Abstract
Four canine coronavirus type II (CCoV-II) strains were identified in the guts and internal organs of pups which had died of acute gastroenteritis. The CCoV-II strains were strictly related to porcine transmissible gastroenteritis virus (TGEV) in the N-terminal domain of the spike protein, whereas in the other parts of the genome, a higher genetic relatedness to recent CCoV-II isolates was observed. Experimental infection of dogs with a TGEV-like isolate induced mild gastroenteritis without any systemic involvement. By virus neutralization tests, antigenic differences between reference and TGEV-like CCoVs were found. Our data support the potential recombinant origin of the TGEV-like CCoVs.
Coronaviruses (CoVs) are able to cause a variety of different clinical forms of disease in a wide range of species (11). Currently, they are classified into three groups based on antigenic and genetic relatedness. CoVs infecting dogs have been reported that belong to groups 1 and 2, namely, canine CoV (CCoV) and canine respiratory CoV, respectively (3). CCoV is an enteropathogen which has been known since the early 1970s (1) and includes two different genotypes, CCoV-I and CCoV-II, with different genetic and biological properties (19, 22). CCoV-II strains with uncommon virulence have been described (12, 13, 24), including a pantropic variant causing systemic disease in pups (2, 4, 8). In addition, recombinant viruses have been reported between CCoV-II and CCoV-I (12) or porcine transmissible gastroenteritis virus (TGEV) (27). In this paper, we report the genomic, biological, and antigenic characterization of four type II CCoVs with the N-terminal domain of the S protein highly divergent from classical CCoV strains but strictly related to TGEV.
Identification of TGEV-like CCoV strains.
Between December 2005 and March 2008, four dogs which had died of gastroenteritis were submitted to our laboratory for routine diagnostic investigations. The dogs were a 14-week-old great dane pup (341/05), a 10-week-old chihuahua pup (174/06), an 11-week-old mixed-breed pup (430/07), and a 13-week-old maltese pup (119/08). Dogs 174/06 and 119/08 had been imported from Hungary a few days before the onset of clinical signs. Intestinal contents and tissue samples collected from the dead dogs were tested by conventional or real-time PCR assays for the detection of the main viral pathogens of dogs as previously described (8). CCoV was detected in the fecal samples or intestinal contents of all of the pups examined, with viral RNA titers ranging from 1.37 × 105 to 2.38 × 107 μl−1 of template. Further genotyping by type-specific TaqMan assays (10) showed the presence of both CCoV types I and II in the guts of dogs 430/07 and 119/08, whereas the specimens from the other two dogs were found to contain only genotype II. Surprisingly, CCoV-II RNA was also detected in the internal organs of all of the dogs, albeit with variable tissue distribution (data not shown). It is noteworthy that all of the pups were positive for canine parvovirus (CPV) by real-time PCR (7). Subsequent characterization by means of type-specific minor-groove binder probe assays (5, 6, 9) showed that dogs 341/05, 430/07, and 119/08 were coinfected with CPV-2a, whereas a classical CPV-2 (vaccinal) strain was detected in the gut of pup 174/06.
The 3′ end of the genome of the four CCoV-II strains detected in the lung samples was amplified and analyzed as previously described (8), and the nucleotide sequences were deposited in GenBank under accession numbers EU856361 to EU856362 and EU924790 to EU924791. As expected, all of the predicted genes but open reading frame 3b (ORF3b), ORF3c, and ORF7b were preceded by the repeated intergenic sequence CTAAAC. The spike (S) protein was 1,457 amino acids (aa) long in all of the strains that were analyzed, in contrast to classical type II CCoVs and feline CoVs (FCoVs), which displayed a shorter protein (1,451 to 1,454 aa). In the S protein, the amino acid identities among the CCoV strains sequenced in this study ranged between 95.1 and 98.9%, and the identity of these strains to other type II CCoVs was only 79.9 to 82.8%. Surprisingly, a higher genetic relatedness to TGEV was found, whereas other group 1a CoVs were proven to be less related. An exceptionally high identity to TGEV was evident in the N terminus (Table 1). Analysis of the other structural proteins, including the small envelope (E), membrane (M), and nucleocapsid (N) proteins, did not show atypical findings, with the exception of the E protein of strain 430/07, which was 75 instead of 82 aa long, due to a 21-nt deletion in the 5′ end of the encoding gene. Analogously, the nonstructural proteins were conserved with respect to CCoV-II, except for a 154-nt deletion in ORF7b of strain 341/05 leading to a shorter accessory protein 7b.
TABLE 1.
Amino acid identities of TGEV-like CCoVs to group 1a CoV reference strains in main nonstructural and structural proteins
| CoV strain | Accession no. | Amino acid identity (%) to TGEV-like CCoVs
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Pp1aba | Pp1aa | S | S N terminusb | S C terminusc | E | M | N | ||
| TGEV-Purdue | NC_002306 | 98.0-98.2 | 97.4 | 91.5-92.1 | 80.2-81.9 | 94.3-94.9 | 89.0-96.3 | 91.9-95.0 | 95.0-95.8 |
| TGEV-TS | DQ201447 | NDd | NAe | 91.2-91.9 | 80.5-82.3 | 93.9-94.7 | 89.0-97.5 | 91.9-93.8 | 94.7-95.5 |
| TGEV-96-933 | AF104420 | NA | NA | 89.0-89.3 | 76.3-77.7 | 92.0-92.5 | 87.8-95.1 | 90.8-91.6 | 93.1-93.7 |
| PRCoV-RM4 | Z24675 | NA | NA | 77.0-77.5 | 12.0-12.7 | 92.8-93.4 | 89.0-97.5 | 91.9-93.1 | 93.1-93.9 |
| PRCoV-86-37004 | X60089 (S), X55980 (E, M, N) | NA | NA | 77.2-77.7 | 12.7-13.4 | 92.8-93.4 | 89.0-97.5 | 92.3-93.5 | 93.4-94.2 |
| CCoV-II-UCD1 | AF116248 | NA | NA | NA | 81.6-82.3 | NA | NA | NA | NA |
| CCoV-II-Insavc-1 | D13096 | NA | NA | 79.9-81.1 | 22.6-24.3 | 93.6-95.1 | 86.5-95.1 | 90.0-90.4 | 91.8-92.1 |
| CCoV-II-CB/05 | NA (pp1ab, pp1a), DQ112226 (S, E, M, N) | 98.7 | 97.9-98.3 | 81.5-82.8 | 21.9-24.0 | 95.7-98.0 | 91.4-100 | 94.6-99.2 | 97.6-100 |
| CCoV-II-BGF10 | AY342160 | NA | NA | 80.3-80.9 | 24.0-36.4 | 93.9-94.7 | 79.2-87.8 | 89.7-91.2 | 92.6-92.9 |
| CCoV-I-Elmo/02 | AY307020 (S), AY426983 (E); NA (M, N) | NA | NA | 41.3-41.9 | 17.9-19.2 | 48.9-49.1 | 79.2-85.3 | 87.9-89.0 | 88.4-89.0 |
| CCoV-I-23/03 | AY307021 (S), AY426984 (E), AY548235 (M, N) | NA | NA | 41.6-42.1 | 15.5-17.4 | 49.1-49.3 | 80.4-86.5 | 87.9-89.0 | 88.4-89.0 |
| FCoV-II-79-146 | NC_007025 | 94.5-94.8 | 87.8-88.1 | 80.5-81.5 | 21.3-24.0 | 95.3-95.7 | 71.9-78.0 | 83.6-84.4 | 76.7-77.2 |
| FCoV-II-79-683 | X80799 (S), FCY13921 (E, M, N) | NA | NA | 81.1-81.8 | 21.9-24.3 | 95.8-96.1 | 89.0-97.5 | 85.6-87.9 | 78.0-78.5 |
| FCoV-I-KU-2 | D32044 (S), AAB47501 (M), AB086881 (N) | NA | NA | 41.3-41.8 | 17.4-18.4 | 48.0-48.4 | NA | 82.1-82.8 | 75.3-75.6 |
| FCoV-I-Black | EU186072 | 92.9-93.2 | 88.2-88.5 | 40.8-41.3 | 17.7-18.7 | 47.5-47.8 | 71.9-78.0 | 82.5-84.0 | 76.1-76.4 |
| FCoV-I-UCD1 | AB088222 (S), AB086902 (M, N) | NA | NA | 41.3-41.7 | 17.8-19.1 | 48.1-48.5 | NA | 81.3-81.7 | 76.1-77.2 |
Only partial C-terminal sequences of strains 341/05 and 174/06 were analyzed.
Residues 1 to 282 of the S protein of TGEV-like CCoVs.
Residues 283 to 1457 of the S protein of TGEV-like CCoVs.
ND, not done.
NA, not available.
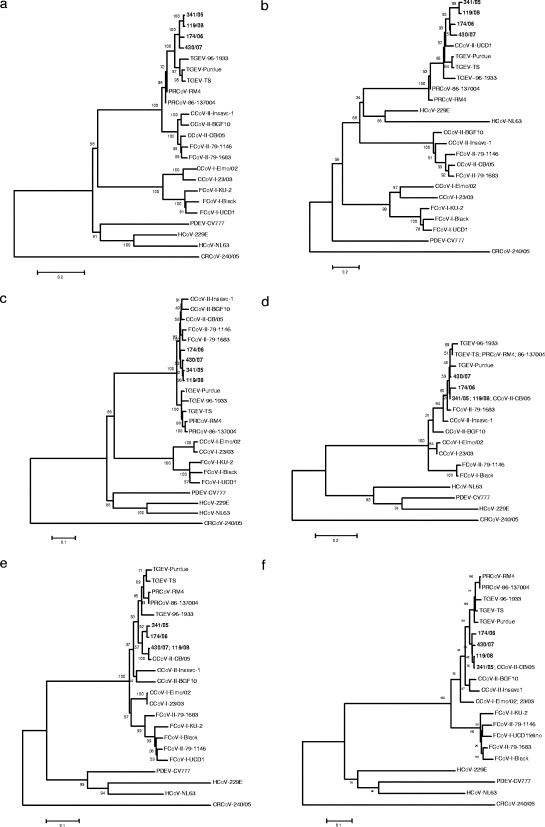
By phylogenetic analysis of the full-length sequence of the S protein conducted with Mega3 (17), group 1a CoVs clustered into two main clades. The first clade included CCoV/FCoV-I, whereas swine CoVs and CCoV/FCoV-II segregated into two separate clusters within the same clade (Fig. 1a). Interestingly, the four TGEV-like CCoVs formed a monophyletic group within the same cluster of TGEV and porcine respiratory CoV, which was separated from other type II CCoVs. This pattern of segregation was more evident in the N terminus, where the atypical CCoVs segregated together with the old CCoV strain UCD1 within the cluster including swine CoVs (Fig. 1b). In contrast, in the C terminus, the TGEV-like CCoVs clustered together with extant CCoV-II isolates and separately from TGEV/porcine respiratory CoV (Fig. 1c). Analysis of the E, M, and N proteins showed a constant segregation of the TGEV-like CCoVs with typical CCoV-II strains (Fig. 1d to f).
FIG. 1.
Phylogenetic analysis of group 1 CoVs. Neighbor-joining trees based on the spike protein full-length (a), N-terminal (b), and C-terminal (c) sequences and the envelope (d), membrane (e), and nucleocapsid (f) proteins of group 1 CoVs. For phylogenetic tree construction, the group 1a CoVs listed in Table 1 and the following CoV strains were used (GenBank accession numbers are in parentheses): human CoV (HCoV) 229E (NC_002645), porcine epidemic diarrhea virus (PDEV) CV777 (NC_003436), and HCoV-NL63 (NC_005831). The tree is rooted on the group 2 CoV canine respiratory CoV 240/05 (EU999954). Statistical support was provided by bootstrapping more than 1,000 replicates. The scale bars indicate the estimated numbers of amino acid substitutions per site.
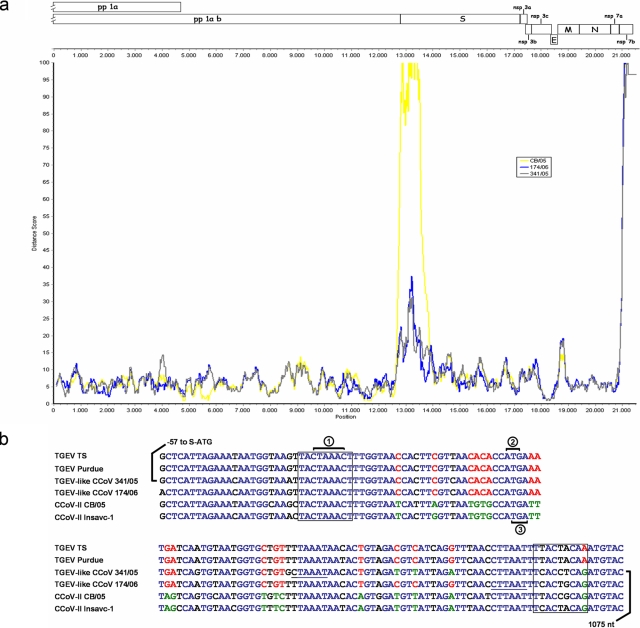
The partial nucleotide sequence of ORF1a and the complete nucleotide sequence of ORF1b of strains 341/05 and 174/06 (accession numbers EU856361 to EU856362) were determined with primers amplifying overlapping fragments. In total, 21,347 and 21,395 nucleotides (nt) were sequenced from strains 341/05 and 174/06, respectively, representing about three-fourths of the entire genome of group 1a CoVs. In the partial C-terminal domain of pp1ab (4,239 aa), strains 341/05 and 174/06 were 98.8% identical and displayed approximately the same identity to CCoV-II-CB/05. In contrast, less similarity to TGEV, FCoV-II, and FCoV-I was found (Table 1). Analysis of the nearly full-length genome (more than 21,000 nt) confirmed the higher genetic relatedness to CCoV-II-CB/05 (95.8% nucleotide sequence identity). By a SimPlot analysis (18), the potential recombinant origin of the TGEV-like CCoVs was evident. In fact, their genetic distance from TGEV was approximately the same as that from CCoV in the entire sequence, except at the 5′ end of the S gene (Fig. 2a). By visual inspection of the S-gene alignments, the putative acceptor and donor sites involved in the double recombination between CCoV-II and TGEV were identified (Fig. 2b).
FIG. 2.
Recombinant origin of the TGEV-like CCoVs. (a) SimPlot analysis of the nucleotide sequences of the nearly full-length genome of the TGEV-like CCoVs. Each point plotted is the percent genetic similarity within a 200-nt-wide sliding window centered on the position plotted with a step size of 20 nt, and Hamming correction on each curve represents a comparison of the sequence data of TGEV-like CCoVs 341/05 and 174/06 and CCoV-II-CB/05 to the reference sequence data of TGEV-Purdue. (b) Nucleotide sequence alignment of TGEV, TGEV-like CCoV, and CCoV-II genomes at the 5′ end of the S-encoding gene. Indicated are the S-gene transcription-regulating sequence (circled number 1), the S-gene initiation translation codon (circled number 2), and the ORF1b termination codon (circled number 3). The potential acceptor and donor sequences involved in the recombination event are boxed or underlined. Blue, red, and green letters indicate nucleotides conserved among all viruses, nucleotides conserved among TGEV and TGEV-like CCoV genomes, and typical nucleotides of CCoV origin, respectively. A putative location of the acceptor site is the sequence 22-TTACTAAAC-30, which has high identity (seven out of nine nucleotides) with that present in the proposed donor site (1061-TTACTACAA-1069). Alternatively, small sequence domains present in the TGEV-like CCoVs 341/05 (1021-CTAAAT-1026) and 174/06 (1054-CTTAAT-1059) are observed in the potential donor sites that could act as a slow-down signal and promote recombination between these sequences and others with high identity that are present at the acceptor sites, such as the sequence 25-CTAAAC-30.
Biological and antigenic characterization of the TGEV-like CCoV strains.
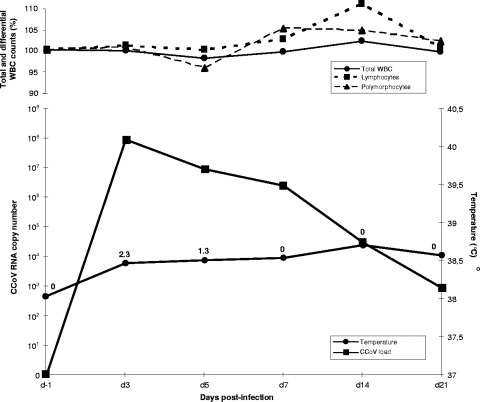
Virus isolation attempts from lung samples with canine fibroma A-72 cells (8) were successful with all TGEV-like CCoVs, except for strain 119/08, which possessed lower viral RNA titers in the internal organs and intestinal content of the dead dog. All virus isolates induced a cytopathic effect in the inoculated monolayers and were recognized by a monoclonal antibody (MAb) that bound the N protein of CCoV, FCoV, and TGEV. In contrast, no viral growth was observed when using swine testicular cells that were permissive for the reference TGEV strain, Purdue. In order to evaluate the pathogenic potential of TGEV-like CCoVs in dogs, the plaque-purified isolate from sample 341/05 was used to infect experimentally three antibody-defined beagle pups, whereas an additional pup served as a control. All inoculated pups shed virus with their feces during the observation period (Fig. 3), whereas no trace of viral RNA was detected in the blood or nasal samples. Clinical signs observed in the infected dogs were indicative of a classical enteric coronavirosis, consisting of mild diarrhea lasting 2 to 3 days. Neither leukopenia nor other signs of systemic involvement were observed in any infected pup.
FIG. 3.
Results of experimental infection of dogs with a TGEV-like CCoV. Three 12-week-old beagle pups were inoculated oronasally with 2 ml of strain 341/05 (titer of 106.25 50% tissue culture infective doses ml−1) and monitored for up to 21 days for total white blood cell (WBC), lymphocyte, and polymorphocyte counts (top graph). In addition, fever, viral RNA shed in feces, and clinical scores were determined (bottom graphs). Total WBC, lymphocyte, and polymorphocyte counts are presented as percentages of the cell counts determined at day 0. Viral RNA titers as determined by real-time RT-PCR are expressed as copy numbers per microliter of template. Clinical scores were calculated as previously described (4) and are reported for each day with reference to the temperature curves.
The sera obtained from dogs experimentally infected with strain 341/05 or with the classical CCoV-II enteric strain S378 (10) were tested against homologous and heterologous viruses with a virus neutralization test (21). Poor cross-protection between homologous and heterologous antibody titers was evident. In fact, in dogs infected with strain 341/05, the homologous VN titer (reciprocal of the geometric mean) was 20.16 and the heterologous titer was 6.35, whereas dogs infected with strain S378 displayed homologous and heterologous antibody titers of 40.32 and 10.08, respectively. In addition, the three TGEV-like CCoV plaque-purified isolates were characterized by using a panel of TGEV MAbs previously developed (14, 16). The viruses reacted against three MAbs, two of which neutralize both TGEV and CCoV, whereas the third MAb had been shown to bind TGEV but not CCoV (23).
We have identified CCoVs with a potential double-recombinant origin through partial S-gene exchange with TGEV. Recombination involving the S gene has been previously described for FCoV (15) and CCoV (12, 27). A TGEV-like CCoV, strain UCD1, has been previously reported, but only partial S-gene sequences were determined, thus preventing a complete genomic characterization (27). In contrast, we have determined and analyzed the 3′ end of four TGEV-like CCoVs and the nearly full-length genome of two of those strains. In addition, strain UCD1 was detected in the feces of a dog with diarrhea, whereas we detected the recombinant viruses in the feces/intestinal contents and in the internal organs as well.
A major mechanism driving CoV genetic evolution is represented by the high frequency of homologous RNA recombination, which is frequently mediated by a copy choice mechanism based on sequence homology flanking the recombination sites (20, 28). In addition, the necessary discontinuous RNA synthesis required during RNA transcription to generate the subgenomic mRNAs in CoVs is associated with highly conserved sequences preceding each gene and representing a slow-down or stop signal for RNA synthesis (25). This sequence, in the case of group 1a CoVs, is CTAAAC (26, 28). Therefore, in the sequence domains where recombination has taken place, it is likely to be small segments of sequence identity between the donor and the acceptor sequences. In fact, observation of the sequence domains involved in the recombination event revealed the presence of segments of sequence homology (Fig. 2b), although single well-defined crossover points could not be definitively determined.
Although the TGEV-like CCoVs were detected in the internal organs of naturally infected dogs, experimental infection failed to cause systemic involvement or virus dissemination through the blood. Thus, the CPV coinfection observed in the natural outbreaks could have played a certain role in TGEV-like CCoV spreading to internal organs. In addition, the antigenic differences observed between reference and recombinant CCoVs may have some implications for prophylaxis programs, as dogs administered classical CCoV vaccines may be susceptible to infection (disease?) caused by TGEV-like CCoVs. The detection of these viruses in different time periods (at least 4 years) and geographic areas (at least Italy and Hungary) has let us to presume that TGEV-like CCoVs are effectively circulating in the dog population. Taking into account the genetic and antigenic differences between classical and recombinant viruses, we propose the further division of CCoV-II into two different subtypes, CCoV-IIa and CCoV-IIb, including reference and TGEV-like CCoV-II isolates, respectively.
Acknowledgments
We are grateful to undergraduate student Mauro Di Pilato for his excellent assistance with part of the experimental work and to P. J. Collins (CIT, Department of Biology, Cork, Ireland) for the English revision of the manuscript. The MAbs used for the immunofluorescence assay and CCoV strain S378 were kindly donated by G. Chappuis (Merial, Lyon, France) and L. E. Carmichael (James Baker Institute for Animal Health, Cornell University, Ithaca, NY), respectively.
This work was supported by grants from the Italian Ministry of Health, Ricerca finalizzata 2008, project Mammalian Coronaviruses: Molecular Epidemiology, Vaccine Development and Implications for Animal and Human Health (Nicola Decaro), and from the Italian Ministry of Education, University and Research, PRIN 2006, project Infezione del Cane da Coronavirus Pantropico: Aspetti Epidemiologici, Patogenetici e Molecolari (Canio Buonavoglia).
Footnotes
Published ahead of print on 26 November 2008.
REFERENCES
- 1.Binn, L. N., E. C. Lazar, K. P. Keenan, D. L. Huxsoll, R. H. Marchwicki, and A. J. Strano. 1974. Recovery and characterization of a coronavirus from military dogs with diarrhea. Proc. Annu. Meet. U. S. Anim. Health Assoc. 78359-366. [PubMed] [Google Scholar]
- 2.Buonavoglia, C., N. Decaro, V. Martella, G. Elia, M. Campolo, C. Desario, M. Castagnaro, and M. Tempesta. 2006. Canine coronavirus highly pathogenic for dogs. Emerg. Infect. Dis. 12492-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Decaro, N., and C. Buonavoglia. 2008. An update on canine coronaviruses: viral evolution and pathobiology. Vet. Microbiol. 132221-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Decaro, N., M. Campolo, A. Lorusso, C. Desario, V. Mari, M. L. Colaianni, G. Elia, V. Martella, and C. Buonavoglia. 2008. Experimental infection of dogs with a novel strain of canine coronavirus causing systemic disease and lymphopenia. Vet. Microbiol. 128253-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Decaro, N., G. Elia, C. Desario, S. Roperto, V. Martella, M. Campolo, A. Lorusso, A. Cavalli, and C. Buonavoglia. 2006. A minor groove binder probe real-time PCR assay for discrimination between type 2-based vaccines and field strains of canine parvovirus. J. Virol. Methods 13665-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Decaro, N., G. Elia, V. Martella, M. Campolo, C. Desario, M. Camero, F. Cirone, E. Lorusso, M. S. Lucente, D. Narcisi, P. Scalia, and C. Buonavoglia. 2006. Characterisation of the canine parvovirus type 2 variants using minor groove binder probe technology. J. Virol. Methods 13392-99. [DOI] [PubMed] [Google Scholar]
- 7.Decaro, N., G. Elia, V. Martella, C. Desario, M. Campolo, L. D. Trani, E. Tarsitano, M. Tempesta, and C. Buonavoglia. 2005. A real-time PCR assay for rapid detection and quantitation of canine parvovirus type 2 in the feces of dogs. Vet. Microbiol. 10519-28. [DOI] [PubMed] [Google Scholar]
- 8.Decaro, N., V. Martella, G. Elia, M. Campolo, C. Desario, F. Cirone, M. Tempesta, and C. Buonavoglia. 2007. Molecular characterisation of the virulent canine coronavirus CB/05 strain. Virus Res. 12554-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Decaro, N., V. Martella, G. Elia, C. Desario, M. Campolo, D. Buonavoglia, A. L. Bellacicco, M. Tempesta, and C. Buonavoglia. 2006. Diagnostic tools based on minor groove binder probe technology for rapid identification of vaccinal and field strains of canine parvovirus type 2b. J. Virol. Methods 13810-16. [DOI] [PubMed] [Google Scholar]
- 10.Decaro, N., V. Martella, D. Ricci, G. Elia, C. Desario, M. Campolo, N. Cavaliere, L. Di Trani, M. Tempesta, and C. Buonavoglia. 2005. Genotype-specific fluorogenic RT-PCR assays for the detection and quantitation of canine coronavirus type I and type II RNA in faecal samples of dogs. J. Virol. Methods 13072-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enjuanes, L., D. Brian, D. Cavanagh, K. Holmes, M. M. C. Lai, H. Laude, P. Masters, P. Rottier, S. Siddell, W. J. M. Spaan, F. Taguchi, and P. Talbot. 2000. Coronaviridae, p. 835-849. In M. H. V. van Regenmortel, C. M. Fauquet, D. H. L. Bishop, E. B. Carstens, M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wickner (ed.), Virus taxonomy, classification and nomenclature of viruses. Academic Press, Inc., New York, NY.
- 12.Escutenaire, S., M. Isaksson, L. H. Renstrom, B. Klingeborn, C. Buonavoglia, M. Berg, S. Belak, and P. Thoren. 2007. Characterization of divergent and atypical canine coronaviruses from Sweden. Arch. Virol. 1521507-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evermann, J. F., J. R. Abbott, and S. Han. 2005. Canine coronavirus-associated puppy mortality without evidence of concurrent canine parvovirus infection. J. Vet. Diagn. Investig. 17610-614. [DOI] [PubMed] [Google Scholar]
- 14.Gebauer, F., W. P. Posthumus, I. Correa, C. Suñé, C. Smerdou, C. M. Sánchez, J. A. Lenstra, R. H. Meloen, and L. Enjuanes. 1991. Residues involved in the antigenic sites of transmissible gastroenteritis coronavirus S glycoprotein. Virology 183225-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrewegh, A. A., I. Smeenk, M. C. Horzinek, P. J. Rottier, and R. J. de Groot. 1998. Feline coronavirus type II strains 79-1683 and 79-1146 originate from a double recombination between feline coronavirus type I and canine coronavirus. J. Virol. 724508-4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiménez, G., I. Correa, M. P. Melgosa, M. J. Bullido, and L. Enjuanes. 1986. Critical epitopes in transmissible gastroenteritis virus neutralization. J. Virol. 60131-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5150-163. [DOI] [PubMed] [Google Scholar]
- 18.Lole, K. S., R. C. Bollinger, R. S. Paranjape, D. Gadkari, S. S. Kulkarni, N. G. Novak, R. Ingersoll, H. W. Sheppard, and S. C. Ray. 1999. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 73152-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lorusso, A., N. Decaro, P. Schellen, P. J. Rottier, C. Buonavoglia, B. J. Haijema, and R. J. de Groot. 2008. Gain, preservation, and loss of a group 1a coronavirus accessory glycoprotein. J. Virol. 8210312-10317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasternak, A. O., E. van der Born, W. J. Spaan, and E. J. Snijder. 2001. Sequence requirements for RNA strand transfer during nidovirus discontinuous subgenomic RNA synthesis. EMBO J. 207220-7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pratelli, A., G. Elia, V. Martella, A. Palmieri, F. Cirone, A. Tinelli, M. Corrente, and C. Buonavoglia. 2002. Prevalence of canine coronavirus antibodies by an enzyme-linked immunosorbent assay in dogs in the south of Italy. J. Virol. Methods 10267-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pratelli, A., V. Martella, N. Decaro, A. Tinelli, M. Camero, F. Cirone, G. Elia, A. Cavalli, M. Corrente, G. Greco, D. Buonavoglia, M. Gentile, M. Tempesta, and C. Buonavoglia. 2003. Genetic diversity of a canine coronavirus detected in pups with diarrhoea in Italy. J. Virol. Methods 1109-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sánchez, C. M., G. Jiménez, M. D. Laviada, I. Correa, C. Suñé, M. Bullido, F. Gebauer, C. Smerdou, P. Callebaut, J. M. Escribano, and L. Enjuanes. 1990. Antigenic homology among coronaviruses related to transmissible gastroenteritis virus. Virology 174410-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez-Morgado, J. M., S. Poynter, and T. H. Morris. 2004. Molecular characterization of a virulent canine coronavirus BGF strain. Virus Res. 10427-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawicki, D. L., and S. G. Sawicki. 1998. Role of the nonstructural polyproteins in alphavirus RNA synthesis. Adv. Exp. Med. Biol. 440187-198. [DOI] [PubMed] [Google Scholar]
- 26.Sola, I., J. L. Moreno, S. Zuniga, S. Alonso, and L. Enjuanes. 2005. Role of nucleotides immediately flanking the transcription-regulating sequence core in coronavirus subgenomic mRNA synthesis. J. Virol. 792506-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wesley, R. D. 1999. The S gene of canine coronavirus, strain UCD-1, is more closely related to the S gene of transmissible gastroenteritis virus than to that of feline infectious peritonitis virus. Virus Res. 61145-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zúñiga, S., S. I. S. Alonso, and L. Enjuanes. 2004. Sequence motifs involved in the regulation of discontinuous coronavirus subgenomic RNA synthesis. J. Virol. 78980-994. [DOI] [PMC free article] [PubMed] [Google Scholar]