Abstract
Antibody neutralization is an important component of protective immunity against vaccinia virus (VACV). Two distinct virion forms, mature virion and enveloped virion (MV and EV, respectively), possess separate functions and nonoverlapping immunological properties. In this study we examined the mechanics of EV neutralization, focusing on EV protein B5 (also called B5R). We show that neutralization of EV is predominantly complement dependent. From a panel of high-affinity anti-B5 monoclonal antibodies (MAbs), the only potent neutralizer in vitro (90% at 535 ng/ml) was an immunoglobulin G2a (IgG2a), and neutralization was complement mediated. This MAb was the most protective in vivo against lethal intranasal VACV challenge. Further studies demonstrated that in vivo depletion of complement caused a >50% loss of anti-B5 IgG2a protection, directly establishing the importance of complement for protection against the EV form. However, the mechanism of protection is not sterilizing immunity via elimination of the inoculum as the viral inoculum consisted of a purified MV form. The prevention of illness in vivo indicated rapid control of infection. We further demonstrate that antibody-mediated killing of VACV-infected cells expressing surface B5 is a second protective mechanism provided by complement-fixing anti-B5 IgG. Cell killing was very efficient, and this effector function was highly isotype specific. These results indicate that anti-B5 antibody-directed cell lysis via complement is a powerful mechanism for clearance of infected cells, keeping poxvirus-infected cells from being invisible to humoral immune responses. These findings highlight the importance of multiple mechanisms of antibody-mediated protection against VACV and point to key immunobiological differences between MVs and EVs that impact the outcome of infection.
Vaccines are one of the most cost-effective medical treatments in modern civilization (80). A smallpox vaccine was the first human vaccine, and the modern smallpox vaccine, live vaccinia virus (VACV), is the most successful human vaccine, bringing about the worldwide eradication of smallpox disease due to a heroic World Health Organization campaign in the 1960s and 1970s (27). Elucidating the immunobiology underlying the protection provided by the smallpox vaccine will continue to reveal vaccinology principles that can be applied to future vaccine development against other infectious scourges (95). Neutralizing antibodies are of primary importance in the protection from smallpox provided by the smallpox vaccine in animal models (6, 25, 33, 64) and humans (4). Vaccinia immune globulin (VIG) has been shown to be an effective treatment against smallpox (45) as it was able to reduce the number of smallpox cases by ∼80% among exposed individuals in four case-controlled studies (42, 45, 50, 51, 66).
Neutralizing antibodies confer protection mainly through the recognition of structures on the surface of virus particles, and therefore antiviral antibodies directed against the surface of virions are of primary interest. There are several interesting features and problems of the antibody response to variola and related poxviruses, including the large size of the viral particles and the abundance of distinct surface proteins (>25 total) (17, 70, 88). Poxviruses (vaccinia, variola/smallpox, and monkeypox) have two virion forms, intracellular mature virions (MV) and extracellular enveloped virions (EV), each with distinct biology (17, 88). For this reason, an understanding of the virion structures is required to develop knowledge regarding the targets of protective antibodies. MV and EV forms express mutually exclusive sets of viral proteins on the surface (17, 70, 88). The most abundant particle is the MV, which accumulates in infected cells and is released as cells die (70). The relative roles of antibodies against MV and EV in protective immunity still remain somewhat unclear. A number of groups have discovered neutralizing antibody targets of poxviruses in animals and humans (4). Animal model studies have clearly shown that antibodies against either the MV or EV form can be protective (4). Neutralization of MV is relatively well characterized. Neutralization of EV remains more enigmatic. There are compelling data that antibodies against MV (4, 21, 31, 43, 64, 79, 84) or EV are sufficient for protection (14, 15, 33, 64), and a combination of antibodies against both targets is most protective (64). It remains controversial as to whether antibodies to one virion form are more important than antibodies to the other (57, 64).
The focus of the study reported here is to elucidate the mechanics of EV neutralization, focusing on the EV protein B5 (also called B5R or WR187). Our overall goal is to understand underlying immunobiological and virological parameters that determine the emergence of protective immune responses, utilizing the smallpox vaccine to come to such an understanding.
B5 was identified as a protective antigen in early DNA immunization studies, and the available evidence indicated that the protection was mediated by anti-B5 antibodies (33). Since then, a series of studies has examined B5 as a potential recombinant vaccine antigen or as a target of therapeutic monoclonal antibodies (MAbs) (1, 15, 39, 44, 64, 101). It is known that humans immunized with the smallpox vaccine make antibodies against B5 (5, 22, 59, 77). It is also known that animals receiving the smallpox vaccine generate antibodies against B5 (20, 25). Furthermore, available neutralization assays have indicated that it is only the antibody response to B5 that is responsible for neutralization of VACV EV (5, 78).
We set out to further understand the mechanics of poxvirus neutralization, and we generated a new panel of anti-B5 MAbs to do so. This panel of anti-B5 MAbs has also served as a starting point for the generation of fully human MAbs against B5 that can potentially be used as a therapeutic agent against vaccinia, monkeypox, or variola.
MATERIALS AND METHODS
Virus strains.
VACV Western Reserve was used unless otherwise stated. VACVNYCBOH stocks were generated as low-passage stocks from commercial Dryvax, using MV preparation conditions. VACVIHDJ was obtained from Stuart Isaacs (University of Pennsylvania), and stocks were grown using MV preparation conditions. Recombinant VACV B5-green fluorescent protein ([GFP] VACV/B5-GFP) was obtained from Bernard Moss (NIH) (96) (97), and stocks were prepared following the method of preparation of VACV MV.
VACV MV preparations.
VACV stocks were grown on HeLa cells in D-10 medium (Dulbecco's modified Eagle's medium [DMEM] supplemented with 10% fetal calf serum [FCS] and penicillin/streptomycin and glutamine) in T175 flasks (Falcon, Becton Dickinson) to 80 to 90% confluence, and cells were infected at a multiplicity of infection (MOI) of 0.5. FCS used in all experiments was heat inactivated (at 56°C for 30 to 60 min) prior to use to eliminate complement activity. Cells were harvested at 2.5 days, and virus was isolated by rapidly freeze-thawing the cell pellet three times in a volume of 2.3 ml of DMEM supplemented with 1% heat-inactivated FCS. Cell debris was removed by centrifugation (at 700 × g for 8 min). Virus titers at this stage were in the following ranges: VACV, 2 × 108 to 8 × 108 PFU/ml; VACVNYCBOH, 1 × 108 to 2 × 108 PFU/ml; VACVIHDJ, 2 × 108 to 4 × 108 PFU/ml; VACV/B5-GFP, 5 × 108 PFU/ml. Purified VACV stocks were made via centrifugation through a sucrose cushion. Virus was sonicated (for 40 s) using a water sonicator (Branson Ultrasonics, CT) and carefully layered over 36% sucrose in 17 ml of TM buffer (10 mM Tris-HCl, pH 7.4, 5 mM MgCl2) containing 36% sucrose. VACV was centrifuged (in an SW28 rotor) at 13,500 rpm (33,000 × g) for 80 min at 4°C (16). The VACV pellet was resuspended in 1 ml of TM buffer and then brought up to 10 ml with DMEM supplemented with 1% heat-inactivated FCS. Purified VACV was stored at −80°C.
VACV EV.
EV of VACV were prepared using HeLa cells in D-10 medium in T75 flasks (Falcon; Becton Dickinson) at 90% confluence, and cells were infected at an MOI of 0.5. FCS used in all experiments was heat inactivated (at 56°C for 30 to 60 min) prior to use. The medium containing the EV form was harvested at 2 days, and virus was isolated by centrifugation twice (450 × g for 8 min) to remove cells. Clarified supernatant was used immediately or stored at 4°C for a maximum of 3 weeks (1). VACV EV titers were determined on Vero cells (∼5 × 105 PFU/ml).
Baculovirus-expressed rB5 protein.
Viral DNA was isolated from VACVNYCBOH-infected HeLa cells using a QIAamp DNA Mini Kit (Qiagen Inc., Valencia, CA) following the manufacturer's instructions. The sequence encoding the extracellular domain of vaccinia B5 (amino acids 1 to 312) was amplified by reverse transcription-PCR using primers B5-Ftopo dir gate (CACCATGAAAACGATTTCCGTTGTTA) and B5-Rtopo dir gate (TTCTAACGATTCTATTTCTTGTTCATATTGTAC). The product was subcloned into the pENTR/D-TOPO Gateway entry vector (Invitrogen Corp, Carlsbad, CA). The cloned PCR-amplified product was sequenced and confirmed to be identical to the published sequence of the B5 protein of the VACV NYCBOH strain. Recombinant baculovirus was generated that encoded the C-terminal six-His-tagged vaccinia B5 (rB5) protein by performing the recombination reaction between the Gateway pENTR/D-TOPO B5-His and the Gateway BaculoDirect C-terminal linear DNA (Invitrogen Corp, Carlsbad, CA). Trichoplusia ni High-Five BTI-TN-5b1-4 insect cells (Invitrogen Corp., Carlsbad, CA) were infected with the B5 recombinant baculovirus for protein production. The supernatant was harvested 4 days after the infection of BTI-TN-5b1-4 cells with the recombinant baculovirus and stored at −80°C until purification. Protein was concentrated and diafiltered into phosphate-buffered saline (PBS) by tangential flow filtration using a Sartocon cassette with Hydrosart membrane (Sartorius; 10-kDa-molecular-weight cutoff). rB5 protein was purified by nickel chelate affinity chromatography with Ni-Sepharose 6 Fast Flow resin (GE Healthcare). Nonspecific binding was reduced with a washing step using 20 mM imidazole for five column volumes, and the target protein was eluted with a 20 to 500 mM imidazole gradient. The B5 peak corresponding to 250 mM imidazole was pooled and subsequently dialyzed against PBS.
Hybridomas.
rB5 protein was mixed with an equal volume of complete Freund's adjuvant (CFA; Sigma), and an emulsion was prepared. BALB/c mice were immunized subcutaneously with 25 to 50 μg of rB5 protein in CFA. Mice were boosted subcutaneously with 10 to 20 μg of protein emulsified in incomplete Freund's adjuvant (IFA; Sigma) at 1- to 2-week intervals for two boosts. The first boost was with 10% CFA-90% IFA; the second boost was with IFA alone. A final intravenous injection of 10 μg of rB5 without adjuvant was given 3 days prior to fusion. Spleens were harvested, and single-cell suspensions were fused to a myeloma cell line (SP2/O-Ag14) (ATCC, Rockville, MD) at a ratio of 5:1 with 50% polyethylene glycol (Boehringer Mannheim, Indianapolis, IN) to generate hybridomas. The fusions were plated into 96-well flat bottom plates at an optimal density and cultured in complete D-10 (DMEM with 10% fetal bovine serum [FBS; Invitrogen, Corp.], 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin sulfate [all from BioWhittaker, Walkersville, MD]), HAT (hypoxanthine, aminopterin, and thymidine) supplement (Sigma), and 10% hybridoma cloning factor (Biovaris, San Diego, CA) in 10% CO2 at 37°C in an incubator. Crude hybridoma supernatants were screened for B5 specificity using an rB5 enzyme-linked immunosorbent assay (ELISA). Positive wells were expanded and subjected to two to three rounds of limiting dilution cloning to obtain MAbs.
For antibody purification, hybridomas were cultured in 2-liter roller bottles at 350 ml to 1 liter/bottle or in a 1-liter Integra system (Integra Bioscience, Inc. Ijamsville, MD) with hybridoma-serum-free medium (Invitrogen, Corp.) supplemented with ultralow immunoglobulin G (IgG) FBS (Invitrogen, Corp.) MAbs were purified from culture medium using recombinant protein A-Sepharose Fast Flow gel (Amersham Biosciences). Conditioned medium generated in roller bottles was first concentrated using an Ultrasette tangential flow system (Pall Corporation, East Hills, NY). The conditioned medium was filtered with a 0.22-μm-pore-size vacuum filter unit (Millipore, Bedford, MA) and loaded onto a protein A-Sepharose Fast Flow column of appropriate size for the amount of human antibody in the medium. The column was washed thoroughly with 20 column volumes of PBS, and the antibody was eluted with 0.1 M Gly-HCl (pH 3.6)-0.15 M NaCl and neutralized with 1 M Tris-HCl, pH 8.0. The fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the positive fractions were pooled and concentrated with a centrifugal concentrator (Vivaspin; 50,000-molecular-weight cutoff; Sartorius, Gettingen, Germany). Sephadex G-25 desalting columns (NAP; Amersham Biosciences) were used for buffer exchange to PBS, pH 7.4. Finally, the antibody was filter sterilized using syringe filters with 0.22-μm-pore-size diameters, and the antibody concentration was determined by the Lowry method. Pyrogen content was determined using a Limulus amebocyte lysate assay (Associates of Cape Cod, Falmouth, MA). The limits of detection of this assay are 0.06 endotoxin units/mg. If the test was negative, the samples were considered endotoxin free.
rB5 ELISA.
Flat-bottomed 96-well microtiter plates (Nunc Maxisorp) were coated overnight at 4°C with 100 μl of 1 μg/ml of rB5 protein in PBS. Plates were washed (PBS plus 0.2% Tween 20) and blocked (PBS plus 0.5% bovine serum albumin and 0.2% Tween 20). Hybridoma supernatants were screened without dilution. For MAbs, standard serial dilution ELISA followed. The secondary antibody was streptavidin-horseradish peroxidase-conjugated goat anti-mouse IgG1 or IgG2a (Caltag Laboratories, Burlingame, CA). The EC50, the half-saturation binding concentration of the antibody corresponding to 50% maximum optical density, for each individual anti-B5 MAb was determined by sigmoidal dose-response nonlinear regression using a variable slope (Prism 5.0).
Whole VACV ELISA.
The purified VACV MV stock was tested for VACV EV antigen using a whole VACV MV ELISA. Direct ELISA was done using Nunc Maxisorp flat-bottomed 96-well plates coated overnight at 4°C with 100 μl/well of UV-inactivated MV of VACV (5 × 107 PFU/ml and 51 μg/ml, prior to 1:25 dilution in PBS), as described previously (18, 89).
Protein synthesis and proteome arrays.
VACV protein microarrays were produced and used as described previously (7). The secondary antibody was Cy3-conjugated goat anti-mouse IgG gamma chain Fc-region-specific Ig (Jackson Immunoresearch). Signal strength was quantified as total 532-nm fluorescence intensity of each spot. Background signal was subtracted out using relevant matched control samples (e.g., rapid translation system reaction without plasmid or buffer alone), and background-subtracted signal was converted to the final IgG-relative units via 10−6 transformation.
Sera.
Rabbit-anti L1 serum (n = 2) was generated by immunizing two rabbits with recombinant L1 protein (200 μg of recombinant L1/dose) emulsified in CFA and boosted three times at weeks 3, 6, and 10 with antigen (100 μg of recombinant L1/dose) in IFA (ProSci, Poway, CA). Mouse anti-VACV serum was obtained from C57BL/6J mice (Jackson Laboratory) at various time points after VACV intraperitoneal (i.p.) infection (2 × 106 or 1 × 107 PFU). Mouse and rabbit sera were heat inactivated prior to use to eliminate complement activity (at 56°C for 30 and 60 min, respectively). Human VIG (50 mg/ml; Cangene Corp., Winnipeg, Canada) was stored at −80°C.
VACV EV neutralization.
Vero E6 cells were seeded at 1.5 × 105 cells/well into 24-well Costar plates (Corning Inc., Corning, NY) and used the following day (75 to 90% confluence). Antibody or serum samples in D-10 medium were used in the absence or the presence of 10% sterile baby rabbit complement (Cedarlane Laboratories, Ontario, Canada). Diluted antibody samples (sera or MAbs) were then incubated at 37°C in an equal volume (50 μl) of VACV EV (1:100 to 1:400 dilution from stock), supplemented with rabbit anti-L1 (1:25 to 1:100 final) for 30 min. All serum samples were heat inactivated prior to use to eliminate complement function. Rabbit anti-L1 was used to block the MV present in the EV stock (1, 33, 91), except in the experiment shown in Fig. 3E to G, where anti-L1 was included or excluded as described below. In all experiments with serial dilutions, twofold dilutions of serum or MAb samples were used. VACV EV samples supplemented with anti-L1 antibody alone were regularly used in each assay, with or without baby rabbit complement as negative controls. Medium from 24-well plates was aspirated, and samples were added and allowed to adsorb for 45 min at 37°C. Cells were rinsed with warm PBS. One milliliter of D-10 medium was then added, and the plates were incubated for 40 to 48 h. Cells were fixed and stained with 0.1% crystal violet in 20% reagent alcohol (90% ethanol, 5% methanol, and 5% isopropanol), and plaques were quantified. A 50% plaque reduction was calculated from a VACV EV sample with anti-L1 alone. In titration experiments, 90% and 50% EV neutralization rates were calculated based on sigmoidal dose-response nonlinear regression (Prism software, version 5.0). For the time course of VACV EV neutralization (see Fig. 7), VACV EV samples (with anti-L1 at a final concentration of 1:50) were added to MAb with rabbit complement (10% final) for various time periods at 37°C in 5% CO2. The VACV EV plaque assay was developed as described above. The plaque numbers were quantified in each time point and plotted. Rabbit complement was used in all complement assays except for the experiment shown in Fig. 7C (see below). In a separate series of experiments, mouse complement was used in EV neutralization assays with similar results though maximal neutralization was 75% with the use of mouse complement in vitro. Mouse complement was prepared by retro-orbital bleed of C57BL/6 mice with heparin-free Pasteur pipettes on ice, followed by centrifugation (at >12,000 × g) at 4°C to remove red blood cells; then supernatant (mouse complement fraction) was frozen at −80 C until use.
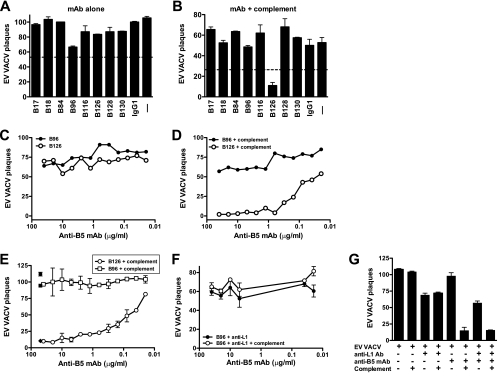
FIG. 3.
Complement-dependent MAb neutralization of EV. VACV EV neutralization is dependent on complement and anti-B5 monoclonal antibody isotype. (A and B) VACV EV neutralization activity of the full panel of MAbs against B5 at 10 μg/ml in the absence (A) or the presence (B) of complement. EV alone (− lane) and mouse anti-dinitrophenol control IgG1 plus EV (IgG1) were negative controls. (C and D) Titrated VACV EV neutralization activity of murine anti-B5 MAbs B126 (IgG2a) and B96 (IgG1) in the absence (C) or the presence (D) of complement. Data are represented as plaque numbers. The 50% and 90% neutralization titers of B126 (107 ng/ml and 535 ng/ml, respectively) were determined based on sigmoidal dose-response nonlinear regression. All data are representative of three or more experiments, each of which was done in the presence of anti-L1 antibody. Error bars indicate SEM in each condition. (E to G) Titrated VACV EV neutralization activity of murine anti-B5 MAbs B126 (IgG2a) and B96 (IgG1) in the absence or the presence of complement and with or without rabbit anti-L1 antibody. (E) In the absence of anti-L1 antibody, the neutralization activity of B126 plus complement or B96 plus complement was titrated. Control samples are shown at single dilutions: B126 alone (filled circle), B96 alone (filled square), and B126 with complement and rabbit anti-L1 antibody (filled diamond). (F) Titration of B96 plus anti-L1 antibody and B96 plus anti-L1 antibody and complement. (G) Quantitation of conditions described in panels E and F and additional controls, with anti-B5 MAb B126 at 10 μg/ml. Data in panels E to G are representative of three independent experiments. Error bars indicate SEM for each condition.
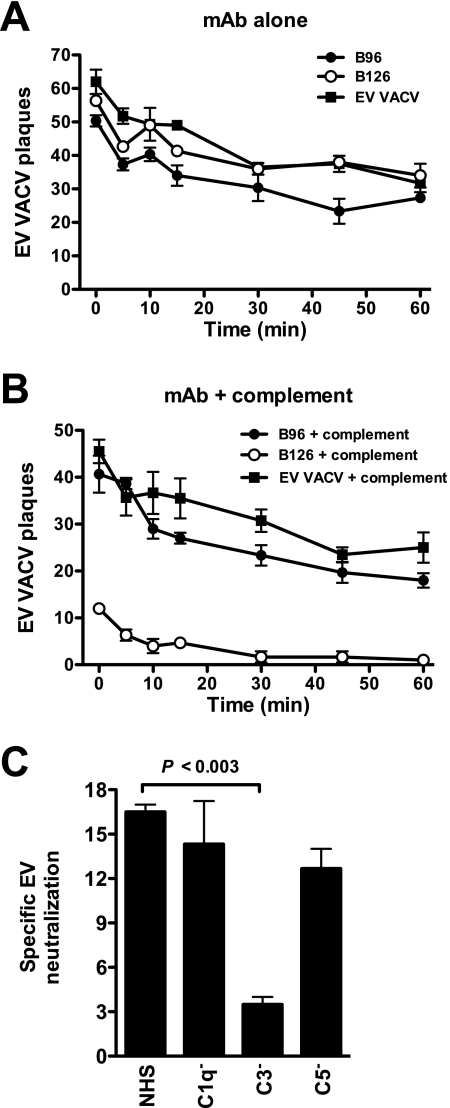
FIG. 7.
Complement kinetics and components. (A and B) Kinetics of VACV EV neutralization activity in vitro of murine anti-B5 MAbs (B126 and B96) at 10 μg/ml in the absence or the presence of rabbit complement. Data are shown as plaque numbers. One of three independent experiments is shown. VACV EV is fully neutralized by anti-B5 MAb B126 within 5 min of the addition of complement. (C) VACV EV neutralization by the anti-B5 MAb and the addition of normal human serum (NHS) complement or C1q-, C3-, or C5-depleted serum. Specific VACV EV neutralization in the absence of C3 was statistically significant compared to the value for normal human serum (P < 0.003). One of three independent experiments is shown. Error bars indicate SEM for each group.
Comet tail plaque formation.
Vero E6 cells were seeded at 5 × 105 cells/well into six-well Costar plates and used the following day (75 to 90% confluence). Vero E6 cells were infected with the VACVIHDJ strain (20 to 60 PFU/well) for 60 min at 37°C in 5% CO2 and then washed and overlaid with 2 ml of medium containing 20 μg/ml of anti-B5 MAbs or a 1/400 dilution of serum from VACV-immunized mice (45 days postinfection) or naïve mouse serum. Anti-mouse IgG was used as an isotype control at 20 μg/ml. Plates were tilted at approximately a 15° angle during incubation to enhance convection currents (56). After 50 to 52 h of incubation without disturbance, cells were stained with crystal violet.
Mice.
Female BALB/cByJ mice (Jackson Laboratory) 8 to 12 weeks of age were used in all experiments unless otherwise noted. Serum studies in shown in Fig. 2 and 4 used C57BL/6J females (Jackson Laboratory). The SCID model used 8- to 12-week-old female SCID mice on a BALB/c background (Jackson Laboratory or Taconic).
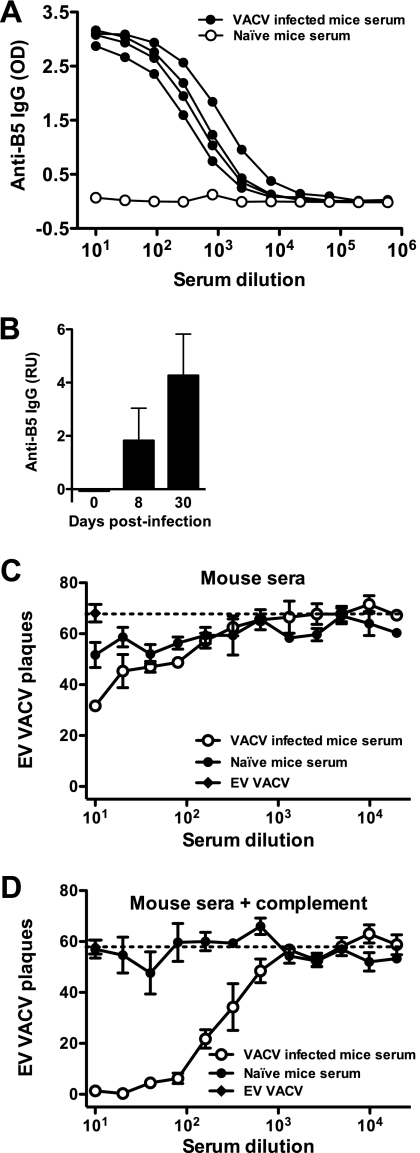
FIG. 2.
Anti-B5 IgG responses in VACV-infected mice. (A) B5 ELISA of serum from VACV-infected mice at day 8 postinfection. (B) Quantitation of anti-B5 IgG by protein microarray at days 8 and 30 postinfection. (C and D) VACV EV neutralizing antibody activity of the day 30 mouse serum described above in the absence (C) or presence (D) of complement. Plaque assay results are graphed. A dashed line indicates the plaque number of VACV EV alone (C) or in the presence of complement without antibody (D). Data are representative of two experiments. Error bars indicate SEM for each condition. OD, optical density; RU, relative units.
Vaccinia intranasal infection protection studies.
Age-matched female BALB/cByJ mice were used in all experiments. To infect the mice, a Pipetman was used to place 5 μl of VACV on each nare of an isofluorane-anesthetized mouse (total volume, 10 μl), and the liquid was rapidly inhaled by the mouse. Mice were weighed daily to assess disease progression. After intranasal infection with VACV, mice develop a systemic infection and exhibit severe weight loss. Mice were euthanized if and when 30% weight loss occurred. A dose of 5 × 104 PFU of VACV was the standard lethal dose given to 8-week-old BALB/c females. For B5 MAb protection studies, mice were treated i.p. with various doses (5 to 100 μg) of anti-B5 antibody 1 day before infection. Control mice received PBS alone. An additional group received 1.25 mg of VIG, the human mg/kg of body weight dose equivalent.
Vaccinia intravenous infection protection studies.
To infect mice, 1 × 103 PFU of VACV was injected intravenously via the tail vein. Mice were weighed daily to assess disease progression. For B5 MAb protection studies, mice were inoculated i.p. with 100 μg of anti-B5 antibody 1 day before infection. Control mice received PBS alone. An additional group received 1.25 mg of VIG, the human mg/kg of body weight dose equivalent.
In vivo complement depletion studies.
To assess the functionality of the anti-B5 MAbs in the absence of complement, cobra venom factor (CVF) was used to deplete complement in vivo (9, 90). Native CVF from Naja naja kaouthia (EMD Chemicals, Gibbstown, NJ) was administered i.p. to age-matched BALB/cByJ female mice in 30-μg doses at days −1, 2, and 5. Complement depletion was confirmed by C3 ELISA. Mice were treated with anti-B5 MAbs at day −1 and infected intranasally with VACV at day 0 as above. Similar results were observed with 2 μg of CVF per dose per mouse (data not shown). Complement could be depleted for 6 days. More prolonged depletion was not attainable, even after multiple CVF injections of various amounts and on various administration schedules. We interpreted these results as due to the rapid generation of neutralizing antibodies to CVF in the mice, which led to recovery of normal serum complement levels by day 7 after the initiation of CVF treatment.
Complement pathway.
Vero E6 cells were seeded at 5 × 105 cells/well into six-well Costar plates and used the following day (75 to 90% confluence). Murine MAbs (B126 or B96) against B5 at 20 μg/ml in D-10 medium were used in the absence or the presence of 10% of rabbit complement (positive control), normal human serum complement, or human serum depleted of C1q, C3, or C5 (Quidel, San Diego, CA). VACV EV supplemented with anti-L1 antibody was also used in the absence or the presence of each complement as a negative control. Samples were then incubated in an equal volume of VACV EV (100 μl) at a 1:100 dilution of EV (with a 1:50 final concentration of anti-L1 antibody) for 30 min at 37°C in 5% CO2. The VACV EV plaque assays were developed as described above. Plaque numbers were quantified, and specific VACV EV neutralization was used for each complement sample tested: PNIgG1 − PNIgG2a, where PNIgG1 is the plaque number after treatment with IgG1 B96, and PNIgG2a is the plaque number after treatment with IgG2a anti-B5 MAb B126.
Surface staining of B5.
For the experiment shown in Fig. 1C, Vero E6 cell monolayers (1 × 106 cells/well) were infected with VACV/B5-GFP for 12 h at an MOI of 5 at 37°C in 5% CO2. Infected cells were washed twice with plain DMEM and treated with a standard serial dilution of murine anti-B5 MAbs B126 (IgG2a), B96 (IgG1), and B116 for 45 min at a starting concentration of 40 μg/ml or with D-10 medium alone. Cells were briefly trypsinized in each well and washed twice with fluorescence-activated cell sorter (FACS) buffer (PBS plus 0.5% bovine serum albumin). Cells were incubated with a 1:100 dilution of a phycoerythrin-conjugated anti-mouse IgG(H+L) antibody (Jackson Immunoresearch, West Grove, PA) in FACS buffer at 100 μl/well for 20 min at 4°C. Cells were washed twice with FACS buffer, fixed with 2% paraformaldehyde, and collected using a FACSCalibur instrument (BD). Data were analyzed, and mean fluorescence intensity (MFI) of surface B5 expression was determined using FlowJo software.
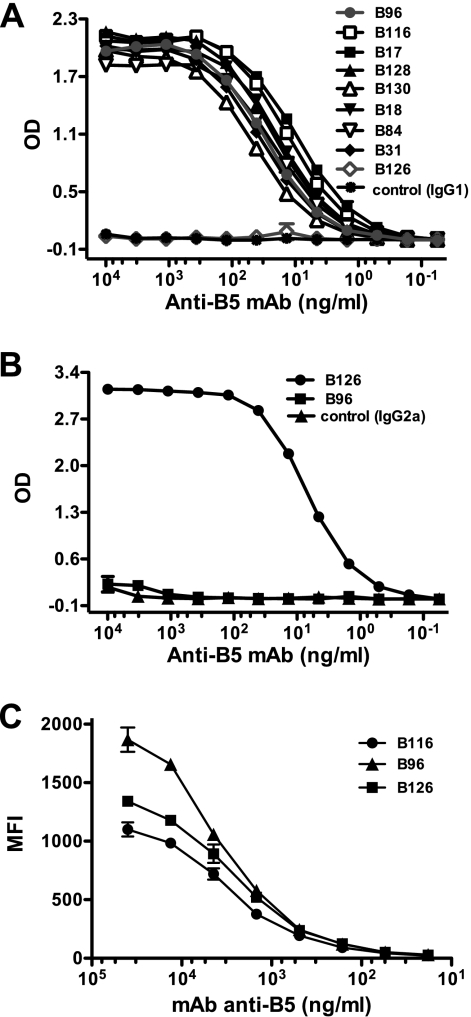
FIG. 1.
Binding affinities of anti-B5 MAbs. Titration of anti-B5 MAbs using IgG1-specific (A) and IgG2a-specific (B) ELISAs. (C) Mean fluorescence intensity (MFI) of cell-based ELISA quantitating surface-bound anti-B5 MAbs to native B5 on VACV-infected cells. Serial dilution of each anti-B5 MAb was performed. Data are representative of three independent experiments. OD, optical density.
For the experiment shown in Fig. 9A, Vero E6 cell monolayers were infected with VACV or VACV/B5-GFP for 2, 4, 8, and 10 h at an MOI of 4 to 8 at 37°C in 5% CO2. Infected cells were briefly trypsinized and then washed twice with FACS buffer. B5 expression was tested with rabbit anti-B5 serum (1:100) or anti-B5 MAbs in FACS buffer for 30 min. Uninfected cells (control cells) are represented as time zero. The secondary antibody was goat anti-rabbit IgG-allophycocyanin (Santa Cruz) or goat anti-mouse IgGγ (Caltag). Cells were washed, incubated for 5 min with 7-aminoactinomycin D and collected using a FACSAria (BD Biosciences, San Jose, CA). Dead cells were excluded from the analysis gate based on forward-scatter and side-scatter profiles and also using the 7-aminoactinomycin D channel. In some experiments GFP expression by VACV/B5-GFP was used as an additional parameter for comparison of total B5 expression.
FIG. 9.
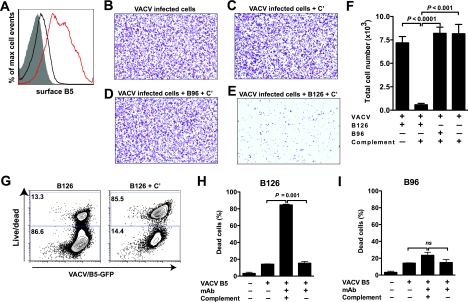
Complement and anti-B5 IgG2a cooperate to efficiently mediate destruction of VACV-infected cells. Anti-B5 antibodies are able to direct complement lysis of VACV-infected cells due to their surface expression of B5. (A) Cell monolayers (Vero E6) were infected with VACV (MOI of 5), and surface expression of B5 was tested at 4 h (black line) and 8 h (red line) after infection by surface-staining infected cells with anti-B5 MAb and performing flow cytometry. Uninfected cells, negative control (filled curve). (B to F) Anti-B5 directed complement lysis of infected cells. Virus-infected Vero E6 monolayer cells (crystal violet stained) at a magnification of ×40. VACV-infected cells were treated with medium (B) or complement (+ C′) (C to E) in the absence (C) or presence of anti-B5 IgG1 MAb B96 (D) or IgG2a MAb B126 (E). VACV-infected cells were completely and specifically destroyed by anti-B5 IgG2a and complement. (F) Quantitation of live cell numbers. Destruction of VACV-infected cells was highly statistically significant in the presence of anti-B5 MAb B126 and complement versus B126 alone (P < 0.0001), complement alone (P < 0.001), or B96 plus complement (P < 0.0001). No killing was observed for IgG1 B96 in the absence or presence of complement (P ≫ 0.05; nonsignificant). (G to I) Anti-B5 directed killing of virus-infected cells was assessed in a separate series of experiments using flow cytometric assays. Cells were infected with VACV-B5-GFP and treated with anti-B5 MAb B126 in the absence (left panel) or presence (right panel) of complement, and cell killing was determined by measuring the uptake of a viability dye (live/dead) by damaged cells (G). Killed infected cells exhibit intense live/dead fluorescence staining (y axis). Infected cells were identified by B5-GFP expression (x axis). Complement-mediated cell killing by anti-B5 IgG2a B126 was effective and highly statistically significant (P = 0.001 versus B126 alone or complement alone) (H). When IgG1 MAb B96 in the absence or presence of complement was used, no statistically significant (ns) differences were observed (I). Error bars indicate SEM for each group. One of three independent experiments is shown in panels A to F. One of two independent experiments is shown in panels G to I.
Complement lysis assay, plate bound.
Vero E6 monolayer cells (1 × 106 cells/well) were infected with VACV at an MOI of 4 to 8 and incubated for 12 h at 37°C in 5% CO2. Infected cells were washed twice and pretreated with D-10 medium alone or with medium containing 10 μg/ml anti-B5 MAb B126 or B96 (final concentration) for 30 min at 37°C in 5% CO2. After the preincubation, infected or uninfected cells were treated with plain DMEM or plain DMEM plus 20% rabbit complement (final concentration) for 40 min at 37°C in 5% CO2. Medium was removed, and cells were fixed and stained with 0.1% crystal violet in 20% reagent alcohol. Representative areas in each well were examined using a Nikon Eclipse E1000 microscope at magnifications of ×40 and ×100. Quantitation of cell numbers was determined using Imaris File Converter, version 6.1.3, software (Bit plane AG, 2008).
Complement lysis assay: flow cytometry.
Vero E6 monolayer cells (1 × 106 cells/well) were infected with VACV/B5-GFP or VACV at an MOI of 4 to 8 and incubated for 12 h at 37°C in 5% CO2. Antibody and complement treatment was done as described above. For surface B5 and live/dead cell staining, cells were collected from each well using trypsin and washed twice in FACS buffer. Cells were incubated with a 1:100 dilution of a phycoerythrin-conjugated anti-mouse IgG(H+L) antibody (Jackson Immunoresearch, West Grove, PA) in FACS buffer at 100 μl/well for 20 min at 4°C. Cells were washed and stained using a live/dead fixable dead cell stain kit (Invitrogen-Molecular Probes, Eugene, OR), according the manufacturer's protocol with a small modification. Cells were washed with cold PBS one time and stained with amine-reactive viability dye (1:500) for 30 min in a 500-μl volume. Cells were washed with FACS buffer and then fixed for 15 min in 3.5% paraformaldehyde. Cells were washed and collected using a FACSCalibur instrument (BD). Data were analyzed using FlowJo software. Viability was assessed by the reaction of the fluorescence-reactive dye with amine groups in the cell membrane, which produces weak or low levels of staining. After the killing assay using rabbit complement, the dye penetrates and reacts with amine groups in the cytoplasm, resulting in an increase in the fluorescence intensity of damaged or dead cells. The percentages of live and dead cells were determined after gating each population.
C3 ELISA.
Serum was collected from CVF-treated mice on days 1, 3, 5, and 10 and assayed for C3 in an ELISA. Briefly, Maxisorp plates were coated overnight with a 1:1,000 dilution of a goat IgG fraction to mouse complement C3 (MP Biomedical, Solon, OH). After samples were blocked with PBS plus 5% FBS plus 0.5% Tween 20, serial dilutions of serum samples were incubated for 90 min at room temperature. Horseradish peroxidase-conjugated goat anti-mouse C3 (Innovative Research, Novi, Michigan) diluted 1:1,000 in PBS plus 5% FBS plus 0.5% Tween 20 was used for detection. The plates were developed, and the optical density was read as described above in “rB5 ELISA.”
Statistical analysis.
Tests were performed using Prism software, version 4.0 or 5.0 (GraphPad, San Diego, CA). Statistics were done using a two-tailed, unpaired t test with 95% confidence bounds unless otherwise stated. Error bars for graphs are ± 1 standard error of the mean (SEM). Error bars for protein microarrays represent the full data range.
RESULTS
As part of our efforts to understand the immunobiology of the smallpox vaccine, we have been studying the mechanics of poxvirus neutralization. Knowing that B5 is a target of neutralizing antibodies against VACV (1, 5, 33, 64), we generated a panel of neutralizing antibodies against B5 to facilitate a better understanding of poxvirus neutralization. The immunogen used was B5 ectodomain (rB5) synthesized in insect cells using conventional baculovirus expression vector technology (see Materials and Methods). Hybridomas were screened for B5 specificity using an rB5 ELISA. Subsequent to hybridoma subcloning, the relative affinity of each clone was determined. All clones bound B5 with EC50s of better than 1 × 10−10 M, indicative of subnanomolar Kd (dissociation constant) values (Table 1 and Fig. 1). In addition, MAbs bound native B5 expressed on the surface of VACV-infected cells (Fig. 1C).
TABLE 1.
Anti-B5 MAb IgG binding affinities
| Clone | Isotype | EC50 (nM) |
|---|---|---|
| B17 | IgG1 | 0.054 |
| B18 | IgG1 | 0.099 |
| B31 | IgG1 | 0.198 |
| B84 | IgG1 | 0.115 |
| B96 | IgG1 | 0.169 |
| B116 | IgG1 | 0.077 |
| B126 | IgG2a | 0.042 |
| B128 | IgG1 | 0.131 |
| B130 | IgG1 | 0.274 |
Positive hybridomas in that primary screen were then selected for further examination. Identifying MAbs with neutralizing activity presented several technical challenges. First, direct neutralization of EV—performed with a mixture of EV plus Ig, followed by incubation with target cells and quantitation of plaque number reduction—is generally poor, with maximal neutralization of 40 to 60% even at high antibody concentrations (Fig. 2; see also Fig. 3) (1, 3, 91, 93) though in some cases higher neutralization has been obtained with polyclonal serum (5, 33, 57). Therefore, screening B5 hybridomas for neutralization activity presented a signal-to-noise challenge, as well as biological concerns about the neutralization resistance of the virions. Second, the most commonly used EV neutralization assay, comet tail inhibition (reduction of VACVIHDJ spread in culture supernatant) (1, 33, 56), requires high concentrations and large amounts of Ig (∼20 to 40 μg/ml of culture) for clear results, which can be difficult to attain using samples from early hybridoma cultures. Thirdly, we found that the presence of nucleotide analogs in hybridoma HAT had significant, deleterious effects on comet tail assays. In light of these complications and limitations, we developed a new EV neutralization assay that was convenient, provided a high signal-to-noise ratio, only required small amounts of antibody, and had obvious biological relevance.
Complement required for EV neutralization in vitro.
The new EV neutralization assay was first developed in the course of studying the immune responses of mice immunized with VACV. Mice rapidly develop neutralizing antibodies to the MV form of VACV, as measured by direct mixture of VACV MV plus serial dilutions of serum followed by plaque assay (86, 100). In contrast, we found that even as late as 30 days postimmunization, when MV-neutralizing antibody titers had peaked, minimal anti-EV neutralization activity was measurable using a comparable assay (using a direct mixture of VACV EV plus serial dilutions of serum followed by plaque assay) (Fig. 2C). Substantial antibody responses to B5 were measurable in VACV-immunized mice as early as day 8 postinfection (Fig. 2A and B), and with strong responses maintained for greater than 30 days postinfection (Fig. 2B; also unpublished data), consistent with previous observations (23, 86). Given the presence of high antibody responses to B5, why was strong EV neutralization not observed? We reasoned that complement might be required for neutralization of the EV form. To test the hypothesis that complement is required for EV neutralization, serum from immunized mice was either mixed directly with EV or supplemented with complement. While no substantial neutralization of EV was observed in the absence of complement (<50% antibody-specific neutralization even at the highest Ig concentrations) (Fig. 2C), addition of complement revealed a strong EV neutralization activity in immunized mice (Fig. 2D). Given that complement is constitutively available in vivo, we refer to this assay as a “physiological neutralization assay.”
This physiological EV neutralization assay was then applied to the screening of B5-specific hybridomas. None of the MAbs exhibited substantial direct B5 neutralization activity in the absence of complement (Fig. 3A), but screening in the presence of complement revealed one strongly neutralizing antibody, B126 (Fig. 3B). B126 potently neutralized EV, with a 50% plaque neutralization titer as assessed by a plaque reduction neutralization test (PRNT50) of 107 ng/ml and a 90% plaque neutralization titer (PRNT90) of 535 ng/ml (Fig. 3C and D).
EV neutralization assays are regularly performed in the presence of an anti-MV IgG to eliminate contaminating MV particles in EV stocks (1, 33, 56, 91) (see Materials and Methods). Plaque numbers from EV stocks in the presence of anti-L1 IgG, with or without complement, were reduced ∼25%, consistent with a moderate level of contamination with MV particles (Fig. 3G). All experiments shown in Fig. 3A to D were performed in the presence of anti-L1 IgG to eliminate MV. To determine whether the anti-MV IgG was influencing the EV neutralization being measured for anti-B5 MAbs, the series of neutralization assays was repeated with and without anti-L1 IgG and with and without complement. Complement-dependent IgG2a anti-B5 (B126) neutralization of EV was not dependent on the presence of anti-MV IgG (Fig. 3E), and EV neutralization was quantitatively comparable, irrespective of the presence or absence of anti-MV IgG (Fig. 3G). IgG1 anti-B5 MAb B96 was used as a negative control as it binds native B5 (Fig. 1C) but does not exhibit complement-dependent EV neutralization (Fig. 3E to G). In addition, a series of neutralization experiments was performed with mouse complement to confirm that mouse complement actively neutralizes VACV EV in the presence of anti-B5 IgG2a. Neutralization was again observed only in the presence of anti-B5 IgG2a in combination with complement and was not observed for anti-B5 IgG1 plus complement or for MAb alone or complement alone (data not shown), though neutralization in assays using mouse complement was less robust due to the commonly observed reduction in in vitro activity when species-matched complement was used.
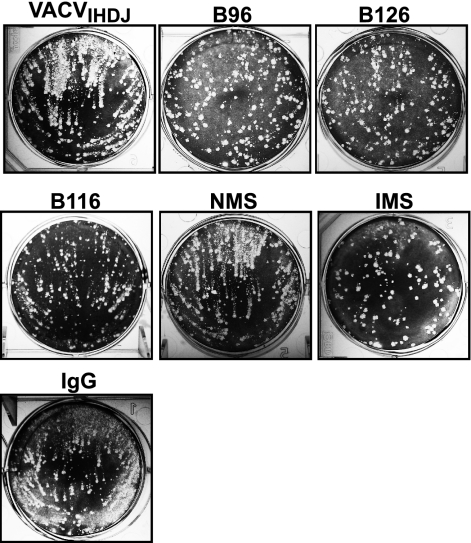
In later studies, purified MAbs were tested for comet tail inhibition, limiting VACVIHDJ dispersal in vitro. All MAbs exhibited comet tail inhibition activity at high concentrations, irrespective of isotype (Fig. 4).
FIG. 4.
Comet tail plaque inhibition. All anti-B5 MAbs exhibited comet tail inhibition activity in vitro, independent of isotype. Vero E6 cells were infected with VACVIHDJ and then cultured in the absence of antibody (VACVIHDJ) or with anti-B5 MAbs (B96, B126, or B116), naïve mouse serum (NMS), immune mouse serum (IMS), or irrelevant MAb (IgG).
MAb protection in vivo.
Were these B5 binding MAbs effective at protecting against VACV in vivo? The three different neutralization assays tested make three divergent predictions. The direct neutralization assay predicts no in vivo protection by the MAbs as none of the MAbs was effective in that assay. The comet tail inhibition assay predicts that all of the MAbs would be protective in vivo as all of the MAbs were effective at comet tail inhibition in vitro. The physiological neutralization assay predicts that only MAb B126 would be protective in vivo as that MAb was the only anti-B5 antibody with strong neutralization activity. To test these predictions, we tested the full panel of anti-B5 MAbs for efficacy in vivo.
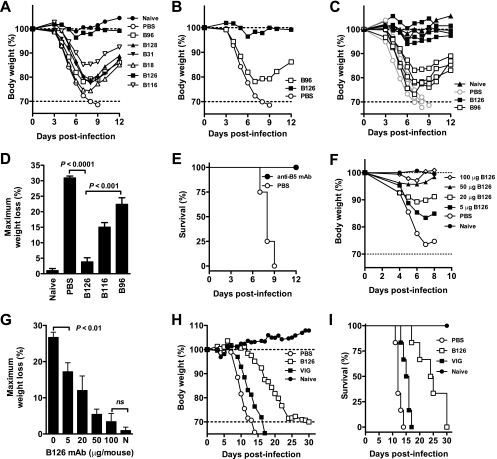
Antibodies can be protective against a lethal VACV intranasal challenge (33, 64), which is used as a small-animal model of human inhalation smallpox for vaccinations and therapeutic treatments (6, 15, 64, 100), based on genetic relationship and intranasal transmission; however, the model is limited by the distinctly different forms of lung disease and lack of an obligatory secondary systemic phase (57, 98). We previously showed that anti-H3 antibodies are protective using the VACV intranasal challenge model (21), and it has previously been shown in the literature that antibodies against B5 can be protective in the intranasal model (64). Here, mice were treated with individual B5 MAbs and then administered an intranasal challenge with a lethal dose of VACV (Fig. 5). For most of the MAbs the protective effect was marginal, as severe weight loss was observed after infection (Fig. 5A) though protection against mortality was achieved (Fig. 5E) (P < 0.01). Importantly, one MAb was significantly more effective than all other MAbs identified (Fig. 5A to D) (MAb B126; P < 0.0001 versus PBS and P < 0.002 versus MAb B96, B116, B128, B31, or B18 individually). MAb B126 was so effective that virtually no weight loss or clinical disease was observed in mice after viral challenge (P > 0.05 versus uninfected control mice) (Fig. 5B to D and G), and this was the one MAb with potent virus neutralization activity in the in vitro physiological neutralization assay (Fig. 3B). Therefore, the physiological neutralization assay was the best predictor of efficacy in vivo.
FIG. 5.
Protection against a lethal VACV infection. All anti-B5 MAbs exhibited some protective effect in vivo, and B126 IgG2a was highly protective. (A to E) Groups of BALB/c mice were inoculated i.p. with 100 μg of anti-B5 MAb (B18, B31, B96, B116, B126, or B128). Control mice received PBS. Naïve mice were unchallenged. One day later, mice were challenged intranasally with 5 × 104 PFU of purified VACV. Body weight was tracked, and average weights were graphed (A). Dotted lines indicate starting body weight (upper) and terminal body weight (bottom). B126 and B96 group means are shown again for clarity (B), with all individual animal curves are shown (C), and weight loss nadirs were calculated for specific experimental groups (D). B126-treated mice exhibited no significant weight loss compared to uninfected mice (P ≫ 0.05) and were significantly better protected than mice treated with any other MAb (P < 0.001; n = 4/group). Panel E shows survival after intranasal challenge. All MAb-treated mice were protected from death compared to mock-treated mice (P < 0.01, for each individual MAb versus PBS). One of four independent experiments is shown. (F and G) Dose titrations of MAb B126 were tested against intranasal challenge with VACV as described above. Weight loss curves for each group (F) and percent maximum weight loss (G) were plotted. Five micrograms of anti-B5 MAb B126 completely prevented mortality, and mice exhibited significantly ameliorated weight loss versus no MAb (P < 0.01; n = 6/group). A 100-μg dose provided excellent protection, with virtually no weight loss observed (P = 0.5; ns). One of five independent experiments is shown. (H and I) SCID mice were inoculated with 100 μg of anti-B5 MAb B126 or 1.25 mg of VIG. Body weight was tracked after intravenous infection with 1 × 103 PFU VACV. Mean percentage weight loss (H) and survival curves (I) were plotted for each experimental group. Anti-B5 MAb B126 protected substantially better than VIG, as determined by weight loss (P < 0.0005 versus no treatment; P < 0.0014 versus VIG; n = 6/group) and survival (P < 0.001 versus no treatment; P < 0.01 versus VIG). Bar graph error bars indicate SEM for each condition.
Dose titrations of MAb B126 were tested in vivo, and doses as low as 5 μg completely prevented mortality and substantially prevented morbidity (P < 0.01) (Fig. 5F and G). A 5-μg dose results in a calculated maximum serum concentration of less than 2.5 μg/ml, which is no more than five times higher than the B126 physiological neutralization assay PRNT90.
SCID mice were used as a second in vivo immunity model. This is a model system used for testing the efficacy of human VIG or related polyclonal antibodies (35, 38, 87). Studies have shown that VIG administered before infection can provide partial immunity to SCID and delay time to death. SCID mice infected intravenously with VACV lost weight and died over the course of 2 weeks (Fig. 5H). VIG can provide a modest degree of protection against VACV, extending median time to death by 3 days (Fig. 5H and I) (P < 0.0051). A single 100-μg dose of anti-B5 MAb B126 provided much better protection, extending median time to death by 12 days (Fig. 5H and I) (P < 0.0005 versus no treatment and P < 0.0014 versus VIG).
Requirement for complement in vivo.
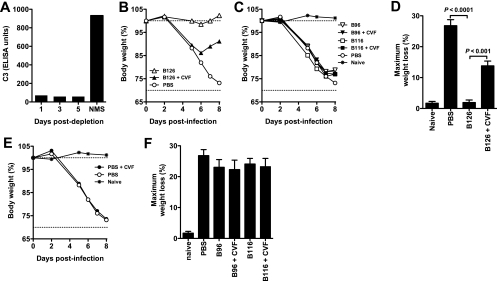
B126 was distinguished from all other anti-B5 MAbs by its ability to neutralize EV in vitro. The neutralization assay incorporated complement. B126 was also the only IgG2a clone identified, and IgG2a is the most efficient complement-binding murine isotype (36, 72). The other anti-B5 MAbs identified were all IgG1 (Table 1). These findings indicated that the potent protective efficacy of B126 in vivo was due to its ability to fix complement. We tested this hypothesis by testing the efficacy of B126 after depleting complement in vivo. Complement was depleted by administering CVF. Complement depletion was confirmed by measuring serum levels of C3 (94% depletion) (Fig. 6A). Two different anti-B5 IgG1 MAbs were used for comparison to B126: B116 and B96. Complement depletion did not affect the modest protection provided by either B96 or B116 (average nadir weight loss, 22 to 24%) (Fig. 6C and F). Nor did depletion of complement affect pathogenesis or kinetics of disease in untreated mice infected with VACV (Fig. 6E). In contrast, depletion of complement ablated B126 activity by more than 50% (Fig. 6B and D). Mice treated with B126 and infected with VACV lost no weight (average nadir weight loss, 1%) (Fig. 6B and D), but complement-depleted mice had a specific loss in B126 MAb protection (average nadir weight loss, 14%) (Fig. 6B and D). This demonstrated that the majority of the protection against VACV provided by the IgG2a anti-B5 MAb B126 requires complement (P < 0.001). However, some of the protection was still present in the absence of complement, and B126 was still more protective than IgG1 isotype B5 antibodies of comparable affinity (P < 0.02), implicating additional Fc-mediated functions (see Discussion).
FIG. 6.
Anti-B5 protection in vivo is complement dependent. Complement depletion in vivo abrogated the majority of anti-B5 protection against VACV. (A) Complement C3 levels in CVF-treated mice at days 1 to 5 after treatment. Serum from mice prior to treatment (naïve mouse serum [NMS]) was used as control. (B to D) Complement-depleted (+CVF) or nondepleted mice were treated with 100 μg of anti-B5 MAb (B96, B116, or B126) or PBS at day −1 and challenged intranasally with 5 × 104 PFU of purified VACV at day 0. Mean weight loss kinetics in each group (B and C), and maximum weight loss (weight nadir) (D) are shown. As shown in panel B, VACV-infected mice treated with B126 were fully protected compared with untreated infected mice (P < 0.0001), but complement-depleted mice had a >50% specific loss in B126 MAb protection. As shown in panel C, B96 and B116 provided minimal protection against disease, and neither B96 nor B116 was affected by CVF treatment. Abrogation of anti-B5 B126 protection by complement-depletion was highly statistically significant (P < 0.0001, for B126 plus CVF versus B126 in complement-sufficient recipients B126), while there were no significant effect of complement depletion on any other experimental group (P ≫ 0.05; nonsignificant) (D). Complement depletion in vivo (CVF treatment) did not affect disease in untreated mice (E) or the modest activities of B96 or B116 (F). One of three independent experiments is shown. Error bars indicate SEM for each group.
Complement factors involved in VACV EV neutralization.
Given that the in vitro physiological neutralization assay was predictive of protective efficacy in vivo and that complement activity was required in vivo for strong protection, we examined which complement factors were involved in VACV EV neutralization in vitro. VACV EV is fully neutralized by the anti-B5 MAb within 5 min of the addition of complement (Fig. 7A and B). Depletion experiments revealed reductions in EV neutralization when C3 was removed (Fig. 7C). However, absence of C5 did not abrogate virus neutralization (Fig. 7C). These experiments suggest that the primary mechanism of neutralization was opsonization—coating of the EV particles with antibody and complement C3, sterically preventing EV from binding to cells—while direct complement lysis of EV viral particles (virolysis) did not appear to be a major pathway of neutralization. Lustig et al. demonstrated a membrane lysis model of EV neutralization whereby complement plus anti-A33 IgG did not neutralize EV but did lyse the EV membrane and thereby release the internal MV (65). The released MV was then susceptible to neutralization by anti-MV IgG (65). Herein we show a different mechanism of neutralization. Anti-B5 IgG2a neutralizes EV in a complement-dependent manner (Fig. 3A to D), but the neutralization is only dependent on anti-B5 and does not require the presence of an anti-MV Ig (Fig. 3E to G). Therefore, virolysis and release of MV are not the mechanisms of action of complement-dependent anti-B5 EV neutralization. This is consistent with the complement depletion experiments described above (Fig. 7C), showing that complement components necessary for opsonization (C3) are involved but that complement components required for membrane lysis (C5) are not required. This is also consistent with the in vivo results, where the only anti-VACV-specific IgG present at the time of virus challenge was anti-B5 (Fig. 5 and 6).
Killing of VACV-infected cells by B5 antibodies.
The physiological EV neutralization assay in vitro demonstrated that anti-B5 antibodies can efficiently neutralize EV, and this functionality was predictive of protective efficacy in vivo (Fig. 3, 5, and 6). Complement can function in multiple ways, and the observation that B126 IgG2a MAb was so effective in vivo that mice developed no clinical symptoms suggested that the VACV infection could be stopped very rapidly. Mouse infections were done using purified VACV MV stock. Given that EV and B5 are undetectable in the purified MV stock used for inoculation (Fig. 8), sterilizing immunity cannot be accounted for by direct neutralization of the virus inoculum by anti-B5 MAb. These observations raised the possibility that anti-B5 antibodies are able to direct complement-mediated destruction of VACV-infected cells, utilizing the membrane attack complex, and thereby rapidly quench the spread of VACV in the lungs, leading to resolution of the infection.
FIG. 8.
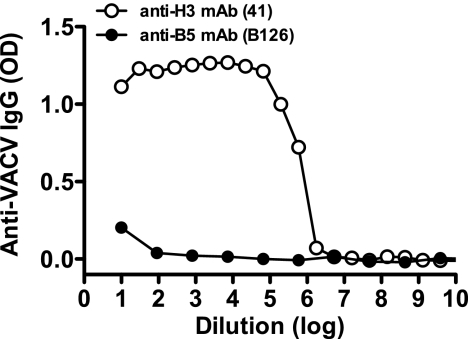
The purified VACV MV stock did not contain EV or B5 antigen. ELISA plates were coated with UV-inactivated VACV MV and then probed with serial dilutions of anti-B5 (B126) or anti-H3 (41) MAbs. Starting concentrations were 0.62 and 0.5 μg/ml, respectively. OD, optical density.
We tested this hypothesis by assessing the ability of anti-B5 MAbs to direct complement lysis of cells infected with VACV. While VACV MV,the most abundant virion form, are produced intracellularly and do not have cell surface expressed proteins, EV are secreted from the plasma membrane (17, 70, 88). VACV-infected cells express B5 on the surface (24, 47, 96) (Fig. 1C). This expression can be detected within 4 h of infection, and is at high levels by 8 to 10 h (MFI in infected cells, 500; MFI in uninfected cells, 16) (Fig. 9A).
Adherent cells were infected with VACV and then tested for susceptibility to antibody-directed complement lysis at time points after infection when virus expression of B5 protein led to an accumulation of B5 on the surface of infected cells (8 to 12 h). Treatment with antibody or complement alone had no effect on infected cells (Fig. 9B, C, and F). In stark contrast, addition of anti-B5 MAb B126 with complement resulted in rapid and complete killing of infected cells (P < 0.0001) (Fig. 9E and F). Treatment with a non-complement-fixing anti-B5 MAb (B96 IgG1) in the presence of complement did not direct cell lysis, again highlighting the importance of antibody isotype in this antiviral function (P ≫ 0.05; nonsignificant) (Fig. 9D and F).
We also made use of a flow cytometric assay as a second approach to demonstrate antibody-directed complement lysis of VACV-infected cells (Fig. 9G to I). A similar approach has recently been published by Girgis and colleagues (34). For the experimental system used here, a genetically engineered VACV that expressed a B5-GFP fusion protein was used to track infected cells and B5 expression (97). Infected cells were killed by anti-B5 B126 in the presence of complement (P < 0.001) (Fig. 9G to I) but were not killed by a non-complement-fixing anti-B5 MAb (B96) (Fig. 9I) though we did observe more experiment-to-experiment variability in killing with this flow cytometric assay. Using both experimental approaches (Fig. 9C to F and G to I), it was clear that antibody-mediated killing of virus-infected cells was specific; killing was observed only in the presence of anti-B5 MAb, was dependent on the presence of complement, was active only against virally infected cells, and was completely absent when a non-complement-fixing anti-B5 IgG1 MAb was used. These results and their consistency with the other in vitro and in vivo data shown indicate that anti-B5 antibody killing of VACV-infected cells in vivo is likely an important protective mechanism of the anti-EV humoral immune response.
DISCUSSION
The smallpox vaccine has led to the eradication of smallpox disease and is generally considered the gold standard of human vaccines. It is therefore important to understand the immunological mechanisms resulting in the vaccine-mediated protection so that we can better assess alternative smallpox vaccines, develop high-quality anti-poxvirus antibody therapeutics, and develop a fundamental immunobiological basis for vaccinology in order to be able to design vaccines to other pathogens of interest in the future. Protection against smallpox is primarily antibody mediated in primates and humans (4, 25), and antibodies are highly effective at providing immunity in mouse poxvirus models (21, 33, 57, 64). Therefore, understanding the specificities and functions of the antibody response to the smallpox vaccine has been an area of intensive research.
VACV and other orthopoxviruses produce two virion forms, MV and EV. These virion forms are both functionally and immunologically distinct. From the perspective of humoral immunity, the pathogen effectively exists as two independent viruses, given that the two virion forms do not share any surface proteins and that, therefore, neutralizing antibodies are specific for either one virion form or the other. This morphological bifurcation is a clever immune evasion strategy that prevents the virus from being stopped by any single antibody response.
While there have been clear correlations between neutralization activity of anti-MV antibodies and protection in vivo, the relationship between EV neutralization by antibodies and protection in vitro and in vivo remains less clear as anti-B5 MAbs and antiserum have generally exhibited weak neutralizing activity in vitro (except by comet tail inhibition) (2, 15, 33, 58, 65, 88, 91, 93). Anti-A33 antibodies are protective in vivo but do not neutralize in vitro in a conventional assay (but do inhibit comet tail formation) (33, 65), additionally confounding efforts to relate anti-EV neutralization in vitro and protection in vivo.
We tested the ability of three different in vitro assays to predict protection in vivo. The complement-mediated neutralization assay was clearly the best predictor of protection. This implied the importance of complement for protection against EV, and subsequent experiments depleting complement in vivo directly demonstrated the role of complement in anti-B5-mediated protection against EV (Fig. 6). Our data indicate that full neutralization (>90%) of EV is difficult, if not impossible, to accomplish in vitro with anti-B5 IgG alone. In contrast, highly efficient EV neutralization by IgG2a occurs in the presence of complement (PRNT90 of 535 ng/ml), which is the physiological condition. Parren and Burton (and Burnet before) have proposed a simple occupancy model for virus neutralization, whereby a virion is neutralized at the point at which the surface of the particle is sufficiently coated with antibody as to prevent binding of the virion to target cells (74). This model effectively predicts neutralizing antibody activities against poliovirus (74), rhabdoviruses (28, 74), West Nile virus (52, 76), and others (61, 81), and this model has highlighted structural neutralization problems of certain viruses such as human immunodeficiency virus (12, 13). In addition, antigen-coating activities of complement are easily incorporated into the model (74). The mass of C1q substantially enlarges the footprint of the bound Ig, and the covalent attachment of numerous copies of C3b to the virion can serve as a potent means of virion inactivation, preventing binding to target cells in vitro and in vivo (10, 74). In vivo C1q and C3b play a more active role, directing opsonization of bound virions, presumably at lower concentrations than what is necessary for complete coating of a virion (29, 71). The in vitro complement-containing neutralization assay is therefore both a direct and a surrogate assay for the in vivo mechanisms. The lack of a requirement for C5 in vitro is consistent with the coating model as C5 is not involved in coating but is the central regulator of the complement membrane attack complex that causes virolysis. While C5-mediated virolysis of virions may occur in vitro or in vivo, our data indicate that virolysis is not necessary for neutralization.
It was intriguing and impressive that mice treated with B126 IgG2a were almost completely protected against a lethal intranasal challenge with VACV, exhibiting virtually no signs of disease. This was somewhat puzzling. The mechanism of protection is not sterilizing immunity via elimination of the inoculum as the viral inoculum was the purified MV form. EV production is important for systemic spread of VACV (75), but the anti-B5 protection data herein highlight and reiterate the findings that EV is also important in vivo for local cell-to-cell virus spreading. Our anti-B5 data are consistent with a model of EV inhibition where direct binding of anti-B5 IgG to EV can result in modest neutralization (≤50%) by agglutination (cross-linking or aggregation). This activity is measured in the comet tail inhibition assay, and this activity by itself can provide a modest degree of protection in vivo (as shown in Fig. 5 and 6 in vivo by the IgG1 comet tail-inhibiting, non-complement-fixing MAbs). However, complement-mediated neutralization of EV is much more effective in vivo, as demonstrated by the in vivo efficacy of B126 anti-B5 IgG2a and the effect of in vivo complement depletion (Fig. 5 and 6). These results are consistent with the small-plaque phenotypes in vitro of mutant viruses containing deletions in genes required for EV formation (11, 26, 56, 97, 99). These results are also consistent with the immunity induced in mice after VACV infection as the predominant isotype of the anti-B5 antibody response is IgG2a after infection (data not shown).
An important second protection mechanism was illuminated by the observation that B5 is expressed on the surface of VACV-infected cells. VACV MV proteins are not available on the surface of infected cells, and therefore free virion binding is the only available mechanism for neutralization of VACV MV by anti-MV antibodies. However, the biology of EV opens additional pathways for Ig antiviral activities. The presence of B5 on the surface of VACV-infected cells relatively early after infection (Fig. 9A) exposes infected cells to antiviral activities of anti-B5 antibodies. We show here that anti-B5 antibodies can bind to VACV-infected cells and efficiently direct complement lysis (Fig. 9). This lysis may occur by targeting cell-associated EV or by targeting B5 on the plasma membrane, which is not associated with virions. Importantly, the lysis is only observed in the presence of a complement-fixing murine Ig isotype IgG2a and not a non-complement-fixing isotype (IgG1). We directly demonstrated that this cell lysis mechanism is highly effective in vitro (Fig. 9). Our in vivo data are also consistent with this mechanism of protective immunity as protection was isotype dependent (Fig. 5), and complement-depleted mice were significantly less protected by B126 IgG2a MAb (Fig. 6).
While our experiments were being completed, Girgis and colleagues recently published the first, demonstrated complement-mediated lysis of VACV-infected target cells using an anti-VACV serum (34). Our work confirms and extends the results published in that study, now demonstrating that lysis can be mediated by antibodies to a single VACV antigen, B5; moreover, the antibody-directed complement lysis is well correlated with EV neutralization in vitro and protection against lethal viral challenges in vivo, indicating that the cell-killing process is likely of substantial importance during a poxvirus infection. B5 has long been the EV antigen of most intensive study, and previous studies have demonstrated the importance of antibodies against B5 (5, 33) and identified B5 MAbs with antiviral activity in vitro, primarily via the comet tail inhibition assay (1, 3, 15, 33). Laboratory-to-laboratory variations in EV neutralization results may be due in part to differences in methods for EV stock production, variations in residual complement activity in FBS or serum samples, or differences in the types of assays. In addition, different cell types used for virus stock preparation may affect the abundance (and species) of inhibitory proteins (91, 92). These various experimental concerns highlight the importance of validation in vivo, and our in vivo data demonstrate the relevance of the in vitro systems utilized in this study, which have identified dramatic effects of complement-mediated neutralization of EV particles and lysis of VACV-infected cells.
The observation that anti-B5 antibodies can lyse infected cells highlights the role antibodies can play in the clearance of an intracellular pathogen. For example, it has been shown that smallpox vaccine-elicited antibodies are necessary and sufficient for protection of primates against a lethal intravenous challenge with monkeypox (25). A simple interpretation is that the antibodies provided sterilizing immunity against the inoculum. Our data indicate that an additional possibility is that anti-B5 antibodies also play a role in the protection by rapid clearance of the viral infection via destruction of infected cells, after perhaps a single round of cell infection in vivo. Cell surface-expressed B5 keeps poxvirus-infected cells from being invisible to the humoral immune response in vivo.
BS-specific MAbs still had a partial protective efficacy when administered to complement-depleted mice prior to infection, and that protection was substantially greater with the IgG2a MAb B126 than the IgG1 MAbs B116 or B96 even though the MAbs possessed comparable affinities. This suggests that the IgG2a isotype is inherently more protective than IgG1 even in the absence of complement. We propose that this is likely due to enhanced Fc receptor binding of IgG2a by effector cells, resulting in more effective macrophage (or neutrophil) phagocytic activity that destroys infected cells and/or in NK cell Fc-mediated antibody-dependent cell-mediated cytotoxicity. Opsonization by C3b receptor (CR1 or CD35)-expressing cells is an additional potential pathway utilized.
After VACV infection of mice, IgG2a is the dominant isotype, and IgG1 is a minor component of the antiviral antibody response (30; also S. Crotty, unpublished data), consistent with the biological relevance of IgG2 isotypes in antiviral protection and our findings using MAbs. Note also that mouse IgM and IgG3 have substantial complement fixation activities and likely contribute to early protective B5 antibody responses after immunization with the smallpox vaccine (unpublished data). Furthermore, human and chimpanzee IgG1s are very effective at complement fixation and are not direct homologs of mouse IgG1, which may explain the potency of chimpanzee anti-B5 IgG1 antibodies in vivo (15).
One successful method for identifying immunological pathways important for the control of a virus is to discover which pathways are targeted by the virus for immune evasion as there is strong pressure on the virus to reduce the efficacy of such pathways. VACV encodes vaccinia complement control protein, VCP (C3L; also called WR025), which binds C3b and C4b (54, 83). VCP was the first viral secreted inhibitor of complement identified (54, 55). It has recently been discovered that VCP is also bound to A56, an EV surface protein (34, 94), suggesting that surface-bound VCP may have similar properties to gC, the surface-expressed complement inhibitor of herpes simplex virus 1 (32, 37). VCP is a known virulence factor of vaccinia (54), and the variola homolog SPICE (for smallpox inhibitor of complement enzyme) is a more potent complement inhibitor (82). It has been proposed that the limited severity of the monkeypox outbreak in the United States in 2003 was because the causative virus was of West African origin (49, 85), and the West African clade of monkeypox is significantly less virulent than Congo basin strains (62). This milder virulence of the West African clade is possibly due to the deletion of the monkeypox VCP homolog (MOSPICE) (63). In the context of B5, poxvirus complement inhibitors putatively inhibit both the neutralization of EV particles and the antibody-directed lysis of infected cells since the membrane attack complex is dependent on C3b and C4b and since neutralization of free EV particles is C3-dependent (Fig. 7C). In our experiments, wild-type VACV, which expresses VCP, was used both in vitro and in vivo. Therefore, the inhibitory effects of VCP are only partial and can be overcome by high-affinity anti-B5 IgG2a in the presence of complement. The observation that VACV mutants with a deletion of VCP have only a moderate loss of virulence is consistent with these observations (46).
Complement is an ancient and highly conserved immunological defense mechanism, and complement has been shown to be important for protection against a variety of viruses via a variety of components and mechanisms (8, 19, 37, 40, 41, 46, 48, 53, 60, 67-69, 73). Our data here demonstrate the importance of complement against vaccinia in vivo, with particular focus pointing to the relevance of complement in vivo against EV and for killing of virus-infected cells.
In summary, we propose that IgG2a B5 antibodies are highly effective because they accomplish both the elimination of infectious virions and infected cells via three activities: (i) direct neutralization of EV in the presence of complement via coating of the virion surface, (ii) anti-B5-directed complement lysis of VACV-infected cells (complement-dependent cytotoxicity), and (iii) Fc-mediated cellular phagocytosis (macrophage) or killing (NK cell antibody-dependent cell-mediated cytotoxicity) of VACV-infected cells bound to anti-B5 IgG2a. These results demonstrate a variety of Ig functionalities that should be kept under consideration in the evaluation of the mechanisms of poxvirus neutralization in vivo.
Acknowledgments
This work was supported in part by NIH NIAID grant AI63107, NIH NIAID grant AI077953, a Pew Scholar Award, and a Cancer Research Institute Award to S.C.
We thank Vincent Liu, Analisa Benedetto, and Sacha Garcia for technical assistance. We thank Kevin Karem (CDC), and Stuart Isaacs (University of Pennsylvania) for technical advice. We thank Bernard Moss, Jonathan Yewdell, Philip Felgner, and Alessandro Sette for virus strains and reagents.
Footnotes
Published ahead of print on 19 November 2008.
REFERENCES
- 1.Aldaz-Carroll, L., J. C. Whitbeck, M. Ponce de Leon, H. Lou, L. Hirao, S. N. Isaacs, B. Moss, R. J. Eisenberg, and G. H. Cohen. 2005. Epitope-mapping studies define two major neutralization sites on the vaccinia virus extracellular enveloped virus glycoprotein B5R. J. Virol. 796260-6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldaz-Carroll, L., J. C. Whitbeck, M. Ponce de Leon, H. Lou, L. K. Pannell, J. Lebowitz, C. Fogg, C. L. White, B. Moss, G. H. Cohen, and R. J. Eisenberg. 2005. Physical and immunological characterization of a recombinant secreted form of the membrane protein encoded by the vaccinia virus L1R gene. Virology 34159-71. [DOI] [PubMed] [Google Scholar]
- 3.Aldaz-Carroll, L., Y. Xiao, J. C. Whitbeck, M. Ponce de Leon, H. Lou, M. Kim, J. Yu, E. L. Reinherz, S. N. Isaacs, R. J. Eisenberg, and G. H. Cohen. 2007. Major neutralizing sites on vaccinia virus glycoprotein B5 are exposed differently on variola virus ortholog B6. J. Virol. 818131-8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amanna, I. J., M. K. Slifka, and S. Crotty. 2006. Immunity and immunological memory following smallpox vaccination. Immunol. Rev. 211320-337. [DOI] [PubMed] [Google Scholar]
- 5.Bell, E., M. Shamim, J. C. Whitbeck, G. Sfyroera, J. D. Lambris, and S. N. Isaacs. 2004. Antibodies against the extracellular enveloped virus B5R protein are mainly responsible for the EEV neutralizing capacity of vaccinia immune globulin. Virology 325425-431. [DOI] [PubMed] [Google Scholar]
- 6.Belyakov, I. M., P. Earl, A. Dzutsev, V. A. Kuznetsov, M. Lemon, L. S. Wyatt, J. T. Snyder, J. D. Ahlers, G. Franchini, B. Moss, and J. A. Berzofsky. 2003. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc. Natl. Acad. Sci. USA 1009458-9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benhnia, M. R., M. M. McCausland, H. P. Su, K. Singh, J. Hoffmann, D. H. Davies, P. L. Felgner, S. Head, A. Sette, D. N. Garboczi, and S. Crotty. 2008. Redundancy and plasticity of neutralizing antibody responses are cornerstone attributes of the human immune response to the smallpox vaccine. J. Virol. 823751-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berry, D. M., and J. D. Almeida. 1968. The morphological and biological effects of various antisera on avian infectious bronchitis virus. J. Gen. Virol. 397-102. [DOI] [PubMed] [Google Scholar]
- 9.Beukelman, C. J., P. C. Aerts, H. Van Dijk, and J. M. Willers. 1987. A one-step isolation procedure for phospholipase A2-free cobra venom factor by fast protein liquid chromatography. J. Immunol. Methods 97119-122. [DOI] [PubMed] [Google Scholar]
- 10.Bindon, C. I., G. Hale, M. Bruggemann, and H. Waldmann. 1988. Human monoclonal IgG isotypes differ in complement activating function at the level of C4 as well as C1q. J. Exp. Med. 168127-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blasco, R., and B. Moss. 1991. Extracellular vaccinia virus formation and cell-to-cell virus transmission are prevented by deletion of the gene encoding the 37,000-Dalton outer envelope protein. J. Virol. 655910-5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burton, D. R. 2002. Antibodies, viruses and vaccines. Nat. Rev. Immunol. 2706-713. [DOI] [PubMed] [Google Scholar]
- 13.Burton, D. R. 2006. Structural biology: images from the surface of HIV. Nature 441817-818. [DOI] [PubMed] [Google Scholar]
- 14.Chen, Z., P. Earl, J. Americo, I. Damon, S. K. Smith, F. Yu, A. Sebrell, S. Emerson, G. Cohen, R. J. Eisenberg, I. Gorshkova, P. Schuck, W. Satterfield, B. Moss, and R. Purcell. 2007. Characterization of chimpanzee/human monoclonal antibodies to vaccinia virus A33 glycoprotein and its variola virus homolog in vitro and in a vaccinia virus mouse protection model. J. Virol. 818989-8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen, Z., P. Earl, J. Americo, I. Damon, S. K. Smith, Y. H. Zhou, F. Yu, A. Sebrell, S. Emerson, G. Cohen, R. J. Eisenberg, J. Svitel, P. Schuck, W. Satterfield, B. Moss, and R. Purcell. 2006. Chimpanzee/human mAbs to vaccinia virus B5 protein neutralize vaccinia and smallpox viruses and protect mice against vaccinia virus. Proc. Natl. Acad. Sci. USA 1031882-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung, C. S., C. H. Chen, M. Y. Ho, C. Y. Huang, C. L. Liao, and W. Chang. 2006. Vaccinia virus proteome: identification of proteins in vaccinia virus intracellular mature virion particles. J. Virol. 802127-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Condit, R. C., N. Moussatche, and P. Traktman. 2006. In a nutshell: structure and assembly of the vaccinia virion. Adv. Virus. Res. 6631-124. [DOI] [PubMed] [Google Scholar]
- 18.Crotty, S., P. Felgner, H. Davies, J. Glidewell, L. Villarreal, and R. Ahmed. 2003. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J. Immunol. 1714969-4973. [DOI] [PubMed] [Google Scholar]
- 19.Da Costa, X. J., M. A. Brockman, E. Alicot, M. Ma, M. B. Fischer, X. Zhou, D. M. Knipe, and M. C. Carroll. 1999. Humoral response to herpes simplex virus is complement-dependent. Proc. Natl. Acad. Sci. USA 9612708-12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies, D. H., X. Liang, J. E. Hernandez, A. Randall, S. Hirst, Y. Mu, K. M. Romero, T. T. Nguyen, M. Kalantari-Dehaghi, S. Crotty, P. Baldi, L. P. Villarreal, and P. L. Felgner. 2005. Profiling the humoral immune response to infection by using proteome microarrays: high-throughput vaccine and diagnostic antigen discovery. Proc. Natl. Acad. Sci. USA 102547-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies, D. H., M. M. McCausland, C. Valdez, D. Huynh, J. E. Hernandez, Y. Mu, S. Hirst, L. Villarreal, P. L. Felgner, and S. Crotty. 2005. Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. J. Virol. 7911724-11733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davies, D. H., D. M. Molina, J. Wrammert, J. Miller, S. Hirst, Y. Mu, J. Pablo, B. Unal, R. Nakajima-Sasaki, X. Liang, S. Crotty, K. L. Karem, I. K. Damon, R. Ahmed, L. Villarreal, and P. L. Felgner. 2007. Proteome-wide analysis of the serological response to vaccinia and smallpox. Proteomics 71678-1686. [DOI] [PubMed] [Google Scholar]
- 23.Davies, D. H., L. S. Wyatt, F. K. Newman, P. L. Earl, S. Chun, J. E. Hernandez, D. M. Molina, S. Hirst, B. Moss, S. E. Frey, and P. L. Felgner. 2008. Antibody profiling by proteome microarray reveals the immunogenicity of the attenuated smallpox vaccine modified vaccinia virus Ankara is comparable to that of Dryvax. J. Virol. 82652-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Earley, A. K., W. M. Chan, and B. M. Ward. 2008. The vaccinia virus B5 protein requires A34 for efficient intracellular trafficking from the endoplasmic reticulum to the site of wrapping and incorporation into progeny virions. J. Virol. 822161-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edghill-Smith, Y., H. Golding, J. Manischewitz, L. R. King, D. Scott, M. Bray, A. Nalca, J. W. Hooper, C. A. Whitehouse, J. E. Schmitz, K. A. Reimann, and G. Franchini. 2005. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 11740-747. [DOI] [PubMed] [Google Scholar]
- 26.Engelstad, M., and G. L. Smith. 1993. The vaccinia virus 42-kDa envelope protein is required for the envelopment and egress of extracellular virus and for virus virulence. Virology 194627-637. [DOI] [PubMed] [Google Scholar]
- 27.Fenner, F., D. A. Henderson, I. Arita, Z. Jezek, and I. D. Ladnyi. 1988. Smallpox and its eradication. World Health Organization, Geneva, Switzerland.
- 28.Flamand, A., H. Raux, Y. Gaudin, and R. W. Ruigrok. 1993. Mechanisms of rabies virus neutralization. Virology 194302-313. [DOI] [PubMed] [Google Scholar]
- 29.Flint, S. J., L. W. Enquist, V. R. Racaniello, and A. M. Skalka. 2004. Principles of virology, 2nd ed. ASM Press, Washington, DC.
- 30.Fogg, C., S. Lustig, J. C. Whitbeck, R. J. Eisenberg, G. H. Cohen, and B. Moss. 2004. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J. Virol. 7810230-10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fogg, C. N., J. L. Americo, P. L. Earl, W. Resch, L. Aldaz-Carroll, R. J. Eisenberg, G. H. Cohen, and B. Moss. 2008. Disparity between levels of in vitro neutralization of vaccinia virus by antibody to the A27 protein and protection of mice against intranasal challenge. J. Virol. 828022-8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedman, H. M., G. H. Cohen, R. J. Eisenberg, C. A. Seidel, and D. B. Cines. 1984. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature 309633-635. [DOI] [PubMed] [Google Scholar]
- 33.Galmiche, M. C., J. Goenaga, R. Wittek, and L. Rindisbacher. 1999. Neutralizing and protective antibodies directed against vaccinia virus envelope antigens. Virology 25471-80. [DOI] [PubMed] [Google Scholar]
- 34.Girgis, N. M., B. C. Dehaven, X. Fan, K. M. Viner, M. Shamim, and S. N. Isaacs. 2008. Cell surface expression of the vaccinia virus complement control protein is mediated by interaction with the viral A56 protein and protects infected cells from complement attack. J. Virol. 824205-4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldsmith, J. C., N. Eller, M. Mikolajczyk, J. Manischewitz, H. Golding, and D. E. Scott. 2004. Intravenous immunoglobulin products contain neutralizing antibodies to vaccinia. Vox Sang. 86125-129. [DOI] [PubMed] [Google Scholar]
- 36.Hardy, R. R. 1986. Complement fixation by monoclonal antibody-antigen complexes, p. 40.1-40.12. In D. M. Weir, L. A Herzenberg, C. Blackwell, and L. A Herzenberg (ed.), Handbook of experimental immunology, vol. 1. 4th ed. Blackwell Science, London, United Kingdom. [Google Scholar]
- 37.Harris, S. L., I. Frank, A. Yee, G. H. Cohen, R. J. Eisenberg, and H. M. Friedman. 1990. Glycoprotein C of herpes simplex virus type 1 prevents complement-mediated cell lysis and virus neutralization. J. Infect. Dis. 162331-337. [DOI] [PubMed] [Google Scholar]
- 38.He, Y., J. Manischewitz, C. A. Meseda, M. Merchlinsky, R. A. Vassell, L. Sirota, I. Berkower, H. Golding, and C. D. Weiss. 2007. Antibodies to the A27 Protein of Vaccinia Virus Neutralize and Protect against Infection but Represent a Minor Component of Dryvax Vaccine-Induced Immunity. J. Infect. Dis. 1961026-1032. [DOI] [PubMed] [Google Scholar]
- 39.Heraud, J. M., Y. Edghill-Smith, V. Ayala, I. Kalisz, J. Parrino, V. S. Kalyanaraman, J. Manischewitz, L. R. King, A. Hryniewicz, C. J. Trindade, M. Hassett, W. P. Tsai, D. Venzon, A. Nalca, M. Vaccari, P. Silvera, M. Bray, B. S. Graham, H. Golding, J. W. Hooper, and G. Franchini. 2006. Subunit recombinant vaccine protects against monkeypox. J. Immunol. 1772552-2564. [DOI] [PubMed] [Google Scholar]
- 40.Hicks, J. T., F. A. Ennis, E. Kim, and M. Verbonitz. 1978. The importance of an intact complement pathway in recovery from a primary viral infection: influenza in decomplemented and in C5-deficient mice. J. Immunol. 1211437-1445. [PubMed] [Google Scholar]
- 41.Hirsch, R. L., D. E. Griffin, and J. A. Winkelstein. 1980. Role of complement in viral infections: participation of terminal complement components (C5 to C9) in recovery of mice from Sindbis virus infection. Infect. Immun. 30899-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hobday, T. L. 1962. Antivaccinial gamma-globulin in the control of smallpox. Lancet 1907-908. [DOI] [PubMed] [Google Scholar]
- 43.Hooper, J. W., D. M. Custer, C. S. Schmaljohn, and A. L. Schmaljohn. 2000. DNA vaccination with vaccinia virus L1R and A33R genes protects mice against a lethal poxvirus challenge. Virology 266329-339. [DOI] [PubMed] [Google Scholar]
- 44.Hooper, J. W., E. Thompson, C. Wilhelmsen, M. Zimmerman, M. A. Ichou, S. E. Steffen, C. S. Schmaljohn, A. L. Schmaljohn, and P. B. Jahrling. 2004. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J. Virol. 784433-4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hopkins, R. J., and J. M. Lane. 2004. Clinical efficacy of intramuscular vaccinia immune globulin: a literature review. Clin. Infect. Dis. 39819-826. [DOI] [PubMed] [Google Scholar]
- 46.Isaacs, S. N., G. J. Kotwal, and B. Moss. 1992. Vaccinia virus complement-control protein prevents antibody-dependent complement-enhanced neutralization of infectivity and contributes to virulence. Proc. Natl. Acad. Sci. USA 89628-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Isaacs, S. N., E. J. Wolffe, L. G. Payne, and B. Moss. 1992. Characterization of a vaccinia virus-encoded 42-kilodalton class I membrane glycoprotein component of the extracellular virus envelope. J. Virol. 667217-7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kapadia, S. B., B. Levine, S. H. Speck, and H. W. t. Virgin. 2002. Critical role of complement and viral evasion of complement in acute, persistent, and latent gamma-herpesvirus infection. Immunity 17143-155. [DOI] [PubMed] [Google Scholar]
- 49.Karem, K. L., M. Reynolds, C. Hughes, Z. Braden, P. Nigam, S. Crotty, J. Glidewell, R. Ahmed, R. Amara, and I. K. Damon. 2007. Monkeypox-induced immunity and failure of childhood smallpox vaccination to provide complete protection. Clin. Vaccine Immunol. 141318-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kempe, C. H. 1960. Studies on smallpox and complications of smallpox vaccination. Pediatrics 25176-189. [PubMed] [Google Scholar]
- 51.Kempe, C. H., T. O. Berge, and B. England. 1956. Hyperimmune vaccinial gamma globulin. Pediatrics 18177-188. [PubMed] [Google Scholar]
- 52.Klasse, P. J., and D. R. Burton. 2007. Antibodies to West Nile virus: a double-edged sword. Cell Host Microbe 187-89. [DOI] [PubMed] [Google Scholar]
- 53.Kopf, M., B. Abel, A. Gallimore, M. Carroll, and M. F. Bachmann. 2002. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nat. Med. 8373-378. [DOI] [PubMed] [Google Scholar]
- 54.Kotwal, G. J., S. N. Isaacs, R. McKenzie, M. M. Frank, and B. Moss. 1990. Inhibition of the complement cascade by the major secretory protein of vaccinia virus. Science 250827-830. [DOI] [PubMed] [Google Scholar]
- 55.Kotwal, G. J., and B. Moss. 1988. Vaccinia virus encodes a secretory polypeptide structurally related to complement control proteins. Nature 335176-178. [DOI] [PubMed] [Google Scholar]
- 56.Law, M., R. Hollinshead, and G. L. Smith. 2002. Antibody-sensitive and antibody-resistant cell-to-cell spread by vaccinia virus: role of the A33R protein in antibody-resistant spread. J. Gen. Virol. 83209-222. [DOI] [PubMed] [Google Scholar]
- 57.Law, M., M. M. Putz, and G. L. Smith. 2005. An investigation of the therapeutic value of vaccinia-immune IgG in a mouse pneumonia model. J. Gen. Virol. 86991-1000. [DOI] [PubMed] [Google Scholar]
- 58.Law, M., and G. L. Smith. 2001. Antibody neutralization of the extracellular enveloped form of vaccinia virus. Virology 280132-142. [DOI] [PubMed] [Google Scholar]
- 59.Lawrence, S. J., K. R. Lottenbach, F. K. Newman, R. M. Buller, C. J. Bellone, J. J. Chen, G. H. Cohen, R. J. Eisenberg, R. B. Belshe, S. L. Stanley, Jr., and S. E. Frey. 2007. Antibody responses to vaccinia membrane proteins after smallpox vaccination. J. Infect. Dis. 196220-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leddy, J. P., R. L. Simons, and R. G. Douglas. 1977. Effect of selective complement deficiency on the rate of neutralization of enveloped viruses by human sera. J. Immunol. 11828-34. [PubMed] [Google Scholar]
- 61.Li, L., K. L. Coelingh, and W. J. Britt. 1995. Human cytomegalovirus neutralizing antibody-resistant phenotype is associated with reduced expression of glycoprotein H. J. Virol. 696047-6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Likos, A. M., S. A. Sammons, V. A. Olson, A. M. Frace, Y. Li, M. Olsen-Rasmussen, W. Davidson, R. Galloway, M. L. Khristova, M. G. Reynolds, H. Zhao, D. S. Carroll, A. Curns, P. Formenty, J. J. Esposito, R. L. Regnery, and I. K. Damon. 2005. A tale of two clades: monkeypox viruses. J. Gen. Virol. 862661-2672. [DOI] [PubMed] [Google Scholar]
- 63.Liszewski, M. K., M. K. Leung, R. Hauhart, R. M. Buller, P. Bertram, X. Wang, A. M. Rosengard, G. J. Kotwal, and J. P. Atkinson. 2006. Structure and regulatory profile of the monkeypox inhibitor of complement: comparison to homologs in vaccinia and variola and evidence for dimer formation. J. Immunol. 1763725-3734. [DOI] [PubMed] [Google Scholar]
- 64.Lustig, S., C. Fogg, J. C. Whitbeck, R. J. Eisenberg, G. H. Cohen, and B. Moss. 2005. Combinations of polyclonal or monoclonal antibodies to proteins of the outer membranes of the two infectious forms of vaccinia virus protect mice against a lethal respiratory challenge. J. Virol. 7913454-13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lustig, S., C. Fogg, J. C. Whitbeck, and B. Moss. 2004. Synergistic neutralizing activities of antibodies to outer membrane proteins of the two infectious forms of vaccinia virus in the presence of complement. Virology 32830-35. [DOI] [PubMed] [Google Scholar]
- 66.Marennikova, S. S. 1962. The use of hyperimmune antivaccinia gamma-globulin for the prevention and treatment of smallpox. Bull. W. H. O. 27325-330. [PMC free article] [PubMed] [Google Scholar]
- 67.Mehlhop, E., C. Ansarah-Sobrinho, S. Johnson, M. Engle, D. H. Fremont, T. C. Pierson, and M. S. Diamond. 2007. Complement protein C1q inhibits antibody-dependent enhancement of flavivirus infection in an IgG subclass-specific manner. Cell Host Microbe 2417-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mehlhop, E., and M. S. Diamond. 2006. Protective immune responses against West Nile virus are primed by distinct complement activation pathways. J. Exp. Med. 2031371-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mehlhop, E., K. Whitby, T. Oliphant, A. Marri, M. Engle, and M. S. Diamond. 2005. Complement activation is required for induction of a protective antibody response against West Nile virus infection. J. Virol. 797466-7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moss, B. 2006. Poxviridae: the viruses and their replication, p. 2849-2883. In D. M. Knipe, P. Howley, D. Giriffin, and R. Lamb (ed.), Fundamental virology, 5 ed. Lippincott Williams & Wilkins, Hagerstown, MD.
- 71.Murphy, K., P. Travers, and M. Walport. 2008. Janeway's immunobiology, 7th ed. Garland Science, New York, NY.
- 72.Neuberger, M. S., and K. Rajewsky. 1981. Switch from hapten-specific immunoglobulin M to immunoglobulin D secretion in a hybrid mouse cell line. Proc. Natl. Acad. Sci. USA 781138-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ochsenbein, A. F., D. D. Pinschewer, B. Odermatt, M. C. Carroll, H. Hengartner, and R. M. Zinkernagel. 1999. Protective T cell-independent antiviral antibody responses are dependent on complement. J. Exp. Med. 1901165-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parren, P. W., and D. R. Burton. 2001. The antiviral activity of antibodies in vitro and in vivo. Adv. Immunol. 77195-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Payne, L. G. 1980. Significance of extracellular enveloped virus in the in vitro and in vivo dissemination of vaccinia. J. Gen. Virol. 5089-100. [DOI] [PubMed] [Google Scholar]
- 76.Pierson, T. C., Q. Xu, S. Nelson, T. Oliphant, G. E. Nybakken, D. H. Fremont, and M. S. Diamond. 2007. The stoichiometry of antibody-mediated neutralization and enhancement of West Nile virus infection. Cell Host Microbe 1135-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Putz, M. M., I. Alberini, C. M. Midgley, I. Manini, E. Montomoli, and G. L. Smith. 2005. Prevalence of antibodies to Vaccinia virus after smallpox vaccination in Italy. J. Gen. Virol. 862955-2960. [DOI] [PubMed] [Google Scholar]
- 78.Putz, M. M., C. M. Midgley, M. Law, and G. L. Smith. 2006. Quantification of antibody responses against multiple antigens of the two infectious forms of vaccinia virus provides a benchmark for smallpox vaccination. Nat. Med. 121310-1315. [DOI] [PubMed] [Google Scholar]
- 79.Ramirez, J. C., E. Tapia, and M. Esteban. 2002. Administration to mice of a monoclonal antibody that neutralizes the intracellular mature virus form of vaccinia virus limits virus replication efficiently under prophylactic and therapeutic conditions. J. Gen. Virol. 831059-1067. [DOI] [PubMed] [Google Scholar]
- 80.Rappuoli, R., H. I. Miller, and S. Falkow. 2002. Medicine. The intangible value of vaccination. Science 297937-939. [DOI] [PubMed] [Google Scholar]
- 81.Roden, R. B., E. M. Weissinger, D. W. Henderson, F. Booy, R. Kirnbauer, J. F. Mushinski, D. R. Lowy, and J. T. Schiller. 1994. Neutralization of bovine papillomavirus by antibodies to L1 and L2 capsid proteins. J. Virol. 687570-7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rosengard, A. M., Y. Liu, Z. Nie, and R. Jimenez. 2002. Variola virus immune evasion design: expression of a highly efficient inhibitor of human complement. Proc. Natl. Acad. Sci. USA 998808-8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sahu, A., S. N. Isaacs, A. M. Soulika, and J. D. Lambris. 1998. Interaction of vaccinia virus complement control protein with human complement proteins: factor I-mediated degradation of C3b to iC3b1 inactivates the alternative complement pathway. J. Immunol. 1605596-5604. [PubMed] [Google Scholar]
- 84.Sakhatskyy, P., S. Wang, T. H. Chou, and S. Lu. 2006. Immunogenicity and protection efficacy of monovalent and polyvalent poxvirus vaccines that include the D8 antigen. Virology 355164-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sejvar, J. J., Y. Chowdary, M. Schomogyi, J. Stevens, J. Patel, K. Karem, M. Fischer, M. J. Kuehnert, S. R. Zaki, C. D. Paddock, J. Guarner, W. J. Shieh, J. L. Patton, N. Bernard, Y. Li, V. A. Olson, R. L. Kline, V. N. Loparev, D. S. Schmid, B. Beard, R. R. Regnery, and I. K. Damon. 2004. Human monkeypox infection: a family cluster in the Midwestern United States. J. Infect. Dis. 1901833-1840. [DOI] [PubMed] [Google Scholar]
- 86.Sette, A., M. Moutaftsi, J. Moyron-Quiroz, M. M. McCausland, D. H. Davies, R. J. Johnston, B. Peters, M. Rafii-El-Idrissi Benhnia, J. Hoffmann, H. P. Su, K. Singh, D. N. Garboczi, S. Head, H. Grey, P. L. Felgner, and S. Crotty. 2008. Selective CD4+ T cell help for antibody responses to a large viral pathogen: deterministic linkage of specificities. Immunity 28847-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shearer, J. D., L. Siemann, M. Gerkovich, and R. V. House. 2005. Biological activity of an intravenous preparation of human vaccinia immune globulin in mouse models of vaccinia virus infection. Antimicrob. Agents Chemother. 492634-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smith, G. L., A. Vanderplasschen, and M. Law. 2002. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 832915-2931. [DOI] [PubMed] [Google Scholar]
- 89.Tsung, K., J. H. Yim, W. Marti, R. M. Buller, and J. A. Norton. 1996. Gene expression and cytopathic effect of vaccinia virus inactivated by psoralen and long-wave UV light. J. Virol. 70165-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Van den Berg, C. W., P. C. Aerts, and H. Van Dijk. 1991. In vivo anti-complementary activities of the cobra venom factors from Naja naja and Naja haje. J. Immunol. Methods 136287-294. [DOI] [PubMed] [Google Scholar]
- 91.Vanderplasschen, A., M. Hollinshead, and G. L. Smith. 1997. Antibodies against vaccinia virus do not neutralize extracellular enveloped virus but prevent virus release from infected cells and comet formation. J. Gen. Virol. 782041-2048. [DOI] [PubMed] [Google Scholar]
- 92.Vanderplasschen, A., E. Mathew, M. Hollinshead, R. B. Sim, and G. L. Smith. 1998. Extracellular enveloped vaccinia virus is resistant to complement because of incorporation of host complement control proteins into its envelope. Proc. Natl. Acad. Sci. USA 957544-7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Viner, K. M., and S. N. Isaacs. 2005. Activity of vaccinia virus-neutralizing antibody in the sera of smallpox vaccinees. Microbes Infect. 7579-583. [DOI] [PubMed] [Google Scholar]
- 94.Wagenaar, T. R., and B. Moss. 2007. Association of vaccinia virus fusion regulatory proteins with the multicomponent entry/fusion complex. J. Virol. 816286-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Walker, B. D., and D. R. Burton. 2008. Toward an AIDS vaccine. Science 320760-764. [DOI] [PubMed] [Google Scholar]
- 96.Ward, B. M., and B. Moss. 2000. Golgi network targeting and plasma membrane internalization signals in vaccinia virus B5R envelope protein. J. Virol. 743771-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ward, B. M., and B. Moss. 2001. Visualization of intracellular movement of vaccinia virus virions containing a green fluorescent protein-B5R membrane protein chimera. J. Virol. 754802-4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Williamson, J. D., R. W. Reith, L. J. Jeffrey, J. R. Arrand, and M. Mackett. 1990. Biological characterization of recombinant vaccinia viruses in mice infected by the respiratory route. J. Gen. Virol. 712761-2767. [DOI] [PubMed] [Google Scholar]
- 99.Wolffe, E. J., S. N. Isaacs, and B. Moss. 1993. Deletion of the vaccinia virus B5R gene encoding a 42-kilodalton membrane glycoprotein inhibits extracellular virus envelope formation and dissemination. J. Virol. 674732-4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wyatt, L. S., P. L. Earl, L. A. Eller, and B. Moss. 2004. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc. Natl. Acad. Sci. USA 1014590-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xiao, Y., L. Aldaz-Carroll, A. M. Ortiz, J. C. Whitbeck, E. Alexander, H. Lou, H. L. Davis, T. J. Braciale, R. J. Eisenberg, G. H. Cohen, and S. N. Isaacs. 2007. A protein-based smallpox vaccine protects mice from vaccinia and ectromelia virus challenges when given as a prime and single boost. Vaccine 251214-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]