular HBcAg versus HBeAg as they are expressed within hepatocytes in vivo.
FIG. 11.
Phenotype of liver injury is dependent on antigen expressed in the liver. A total of 10 × 106 HBcAg-specific CTLs were adoptively transferred i.v. into groups of three to six wild-type, HBcAg-Tg, HBeAg-Tg, or HBc/HBeAg-dbl-Tg mice at day 0. sALT activity was measured at days 0.5, 1, 2, 3, 4, 5, 7, and 14 after adoptive transfer and is expressed as units/liter ± SD. Values of below 100 U/liter were considered normal ALT levels. The inset shows the in vitro lytic activity of the primed HBcAg-specific CTLs prior to transfer.
To further confirm differences between HBcAg and HBeAg as CTL targets in the liver, we performed gene array analysis of liver tissues from HBcAg and HBeAg-Tg mice prior to CTL adoptive transfer (day 0) and 3 days after CTL adoptive transfer (day 3). The data confirm at the mRNA level that the HBeAg-expressing liver is significantly more inflammatory than the HBcAg-expressing liver at day 3 after CTL transfer (Tables 1 and 2). A number of genes involved in inflammation (i.e., those for IFN-γ, Irf-1, Irf-3, Ccl3, Ccl4, Ccl19, Ccl22, IL-1r1, IL-12rb1, IL-15ra, CD8b1, Ltb, and GITR) and apoptosis (i.e., those for Bak1, Bcl2l14, Bok, and Card11) were upregulated in HBeAg-Tg versus HBcAg-Tg liver at day 3 after CTL transfer (Table 1), whereas a number of genes that are relevant to antiapoptotic (i.e., those for Bag1, Bag4, Bcl2l1, and Aven) or antistress (i.e., those for heat shock proteins, Cyp3a11, Cyp3a16, Gpx1, and Gsr) mechanisms were upregulated in HBcAg-Tg liver at day 3 after CTL transfer (Table 2). At day 0 the selected genes were equivalently expressed in HBcAg- and HBeAg-Tg mice.
TABLE 1.
Inflammation and apoptosis genes differentially expressed in HBeAg-Tg versus HBcAg-Tg mice before (day 0) and after (day 3) CTL transfer
| Gene | HBeAg-Tg/HBc-Tg ratio on day:
|
|
|---|---|---|
| 0 | 3 | |
| IFN-γ | 1.7 | 4.5 |
| Irf-1 | 1.0 | 3.8 |
| Irf-3 | 1.1 | 87.2 |
| Mapk8ip3 | 0.9 | 4.6 |
| NF-κbia | 1.4 | 4.3 |
| Ccl3 | 0.5 | 2.9 |
| Ccl4 | 0.8 | 3.9 |
| Ccl5 | 1.5 | 4.2 |
| Ccl19 | 0.9 | 5.2 |
| Ccl22 | 0.3 | 3.4 |
| Cxcl1 | 0.5 | 2.9 |
| Cxcl10 | 0.9 | 1.7 |
| Cxcl11 | 0.9 | 2.9 |
| Ltb | 1.1 | 2.7 |
| Ifnar2 | 1.1 | 3.1 |
| Ifngr1 | 1.0 | 1.8 |
| IL-12rb1 | 1.3 | 3.0 |
| IL-15ra | 0.9 | 2.7 |
| IL-1r1 | 1.4 | 2.8 |
| CD8a | 1.4 | 2.1 |
| CD8b1 | 1.7 | 4.0 |
| NF-κb1 | 0.9 | 2.3 |
| GITR | 1.3 | 2.5 |
| Bak1 | 0.8 | 2.0 |
| Bcl2l14 | 0.2 | 11.0 |
| Bok | 0.9 | 1.5 |
| Card11 | 1.1 | 5.5 |
| Card4 | 1.1 | 3.6 |
TABLE 2.
Antiapoptosis and antistress genes differentially expressed in HBeAg-Tg versus HBcAg-Tg mice before (day 0) and after (day 3) CTL transfer
| Gene | HBeAg-Tg/HBc-Tg ratio on day:
|
|
|---|---|---|
| 0 | 3 | |
| Hspa4 | 1.0 | 0.3 |
| Hspd1 | 1.1 | 0.3 |
| Hspa5 | 1.1 | 0.5 |
| Hspa8 | 1.2 | 0.3 |
| Hspe1 | 1.0 | 0.4 |
| DNAja1 | 1.2 | 0.1 |
| Bag1 | 1.1 | 0.2 |
| Bag4 | 1.2 | 0.3 |
| Bcl2l1 | 0.9 | 0.5 |
| Crp | 1.2 | 0.3 |
| F2 | 0.9 | 0.5 |
| Hp | 1.1 | 0.6 |
| Orm1 | 0.9 | 0.3 |
| Serpina1b | 1.0 | 0.2 |
| Cfh | 1.2 | 0.2 |
| Gpx1 | 0.7 | 0.4 |
| Gsr | 1.3 | 0.4 |
| Ugt1a1 | 1.2 | 0.3 |
| Ugt1a9 | 1.3 | 0.2 |
| Aven | 1.2 | 0.1 |
| Alb1 | 0.9 | 0.2 |
| Fabp1 | 0.7 | 0.3 |
| Cct2 | 1.1 | 0.2 |
| DNAja3 | 1.3 | 0.2 |
| DNAjb4 | 1.1 | 0.2 |
| DNAjb6 | 1.1 | 0.3 |
| Cyp3a11 | 2.5 | 0.1 |
| Cyp3a16 | 3.8 | 0.1 |
DISCUSSION
Although previous investigators have demonstrated that DNA-based immunogens elicited HBc/HBeAg-specific CTL responses (27, 28, 55), the relative efficacy of CTL induction is less studied. Using two separate injections with a pVAX1 vector encoding either HBcAg or HBeAg, we achieved relatively modest CTL induction. Similarly, injecting HBcAg- or HBeAg-expressing tumor cell lines (i.e., RBL-5/C or RBL-5/E) induced even less efficient CTL responses. However, the combination of either two HBc/HBeAg-pVAX1 injections or a single HBc/HBeAg-pVAX1 injection with an RBL-5/C-E tumor cell injection elicited very efficient CTL responses (Fig. 4). The necessity for this rather unique immunization strategy suggests that the HBc/HBeAgs are relatively modest antigens in terms of CTL generation, which is in contrast to their strong immunogenicity for CD4+ T cells and antibody production. The two-step immunization protocol may have enhanced HBc/HBeAg-specific CTL priming because the pVAX1-based immunogens elicited a highly Th1-biased response and the RBL-5 tumor cell-based immunogens elicited a Th2-biased response, and efficient CTL generation may benefit from this mixed Th1-Th2 response. For example, in the Plasmodium falciparum malaria system, IL-4-secreting CD4+ T cells are absolutely required for the development of CD8+ CTL responses to liver stage antigen (9). Further, it has been shown that IL-4 has a strong in vivo and in vitro antiapoptotic effect on activated and resting CD8+ T cells (1). Regardless of whether the pVAX1 vector alone or the two-step protocol for priming CTLs was used, the HBc/HBeAgs appeared to be equivalent in terms of priming a CTL response and in terms of target recognition in vitro, as reported previously (27, 28, 55). However, this was not the case for CTL recognition of the HBc/HBeAgs expressed in hepatocytes in vivo, as discussed below.
Although we have previously shown that the HBc/HBeAgs can induce immune tolerance in CD4+ T cells and that HBeAg is a more efficient tolerogen (12, 13, 35), tolerance at the level of CTL induction has not been as well studied. Here it was shown that the two-step protocol of HBcAg-pVAX1 (twice) and RBL-5/C injections in wild-type mice efficiently induced CTL responses. However, Tg mice expressing either HBcAg or HBeAg did not generate a CTL response after identical immunization (Fig. 8b). Similarly, in the in vivo tumor model, in wild-type mice preimmunized with HBcAg-pVAX1 (twice) the injected RBL-5/C tumor cells did not grow and were rejected by the CD8+ CTL response (i.e., tumor rejection does not occur in CD8-knockout mice). However, due to tolerance at the CTL level, the RBL-5/C tumor grew efficiently in HBeAg-Tg mice whether the HBeAg-Tg mice were immunized or not (Fig. 7a and 8a). In this model system the HBcAg-Tg mice appeared to be somewhat less tolerant, as preimmunization with HBcAg-pVAX1 had a partial effect in reducing tumor growth. However, note that in HBc/HBeAg-dbl-Tg mice there was no effect of HBcAg-pVAX1 preimmunization, and the tumor grew equally in naïve and immunized HBc/HBeAg-dbl-Tg mice (Fig. 8a). Therefore, as previously observed at the level of CD4+ T cells and antibody production, HBeAg appears to be more tolerogenic than HBcAg at the CTL level, possibly because of HBeAg-specific CD4+ T-cell tolerance mediated by secreted HBeAg. Although HBc/HBeAg-specific CD8+ CTL induction using the two-step protocol was not strictly dependent on HBc/HBeAg-specific CD4+ T-cell function, CTL function was enhanced in the presence of CD4+ T-cell help (Fig. 6). Interestingly, if the HBc/HBeAg-pVAX vector alone was used to prime CTLs, the CTL response was totally dependent on HBc/HBeAg-specific CD4+ T cells (data not shown). Therefore, it appears that the strength of CTL induction determines the requirement for CD4+ T-cell help. Therefore, nonresponse to the HBc/HBeAgs at the CTL level may be due to either direct CD8+ T-cell tolerance or an indirect effect of CD4+ T-cell tolerance depending on the strength of the CTL induction signal(s).
Because direct immunization with the HBc/HBeAg-pVAX1 vectors and/or RBL-5 cell lines expressing HBc/HBeAg did not induce liver injury in HBc/HBeAg-Tg mice due to CD4+/CTL tolerance, an adoptive transfer model was used to examine CTL-mediated liver injury and to circumvent immune tolerance. Perhaps the most surprising result was that the adoptive transfer of HBc/HBeAg-specific CTLs elicited different phenotypes of liver injury depending on whether HBcAg or HBeAg was expressed in the liver (Fig. 9 to 11). Tg mice expressing only HBcAg in the liver demonstrated very transient liver injury that peaked at 24 h after CTL transfer, whereas Tg mice expressing only HBeAg demonstrated a more prolonged liver injury which peaked 72 h after CTL transfer. Importantly, Tg mice expressing both HBcAg and HBeAg in the liver (i.e., analogous to a wild-type HBV infection) demonstrated liver injury more similar to that in HBeAg-Tg recipients than to that in HBcAg-Tg recipients of transferred CTLs (Fig. 11). These results suggest that the HBeAg expressed within hepatocytes in vivo serves as a superior target either directly or indirectly for HBc/HBeAg-specific CTL recognition compared to intracellular HBcAg and/or suggest an anti-inflammatory role for hepatic HBcAg in the context of CTL-mediated liver injury. It is not likely that the HBc/HBeAgs differ significantly in terms of processing and presentation in the context of MHC class I molecules for recognition by CTLs, because tumor cell lines expressing the HBc/HBeAgs are equivalently lysed by HBc/HBeAg-specific CTLs in vitro, as observed here and by others (27, 28, 55). It is difficult to compare the levels of HBcAg and HBeAg expressed in Tg mice because HBcAg is exclusively intracellular and HBeAg is very efficiently secreted. The level of accumulated intracellular HBcAg is relatively high (0.2 to 2.0 μg/mg liver protein) and easily detectable by immunohistochemistry and is comparable to that in an HBV-infected liver (22). Therefore, HBcAg expression is unlikely to be limiting for CTL recognition compared to the much lower level (undetectable by immunohistochemistry) of HBeAg protein or its precursors accumulated intracellularly in HBeAg-Tg mice (23). Measurements of Tg HBc/HBeAg-specific mRNA in both HBcAg-Tg and HBeAg-Tg lineages have been made by Northern blotting, and they appear to be similar, as expected because expression of both transgenes is regulated by the same liver-specific, mouse major urinary protein promoter (22, 23). Therefore, the difference between liver injury observed in HBc/HBeAg-Tg mice is more likely the result of indirect effects of the HBc/HBeAgs being expressed within the liver. For example, HBeAg may condition the hepatocyte for a greater sensitivity to HBc/HBeAg-specific CTL-mediated injury or to secondary antigen-nonspecific injury due to recruitment of nonspecific inflammatory cells into the liver compared to HBcAg. Reciprocally, HBcAg may condition the hepatocyte for less sensitivity to HBc/HBeAg-specific or nonspecific liver cell injury compared to HBeAg. Tentatively, we favor the second possibility, because in an analogous system of HBsAg-specific CTLs adoptively transferred into HBsAg-expressing Tg recipients, the typical pattern of liver injury is a 5- to 7-day course of ALT elevations, which peaks at day 3 (4, 21, 25, 50, 51). Although the database is limited, the very transient liver injury observed in HBcAg-Tg recipients of transferred CTLs (i.e., 24 h) appears atypical and is an unexpected result. The HBsAg-Tg mouse model of CTL-induced liver injury has been well characterized. A prominent finding has been that CTL-mediated liver injury occurs in a two- or three-step process and that most of the liver injury is not caused by the direct effect of the HBsAg-specific CTLs (step 1), even though the CTLs are absolutely required to initiate liver injury. Rather, the bulk of the liver injury is mediated by later-acting (steps two and three) antigen-nonspecific, inflammatory cells recruited into the liver by activated CTLs and their chemical mediators (3, 4). By analogy to the HBsAg-Tg model, the liver injury observed after adoptive transfer of HBcAg-specific CTLs (10 × 106 to 20 × 106) into HBcAg-Tg recipients may represent the direct lysis of hepatocytes by CTLs (step 1). However, the secondary antigen-nonspecific inflammatory response (steps two and three) appears to be absent in comparison to the response elicited in HBeAg-Tg recipients and, by analogy, HBsAg recipients. The gene array data are consistent with this hypothesis. Three days after the adoptive transfer of HBcAg-specific CTLs, the HBeAg-expressing liver was significantly more inflammatory than the HBcAg-expressing liver (Tables 1 and 2). Genes involved in inflammation and apoptosis were upregulated in HBeAg-Tg versus HBcAg-Tg liver at 3 days after CTL transfer, whereas genes that are relevant to antiapoptotic or antistress mechanisms were upregulated in the HBcAg-Tg liver. Also note that CD8a and CD8b1 genes are upregulated in the HBeAg-Tg liver relative to the HBcAg-Tg liver at day 3, suggesting that transferred CD8+ T cells persist longer in the HBeAg-Tg liver. Most notable is the upregulation of a number of C-C and CXC chemokine genes in HBeAg-Tg mice compared to HBcAg-Tg mice at 3 days after adoptive transfer of CTL. For example, Ccl4 is a chemokine produced in hepatic cells that recruits inflammatory cells to the liver and maintains inflammation. Further studies will be necessary to determine if these or other genes are causally related to the different phenotypes of liver injury in HBcAg versus HBeAg-Tg recipients. Indirect effects of the HBc/HBeAgs on liver cell sensitivity to direct lysis or secondary nonspecific inflammatory injury would explain the inability to detect differences between the HBc/HBeAgs in previous in vitro CTL assays (27, 28, 55).
Regardless of the mechanism, the observation that expression of HBeAg in the liver results in more severe injury than expression of HBcAg in the liver may have profound implications for how an HBeAg-negative mutant virus is selected during chronic HBV infection. Hepatocytes expressing wild-type HBV (i.e., both HBcAg and HBeAg) may be more susceptible to the direct or indirect effects of CTL-mediated clearance mechanisms than hepatocytes expressing only HBcAg. This would provide the HBeAg-negative mutant with a selective advantage during an immune response. HBV variants that do not produce HBeAg are very common in chronic HBV infections. A stop codon mutation in codon 28 of the precore region is the most common HBeAg-negative variant (8). Mutations in the core promoter can also abolish or reduce HBeAg production (39). Although HBeAg-negative variants often occur in chronic HBV infections, the mechanistic selection process has remained elusive. Historically, it has been problematic to understand the selective advantage of an HBeAg-negative variant at the level of CTL recognition because the infected hepatocyte expresses the CTL-cross-reactive HBcAg regardless of HBeAg coexpression. The observation reported here that differential CTL recognition of the HBc/HBeAgs expressed in hepatocytes in vivo may result in preferential clearance of wild-type HBV could resolve this dilemma. Because hepatocytes do not serve as effective antigen-presenting cells for exogenous antigens and exogenous HBeAg has not been shown to elicit CTLs, it is unlikely that secreted HBeAg is a target of the immune selection process. Indeed, secreted HBeAg likely serves as a counterbalance that moderates HBc/HBeAg-specific liver injury due to its immunoregulatory properties (12, 13, 33-36). It may seem counterintuitive that HBeAg functions both as a tolerogen/immunoregulatory protein and as a viral target for CTL recognition and the mediator of subsequent liver injury. How can a immunoregulatory protein also be the target of immune-mediated selection? In this regard, it may be helpful to discriminate between secreted HBeAg and cytosolic HBeAg. The secreted HBeAg can tolerize HBc/HBeAg-specific CD4+ T cells, and this function is most apparent during the asymptomatic tolerance phase of chronic HBV infection. However, once HBeAg-specific T-cell tolerance is at least partially broken, usually in young adulthood in perinatally infected chronic carriers, cytosolic HBeAg not only may serve as a CTL target but may actually be a more efficient CTL target than intracellular HBcAg in the liver. While secreted HBeAg is advantageous for the virus during the early phase of infection, cytosolic HBeAg becomes a liability for the virus as HBeAg-specific T-cell tolerance wanes. Production of HBeAg-negative CTL escape mutants and their selection by the host immune response may represent another mechanism for viral persistence in later stages of chronic infection. In this way the HBeAg-negative virus escapes the CTL response targeted at cytosolic HBeAg, but the escape mutant is not entirely successful because the virus also loses the function of its secreted immunoregulatory protein, serum HBeAg. Although the emergence of an HBeAg-negative variant may sometimes correlate with reduced liver injury and viral clearance (2, 40, 53), because HBcAg remains as a CTL target and because the immunoregulatory function of secreted HBeAg is lost, the HBeAg-negative immune escape variant may actually become more pathogenic in some circumstances, most likely depending on the viral load and stage of infection (5, 7, 15, 33).
A recent clinical study illustrates the selective advantage of the precore stop codon mutation (57). Chronic HBV patients with preexisting immunity and liver injury infected with wild-type HBV or a mixture of approximately 25% precore mutant virus were treated with a 6-month course of the antiviral drug lamivudine. Lamivudine can reduce the viral load to undetectable levels, and HBc/HBeAg production within hepatocytes can become limiting, which is an ideal environment for selective immune pressure to be analyzed. Within 2 to 3 months after withdrawal of lamivudine therapy and while viral loads were still low to undetectable, the percentage of precore mutant virus rose from 0 to 25% to virtually 100% in all three patients studied (57). This indicates a very rapid selection of the precore mutant in the context of limiting viral load and limiting HBc/HBeAg production. Limiting intracellular HBcAg may be a prerequisite for efficient selection of the HBeAg-negative mutant, as previously suggested (33). Although it was proposed that the mutant must have “outgrown” the wild-type virus, there is no evidence for enhanced replication of the precore stop codon mutant in a number of in vivo experimental infection models (11, 16, 30, 47, 54, 58). We propose as an alternative explanation that the greater sensitivity to liver injury in HBc/HBeAg-expressing versus HBcAg-expressing hepatocytes observed in this CTL adoptive transfer model predicts that wild-type virus would be preferentially cleared by the host immune response in this clinical setting.
In summary, this study reveals the limitation of relying entirely on in vitro CTL assays to examine the function of CTLs. The differences between CTL recognition of the HBc/HBeAgs were discernible only in an in vivo system in which the HBc/HBeAgs were expressed within the liver. In vitro CTL assays cannot reflect either the indirect effects that antigen expression within target tissues may have (i.e., hepatocytes) or aspects of CTL function such as CTL homing to target tissues or cellular recruitment by activated CTLs. Although the observation that HBc/HBeAg-specific CTLs can preferentially elicit liver injury in HBeAg-expressing hepatocytes must be confirmed in other Tg or infectious systems, it provides a mechanistic explanation for how the HBeAg-negative variant can represent a CTL escape mutant.
Acknowledgments
We gratefully acknowledge Francis V. Chisari (The Scripps Research Institute, La Jolla, CA) and all staff in his laboratory, especially Alana Althage, Masanori Isogawa, and Holly Maier, for scientific discussions, excellent help, and technical assistance. We thank Matti Sällberg, Karolinska Institutet, Stockholm, Sweden, for kindly providing the HBcAg-pVAX1 and HBeAg-pVAX1 expression vectors.
This study was supported by NIH grants R01 AI049730 and R01 AI20720. Lars Frelin was supported by grants from the Erik and Edith Fernströms Foundation and the Gålö Foundation/Gemzeús Foundation.
Footnotes
Published ahead of print on 12 November 2008.
REFERENCES
- 1.Acacia de Sa Pinheiro, A., A. Morrot, S. Chakravarty, M. Overstreet, J. H. Bream, P. M. Irusta, and F. Zavala. 2007. IL-4 induces a wide-spectrum intracellular signaling cascade in CD8+ T cells. J. Leukoc. Biol. 811102-1110. [DOI] [PubMed] [Google Scholar]
- 2.Akarca, U. S., S. Greene, and A. S. Lok. 1994. Detection of precore hepatitis B virus mutants in asymptomatic HBsAg-positive family members. Hepatology 191366-1370. [PubMed] [Google Scholar]
- 3.Ando, K., L. G. Guidotti, S. Wirth, T. Ishikawa, G. Missale, T. Moriyama, R. D. Schreiber, H. J. Schlicht, S. N. Huang, and F. V. Chisari. 1994. Class I-restricted cytotoxic T lymphocytes are directly cytopathic for their target cells in vivo. J. Immunol. 1523245-3253. [PubMed] [Google Scholar]
- 4.Ando, K., T. Moriyama, L. G. Guidotti, S. Wirth, R. D. Schreiber, H. J. Schlicht, S. N. Huang, and F. V. Chisari. 1993. Mechanisms of class I restricted immunopathology. A transgenic mouse model of fulminant hepatitis. J. Exp. Med. 1781541-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angus, P. W., S. A. Locarnini, G. W. McCaughan, R. M. Jones, J. S. McMillan, and D. S. Bowden. 1995. Hepatitis B virus precore mutant infection is associated with severe recurrent disease after liver transplantation. Hepatology 2114-18. [PubMed] [Google Scholar]
- 6.Bertoletti, A., C. Ferrari, F. Fiaccadori, A. Penna, R. Margolskee, H. J. Schlicht, P. Fowler, S. Guilhot, and F. V. Chisari. 1991. HLA class I-restricted human cytotoxic T cells recognize endogenously synthesized hepatitis B virus nucleocapsid antigen. Proc. Natl. Acad. Sci. USA 8810445-10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonino, F., F. Rosina, M. Rizzetto, R. Rizzi, E. Chiaberge, R. Tardanico, F. Callea, and G. Verme. 1986. Chronic hepatitis in HBsAg carriers with serum HBV-DNA and anti-HBe. Gastroenterology 901268-1273. [DOI] [PubMed] [Google Scholar]
- 8.Carman, W. F., M. R. Jacyna, S. Hadziyannis, P. Karayiannis, M. J. McGarvey, A. Makris, and H. C. Thomas. 1989. Mutation preventing formation of hepatitis B e antigen in patients with chronic hepatitis B infection. Lancet ii588-591. [DOI] [PubMed] [Google Scholar]
- 9.Carvalho, L. H., G. Sano, J. C. Hafalla, A. Morrot, M. A. Curotto de Lafaille, and F. Zavala. 2002. IL-4-secreting CD4+ T cells are crucial to the development of CD8+ T-cell responses against malaria liver stages. Nat. Med. 8166-170. [DOI] [PubMed] [Google Scholar]
- 10.Chang, C., G. Enders, R. Sprengel, N. Peters, H. E. Varmus, and D. Ganem. 1987. Expression of the precore region of an avian hepatitis B virus is not required for viral replication. J. Virol. 613322-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, H. S., M. C. Kew, W. E. Hornbuckle, B. C. Tennant, P. J. Cote, J. L. Gerin, R. H. Purcell, and R. H. Miller. 1992. The precore gene of the woodchuck hepatitis virus genome is not essential for viral replication in the natural host. J. Virol. 665682-5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, M., M. Sallberg, J. Hughes, J. Jones, L. G. Guidotti, F. V. Chisari, J. N. Billaud, and D. R. Milich. 2005. Immune tolerance split between hepatitis B virus precore and core proteins. J. Virol. 793016-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, M. T., J. N. Billaud, M. Sallberg, L. G. Guidotti, F. V. Chisari, J. Jones, J. Hughes, and D. R. Milich. 2004. A function of the hepatitis B virus precore protein is to regulate the immune response to the core antigen. Proc. Natl. Acad. Sci. USA 10114913-14918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chisari, F. V., and C. Ferrari. 1995. Hepatitis B virus immunopathogenesis. Annu. Rev. Immunol. 1329-60. [DOI] [PubMed] [Google Scholar]
- 15.Chu, C. M., C. T. Yeh, C. S. Lee, I. S. Sheen, and Y. F. Liaw. 2002. Precore stop mutant in HBeAg-positive patients with chronic hepatitis B: clinical characteristics and correlation with the course of HBeAg-to-anti-HBe seroconversion. J. Clin. Microbiol. 4016-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chuang, W. L., M. Omata, T. Ehata, O. Yokosuka, K. Hosoda, F. Imazeki, and M. Ohto. 1994. Coinfection study of precore mutant and wild-type hepatitis B-like virus in ducklings. Hepatology 19569-576. [DOI] [PubMed] [Google Scholar]
- 17.Encke, J., J. zu Putlitz, M. Geissler, and J. R. Wands. 1998. Genetic immunization generates cellular and humoral immune responses against the nonstructural proteins of the hepatitis C virus in a murine model. J. Immunol. 1614917-4923. [PubMed] [Google Scholar]
- 18.Frelin, L., M. Alheim, A. Chen, J. Soderholm, B. Rozell, C. Barnfield, P. Liljestrom, and M. Sallberg. 2003. Low dose and gene gun immunization with a hepatitis C virus nonstructural (NS) 3 DNA-based vaccine containing NS4A inhibit NS3/4A-expressing tumors in vivo. Gene Ther. 10686-699. [DOI] [PubMed] [Google Scholar]
- 19.Ganem, D., R. J. Schneider, D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, B. Roizman, M. A. Martin, and S. E. Straus. 2001. Hepadnaviridae and their replication, p. 2923-2970. In K. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 20.Glynn, J. P., J. L. McCoy, and A. Fefer. 1968. Cross-resistance to the transplantation of syngeneic Friend, Moloney, and Rauscher virus-induced tumors. Cancer Res. 28434-439. [PubMed] [Google Scholar]
- 21.Guidotti, L. G., T. Ishikawa, M. V. Hobbs, B. Matzke, R. Schreiber, and F. V. Chisari. 1996. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity 425-36. [DOI] [PubMed] [Google Scholar]
- 22.Guidotti, L. G., V. Martinez, Y. T. Loh, C. E. Rogler, and F. V. Chisari. 1994. Hepatitis B virus nucleocapsid particles do not cross the hepatocyte nuclear membrane in transgenic mice. J. Virol. 685469-5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guidotti, L. G., B. Matzke, C. Pasquinelli, J. M. Shoenberger, C. E. Rogler, and F. V. Chisari. 1996. The hepatitis B virus (HBV) precore protein inhibits HBV replication in transgenic mice. J. Virol. 707056-7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imai, M., M. Nomura, T. Gotanda, T. Sano, K. Tachibana, H. Miyamoto, K. Takahashi, S. Toyama, Y. Miyakawa, and M. Mayumi. 1982. Demonstration of two distinct antigenic determinants on hepatitis B e antigen by monoclonal antibodies. J. Immunol. 12869-72. [PubMed] [Google Scholar]
- 25.Kakimi, K., T. E. Lane, S. Wieland, V. C. Asensio, I. L. Campbell, F. V. Chisari, and L. G. Guidotti. 2001. Blocking chemokine responsive to gamma-2/interferon (IFN)-gamma inducible protein and monokine induced by IFN-gamma activity in vivo reduces the pathogenetic but not the antiviral potential of hepatitis B virus-specific cytotoxic T lymphocytes. J. Exp. Med. 1941755-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karre, K., H. G. Ljunggren, G. Piontek, and R. Kiessling. 1986. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 319675-678. [DOI] [PubMed] [Google Scholar]
- 27.Kuhober, A., H. P. Pudollek, K. Reifenberg, F. V. Chisari, H. J. Schlicht, J. Reimann, and R. Schirmbeck. 1996. DNA immunization induces antibody and cytotoxic T cell responses to hepatitis B core antigen in H-2b mice. J. Immunol. 1563687-3695. [PubMed] [Google Scholar]
- 28.Kuhrober, A., J. Wild, H. P. Pudollek, F. V. Chisari, and J. Reimann. 1997. DNA vaccination with plasmids encoding the intracellular (HBcAg) or secreted (HBeAg) form of the core protein of hepatitis B virus primes T cell responses to two overlapping Kb- and Kd-restricted epitopes. Int. Immunol. 91203-1212. [DOI] [PubMed] [Google Scholar]
- 29.Lazdina, U., M. Alheim, J. Nystrom, C. Hultgren, G. Borisova, I. Sominskaya, P. Pumpens, D. L. Peterson, D. R. Milich, and M. Sallberg. 2003. Priming of cytotoxic T cell responses to exogenous hepatitis B virus core antigen is B cell dependent. J. Gen. Virol. 84139-146. [DOI] [PubMed] [Google Scholar]
- 30.Ma, Z., Y. Wen, S. Xiong, W. Zhai, L. He, and X. Yao. 1998. Experimental study on pathogenicity of precore mutants in Hepadnaviridae. Chin. Med. J. 111519-523. [PubMed] [Google Scholar]
- 31.Maini, M. K., C. Boni, G. S. Ogg, A. S. King, S. Reignat, C. K. Lee, J. R. Larrubia, G. J. Webster, A. J. McMichael, C. Ferrari, R. Williams, D. Vergani, and A. Bertoletti. 1999. Direct ex vivo analysis of hepatitis B virus-specific CD8(+) T cells associated with the control of infection. Gastroenterology 1171386-1396. [DOI] [PubMed] [Google Scholar]
- 32.McLachlan, A., D. R. Milich, A. K. Raney, M. G. Riggs, J. L. Hughes, J. Sorge, and F. V. Chisari. 1987. Expression of hepatitis B virus surface and core antigens: influences of pre-S and precore sequences. J. Virol. 61683-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milich, D., and T. J. Liang. 2003. Exploring the biological basis of hepatitis B e antigen in hepatitis B virus infection. Hepatology 381075-1086. [DOI] [PubMed] [Google Scholar]
- 34.Milich, D. R. 1997. Influence of T-helper cell subsets and crossregulation in hepatitis B virus infection. J. Viral Hepat. 448-59. [DOI] [PubMed] [Google Scholar]
- 35.Milich, D. R., M. K. Chen, J. L. Hughes, and J. E. Jones. 1998. The secreted hepatitis B precore antigen can modulate the immune response to the nucleocapsid: a mechanism for persistence. J. Immunol. 1602013-2021. [PubMed] [Google Scholar]
- 36.Milich, D. R., J. E. Jones, J. L. Hughes, J. Price, A. K. Raney, and A. McLachlan. 1990. Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero? Proc. Natl. Acad. Sci. USA 876599-6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milich, D. R., A. McLachlan, S. Stahl, P. Wingfield, G. B. Thornton, J. L. Hughes, and J. E. Jones. 1988. Comparative immunogenicity of hepatitis B virus core and E antigens. J. Immunol. 1413617-3624. [PubMed] [Google Scholar]
- 38.Missale, G., A. Redeker, J. Person, P. Fowler, S. Guilhot, H. J. Schlicht, C. Ferrari, and F. V. Chisari. 1993. HLA-A31- and HLA-Aw68-restricted cytotoxic T cell responses to a single hepatitis B virus nucleocapsid epitope during acute viral hepatitis. J. Exp. Med. 177751-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 39.Okamoto, H., F. Tsuda, Y. Akahane, Y. Sugai, M. Yoshiba, K. Moriyama, T. Tanaka, Y. Miyakawa, and M. Mayumi. 1994. Hepatitis B virus with mutations in the core promoter for an e antigen-negative phenotype in carriers with antibody to e antigen. J. Virol. 688102-8110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okamoto, H., S. Yotsumoto, Y. Akahane, T. Yamanaka, Y. Miyazaki, Y. Sugai, F. Tsuda, T. Tanaka, Y. Miyakawa, and M. Mayumi. 1990. Hepatitis B viruses with precore region defects prevail in persistently infected hosts along with seroconversion to the antibody against e antigen. J. Virol. 641298-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ou, J. H., O. Laub, and W. J. Rutter. 1986. Hepatitis B virus gene function: the precore region targets the core antigen to cellular membranes and causes the secretion of the e antigen. Proc. Natl. Acad. Sci. USA 831578-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Penna, A., F. V. Chisari, A. Bertoletti, G. Missale, P. Fowler, T. Giuberti, F. Fiaccadori, and C. Ferrari. 1991. Cytotoxic T lymphocytes recognize an HLA-A2-restricted epitope within the hepatitis B virus nucleocapsid antigen. J. Exp. Med. 1741565-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rehermann, B., P. Fowler, J. Sidney, J. Person, A. Redeker, M. Brown, B. Moss, A. Sette, and F. V. Chisari. 1995. The cytotoxic T lymphocyte response to multiple hepatitis B virus polymerase epitopes during and after acute viral hepatitis. J. Exp. Med. 1811047-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roossinck, M. J., S. Jameel, S. H. Loukin, and A. Siddiqui. 1986. Expression of hepatitis B viral core region in mammalian cells. Mol. Cell. Biol. 61393-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sallberg, M., U. Ruden, L. O. Magnius, E. Norrby, and B. Wahren. 1991. Rapid “tea-bag” peptide synthesis using 9-fluorenylmethoxycarbonyl (Fmoc) protected amino acids applied for antigenic mapping of viral proteins. Immunol. Lett. 3059-68. [DOI] [PubMed] [Google Scholar]
- 46.Schlicht, H. J., J. Salfeld, and H. Schaller. 1987. The duck hepatitis B virus pre-C region encodes a signal sequence which is essential for synthesis and secretion of processed core proteins but not for virus formation. J. Virol. 613701-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schneider, R., D. Fernholz, G. Wildner, and H. Will. 1991. Mechanism, kinetics, and role of duck hepatitis B virus e-antigen expression in vivo. Virology 182503-512. [DOI] [PubMed] [Google Scholar]
- 48.Schodel, F., A. M. Moriarty, D. L. Peterson, J. A. Zheng, J. L. Hughes, H. Will, D. J. Leturcq, J. S. McGee, and D. R. Milich. 1992. The position of heterologous epitopes inserted in hepatitis B virus core particles determines their immunogenicity. J. Virol. 66106-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schodel, F., D. Peterson, J. Zheng, J. E. Jones, J. L. Hughes, and D. R. Milich. 1993. Structure of hepatitis B virus core and e-antigen. A single precore amino acid prevents nucleocapsid assembly. J. Biol. Chem. 2681332-1337. [PubMed] [Google Scholar]
- 50.Sitia, G., M. Isogawa, M. Iannacone, I. L. Campbell, F. V. Chisari, and L. G. Guidotti. 2004. MMPs are required for recruitment of antigen-nonspecific mononuclear cells into the liver by CTLs. J. Clin. Investig. 1131158-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sitia, G., M. Isogawa, K. Kakimi, S. F. Wieland, F. V. Chisari, and L. G. Guidotti. 2002. Depletion of neutrophils blocks the recruitment of antigen-nonspecific cells into the liver without affecting the antiviral activity of hepatitis B virus-specific cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. USA 9913717-13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Street, M., K. Herd, P. Londono, T. Doan, G. Dougan, W. M. Kast, and R. W. Tindle. 1999. Differences in the effectiveness of delivery of B- and CTL-epitopes incorporated into the hepatitis B core antigen (HBcAg) c/e1-region. Arch. Virol. 1441323-1343. [DOI] [PubMed] [Google Scholar]
- 53.Takeda, K., Y. Akahane, H. Suzuki, H. Okamoto, F. Tsuda, Y. Miyakawa, and M. Mayumi. 1990. Defects in the precore region of the HBV genome in patients with chronic hepatitis B after sustained seroconversion from HBeAg to anti-HBe induced spontaneously or with interferon therapy. Hepatology 121284-1289. [DOI] [PubMed] [Google Scholar]
- 54.Tomita, T., O. Yokosuka, M. Tagawa, H. Saisho, S. Tamura, I. Fukuda, and M. Omata. 2000. Decrease of wild-type and precore mutant duck hepatitis B virus replication during lamivudine treatment in white Pekin ducks infected with the viruses. J. Hepatol. 32850-858. [DOI] [PubMed] [Google Scholar]
- 55.Townsend, K., M. Sallberg, J. O'Dea, T. Banks, D. Driver, S. Sauter, S. M. Chang, D. J. Jolly, S. J. Mento, D. R. Milich, and W. T. Lee. 1997. Characterization of CD8+ cytotoxic T-lymphocyte responses after genetic immunization with retrovirus vectors expressing different forms of the hepatitis B virus core and e antigens. J. Virol. 713365-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weimer, T., J. Salfeld, and H. Will. 1987. Expression of the hepatitis B virus core gene in vitro and in vivo. J. Virol. 613109-3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yeh, C. T., W. P. Lin, C. W. Hsu, M. L. Chang, S. M. Lin, and I. S. Sheen. 2006. Emergence and takeover of precore-stop mutant prior to exacerbation of e antigen-negative chronic hepatitis B after withdrawal of lamivudine therapy. J. Med. Virol. 78906-910. [DOI] [PubMed] [Google Scholar]
- 58.Zhang, Y. Y., and J. Summers. 1999. Enrichment of a precore-minus mutant of duck hepatitis B virus in experimental mixed infections. J. Virol. 733616-3622. [DOI] [PMC free article] [PubMed] [Google Scholar]