Abstract
The current annual incidence of sudden cardiac death in the US is likely to be in the range of 180–250,000 per year. Coinciding with the decreased mortality from coronary artery disease, there is evidence pointing toward a significant decrease in rates of sudden cardiac death in the US during the second half of the twentieth century. However the alarming rise in prevalence of obesity and diabetes in the first decade of the new millennium both in the US and worldwide, would indicate that this favorable trend is unlikely to persist. We are likely to witness a resurgence of coronary artery disease and heart failure, as a result of which sudden cardiac death will have to be confronted as a shared and indiscriminate, worldwide public health problem. There is also increasing recognition of the fact that discovery of meaningful and relevant risk stratification and prevention methodologies will require careful prospective community-wide analyses, with access to large archives of DNA, serum and tissue that link with well-phenotyped databases. The purpose of this review is to summarize current knowledge of sudden cardiac death epidemiology. We will discuss the significance and strengths of community-wide evaluations of sudden cardiac death, summarize recent observations from such studies, and finally highlight specific potential predictors that warrant further evaluation as determinants of sudden cardiac death in the general population.
Introduction
Analyses of disease progression patterns performed at the end of the last millennium predicted a globally increased incidence of heart disease by the year 2020 (1–3). Even in the first decade of the new millennium, these predictions are already being realized. In a reversal of trends, the greatest increases in prevalence of diabetes and coronary artery disease are being observed in the developing nations (4). An important consequence of this burgeoning population of patients with coronary disease and heart failure will be an increasing incidence of sudden cardiac death. As a result, sudden cardiac death will have to be confronted as a shared and indiscriminate, worldwide public health problem. The current advancements in resuscitation science notwithstanding, survival from sudden cardiac arrest remains low even in the developed nations (5). At the close of the 20th century, awareness of this important deficiency has focused considerable interest on mechanisms of sudden cardiac death. Despite a renewed focus, the significant delay in development of effective measures of risk stratification and prevention of sudden cardiac death can be attributed directly to a poor understanding of mechanisms involved in fatal arrhythmogenesis (6), particularly at the community-wide level. While longitudinal cohort studies and the multi-center trials of sudden cardiac death prevention have been invaluable, there is increasing recognition of the critical need to view these observations in the context of community-wide analyses, without which discovery of meaningful and relevant risk stratification methodology may not be possible. The purpose of this review is to summarize current knowledge of sudden cardiac death epidemiology. We will discuss the significance and strengths of community-wide evaluations of sudden cardiac death, summarize recent observations from such studies, and finally highlight specific potential predictors that warrant further evaluation as determinants of sudden cardiac death in the general population.
Definition and current burden of sudden cardiac death in the community
The first stage in the process of confronting any community-wide disease condition is the definition of the problem. In essence, the individual we would like to identify is one who suffers a sudden, unexpected pulseless condition of cardiac etiology. The vast majority of these patients are going to have a sudden arrhythmic death. For several reasons assessment of sudden cardiac death has been difficult to accomplish, the first of which being that the condition is a dynamic event that occurs in the general population in an unpredictable manner. Secondly, over the years, a standard definition for identifying the condition has not employed in the large number of studies that have been conducted. The most accepted definition is sudden and unexpected death within an hour of symptom-onset (7, 8). If un-witnessed, subjects should have been observed alive within 24 hours of their death. In addition, whenever possible, it is important to exclude subjects that are likely to have had a non-cardiac cause of sudden death, such as patients that may have had a large pulmonary embolism that resulted in cardiac arrest or those that were known to be suffering from malignancy that is not in remission. Thirdly, an accurate estimate of sudden cardiac death incidence requires prospective ascertainment of cases. Studies that have used retrospective death-certificate based methodology to identify cases of sudden cardiac death are likely to significantly overestimate sudden cardiac death incidence by as much as 200–300 percent (9).
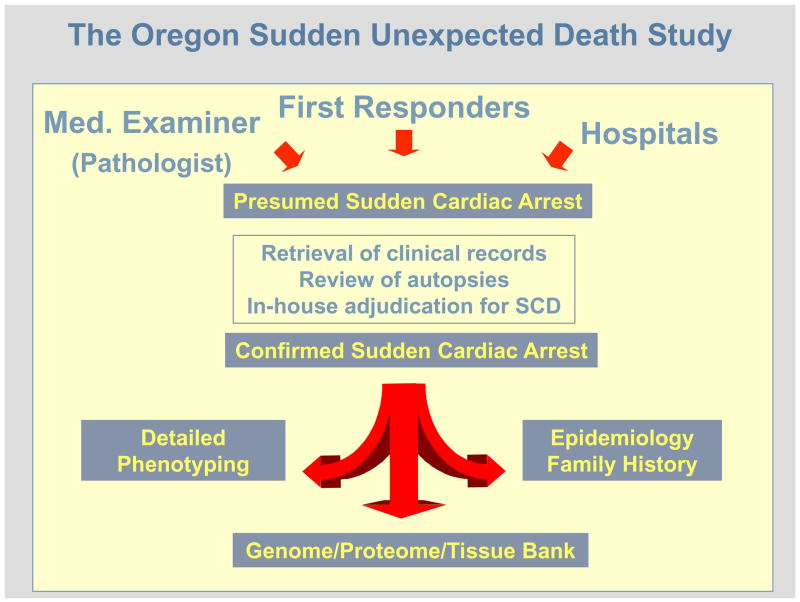
Therefore the US estimates published by the US Centers for Disease Control and Prevention (400–450,000 per year) (10) are likely to be a significant overestimate (5). Prior to the publication of the limited number of community-wide studies that have employed the standard definition, there were several that prospectively examined the community-wide incidence of primary cardiac arrest using data collected by first responders. In these studies, the annual incidence of treated primary cardiac arrest ranged between 41 and 89/100,000 (11–14). However the major limitation of this methodology is that it does not include the significant proportion of sudden cardiac death cases that are un-witnessed. In addition, due to the unavailability of detailed clinical records, important diagnoses that exclude patients (such as a diagnosis of terminal malignancy) could not be made. A prospective study in the Maastricht area of the Netherlands reported an annual incidence of sudden out-of-hospital cardiac arrests of 90–100/100,000 residents age 20–75 years (15). On the other hand, a retrospective study of sudden cardiac death among residents of southern Okinawa, Japan, that employed multiple sources, reported a crude annual incidence rate of 37 per 100,000 residents (16). The Oregon Sudden Unexpected Death Study (Ore-SUDS) is an ongoing prospective community-wide evaluation of sudden cardiac death (5, 17). Originally underwritten by the US Centers for Disease Control and Prevention, this study is now in its seventh year and tracks cases of sudden cardiac death that occur in Multnomah County Oregon (Portland, Oregon metropolitan area) among a population of approximately 1,000,000 residents. A multiple source method of ascertainment is used, in order to capture all cases of sudden cardiac death (Figure 1). Cases are reported by first responders (ambulance and emergency medicine personnel, 70%), the State Medical Examiner or Coroner (25%) and from the area hospitals (approximately 5%). In the first year of this study, the annual incidence of sudden cardiac death was 53 per 100,000 residents and accounted for 5.6 percent of overall deaths (5). It is of interest that an almost identical annual incidence of sudden cardiac death (51.2/100,000) was recently reported in a community of 414,277 in the West of Ireland (18) as well as a national survey of out of hospital cardiac arrests in Canada (56/100,000) (19). Taken together, an estimated annual incidence of sudden cardiac death in the US (total population approx. 300,000,000), would range between 180,000 – 250,000 cases per year. For the world (total population approx. 6,540,000,000), the estimated annual burden of sudden cardiac death would be in the range of 4–5 million cases per year.
Figure 1.
The Oregon Sudden Unexpected Death Study: A prospective, population-based multiple-source evaluation of sudden cardiac arrest, ongoing since Feb 1, 2002.
Age and Gender Trends
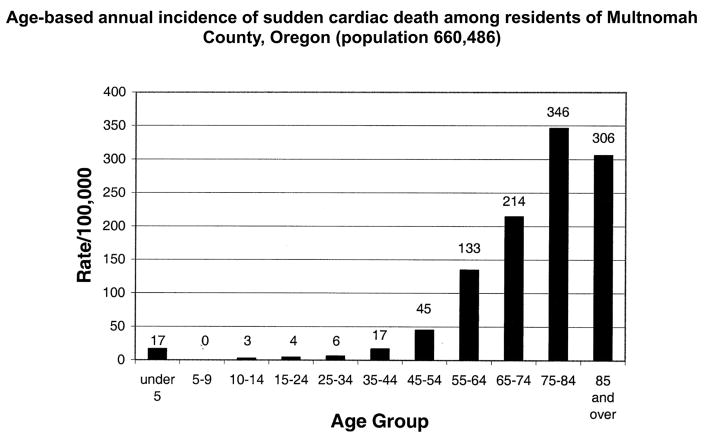
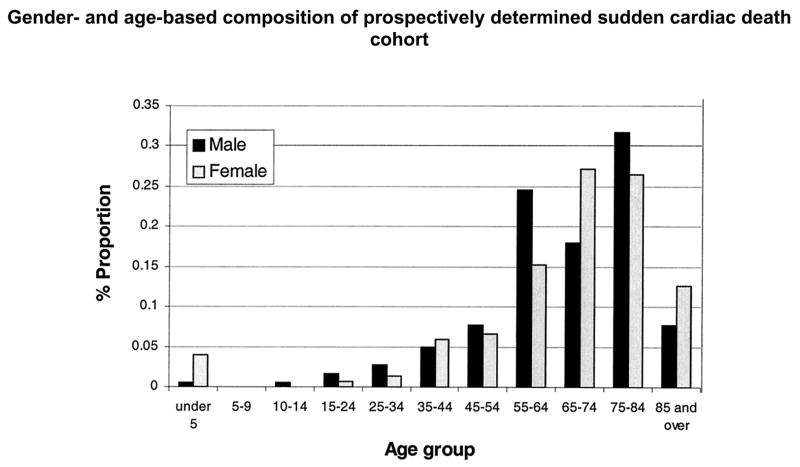
There are two well-established peaks in the age-related prevalence of sudden cardiac death, one during infancy representing the sudden infant death syndrome and the second in the geriatric age group, between ages 75 and 85 years. These trends continue to persist (Figures 2 and 3). However the gender-related trends appear to have shifted, with an increase in the proportion of females who suffer sudden cardiac death. Earlier studies had reported 25:75 ratio of females to males. The Oregon experience shows that females, on a yearly basis, consistently make up approximately 40% of all sudden cardiac death cases (5). While the reasons for this changing trend merit further evaluation, one possibility is that this mirrors the altered gender distribution in prevalence of and mortality from, coronary artery disease (20).
Figure 2.
Age-based annual incidence of sudden cardiac death among residents of Multnomah County, Oregon (population 660,486). From Chugh SS, Jui J, Gunson K, Stecker EC, John BT, Thompson B, Ilias N, Vickers C, Dogra V, Daya M, Kron J, Zheng ZJ, Mensah G, McAnulty J. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Coll Cardiol 2004;44(6):1268–75. Reprinted by permission of the American College of Cardiology Foundation ©2004.
Figure 3.
Gender- and age-based composition of prospectively determined sudden cardiac death population. Adapted from Chugh SS, Jui J, Gunson K, Stecker EC, John BT, Thompson B, Ilias N, Vickers C, Dogra V, Daya M, Kron J, Zheng ZJ, Mensah G, McAnulty J. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Coll Cardiol 2004;44(6):1268–75. Reprinted by permission of the American College of Cardiology Foundation ©2004.
Spectrum of etiologies of sudden cardiac death
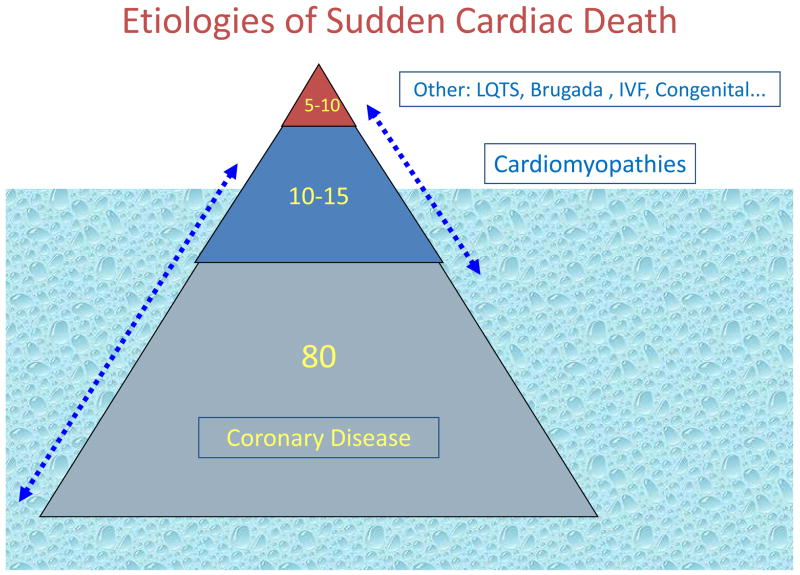
The most common clinical finding associated with sudden cardiac death is coronary artery disease (Figure 4) and approximately 80% of sudden cardiac deaths are attributed to this disease condition (5, 8, 21, 22). Based on our knowledge of the stages of the natural history of coronary artery disease, there are two major mechanisms of fatal ventricular arrhythmias. Acute coronary ischemia usually associated with plaque rupture and occlusion of one or more major coronary arteries is more likely to result in polymorphic ventricular tachycardia. It is likely that such patients have relatively normal LV systolic function or mildly depressed LV function. Those with ischemic cardiomyopathy following one or more myocardial infarctions are more likely to have monomorphic ventricular tachycardias resulting from re-entrant loops around areas of scarred myocardium. Either arrhythmia if untreated will eventually degenerate into ventricular fibrillation.
Figure 4.
Previously silent coronary artery disease is likely to be the predominant disease condition contributing to sudden cardiac death in the general population.
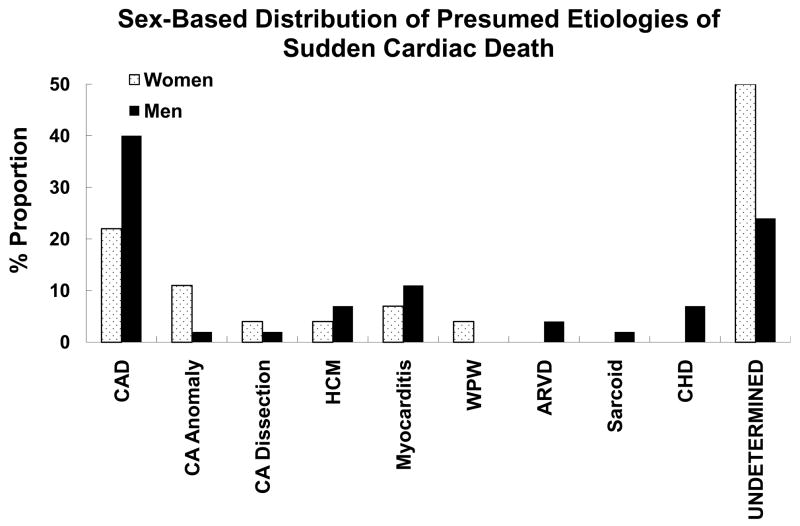
Another 10–15% occur in patients who have cardiomyopathies such as hypertrophic cardiomyopathy, dilated cardiomyopathies, arrhythmogenic right ventricular dysplasia and the myocardial infiltrative diseases (sarcoid, amyloidosis). Given the ubiquitous nature of coronary disease, it is likely that there is significant overlap of this condition with the cardiomyopathies, i.e. there are patients who will have both conditions and it is likely that both etiologies contribute to risk of sudden cardiac death (Figure 4). The remaining 5–10% are composed of either structurally abnormal congenital cardiac conditions (coronary anomalies, cyanotic/non-cyanotic diseases) or patients with structurally normal but electrically abnormal heart. It is likely that some proportion of these patients eventual develop structural heart disease if they survive their sudden cardiac arrest or this event is successfully prevented by an intervention (Figure 4). Besides the relatively rare genetic diseases such as long QT syndrome, Brugada syndrome and catecholaminergic polymorphic ventricular tachycardia, patients with autopsy-negative sudden cardiac death (no genetic abnormalities identified) may comprise a larger part of this subgroup than previously anticipated (9). In fact, while this may not be true in all populations, Behr and co-workers estimated the incidence to be as high as 11/100,000, in a nationwide epidemiological survey of unexpected sudden cardiac death in England, UK, among healthy individuals aged 16–64 years (14, 15). The latter study did not evaluate all cases of sudden cardiac death in the general population – analysis was limited to cases of presumed sudden cardiac death that underwent postmortem evaluation by a sampling of coroners in the UK. Also the incidence is likely to be lower if older age groups, who experience most sudden cardiac deaths were included in this analysis. Subjects with sudden unexplained death have also been termed as having idiopathic ventricular fibrillation or sudden arrhythmic death syndromes (6, 9, 10, 15). It is possible that some proportion of these patients had heritable monogenic disorders such as the long QT syndrome, Brugada syndrome and catecholaminergic polymorphic ventricular tachycardia (6, 20–24). Defects in the SCN5A gene, also causing long QT syndrome 3 and the Brugada syndrome, have been reported in some cases of the sudden infant death syndrome (25, 26). A retrospective postmortem molecular analysis of sudden cardiac death with structurally normal heart was conducted from a sudden cardiac death autopsy series based at the Edwards Registry of Cardiovascular Disease in St Paul, Minnesota. Using a candidate gene-based approach, only 2 of 12 patients (17%) had identifiable defects among five candidate genes tested (13). Behr and co-workers evaluated the families of 32 individuals with unexplained sudden cardiac death (15). Seven of 32 families (22%) were found to have one of several heritable cardiac conditions – the long QT syndrome (n=4), an unidentified non-structural cardiac electrophysiological condition (n=1), myotonic dystrophy (n=1) and hypertrophic cardiomyopathy (n=1). However, in five of the seven probands that led to these families, a genetic abnormality could not be identified. In the Minnesota study, there was a family history of sudden cardiac death in only 3 of 18 cases (17%) – indicating that family history may be a reliable predictor of clinical risk for only a minority of SUD cases in the general population. Similar to overall sudden cardiac death, SUD seems to be more common in males (mean age range 24–32 years), with published autopsy series reporting 63–68% of affected subjects as males (23, 24). However, given the lower overall sudden cardiac death rates in females, SUD cases are likely to comprise a higher proportion of sudden cardiac death cases in women compared to men, particularly among younger adults. From the Edwards Registry 270-patient autopsy series of sudden cardiac death, the age-group 35–44 years contained 72 patients, of which 27 were women (32% of total women) and 45 men (24% of total men) (25). Detailed cardiac pathologic examinations revealed significant gender-related differences in prevalence of SUD. There was a significantly higher rate of SUD in these younger women. Following detailed autopsy, cardiac pathologic examination and analysis of available clinical findings, 50% of women had sudden cardiac death of undetermined etiology compared to 24% of men 18 (Figure 5). In a separate study of even younger women, clinical information was reviewed for 852,300 female army recruits, who entered basic military training from 1977 to 2001 (26). During this period, there were 13 sudden cardiac death cases (median age 19 years, 73% African-American), occurring at a median of 25 days after arrival for training. Of these, eight recruits (53%) suffered SUD, and anomalous coronary origins were found in two (13%). Therefore, SUD was the leading cause of non-traumatic sudden death in young female recruits during military training. These findings suggest that the overall burden of SUD in younger women could be higher than anticipated.
Figure 5.
Sex-based distribution of presumed etiologies of sudden cardiac death. These data represent comparisons between 27 women and 45 men ages 35–44 years. CAD, Coronary artery disease; CA, coronary artery; HCM, hypertrophic cardiomyopathy; WPW, Wolff-Parkinson-White syndrome; ARVD, arrhythmogenic right ventricular dysplasia; CHD, congenital heart disease. Adapted from Chugh SS, Chung K, Zheng ZJ, John B, Titus JL. Cardiac pathologic findings reveal a high rate of sudden cardiac death of undetermined etiology in younger women. Am Heart J 2003;146(4):635–9. Reprinted by permission of Mosby Inc. ©2003.
It is worth mentioning that the importance of a careful postmortem histological examination in patients with sudden death and apparently normal heart cannot be underestimated (21). Besides uncovering relatively mild structural manifestations of rare conditions such as arrhythmogenic right ventricular dysplasia, there is a possibility that novel phenotypes can be uncovered. From the autopsy series at the Edwards Registry described above, several patients with a heart that appeared structurally normal were found to have abnormalities consistent with idiopathic myocardial fibrosis on histologic examination. Following a detailed characterization of this phenotype in six patients (17) we observed a diffuse but heterogeneous increase in myocardial collagen content with a predilection for the left ventricular inferior wall. The increase in collagen was exclusively interstitial, without evidence of myocyte necrosis or stigmata of myocarditis. Transforming growth factor-beta 1 was implicated as a mediator of idiopathic myocardial fibrosis but specific triggers as well as sub-cellular signaling pathways have yet to be determined.
Presenting arrhythmia in patients who suffer sudden cardiac arrest: altered trends
Despite the spectrum of etiologic conditions, in the overwhelming majority of cases sudden cardiac death results from a fatal cardiac arrhythmia, either ventricular tachycardia/fibrillation or severe bradycardia/pulseless electrical activity (27, 28). In the last two decades there have been important alterations in the prevalence trends of each of these presenting arrhythmias. Early studies reported that VF was the initial rhythm in the vast majority of sudden cardiac death cases; 75% in a longitudinal study from Seattle (29) and 84% in a series of patients wearing Holter monitors (30). Studies also consistently demonstrated that VF had the highest likelihood of survival among the lethal arrhythmias, which led to special emphasis on VF detection and treatment in resuscitation protocols. However since 1990, a declining rate of VF and a steady rise in prevalence of PEA has been reported. Initially this was reported only from in-hospital studies (PEA 35–40% of events (31)) but this was soon confirmed in pre-hospital studies as well. Data from Seattle during 1990–96 indicated 41% VF and 24% PEA (32). Another study from Seattle that looked at prevalence trends between 1980–2000 reported a 56% decrease in VF as the first identified rhythm from 1980 to 2000 (from 0.85 to 0.38 per 1000; relative risk [RR], 0.44; 95% confidence interval [CI], 0.37–0.53) (11). Similar reductions occurred in blacks and whites and this phenomenon was most evident in men (57%; RR, 0.43; 95% CI, 0.35–0.53), in whom the baseline incidence was relatively high. An amiodarone vs. lidocaine trial reported VF prevalence among pre-hospital cardiac arrests, of only 26% (33). Similar reports were published from Goteborg, Sweden documenting a decrease in VF prevalence of 39% over 17 years despite significant improvements in response time and rates of bystander cardiopulmonary resuscitation (34). Prevalence of PEA among sudden cardiac arrest cases rose from 6% to 26% during 1980–96 (34). What are the causes of this altered prevalence and why is it important? It is possible that VF is more commonly related to coronary disease and PEA to non-cardiac factors such as lung disease (35). As a consequence the decline in VF prevalence may have occurred due to the significant decrease in age-adjusted mortality from coronary artery disease over the last 50 years (36). Other factors may be involved, but more detailed investigation of PEA is needed before we can identify these. Given significantly divergent survival rates from the two arrhythmias a strong and ongoing focus on improving the understanding of PEA mechanisms and treatment will have significant implications for improving current rates of survival from sudden cardiac arrest in the community.
Findings from Population-based Studies
There are important reasons to make a distinction between subjects who suffer sudden cardiac arrest after having previously identified heart disease vs. those that do not have previously identified heart disease. Among the latter group there are many who may have never had symptoms suggestive of heart disease or were not evaluated for other reasons, such as disparate access to health care. Recent observations such as the recently reported Home Automated External Defibrillation trial (37) have confirmed the present, remarkably low annual mortality rate (2%) among patients with treated acute anterior myocardial infarction who did not meet criteria for an implantable defibrillator. This reduction in mortality may in fact have been the single most important reason that home use of the automated external defibrillator did not benefit this group of patients. These findings underscore the critical need to learn more about patients who suffer sudden cardiac arrest in the community, particularly when they do not have previously identified heart disease.
Coronary artery disease
This remains the condition most commonly associated with sudden cardiac death and identified in at least 80% of overall cases. However among all patients with coronary artery disease, depending on the age group, only 13–20% will have sudden cardiac arrest (38). How then are we to identify this relatively small subgroup of patients at highest risk? It is likely that this risk will be multi-factorial with one component that relates directly to coronary artery disease and a second component that is either related to an associated co-morbidity such as heart failure or an inherent tendency such as genetic risk. There is significant evidence to suggest that this first component could be rupture of the vulnerable plaque. The fact is that mortality from sudden cardiac death was halved in the last 50 years of the past century and this correlated with the overall similar decrease in mortality from coronary artery disease (36), is further evidence in support of this possibility. It is likely that treatment and stabilization of the vulnerable plaque was achieved in large measure by rapid deployment of coronary artery bypass surgery, percutaneous angioplasty-stent, aspirin use, cholesterol-lowering therapies as well as increased awareness of coronary artery disease prevention during this era. However, while mortality from coronary artery disease has plummeted, prevalence of coronary disease has not. Therefore there are ongoing attempts to identify risk markers for sudden rupture of the vulnerable plaque but none that have proven definitive. Several epidemiologic studies have evaluated the potential role of total cholesterol levels in predicting sudden cardiac arrest risk, but results have been mixed, with some studies finding a significant role for this condition (39, 40)) and others finding no significant influence on sudden cardiac arrest occurrence (41). Atherosclerosis is a process driven by atherogenic lipoproteins, and given the central role of low-density lipoprotein (LDL) in coronary artery plaque development (42), others have evaluated the specific role of LDL levels in predicting risk. However, these trends have also not been observed uniformly. An analysis from the Physicians Health Study reported no significant differences in total cholesterol or LDL using a case-control analysis. In contrast, a cohort study of patients with stable angina followed for 10 years, found that LDL was a predictor of sudden cardiac arrest in the short term (less than 2 years, RR 1.8) but not in the long term (>2 years) (43). Identification of novel markers and modalities for the identification of the plaque that is prone to rupture or patients that are prone to aggressive clot formation are the subjects of intensive ongoing investigations that have been reviewed in detail elsewhere (44–46). However, specific risk predictors for vulnerable plaque rupture, that can be utilized clinically are still awaited.
Role of severe left ventricular dysfunction
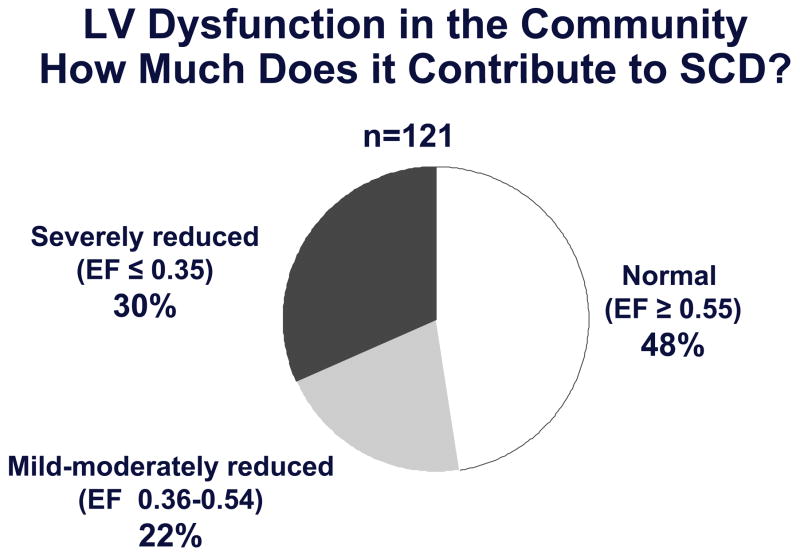
Presently, both in the presence or absence of coronary artery disease, a diagnosis of severe left ventricular dysfunction is the best available predictor of sudden cardiac death risk. Based on data from the multi-center primary prevention trials of sudden cardiac death, the presence of severe left ventricular dysfunction is the major indication for primary prevention with the implantable cardioverter-defibrillator (ICD) (47–50). However, only 20–30% of patients that are implanted with a prophylactic ICD, actually receive appropriate therapies over a follow-up period of 4–5 years (47, 51). When severe left ventricular dysfunction is used as the criterion for a prophylactic ICD, it takes at least 15 ICD recipients to save one life during a one-year follow up period. Furthermore, the high-risk sudden cardiac death patients we see in our clinics and hospital wards are likely to be different from the residents in the community that have sudden cardiac death, often as the very first manifestation of heart disease. Patients who present to health care providers with severely decreased left ventricular dysfunction, constitute a high-risk group that is likely to comprise a small proportion of overall sudden cardiac death cases (52). We and others have observed this to indeed be true in community-wide analyses (15, 17, 53). In the ongoing Oregon Sudden Unexpected Death Study (Ore-SUDS), severe left ventricular dysfunction did predict sudden cardiac death, but was found to affect less than a third of all sudden cardiac death cases in the community (Figure 6) (17). Since only a subgroup of symptomatic individuals tend to present for evaluation this figure is also likely to be an overestimate. Almost half of all sudden cardiac death cases had normal left ventricular function and the remaining 20% had either mildly or moderately decreased left ventricular systolic function (left ventricular ejection fraction >0.35 and <0.50). Similar findings were reported from a community-based study in Maastricht, the Netherlands (15, 53). Among 200 cases of sudden cardiac death with an assessment of left ventricular function available, 101 (51%) had normal left ventricular ejection fraction, defined as >0.50, and 38 (19%) had severely reduced left ventricular ejection fraction, defined as ≤0.30. These findings indicate that while severe left ventricular dysfunction remains a valuable contributor, there is an acute need identify clinical and non-clinical predictors that could enhance the process of risk stratification. What follows is a discussion of some predictors that have potential for enhancing risk stratification either independently or in combination with a diagnosis of severe left ventricular dysfunction.
Figure 6.
Severe LV dysfunction, currently the risk predictor most widely used in clinical practice, is likely to affect less than a third of all cases of sudden cardiac death in the general population.
Diabetes as a potential predictor of sudden cardiac death risk
The independent role of diabetes mellitus in enhancing risk of sudden cardiac death has been investigated in a small number of studies. However, all have consistently identified diabetes as a strong predictor of sudden cardiac death. An analysis was performed among >6,000 middle-aged, healthy male Parisian civil servants who were enrolled in the Paris Prospective Study and followed for over 23 years (39). There were a total of 120 sudden cardiac death cases and separately, there occurred 192 non-sudden death that were related to acute myocardial infarction. In a multivariate analysis, diabetes independently conferred a significant risk for sudden cardiac death (Relative Risk 2.2) controlling for all other variables (age, body mass index, tobacco use, history, heart rate, systolic pressure, cholesterol and triglyceride levels) (39, 54). The US Nurses Study and the Physicians Health Study (41, 55) as well as a retrospective clinical database analysis from a health cooperative in Seattle (56) have reported similar findings. While these findings clearly implicate diabetes as an important factor in pathogenesis of sudden cardiac death, the relationship has yet to be evaluated in prospective, community-wide studies of sudden cardiac death. While little is known about the specific ways in which diabetes-related mechanisms contribute to the pathogenesis of sudden cardiac death, several mechanisms have been postulated. Diabetes increases risk of coronary artery disease, a condition that is commonly found in association with sudden cardiac death. However, there may be diabetes-specific accelerated forms of atherosclerosis with enhanced thrombogenicity (57). In the literature, a case is being made for the existence of a distinct form of cardiac dysfunction that has been termed “diabetic cardiomyopathy” (58, 59). A universal finding among diabetics is a high prevalence of abnormal prolongation of the corrected QT (QTc) interval from the ECG (60). Earlier clinical studies of diabetics have also reported a good correlation between prolonged QTc interval and overall cardiac mortality (61, 62). Several studies have found a significant association between diabetic autonomic dysfunction and prolongation of the QTc interval (63–66). Given the recently confirmed status of prolonged QTc interval as a marker of sudden cardiac death risk in a large, community-based cohort (described below), this parameter has potential for increased significance among diabetics.
Prolonged ventricular repolarization and risk of sudden cardiac death
The relatively rare monogenic, long QT syndromes have long constituted a human model for the causative association between prolonged repolarization and increased risk of sudden cardiac death (67–70). However the Rotterdam study reported that QTc interval was independently associated with sudden cardiac death even in a cohort of unrelated individuals (71). In a cohort of 6,693 patients followed for two years patients without evidence of cardiac dysfunction and QTc >440 ms had a 2.3-fold higher risk of sudden cardiac death compared to those with QTc interval <440 ms. This association was independent of age, gender, history of myocardial infarction (MI), heart rate and drug use. A more recent analysis from the same cohort reports that prolonged QTc interval was an independent risk factor for sudden cardiac death in older adults followed for 6.7 years (72, 73). QTc interval was also found to be a predictor of increased overall cardiovascular morbidity and mortality in several cohort studies (74–78). There is significant potential for prolonged cardiac repolarization to enhance risk stratification for sudden cardiac death among unrelated individuals in the general population and it clearly merits further evaluation.
Socioeconomic status and risk of sudden cardiac death
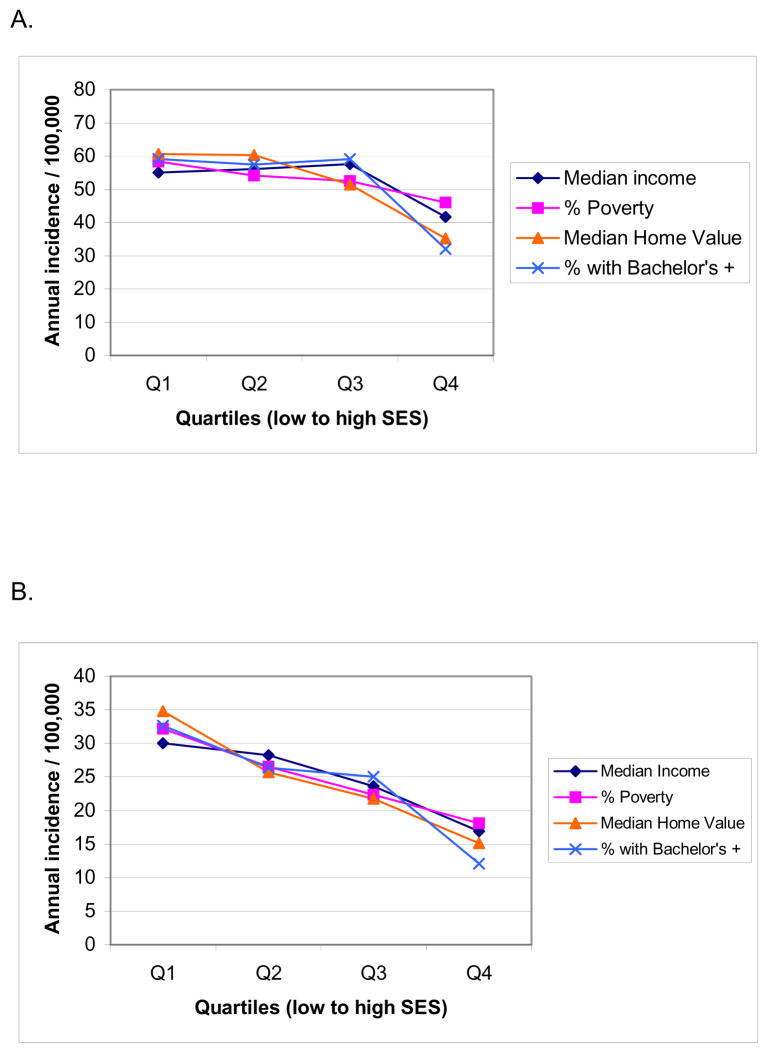
Given the relatively common association between poverty and increased prevalence of human disease conditions, socioeconomic factors are likely to have significant effects on incidence of sudden cardiac death (79–81). Until recently this association had not been evaluated in a prospective, community-wide study. There are some existing analyses of primary cardiac arrest cases, but evaluation was mostly limited to subjects that underwent resuscitation (82). As a result, the 40–50% of overall sudden cardiac death cases that are un-witnessed or do not undergo attempted resuscitation may not have been included in most existing analyses. In the ongoing Oregon Sudden Unexpected Death Study (5), a two-year prospective evaluation of the potential relationship between socioeconomic status and occurrence of sudden cardiac death was performed, based both on address of residence as well as specific geographic location of cardiac arrest (83). Analysis was conducted for both witnessed and un-witnessed sudden cardiac death cases. In this investigation of all cases of sudden cardiac death in a large urban and suburban U.S. county (Population 670,000), incidence of sudden cardiac death based on address of residence was 30% to 80% higher among residents of neighborhoods in the lowest socioeconomic status quartile compared to neighborhoods in the highest socioeconomic status quartile. The gradient of socioeconomic status was steeper for patients under age 65 years vs. over 65 years (Figure 7). Identical and significant effects were observed based on geographic location of sudden cardiac death. In the long term, there are likely to be multiple factors that result in the observed association between socioeconomic status and sudden cardiac death and these merit further evaluation. Risk factors for coronary artery disease such as lack of physical activity, smoking, hyperlipidemia, hypertension, obesity, and DM are more common among individuals with lower socioeconomic status (81, 84, 85). A study conducted in the UK found that incidence of out-of-hospital sudden cardiac death was significantly higher in areas of socioeconomic deprivation, but the same was not true for overall coronary artery disease (86). A contributory role of psychosocial factors as direct triggers of ventricular arrhythmias and consequent sudden cardiac death has also been postulated (80). Given that automated external defibrillators are likely to have a significant beneficial impact on survival from out-of-hospital sudden cardiac arrest (87, 88), these findings would suggest that for deployment of automated external defibrillators in the community, both population density and neighborhood socioeconomic status should be taken into consideration.
Figure 7.
Annual incidence of sudden cardiac arrest based on address of residence in Multnomah County, in census tracts grouped by quartiles of socioeconomic status (SES), low SES to high SES. (A) All ages combined; (B) age 0—64 years; (C) age 65 years and older. Adapted from Reinier K, Stecker EC, Vickers C, Gunson K, Jui J, Chugh SS. Incidence of sudden cardiac arrest is higher in areas of low socioeconomic status: A prospective two year study in a large United States community. Resuscitation 2006;70(2):186–92. Reprinted with permission from Elsevier Ireland Ltd. ©2006.
Triggers of sudden cardiac arrest
The substrate-trigger hypothesis is a traditional and long-held view of fatal ventricular arrhythmogenesis that requires the existence of a substrate (e.g. coronary artery disease) and a trigger (an external factor believed to incite an acute cardiovascular instability in a subject), for occurrence of sudden cardiac arrest. With the rapid and ongoing advancement of tools such as sophisticated imaging techniques and microarray-based studies of genomic variation, our ability to identify the substrate will continue to improve. However triggers are dynamic events and often difficult to measure and quantify. Many have postulated that physical activity and emotional stress are triggers of sudden cardiac arrest and vigorous activity has been shown to be a trigger among men (55, 89–91), but there is a lack of studies performed in community-based settings. Recently, the role of physical activity as a potential trigger of sudden cardiac arrest was evaluated in detail in the ongoing community-wide Oregon Sudden Unexpected Death Study (92). Over 300 subjects who suffered a sudden cardiac arrest were analyzed for level of physical activity just prior to the occurrence of cardiac arrest. A pre-defined estimated metabolic equivalent (MET) score was assigned to each type of physical activity which was classified into five groups: sleep, light activity, moderate activity, heavy activity and sexual activity. A total of 51 (17%) subjects were sleeping, 193 (63%) were performing light activities, 39 (13%) were performing moderate activities, 14 (5%) were performing heavy activities, and 7 (2%) were engaged in sexual activity prior to sudden cardiac arrest. The researchers concluded from these findings that the vast majority (80%) of the adult subjects that suffered sudden cardiac arrest were performing light physical activity or were asleep at the time of the event. Therefore physical activity may have been a trigger in only a small minority of individuals in this study. While the identification of triggers remains important, it is likely that improved identification of the substrate is likely to contribute more to enhancement of sudden cardiac arrest risk stratification. Future studies of sudden cardiac arrest triggers should focus more on the potential role of emotional or behavioral stressors as well as sleep-related disorders.
Potential role of genetic factors in predicting risk of sudden cardiac death
Two large retrospective cohort studies have provided evidence that genetic factors contribute to risk of sudden cardiac death. The potential association between sudden cardiac death and history of sudden cardiac death or coronary artery disease in a first degree relative was analyzed in a cohort of men and women attended by first responders in King County, Washington (235 cases, 374 controls) (93). A separate analysis from the Paris Prospective Study was performed in a cohort of 7,746 asymptomatic middle aged males followed for a mean of 23 years, classifying cardiac deaths as either sudden deaths or non-sudden with MI (39). Multivariate analyses indicated that the occurrence of sudden cardiac death in a parent or first degree relative results in a 1.6–1.8 fold increase in sudden cardiac death susceptibility after controlling for conventional risk factors for coronary artery disease. In a very limited number of cases in the Paris study, where there was a history of both maternal and paternal sudden cardiac death events (n=19), the offspring had a 9-fold increased risk of sudden cardiac death. Familial incidence of sudden cardiac death in the Paris study segregated independently of familial incidence of death due to acute MI. These studies provide evidence of a significant genetic contribution to sudden cardiac death and the next logical step is to identify specific gene defects that could be used for screening and identification of the high-risk patient. Significant advances are continuously being made in gene-finding technology, but some basic issues will need to be resolved before we can perform a search for candidate genes (9). Most patients that suffer sudden cardiac death have multiple associated co-morbidities such as coronary artery disease, DM, obesity and heart failure, each one of which may have genetic risk that could be unrelated to the genetic risk of sudden cardiac death (Figure 8). Therefore genes that contribute to sudden cardiac death occurrence have to be separated from genes that lead to associated conditions. Secondly, even for the so-called “monogenic syndromes” such as the long QT syndrome, there may be additional modifier genes that are involved (50, 94). For the complex phenotype of overall sudden cardiac death, it is quite likely that for an individual patient, screening may have to be conducted for a panel of genes instead of a single gene. From a methodological standpoint, approaches that use a very high-resolution map of single nucleotide polymorphisms (SNPs) (95) to search for associations, linkage disequilibrium or a small shared genomic segment among affected individuals, are likely to be necessary (9). While the search for sudden cardiac death genes had not presently employed community-wide approaches, these studies are likely to be conducted in the very near future (9).
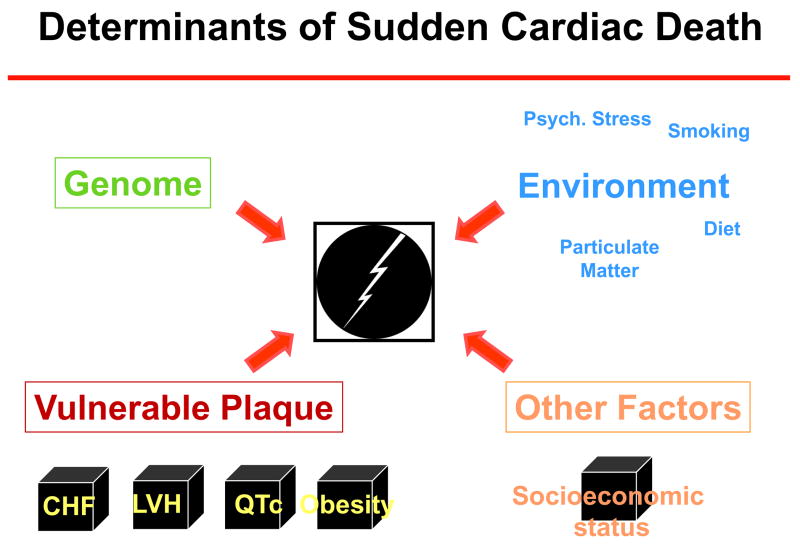
Figure 8.
Sudden cardiac death is a complex phenotype and determinants are likely to be multi-factorial. There appears to be a significant genetic component which has to considered in the context of multiple cardiac conditions, co-morbidities as well as epidemiologic and environmental factors.
Clinical and Research Implications
The current annual incidence of sudden cardiac death in the US is likely to be in the range of 180–250,000 per year. An estimate for global annual incidence of sudden cardiac death would be in the range of 4–5 million cases per year. Coinciding with the decreased mortality from coronary artery disease, there is evidence pointing toward a significant decrease in rates of sudden cardiac death in the US during the second half of the twentieth century. This has likely resulted from a strong focus on prevention of mortality from coronary disease with modalities such as aspirin, coronary interventions, diet modification and lifestyle changes. However the alarming rise in prevalence of obesity and diabetes in the first decade of the new millennium both in the US and worldwide, would indicate that this favorable trend is unlikely to persist. We are likely to witness a resurgence of coronary artery disease and heart failure, as a result of which sudden cardiac death will have to be confronted as a shared and indiscriminate, worldwide public health problem. In coming years, as incidence of ventricular fibrillation among cases of sudden cardiac arrest continues to fall and pulseless electrical activity continues to rise, advances in resuscitation efforts are likely to focus on mechanisms and treatment of pulseless electrical activity. The important goal of preventing sudden cardiac death however will be heavily dependent on enhancement of risk stratification techniques. Therefore discovery of novel risk stratification markers and methods has become the top priority in the field of sudden cardiac arrest investigation. We are also learning that while severe left ventricular dysfunction is a useful predictor for a subgroup of patients who will have future sudden cardiac death, the specificity of this predictor is significantly lower than anticipated and that we must extend our search beyond the ejection fraction. Among the strategies previously used to identify predictors of sudden cardiac death, there has been a significant lack of community-wide analyses. As illustrated by the findings related to severe left ventricular dysfunction in the Portland, Oregon USA and Maastricht, the Netherlands community-wide studies, this trend will have to be reversed. Studies that employ hospital and clinic based ascertainment of sudden cardiac death patients will still have a role, but meaningful predictors of sudden cardiac death are most likely to be discovered by prospective, community wide investigations. There are several clinical and non-clinical predictors that have already shown promise in longitudinal cohort or community-wide studies. Specific examples from relatively recent studies are a diagnosis of diabetes mellitus, prolonged ventricular repolarization (QTc interval) on the ECG and low socioeconomic status. However sudden cardiac arrest in patients with coronary artery disease that was previously silent still constitutes the large and hidden portion of the sudden cardiac death iceberg (Figure 4). Factors that determine rupture of the vulnerable plaque have been the target of intensive ongoing investigation and are likely to still have the highest yield for determinants of sudden cardiac arrest. It is clear that genetic factors play a role in the occurrence of sudden cardiac death and rapid advancements in the field of gene finding technology indicate that availability of potential genetic predictors is imminent. For risk stratification of sudden cardiac death to be comprehensive, factors as diverse as genomics and socioeconomic status may have to be taken in consideration (Figure 8). All of these predictors as well as those discovered in the future will require validation in multiple and diverse groups of individuals in order to ensure their relevance across populations. This will only be accomplished by the assembly of research consortiums that bring together archives of DNA, serum and tissue linked to well-phenotyped databases. Triggers of sudden cardiac death are dynamic and elusive, and investigation of these will require a shift in focus toward less quantifiable entities such as psychosocial factors and sleep abnormalities. The ultimate goal of preventing sudden cardiac death is likely to be attained largely by a continued, focused refinement of complex interactions between substrate (whether phenotype or genotype) and clinical manifestations, particularly in the context of the general population.
Acknowledgments
Funded by National Heart Lung and Blood Institute R01HL088416 and a Hopkins-Reynolds Clinical Cardiovascular Research Center Grant to Dr Sumeet S. Chugh.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349(9064):1498–504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 2.Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349(9063):1436–42. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 3.Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349(9061):1269–76. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 4.Okrainec K, Banerjee DK, Eisenberg MJ. Coronary artery disease in the developing world. Am Heart J. 2004;148(1):7–15. doi: 10.1016/j.ahj.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 5.Chugh SS, Jui J, Gunson K, Stecker EC, John BT, Thompson B, Ilias N, Vickers C, Dogra V, Daya M, Kron J, Zheng ZJ, Mensah G, McAnulty J. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Coll Cardiol. 2004;44(6):1268–75. doi: 10.1016/j.jacc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 6.Myerburg RJ. Scientific gaps in the prediction and prevention of sudden cardiac death. J Cardiovasc Electrophysiol. 2002;13(7):709–23. doi: 10.1046/j.1540-8167.2002.00709.x. [DOI] [PubMed] [Google Scholar]
- 7.Report of a Working Group on Ischaemic Heart Disease Registers. Euro 5010 1969. Copenhagen: 1969. Parts I and II. Regional Office for Europe WHO. [Google Scholar]
- 8.Myerburg RJ, Castellanos A. Cardiac Arrest and Sudden Cardiac Death. In: Zipes DP, Libby P, Bonow RO, Braunwald E, editors. Braunwald’s Heart Disease. A Textbook of Cardiovascular Medicine. Philadelphia: Elsevier Saunders; 2005. pp. 865–908. [Google Scholar]
- 9.Arking DE, Chugh SS, Chakravarti A, Spooner PM. Genomics in sudden cardiac death. Circ Res. 2004;94(6):712–23. doi: 10.1161/01.RES.0000123861.16082.95. [DOI] [PubMed] [Google Scholar]
- 10.Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104(18):2158–63. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 11.Cobb LA, Fahrenbruch CE, Olsufka M, Copass MK. Changing incidence of out-of-hospital ventricular fibrillation, 1980–2000. Jama. 2002;288(23):3008–13. doi: 10.1001/jama.288.23.3008. [DOI] [PubMed] [Google Scholar]
- 12.Kass LE, Eitel DR, Sabulsky NK, Ogden CS, Hess DR, Peters KL. One-year survival after prehospital cardiac arrest: the Utstein style applied to a rural-suburban system. Am J Emerg Med. 1994;12(1):17–20. doi: 10.1016/0735-6757(94)90190-2. [DOI] [PubMed] [Google Scholar]
- 13.Lombardi G, Gallagher J, Gennis P. Outcome of out-of-hospital cardiac arrest in New York City. The Pre-Hospital Arrest Survival Evaluation (PHASE) Study. Jama. 1994;271(9):678–83. [PubMed] [Google Scholar]
- 14.Westal RE, Reissman S, Doering G. Out-of-hospital cardiac arrests: an 8-year New York City experience. Am J Emerg Med. 1996;14(4):364–8. doi: 10.1016/S0735-6757(96)90050-9. [DOI] [PubMed] [Google Scholar]
- 15.de Vreede-Swagemakers JJ, Gorgels AP, Dubois-Arbouw WI, van Ree JW, Daemen MJ, Houben LG, Wellens HJ. Out-of-hospital cardiac arrest in the 1990’s: a population-based study in the Maastricht area on incidence, characteristics and survival. J Am Coll Cardiol. 1997;30(6):1500–5. doi: 10.1016/s0735-1097(97)00355-0. [DOI] [PubMed] [Google Scholar]
- 16.Tokashiki T, Muratani A, Kimura Y, Muratani H, Fukiyama K. Sudden death in the general population in Okinawa: incidence and causes of death. Jpn Circ J. 1999;63(1):37–42. doi: 10.1253/jcj.63.37. [DOI] [PubMed] [Google Scholar]
- 17.Stecker EC, Vickers C, Waltz J, Socoteanu C, John BT, Mariani R, McAnulty JH, Gunson K, Jui J, Chugh SS. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol. 2006;47(6):1161–6. doi: 10.1016/j.jacc.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 18.Byrne R, Constant O, Smyth Y, Callagy G, Nash P, Daly K, Crowley J. Multiple source surveillance incidence and aetiology of out-of-hospital sudden cardiac death in a rural population in the West of Ireland. Eur Heart J. 2008 doi: 10.1093/eurheartj/ehn155. [DOI] [PubMed] [Google Scholar]
- 19.Vaillancourt C, Stiell IG. Cardiac arrest care and emergency medical services in Canada. Can J Cardiol. 2004;20(11):1081–90. [PubMed] [Google Scholar]
- 20.Gerber Y, Jacobsen SJ, Frye RL, Weston SA, Killian JM, Roger VL. Secular trends in deaths from cardiovascular diseases: a 25-year community study. Circulation. 2006;113(19):2285–92. doi: 10.1161/CIRCULATIONAHA.105.590463. [DOI] [PubMed] [Google Scholar]
- 21.Chugh SS, Kelly KL, Titus JL. Sudden cardiac death with apparently normal heart. Circulation. 2000;102(6):649–54. doi: 10.1161/01.cir.102.6.649. [DOI] [PubMed] [Google Scholar]
- 22.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345(20):1473–82. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 23.Behr E, Wood DA, Wright M, Syrris P, Sheppard MN, Casey A, Davies MJ, McKenna W. Cardiological assessment of first-degree relatives in sudden arrhythmic death syndrome. Lancet. 2003;362(9394):1457–9. doi: 10.1016/s0140-6736(03)14692-2. [DOI] [PubMed] [Google Scholar]
- 24.Wisten A, Forsberg H, Krantz P, Messner T. Sudden cardiac death in 15–35-year olds in Sweden during 1992–99. J Intern Med. 2002;252(6):529–36. doi: 10.1046/j.1365-2796.2002.01038.x. [DOI] [PubMed] [Google Scholar]
- 25.Chugh SS, Chung K, Zheng ZJ, John B, Titus JL. Cardiac pathologic findings reveal a high rate of sudden cardiac death of undetermined etiology in younger women. Am Heart J. 2003;146(4):635–9. doi: 10.1016/S0002-8703(03)00323-5. [DOI] [PubMed] [Google Scholar]
- 26.Eckart RE, Scoville SL, Shry EA, Potter RN, Tedrow U. Causes of sudden death in young female military recruits. Am J Cardiol. 2006;97(12):1756–8. doi: 10.1016/j.amjcard.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 27.Myerburg RJ, Castellanos A. Cardiac Arrest and Sudden Cardiac Death. In: Braunwald E, editor. Heart Disease: A Textbook of Cardiovascular Medicine. 5. Philadelphia: W. B. Saunders; 1997. pp. 742–79. [Google Scholar]
- 28.Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98(21):2334–51. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 29.Greene HL. Sudden arrhythmic cardiac death--mechanisms, resuscitation and classification: the Seattle perspective. Am J Cardiol. 1990;65(4):4B–12B. doi: 10.1016/0002-9149(90)91285-e. [DOI] [PubMed] [Google Scholar]
- 30.Bayes de Luna A, Coumel P, Leclercq JF. Ambulatory sudden cardiac death: mechanisms of production of fatal arrhythmia on the basis of data from 157 cases. Am Heart J. 1989;117(1):151–9. doi: 10.1016/0002-8703(89)90670-4. [DOI] [PubMed] [Google Scholar]
- 31.Cooper S, Cade J. Predicting survival, in-hospital cardiac arrests: resuscitation survival variables and training effectiveness. Resuscitation. 1997;35(1):17–22. doi: 10.1016/s0300-9572(97)00020-8. [DOI] [PubMed] [Google Scholar]
- 32.Cobb LA, Fahrenbruch CE, Walsh TR, Copass MK, Olsufka M, Breskin M, Hallstrom AP. Influence of cardiopulmonary resuscitation prior to defibrillation in patients with out-of-hospital ventricular fibrillation. Jama. 1999;281(13):1182–8. doi: 10.1001/jama.281.13.1182. [DOI] [PubMed] [Google Scholar]
- 33.Dorian P, Cass D, Schwartz B, Cooper R, Gelaznikas R, Barr A. Amiodarone as compared with lidocaine for shock-resistant ventricular fibrillation. N Engl J Med. 2002;346(12):884–90. doi: 10.1056/NEJMoa013029. [DOI] [PubMed] [Google Scholar]
- 34.Herlitz J, Andersson E, Bang A, Engdahl J, Holmberg M, lindqvist J, Karlson BW, Waagstein L. Experiences from treatment of out-of-hospital cardiac arrest during 17 years in Goteborg. Eur Heart J. 2000;21(15):1251–8. doi: 10.1053/euhj.2000.2150. [DOI] [PubMed] [Google Scholar]
- 35.Parish DC, Dinesh Chandra KM, Dane FC. Success changes the problem: why ventricular fibrillation is declining, why pulseless electrical activity is emerging, and what to do about it. Resuscitation. 2003;58(1):31–5. doi: 10.1016/s0300-9572(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 36.Fox CS, Evans JC, Larson MG, Kannel WB, Levy D. Temporal trends in coronary heart disease mortality and sudden cardiac death from 1950 to 1999: the Framingham Heart Study. Circulation. 2004;110(5):522–7. doi: 10.1161/01.CIR.0000136993.34344.41. [DOI] [PubMed] [Google Scholar]
- 37.Bardy GH, Lee KL, Mark DB, Poole JE, Toff WD, Tonkin AM, Smith W, Dorian P, Packer DL, White RD, Longstreth WT, Jr, Anderson J, Johnson G, Bischoff E, Yallop JJ, McNulty S, Ray LD, Clapp-Channing NE, Rosenberg Y, Schron EB. Home use of automated external defibrillators for sudden cardiac arrest. N Engl J Med. 2008;358(17):1793–804. doi: 10.1056/NEJMoa0801651. [DOI] [PubMed] [Google Scholar]
- 38.Kannel WB, Cupples LA, D’Agostino RB. Sudden death risk in overt coronary heart disease: the Framingham Study. Am Heart J. 1987;113(3):799–804. doi: 10.1016/0002-8703(87)90722-8. [DOI] [PubMed] [Google Scholar]
- 39.Jouven X, Desnos M, Guerot C, Ducimetiere P. Predicting sudden death in the population: the Paris Prospective Study I. Circulation. 1999;99(15):1978–83. doi: 10.1161/01.cir.99.15.1978. [DOI] [PubMed] [Google Scholar]
- 40.Thorgeirsson G, Thorgeirsson G, Sigvaldason H, Witteman J. Risk factors for out-of-hospital cardiac arrest: the Reykjavik Study. Eur Heart J. 2005;26(15):1499–505. doi: 10.1093/eurheartj/ehi179. [DOI] [PubMed] [Google Scholar]
- 41.Albert CM, Chae CU, Grodstein F, Rose LM, Rexrode KM, Ruskin JN, Stampfer MJ, Manson JE. Prospective study of sudden cardiac death among women in the United States. Circulation. 2003;107(16):2096–101. doi: 10.1161/01.CIR.0000065223.21530.11. [DOI] [PubMed] [Google Scholar]
- 42.Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006;47(8 Suppl):C7–12. doi: 10.1016/j.jacc.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 43.Benchimol D, Dubroca B, Bernard V, Lavie J, Paviot B, Benchimol H, Couffinhal T, Pillois X, Dartigues J, Bonnet J. Short- and long-term risk factors for sudden death in patients with stable angina. Int J Cardiol. 2000;76(2–3):147–56. doi: 10.1016/s0167-5273(00)00370-3. [DOI] [PubMed] [Google Scholar]
- 44.Hellings WE, Peeters W, Moll FL, Pasterkamp G. From vulnerable plaque to vulnerable patient: the search for biomarkers of plaque destabilization. Trends Cardiovasc Med. 2007;17(5):162–71. doi: 10.1016/j.tcm.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 45.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, Fayad Z, Stone PH, Waxman S, Raggi P, Madjid M, Zarrabi A, Burke A, Yuan C, Fitzgerald PJ, Siscovick DS, de Korte CL, Aikawa M, Airaksinen KE, Assmann G, Becker CR, Chesebro JH, Farb A, Galis ZS, Jackson C, Jang IK, Koenig W, Lodder RA, March K, Demirovic J, Navab M, Priori SG, Rekhter MD, Bahr R, Grundy SM, Mehran R, Colombo A, Boerwinkle E, Ballantyne C, Insull W, Jr, Schwartz RS, Vogel R, Serruys PW, Hansson GK, Faxon DP, Kaul S, Drexler H, Greenland P, Muller JE, Virmani R, Ridker PM, Zipes DP, Shah PK, Willerson JT. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part II. Circulation. 2003;108(15):1772–8. doi: 10.1161/01.CIR.0000087481.55887.C9. [DOI] [PubMed] [Google Scholar]
- 46.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, Fayad Z, Stone PH, Waxman S, Raggi P, Madjid M, Zarrabi A, Burke A, Yuan C, Fitzgerald PJ, Siscovick DS, de Korte CL, Aikawa M, Juhani Airaksinen KE, Assmann G, Becker CR, Chesebro JH, Farb A, Galis ZS, Jackson C, Jang IK, Koenig W, Lodder RA, March K, Demirovic J, Navab M, Priori SG, Rekhter MD, Bahr R, Grundy SM, Mehran R, Colombo A, Boerwinkle E, Ballantyne C, Insull W, Jr, Schwartz RS, Vogel R, Serruys PW, Hansson GK, Faxon DP, Kaul S, Drexler H, Greenland P, Muller JE, Virmani R, Ridker PM, Zipes DP, Shah PK, Willerson JT. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108(14):1664–72. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 47.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 48.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341(25):1882–90. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 49.Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335(26):1933–40. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 50.Benhorin J, Moss AJ, Bak M, Zareba W, Kaufman ES, Kerem B, Towbin JA, Priori S, Kass RS, Attali B, Brown AM, Ficker E. Variable expression of long QT syndrome among gene carriers from families with five different HERG mutations. Ann Noninvasive Electrocardiol. 2002;7(1):40–6. doi: 10.1111/j.1542-474X.2001.tb00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 52.Myerburg RJ, Mitrani R, Interian A, Jr, Castellanos A. Interpretation of outcomes of antiarrhythmic clinical trials: design features and population impact. Circulation. 1998;97(15):1514–21. doi: 10.1161/01.cir.97.15.1514. [DOI] [PubMed] [Google Scholar]
- 53.Gorgels AP, Gijsbers C, de Vreede-Swagemakers J, Lousberg A, Wellens HJ. Out-of-hospital cardiac arrest--the relevance of heart failure. The Maastricht Circulatory Arrest Registry. Eur Heart J. 2003;24(13):1204–9. doi: 10.1016/s0195-668x(03)00191-x. [DOI] [PubMed] [Google Scholar]
- 54.Balkau B, Jouven X, Ducimetiere P, Eschwege E. Diabetes as a risk factor for sudden death. Lancet. 1999;354(9194):1968–9. doi: 10.1016/S0140-6736(99)04383-4. [DOI] [PubMed] [Google Scholar]
- 55.Albert CM, Mittleman MA, Chae CU, Lee IM, Hennekens CH, Manson JE. Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med. 2000;343(19):1355–61. doi: 10.1056/NEJM200011093431902. [DOI] [PubMed] [Google Scholar]
- 56.Jouven X, Lemaitre RN, Rea TD, Sotoodehnia N, Empana JP, Siscovick DS. Diabetes, glucose level, and risk of sudden cardiac death. Eur Heart J. 2005;26(20):2142–7. doi: 10.1093/eurheartj/ehi376. [DOI] [PubMed] [Google Scholar]
- 57.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. Jama. 2002;287(19):2570–81. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 58.Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev. 2004;25(4):543–67. doi: 10.1210/er.2003-0012. [DOI] [PubMed] [Google Scholar]
- 59.Govind S, Saha S, Brodin LA, Ramesh SS, Arvind SR, Quintana M. Impaired Myocardial Functional Reserve in Hypertension and Diabetes Mellitus Without Coronary Artery Disease: Searching for the Possible Link With Congestive Heart Failure in the Myocardial Doppler in Diabetes (MYDID) Study II. Am J Hypertens. 2006;19(8):851–7. doi: 10.1016/j.amjhyper.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 60.Veglio M, Borra M, Stevens LK, Fuller JH, Perin PC. The relation between QTc interval prolongation and diabetic complications. The EURODIAB IDDM Complication Study Group. Diabetologia. 1999;42(1):68–75. doi: 10.1007/s001250051115. [DOI] [PubMed] [Google Scholar]
- 61.Cardoso CR, Salles GF, Deccache W. QTc interval prolongation is a predictor of future strokes in patients with type 2 diabetes mellitus. Stroke. 2003;34(9):2187–94. doi: 10.1161/01.STR.0000085084.15144.66. [DOI] [PubMed] [Google Scholar]
- 62.Rana BS, Lim PO, Naas AA, Ogston SA, Newton RW, Jung RT, Morris AD, Struthers AD. QT interval abnormalities are often present at diagnosis in diabetes and are better predictors of cardiac death than ankle brachial pressure index and autonomic function tests. Heart. 2005;91(1):44–50. doi: 10.1136/hrt.2003.017632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lloyd-Mostyn RH, Watkins PJ. Defective innervation of heart in diabetic autonomic neuropathy. Br Med J. 1975;3(5974):15–7. doi: 10.1136/bmj.3.5974.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nesto RW. Correlation between cardiovascular disease and diabetes mellitus: current concepts. Am J Med. 2004;116(Suppl 5A):11S–22S. doi: 10.1016/j.amjmed.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 65.Pourmoghaddas A, Hekmatnia A. The relationship between QTc interval and cardiac autonomic neuropathy in diabetes mellitus. Mol Cell Biochem. 2003;249(1–2):125–8. [PubMed] [Google Scholar]
- 66.Veglio M, Chinaglia A, Borra M, Perin PC. Does abnormal QT interval prolongation reflect autonomic dysfunction in diabetic patients? QTc interval measure versus standardized tests in diabetic autonomic neuropathy. Diabet Med. 1995;12(4):302–6. doi: 10.1111/j.1464-5491.1995.tb00481.x. [DOI] [PubMed] [Google Scholar]
- 67.Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80(5):795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 68.Keating MT, Sanguinetti MC. Molecular and cellular mechanisms of cardiac arrhythmias. Cell. 2001;104(4):569–80. doi: 10.1016/s0092-8674(01)00243-4. [DOI] [PubMed] [Google Scholar]
- 69.Moss AJ, Schwartz PJ, Crampton RS, Tzivoni D, Locati EH, MacCluer J, Hall WJ, Weitkamp L, Vincent GM, Garson A, Jr, et al. The long QT syndrome. Prospective longitudinal study of 328 families. Circulation. 1991;84(3):1136–44. doi: 10.1161/01.cir.84.3.1136. [DOI] [PubMed] [Google Scholar]
- 70.Splawski I, Tristani-Firouzi M, Lehmann MH, Sanguinetti MC, Keating MT. Mutations in the hminK gene cause long QT syndrome and suppress IKs function. Nat Genet. 1997;17(3):338–40. doi: 10.1038/ng1197-338. [DOI] [PubMed] [Google Scholar]
- 71.Algra A, Tijssen JG, Roelandt JR, Pool J, Lubsen J. QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation. 1991;83(6):1888–94. doi: 10.1161/01.cir.83.6.1888. [DOI] [PubMed] [Google Scholar]
- 72.Goldenberg I, Moss AJ, Zareba W. QT interval: how to measure it and what is “normal”. J Cardiovasc Electrophysiol. 2006;17(3):333–6. doi: 10.1111/j.1540-8167.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- 73.Straus SM, Kors JA, De Bruin ML, van der Hooft CS, Hofman A, Heeringa J, Deckers JW, Kingma JH, Sturkenboom MC, Stricker BH, Witteman JC. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47(2):362–7. doi: 10.1016/j.jacc.2005.08.067. [DOI] [PubMed] [Google Scholar]
- 74.Dekker JM, Schouten EG, Klootwijk P, Pool J, Kromhout D. Association between QT interval and coronary heart disease in middle-aged and elderly men. The Zutphen Study. Circulation. 1994;90(2):779–85. doi: 10.1161/01.cir.90.2.779. [DOI] [PubMed] [Google Scholar]
- 75.Elming H, Holm E, Jun L, Torp-Pedersen C, Kober L, Kircshoff M, Malik M, Camm J. The prognostic value of the QT interval and QT interval dispersion in all-cause and cardiac mortality and morbidity in a population of Danish citizens. Eur Heart J. 1998;19(9):1391–400. doi: 10.1053/euhj.1998.1094. [DOI] [PubMed] [Google Scholar]
- 76.Goldberg RJ, Bengtson J, Chen ZY, Anderson KM, Locati E, Levy D. Duration of the QT interval and total and cardiovascular mortality in healthy persons (The Framingham Heart Study experience) Am J Cardiol. 1991;67(1):55–8. doi: 10.1016/0002-9149(91)90099-7. [DOI] [PubMed] [Google Scholar]
- 77.Karjalainen J, Reunanen A, Ristola P, Viitasalo M. QT interval as a cardiac risk factor in a middle aged population. Heart. 1997;77(6):543–8. doi: 10.1136/hrt.77.6.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schouten EG, Dekker JM, Meppelink P, Kok FJ, Vandenbroucke JP, Pool J. QT interval prolongation predicts cardiovascular mortality in an apparently healthy population. Circulation. 1991;84(4):1516–23. doi: 10.1161/01.cir.84.4.1516. [DOI] [PubMed] [Google Scholar]
- 79.Escobedo LG, Zack MM. Comparison of sudden and nonsudden coronary deaths in the United States. Circulation. 1996;93(11):2033–6. doi: 10.1161/01.cir.93.11.2033. [DOI] [PubMed] [Google Scholar]
- 80.Hemingway H, Malik M, Marmot M. Social and psychosocial influences on sudden cardiac death, ventricular arrhythmia and cardiac autonomic function. Eur Heart J. 2001;22(13):1082–101. doi: 10.1053/euhj.2000.2534. [DOI] [PubMed] [Google Scholar]
- 81.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111(10):1233–41. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 82.Feero S, Hedges JR, Stevens P. Demographics of cardiac arrest: association with residence in a low-income area. Acad Emerg Med. 1995;2(1):11–6. doi: 10.1111/j.1553-2712.1995.tb03071.x. [DOI] [PubMed] [Google Scholar]
- 83.Reinier K, Stecker EC, Vickers C, Gunson K, Jui J, Chugh SS. Incidence of sudden cardiac arrest is higher in areas of low socioeconomic status: A prospective two year study in a large United States community. Resuscitation. 2006;70(2):186–92. doi: 10.1016/j.resuscitation.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 84.Jaglal SB, Goel V. Social inequity in risk of coronary artery disease in Ontario. Can J Cardiol. 1994;10(4):439–43. [PubMed] [Google Scholar]
- 85.Rozanski A, Blumenthal JA, Davidson KW, Saab PG, Kubzansky L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. J Am Coll Cardiol. 2005;45(5):637–51. doi: 10.1016/j.jacc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 86.Soo L, Huff N, Gray D, Hampton JR. Geographical distribution of cardiac arrest in Nottinghamshire. Resuscitation. 2001;48(2):137–47. doi: 10.1016/s0300-9572(00)00248-3. [DOI] [PubMed] [Google Scholar]
- 87.Hallstrom AP, Ornato JP, Weisfeldt M, Travers A, Christenson J, McBurnie MA, Zalenski R, Becker LB, Schron EB, Proschan M. Public-access defibrillation and survival after out-of-hospital cardiac arrest. N Engl J Med. 2004;351(7):637–46. doi: 10.1056/NEJMoa040566. [DOI] [PubMed] [Google Scholar]
- 88.Hazinski MF, Idris AH, Kerber RE, Epstein A, Atkins D, Tang W, Lurie K. Lay rescuer automated external defibrillator (“public access defibrillation”) programs: lessons learned from an international multicenter trial: advisory statement from the American Heart Association Emergency Cardiovascular Committee; the Council on Cardiopulmonary, Perioperative, and Critical Care; and the Council on Clinical Cardiology. Circulation. 2005;111(24):3336–40. doi: 10.1161/CIRCULATIONAHA.105.165674. [DOI] [PubMed] [Google Scholar]
- 89.Siscovick DS, Weiss NS, Fletcher RH, Lasky T. The incidence of primary cardiac arrest during vigorous exercise. N Engl J Med. 1984;311(14):874–7. doi: 10.1056/NEJM198410043111402. [DOI] [PubMed] [Google Scholar]
- 90.Thompson PD, Funk EJ, Carleton RA, Sturner WQ. Incidence of death during jogging in Rhode Island from 1975 through 1980. Jama. 1982;247(18):2535–8. [PubMed] [Google Scholar]
- 91.Willich SN, Maclure M, Mittleman M, Arntz HR, Muller JE. Sudden cardiac death. Support for a role of triggering in causation. Circulation. 1993;87(5):1442–50. doi: 10.1161/01.cir.87.5.1442. [DOI] [PubMed] [Google Scholar]
- 92.Reddy PR, Reinier K, Singh T, Mariani R, Gunson K, Jui J, Chugh SS. Physical activity as a trigger of sudden cardiac arrest: The Oregon Sudden Unexpected Death Study. Int J Cardiol. 2008 doi: 10.1016/j.ijcard.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Friedlander Y, Siscovick DS, Weinmann S, Austin MA, Psaty BM, Lemaitre RN, Arbogast P, Raghunathan TE, Cobb LA. Family history as a risk factor for primary cardiac arrest. Circulation. 1998;97(2):155–60. doi: 10.1161/01.cir.97.2.155. [DOI] [PubMed] [Google Scholar]
- 94.Crotti L, Lundquist AL, Insolia R, Pedrazzini M, Ferrandi C, De Ferrari GM, Vicentini A, Yang P, Roden DM, George AL, Jr, Schwartz PJ. KCNH2-K897T is a genetic modifier of latent congenital long-QT syndrome. Circulation. 2005;112(9):1251–8. doi: 10.1161/CIRCULATIONAHA.105.549071. [DOI] [PubMed] [Google Scholar]
- 95.Lander ES. The new genomics: global views of biology. Science. 1996;274(5287):536–9. doi: 10.1126/science.274.5287.536. [DOI] [PubMed] [Google Scholar]