Abstract
On solid media, the reproductive growth of Streptomyces involves antibiotic biosynthesis coincident with the erection of filamentous aerial hyphae. Following cessation of growth of an aerial hypha, multiple septation occurs at the tip to form a chain of unigenomic spores. A gene, crgA, that coordinates several aspects of this reproductive growth is described. The gene product is representative of a well-conserved family of small actinomycete proteins with two C-terminal hydrophobic-potential membrane-spanning segments. In Streptomyces avermitilis, crgA is required for sporulation, and inactivation of the gene abolished most sporulation septation in aerial hyphae. Disruption of the orthologous gene in Streptomyces coelicolor indicates that whereas CrgA is not essential for sporulation in this species, during growth on glucose-containing media, it influences the timing of the onset of reproductive growth, with precocious erection of aerial hyphae and antibiotic production by the mutant. Moreover, CrgA subsequently acts to inhibit sporulation septation prior to growth arrest of aerial hyphae. Overexpression of CrgA in S. coelicolor, uncoupling any nutritional and growth phase-dependent regulation, results in growth of nonseptated aerial hyphae on all media tested, consistent with a role for the protein in inhibiting sporulation septation.
Reproductive growth of gram-positive Streptomyces involves the formation of filamentous aerial hyphae that metamorphose into chains of unigenomic spores, as exemplified by the model species, Streptomyces coelicolor (5, 6). The growth of aerial hyphae is away from the nutrient source, fueled partly by cannibalization of lysing substrate mycelia. In plate-grown cultures, antibiotic production (physiological differentiation) is generally coincident with morphological differentiation, and released antibiotics may serve to protect a differentiating colony from predation in a natural soil habitat. Mutants affected in early reproductive growth fail to erect aerial hyphae, and the corresponding bld genes often pleiotropically influence antibiotic production. Genes involved in later stages of spore formation include the whi genes, so named because mutations in these genes prevent either formation of spore compartments (early whi genes) or subsequent spore maturation events (late whi genes), which include production of a grey-brown spore-associated pigment (reviewed in reference 6). Most of these whi genes appear to encode regulatory functions. Among the early whi genes, whiG encodes an alternative sigma factor (29); whiA encodes a protein of unknown function (2); whiB encodes a small, highly charged, and cysteine-rich protein of unknown function (20); whiH encodes a member of the GntR family of transcription factors (24); and whiI encodes a protein resembling the response regulator of a two-component sensor-regulator system, although there is no adjacent recognizable kinase gene and WhiI itself lacks important amino acid residues normally needed for phosphorylation to take place (1). On the basis of sequence information and limited analysis of gene function, the whi genes appear to be well conserved among streptomycetes. For example, whiG has a homologue, rpoZ, in the phylogenetically distant Streptomyces aureofaciens. As in S. coelicolor, this sigma factor is required for transcription of whiH (17). These similarities indicate extensive conservation of the way in which streptomycete sporulation is regulated and suggest monophyletic evolution of the process.
Critical aspects that distinguish the growth of aerial and substrate hyphae are the positioning, type, and number of septa. Indeed, as prokaryotic cell division has been largely studied in rod-shaped unicellular bacteria, investigations into cytokinesis in filamentous organisms are likely to provide novel insights into the process. Differentiation of a single filamentous aerial hyphal cell involves synchronous polymerization of the tubulin-like FtsZ protein on the inner surface of the cytoplasmic membrane, forming regularly spaced multiple ringlike structures, precursors of sporulation septa (27). An ftsZ null mutant of S. coelicolor that produces no cross-walls at all is still viable but can only grow vegetatively (19). Multiple septation of an aerial hyphal cell is achieved, at least partly, by developmental control of ftsZ expression, involving upregulation of transcription of the gene specifically in the reproductive hyphae (9). Moreover, a C-terminal substitution in FtsZ leads to loss of sporulation septa but not vegetative cross-walls (11), suggesting mechanistic differences and developmental control of cytokinesis in the different hyphae.
To extend the comparison of sporulation on solid media between phylogenetically distinct streptomycetes, we have been investigating the process in the avermectin producer Streptomyces avermitilis. Superficially at least, the reproductive growth of S. avermitilis follows a pattern similar to that of S. coelicolor, albeit delayed, with the erection of white aerial hyphae on the surface of cultures grown for 4 days on sporulation media. These aerial hyphae subsequently differentiate, with spore maturation accompanied by production of a grey-brown spore-associated pigment. During the screening of a small Tn1792 transposon library of S. avermitilis, we previously identified a putative novel whi gene (22). In this paper, we characterize the role of this gene in the differentiation of aerial hyphae in S. avermitilis and compare its function in S. coelicolor, in which it is conditionally required for normal sporulation. The gene product, which coordinates reproductive growth and cell division, is representative of a new family of conserved actinomycete-specific proteins.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and conjugal transfer from E. coli to Streptomyces.
The bacterial strains used in this study are listed in Table 1. Streptomyces was cultured on NE (1% glucose, 0.2% yeast extract, 0.2% meat extract, 0.2% Casamino Acids, and 2% agar, pH 7), R2YE, MS agar, or NMMP agar, omitting polyethylene glycol 6000 and supplemented with glucose or mannitol (final concentration, 0.5%) (16). Escherichia coli ET12567 containing pUZ8002 was used for intergeneric conjugation of plasmids into S. coelicolor (24). pUZ8002 supplies transfer functions to oriT-containing plasmids but is not efficiently transferred itself because of a mutation in its own oriT.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Characteristics | Reference or source |
|---|---|---|
| S. avermitilis 12804 | Prototrophic; pSA1 pSA2 | NCIMB, United Kingdom |
| S. avermitilis DM219 | 12804 crgA::Tn1792 | This study |
| S. coelicolor M145 | Prototrophic; SCP1− SCP2− Pg1+ | 12 |
| S. coelicolor DC3845 | M145 ΔcrgA::tsr | This study |
| E. coli JM109 | F′ traD36 proA+B+laclq Δ(lacZ)M15/Δ(lac-proAB) glnV44 e14−gyrA96 recA1 relA1 endA1 thi hsdR17 | 30 |
| E. coli ET12567(pUZ8002) | dam13::Tn9 dcm-6 hsdM hsdR recF143 zjj-201::Tn10 galK2 galT22 ara14 lacY1 xyl-5 leuB6 thi-1 tonA31 rpsL136 hisG4 tsx-78 mtlI glnV44, containing the nontransmissible oriT mobilizing plasmid, pUZ8002 | 18, 21 |
| SCH69 | Supercos containing chromosomal DNA from S. coelicolor; bla Km | 23 |
| pOJ260 | Suicide vector for Streptomyces; oriT(RK2) aac(3)IV | 4 |
| pOJ69 | pOJ260 containing ΔcrgA::tsr | This study |
| pSET152 | Integrative vector for Streptomyces; oriT(RK2) int attP (φC31) aac(3)IV | 4 |
| pSET219 | pSET152 containing crgASa | This study |
| pSC3854 | pSET152 containing crgASc | This study |
| pIJ8600 | Integrative tipAp expression vector for Streptomyces; oriT(RK2) int attP (φC31) aac(3)IV | 28 |
| pME41 | pIJ8600 containing tipAp crgA fusion cloning vector for E. coli; aac1 tsr | This study |
| pGB1 | P. Herron, unpublished data | |
| pIJ2925 | Cloning vector for E. coli; bla | 15 |
| pME38 | pIJ2925 containing crgA | This study |
| pME40 | pME38 containing crgA with 5′ NdeI site | This study |
Plasmid constructions.
The plasmids used are listed in Table 1; PCR primers and mutagenesis oligonucleotides are listed in Table 2. General procedures for DNA manipulation were used (25). All DNA manipulations were carried out using E. coli JM109 as the host. Plasmid constructs were verified by DNA sequencing. A 628-bp DNA fragment containing crgASa was amplified by PCR using oligonucleotides M219SCS and M219SCA and genomic DNA from S. avermitilis 12804 as a template. The PCR product, digested with XbaI and EcoRI, was cloned into pSET152, resulting in pSET219. Primers H69LHEcoRI and H69LHKpnI were used to amplify a 1,236-bp fragment containing upstream sequence and the first 70 bp of crgASc from cosmid SCH69 (23). The PCR product was digested with EcoRI and KpnI and subcloned upstream of the tsr gene on pGB1, generating pGBL1. A second, 955-bp PCR product amplified using primers H69RHXbaI and H69RHPstI (containing the last 60 bp of the open reading frame and downstream sequence) was digested with XbaI and PstI and ligated to pGBL1 downstream of the tsr gene. The resulting plasmid, pGBLR1, was digested with EcoRI and PstI, and the fragment containing the disrupted version of crgA was subcloned into the suicide vector pOJ260, generating pOJ69. A full-length copy of crgASc and upstream sequence was amplified from the cosmid SCH69 using primers SCH69S1 and SCH69A1 (complementary to sequences 4238739 to 4238756 and 4239765 to 4239783 [S. coelicolor web server; http://jiio16.jic.bbsrc.ac.uk/S.coelicolor/]). The 1,055-bp fragment was digested with XbaI and EcoRI and ligated with similarly digested pSET152 and pIJ2925, generating pSC3854 and pME38, respectively. An NdeI site was introduced in the start codon of crgA cloned in pME38 by site-directed mutagenesis using a Quick Change XL Site Directed Mutagenesis kit (Stratagene) and the oligonucleotides crgA1 and crgA2, generating pME40 (the wild-type sequence CTCGTG was changed to CATATG). Restriction of pME40 with NdeI and BglII released the coding sequence of crgA, which was cloned into pIJ8600, generating pME41.
TABLE 2.
Oligonucleotides useda
| Use | Name | Sequence |
|---|---|---|
| PCR | M219SCS | 5′ ACGTCTAGATGGTGCCTTTGGCGG TGT |
| M219SCA | 5′ CGGAATTCAAGCCTGTGGATAAC TCGGT | |
| H69LHEcoRI | 5′ GCGGAATTCTTGTCCACCGTCAC ATCGTT | |
| H69LHKpnI | 5′ CGCGGTACCTGATGCTGGTCGC CTGTCT | |
| H69RHXbaI | 5′ GGCGTCTAGATCGTGGTGGGCT TCGGTTT | |
| H69RHPstI | 5′ GGCCTGCAGCCGCACCTCGTCA CCAAGAT | |
| SCH69S1 | 5′ ACGTCTAGATGACCAGGTAGTCG GGCT | |
| SCH69A1 | 5′ CGGAATTCGCTGACCAGTGTTAT CGCA | |
| Site-directed mutagenesis | crgA1 | 5′ CGACAGGAGAGACCCATATG CCGAAGTCACG |
| crgA2 | 5′ CGTGACTTCGGCATATGGGTC TCTCCTGTCG |
Restriction sites are in boldface.
Microscopical methods.
Unless otherwise stated, cultures for light and fluorescence microscopy were set up by inoculating spore suspensions in the acute angle of a sterile coverslip inserted at a 45° angle in the agar medium. After the desired incubation time, the coverslip was removed and cells on its surface were stained for the cell wall with fluorescein-conjugated wheat germ agglutinin (Fluo-WGA) (SlowFade light antifade kit; Molecular Probes) and with propidium iodide (Sigma) for DNA as described previously (27).
Computer analysis.
The BLAST search engines (3) were used to perform database searches. Figure 1 was produced using the Boxshade program (http://www.ch.embnet.org/software/BOX_form.html). The Simple Modular Architecture Research Tool (http://smart.embl-heidelberg.de) was used to predict transmembrane segments and topology (26).
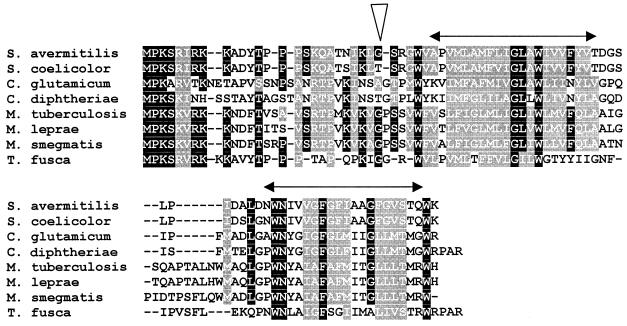
FIG. 1.
Alignment of amino acid sequences of actinomycete CrgA-like proteins. The sequence of the 84-amino-acid protein encoded by crgA (SAV4331) is shown. The triangle indicates the position of insertion of Tn1792 in the S. avermitilis crgA mutant. The double-ended arrows indicate the extents of the two predicted transmembrane domains. Amino acid identities and similarities to S. avermitilis CrgA (accession number CAC47962) are as follows: S. coelicolor CAB45221.1, 92 and 96%; Corynebacterium glutamicum NP_599292, 35 and 53%; Corynebacterium diphtheriae, 16 and 33%; M tuberculosis CAB02432.1, 41 and 62%; M. leprae CAC29521, 41 and 62%; Mycobacterium smegmatis, 32 and 50%; and Thermobifida fusca, 45 and 62%. The M. smegmatis sequence was obtained from the website of The Institute for Genomic Research (http://www.tigr.org/), and the T. fusca sequence was obtained from the Department of Energy Joint Genome Institute Website (http://www.jgi.doe.gov/JGI_microbial/html/thermobifida/themom_homepage.html/). Solid and shaded boxes represent amino acid identity and similarity, respectively.
RESULTS
Isolation of the S. avermitilis crgA gene.
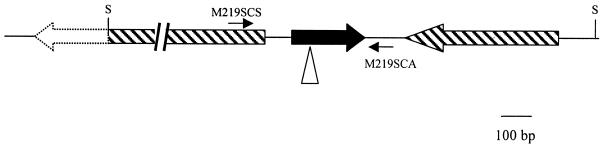
A small library of 500 strains obtained after transposon mutagenesis with Tn1792 was screened for mutants whose morphological development and oligomycin production were affected (22). One of these mutants, M219, had a Whi phenotype on MS medium after 7 days of growth. A 5.3-kb Tn1792-tagged SalI fragment was cloned from this mutant and sequenced. FramePlot (14) analysis of the sequence revealed that the insertion was 78 bp downstream from the translation initiation codon of an open reading frame, identified as SAV4331 in the annotated S. avermitilis sequence (13), encoding a hypothetical protein of 84 amino acids (Fig. 1). The non-Tn1792 sequenced DNA showed 100% identity with the corresponding segment of the completed S. avermitilis genome sequence. Analysis of the S. avermitilis sequence surrounding SAV4331 revealed extensive homology with the corresponding S. coelicolor sequence (centered around the S. coelicolor ortholog of SAV4331, namely, SCO3854), indicating that the upstream open reading frame is divergently transcribed and the downstream gene is convergently transcribed with respect to SAV4331 (Fig. 2). The architecture of the surrounding genes, which is conserved in other actinomycetes, excludes the possibility of any polar effects of the Tn1792 insertion on neighboring genes. To confirm that the Whi phenotype associated with M219 was the result of the transposon insertion, the 5.3-kb SalI fragment was subcloned into pOJ260 (4), and the resulting plasmid was introduced by intergeneric conjugation into wild-type S. avermitilis. On the basis of their antibiotic resistance phenotypes, 12 mutants in which a double crossover replacing the wild-type gene had taken place were identified. The disruption of SAV4331 in these strains was confirmed by Southern hybridization (results not shown). When grown on MS medium, all 12 mutants exhibited Whi phenotypes similar to that of the original mutant, M219. One of these SAV4331 disruption mutants, DM219 (Fig. 3A), was used in subsequent analysis.
FIG. 2.
Architecture of crgA and surrounding genes. The S. avermitilis crgA gene (SAV4331) is represented by the solid arrow. The position of insertion of Tn1792 in the crgA mutant is indicated by the triangle. The adjacent sequenced open reading frames are indicated by hatched arrows. The positions of the SalI sites used to clone out the tagged copy of crgA are indicated (S). The sequence beyond the left-hand SalI site was not determined, and the extent of the divergently transcribed gene is an estimate based on the corresponding orthologous gene in the S. coelicolor genome. The positions of the sequences complementary to the PCR primers used to obtain the full-length crgA gene for complementation (M219SCS and M219SCA) are shown.
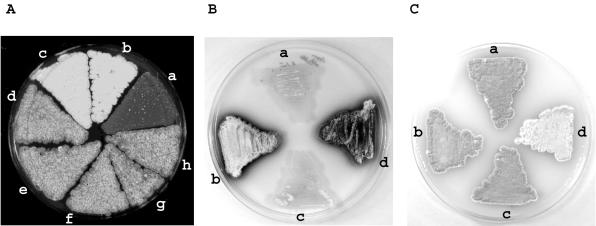
FIG. 3.
crgA influences reproductive growth in Streptomyces. (A) Surface view of cultures grown on MS agar for 7 days. a, S. avermitilis 12804 (wild type); b, S. avermitilis DM219; c, S. avermitilis DM219(pSET152); d to h, S. avermitilis DM219(pSET219). (B) Cultures were grown on NE medium for 3 days. a, S. coelicolor M145; b, S. coelicolor DC3854; c, S. coelicolor DC3854/pSC3854; d, S. coelicolor DC3854/pSET152. (C) Cultures were grown on MS medium containing 2.5 μg of thiostrepton ml−1 for 5 days. a, S. coelicolor M145; b, S. coelicolor M145/pIJ8600; c, S. coelicolor DC3854; d, S. coelicolor M145/pME41.
Further confirmation that the disruption of SAV4331 was responsible for the Whi phenotype was obtained by complementation. Complementing DNA was obtained by PCR amplification of a 628-bp sequence containing SAV4331 and its upstream promoter region from the wild type (Fig. 2). The complementing plasmid pSET219 was introduced into S. avermitilis DM219. All five transconjugants analyzed produced a grey aerial surface color (Fig. 3A), although the phenotype of these complemented strains was less grey than that of the wild-type. This may be a consequence of the different chromosomal location of the gene integrated at the ΦC31 attB site or the presence of more than one integrated copy of the plasmid, as has been commented on previously with respect to using pSET152-based integrative vectors (11). The development of the wild-type strain was unaffected by the introduction of pSET152 (results not shown). This gene represents a new developmental locus that we have called crgA (see Discussion).
A single orthologous gene is found in all fully or partially sequenced actinomycete genomes, including those of Mycobacterium tuberculosis and Mycobacterium leprae (Fig. 1), but not in other bacteria. A hydropathy plot of each member of this actinomycete-specific family of proteins revealed two hydrophobic putative transmembrane domains at the C terminus; the N terminus is predicted to be cytoplasmic.
crgA is required for the formation of sporulation septa in S. avermitilis.
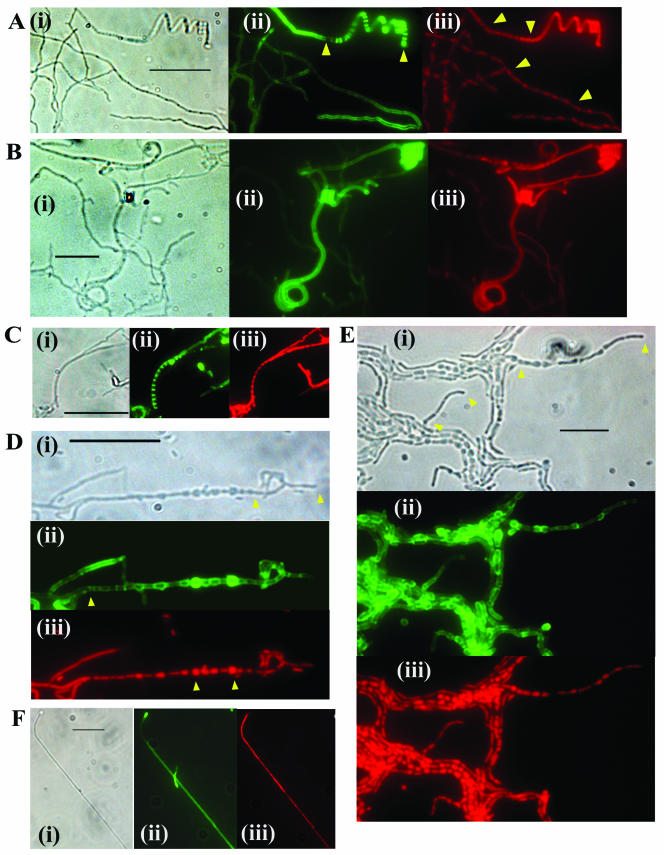
To examine the morphology of aerial hyphae, lysozyme-treated preparations were stained with Fluo-WGA to reveal cell walls and with propidium iodide to indicate the distribution of nucleoids. Cytological comparison of the aerial mycelia of S. avermitilis and S. coelicolor grown on MS medium revealed notable differences. Contrary to the paradigm established with S. coelicolor (27), nucleoid condensation occurred in regions distal to the tips of S. avermitilis hyphae in the absence of septation, whereas in young (after 4 days of growth) apical regions, the nucleoids remained diffuse and not condensed (results not shown). The same degree of nucleoid condensation without septation was also apparent in 7-day-old hyphae (Fig. 4A). Differentiation of the apical regions of these older hyphae involved nucleoid condensation accompanied by septation and resulting in spore chains that tended to be more tightly coiled than in S. coelicolor (Fig. 4A). Comparison of the crgA mutant DM219 with either wild-type S. avermitilis or DM219 complemented with crgA, grown for 7 days on MS medium, revealed a very low abundance of spore chains in the mutant, and these were generally not so tightly coiled. Much more abundant in the mutant were long irregularly septated filamentous hyphae that were tightly coiled at the apices. Uncondensed diffuse nucleoids were present in these nonseptated apical coils (Fig. 4B). These terminally undifferentiated hyphae were largely absent in the wild-type and complemented mutant strains. MS medium was the only medium tested that supported aerial hyphal development of S. avermitilis; no differentiation was evident on glucose-containing media.
FIG.4.
Influence of crgA on aerial hyphal development in S. avermitilis and S. coelicolor. Each panel illustrates a representative field of hyphae under phase-contrast microscopy (i), Fluo-WGA cell wall staining (ii), and propidium iodide DNA staining (iii). (A) S. avermitilis 12804 (wild type) grown for 7 days on MS medium; the arrowheads indicate the regions of a hypha that have undergone sporulation septation (ii) and, in the respective red field, regions of hyphae where nucleoid condensation is evident in the absence of regular septation (iii). (B) S. avermitilis DM219 grown for 7 days on MS medium. (C) S. coelicolor M145 (wild type) grown for 7 days on NMMP-glucose. (D) S. coelicolor DC3854 grown on NMMP-glucose for 3 days; the arrowheads indicate the apical region of an aerial hypha containing elongated cells (i), the septum separating the filamentous region of the hypha from the multiply septated region (ii), and two large swollen cells in the chain that stained intensely with propidium iodide (iii). (E) S. coelicolor DC3854 grown on NMMP-glucose for 7 days; the arrowheads indicate tips of hyphae consisting of elongated cells (i). (F) S. coelicolor M145 containing plasmid pME41 grown for 7 days on MS medium. Bars, 10 μm.
Mutation and complementation of S. coelicolor crgA (orf3854).
Comparison of the amino acid sequences of the translated products of crgASa and the orthologous S. coelicolor gene, crgASc, revealed strong end-to-end similarity (92% identity). To disrupt the gene in S. coelicolor, two PCR products containing sequences flanking the locus were generated and cloned on either side of a thiostrepton resistance marker, resulting in plasmid pOJ260, which was conjugated into S. coelicolor M145. Thiostrepton-resistant double-crossover disruption mutants of crgA were obtained only after strains containing a single crossover were initially isolated; single- and double-crossover strains were verified by Southern hybridization. The adjacent genes on either side of crgA are both oriented opposite to the mutated gene, and consequently no polar effects could be expected as a result of the disruption. The morphological phenotypes of four independently obtained disruption mutants were monitored during growth on MS medium, revealing no significant macroscopic difference in aerial mycelial development and spore maturation between the mutants and M145. This is illustrated for one representative mutant, DC3854 (Fig. 3C). However, comparison of growth on other complete media, notably R2YE and NE, which both support less reproductive growth of the wild type than MS medium, revealed precocious production of actinorhodin and aerial hyphae by the mutant (Fig. 3B). Both aspects of differentiation of DC3854 were accelerated by at least 24 h. On defined minimal media used to compare responses to different carbon sources, NMMP-glucose and NMMP-mannitol, there was only sparse growth of reproductive aerial hyphae by the wild type. DC3854, however, exhibited earlier antibiotic production and aerial hyphal growth on glucose, although less marked than in the case of complete media.
To complement the mutant, plasmid pSC3854 was introduced into DC3854. In terms of the timing of actinorhodin production and reproductive growth on all media tested, the complemented strain resembled the wild-type M145 (Fig. 3B and C).
Abnormal morphology of aerial hyphae of the S. coelicolor crgA mutant.
To examine the cytological effects of disruption of crgA, aerial hyphae were analyzed by phase-contrast microscopy and by staining lysozyme-treated cell walls with Fluo-WGA and propidium iodide. In young, undifferentiated surface cultures grown on all medium types tested, there were no apparent differences in hyphal morphology between the mutant and wild type. In older surface cultures grown on MS medium, no significant differences between the spore chains of the wildtype and mutant were noted (data not shown). Direct comparison of similarly aged cultures grown on glucose revealed more abundant unbranched, multiply septated aerial hyphae in samples prepared from the mutant, consistent with precocious reproductive growth. Consequently, we examined samples prepared over a range of time points to compare wild-type and mutant hyphae. The most apparent differences were between the aerial hyphae of cultures grown on NMMP-glucose. After 7 days of growth, maturing wild-type aerial hyphae that possessed multiple, regularly spaced septa defining prespore compartments were observed, although they were not abundant (Fig. 4C). Differentiating aerial hyphae were present in similar abundance in 3-day-old cultures of the mutant. However, these hyphae consisted of chains of abnormally shaped cells separated by well-defined septa (Fig. 4D). The majority of the cells, distal to the tip, were swollen, each to a different extent, and spherical. Apical cells, in contrast, were elongated rather than spherical. In older cultures, the abundance and length of chains of abnormal cells greatly increased (Fig. 4E), each chain consisting characteristically of large spherical cells with elongated cells at the tip. Propidium iodide staining revealed the presence of DNA in each of the swollen cells, with larger cells staining most intensely (these cells also appeared more intense in the corresponding green fields, which may be due to some excitation and emission arising from propidium iodide). The distribution of DNA in the elongated tip cells was not uniform, with the most intense staining close to septa. Under phase-contrast microscopy, the abnormally shaped large cells were darker than adjacent filamentous hyphae, but not as dark as regular spore chains. Individual swollen cells that had presumably separated from chains during sample preparation were also evident in many fields. Swollen cells were also evident in untreated samples viewed under phase-contrast microscopy, indicating that they were not artifacts of lysozyme treatment (results not shown). Cytological examination of the complemented mutant grown on NMMP-glucose revealed only infrequent, regular chains of prespore compartments (data not shown).
The abnormal morphology of the mutant in glucose-grown cultures was specific to aerial hyphae, as it was only observed in differentiating cultures grown on solid media; submerged cultures of the mutant grown in liquid NMMP-glucose resembled the wild type with respect to the frequency of hyphal cross-walls and overall morphology (data not shown).
Overexpression of crgASc inhibits sporulation septation.
To overexpress crgASc, the gene was placed under the control of the thiostrepton-inducible tipA promoter in plasmid pIJ8600. The resulting plasmid, pME41, was introduced into wild-type S. coelicolor, and cultures were grown on a variety of media containing either glucose or mannitol as a carbon source, with or without the addition of the inducer thiostrepton. Induction of the promoter resulted in a significant delay (24 h or more) in both morphological differentiation and antibiotic production on all media tested. The addition of an inducer did not affect the growth of the wild type containing pIJ8600. After extended incubation, the cultures in which overexpression of crgASc was induced were white, being impaired in the production of grey spore chains. This Whi phenotype was independent of the medium and carbon source; the difference between the mutant and M145 or M145 containing pIJ8600 was most evident on MS and R2YE media (Fig. 3C). For cytological analysis, to be confident of observing predominantly aerial hyphae, samples were prepared using coverslip impressions on 7-day-old white cultures. Microscopy of Fluo-WGA-stained aerial hyphae from these cultures in which crgA was overexpressed revealed long, unbranched hyphae lacking sporulation septa (Fig. 4F); impressions from wild-type cultures revealed abundant spore chains with morphologies similar to the example illustrated in Fig. 4C. DNA in these hyphae was not condensed, as observed in the aerial hyphae of other whi mutants impaired in sporulation septation (8). In parallel experiments, we observed that crgASa overexpressed in S. avermitilis also resulted in a Whi phenotype and that the resulting aerial hyphae lacked sporulation septa (data not shown).
DISCUSSION
We have identified a new gene required for sporulation in S. avermitilis. Following the convention established in S. coelicolor, we originally named the locus whiP. However, sporulation is not overtly affected in an S. coelicolor mutant in which the orthologous gene is disrupted. Instead, depending on the growth medium, the mutant exhibits precocious antibiotic production and erection of aerial hyphae. Moreover, subsequent development of the aerial hyphae can be affected, resulting in chains of cells with abnormal morphology. To reflect the disparate roles of the gene in the two species that impact on the coordination of reproductive growth, the gene was renamed crgA. The apparent differences in the functions of this gene in the two organisms may reflect species-specific aspects of the regulatory cascade controlling sporulation. Indeed, cytological evidence shows crucial differences in reproductive growth between the two species. For example, nucleoid condensation occurs in the absence of septation in the aerial hyphae of S. avermitilis but is normally coincident with septation of the aerial hyphae of S. coelicolor (27). Differences in the regulation of reproductive growth are also apparent in that sporulation of S. avermitilis is suppressed on a glucose carbon source.
Cytological examination of aerial hyphae indicates that CrgA may have related roles in coordinating cell division with growth in both species. In the absence of CrgA in S. avermitilis, the aerial hyphae form coils at the tips and are similar in length to those of the wild type. This suggests that the protein is not required for cessation of growth but for the formation of sporulation septa subsequent to growth. This phenotype is reminiscent of those of S. coelicolor whiH and whiI mutants; these genes are postulated to define a developmental checkpoint that links growth arrest with septation (6, 8). In contrast, one aspect of the growth of the S. coelicolor crgA mutant on NMMP-glucose was early development of aerial hyphae and the subsequent formation of chains of irregular large cells with abnormal morphology. The sizes of these cells may indicate irregular placement of septa and continued growth after septation. Cells distal to a hyphal tip were characteristically spherical, while those at the apex were elongated. This may be a consequence of the continuation of two types of growth, as has been postulated to account for the exponential increase in the total length of an unbranched aerial hypha (5). Intercalary growth after septation will result in large spherical cells. Similar extension of the lateral walls of compartments closer to the tips, prior to closure of septa, coupled with apical extension can generate elongated cells at the tip. Continued growth was also suggested by more intense staining of the largest cells by propidium iodide, possibly due to continued replication of the genome. CrgA in S. coelicolor may therefore function conditionally to coordinate septation with growth cessation. Prior to the arrest of hyphal extension, CrgA would act either directly or indirectly as a conditional inhibitor of septation. A test of this inhibiting function was to overexpress the gene, uncoupling any carbon source and growth phase-dependent regulation. This resulted in a Whi phenotype on all growth media tested. The aerial hyphae were long, uncoiled, and filamentous, with no evidence of sporulation septa.
The dependence on a carbon source for the manifestation of the effects of disruption of crgA in S. coelicolor suggests that other functions coordinating the growth and septation of aerial hyphae may be under glucose repression. The implication that development of the aerial hyphae is regulated in different ways depending on the carbon source is reminiscent of the functions of the bld genes. A shared trait of most S. coelicolor bld mutants is that they fail to erect aerial mycelia on glucose-containing media but form sporulating aerial hyphae on other carbon sources, including mannitol.
In conclusion, crgA is a new developmental gene that encodes a small 84-amino-acid predicted transmembrane protein that is representative of a new family of actinomycete-specific proteins. A single orthologous gene is located very close to the chromosomal origin of replication, oriC, in all fully sequenced actinomycete genomes. The presence of a crgA ortholog in mycobacteria and, in particular, in the genome of M. leprae, a product of extensive reductive evolution that is suggested to contain a minimal gene set (7), could imply an important role for the protein in controlling the growth and cell division of these pathogenic bacteria. CrgA is another example of an actinomycete-specific protein implicated in development and cell division, as demonstrated for the unrelated WhiB-like class of proteins (10, 20).
Acknowledgments
This project was supported by the BBSRC. A.P. was supported by a CASE studentship awarded by the BBSRC and Pfizer Central Research; R.D.S. was supported by a British Council Chevening training scholarship.
We are grateful to Meirwyn Evans and Sue Fielding for technical assistance.
REFERENCES
- 1.Aínsa, J. A., H. D. Parry, and K. F. Chater. 1999. A response regulator-like protein that functions at an intermediate stage of sporulation in Streptomyces coelicolor A3(2). Mol. Microbiol. 34:607-619. [DOI] [PubMed] [Google Scholar]
- 2.Aínsa, J. A., N. J. Ryding, N. Hartley, K. C. Findlay, C. J. Bruton, and K. F. Chater. 2000. WhiA, a protein of unknown function conserved among gram-positive bacteria, is essential for sporulation in Streptomyces coelicolor A3(2). J. Bacteriol. 182:5470-5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bierman, M., R. Logan, K. O'Brien, E. T. Seno, R. N. Rao, and B. E. Schoner. 1992. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116:43-49. [DOI] [PubMed] [Google Scholar]
- 5.Chater, K. F. 2000. Developmental decisions during sporulation in the aerial mycelium of Streptomyces, p. 33-48. In Y. V. Brun and L. J. Shimkets (ed.), Prokaryotic development. American Society for Microbiology, Washington, D.C.
- 6.Chater, K. F. 2001. Regulation of sporulation in Streptomyces coelicolor A3(2): a checkpoint multiplex? Curr. Opin. Microbiol. 4:667-673. [DOI] [PubMed] [Google Scholar]
- 7.Cole, S. T., K. Eiglmeier, J. Parkhill, K. D. James, N. R. Thomson, P. R. Wheeler, N. Honore, T. Ganier, C. Churcher, D. Harris, K. Mungall, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. M. Davies, K. Devlin, S. Duthoy, T. Feltwell, A. Fraser, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, C. Lacroix, J. Maclean, S. Moule, L. Murphy, K. Oliver, M. A. Quail, M.-A. Rajandream, K. M. Rutherford, S. Rutter, K. Seeger, S. Simon, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, K. Taylor, S. Whitehead, J. R. Woodward, and B. G. Barrell. 2001. Massive gene decay in the leprosy bacillus. Nature 409:1007-1011. [DOI] [PubMed] [Google Scholar]
- 8.Flärdh, K., K. C. Findlay, and K. F. Chater. 1999. Association of early sporulation genes with suggested developmental decision points in Streptomyces coelicolor A3(2). Microbiology 145:2229-2243. [DOI] [PubMed] [Google Scholar]
- 9.Flärdh, K., E. Leibovitz, M. J. Buttner, and K. F. Chater. 2000. Generation of a non-sporulating strain of Streptomyces coelicolor A3(2) by the manipulation of a developmentally controlled ftsZ promoter. Mol. Microbiol. 38:737-749. [DOI] [PubMed] [Google Scholar]
- 10.Gomez, J. E., and W. R. Bishai. 2000. whmD is an essential mycobacterial gene required for proper septation and cell division. Proc. Natl. Acad. Sci. USA 97:8554-8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grantcharova, N., W. Ubhayasekara, S. L. Mowbray, J. R. McCormick, and K. Flärdh. 2003. A missense mutation in ftsZ differentially affects vegetative and developmentally controlled cell division in Streptomyces coelicolor A3(2). Mol. Microbiol. 47:645-656. [DOI] [PubMed] [Google Scholar]
- 12.Hopwood, D. A., M. J. Bibb, K. F. Chater, T. Kieser, C. J. Bruton, H. M. Kieser, D. P. Lydiate, C. P. Smith, J. M. Ward, and H. Schrempf. 1985. Genetic manipulation of Streptomyces: a laboratory manual. John Innes Foundation, Norwich, United Kingdom.
- 13.Ikeda, H., J. Ishikawa, A. Hanamoto, M. Shinose, H. Kikuchi, T. Shiba, Y. Sakaki, M. Hattori, and S. mura. 2003. Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis. Nat. Biotechnol. 21:526-531. [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa, J., and K. Hotta. 1999. FramePlot: a new implementation of the frame analysis for predicting protein-coding regions in bacterial DNA with a high G + C content. FEMS Microbiol. Lett. 174:251-253. [DOI] [PubMed] [Google Scholar]
- 15.Janssen, G. R., and M. J. Bibb. 1993. Derivatives of pUC18 that have BglII sites flanking a modified multiple cloning site and that retain the ability to identify recombinant clones by visual screening of Escherichia coli colonies. Gene 124:133-134. [DOI] [PubMed] [Google Scholar]
- 16.Kieser, T., M. J. Bibb, M. J. Buttner, K. F. Chater, and D. A. Hopwood. 2000. Practical Streptomyces genetics. John Innes Foundation, Norwich, United Kingdom.
- 17.Kormanec, J., R. Nováková, D. Homerová, and B. Sevcíková. 1999. The Streptomyces aureofaciens homologue of the sporulation gene whiH is dependent on rpoZ-encoded sigma factor. Biochim. Biophys. Acta 1444:80-84. [DOI] [PubMed] [Google Scholar]
- 18.MacNeil, D. J., J. L. Occi, K. M. Gewain, T. MacNeil, P. H. Gibbons, C. L. Ruby, and S. L. Danis. 1992. Complex organization of the Streptomyces avermitilis genes encoding the avermectin polyketide synthase. Gene 115:119-125. [DOI] [PubMed] [Google Scholar]
- 19.McCormick, J. R., E. P. Su, A. Driks, and R. Losick. 1994. Growth and viability of Streptomyces coelicolor mutant for the cell division gene ftsZ. Mol. Microbiol. 14:243-254. [DOI] [PubMed] [Google Scholar]
- 20.Molle, V., W. J. Palframan, K. C. Findlay, and M. J. Buttner. 2000. WhiD and WhiB, homologous proteins required for different stages of sporulation in Streptomyces coelicolor A3(2). J. Bacteriol. 182:1286-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paget, M. S., L. Chamberlin, A. Atrih, S. J. Foster, and M. J. Buttner. 1999. Evidence that the extracytoplasmic function sigma factor sigmaE is required for normal cell wall structure in Streptomyces coelicolor A3(2). J. Bacteriol. 181:204-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pitman, A., P. Herron, and P. Dyson. 2002. Cointegrate resolution following transposition of Tn1792 in Streptomyces avermitilis facilitates analysis of transposon-tagged genes. J. Microbiol. Methods 49:89-96. [DOI] [PubMed] [Google Scholar]
- 23.Redenbach, M., H. M. Kieser, D. Denapaite, A. Eichner, J. Cullum, H. Kinashi, and D. A. Hopwood. 1996. A set of ordered cosmids and a detailed genetic and physical map for the 8 Mb Streptomyces coelicolor A3(2) chromosome. Mol. Microbiol. 21:77-96. [DOI] [PubMed] [Google Scholar]
- 24.Ryding, N. J., G. H. Kelemen, C. A. Whatling, K. Flärdh, M. J. Buttner, and K. F. Chater. 1998. A developmentally regulated gene encoding a repressor-like protein is essential for sporulation in Streptomyces coelicolor A3(2). Mol. Microbiol. 29:343-357. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 26.Schultz, J., F. Milpetz, P. Bork, and C. P. Ponting. 1998. SMART, a simple modular architecture research tool: identification of signaling domains. Proc. Natl. Acad. Sci. USA 95:5857-5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwedock, J., J. R. McCormick, E. R. Angert, J. R. Nodwell, and R. Losick. 1997. Assembly of the cell division protein FtsZ into ladder-like structures in the aerial hyphae of Streptomyces coelicolor. Mol. Microbiol. 25:847-858. [DOI] [PubMed] [Google Scholar]
- 28.Sun, J., G. H. Kelemen, J. M. Fernández-Abalos, and M. J. Bibb. 1999. Green fluorescent protein as a reporter for spatial and temporal gene expression in Streptomyces coelicolor A3(2). Microbiology 145:2221-2227. [DOI] [PubMed] [Google Scholar]
- 29.Tan, H., H. Yang, Y. Tian, W. Wu, C. A. Whatling, L. C. Chamberlin, M. J. Buttner, J. Nodwell, and K. F. Chater. 1998. The Streptomyces coelicolor sporulation-specific sigma WhiG form of RNA polymerase transcribes a gene encoding a ProX-like protein that is dispensable for sporulation. Gene 212:137-146. [DOI] [PubMed] [Google Scholar]
- 30.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]