Abstract
A vaccine for the prevention of human immunodeficiency virus (HIV) infection is desperately needed to control the AIDS pandemic. To address this problem, we developed vesicular stomatitis virus glycoprotein-pseudotyped replication-defective simian immunodeficiency viruses (dSIVs) as an AIDS vaccine strategy. The dSIVs retain characteristics of a live attenuated virus without the drawbacks of potential virulence caused by replicating virus. To improve vaccine immunogenicity, we incorporated CD40 ligand (CD40L) into the dSIV envelope. CD40L is one of the most potent stimuli for dendritic cell (DC) maturation and activation. Binding of CD40L to its receptor upregulates expression of major histocompatibility complex class I, class II, and costimulatory molecules on DCs and increases production of proinflammatory cytokines and chemokines, especially interleukin 12 (IL-12). This cytokine polarizes CD4+ T cells to Th1-type immune responses. DC activation and mixed lymphocyte reaction (MLR) studies were performed to evaluate the immunogenicity of CD40L-dSIV in vitro. Expression levels of CD80, CD86, HLA-DR, and CD54 on DCs transduced with the dSIV incorporating CD40L (CD40L-dSIV) were significantly higher than on those transduced with dSIV. Moreover, CD40L-dSIV-transduced DCs expressed up to 10-fold more IL-12 than dSIV-transduced DCs. CD40L-dSIV-transduced DCs enhanced proliferation and gamma interferon secretion by naive T cells in an MLR. In addition, CD40L-dSIV-immunized mice exhibited stronger humoral and cell-mediated immune responses than dSIV-vaccinated animals. The results show that incorporating CD40L into the dSIV envelope significantly enhances immunogenicity. As a result, CD40L-dSIVs can be strong candidates for development of a safe and highly immunogenic AIDS vaccine.
More than twenty-five years into the AIDS pandemic, a safe and effective vaccine has not been developed to prevent human immunodeficiency virus (HIV) infection (19). To date, the most effective vaccine developed, using the simian immunodeficiency virus (SIV)/rhesus macaque model, is a live attenuated virus with a deletion in the nef gene (SIVΔnef) (15). However, safety remains a major concern for this vaccine, since it is pathogenic to neonatal macaques (2). In addition, this vaccine can cause AIDS in some adult macaques anywhere from several months to years after vaccination, apparently the result of a restoration of the pathogenic phenotype after constitutive replication (3). Tremendous efforts have been put forth toward developing a safer vaccine strategy. Several groups have constructed replication-defective SIVs whose infection is limited to a single round of replication to reduce the risk of reversion to virulence while simultaneously maintaining efficacy similar to that of live attenuated vaccines (17, 36). Our laboratory and others have constructed vesicular stomatitis virus glycoprotein (VSV-G)-pseudotyped SIVs (dSIV) (55, 64). Pseudotyping with VSV-G expands tissue tropism, potentially enhances immune responses, and stabilizes the viral structure, allowing ultracentrifugation and ultrafiltration without losing infectivity (10). In macaque studies, dSIV-vaccinated animals had a 1- to 3-log reduction in primary viremia compared to unvaccinated animals; however, viral loads in both groups were indistinguishable in the chronic phase of infection (17, 36). In a rat study conducted by our laboratory, animals vaccinated with dSIV expressing gamma interferon (IFN-γ) had humoral and cell-mediated immune responses to Gag but only partially controlled replication of a recombinant vaccinia virus expressing SIV Gag-Pol used as a surrogate challenge (55a). To improve the efficacy of this vaccine, we therefore developed a dSIV with human CD40 ligand (CD40L) as well as VSV-G incorporated into the virus envelope.
CD40L (CD154), a 39-kDa type II membrane glycoprotein, belongs to the tumor necrosis factor (TNF) family. CD40L is transiently expressed on activated CD4+ T cells, CD8+ T cells, γδ T cells, mast cells, and interleukin 2 (IL-2)-activated natural killer cells (23). Its receptor, CD40, a member of the TNF receptor superfamily, is constitutively expressed on epithelial cells, endothelial cells, and all antigen-presenting cells (APCs), including dendritic cells (DCs), macrophages, and B lymphocytes (66). Binding of the CD40 protein on immature DCs triggers DC activation and maturation (44), resulting in increased expression of costimulatory molecules and enhancing the DCs' ability to activate naive T cells. In addition, the CD40/CD40L interaction upregulates Bcl-2 and Bcl-xL expression, increasing DC survival (52). CD40L also upregulates production of proinflammatory cytokines and chemokines by DCs, especially IL-12, a cytokine responsible for polarizing CD4+ T cells to Th1-type immune responses (12). Moreover, activation of DCs through CD40/CD40L signaling allows DCs to cross-present exogenous antigen and thus cross-prime CD8+ cytotoxic T cells without CD4+ T-helper cells (51).
CD40L has been shown to improve immunogenicity in several therapeutic cancer (40, 58) and prophylactic vaccine (20, 46) studies. Skountzou et al. demonstrated that incorporating CD40L into an SIV virus-like particle enhances humoral and cellular immune responses (61). However, it requires extremely high doses to induce adequate immune responses when administered locally. In this study, we took advantage of the immunoregulatory characteristics of CD40L and the wide cell tropism of VSV-G by incorporating both into the envelope of pseudotyped viral particles to enhance the immunogenicity of dSIVs. We evaluated the immunogenicity of CD40L-dSIV in vitro using monocyte-derived DCs with a phenotype comparable to that of interstitial DCs (5). The results suggested that CD40L, as a vaccine adjuvant, significantly enhanced the ability of dSIV to activate DCs and prime naive T cells in vitro. The immunogenicity of CD40L-dISV was further confirmed in vivo. CD40L-dSIV-immunized mice had higher antibody and cell-mediated immune (CMI) responses than dSIV-immunized animals, suggesting that this strategy could increase the immune responses required for controlling HIV infection.
MATERIALS AND METHODS
Cells and mediums.
HeLa and embryonic kidney (293T) cells were grown at 37°C in 5% CO2 in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum and 2 mM l-glutamine. Rhesus macaque peripheral blood mononuclear cells (PBMCs) (38) were prepared by Ficoll-Paque density centrifugation. PBMCs were maintained in AIM-V medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum.
Plasmid construction.
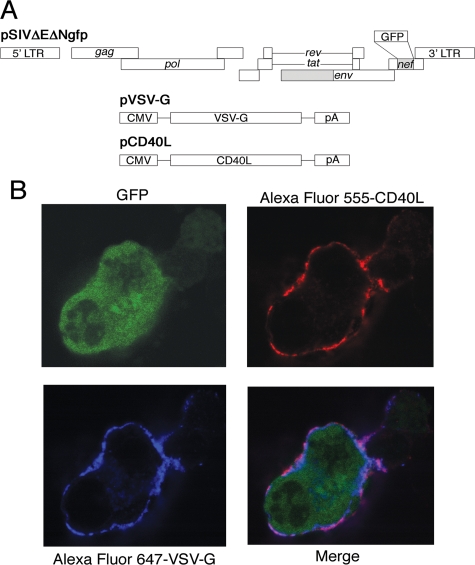
The human CD40L gene was obtained from the plasmid pBS-hCD40L-6A9 (American Type Culture Collection, Manassas, VA) by restriction enzyme digestion at the EcoRI and BamHI sites (63). The resulting EcoRI-BamHI fragment containing the CD40L gene was cloned into the EcoRI-BamHI sites of pcDNA3.1/Zeo (−) (Invitrogen) to generate pCD40L (Fig. 1), placing the CD40L gene under the control of the cytomegalovirus (CMV) immediate-early promoter.
FIG. 1.
Generation of CD40L-pseudotyped SIVs. (A) CD40L-dSIV was generated by transient cotranfection of pCD40L, pVSV-G, and pSIVΔEΔNgfp into 293T cells. pSIVΔEΔNgfp is a plasmid encoding the full-length SIVmac239 proviral DNA with deletions in the env and nef genes and an insertion of the GFP gene in the nef region. pVSV-G is the plasmid containing the VSV-G gene under a CMV promoter. pCD40L contains the CD40L gene under a CMV promoter. Deletions are indicated by shaded regions, the inserted GFP gene is shown as an open box, and pA refers to the polyadenylation signals from the expression plasmids. (B) 293T cells cotransfected with pCD40L, pVSV-G, and pSIVΔEΔNgfp were able to express all three genes (CD40L, VSV-G, and GFP). Cells were fixed, permeabilized, and double stained with anti-CD40L conjugated with Alexa Fluor 555 and anti-VSV-G conjugated with Alexa Fluor 647. Protein expression was visualized by confocal microscopy.
Generation of pseudotyped SIV stocks.
dSIVs were prepared as follows: 293T cells (90% confluent in 150-cm2 flasks) were cotransfected with pSIVΔEΔNgfp (36 μg) and pVSV-G (18 μg; Clontech, California). pSIVΔEΔNgfp contains the SIVmac239 genome with a partial deletion of the env gene and with the nef gene replaced with the green fluorescent protein (GFP) gene (55) (Fig. 1A). pVSV-G expresses the VSV-G gene (Indiana serotype) under the control of the CMV immediate-early promoter (71) (Fig. 1A). For dSIV incorporating CD40L (CD40L-dSIV), pSIVΔEΔNgfp (26.5 μg), pVSV-G (13.25 μg), and pCD40L (13.25 μg) (Fig. 1A) were cotransfected into 293T cells and viral particle-containing mediums were collected 48, 72, and 96 h after transfection, pooled, clarified by centrifugation at 500 × g for 10 min, and filtered through a 0.45-μm-pore-size membrane (Millipore, Billerica, MA). To prepare high-titer stocks, viral particles were concentrated by centrifugation with Centricon Plus-70 filter units (Millipore). Viral particles were pelleted by ultracentrifugation at 141,000 × g for 2 h (Beckman SW 28 rotor). The viral particle pellets were resuspended in phosphate-buffered saline (PBS) and stored at −80°C. A transduction assay using HeLa cells was performed to determine viral titers. The titer was calculated as green-forming units/ml, according to the formula described by Zufferey (75).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting.
Viral particle stocks were run on a 7.5% sodium dodecyl sulfate-polyacrylamide gel (Bio-Rad, Hercules, CA). Proteins were then transferred to nitrocellulose membranes (0.22 μm; GE Osmonics, Minnetonka, MN) and blocked (5% milk in PBS-0.2% Tween 20). The membranes were incubated individually with primary antibody overnight at 4°C. These antibodies included the following: (i) 1:250 dilution of rabbit anti-CD40L rabbit polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) (59), (ii) 1:800 dilution of rabbit anti-VSV-G polyclonal antibody (6), and (iii) 1:2,000 dilution of mouse anti-Gag p27 antibody, obtained through the National Institutes of Health AIDS Research and Reference Reagent Program (Germantown, MD) (SIVmac251 Gag monoclonal [KK64], catalogue no. 2321, from Karen Kent and Caroline Powell). Membranes were washed with PBS-0.2% Tween 20 and incubated with either horseradish peroxidase (HRP)-conjugated goat antirabbit or antimouse antibody (Pierce, Rockford, IL) at a 1:5,000 dilution in blocking buffer. Following incubation in the secondary antibody, the membranes were washed and then incubated in HRP substrate (Pico chemiluminescence; Pierce). Membranes were placed on Whatman 3MM filter paper and exposed to film (BioMax; Kodak, Rochester, NY).
Gradient fraction analysis.
To show that CD40L is incorporated into the virus particles, we examined its colocalization with VSV-G and Gag protein on both sucrose density equilibrium and iodixanol (OptiPrep; Invitrogen) velocity sedimentation gradients (16, 28). Briefly, sucrose gradients were prepared in PBS in 4% increments ranging from 20 to 60%. Viral particle stocks (0.5 ml) were layered on top of the gradients and centrifuged for 14 h at 95,000 × g. Iodixanol gradients were prepared in PBS in 1.2% increments ranging from 6 to 18%. Viral particle stocks (0.5 ml) were layered on top of the gradient and centrifuged for 1 h at 246,000 × g. A total of 20 gradient fractions were collected from the bottom of the gradient (about 250 μl per fraction). An aliquot of each fraction was examined for the concentration of Gag p27, VSV-G, and CD40L by enzyme-linked immunosorbent assay (ELISA). A p27 ELISA kit (Beckman Coulter, Fullerton, CA) was used to measure the Gag p27 concentration, and a human CD40L ELISA kit (R&D Systems, Minneapolis, MN) was used to measure the CD40L concentration, as described in the manufacturer's protocol. To quantitate VSV-G, samples were diluted in coating buffer (KPL, Gaithersburg, MD) and adsorbed on ELISA plates (MaxiSorp; Nunc, Rochester, NY) overnight at 4°C. The plates were washed with PBS-0.05% Tween 20 and then blocked with PBS-5% bovine serum albumin. Plates then were washed and incubated with a 1:10,000 dilution of HRP-conjugated anti-VSV-G antibody (Novus, Littleton, CO). After washing with PBS-0.05% Tween 20, a tetramethylbenzidine substrate (KPL) was added to each well. The reaction was stopped by adding the same volume of stop solution (KPL), and the optical density was read at 450 nm.
Preparation and transduction of monocyte-derived DCs.
A standard protocol was used to prepare monocyte-derived DCs (26, 27). PBMCs from healthy donor rhesus macaques were isolated from whole blood using Accu-Paque (Accurate Chemical & Scientific Corporation, Westbury, NY) gradient centrifugation. CD14+ cells were positively selected from PBMCs using magnetic beads according to the manufacture's protocol (Miltenyi Biotec, Albon, CA). Selected monocytes were cultured in a six-well plate (3 × 106 cells/well) in AIM-V medium supplemented with 100 ng/ml granulocyte-macrophage colony-stimulating factor and 50 ng/ml IL-4 (R&D Systems) for 6 days. Half of the medium was replaced on days 2 and 4 with fresh medium containing the same concentrations of cytokines. On day 6, immature DCs were then transduced by different viral particles at a multiplicity of infection (MOI) of 0.1 or left untransduced and cultured in the cytokine medium as described above for 2 days. The culture mediums of transduced DCs were measured for IL-12 concentration by ELISA (Invitrogen), performed according to the manufacture's protocol. DCs were harvested by gently resuspending the cells and staining with anti-CD54, anti-CD80, anti-CD83, or anti-CD86-phycoerythrin (PE) or with anti-CD11c-APC or anti-HLA-DR-APC-Cy7 (Becton Dickinson, Franklin Lakes, NJ) in fluorescence-activated cell sorter buffer (PBS supplemented with 3% fetal calf serum and 0.02% sodium azide). The expression level of surface molecules on DCs was monitored by flow cytometric analysis using a FACSArray bioanalyzer (Becton Dickinson) and analyzed using the FlowJo software program (Tree Star, San Carlos, CA).
Confocal microscopy.
Plasmid-transfected 293T cells were fixed with 4% paraformaldehyde 2 days posttransfection, permeablized with 0.1% of Triton X-100 in PBS, and subsequently blocked with PBS containing 1% bovine serum albumin and 0.5% Tween 20. Cells were incubated with goat anti-human CD40L polyclonal antibody (R&D Systems) and rabbit anti-VSV-G polyclonal antibody. Cells were then stained with Alexa Fluor 555-conjugated donkey antirabbit antibody and Alexa Fluor 647-conjugated donkey antigoat antibody (Invitrogen).
The virus particle-transduced DCs described above were fixed, permeablized, and subsequently blocked. Cells were incubated with the mouse anti-Gag p27 antibody described earlier. The cells were stained with Alexa Fluor 647-conjugated goat antimouse antibody (Invitrogen). Images were taken using a Zeiss Radiance 2100 confocal microscope system.
Allogeneic MLR.
PBMCs from healthy donor rhesus macaques were isolated from whole blood by Accu-Paque gradient centrifugation. T cells as responder cells were enriched using the Pan T cell isolation kit (Miltenyi Biotec) according to the manufacturer's protocol. Stimulator cells (DCs), isolated from another donor monkey, were transduced with viral particles and cultured for 2 days with 0.1 ng/ml lipopolysaccharide (Sigma-Aldrich, St. Louis, MO). Enriched allogeneic T cells (106) were incubated with transduced DCs (105) in 48-well plates in triplicate for 5 days. Bromodeoxyuridine (BrdU) was added to the culture medium to a final concentration of 10 μM 18 h before the cells were harvested. The culture medium from mixed lymphocyte reactions (MLR) was collected before addition of BrdU, and the concentrations of IFN-γ, IL-2, and IL-4 were measured using ELISAs (Invitrogen), performed according to the manufacturer's protocols. T cells from MLR were restimulated with anti-CD3 (1 μg/ml) and anti-CD28 (1 μg/ml) in medium containing brefeldin A (Sigma-Aldrich) and GolgiStop (Becton Dickinson) 6 h before harvesting. T cells were then stained with anti-CD8-APC-Cy7, anti-CD4-PE-Cy7, anti-BrdU-fluorescein isothiocyanate, anti-IL-2-APC (Becton Dickinson), anti-IL-4-PE (Miltenyi Biotec), and anti-IFN-γ-Pacific Blue (eBioscience, San Diego, CA) using a BrdU-FITC Flow kit (Becton Dickinson). T cells were analyzed on an LSRII flow cytometer (Becton Dickinson).
Animals and immunization.
Female CB6F1 mice were purchased from Harlan Sprague Dawley (Indianapolis, IN). All animals were maintained according to National Institutes of Health guidelines. Animal care protocols were approved by the Animal Use and Care Administrative Advisory Committee at the University of California, Davis. Groups of mice (4- to 5-week-old females) were immunized intradermally (i.d.) with 2 × 106 PFU of dSIVs or CD40L-dSIV in a final volume of 50 μl of sterile PBS under light anesthesia. Two weeks later, animals were boosted i.d. with the same dose of dSIVs.
Humoral studies.
Mice were bled at 0, 1, 2, and 4 weeks after immunization. Sera were pooled for the 2-week samples in each group. Titers of antibody to SIV-Gag and VSV were determined by ELISA and neutralization assay, respectively.
IFN-γ ELISPOT assay.
Enzyme-linked immunospot (ELISPOT) assays for IFN-γ-secreting cells were performed using a commercial kit (Becton Dickinson) according to the manufacturer's protocol. Briefly, spleens from immunized mice were harvested at 1 or 4 weeks postimmunization. After homogenization, splenocytes were depleted of CD4+ cells using magnetic beads (Miltenyi Biotec). Freshly isolated splenocytes (from 2.5 × 105 to 1 × 106) from each mouse were seeded in triplicate in 96-well plates precoated with anti-IFN-γ monoclonal antibody. Cells were pulsed for 24 h at 37°C with a peptide pool consisting of overlapping 15-mer peptides spanning the entire SIV Gag (with 11-amino-acid overlaps) at a final concentration 1 μg/ml of each peptide (AIDS Research and Reference Reagent Program, National Institutes of Health). Cells were removed from plates by washing with PBS. Aliquots of 100 μl of anti-IFN-γ-biotin were added to each well and incubated for 2 h at room temperature. After washing with PBS, 100 μl of streptavidin-alkaline phosphatase was added to each well and incubated for 1 h at room temperature. A chromogenic substrate was then added to each well for 15 min to allow color development and formation of spots. The color reaction was stopped by the addition of water. Wells were then air dried. Spot-forming cells (SFC) were counted using a dissecting microscope and normalized as SFC/106 cells.
Data analysis.
Statistical analyses were performed using the statistical software program GraphPad Prism, version 4.0 (GraphPad Software Inc., San Diego, CA). Data were expressed as the means ± the standard errors of the means, and a P value of <0.05 was considered significant.
RESULTS
Generation of pseudotyped SIVs with human CD40L incorporated into viral membranes.
We obtained the human CD40L gene from the plasmid pBS-hCD40L-6A9, which was cloned and characterized by Gauchat et al. (23). The full human CD40L gene was subsequently cloned into the EcoRI-BamHI site of vector pcDNA3.1/Zeo (−). To generate CD40L-dSIV viral particles, we cotransfected 293T cells with three plasmids, pCD40L, pVSV-G, and pSIVΔEΔNgfp, simultaneously (Fig. 1A). Images from confocal microscopy show that 293T cells successfully expressed all three proteins at the same time, CD40L and VSV-G on the cell surface and GFP intracellularly (Fig. 1B).
CD40L-dSIV particles incorporate CD40L.
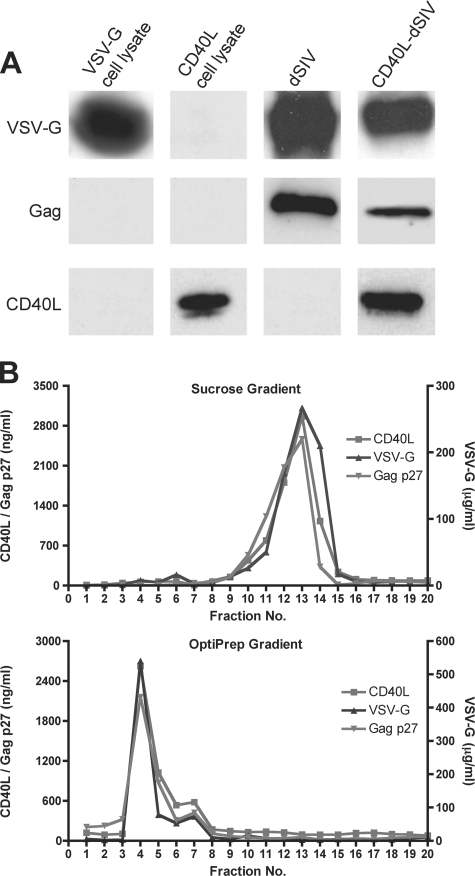
The CD40L-dSIV viral particle stock was generated by collecting the medium of transfected cells and purifying viral particles by using a sucrose gradient. The results of the Western blot show that the VSV-G, Gag, and CD40L proteins were present in CD40L-dSIV particles (Fig. 2A). In order to rule out potential contamination with cell debris and vesicles and further prove that CD40L is indeed present in the virus particle, we examined the viral stock using both density and velocity gradients. The proteins contained in the viral stocks were fractioned according to either their density or their sedimentation coefficient. Therefore, we could separate viral particles from cell debris and microvesicles. We measured the concentrations of VSV-G, Gag, and CD40L in each fraction and found that the highest concentrations of these proteins colocalized to the same fraction(s) (Fig. 2B). The gradients were run in duplicate, and analysis of the fractions gave consistent results. The combined data from Western blot and gradient analyses demonstrated that human CD40L was incorporated into the virus particles.
FIG. 2.
Verification of proteins present in CD40L-dSIV as determined by Western blotting. (A) Purified CD40L-dSIV particles were analyzed by Western blotting. Lysates from cells transfected with either pCD40L or pVSV-G served as controls. VSV-G and Gag p27 were present in both dSIV and CD40L-dSIV particles, while CD40L was present only in the CD40L-dSIV particles. Blots were stained with anti-VSV-G (upper panels), anti-Gag (middle panels), or anti-CD40L (lower panels) antibodies. (B) CD40L-dSIV viral stock was run on either a continuous 20 to 60% sucrose gradient or a 6 to 18% iodixanol (OptiPrep) gradient, and 20 fractions were collected from the bottom of the tube. Each fraction was analyzed by ELISA for human CD40L, SIV Gag p27, and VSV-G. The highest concentrations of these three proteins in both gradients were found in the same fraction in two separate experiments (duplicates not shown).
Transduction of DCs by CD40L-dSIV results in greater DC activation.
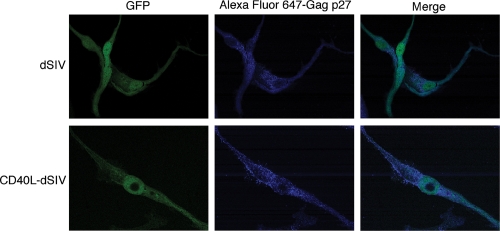
The immunogenic potential of CD40L-dSIV was tested by its ability to activate DCs derived from monocytes of eight macaques. The time line and MOI used in this assay were optimized before the actual experiments were conducted. Both dSIV and CD40L-dSIV-transduced DCs underwent typical morphological changes, including extended dendrites, which increase the cell's surface area and its ability to interact with T cells (data not shown). Also, virus-transduced DCs expressed viral proteins, such as Gag (Fig. 3), which should allow these DCs to present the viral proteins via the major histocompatibility complex (MHC) class I pathway.
FIG. 3.
Expression of viral proteins in transduced DCs. Viral protein expression was visualized by confocal microscopy. Virus particle-transduced DCs were fixed, permeabilized, and stained with anti-Gag p27 conjugated with Alexa Fluor 647. Upper panels show DCs transduced with dSIV, and lower panels show DCs transduced with CD40L-dSIV.
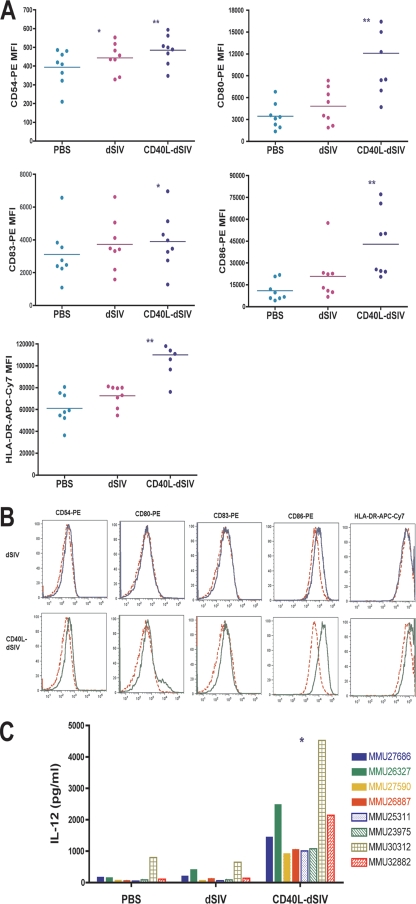
The expression levels of the surface molecules CD54 (ICAM-1), CD80 (B7.1), CD86 (B7.2), and HLA-DR on virus-transduced DCs were evaluated by flow cytometry 2 days after transduction. CD40L-dSIV-transduced DCs had significantly higher levels of these markers than the PBS controls or dSIV-transduced DCs (Fig. 4A and B). Interestingly, the expression levels of CD83, the DC maturation marker, were similar for both CD40L-dSIV and dSIV-transduced DCs although it was significantly higher than that for the PBS control group. The expression level of CD83 on virus particle-transduced DCs was not as high as that on cytokine cocktail-maturated DCs (data not shown). This result suggests that the activation signal provided by CD40L-dSIV is strong enough to initiate activation but not enough to reach full maturation.
FIG. 4.
Transduction of DCs by CD40L-dSIV results in greater DC activation. (A) The expression level of surface molecules on virus particle-transduced DCs was examined 2 days after transduction by flow cytometry with cells derived from eight different animal donors. Transduction with CD40L-dSIVs resulted in activated DCs and significantly increased levels of CD54, CD80, CD86, and HLA-DR expression compared to results for the PBS and dSIV-transduced groups. The expression of CD83 on CD40L-dSIV-transduced DCs is significantly increased compared to results for the PBS group, but there is no difference from the dSIV-transduced group. DCs were gated using forward scatter, side scatter, and CD11c. Results show the mean florescent intensity of CD11c+ cells in different channels. The expression levels of surface molecules on transduced DCs for all the experimental animals are presented as mean fluorescence intensity (MFI). Data were analyzed with the paired t test: *, P < 0.04 compared with results for the PBS group; **, P < 0.004 compared individually with results for the dSIV and PBS groups (n = 8). Bars represent mean values. (B) Flow cytometric data from cells derived from a representative animal donor (out of eight). PBS (red dashed line), dSIV (blue solid line), and CD40L-dSIV (green solid line) groups are shown. (C) IL-12 production is significantly enhanced in CD40L-dSIV-transduced DCs. DC culture supernatants were collected 2 days after transduction with dSIVs and evaluated for the IL-12 p40 concentration using ELISA. The IL-12 concentration in the culture supernatants of CD40L-dSIV-transduced DCs is as much as 6 to 18 times more than that measured in the culture supernatant of dSIV- and PBS-transduced DCs. The assay was done with eight different animals, and a two-tail paired t test was used for data analysis: *, P < 0.003 compared to results for PBS and dSIV individually.
We next examined the concentration of IL-12 p40 in the culture medium, since one of the major characteristics of DCs that are activated by CD40L is increased production of IL-12, a Th1 regulatory cytokine. Additionally, production of IL-23, which shares the IL-12 p40 subunit, is also a marker of DC activation. DC culture supernatants were collected 2 days after transduction and analyzed by ELISA. The result shows that IL-12 p40 production by CD40L-dSIV-transduced DCs is 6 to 18 times more than that produced by PBS or dSIV-transduced DCs (Fig. 4C). These results further confirm that CD40L-dSIVs indeed have an increased ability to activate DCs in vitro. In conclusion, these data show that incorporating CD40L into dSIV significantly enhances its ability to activate DCs.
CD40L-dSIV-transduced DCs activated naive allogeneic T cells more efficiently in MLRs.
Activated DCs are known to be able to induce activation and proliferation of naive T cells in primary MLRs (60). Thus, we performed MLRs to examine the ability of PBS controls or dSIV- or CD40L-dSIV-transduced DCs to stimulate T-cell proliferation and cytokine secretion. In the DC activation assay described above, we found that DCs did not become fully mature. Reports have shown that tolerant, immature, or semi-immature DCs are prone to inhibiting MLRs (9, 49). Thus, we boosted DC maturation by adding lipopolysaccharide in the virus particle-containing medium at the concentration which would not affect IL-12 production but would assist DC maturation (11). The MLR assay was repeated 10 times, with each set differing in the combination of rhesus macaque donor cells used as stimulators (DCs) and responders (allogeneic T cells).
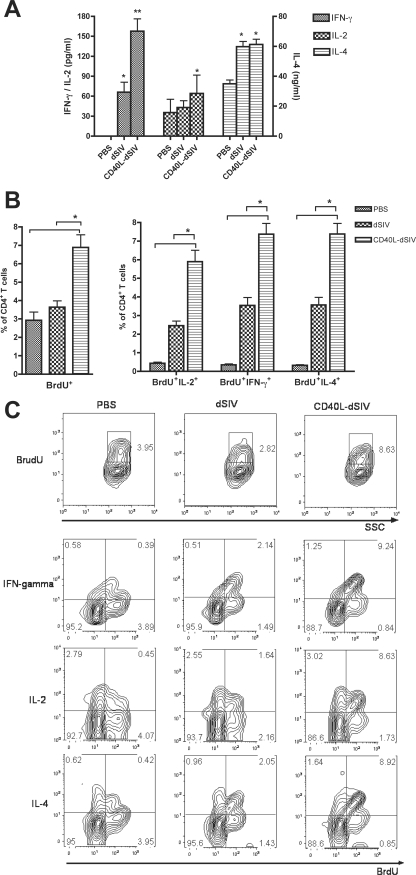
First, we investigated the cytokine profile of the stimulated T cells using ELISA to measure cytokine concentrations in the medium collected 4 days after MLR. The concentration of IFN-γ in supernatants taken from CD40L-dSIV-transduced DCs mixed with responder T cells was two to four times higher than that found in the dSIV-transduced group (Fig. 5A). However, there were no significant differences in IL-2 and IL-4 levels between these groups. This result suggests that CD40L specifically promotes the production of IFN-γ during DC T-cell activation, indicating a Th1 phenotype. At day 5, T cells were labeled with BrdU for 18 h and restimulated with anti-CD3 and anti-CD28 monoclonal antibodies 6 h before harvesting. The BrdU incorporation was examined by flow cytometry as an indication for the intensity of T-cell proliferation. The percentage of proliferating (CD4+ BrdU+) T cells activated by CD40L-dSIV-transduced DCs was significantly higher than that of cells activated by PBS or dSIV-transduced DCs (Fig. 5B and C), while there was no significant difference between the PBS and dSIV groups. The cytokine profile of the T cells was further confirmed by intracellular cytokine staining. The percentages of CD4+ T cells secreting IL-2 and IFN-γ were significant higher in the CD40L-dSIV group, which indicates a Th1 response (Fig. 5B and C). Interestingly, the percentage of CD4+ IL-4+ T cells was also significantly higher in the CD40L-dSIV group than in the PBS and dSIV groups. This is consistent with the results from research in other laboratories, which suggest that CD40L, as a DC activation stimulus, may have a special ability in regulating and balancing Th1 and Th2 responses (30, 56). In conclusion, these results show that CD40L-dSIV-transduced DCs are superior in inducing naive allogeneic CD4+ T-cell proliferation and may have the ability to induce not only Th1 but also Th2 immune responses.
FIG. 5.
CD40L-dSIV-transduced DCs activated naive allogeneic CD4+ T cells more efficiently in a MLR assay. CD40L-dSIV-transduced DCs have a stronger capacity to activate naive allogeneic CD4+ T cells in an MLR assay. T cells were incubated with virus particle-transduced DCs at a ratio of 1:10 and cultured for 5 days. BrdU was added 18 h before harvesting, and T cells were restimulated to boost cytokine secretion 6 h before harvesting. (A) MLR culture mediums were collected before addition of BrdU and evaluated for IFN-γ, IL-2, and IL-4 concentrations using ELISA. The IFN-γ concentration in the medium of CD40L-dSIV-transduced DCs was two to four times more than that measured in the culture mediums of dSIV, while they were no different in the concentrations of IL-2 and IL-4. Bars represent means ± standard errors. A two-tail paired t test was used for data analysis: *, P < 0.003 compared to results with PBS; **, P < 0.003 compared to results with PBS and dSIV individually (n = 10). (B) T cells were analyzed by flow cytometry and gated using forward scatter, side scatter, and CD4. BrdU incorporation was used to evaluate T-cell proliferation. The percentage of CD4+ BrdU+ T cells in the CD40L-dSIV group was as much as twice that of CD4+ T cells in the PBS- and dSIV-transduced DC groups. The percentage of CD4+ BrdU+ T cells that secreted IL-2, IFN-γ, or IL-4 was also significantly higher than those in the PBS- and dSIV-transduced groups. Data were analyzed with two-tailed paired t tests: *, P < 0.0004 compared individually with results for dSIV and PBS groups (n = 10). (C) Representative data from 1 set of MLR experiments out of 10 sets differing in the combination of rhesus macaque donor cells used as stimulators (DCs) and responders (allogeneic T cells). Bars represent means ± standard errors.
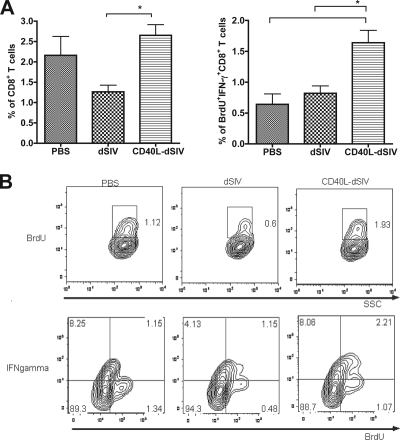
The CD8+ T-cell responses induced by virus-transduced DCs were also measured by MLR. The effector function of CD8+ T cells was evaluated by IFN-γ secretion and proliferation in response to specific antigen. The percentage of CD8+ IFN-γ+/BrdU+ T cells in the CD40L-dSIV group was significantly higher than those in the PBS and dSIV groups (Fig. 6A). This result shows that CD40L-dSIV-transduced DCs are able to induce activation of naive allogeneic CD8+ T cells. Taken together, the data further confirm that transduction of DCs with CD40L-dSIVs enhances DC activation and maturation compared to results with dSIVs. As a result, CD40L-dSIV-transduced DCs have a functional phenotype that should activate naive T cells better than dSIV alone.
FIG. 6.
CD40L-dSIV-transduced DCs activated naive allogeneic CD8+ T cells more efficiently in a MLR assay. T cells were incubated with virus particle-transduced or PBS control DCs at a ratio of 1:10 and cultured for 5 days. BrdU was added 18 h before harvesting, and T cells were restimulated to boost cytokine secretion 6 h before harvesting. (A) T cells were analyzed by flow cytometry and gated using forward scatter, side scatter, and CD8. BrdU incorporation was used to evaluate T-cell proliferation. The percentage of CD8+ BrdU+ T cells in the CD40L-dSIV group was up to twice that of CD8+ T cells in the PBS and dSIV groups. The percentage of CD8+ BrdU+ T cells secreting IFN-γ in the CD40L-dSIV group was also significantly higher than those in the PBS and dSIV groups. Mean data from all of the experiments are shown in the percentages of CD8+ BrdU+ T cells and CD8+ BrdU+ IFN-γ+ T cells. Bars represent means ± standard errors. Data were analyzed with two-tail paired t tests: *, P < 0.03, compared individually with results for the dSIV and PBS groups (n = 10). (B) Representative data from 1 set of MLR experiments out of 10.
CD40L-dSIVs induced stronger humoral and CMI responses in mice.
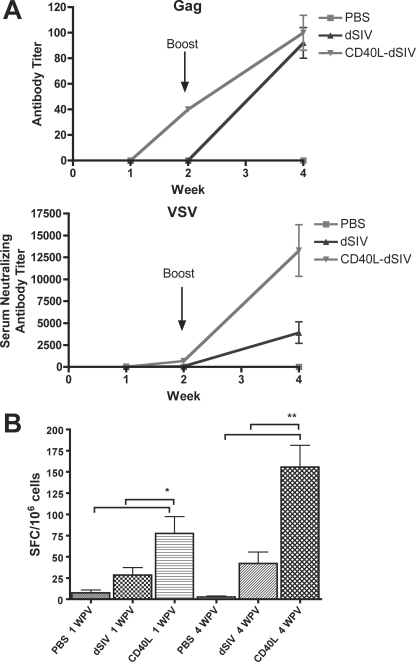
To investigate the immunogenicity of CD40L-dSIV in vivo, groups of mice were immunized i.d. with CD40L-dSIV, dSIV, or PBS and boosted with the same dose i.d. 2 weeks postvaccination. Sera were collected at 1, 2, and 4 weeks postvaccination, and titers of antibody to SIV-Gag and VSV were determined by ELISA and neutralization assay, respectively. The sera from CD40L-dSIV-immunized mice exhibited SIV Gag-specific antibody at 2 weeks postvaccination, while no SIV Gag-specific antibody was detectable in the dSIV group (Fig. 7A). However, the antibody titer was not significantly different at 4 weeks postvaccination (2 weeks postboost). In contrast, the titer of anti-VSV antibody 2 weeks postvaccination was higher in the CD40L-dSIV-vaccinated group, but this was not statistically significant compared to results for the dSIV-vaccinated animals. However, by 4 weeks postvaccination, the titer in the CD40L-SIV group was four times higher than that in the SIV group (Fig. 7A). Next, we examined the SIV Gag-specific CMI response using an IFN-γ ELISPOT assay. Splenocytes taken from mice 1 and 4 weeks postvaccination were depleted of CD4+ cells and stimulated with a SIV Gag peptide pool. Splenocytes from CD40L-dSIV-immunized mice had three to four times more IFN-γ-secreting cells than dSIV-immunized mice (Fig. 7B). The results of animal study show CD40L enhanced the immunogenicity of dSIV in vivo and induced stronger humoral and CMI responses in mice than dSIV alone.
FIG. 7.
CD40L-dSIVs induced stronger humoral and CMI responses in mice. (A) Sera collected from animals were analyzed by ELISA for SIV Gag-specific antibodies and by neutralization assay for anti-VSV antibodies. Sera were pooled for the 2-week samples in each group. “Week” refers to weeks postvaccination. (B) CD4+-cell-depleted splenocytes from each mouse set were seeded in triplicate into 96-well plates precoated with anti-IFN-γ monoclonal antibody. Cells were pulsed with a Gag peptide pool (1 μg/ml) for 24 h at 37°C. IFN-γ-secreting cells were counted as SFC and normalized as SFC/106 cells. Bars represent means ± standard errors. A two-tailed paired t test was used for data analysis: *, P < 0.05 compared to results with PBS; **, P < 0.01 compared to results with PBS and dSIV individually (n = 5 for groups at week one; n = 10 for groups at week four).
DISCUSSION
We have been investigating methods to develop safe and efficacious vaccines by incorporating adjuvant and attenuating genes. Previously we demonstrated that expression of IFN-γ, a Th1 cytokine, in vaccinia virus and SIV vectors increases attenuation as well as enhancing host immune responses (24, 25, 39, 55). The adaptive immune response is initiated when naive T cells interact with DCs, dictating the development of a Th1 or Th2 immune response (34). In the SIV/macaque model and in HIV-infected humans, a Th1 response correlates with control of virus replication (74). Thus, we developed single-cycle, VSV-G-pseudotyped SIV viral particles expressing IFN-γ and showed that transduction with these constructs increased DC maturation and their ability to activate CD4+ and CD8+ lymphocytes (55). In the present study, we incorporated CD40L, one of the most potent stimuli for DCs, into the membrane of these viral particles to further enhance activation of DCs, thus increasing protective immune responses (29, 33, 50). Insertion of the protein in the membrane as a trimer decreases the chances of inflammatory responses to CD40L. Binding of CD40L to its receptor on immature DCs triggers DC activation and maturation and increases DC survival (43, 50). One of the cytokines upregulated in DCs activated by CD40L binding is IL-12, a cytokine responsible for polarizing CD4+ T cells to a Th1 phenotype (12). Previous research with DNA vaccines showed that increasing the activation level of DCs through CD40-CD40L interactions significantly enhances the intensity of CMI and humoral immune responses (29, 33). The membrane-associated trimeric form, the natural form on activated CD4+ T cells, has the strongest bioactivity, while the soluble dimer and monomer have the lowest (18, 67). Moreover, the soluble form may diffuse into circulating blood and cause systemic toxicity (21, 22). Therefore, incorporation of the CD40L protein was carefully designed to ensure that the protein was incorporated into the membrane as a trimer, presented in the same microenvironment as antigens, and was not secreted.
The immunogenicity of CD40L-dSIV was evaluated in vitro by measuring the expression levels of cell surface markers on virus particle-transduced DCs. Transduction of DCs with CD40L-dSIVs significantly increased the expression levels of CD54, CD80, CD86, and HLA-DR as well as secretion of IL-12 compared to results for transduction with dSIV (Fig. 4). However, the levels of CD83 expression were similar in dSIV- and CD40L-dSIV-transduced-cells and was lower than expected for mature DCs, suggesting that transduction with dSIV-CD40L did not induce complete maturation of the DCs in vitro (42, 53). This was not unexpected, since we intentionally excluded other activating stimuli in the culture medium to increase the sensitivity of the assay (41, 73). The concentration of CD40L presented in CD40L-dSIV at this MOI (∼1 ng/ml) was also low, about 1,000 times less than the concentration often used to activate DCs in vitro (14, 45). Under these tightly controlled and stringent conditions, transduction with CD40L-dSIV resulted in production of as much as 10 times more IL-12 than did transduction with dSIV. Since IL-12 stimulates IFN-γ production, proliferation of T cells, and generation of cytotoxic T lymphocytes (43, 65), utilizing CD40L-dSIV as an immunogen should result in increased DC activation and a strong primary Th1 immune response.
The immunogenicity of CD40L-dSIV was further confirmed by primary allogeneic MLR (60). The results show that the proliferation and effector function of activated T cells are stronger when induced by CD40L-dSIV-transduced DCs (Fig. 4). Since DCs expressing higher levels of costimulatory molecules and IL-12 are superior in activating allogeneic T cells in the MLR (13, 35), this confirmed that CD40L-dSIV-transduced DCs have higher activation levels than those transduced by dSIV. Surprisingly, the percentage of CD4+ T cells secreting IL-4, which is a Th2 cytokine, was also higher in the CD40L-dSIV group. Since CD40L-dSIV-transduced DCs secreted a higher level of IL-12, a Th1 response regulatory cytokine, we expected to see a Th1 cytokine profile. There are several possible explanations for this observation. First, immature DCs are prone to inducing Th2 cytokines (9, 49). However, Th2 signaling generally results in downregulation of the expression of IFN-γ via inhibition of transcription of the gene by the transcription factor GATA-3, which is induced by Th2 signaling (1, 37). Since the level of IFN-γ secreted by CD4+ T cells activated by CD40L-dSIV-transduced DCs was significantly higher than that for the other groups (Fig. 5A), this explanation is unlikely to be correct. Alternatively, it may be the result of upregulation of the inducible costimulatory molecule (ICOS), a member of the CD28/CTLA-4 family of T-cell costimulatory molecules that is induced and upregulated on activated T cells (32, 72). Its receptor, B7RP-1, is expressed on APCs and endothelial cells (62, 72). ICOS-ICOSL interaction upregulates the secretion of IL-4, IL-5, IL-10, IFN-γ, and TNF-α (32) but not IL-2 (47) and is essential in germinal center formation (8) and antibody class switching (48). ICOS is essential in generating both Th1 and Th2 responses during infection (7, 70), and one of its main functions is to balance Th1/Th2 responses. A report suggested that the expression level of ICOS on activated T cells is enhanced by IL-12 and IL-23 (68). Since CD40L-dSIV-transduced DCs produced the largest amount of IL-12, this would upregulate the expression of ICOS on T cells and increase secretion of IL-4. If this is true, then CD40L-dSIV may be able to induce a balanced Th1 and Th2 response. Since the inability to generate effective neutralizing antibodies is a major concern in SIV vaccine design, the ability of CD40L-dSIV-transduced DCs to induce both Th1 and Th2 immune responses is an encouraging result.
The immunogenicity of CD40L-dSIV was tested in vivo using a mouse model, since human CD40L has been shown to function in murine cells (31). Animals were immunized i.d. to enhance interaction of CD40L and APCs. Antibody responses to Gag were detectable at 2 weeks postimmunization only in the group vaccinated with CD40L-SIV, indicating that this construct was a better inducer of primary immune responses despite low levels of antigen used for immunization. However, antibody to Gag was equivalent in both groups by 4 weeks postvaccination (2 weeks postboost). Sufficient amounts of antigen must be present in secondary lymphoid organs to stimulate B cells in the marginal zone to induce specific antibody responses (54); a single dose of dSIV was not sufficient to induce detectable humoral immune responses. In contrast, CD40L may enhance the activation level of marginal zone macrophages and thus might have been able to provide sufficient activation signals to B cells with a single dose of vaccine. On the other hand, VSV-G is known to induce antibody efficiently with small amounts of antigen due to its repetitive form (4). Thus, the levels of anti-VSV-G antibody were higher than those of the anti-SIV Gag antibody. The CD8+ T-cell responses to SIV-Gag peptides were significantly higher in the CD40L-dSIV-vaccinated group, which indicates that CD40L-dSIV overcomes the restriction of viral peptides presented via the MHC class I pathway due to the limitation of HIV and SIV genes in murine cells. This is not surprising, since CD40L-activated APCs have the ability to cross-present exogenous peptides on MHC class I and would allow increased stimulation of CD8+ T-cell responses.
Pseudotyped lentiviruses are an attractive alternative vaccine strategy due to increased safety, wider cell tropism, and natural presentation of antigen. However, studies in vitro and in vivo have not been promising due to the limited immune responses induced by these constructs. In this project, we tried to enhance these responses by incorporating CD40L into the dSIV envelope. This strategy not only preserves the native trimeric form of the protein but also ensures that CD40L and virus are present in the vaccination site simultaneously, generating a perfect microenvironment for DC activation and maturation. Thus, DCs receive the first activation signal (viral infection), followed by a second signal (proinflammatory cytokines) and further stimulus from expression of CD40L. The increased density of peptide-MHC complexes, costimulatory molecules, and adhesion molecules on DC surfaces, as well as the increased production of IL-12, should enhance the intensity of DC-T-cell interactions and commit naive T cells to differentiation into Th1 cells. Enhanced immune responses would therefore increase the quantity of memory T cells (57, 69) and protective secondary immune responses. In conclusion, this study shows that CD40L, as a vaccine adjuvant, significantly enhances the immunogenicity of VSV-G-pseudotyped SIV in vitro and in vivo. Future studies will be done with macaques to test immunogenicity, efficacy, and any side effects.
Acknowledgments
We thank Judy Van de Water, Michael McChesney, and Nicole Baumgarth for helpful discussions and Barbara Shacklett for developing the intracellular cytokine staining protocol used in this study.
This work was supported by National Institutes of Health grants AI047025, AI053811, AI054951, and AI066344 (to T.D.Y.) and AI059185 (to P.H.V.).
Footnotes
Published ahead of print on 26 November 2008.
REFERENCES
- 1.Ansel, K. M., D. U. Lee, and A. Rao. 2003. An epigenetic view of helper T cell differentiation. Nat. Immunol. 4616-623. [DOI] [PubMed] [Google Scholar]
- 2.Baba, T. W., Y. S. Jeong, D. Pennick, R. Bronson, M. F. Greene, and R. M. Ruprecht. 1995. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science 2671820-1825. [DOI] [PubMed] [Google Scholar]
- 3.Baba, T. W., V. Liska, A. H. Khimani, N. B. Ray, P. J. Dailey, D. Pennick, R. Bronson, M. F. Greene, H. M. McClure, L. N. Martin, and R. M. Ruprecht. 1999. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat. Med. 5194-203. [DOI] [PubMed] [Google Scholar]
- 4.Bachmann, M. F., H. Hengartner, and R. M. Zinkernagel. 1995. T helper cell-independent neutralizing B cell response against vesicular stomatitis virus: role of antigen patterns in B cell induction? Eur. J. Immunol. 253445-3451. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18767-811. [DOI] [PubMed] [Google Scholar]
- 6.Barratt-Boyes, S. M., M. I. Zimmer, L. A. Harshyne, E. M. Meyer, S. C. Watkins, S. Capuano III, M. Murphey-Corb, L. D. Falo, Jr., and A. D. Donnenberg. 2000. Maturation and trafficking of monocyte-derived dendritic cells in monkeys: implications for dendritic cell-based vaccines. J. Immunol. 1642487-2495. [DOI] [PubMed] [Google Scholar]
- 7.Bertram, E. M., A. Tafuri, A. Shahinian, V. S. Chan, L. Hunziker, M. Recher, P. S. Ohashi, T. W. Mak, and T. H. Watts. 2002. Role of ICOS versus CD28 in antiviral immunity. Eur. J. Immunol. 323376-3385. [DOI] [PubMed] [Google Scholar]
- 8.Bossaller, L., J. Burger, R. Draeger, B. Grimbacher, R. Knoth, A. Plebani, A. Durandy, U. Baumann, M. Schlesier, A. A. Welcher, H. H. Peter, and K. Warnatz. 2006. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th Cells. J. Immunol. 1774927-4932. [DOI] [PubMed] [Google Scholar]
- 9.Buonocore, S., V. Flamand, M. Goldman, and M. Y. Braun. 2003. Bone marrow-derived immature dendritic cells prime in vivo alloreactive T cells for interleukin-4-dependent rejection of major histocompatibility complex class II antigen-disparate cardiac allograft. Transplantation 75407-413. [DOI] [PubMed] [Google Scholar]
- 10.Burns, J. C., T. Friedmann, W. Driever, M. Burrascano, and J.-K. Yee. 1993. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc. Natl. Acad. Sci. USA 908033-8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caron, G., Y. Delneste, E. Roelandts, C. Duez, J. Y. Bonnefoy, J. Pestel, and P. Jeannin. 2001. Histamine polarizes human dendritic cells into Th2 cell-promoting effector dendritic cells. J. Immunol. 1673682-3686. [DOI] [PubMed] [Google Scholar]
- 12.Cella, M., D. Scheidegger, K. Palmer-Lehmann, P. Lane, A. Lanzavecchia, and G. Alber. 1996. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 184747-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakraborty, A., L. Li, N. G. Chakraborty, and B. Mukherji. 2000. Stimulatory and inhibitory differentiation of human myeloid dendritic cells. Clin. Immunol. 9488-98. [DOI] [PubMed] [Google Scholar]
- 14.Chen, X., T. Murakami, J. J. Oppenheim, and O. M. Howard. 2005. Triptolide, a constituent of immunosuppressive Chinese herbal medicine, is a potent suppressor of dendritic-cell maturation and trafficking. Blood 1062409-2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daniel, M. D., F. Kirchhoff, S. C. Czajak, P. K. Sehgal, and R. C. Desrosiers. 1992. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science 2581938-1941. [DOI] [PubMed] [Google Scholar]
- 16.Dettenhofer, M., and X.-F. Yu. 1999. Highly purified human immunodeficiency virus type 1 reveals a virtual absence of Vif in virions. J. Virol. 731460-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans, D. T., J. E. Bricker, H. B. Sanford, S. Lang, A. Carville, B. A. Richardson, M. J. Piatak, J. D. Lifson, K. G. Mansfield, and R. C. Desrosiers. 2005. Immunization of macaques with single-cycle simian immunodeficiency virus (SIV) stimulates diverse virus-specific immune responses and reduces viral loads after challenge with SIVmac239. J. Virol. 797707-7720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fanslow, W. C., S. Srinivasan, R. Paxton, M. G. Gibson, M. K. Spriggs, and R. J. Armitage. 1994. Structural characteristics of CD40 ligand that determine biological function. Semin. Immunol. 6267-278. [DOI] [PubMed] [Google Scholar]
- 19.Fauci, A. S. 2008. 25 years of HIV. Nature 453289-290. [DOI] [PubMed] [Google Scholar]
- 20.Feder-Mengus, C., E. Schultz-Thater, D. Oertli, W. R. Marti, M. Heberer, G. C. Spagnoli, and P. Zajac. 2005. Nonreplicating recombinant vaccinia virus expressing CD40 ligand enhances APC capacity to stimulate specific CD4+ and CD8+ T cell responses. Hum. Gene Ther. 16348-360. [DOI] [PubMed] [Google Scholar]
- 21.Funakoshi, S., D. D. Taub, M. R. Anver, A. Raziuddin, O. Asai, V. Reddy, H. Rager, W. C. Fanslow, D. L. Longo, and W. J. Murphy. 1997. Immunologic and hematopoietic effects of CD40 stimulation after syngeneic bone marrow transplantation in mice. J. Clin. Investig. 99484-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia de Vinuesa, C., I. C. MacLennan, M. Holman, and G. G. Klaus. 1999. Anti-CD40 antibody enhances responses to polysaccharide without mimicking T cell help. Eur. J. Immunol. 293216-3224. [DOI] [PubMed] [Google Scholar]
- 23.Gauchat, J.-F., G. Mazzei, P. Life, S. Henchoz, M. C. Peitsch, J.-P. Aubry, T. Jomotte, and J.-Y. Bonnefoh. 1994. Human CD40 ligand: molecular cloning, cellular distribution and regulation of IgE synthesis. Res. Immunol. 145240-249. [DOI] [PubMed] [Google Scholar]
- 24.Giavedoni, L., S. Ahmad, L. Jones, and T. Yilma. 1997. Expression of gamma interferon by simian immunodeficiency virus increases attenuation and reduces postchallenge virus load in vaccinated rhesus macaques. J. Virol. 71866-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giavedoni, L. D., L. Jones, M. B. Gardner, H. L. Gibson, C. T. Ng, P. J. Barr, and T. Yilma. 1992. Vaccinia virus recombinants expressing chimeric proteins of human immunodeficiency virus and gamma interferon are attenuated for nude mice. Proc. Natl. Acad. Sci. USA 893409-3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Granelli-Piperno, A., L. Zhong, P. Haslett, J. Jacobson, and R. M. Steinman. 2000. Dendritic cells, infected with vesicular stomatitis virus-pseudotyped HIV-1, present viral antigens to CD4+ and CD8+ T cells from HIV-1-infected individuals. J. Immunol. 1656620-6626. [DOI] [PubMed] [Google Scholar]
- 27.Gruber, A., J. Kan-Mitchell, K. L. Kuhen, T. Mukai, and F. Wong-Staal. 2000. Dendritic cells transduced by multiply deleted HIV-1 vectors exhibit normal phenotypes and functions and elicit an HIV-specific cytotoxic T-lymphocyte response in vitro. Blood 961327-1333. [PubMed] [Google Scholar]
- 28.Gurer, C., A. Cimarelli, and J. Luban. 2002. Specific incorporation of heat shock protein 70 family members into primate lentiviral virions J. Virol. 764666-4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gurunathan, S., K. R. Irvine, C. Y. Wu, J. I. Cohen, E. Thomas, C. Prussin, N. P. Restifo, and R. A. Seder. 1998. CD40 ligand/trimer DNA enhances both humoral and cellular immune responses and induces protective immunity to infectious and tumor challenge. J. Immunol. 1614563-4571. [PMC free article] [PubMed] [Google Scholar]
- 30.Harcourt, J. L., M. P. Brown, L. J. Anderson, and R. A. Tripp. 2003. CD40 ligand (CD154) improves the durability of respiratory syncytial virus DNA vaccination in BALB/c mice. Vaccine 212964-2979. [DOI] [PubMed] [Google Scholar]
- 31.Hirano, A., D. L. Longo, D. D. Taub, D. K. Ferris, L. S. Young, A. G. Eliopoulos, A. Agathanggelou, N. Cullen, J. Macartney, W. C. Fanslow, and W. J. Murphy. 1999. Inhibition of human breast carcinoma growth by a soluble recombinant human CD40 ligand. Blood 932999-3007. [PubMed] [Google Scholar]
- 32.Hutloff, A., A. M. Dittrich, K. C. Beier, B. Eljaschewitsch, R. Kraft, I. Anagnostopoulos, and R. A. Kroczek. 1999. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 397263-266. [DOI] [PubMed] [Google Scholar]
- 33.Ihata, A., S. Watabe, S. Sasaki, A. Shirai, J. Fukushima, K. Hamajima, J. Inoue, and K. Okuda. 1999. Immunomodulatory effect of a plasmid expressing CD40 ligand on DNA vaccination against human immunodeficiency virus type-1. Immunology 98436-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kapsenberg, M. L. 2003. Dendritic-cell control of pathogen-driven T-cell polarization. Nat. Rev. Immunol. 3984-993. [DOI] [PubMed] [Google Scholar]
- 35.Kohka, H., H. Iwagaki, T. Yoshino, K. Kobashi, N. Urushihara, T. Yagi, T. Tanimoto, M. Kurimoto, T. Akagi, and N. Tanaka. 1999. Involvement of interleukin-18 (IL-18) in mixed lymphocyte reactions (MLR). J. Interferon Cytokine Res. 191053-1057. [DOI] [PubMed] [Google Scholar]
- 36.Kuate, S., C. Stahl-Hennig, P. T. Haaft, J. Heeney, and K. Uberla. 2003. Single-cycle immunodeficiency viruses provide strategies for uncoupling in vivo expression levels from viral replicative capacity and for mimicking live-attenuated SIV vaccines. Virology 313653-662. [DOI] [PubMed] [Google Scholar]
- 37.Lee, H. J., N. Takemoto, H. Kurata, Y. Kamogawa, S. Miyatake, A. O'Garra, and N. Arai. 2000. GATA-3 induces T helper cell type 2 (Th2) cytokine expression and chromatin remodeling in committed Th1 cells. J. Exp. Med. 192105-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee, M. E., S. Z. Bucur, T. W. Gillespie, J. W. Adams, A. T. Barker, E. K. Thomas, J. D. Roback, and C. D. Hillyer. 1999. Recombinant human CD40 ligand inhibits simian immunodeficiency virus replication: a role for interleukin-16. J. Med. Primatol. 28190-194. [DOI] [PubMed] [Google Scholar]
- 39.Legrand, F. A., P. H. Verardi, K. S. Chan, Y. Peng, L. A. Jones, and T. D. Yilma. 2005. Vaccinia viruses with a serpin gene deletion and expressing IFN-gamma induce potent immune responses without detectable replication in vivo. Proc. Natl. Acad. Sci. USA 1022940-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu, K. J., L. F. Lu, H. T. Cheng, Y. M. Hung, S. R. Shiou, J. Whang-Peng, and S. H. Juang. 2004. Concurrent delivery of tumor antigens and activation signals to dendritic cells by irradiated CD40 ligand-transfected tumor cells resulted in efficient activation of specific CD8+ T cells. Cancer Gene Ther. 11135-147. [DOI] [PubMed] [Google Scholar]
- 41.Lopez, C. B., A. Garcia-Sastre, B. R. Williams, and T. M. Moran. 2003. Type I interferon induction pathway, but not released interferon, participates in the maturation of dendritic cells induced by negative-strand RNA viruses. J. Infect. Dis. 1871126-1136. [DOI] [PubMed] [Google Scholar]
- 42.Lore, K., M. R. Betts, J. M. Brenchley, J. Kuruppu, S. Khojasteh, S. Perfetto, M. Roederer, R. A. Seder, and R. A. Koup. 2003. Toll-like receptor ligands modulate dendritic cells to augment cytomegalovirus- and HIV-1-specific T cell responses. J. Immunol. 1714320-4328. [DOI] [PubMed] [Google Scholar]
- 43.Macatonia, S. E., N. A. Hosken, M. Litton, P. Vieira, C. S. Hsieh, J. A. Culpepper, M. Wysocka, G. Trinchieri, K. M. Murphy, and A. O'Garra. 1995. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J. Immunol. 1545071-5079. [PubMed] [Google Scholar]
- 44.Mackey, M. F., J. R. Gunn, C. Maliszewski, H. Kikutani, R. J. Noelle, and R. Barth. 1998. Dendritic cells require maturation via CD40 to generate protective antitumor immunity. J. Immunol. 1612094-2098. [PubMed] [Google Scholar]
- 45.Majumder, B., M. L. Janket, E. A. Schafer, K. Schaubert, X. L. Huang, J. Kan-Mitchell, C. R. Rinaldo, Jr., and V. Ayyavoo. 2005. Human immunodeficiency virus type 1 Vpr impairs dendritic cell maturation and T-cell activation: implications for viral immune escape. J. Virol. 797990-8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manoj, S., P. J. Griebel, L. A. Babiuk, and S. van Drunen Littel-van den Hurk. 2004. Modulation of immune responses to bovine herpesvirus-1 in cattle by immunization with a DNA vaccine encoding glycoprotein D as a fusion protein with bovine CD154. Immunology 112328-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McAdam, A. J., T. T. Chang, A. E. Lumelsky, E. A. Greenfield, V. A. Boussiotis, J. S. Duke-Cohan, T. Chernova, N. Malenkovich, C. Jabs, V. K. Kuchroo, V. Ling, M. Collins, A. H. Sharpe, and G. J. Freeman. 2000. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. J. Immunol. 1655035-5040. [DOI] [PubMed] [Google Scholar]
- 48.McAdam, A. J., R. J. Greenwald, M. A. Levin, T. Chernova, N. Malenkovich, V. Ling, G. J. Freeman, and A. H. Sharpe. 2001. ICOS is critical for CD40-mediated antibody class switching. Nature 409102-105. [DOI] [PubMed] [Google Scholar]
- 49.Min, W. P., D. Zhou, T. E. Ichim, G. H. Strejan, X. Xia, J. Yang, X. Huang, B. Garcia, D. White, P. Dutartre, A. M. Jevnikar, and R. Zhong. 2003. Inhibitory feedback loop between tolerogenic dendritic cells and regulatory T cells in transplant tolerance. J. Immunol. 1701304-1312. [DOI] [PubMed] [Google Scholar]
- 50.Moll, H. 2003. Dendritic cells as a tool to combat infectious diseases. Immunol. Lett. 85153-157. [DOI] [PubMed] [Google Scholar]
- 51.Morel, Y., A. Truneh, R. W. Sweet, D. Olive, and R. T. Costello. 2001. The TNF superfamily members LIGHT and CD154 (CD40 ligand) costimulate induction of dendritic cell maturation and elicit specific CTL activity. J. Immunol. 1672479-2486. [DOI] [PubMed] [Google Scholar]
- 52.Morel, Y., A. Truneh, R. W. Sweet, D. Olive, and R. T. Costello. 2000. TRANCE, a tumor necrosis factor family member, enhances the longevity and adjuvant properties of dendritic cells in vivo. J. Exp. Med. 191490-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakamura, I., K. Kajino, H. Bamba, F. Itoh, M. Takikita, and K. Ogasawara. 2004. Phenotypic stability of mature dendritic cells tuned by TLR or CD40 to control the efficiency of cytotoxic T cell priming. Microbiol. Immunol. 48211-219. [DOI] [PubMed] [Google Scholar]
- 54.Ochsenbein, A. F., P. Klenerman, U. Karrer, B. Ludewig, M. Pericin, H. Hengartner, and R. M. Zinkernagel. 1999. Immune surveillance against a solid tumor fails because of immunological ignorance. Proc. Natl. Acad. Sci. USA 962233-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peng, Y., F. C. Lin, P. H. Verardi, L. A. Jones, M. B. McChesney, and T. D. Yilma. 2007. Pseudotyped single-cycle simian immunodeficiency viruses expressing gamma interferon augment T-cell priming responses in vitro. J. Virol. 812187-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55a.Yue Peng, Fan-ching Lin, Paulo H. Verardi, Leslie A. Jones, and Tilahun D. Yilma. Lower levels of gamma interferon expressed by a pseudotyped single-cycle simian immunodeficiency virus enhance immunogenicity in rats. J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 56.Poudrier, J., D. van Essen, S. Morales-Alcelay, T. Leanderson, S. Bergthorsdottir, and D. Gray. 1998. CD40 ligand signals optimize T helper cell cytokine production: role in Th2 development and induction of germinal centers. Eur. J. Immunol. 283371-3383. [DOI] [PubMed] [Google Scholar]
- 57.Pulendran, B., and R. Ahmed. 2006. Translating innate immunity into immunological memory: implications for vaccine development. Cell 124849-863. [DOI] [PubMed] [Google Scholar]
- 58.Sato, T., M. Terai, R. Yasuda, R. Watanabe, D. Berd, M. J. Mastrangelo, and K. Hasumi. 2004. Combination of monocyte-derived dendritic cells and activated T cells which express CD40 ligand: a new approach to cancer immunotherapy. Cancer Immunol. Immunother. 5353-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schönbeck, U., G. K. Sukhova, N. Gerdes, and P. Libby. 2002. TH2 predominant immune responses prevail in human abdominal aortic aneurysm. J. Pathol. 161499-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schreurs, M. W., A. A. Eggert, A. J. de Boer, C. G. Figdor, and G. J. Adema. 1999. Generation and functional characterization of mouse monocyte-derived dendritic cells. Eur. J. Immunol. 292835-2841. [DOI] [PubMed] [Google Scholar]
- 61.Skountzou, I., F. S. Quan, S. Gangadhara, L. Ye, A. Vzorov, P. Selvaraj, J. Jacob, R. W. Compans, and S. M. Kang. 2007. Incorporation of glycosylphosphatidylinositol-anchored granulocyte-macrophage colony-stimulating factor or CD40 ligand enhances immunogenicity of chimeric simian immunodeficiency virus-like particles. J. Virol. 811083-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Swallow, M. M., J. J. Wallin, and W. C. Sha. 1999. B7h, a novel costimulatory homolog of B7.1 and B7.2, is induced by TNFalpha. Immunity 11423-432. [DOI] [PubMed] [Google Scholar]
- 63.Takahashi, S., R. F. Rousseau, P. Yotnda, Z. Mei, G. Dotti, D. Rill, R. Hurwitz, F. Marini, M. Andreeff, and M. K. Brenner. 2001. Autologous antileukemic immune response induced by chronic lymphocytic leukemia B cells expressing the CD40 ligand and interleukin 2 transgenes. Hum. Gene Ther. 12659-670. [DOI] [PubMed] [Google Scholar]
- 64.Tang, Y., and R. Swanstrom. 2008. Development and characterization of a new single cycle vaccine vector in the simian immunodeficiency virus model system. Virology 37272-84. [DOI] [PubMed] [Google Scholar]
- 65.Trinchieri, G. 1994. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood 844008-4027. [PubMed] [Google Scholar]
- 66.van Kooten, C., and J. Banchereau. 2000. CD40-CD40 ligand. J. Leukoc. Biol. 672-17. [DOI] [PubMed] [Google Scholar]
- 67.Vidalain, P. O., O. Azocar, C. Servet-Delprat, C. Rabourdin-Combe, D. Gerlier, and S. Manie. 2000. CD40 signaling in human dendritic cells is initiated within membrane rafts. EMBO J. 193304-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wassink, L., P. L. Vieira, H. H. Smits, G. A. Kingsbury, A. J. Coyle, M. L. Kapsenberg, and E. A. Wierenga. 2004. ICOS expression by activated human Th cells is enhanced by IL-12 and IL-23: increased ICOS expression enhances the effector function of both Th1 and Th2 cells. J. Immunol. 1731779-1786. [DOI] [PubMed] [Google Scholar]
- 69.Whitmire, J. K., and R. Ahmed. 2000. Costimulation in antiviral immunity: differential requirements for CD4(+) and CD8(+) T cell responses. Curr. Opin. Immunol. 12448-455. [DOI] [PubMed] [Google Scholar]
- 70.Wilson, E. H., C. Zaph, M. Mohrs, A. Welcher, J. Siu, D. Artis, and C. A. Hunter. 2006. B7RP-1-ICOS interactions are required for optimal infection-induced expansion of CD4+ Th1 and Th2 responses. J. Immunol. 1772365-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yee, J. K., A. Miyanohara, P. LaPorte, K. Bouic, J. C. Burns, and T. Friedmann. 1994. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc. Natl. Acad. Sci. USA 919564-9568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoshinaga, S. K., J. S. Whoriskey, S. D. Khare, U. Sarmiento, J. Guo, T. Horan, G. Shih, M. Zhang, M. A. Coccia, T. Kohno, A. Tafuri-Bladt, D. Brankow, P. Campbell, D. Chang, L. Chiu, T. Dai, G. Duncan, G. S. Elliott, A. Hui, S. M. McCabe, S. Scully, A. Shahinian, C. L. Shaklee, G. Van, T. W. Mak, and G. Senaldi. 1999. T-cell co-stimulation through B7RP-1 and ICOS. Nature 402827-832. [DOI] [PubMed] [Google Scholar]
- 73.Zou, W., J. Borvak, S. Wei, T. Isaeva, D. T. Curiel, and T. J. Curiel. 2001. Reciprocal regulation of plasmacytoid dendritic cells and monocytes during viral infection. Eur. J. Immunol. 313833-3839. [DOI] [PubMed] [Google Scholar]
- 74.Zou, W., A. A. Lackner, M. Simon, I. Durand-Gasselin, P. Galanaud, R. C. Desrosiers, and D. Emilie. 1997. Early cytokine and chemokine gene expression in lymph nodes of macaques infected with simian immunodeficiency virus is predictive of disease outcome and vaccine efficacy. J. Virol. 711227-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zufferey, R. 2000. Production of high-titer lentiviral vectors. In Current protocols in neuroscience, p. 4.21. John Wiley & Sons, New York, NY.