Summary
Myosin VI has been implicated in many cellular processes including endocytosis, secretion, membrane ruffling and cell motility. We carried out a yeast two-hybrid screen and identified TRAF6-binding protein (T6BP) and nuclear dot protein 52 (NDP52) as myosin VI binding partners. Myosin VI interaction with T6BP and NDP52 was confirmed in vitro and in vivo and the binding sites on each protein were accurately mapped. Immunofluorescence and electron microscopy showed that T6BP, NDP52 and myosin VI are present at the trans side of the Golgi complex, and on vesicles in the perinuclear region. Although the SKICH domain in T6BP and NDP52 does not mediate recruitment into membrane ruffles, loss of T6BP and NDP52 in RNAi knockdown cells results in reduced membrane ruffling activity and increased stress fibre and focal adhesion formation. Furthermore, we observed in these knockdown cells an upregulation of constitutive secretion of alkaline phosphatase, implying that both proteins act as negative regulators of secretory traffic at the Golgi complex. T6BP was also found to inhibit NF-κB activation, implicating it in the regulation of TRAF6-mediated cytokine signalling. Thus myosin VI-T6BP interactions may link membrane trafficking pathways with cell adhesion and cytokine-dependent cell signalling.
Keywords: NDP52, T6BP, Myosin VI
Introduction
Myosins are a superfamily of motor proteins found virtually throughout the eukaryota (Foth et al., 2006) and, harnessing the energy liberated by ATP hydrolysis, they move along the actin filament cytoskeleton and instigate a plethora of cellular processes ranging from cell migration to membrane traffic (Coureux et al., 2004; De La Cruz and Ostap, 2004; Tuxworth and Titus, 2000). Although their biophysical and kinetic properties have been intensively studied, they remain less well characterised at a cellular level and the molecular details of many of their various tasks remain to be elucidated.
The myosins in class VI are unique because unlike all the other classes of myosin, they move towards the minus end of actin filaments (Wells et al., 1999) and have thus been implicated in cellular processes, such as endocytosis, secretion and membrane ruffling (Buss et al., 1998; Buss et al., 2001; Warner et al., 2003; Aschenbrenner et al., 2003). There is also a growing body of literature linking myosin VI with cell migration and cancer progression (Dunn et al., 2006; Yoshida et al., 2004). An intriguing question is: how is myosin VI able to achieve such a multitude of functions? One way is by interacting with a wide variety of binding partners and the mechanisms behind these associations are slowly beginning to be understood (Buss et al., 2004). Binding partners, such as disabled homolog 2 (DAB2), GAIP-interacting protein C (GIPC) and the synapse-associated protein 97 (SAP97), link myosin VI to endocytosis and the transport of cargoes such as the transferrin receptor, the cystic fibrosis transmembrane conductance receptor and AMPA receptor (Buss et al., 2001; Morris et al., 2002; Wu et al., 2002; Aschenbrenner et al., 2003; Swiatecka-Urban et al., 2004). In the exocytic pathway, optineurin and myosin VI are found at the Golgi complex and the recycling endosome, where they play a role in the delivery of newly synthesised proteins to the plasma membrane (Sahlender et al., 2005; Au et al., 2007).
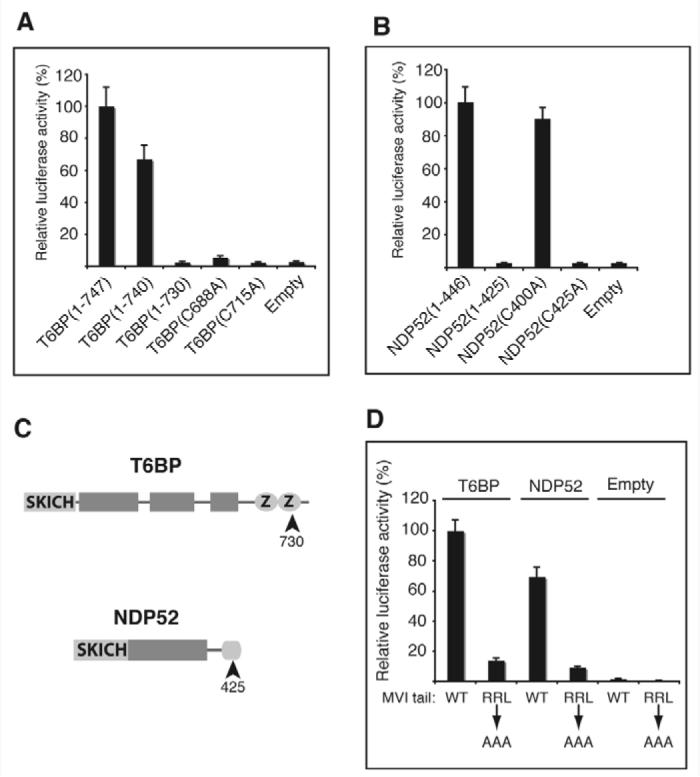
We have identified and characterised two new myosin VI binding partners: TRAF6-binding protein (T6BP) and nuclear dot protein 52 (NDP52). T6BP (also known as TAX1BP1 and TXBP151) is a 747-amino acid protein with three large coiled-coil regions, two C2H2-type zinc fingers at its C-terminus and a region at its N-terminus with homology to the recently defined skeletal muscle and kidney-enriched inositol phosphatase (SKIP) carboxyl homology (SKICH) domain (Gurung et al., 2003) (see cartoon in Fig. 2C). In SKIP this SKICH domain is required for its recruitment into membrane ruffles in COS 7 cells upon epidermal growth factor (EGF) stimulation (Gurung et al., 2003). T6BP was first identified as a target of the human T-cell leukaemia virus protein Tax (Gachon et al., 1998) and was shown to bind to A20, a negative regulator of NF-κB signalling with cell-line-specific anti-apoptotic properties (De Valck et al., 1999). T6BP also interacts with TNFα-receptor-associated factor 6 (TRAF6), a key regulator of NF-κB activation, in an interleukin-1 (IL1)-inducible manner, but a physiological role for this association was not established (Ling and Goeddel, 2000; Chung et al., 2002). NDP52 is a 446-amino acid protein with a high level of similarity to T6BP. Like T6BP, it consists of an N-terminal SKICH domain, an intervening region of coiled-coil, and a zinc finger arrangement at its C-terminus usually referred to as a LIM-like domain (see cartoon in Fig. 2C). NDP52 is clearly a cytosolic protein (Sternsdorf et al., 1997), although initial studies identified it as a protein present in nuclear dots (Korioth et al., 1995). It has latterly been shown to interact with human hepatocellular-carcinoma-associated protein 1 (HCAP1, also known as RNGTT) in COS 7 cells (Di et al., 2003) but its cellular function is not known.
Fig. 2.
Mapping the binding sites on T6BP, NDP52 and myosin VI. (A) The zinc fingers of T6BP bind to myosin VI. Expression plasmids encoding myosin VI tail constructs were used as bait and either T6BP truncation constructs or site-directed mutagenesis constructs as prey. (B) The LIM-like domain of NDP52 binds to myosin VI. Expression plasmids used were as in A but as prey either NDP52 truncation constructs or site-directed mutagenesis constructs were used. (C) T6BP and NDP52 cartoons showing the N-terminal SKICH domains, shaded boxes predicting regions of α-helical coiled coil and the zinc fingers (Z). (D) T6BP and NDP52 interact with the RRL motif in the myosin VI tail. Expression plasmids used were as in A except that those encoding myosin VI wild type (WT) and mutant (RRL to AAA) tail constructs were the bait and T6BP or NDP52 constructs were the prey.
In a yeast two-hybrid screen using the myosin VI tail as bait we identified T6BP and NDP52 as new binding partners for myosin VI. We confirmed that T6BP and NDP52 interact with myosin VI using a series of binding assays, and mapped their binding sites to single-residue accuracy. At the cellular level, we localised both proteins to cytoplasmic vesicles at the trans side of the Golgi complex, and both proteins colocalised with myosin VI on these vesicles in the perinuclear region of the cell. Interestingly T6BP and NDP52 were able to interact with one another as well as with myosin VI. Even though T6BP and NDP52 contain a SKICH domain, contrary to expectations, neither was recruited into membrane ruffles at the leading edge of the cell in response to EGF stimulation. However, functional RNA interference (RNAi) knockdown (KD) experiments show that both proteins modulate actin filament organisation. Loss of either T6BP or NDP52 leads to a decrease in the number of membrane ruffles at the plasma membrane and an increase in stable actin stress fibres and focal adhesions. Furthermore, both proteins appear to have a role in regulating the secretion of the enzyme alkaline phosphatase from the Golgi complex to the plasma membrane. T6BP was also shown to be a potent inhibitor of TRAF6-mediated NF-κB activation. These results suggest that myosin VI-T6BP-NDP52 complexes may play a role in coordinating cytokine signalling and membrane transport pathways with actin filament organisation and cell adhesion.
Results
Identification of T6BP and NDP52 as myosin VI binding partners
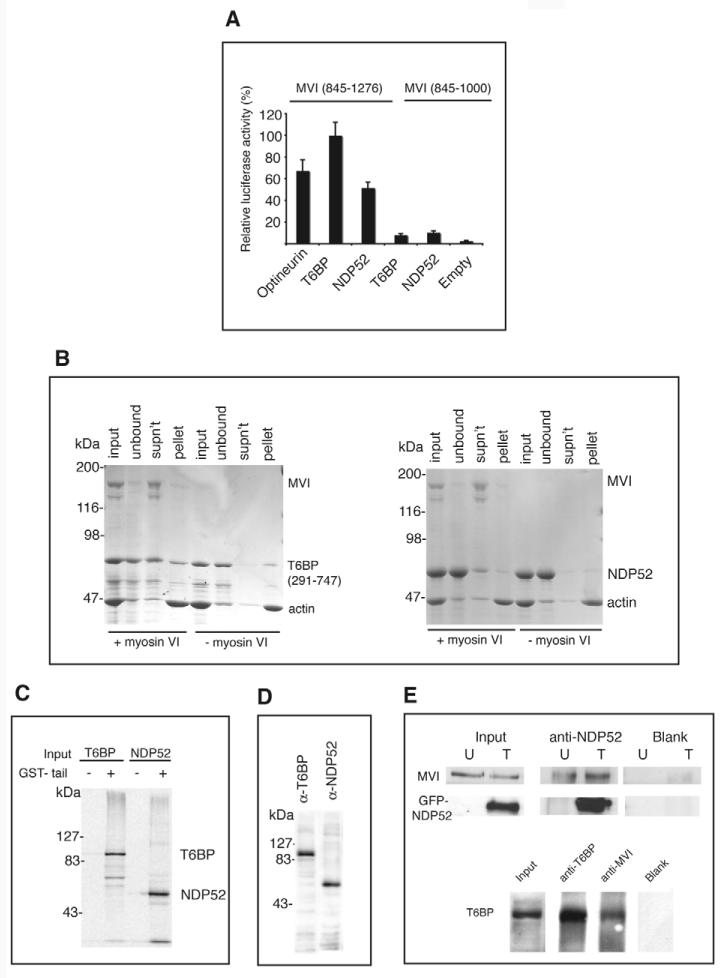
To identify novel binding partners for myosin VI, we used a yeast two-hybrid assay to screen a human endothelial cell cDNA library using the chicken myosin VI tail as bait. A C-terminal fragment of NDP52 (187 amino acids) and seven C-terminal fragments of T6BP ranging in size from 290 to 456 amino acids were identified. The full-length cDNAs of T6BP and NDP52 was amplified by PCR from a human liver cDNA library. To confirm that the two proteins interacted with myosin VI in a mammalian cellular environment, we carried out a series of two-hybrid assays in Chinese hamster ovary (CHO) cells (Fig. 1A). Both full-length proteins interacted with the tail domain (amino acids 845-1276) of myosin VI; the previously characterised binding partner optineurin was included as a positive control. The interactions were shown to be specific to the globular domain of the myosin VI tail, because a truncated construct (amino acids 845-1000) of the myosin VI tail did not bind to either T6BP or NDP52.
Fig. 1.
T6BP and NDP52 are myosin VI binding partners. (A) T6BP and NDP52 interact with the myosin VI C-terminal tail in the mammalian two-hybrid assay. Plasmids encoding myosin VI tail constructs were in the bait vector and T6BP or NDP52 were in the prey vector. (B) T6BP and NDP52 interact with myosin VI in an F-actin pelleting assay. Each binding partner was mixed with full-length myosin VI and then with F-actin (input). After centrifugation, to remove the unbound fraction (unbound), the actomyosin pellet was resuspended in salt-ATP solution to dissociate myosin VI from F-actin. A second centrifugation step partitioned myosin VI and T6BP/NDP52 into the supernatant (SN) and F-actin into the pellet (P). Control samples without myosin VI show that T6BP and NDP52 do not bind to F-actin. (C) NDP52 and full-length T6BP bind the myosin VI tail in a pull-down assay using GST-myosin VI tail and in-vitro-translated T6BP and NDP52. GST was used as a control. (D) HeLa cell lysates probed with our affinity-purified rabbit polyclonal antibodies raised against T6BP or NDP52. (E) T6BP or NDP52 co-immunoprecipitate with myosin VI. HeLa cells either untransfected (U) or overexpressing GFP-NDP52 (T) were lysed and immunoprecipitation was performed using no antibody (Blank) or anti-NDP52. Immunoblots were tested using anti-myosin VI or anti-NDP52 antibodies. A431 cells were used for the T6BP-myosin VI co-immunoprecipitation with antibodies shown. Immunoblots were stained with anti-T6BP antibodies.
To confirm that myosin VI interacted with T6BP and NDP52 we tested their binding using recombinant proteins in vitro. Despite using a variety of strategies and bacterial cell strains we were unable to successfully express full-length T6BP, so a truncation construct (amino acids 291-747) corresponding to the largest fragment identified in the yeast two-hybrid screen was used instead. Binding was first tested using an F-actin pelleting assay (Fig. 1B). Purified T6BP or NDP52 were mixed with myosin VI and F-actin, and the mixtures were centrifuged and the unbound supernatant fractions removed. The actomyosin pellet containing any attached binding partners was then resuspended in a salt-ATP solution. Mg-ATP binding caused the myosin to dissociate from actin and a second centrifugation step separated the myosin into the supernatant. T6BP and NDP52 were found to co-fractionate with the myosin VI in the supernatant (supernatant lane). To check whether T6BP and NDP52 bind to F-actin, both proteins were incubated with F-actin in the absence of myosin VI. Under these conditions very little T6BP or NDP52 pellets with F-actin and is released from the pellet into the supernatant by salt-ATP solution. As a further in vitro test of binding, we carried out GST pull-down assays using [35S]methionine-labelled in vitro-translated T6BP and NDP52. Both proteins bound to the GST-myosin VI tail but not GST alone (Fig. 1C). The smaller band in the T6BP lane is probably the result of initiating translation at an internal ATG codon (De Valck et al., 1999).
When we immunoblotted HeLa cell lysates using affinity-purified rabbit polyclonal antibodies raised against the N-terminus of human T6BP, a 86 kDa band was detected. A polyclonal antibody raised against full-length human NDP52 detected a single 52 kDa protein band (Fig. 1D). T6BP was also detected in the lysates of A431, COS 7, HEK293T, CHO and NRK cell lines by immunoblotting; NDP52 was detected in A431, COS 7 and HEK293T but our antibody raised against human NDP52 did not detect NDP52 in any rodent cell line, consistent with the lower sequence identity of its orthologue in these species (data not shown).
To confirm that myosin VI, T6BP and NDP52 interact in vivo we co-immunoprecipitated myosin VI, T6BP and NDP52 from cell lysates. Since NDP52 has a molecular mass of 52 kDa it cannot be resolved from the immunoglobulin heavy chain on SDS-PAGE gels, so the immunoprecipitations were performed using untransfected HeLa cells (U) and also cells expressing GFP-NDP52 (T) (Fig. 1E). Myosin VI co-immunoprecipitates with endogenous as well as expressed GFP-NDP52. Overexpression of NDP52 results in more myosin VI immunoprecipitated using antibodies to NDP52. Finally, endogenous T6BP was immunoprecipitated by anti-myosin VI antibodies from the cytosol of A431 cells (Fig. 1E). These results clearly demonstrate that, in vitro and also in vivo, T6BP and NDP52 bind to myosin VI.
Mapping the binding sites of T6BP, NDP52 and myosin VI
To determine the regions of T6BP and NDP52 that bind to myosin VI, we used a mammalian two-hybrid binding assay. We tested whether a panel of T6BP truncation mutants bound to the myosin VI tail and found that the C-terminus was required for interaction because removal of the last 17 amino acids, which form the second predicted zinc finger, abolished binding. Further site-directed mutagenesis on the two zinc fingers in T6BP, by converting the first cysteine in each zinc finger into alanine T6BP(C688A) and T6BP(C715A), revealed that neither of these constructs was able to bind to the myosin VI tail, suggesting that both intact zinc fingers are required for binding (Fig. 2A). An identical approach was taken with NDP52. The N-terminal region could be removed without loss of binding, but removal of the C-terminal 21 amino acids abolished binding to myosin VI. The C-terminus of NDP52 contains several cysteine and histidine residues predicted to form a zinc finger-like structure called a LIM domain. Mutating two of the cysteines to alanines in this region showed that Cys425, but not Cys400, is essential for binding to myosin VI (Fig. 2B). These results suggest that the zinc finger regions of T6BP and NDP52 are protein-protein interaction zones involved in binding to myosin VI.
In a converse set of experiments, we determined which region(s) of the myosin VI tail were required for interaction with T6BP and NDP52. A series of truncation constructs showed that both binding partners interacted with the region of the myosin VI tail lying between amino acids 1094 and 1117 (data not shown). This region contains the RRL motif, an interaction hotspot that we have previously shown to be necessary for binding to optineurin and GIPC (Sahlender et al., 2005; Spudich et al., 2007). Using a myosin VI tail construct, in which the RRL sequence had been mutated to alanines, we found that T6BP and NDP52 also bind to this site (Fig. 2D).
Localisation of T6BP and NDP52 in HeLa cells
To gain insight into the intracellular functions of these proteins we localised endogenous T6BP and NDP52 in HeLa cells using affinity-purified antibodies (Fig. 1D). We observed that endogenous T6BP was present in the perinuclear region around the Golgi complex, on a number of cytoplasmic vesicles and as a diffuse cytoplasmic staining (Fig. 3Aa,b). One of our anti-T6BP antibodies also showed some nuclear staining, but we believe this is a nonspecific crossreaction for the following reasons: (1) T6BP has no predicted nuclear localisation sequence; (2) a second anti-T6BP antibody showed no nuclear staining (Fig. 3Ab); (3) transiently transfected GFP-T6BP showed no nuclear staining (Fig. 3Ac); (4) when used in cells transiently overexpressing GFP-T6BP, the anti-T6PB antibody did not give any nuclear staining, presumably because the excess T6BP protein absorbed all of the antibodies; (5) directed depletion of T6BP by using RNAi had no effect on the nuclear stain, despite showing complete silencing of protein levels by immunoblot (data not shown). When the cells were pre-permeabilised with saponin prior to fixation (in order to remove the cytoplasmic pool of T6BP), the vesicular perinuclear T6BP staining was close to and partially colocalised with the trans-Golgi complex marker TGN46 (Fig. 3Ad-f,d′-f′). To investigate whether T6BP is associated with focal adhesions at the plasma membrane, because loss of T6BP and NDP52 increases the number of large focal adhesions in RNAi KD cells (see Fig. 7), we performed double labelling experiments in mouse fibroblasts with antibodies against T6BP and vinculin, a marker protein for focal adhesions. In these fibroblasts, T6BP localises to vesicles/dots that show significant overlap with vinculin patches at the plasma membrane (Fig. 3g-i). These results indicate that a fraction of the T6BP associates with vesicular structures at or near focal adhesion sites in the plasma membrane.
Fig. 3.
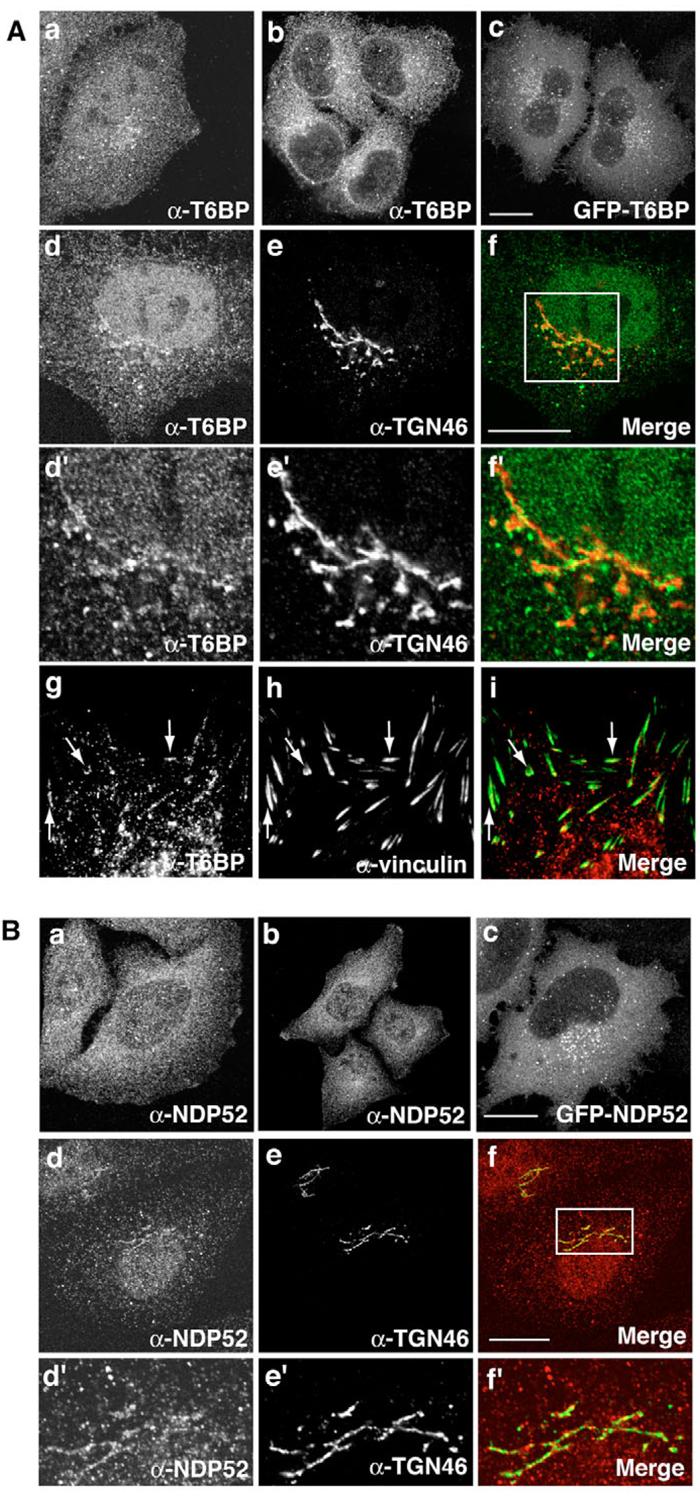
Intracellular localisation of T6BP and NDP52 in HeLa cells. (A) T6BP is localised at the Golgi complex and in the perinuclear region of the cell using anti-T6BP antibody (a and b) or by GFP-T6BP expression (c). Cells in d-f were pre-permeabilised with saponin and double labelled with antibody against TGN46 (e and e′), the box area in panel f is shown enlarged in panels d′-f′. Cells in panels g-i were double labelled with antibodies against T6BP and vinculin. (B) NDP52 is localised at the Golgi complex and in the perinuclear region of the cell by anti-NDP52 antibodies (a and b) or by GFP-NDP52 expression (c). To localise NDP52 around the Golgi complex, cells in panels d-f were pre-permeabilised with saponin and double labelled with TGN46 antibodies (e and e′). The boxed area in panel f is shown enlarged in panels d′-f′. Bars, 10 μm.
Fig. 7.
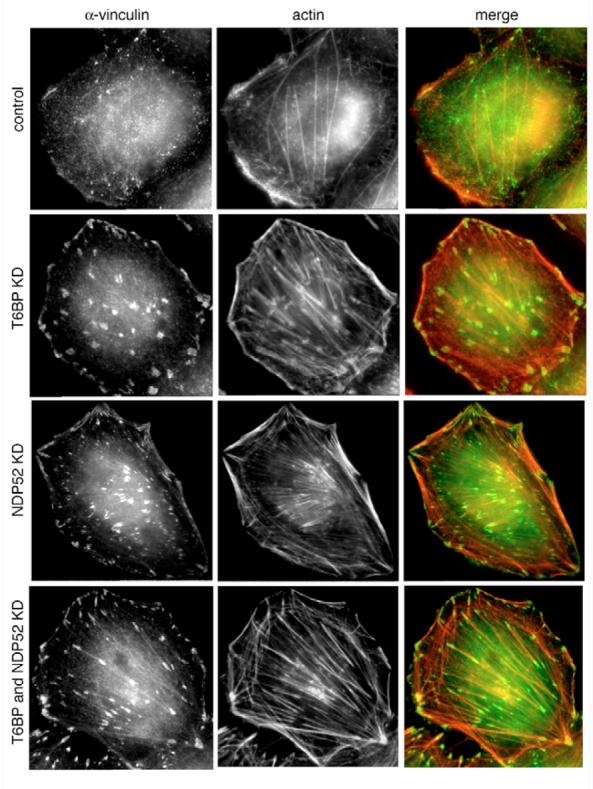
Loss of T6BP and NDP52 leads to dramatic changes in actin filament organisation and focal adhesion formation. HeLa cells were depleted of T6BP, NDP52 or both proteins by transfection with siRNA and stained for immunofluorescence microscopy using Rhodamine-phalloidin and antibodies against vinculin. Whereas control cells show membrane ruffles at the plasma membrane but few focal adhesions and stress fibres, the KD cells have lost membrane ruffles and display a substantial increase in size and number of focal adhesions and stress fibres, respectively.
We observed that, using our anti-NDP52 antibodies, NDP52 displayed a very similar distribution to T6BP, and was present in the perinuclear region and in vesicles distributed throughout the cell (Fig. 3Ba,b). Pre-permeabilisation with saponin also highlights the partial colocalisation of NDP52 with TGN46 (Fig. 3Bd-f,d′-f′). Our results confirmed previous studies that showed NDP52 concentrated in vesicles in the perinuclear area of the cell (Di et al., 2003; Sternsdorf et al., 1997). This association of T6BP and NDP52 with vesicles in the perinuclear region of the cell was confirmed by overexpressing these proteins tagged with GFP (Fig. 3Ac,Bc).
Colocalisation of T6BP and NDP52 with myosin VI
To further investigate the interaction between myosin VI and T6BP or NDP52 in vivo, we compared their localisation in HeLa cells. Using our T6BP or NDP52 antibodies on cells transiently transfected with the GFP-myosin VI tail domain we observed colocalisation of T6BP and NDP52 with myosin VI in vesicles adjacent to the Golgi complex in the perinuclear region of the cell (Fig. 4Aa-d and e-h). At the ultrastructural level, we labelled cryo-sections of HeLa cells with T6BP and NDP52 antibodies and also performed double-labelling experiments with antibodies to myosin VI. Both T6BP and NDP52 are associated with small vesicles surrounding the Golgi complex (Fig. 4Ba,c and enlarged images) and in the double-labelling experiment colocalisation of T6BP and NDP52 with myosin VI was observed (see arrows in Fig. 4Bb,d). So myosin VI and both partners show close association with vesicles at the trans side of the Golgi complex.
Fig. 4.

T6BP and NDP52 colocalise with myosin VI in vesicles in the perinuclear region around the Golgi complex in HeLa cells. (A) Cells transiently overexpressing GFP-tagged myosin VI tail (a-d, e-h) were pre-permeabilised with saponin, fixed, double labelled with the antibodies against T6BP (a-d) and NDP52 (e-h), and analysed by immunofluorescence. The boxed areas in panels a and e are shown enlarged in panels panels b-d and f-h, respectively. Arrows indicate examples of colocalisation. Bars, 10 μm. (B) Immuno-electron microscopy shows endogenous T6BP (a,b) and endogenous NDP52 (c,d). Myosin VI and T6BP (b) or NDP52 (d) colocalise in vesicles near the Golgi complex. The number of gold particles observed in these sections reflects the expected levels of these endogenous proteins. Enlarged images highlight T6BP and NDP52 associated with vesicles. Bar, 450 nm.
T6BP and NDP52 form a protein complex and colocalise in vivo
T6BP and NDP52 are found in vesicles surrounding the trans-Golgi network (TGN) where they colocalise with myosin VI. To establish whether both proteins are found in exactly the same intracellular compartment or whether they associate with different vesicle populations, we transiently expressed GFP-T6BP and double labelled with antibodies against NDP52, and conversely expressed GFP-NDP52 and double labelled with α-T6BP (Fig. 5Aad and e-h). Both proteins are present in the same vesicular structures (see arrows in Fig. 5A) and show very close association at the ultrastructural level in a double labelling with antibodies against T6BP and NDP52 (see arrows in Fig. 5B).
Fig. 5.
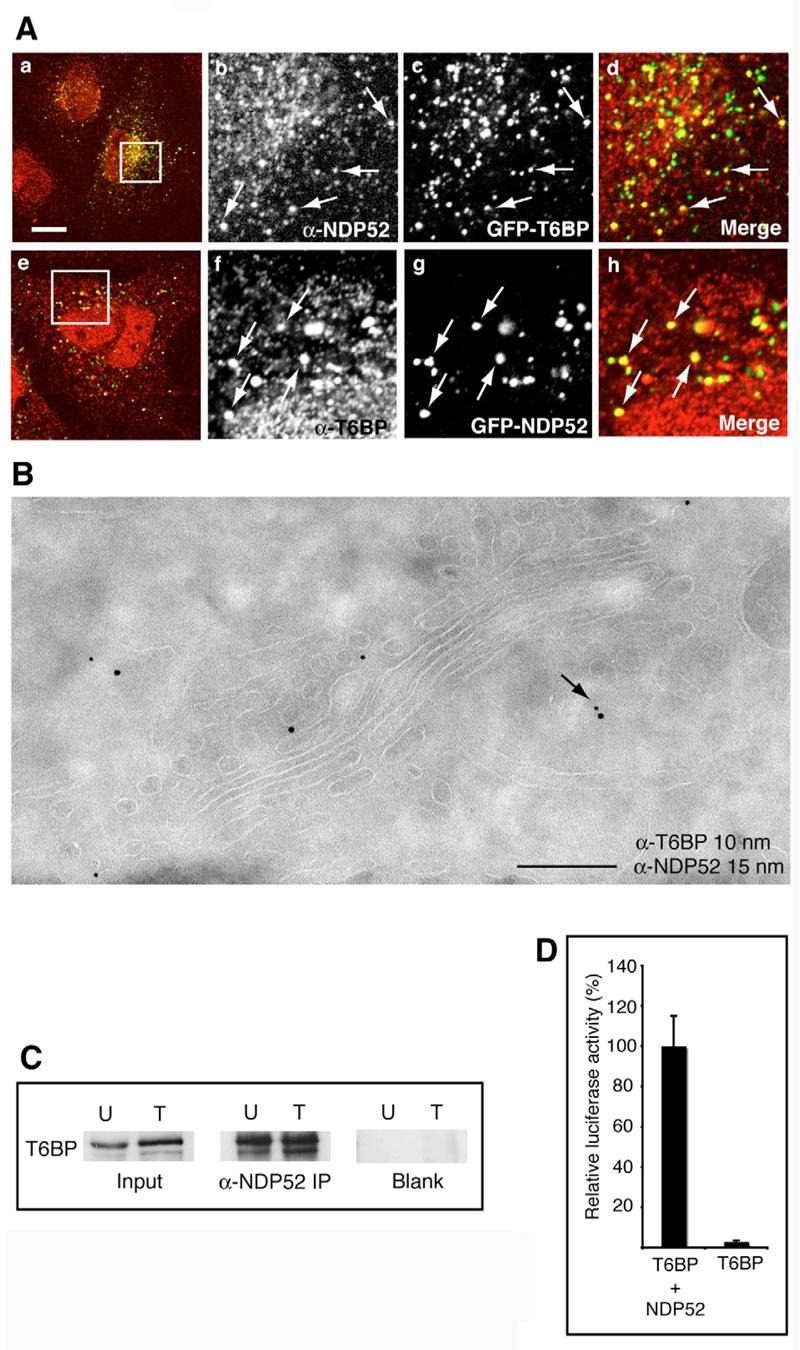
T6BP and NDP52 colocalise and form a complex in vivo. (A) T6BP and NDP52 colocalise in vesicles around the Golgi complex in HeLa cells. Cells transiently overexpressing GFP-T6BP (a-d) or GFP-NDP52 (e-g) were double labelled with antibodies to NDP52 (a-d) and T6BP (e-h). The boxed areas in panels a and e are shown enlarged in adjacent panels b-d and f-h, respectively. Arrows indicate colocalisation. Bar, 10 μm. (B) Immuno-electron microscopy shows that endogenous T6BP and NDP52 colocalise on vesicles on the trans-side of the Golgi complex. Bar, 370 nm. (C) T6BP co-immunoprecipitates with NDP52. HeLa cells either untransfected (U) or overexpressing GFP-NDP52 (T) were lysed and immunoprecipitations were performed using no antibody (Blank) or anti-NDP52. Immunoprecipitates were immunoblotted using anti-T6BP antibodies. (D) T6BP and NDP52 interact in the mammalian two-hybrid assay. T6BP constructs were used as the bait and NDP52 constructs as the prey.
To test whether T6BP and NDP52 directly interact in vivo we immunoprecipitated endogenous NDP52 and GFP-NDP52 from either untransfected (U) HeLa cells or HeLa cells transfected (T) with GFP-NDP52 and observed that both proteins are present in one immuno-complex from both cells (Fig. 5C). These immuno-complexes also contained myosin VI (data not shown), indicating that T6BP–NDP52–myosin-VI complexes can occur in addition to T6BP–myosin-VI and NDP52–myosin-VI complexes. The interaction between T6BP and NDP52 was confirmed using the mammalian two-hybrid assay (Fig. 5D).
T6BP and NDP52 are not recruited into membrane ruffles but are required for actin filament organisation and membrane ruffle formation
The SKICH domain present in the C-terminal region of the phosphatase SKIP and proline-rich inositol-polyphosphate 5-phosphatase (PIPP) has been shown to be important for plasma membrane localisation in resting and in growth-factor-stimulated cells (Gurung et al., 2003). Does the SKICH domain present at the N-terminus of T6BP and NDP52 have a similar function? Since we have shown previously that myosin VI is recruited into membrane ruffles in response to EGF stimulation (Buss et al., 1998), this seemed to be an obvious physiological process that might involve T6BP and NDP52. We used A431 cells, a human epidermoid carcinoma cell line that overexpresses the EGF receptor and, therefore, responds with extensive membrane ruffling to growth factor stimulation. Myosin VI was efficiently recruited into membrane ruffles 5 minutes after EGF stimulation, whereas neither T6BP nor NDP52 were recruited into membrane ruffles (Fig. 6). So, we investigated ruffle formation and F-actin organisation in T6BP and NDP52 RNAi KD cells. In mock-transfected control HeLa cells, where F-actin structures were visualised with Rhodamine-phalloidin and focal adhesions by using vinculin antibodies, we observed membrane ruffles at the cell edge, a few stress fibres and small focal adhesions throughout the cell (Fig. 7). However, in T6BP, NDP52 or double-KD cells we observed a dramatic change in actin organisation with an increase in thick actin stress fibre bundles, very prominent large focal adhesions, but no membrane ruffles at the plasma membrane (Fig. 7). Although neither proteins are recruited into membrane ruffles these observations suggest a role for T6BP and NDP52 in ruffle formation and actin cytoskeleton organisation.
Fig. 6.
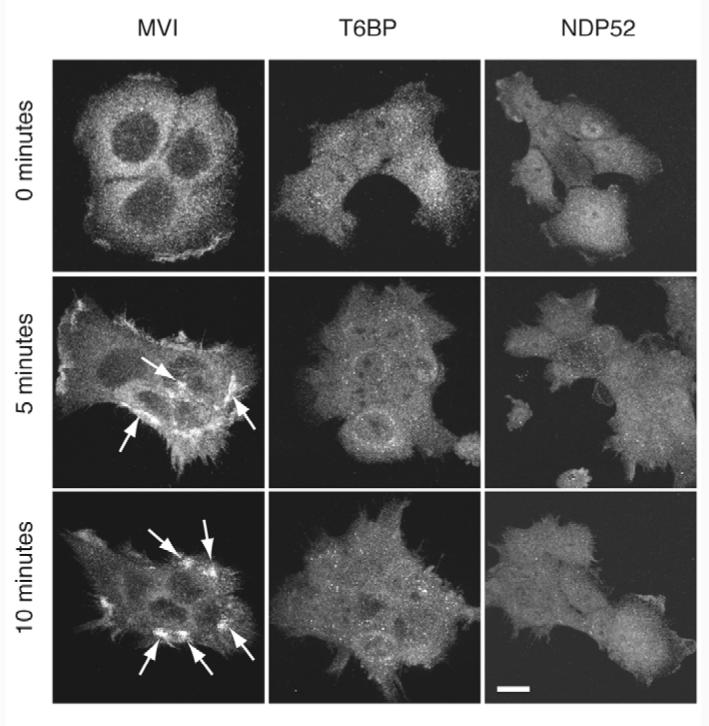
T6BP and NDP52 are not recruited into membrane ruffles upon EGF stimulation. A431 cells were stimulated with EGF and labelled with antibodies against myosin VI (MVI), T6BP or NDP52 after 0, 5 and 10 minutes. After 5-minute stimulation myosin VI is recruited into membrane ruffles (see arrows) whereas T6BP and NDP52 are not recruited into these membrane ruffles. Bar, 10 μm.
T6BP and NDP52 are negative regulators of secretion
Since myosin VI and its binding partner optineurin are involved in secretion from the Golgi complex to the plasma membrane (Warner et al., 2003; Sahlender et al., 2005), and both T6BP and NDP52 localise to vesicles in the trans-Golgi region, we tested whether they played any role in secretion. We silenced the expression of either myosin VI, T6BP or NDP52 by using RNAi in a HeLa cell line stably transfected with the human placental secreted alkaline phosphatase (SEAP) gene, which constitutively secretes the SEAP enzyme into the growth medium. After a double transfection of the small interfering RNA (siRNA) duplexes over a period of 4 days, the expression levels of all three proteins were <10% of control levels as shown by western blotting (Fig. 8A). Loss of myosin VI expression caused a 80% reduction in active SEAP secreted into medium (Fig. 8B), confirming our previous results that myosin VI is required for SEAP secretion (Warner et al., 2003). By contrast, the supernatants of T6BP or NDP52 RNAi KD cells, either transfected with a single siRNA duplex (T6BP P1 or NDP52 P1) or with a smart pool of four siRNA duplexes (T6BP SP or NDP52 SP), contained significantly higher levels of SEAP activity (30-90% increase) (Fig. 8B), This suggests that T6BP and NDP52 negatively regulate constitutive secretion.
Fig. 8.
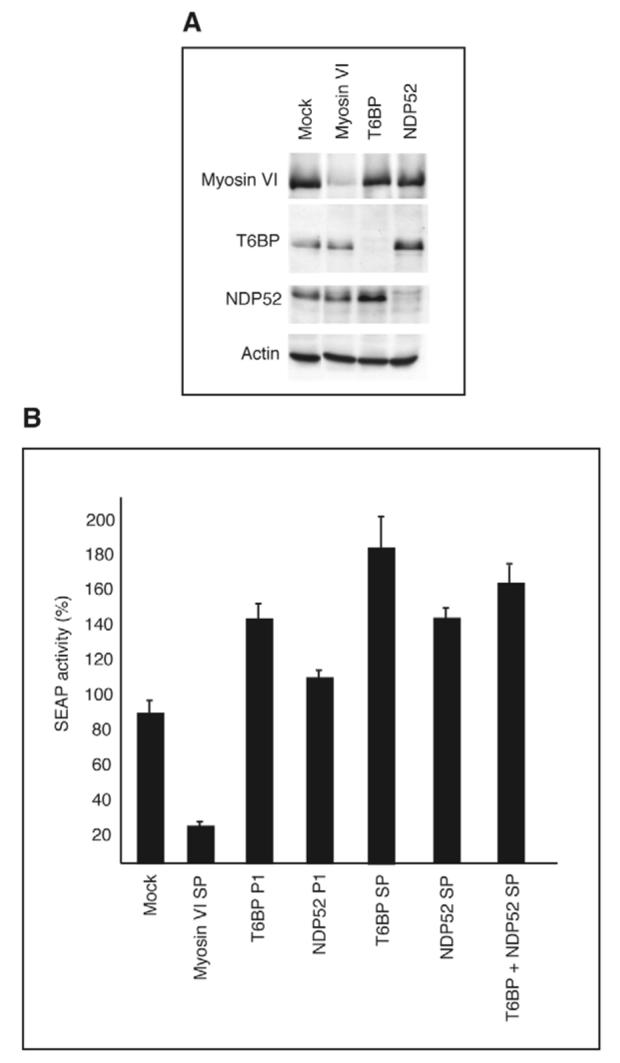
Depleting T6BP or NDP52 by RNAi KD increases the rate of constitutive secretion in HeLa cells. (A) HeLa cells expressing SEAP were either mock-transfected or transfected with siRNAs specifically targeting myosin VI, T6BP or NDP52 and were analysed by immunoblotting using anti-actin antibodies as a loading control. Myosin VI, T6BP and NDP52 expression was <10% of normal levels. (B) In HeLa cells depletion of T6BP or NDP52 or both proteins by single siRNA (P1) or SMARTpool (SP) primers causes a significant increase in SEAP secretion (30-90% increase), whereas depletion of myosin VI leads to a 80% decrease in secretion. The amount of SEAP activity in the growth medium was analysed by chemiluminescence and normalised relative to cell density.
T6BP inhibits TRAF6-dependent NF-κB activation
T6BP was identified as a binding partner of the immune signalling modulator TRAF6, which participates in NF-κB activation, operating downstream of receptors from both the interleukin-1 receptor/toll-like receptor (IL1R/TLR) and tumour necrosis factor α (TNFα) receptor superfamilies (Ling and Goeddel, 2000). TRAF6 exerts a dominant-positive effect when overexpressed (Cao et al., 1996) and, thus, HEK293 ET cells transiently transfected with a TRAF6 expression plasmid show NF-κB activation (Fig. 9A). Since a direct interaction between T6BP and TRAF6 has been reported (De Valck et al., 1999), we investigated the role of T6BP on NF-κB signalling. Coexpression of GFP-T6BP with TRAF6 resulted in a strong inhibition of NF-κB activation. The T6BP point mutant (C688A in the first zinc finger) was able to partially relieve this inhibition, whereas the mutation in the second zinc finger (C715A) in T6BP had a co-stimulatory effect on TRAF6-mediated NF-κB activation. NDP52 exhibited only a slight inhibition, whereas myosin VI exerted no significant inhibitory effect (Fig. 9A).
Fig. 9.
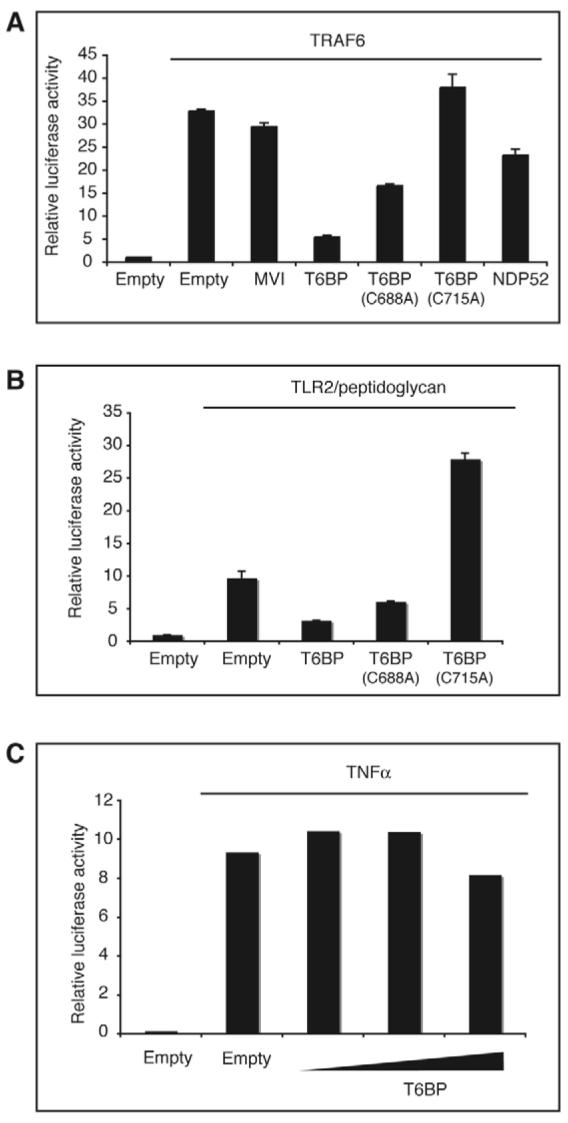
T6BP is an inhibitor of TRAF6-mediated NF-κB activation. (A) Overexpression of T6BP inhibits NF-κB activation induced by TRAF6. HEK293 ET cells were transfected with the plasmids encoding either GFP or wild-type or mutant T6BP, NDP52 or myosin VI (400 ng per well) and plasmids encoding TRAF6 (200 ng per well). An empty pEGFP vector was included as a control. At 24 hours after transfection the luciferase activities in cell lysates were measured. T6BP inhibits TRAF6-mediated NF-κB activation, whereas myosin VI and NDP52 have no significant effect. (B) Overexpression of T6BP inhibits NF-κB activation downstream of a TRAF6-dependent pathway. HEK293 ET cells were transfected with the plasmids encoding either GFP or wild-type or mutant T6BP (600 ng per well) and plasmids encoding TLR2 (100 ng per well). At 24 hours after transfection, cells were stimulated for 5 hours with 10 μg/ml of peptidoglycan. The luciferase activities in cell lysates were then measured. (C) NF-κB activation via a TRAF6-independent pathway is unaffected by T6BP overexpression. HEK293 ET cells were transfected with the GFP plasmid or increasing amounts (200, 400 and 800 ng per well) of T6BP plasmid. At 24 hours after transfection cells were stimulated for 5 hours with 10 ng/ml of TNF-α and then the luciferase activities in the cell lysates were measured.
We next tested whether the T6BP inhibitory effect applied to all NF-κB pathways or only to those that are dependent on TRAF6. Toll-like receptor 2 (TLR2) signalling, which leads to NF-κB activation, is strictly TRAF6 dependent. In HEK293 ET cells transfected with TLR2 and stimulated with peptidoglycan, T6BP inhibited NF-κB activation. Similar to the picture seen in TRAF6-overexpressing cells, zinc finger mutation C688A alleviated the inhibitory effect of T6BP, whereas the T6BP(C715A) mutant had a co-stimulatory effect (Fig. 9B). Since TRAF6 is not required for signalling downstream of the TNFα receptor (Cao et al., 1996), we looked to see whether T6BP affected TRAF6-independent NF-κB activation. In HEK293 ET cells stimulated with TNFα, we found that T6BP did not significantly affect NF-κB activation (Fig. 9C), therefore strongly indicating that T6BP inhibits NF-κB activating pathways at the level of TRAF6.
So far, there is no clear-cut evidence linking NDP52 to a role in cytokine signalling, but its high degree of similarity to T6BP in terms of its domain organisation and cellular localisation makes it a good candidate. Similarly, myosin VI has not been directly implicated in signal transduction, although it was shown to be involved in receptor mediated endocytosis (Swiatecka-Urban et al., 2004; Osterweil et al., 2005), and there is a conceivable need for motor-cargo complexes in the propagation of signals from the plasma membrane to the cell interior and nucleus and vice-versa.
Discussion
One approach to determine the cellular functions of myosin VI is to identify and characterise its interacting proteins. So far, myosin VI has been shown to participate in endocytosis via its interactions with Dab2 and GIPC (Morris et al., 2002; Reed et al., 2005), and to be involved in secretion and protein sorting to the basolateral domain in polarised cells through binding to optineurin (Sahlender et al., 2005; Au et al., 2007). Here, we show that T6BP and NDP52 are myosin VI binding partners that might participate in secretion, but also modulate cell adhesion and actin organisation. In addition, T6BP appears to function in signal transduction, acting as an inhibitor of TRAF6-mediated NF-κB activation.
The binding sites for myosin VI in both proteins are in their extreme C-termini. In the case of T6BP, there are two well-defined C2H2-type zinc fingers in this region, and mutagenesis of cysteine residues in either zinc finger abolished binding to myosin VI. The C-terminus of NDP52 is less well defined but it contains cysteine and histidine residues that fall into the pattern C3HC3HCH, which can be classified as a LIM-like domain (consensus C2HC3) (Korioth et al., 1995), capable of coordinating two zinc ions and involved in protein-protein interactions (Bach, 2000). In the myosin VI tail two interaction hotspots for its binding partners both consisting of three amino acids, the RRL site and the WWY site, have been identified (Spudich et al., 2007). T6BP and NDP52 both bind to the RRL site. Perhaps significantly, optineurin – a protein previously shown to play a role in secretion (Sahlender et al., 2005) – also binds at this site. The observation that these three binding partners bind to such a short stretch of amino acids emphasises the inherently weak, transient nature of these interactions. It is now recognised that many cellular multi-component complexes do not exist as strong, stable entities but, instead, are assembled in a more stochastic manner with transient encounter complexes being progressively buttressed by the combinatorial addition of further elements or ligands (Tang et al., 2006). In this model, weakly associating complexes are precursors for larger functional assemblies and myosin VI would seem to conform to this type. One can readily envisage how myosin VI could bind to T6BP, NDP52 or optineurin at the Golgi complex, and how such interactions would ensure the presence of a pool of myosin VI there at all times. Sidestepping the need to find myosin VI in the thick ‘cytoplasmic/cytoskeletal soup’, these small encounter complexes could serve as the nuclei for larger transport complexes, within which multiple weak interactions combine to preserve a cohesive whole.
T6PB and NDP52 are both present on a number of cytoplasmic vesicles in the perinuclear region around the Golgi complex. Neither T6BP nor NDP52 have transmembrane domains, and their additional presence in a cytoplasmic pool implies that they are recruited to these vesicular structures from the cytosol by an as-yet-unidentified membrane anchor. At what point they begin their association with the secretory pathway is unclear. Immunofluorescence microscopy shows that both T6BP and NDP52 colocalise with the trans-Golgi marker TGN46, and at the greater resolution afforded by electron microscopy most of the T6BP and NDP52 are associated with small vesicles at the trans face of the Golgi complex. Movies showing GFP-T6BP- and GFP-NDP52-associated vesicles moving in HeLa cells are in supplementary material (Movies 1 and 2). These vesicles are most likely to be either TGN-derived vesicles or vesicles belonging to the perinuclear recycling compartment; however, their exact cargo and intracellular destination (whether it be late endosomes/lysosomes or the plasma membrane) needs to be established.
T6BP and NDP52 colocalise with myosin VI on vesicles at the trans face of the Golgi complex and there is now strong evidence that myosin VI in this cellular location plays a role in constitutive secretion (Warner et al., 2003). In HeLa cells depleted of the myosin VI binding partner optineurin, myosin VI is lost from the Golgi complex and transport of the reporter molecule VSV-G to the plasma membrane is reduced (Sahlender et al., 2005). Since T6BP, NDP52, and optineurin all compete for the same RRL-binding site on the myosin VI tail, decreasing the levels of T6BP and NDP52 by using siRNAs could mean that more myosin VI molecules are available for interaction with optineurin, resulting in enhanced constitutive secretion. In polarised epithelial cells, myosin VI and optineurin are required for sorting of membrane proteins with tyrosine-motifs to the basolateral domain (Au et al., 2007). Sorting of cargo after exit from the TGN and on route to the plasma membrane occurs in recycling endosomes that contain myosin VI, optineurin and Rab8. However, whether T6BP, NDP52 and myosin VI regulate exocytosis and delivery of cargo to the plasma membrane at the TGN or in the recycling compartment is unknown.
There is circumstantial evidence that links T6BP to cytokine signalling because it has been identified as a binding partner of TRAF6 and A20 (De Valck et al., 1999), both of which are known to participate in signal transduction cascades controlled by the ubiquitous transcription factor NF-κB. This factor is a key component of the immune system, marshalling the expression of various pro-inflammatory molecules and also guiding tissue repair and regeneration (Youssef and Steinman, 2006). Since NF-κB has such potent pleiotropic effects, it is crucial that its activation is tightly regulated. TRAF6 is one of the main activators of NF-κB, being recruited early on in signal cascades (Moynagh, 2005), whereas A20 is a negative regulator of NF-κB, and it is possible that it accomplishes this function by acting on TRAF6 (Boone et al., 2004; Heyninck and Beyaert, 1999). By being able to interact with both TRAF6 and A20, T6BP could provide a regulatory function or orchestrate their association. Indeed, one possible explanation for the co-stimulatory effect on NF-κB activation seen with the T6BP mutagenesis constructs (Fig. 9) is that they sequester A20 and render it incapable of checking TRAF6-mediated NF-κB activation. Whatever the mechanism, it seems that T6BP is a potent inhibitor of TRAF6 signalling pathways, as shown by its effect on TLR2 activation. No effect on NF-κB activation was seen upon overexpression of NDP52. This seems somewhat surprising given the high level of similarity between NDP52 and T6BP in their primary structure and cellular localisation. The fact that they colocalise and directly interact with one another also hints at a shared function. It may be that there is a degree of redundancy and that T6BP is able to compensate for loss of NDP52, but not vice versa.
The role of T6BP and NDP52 in exocytosis and also in cytokine signalling might be closely linked to the dramatic reorganisation of actin filaments seen in T6BP and NDP52 KD cells. Increased exocytosis either from the Golgi complex or the recycling compartment of cell-substrate adhesion molecules such as integrins (Caswell and Norman, 2006) might explain the increase in size and number of focal adhesions at the plasma membrane accompanied by increased actin stress fibres in KD cells. Alternatively, the SKICH domain present in T6BP and NDP52, which can mediate membrane ruffle localisation, might link T6BP and NDP52 to proteins modulating actin filament organisation underneath the plasma membrane. Loss of T6BP or NDP52 might, therefore, result in loss of these dynamic actin structures leading to an increase in stable stress fibres. Future experiments will aim at exploring the motile behaviour of T6BP and NDP52 KD cells.
Our results provide a strong basis for future work on T6BP, NDP52 and their association with myosin VI. Since T6BP and NDP52 appear to regulate secretion, a process involving myosin VI and optineurin, they may accomplish regulation simply by competing with optineurin for myosin VI binding. An optineurin–myosin-VI complex promotes exocytosis of cargo whereas a T6BP–NDP52–myosin-VI complex results in tethering of the vesicles in the perinuclear area and a reduction in the rate of secretion. Interestingly, we observed in vitro and in vivo that T6BP can self-associate to form a relatively stable dimer (data not shown). In view of the controversy whether myosin VI functions in the cell as a monomer or a dimer (Buss et al., 2004; O'Connell et al., 2006; Sweeney and Houdusse, 2007), the first demonstration that one of its binding partners forms a stable dimer may be significant. Exactly what is the cargo within the T6BP/NDP52 vesicles is an interesting but, so far, unanswered question. T6BP also functions as an inhibitor of TRAF6-mediated NF-κB activation, consistent with its published interactions with TRAF6 and A20. Could the two regulatory roles, in secretion and NF-κB activation, be linked? The activation of NF-κB within cells is known to cause the release of cytokines into the growth media, and a concurrent upregulation of cell surface proteins. Both these processes require the exocytic pathway and it is tempting to speculate that T6BP/NDP52-associated vesicles may harbour proinflammatory cargo. Is the interaction of T6BP and NDP52 with myosin VI limited to regulating secretion? Myosin VI has been shown to be upregulated by p53 in response to DNA damage as part of a pro-survival pathway (Jung et al., 2006) and there is extensive integration between the p53 and NF-κB signalling networks, so T6BP/NDP52 interaction with myosin VI may even act to facilitate this cross-talk. The reported anti-apoptotic activity of T6BP (De Valck et al., 1999) might be a clue in this regard and it will be fascinating to explore these and other possibilities in the future.
Materials and Methods
Constructs, antibodies and reagents
The human T6BP and NDP52 genes were amplified from a human liver cDNA library by PCR and subcloned into the pEGFP, pM and pVP16 vectors (Clontech, Saint Germain-en-Laye, France), pRSET and pcDNA3 vectors (Invitrogen, Paisley, UK), the pGEX vector (GE Healthcare, Amersham, UK) and mutagenesis carried out using the Quikchange Site-Directed Mutagenesis Kit (Stratagene, Amsterdam, NL). For mammalian two-hybrid assays, chicken myosin VI tail fragments (Spudich et al., 2007) were inserted into the pM vector. The optineurin, GST-myosin VI tail and the TLR2 constructs were as described previously (Randow and Seed, 2001; Sahlender et al., 2005). Human TRAF6 was inserted into the pEAK-8 plasmid, which was kindly provided by A. Ting (Mount Sinai School of Medicine, USA). Rabbit polyclonal antibodies were raised against His-tagged T6BP(1-423), T6BP(425-747) and NDP52 by Harlan Sera-Lab Limited (Loughborough, UK) and affinity-purified. Other primary antibodies were rabbit polyclonal myosin VI tail antibody (Buss et al., 1998), mouse monoclonal GFP antibody (Roche Diagnostics, Lewes, UK) and sheep polyclonal TGN46 antibody (Serotec). Secondary antibodies were from Molecular Probes (Cambridge BioSciences, Cambridge, UK) and recombinant human Epidermal Growth Factor (EGF) (Invitrogen), TNFα (R&D Systems, Abingdon, UK) and peptidoglycan (Fluka-Sigma, Gillingham, UK).
Cell culture and treatments
HeLa, A431 and HEK293 ET cells were cultured in DMEM (GibcoBRL, Paisley, UK) and CHO cells in MEM-alpha medium (GibcoBRL) all containing 10% FCS. For EGF treatment, A431 cells were serum-starved for 2 hours and then stimulated with 200 ng/ml EGF. Peptidoglycan was added to HEK293 ET cells at 10 μg/ml for 5 hours. TNFα stimulation was at 10 ng/ml for 5 hours. Transfections of HeLa cells were carried out using FuGENE6 (Roche) and transfections of HEK293 ET cells using LipofectAMINE2000 (Invitrogen), both according to the manufacturer's instructions.
Yeast two-hybrid screen
Chicken brush border myosin VI tail was used to screen a human umbilical vein endothelial cell cDNA library as described previously (Morris et al., 2002) using the Proquest Two-hybrid System (Invitrogen). 3-amino-1,2,4-TRIzol (Sigma, Gillingham, UK) was used to titrate out the propensity of the myosin VI tail to self-activate, and transformants were screened for their ability to grow on plates lacking leucine, tryptophan and histidine and positive colonies were then tested for induction of the three reporter genes URA3, HIS3 and lacZ.
Mammalian two-hybrid assay
CHO cells (2×105) were transfected with 0.5 μg each of (1) the bait construct in the pM vector, (2) the prey construct cloned into the pVP16 vector, (3) the constitutively active pRL-CMV plasmid (Promega, Southampton, UK) encoding Renilla luciferase and (4) the inducible pG5luc plasmid (Clontech) encoding firefly luciferase. After a 48-hour incubation, the cells were lysed using passive lysis buffer (Promega) and relative firefly and luciferase activities measured using the Dual-Luciferase Reporter Assay System (Promega). Three independent transfections were carried out for each experiment and the results compiled. Error bars are s.e.m. for the whole dataset.
Actin pelleting assay
His-tagged T6BP (amino acids 291-747) and NDP52 were expressed and purified by the protocol described (Spudich et al., 2007). Full-length myosin VI was expressed in Sf9 insect cells using the Baculovirus expression system and purified (Lister et al., 2004), and F-actin was prepared from chicken skeletal muscle. 10 μg of F-actin was mixed with 5 μg of binding partner and myosin VI in binding buffer (40 mM NaCl, 1 mM MgCl2, 1 mM EGTA, 0.25 mM DTT, 20 mM Tris-HCl pH 7.5) in a volume of 50 μl and incubated for 30 minutes on ice. The actomyosin complexes with associated binding partners were sedimented at 190,000 g for 10 minutes at 4°C. The supernatant containing unbound protein was removed and the pellet resuspended in 50 μl dissociation buffer (300 mM NaCl, 5 mM MgCl2, 5 mM MgATP, 1 mM EGTA, 20 mM Tris-HCl pH 7.5) and incubated on ice for 15 minutes. This allows active myosin to dissociate from the F-actin, along with any attached binding partners. The mixture was centrifuged at 190,000 g for 10 minutes at 4°C to pellet the F-actin. Samples of the final supernatant and pellet, together with the initial solution and unbound fraction, were analysed by 6-20% acrylamide SDS-PAGE. Controls without myosin VI were run to exclude direct binding to F-actin.
GST pull-down assay
Full-length T6BP and NDP52 cloned into the pcDNA3 vector were in-vitro-translated with [35S]methionine incorporation using the TNT-coupled Reticulocyte Lysate System (Promega). The radiolabelled protein was incubated with GST or GST–myosin-VI-tail (Buss et al., 1998) coupled to glutathione beads in binding buffer (150 mM NaCl, 1% Triton X-100, 10 mM Hepes pH 7.4) for 2 hours at 4°C. Following several washes, the bound proteins were separated by SDS-PAGE and analysed by autoradiography.
Immunoblotting and immunoprecipitation
For immunoprecipitation, HeLa or A431 cells were lysed in 100 mM NaCl, 1% glycerol, 1% Triton X-100, 5 mM MgATP, 5 mM EGTA, Complete™ protease inhibitor cocktail (Roche), 50 mM Tris-HCl pH 7.5 and processed as described (Buss et al., 1998). The immunoprecipitated proteins were run on 10% acrylamide SDS-PAGE gels, transferred to 0.45 μm nitrocellulose membranes, blocked with 10% nonfat dried milk, 0.3% TWEEN-20, PBS and probed using the relevant affinity-purified rabbit polyclonal antisera followed by goat anti-rabbit coupled to horseradish peroxidase (Sigma). The bands were visualised using the ECL Western Blotting Detection Reagents (GE Healthcare).
Indirect immunofluorescence and digital image analysis
The cells were fixed, stained (Buss et al., 1998) and, when required, pre-permeabilised with 0.01% saponin in cytosolic buffer (25 mM KCl, 2.5 mM MgAc, 150 mM glutamate, 5 mM EGTA, 25 mM Hepes pH 7.4). Cells were analysed and imaged using a Radiance confocal microscope (Bio-Rad) using Lasersharp 2000 software, and any further processing was carried out using Adobe Photoshop.
Electron microscopy
HeLa cells were fixed in a mixture of 2% paraformaldehyde/0.2% glutaraldehyde in PBS for 2 hours. After washing with PBS/0.02M glycine, the cells were scraped into PBS/12% gelatine, centrifuged and embedded in the same gelatine solution. Small blocks of less that 1 mm3 were cut, embedded in 2.3M sucrose cryoprotection solution overnight, mounted on aluminium pins and frozen in liquid nitrogen. Ultrathin 70-nm thick cryosections were processed for single and double labelling following previously described protocols (Puri et al., 2005).
RNA interference
To silence T6BP, NDP52 or myosin VI expression, cells were transfected with siRNA duplexes using single or ON-TARGETplus SMARTpool primers (Dharmacon Research UK, Cramlington, UK) using OligofectAMINE (Invitrogen) according to the manufacturer's instructions. Silencing was achieved by two transfections of siRNA duplexes 48 hours apart over a period of 4 days. At the end of this period the cells were lysed and the degree of knockdown determined by immunoblotting and densitometry using a Personal Densitometer SI (Molecular Dynamics, Amersham, UK) and ImageQuant software.
Secreted alkaline phosphatase (SEAP) expression and assay
The SEAP gene was amplified from the pSEAP plasmid (Clontech) by PCR and ligated into the pIRESneo2 vector. HeLa cells were stably transfected with the gene and single cell clones were established. For the knockdown assay, the cells were transfected with siRNAs targeting T6BP, NDP52 or myosin VI as described above. To measure SEAP activity (Warner et al., 2003), the cell culture medium was collected and incubated for 1 hour at 60°C to inactivate endogenous phosphatase. They were then incubated with 1 mg/ml para-nitrophenylphosphate (Sigma) in assay buffer (1 M diethanolamine, 1 mM MgCl2) for 20 minutes at room temperature and the absorption at 405 nm was measured on a plate luminometer (Berthold Detection Systems, Geneflow, Fradley, UK) and normalised on the basis of cell density.
Reporter gene assays
HEK 293 ET cells were seeded with 5×104 cells per well in 24-well plates and transfected the next day with various amounts of DNA up to 800 ng per well using LipofectAMINE2000 (Invitrogen). A pNF-κB-Luc plasmid containing the NF-κB-driven firefly luciferase gene was used to monitor NF-κB activity. The pRL-TK plasmid (Promega) encoding constitutively expressed Renilla luciferase was used to normalise for cellular DNA uptake. 50 ng of each of the luciferase plasmids was added per well. At 24 hours after transfection and subsequent stimulations with various agonists (depending on the assay) cells were lysed and the activities of both luciferases measured using the Dual-Luciferase reporter assay (Promega). All data shown are average values ± s.d. from a representative experiment.
Supplementary Material
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/120/15/2574/DC1
Acknowledgments
The work was funded by MRC PhD studentships (B.M., R.C.R., G.R.), a Wellcome Trust PhD studentship (C.D.), a CRUK project grant (C.P.), a Wellcome Trust Senior Fellowship (F.B.) and was supported by the MRC. The Cambridge Institute for Medical Research (CIMR) is in receipt of a strategic award from the Wellcome Trust.
References
- Aschenbrenner L, Lee T, Hasson T. Myo6 facilitates the translocation of endocytic vesicles from cell peripheries. Mol. Biol. Cell. 2003;14:2728–2743. doi: 10.1091/mbc.E02-11-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au J, Puri C, Ihrke G, Kendrick-Jones J, Buss F. Myosin VI is required for sorting of tyrosine motif containing cargo to the basolateral domain in polarized MDCK cells. J. Cell Biol. 2007;177:103–114. doi: 10.1083/jcb.200608126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach I. The LIM domain: regulation by association. Mech. Dev. 2000;91:5–17. doi: 10.1016/s0925-4773(99)00314-7. [DOI] [PubMed] [Google Scholar]
- Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat. Immunol. 2004;5:1052–1060. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- Buss F, Kendrick-Jones J, Lionne C, Knight AE, Cote GP, Luzio JP. The localization of myosin VI at the golgi complex and leading edge of fibroblasts and its phosphorylation and recruitment into membrane ruffles of A431 cells after growth factor stimulation. J. Cell Biol. 1998;143:1535–1545. doi: 10.1083/jcb.143.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss F, Arden SD, Lindsay M, Luzio JP, Kendrick-Jones J. Myosin VI isoform localized to clathrin-coated vesicles with a role in clathrin-mediated endocytosis. EMBO J. 2001;20:3676–3684. doi: 10.1093/emboj/20.14.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss F, Spudich G, Kendrick-Jones J. Myosin VI: cellular functions and motor properties. Annu. Rev. Cell Dev. Biol. 2004;20:649–676. doi: 10.1146/annurev.cellbio.20.012103.094243. [DOI] [PubMed] [Google Scholar]
- Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel DV. TRAF6 is a signal transducer for interleukin-1. Nature. 1996;383:443–446. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- Caswell PT, Norman JC. Integrin trafficking and control of cell migration. Traffic. 2006;7:14–21. doi: 10.1111/j.1600-0854.2005.00362.x. [DOI] [PubMed] [Google Scholar]
- Chung JY, Park YC, Ye H, Wu H. All TRAFs are not created equal: common and distinct molecular mechanisms of TRAF-mediated signal transduction. J. Cell Sci. 2002;115:679–688. doi: 10.1242/jcs.115.4.679. [DOI] [PubMed] [Google Scholar]
- Coureux PD, Sweeney HL, Houdusse A. Three myosin V structures delineate essential features of chemo-mechanical transduction. EMBO J. 2004;23:4527–4537. doi: 10.1038/sj.emboj.7600458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Cruz EM, Ostap EM. Relating biochemistry and function in the myosin superfamily. Curr. Opin. Cell Biol. 2004;16:61–67. doi: 10.1016/j.ceb.2003.11.011. [DOI] [PubMed] [Google Scholar]
- De Valck D, Jin DY, Heyninck K, Van de Craen M, Contreras R, Fiers W, Jeang KT, Beyaert R. The zinc finger protein A20 interacts with a novel anti-apoptotic protein which is cleaved by specific caspases. Oncogene. 1999;18:4182–4190. doi: 10.1038/sj.onc.1202787. [DOI] [PubMed] [Google Scholar]
- Di Y, Li J, Zhang Y, He X, Lu H, Xu D, Ling J, Huo K, Wan D, Li YY, et al. HCC-associated protein HCAP1, a variant of GEMIN4, interacts with zinc-finger proteins. J. Biochem. 2003;133:713–718. doi: 10.1093/jb/mvg091. [DOI] [PubMed] [Google Scholar]
- Dunn TA, Chen S, Faith DA, Hicks JL, Platz EA, Chen Y, Ewing CM, Sauvageot J, Isaacs WB, De Marzo AM, et al. A novel role of myosin VI in human prostate cancer. Am. J. Pathol. 2006;169:1843–1854. doi: 10.2353/ajpath.2006.060316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foth BJ, Goedecke MC, Soldati D. New insights into myosin evolution and classification. Proc. Natl. Acad. Sci. USA. 2006;103:3681–3686. doi: 10.1073/pnas.0506307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachon F, Peleraux A, Thebault S, Dick J, Lemasson I, Devaux C, Mesnard JM. CREB-2, a cellular CRE-dependent transcription repressor, functions in association with Tax as an activator of the human T-cell leukemia virus type 1 promoter. J. Virol. 1998;72:8332–8337. doi: 10.1128/jvi.72.10.8332-8337.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung R, Tan A, Ooms LM, McGrath MJ, Huysmans RD, Munday AD, Prescott M, Whisstock JC, Mitchell CA. Identification of a novel domain in two mammalian inositol-polyphosphate 5-phosphatases that mediates membrane ruffle localization. The inositol 5-phosphatase skip localizes to the endoplasmic reticulum and translocates to membrane ruffles following epidermal growth factor stimulation. J. Biol. Chem. 2003;278:11376–11385. doi: 10.1074/jbc.M209991200. [DOI] [PubMed] [Google Scholar]
- Heyninck K, Beyaert R. The cytokine-inducible zinc finger protein A20 inhibits IL-1-induced NF-kappaB activation at the level of TRAF6. FEBS Lett. 1999;442:147–150. doi: 10.1016/s0014-5793(98)01645-7. [DOI] [PubMed] [Google Scholar]
- Jung EJ, Liu G, Zhou W, Chen X. Myosin VI is a mediator of the p53-dependent cell survival pathway. Mol. Cell. Biol. 2006;26:2175–2186. doi: 10.1128/MCB.26.6.2175-2186.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korioth F, Gieffers C, Maul GG, Frey J. Molecular characterization of NDP52, a novel protein of the nuclear domain 10, which is redistributed upon virus infection and interferon treatment. J. Cell Biol. 1995;130:1–13. doi: 10.1083/jcb.130.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Goeddel DV. T6BP, a.TRAF6-interacting protein involved in IL-1 signaling. Proc. Natl. Acad. Sci. USA. 2000;97:9567–9572. doi: 10.1073/pnas.170279097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister I, Schmitz S, Walker M, Trinick J, Buss F, Veigel C, Kendrick-Jones J. A monomeric myosin VI with a large working stroke. EMBO J. 2004;23:1729–1738. doi: 10.1038/sj.emboj.7600180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SM, Arden SD, Roberts RC, Kendrick-Jones J, Cooper JA, Luzio JP, Buss F. Myosin VI binds to and localises with Dab2, potentially linking receptor-mediated endocytosis and the actin cytoskeleton. Traffic. 2002;3:331–341. doi: 10.1034/j.1600-0854.2002.30503.x. [DOI] [PubMed] [Google Scholar]
- Moynagh PN. The NF-kappaB pathway. J. Cell Sci. 2005;118:4589–4592. doi: 10.1242/jcs.02579. [DOI] [PubMed] [Google Scholar]
- O'Connell CB, Tyska MJ, Mooseker MS. Myosin at work: motor adaptations for a variety of cellular functions. Biochim. Biophys. Acta. 2006;1773:615–630. doi: 10.1016/j.bbamcr.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Osterweil E, Wells DG, Mooseker MS. A role for myosin VI in postsynaptic structure and glutamate receptor endocytosis. J. Cell Biol. 2005;168:329–338. doi: 10.1083/jcb.200410091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri C, Tosoni D, Comai R, Rabellino A, Segat D, Caneva F, Luzzi P, Di Fiore PP, Tacchetti C. Relationships between EGFR signaling-competent and endocytosis-competent membrane microdomains. Mol. Biol. Cell. 2005;16:2704–2718. doi: 10.1091/mbc.E04-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randow F, Seed B. Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat. Cell Biol. 2001;3:891–896. doi: 10.1038/ncb1001-891. [DOI] [PubMed] [Google Scholar]
- Reed BC, Cefalu C, Bellaire BH, Cardelli JA, Louis T, Salamon J, Bloecher MA, Bunn RC. GLUT1CBP(TIP2/GIPC1) interactions with GLUT1 and myosin VI: evidence supporting an adapter function for GLUT1CBP. Mol. Biol. Cell. 2005;16:4183–4201. doi: 10.1091/mbc.E04-11-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlender DA, Roberts RC, Arden SD, Spudich G, Taylor MJ, Luzio JP, Kendrick-Jones J, Buss F. Optineurin links myosin VI to the Golgi complex and is involved in Golgi organization and exocytosis. J. Cell Biol. 2005;169:285–295. doi: 10.1083/jcb.200501162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich G, Chibalina MV, Au JS-Y, Arden SD, Buss F, Kendrick-Jones J. Myosin VI targeting to clathrin-coated structures and dimerization is mediated by binding to Disabled-2 and PtdIns(4,5)P2. Nat. Cell Biol. 2007;9:176–183. doi: 10.1038/ncb1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternsdorf T, Jensen K, Zuchner D, Will H. Cellular localization, expression, and structure of the nuclear dot protein 52. J. Cell Biol. 1997;138:435–448. doi: 10.1083/jcb.138.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney HL, Houdusse A. What can myosin VI do in cells? Curr. Opin. Cell Biol. 2007;19:57–66. doi: 10.1016/j.ceb.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Swiatecka-Urban A, Boyd C, Coutermarsh B, Karlson KH, Barnaby R, Aschenbrenner L, Langford GM, Hasson T, Stanton BA. Myosin VI regulates endocytosis of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 2004;279:38025–38031. doi: 10.1074/jbc.M403141200. [DOI] [PubMed] [Google Scholar]
- Tang C, Iwahara J, Clore GM. Visualization of transient encounter complexes in protein-protein association. Nature. 2006;444:383–386. doi: 10.1038/nature05201. [DOI] [PubMed] [Google Scholar]
- Tuxworth RI, Titus MA. Unconventional myosins: anchors in the membrane traffic relay. Traffic. 2000;1:11–18. doi: 10.1034/j.1600-0854.2000.010103.x. [DOI] [PubMed] [Google Scholar]
- Warner CL, Stewart A, Luzio JP, Steel KP, Libby RT, Kendrick-Jones J, Buss F. Loss of myosin VI reduces secretion and the size of the Golgi in fibroblasts from Snell's waltzer mice. EMBO J. 2003;22:569–579. doi: 10.1093/emboj/cdg055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AL, Lin AW, Chen LQ, Safer D, Cain SM, Hasson T, Carragher BO, Milligan RA, Sweeney HL. Myosin VI is an actin-based motor that moves backwards. Nature. 1999;401:505–508. doi: 10.1038/46835. [DOI] [PubMed] [Google Scholar]
- Wu H, Reuver SM, Kuhlendahl S, Chung WJ, Garner CC. Subcellular targeting and cytoskeletal attachment of SAP97 to the epithelial lateral membrane. J. Cell Sci. 1998;111:2365–2376. doi: 10.1242/jcs.111.16.2365. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Cheng W, Hung J, Montell D, Geisbrecht E, Rosen D, Liu J, Naora H. Lessons from border cell migration in the Drosophila ovary: a role for myosin VI in dissemination of human ovarian cancer. Proc. Natl. Acad. Sci. USA. 2004;101:8144–8149. doi: 10.1073/pnas.0400400101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef S, Steinman L. At once harmful and beneficial: the dual properties of NF-kappaB. Nat. Immunol. 2006;7:901–902. doi: 10.1038/ni0906-901. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.