Abstract
Integrin αDβ2 (CD11d/CD18) is a multiligand macrophage receptor with recognition specificity identical to that of the major myeloid cell-specific integrin αMβ2 (CD11b/CD18, Mac-1). Despite its prominent upregulation on inflammatory macrophages the role of αDβ2 in monocyte and macrophage migration is unknown. In this study, we have generated model and natural cell lines expressing different densities of αDβ2 and examined their migration to various extracellular matrix proteins. When expressed at a low density, αDβ2 on the surface of recombinant HEK293 cells and murine IC-21 macrophages cooperates with β1/β3 integrins to support cell migration. However, its increased expression on the αDβ2-expressing HEK293 cells and its upregulation by PMA on the IC-21 macrophages results in increased cell adhesiveness and inhibition of cell migration. Furthermore, ligation of αDβ2 with anti-αD blocking antibodies restores β1/β3-driven cell migration by removing the excess αDβ2-mediated adhesive bonds. Consistent with in vitro data, increased numbers of inflammatory macrophages were recovered from the inflamed peritoneum of mice after the administration of anti-αD antibody. These results demonstrate that the density of αDβ2 is critically involved in modulating macrophage adhesiveness and their migration, and suggest that low levels of αDβ2 contribute to monocyte migration while αDβ2 upregulation on differentiated macrophages may facilitate their retention at sites of inflammation.
Keywords: integrin αDβ2, CD11d/CD18, macrophage, cell migration, inflammation
Introduction
The β2 integrins are heterodimeric adhesion receptors expressed on leukocytes which play an important role in the immune-inflammatory response. The group consists of four members, αLβ2 (CD11a/CD18, LFA-1), αMβ2 (CD11b/CD18, Mac-1), αxβ2 (CD11c/CD18, p150, 95) and αDβ2 (CD11d/CD18), which have a common β2 subunit and different, albeit homologous, α subunits. The two related members of the group, αMβ2 and αDβ2, are expressed predominantly on myeloid leukocytes and are strongly upregulated after agonist stimulation on neutrophils and monocytes, respectively. A large number of studies have been performed both in vitro and in vivo to understand the role of the major myeloid cell-specific integrin αMβ2 in neutrophil migration [1]. In the current model of leukocyte trafficking during the inflammatory reaction, αMβ2 does not appear to be important for adhesion to the endothelium but cooperates with αLβ2 in leukocyte emigration from the vessel [2,3]. The same integrins, together with α4β1, have also been implicated in migration of monocytes although αMβ2 and αLβ2 appear to be less important than α4β1 [4,5]. While αMβ2 is required for transendothelial extravasation, it does not support neutrophil migration through the interstitial extracellular matrix (ECM). Instead, neutrophils appear to utilize β1 integrins for migration through tissues [6,2,7,8]. Nevertheless, αMβ2 may have a unique role in neutrophils migration. As studies in different models of inflammation have shown, neutrophils from the αMβ2-deficient mice had enhanced migratory properties since the leukocyte influx was increased ~2–3-fold in the αMβ2-deficient mice compared with wild-type counterparts [9,10,3,11]. Furthermore, using model cells expressing different levels of αMβ2, we have demonstrated that the progressive increase of αMβ2 density inhibited cell migration mediated by β1 integrins [12]. These observations suggest that the major function of αMβ2 is not to support migration but rather to serve as a brake during neutrophil migration through the interstitial space. The basis for this function of αMβ2 is thought to be its unusual stickiness and its ability to bind the same ECM proteins as the β1 integrins. For example, both αMβ2 and α5β1 can adhere to fibronectin [12]. Another important characteristic of αMβ2 is that this receptor can be upregulated ~7–10-fold on the surface of neutrophils in a stepwise manner[1] while the levels of β1 integrins increase modestly [7]. Accordingly, when the density of αMβ2 exceeds that required for optimal β1-driven cell locomotion, migration halts. These observations would be consistent with the idea that the major function of αMβ2 is not migration but neutrophil adhesion and the control of adhesion-dependent leukocyte responses such as degranulation, oxidative burst and phagocytosis at sites of inflammation [10].
We have recently demonstrated that another member of the subfamily, the most recently discovered integrin αDβ2, exhibits multiligand binding properties and has recognition specificity overlapping that of αMβ2 [13]. Specifically, αDβ2 is capable of supporting cell adhesion to various ECM proteins, including fibronectin, vitronectin, fibrinogen, CCN1 (Cyr61) and others. We have also shown that in αDβ2, the αD I-domain is responsible for the binding function and that the mechanism whereby αD I-domain recognizes its ligands is similar to that utilized by αMβ2 [13]. The finding that αDβ2 and αMβ2 have similar recognition specificity and bind a broad assortment of proteins in the ECM suggests that αDβ2 might perform an analogous function in leukocyte migration. Like αMβ2, integrin αDβ2 is poorly expressed on peripheral blood leukocytes [14] and can be rapidly upregulated in response to chemotactic stimulation as a result of its transport to the cell surface from internal secretory granules [14]. However, its expression in neutrophils is much lower than that of αMβ2 [14]. In contrast, αDβ2 levels are gradually increased by de novo synthesis upon monocyte differentiation into macrophages, and oxidized LDL and AcLDL further upregulate its expression [15]. Furthermore, αDβ2 (but not αMβ2) is strongly upregulated on macrophages within atherosclerotic plaques [14]. Such a difference in upregulation of αMβ2 and αDβ2 on neutrophils and monocytes/macrophages, respectively, might indicate their distinct roles in acute and chronic inflammatory responses. The unique pattern of αDβ2 up-regulation by atherogenic lipoproteins suggests a potential role for this integrin in the regulation of monocyte/macrophage migration to extravascular sites and, thus, in the development of atheroscerosis. However, exactly how αDβ2 contributes to monocyte/macrophage migration is still unknown.
In the present study we have examined the migratory properties of αDβ2. We have generated model and natural monocytic cell lines expressing different levels of αDβ2 and tested their migration to various ECM proteins. In addition, a mouse model of inflammation has been used to further clarify the role of αDβ2 in monocyte/macrophage migration in vivo.
Materials and Methods
Proteins and antibodies
Human fibrinogen was obtained from Enzyme Research Laboratories (South Bend, IN). Fibronectin was isolated from fresh human plasma by gelatin-agarose affinity chromatography [16]. Vitronectin was isolated from outdated plasma treated with 8M urea using heparin-agarose affinity chromatography [17]. CCN1 (connective tissue growth factor, Cyr61) was purified as described [18]. The mAb IB4 directed against the β2 integrin subunit and mAb M1/70 which recognizes the mouse αM integrin subunit were purified from the conditioned media of the hybridoma cell line obtained from American Type Culture Collection (Manassas, VA) using protein A agarose (GE Healthcare, Piscataway, NJ). F(ab’)2 preparations of Mab M1/70 were isolated after digestion with pepsin (1:200 w/w). F(ab’)2 retained its full immunoreactivity as assessed by FACS analyses and did not contain intact mAb molecules or its Fc fragments as assessed with goat anti-rat IgG Fc alkaline phosphatase-conjugated antibody (Pierce). Polyclonal antibodies 1950 and 1932 directed against the β1 and β3 integrin subunits, respectively, were purchased from Chemicon Int. (Temecula, CA). MAbs against murine CD14 (PE conjugated), F4/80 (PE conjugated) and αX integrin (FITC conjugated) were from eBioscience (San Diego, CA). Monoclonal antibody against the mouse αV integrin (clone RMV-7) was purchased from Fitzgerald Industries International Inc. (Concord, MA). Polyclonal antibody against the αDI-domain was described previously [13]. The antibody recognizes both human and mouse αDI-domains and has no cross-reactivity with recombinant human and mouse αM, αX and αL I-domains. The antibody was isolated from rabbit serum by affinity chromatography using αDI-domain-Sepharose. Fab preparations were isolated after enzymatic digestion with papain (Sigma-Aldrich, St. Louis, MO) followed by purification by protein A agarose.
Cells
The IC-21 macrophage cell line was obtained from the ATCC. Cells were cultured in RPMI-1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% FBS, 0.1 mg/ml streptomycin, 0.1 unit/ml penicillin, and 2 mM L-glutamine. The αDβ2-expressing HEK 293 cells were generated as described previously [13] and maintained in DMEM/F-12 (Invitrogen) supplemented with 10% FBS, 2 mM glutamine, 15 mM HEPES, 0.1 mg/ml streptomycin, and 0.1 unit/ml penicillin. Populations of cells expressing low and high density of αDβ2 integrin were selected by fluorescence activated cell sorting using a BD Biosciences FACS Vantage Instrument.
Flow cytometry
FACS analyses were performed to assess the expression of integrins on the surface of the αDβ2 transfected HEK 293 cells and the mouse IC-21 macrophage cell line. The cells were incubated with integrin-specific antibodies and analyzed using a FACScan™ (Beckton Dickinson) as described previously [19]. To determine integrin expression on murine peripheral blood monocytes, a population of cells isolated from whole blood by Ficoll fractionation which contained both monocytes and lymphocytes was incubated with a mixture of phycoerythrin-conjugated anti-CD14 (5 µg/ml) and selected FITC-conjugated anti-integrin antibodies (10 µg/ml). Likewise, cells isolated from the mouse peritoneal cavity were incubated with a mixture of the phycoerythrin-conjugated macrophage-specific marker F4/80 (5 µg/ml) and FITC-conjugated anti-integrin antibodies. The levels of integrins were determined using back-gaiting with anti-CD14 and F4/80 mAbs for peripheral blood monocytes and peritoneal macrophages, respectively.
Adhesion and migration assays
For adhesion assays, 96-well tissue culture plates (Costar, Cambridge, MA) were coated with different concentrations of vitronectin for 3 h at 37°C. The wells were post-coated with 0.5% PVA for 1 h at 22°C. The αDβ2-expressing cells were labeled with 10 µM Calcein AM (Molecular Probes, Eugene, OR) and assays were performed as described previously. [12]
Cell migration assays with calcein-labeled cells were performed under sterile conditions using 24-well transwell plates (Costar, Corning, NY) with 8-µM pore size uncoated polycarbonate filters as previously described [20,12]. Lower wells contained 600 µl of vitronectin or other ECM proteins in DMEM/F-12 which during the assay coat both sides of the filter. Upper wells, inserted into the lower wells, contained a final volume of 250 µl after the addition of cells. To begin the assay, 150 µl of cells (2.5 × 106/ml) in medium, with or without mAbs, was added to upper wells and cells were allowed to migrate for 18 h at 37°C in a 5% CO2 humidified atmosphere. In this format, migration is largely directional and cells migrate from the upper chamber to the underside of the filter coated with a ligand [20]. Two hours prior to the completion of the migration assay, cells were labeled with calcein AM which was added to the lower chamber at 10 µM final concentration. Assays were stopped by removing cells from the upper surface of the polycarbonate membrane by wiping the surface twice with a cotton-tipped applicator. Cells migrating to the underside of the filter were detected using a CytoFluorII fluorescence plate reader (Applied Biosystems, Farmington, MA).
Peritoneal Inflammation
Peritonitis was induced by intraperitoneal injection of 0.5 ml of 3% Brewer thioglycollate medium (Sigma-Aldrich, St. Louis, MO) in C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME). After 72 hours the mice were euthanized by isoflurane inhalation and the peritoneal cavities were lavaged with 5 ml PBS. In experiments with antibodies, 100 µg of purified anti-αD polyclonal antibody, Mab M1/70 or normal mouse IgG diluted in PBS were injected intraperitoneally 4 hours before thioglycollate injection. In selected experiments, mice were injected with F(ab’)2 preparations of Mab M1/70 (100 µg). Macrophages were counted by nonspecific esterase assay as described [21]. In selected experiments, the number of cells in total lavage fluid was counted in a hemacytometer and macrophage number was determined by performing differential counts after cytospin and Wright stain. In each experiment, 7 mice were used for a control (non-treated) and 7 mice were used for the treated group.
Statistical analysis
Results are presented as the means ± SD. Statistical analyses were performed using Student’s t test. The value of P<0.05 was considered significant.
Results
Generation of the αDβ2 expressing cells with different densities of receptors and characterization of their adhesive and migratory properties
To examine the role of αDβ2 in migration, we initially examined HEK 293 cells stably transfected with αD and β2 integrin subunits [13]. To generate cells with different levels of αDβ2, the initial heterogeneous population of the αDβ2-expressing cells was sorted into two fractions. Surface expression of αDβ2 was determined by flow cytometry using mAbs directed against the αD and β2 subunits (Fig.1). The mean fluorescence intensities of the two cell lines differed by ~10-fold, indicating that αDβ2 was expressed at different densities. Moderate levels of endogenous integrins α5β1, α2β1, α3β1, α4β1, α6β1 and αVβ1 were detected on the surface of transfected cells in agreement with previous data [12] (not shown). Because adhesion is necessary for cells to obtain traction upon migration, we examined the adhesive capacity of the generated cell lines. In these experiments, vitronectin was selected as a representative ECM ligand and the maximal levels of adhesion were determined. Consistent with the αDβ2 density differential, adhesion of cells expressing low levels of integrin was ~ 2-fold lower than that of high expressors (Fig.2). In previous studies we demonstrated that in the αDβ2-expressing cells both αDβ2 and β1 integrins (apparently αVβ1, a vitronectin receptor in HEK 293 cells) contribute to adhesion to vitronectin [13]. In agreement with these observations, anti-αD function blocking antibody partially inhibited cell adhesion of both cell lines and this effect correlated with the level of αDβ2 expression. Accordingly, anti-αD inhibited adhesion of cells expressing high and low densities of αDβ2 by ~80% and 50%, respectively (Fig.2). Furthermore, mock-transfected HEK 293 cells adhered to vitronectin; however, the adhesion was low because these cells rely only on endogenous αVβ1 for binding to this ligand.
Fig.1. Characterization of HEK 293 cells expressing different levels of αDβ2.
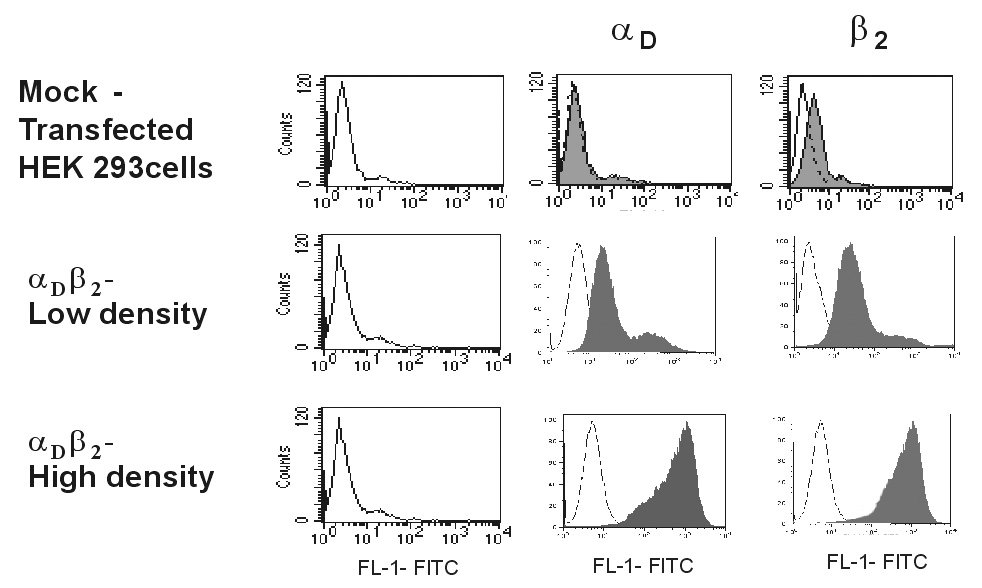
Binding of anti-αD polyclonal antibody (10 µg/ml) and anti-β2 mAb IB4 (10 µg/ml) to mock-transfected and the αDβ2-expressing cells was analyzed by flow cytometry. Results are presented as histograms with the logarithm of fluorescence intensity on the abscissa and the cell number on the ordinate. The level of integrin expression is shown as filled histograms. Control cells incubated with the Alexa Fluor-488 conjugated secondary antibody are shown as open histograms.
Fig.2. Adhesion of HEK 293 cells expressing different densities of αDβ2 and mock-transfected HEK 293 cells to vitronectin.
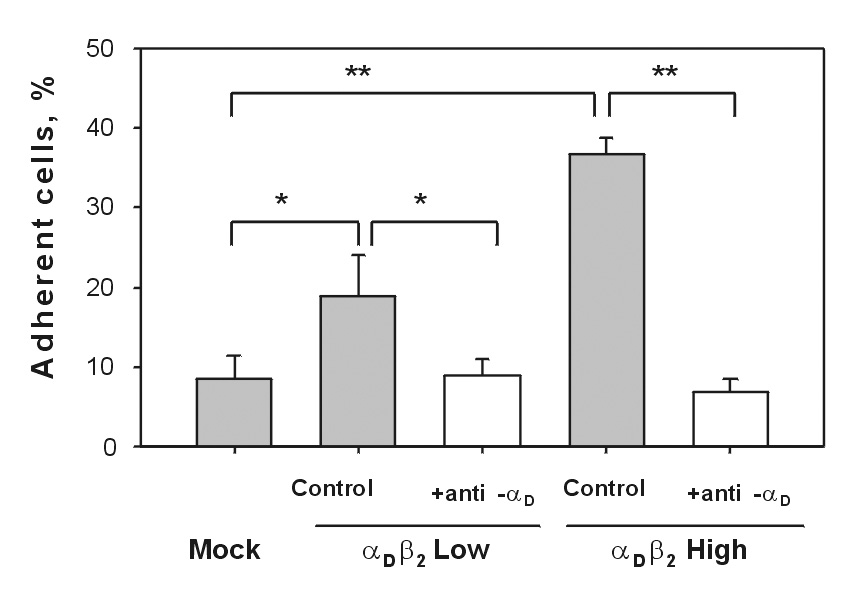
Aliquots of calcein-labeled cells (5×104) were allowed to adhere to wells of 96-well plates coated with 5 µg/ml vitronectin in the absence or presence of blocking anti-αDβ2 antibody (10 µg/ml). After incubation for 30 min at 37°C in 5% CO2, the nonadherent cells were removed by two washes with PBS and fluorescence was measured. The number of adherent cells was calculated by using the fluorescence of aliquots with a known number of labeled cells. Data are expressed as a percentage of added cells and are the mean ± SD of three individual experiments (* denotes P<0.05; ** denotes P<0.01).
To investigate the relationship between the αDβ2 density and cell migration, we compared the ability of cells expressing different levels of integrin to migrate toward vitronectin in a Transwell system. In these experiments, vitronectin (50 µg/ml) was placed in the lower chamber and migration was initiated by adding cells to the upper chamber. Migration was allowed to proceed for 18 h at 37°C, at which time the number of cells adherent to the underside of the filter coated with vitronectin was determined. Migration of the two cell lines to vitronectin was very different (Fig.3A). Both mock-transfected cells and the cell line expressing a low density of αDβ2 migrated efficiently, with migration of the latter cells being 1.7-fold higher. However, migration of cells expressing a high density of αDβ2 was suppressed (by ~3-fold) compared to mock-transfected cells. To examine the contribution of αDβ2 and αvβ1 to migration, cells were preincubated with 20 µg/ml of either anti-αD or anti-β1 function-blocking antibodies before their addition to the upper chamber of the transwell system. Migration of cells with a low density of αDβ2 was inhibited by ~50% and 85%, respectively (Fig.3B). Thus, both αDβ2 and αvβ1 support migration of these cells. However, preincubation of cells expressing a high-density of αDβ2 with anti-αD produced the opposite effect (Fig.3C), i.e., the antibody did not inhibit migration but instead enhanced migration by ~4-fold. At the same time, anti-β1 still blocked migration. Hence, ligation of αDβ2 with anti-αD in these cells could restore migration to the level observed with mock-transfected cells. Since the level of β1 integrins in the two cell lines expressing different densities of αDβ2 is the same, the difference in their migratory ability appears to arise from the dissimilarity in the density of αDβ2. Thus, these results suggest that in both cell lines migration is mediated by αvβ1, and that αDβ2 expressed at a low density contributes to migration. However, when the density of αDβ2 increases it suppresses migration.
Fig.3. Migration of the αDβ2-expresing HEK 293 cells to vitronectin.
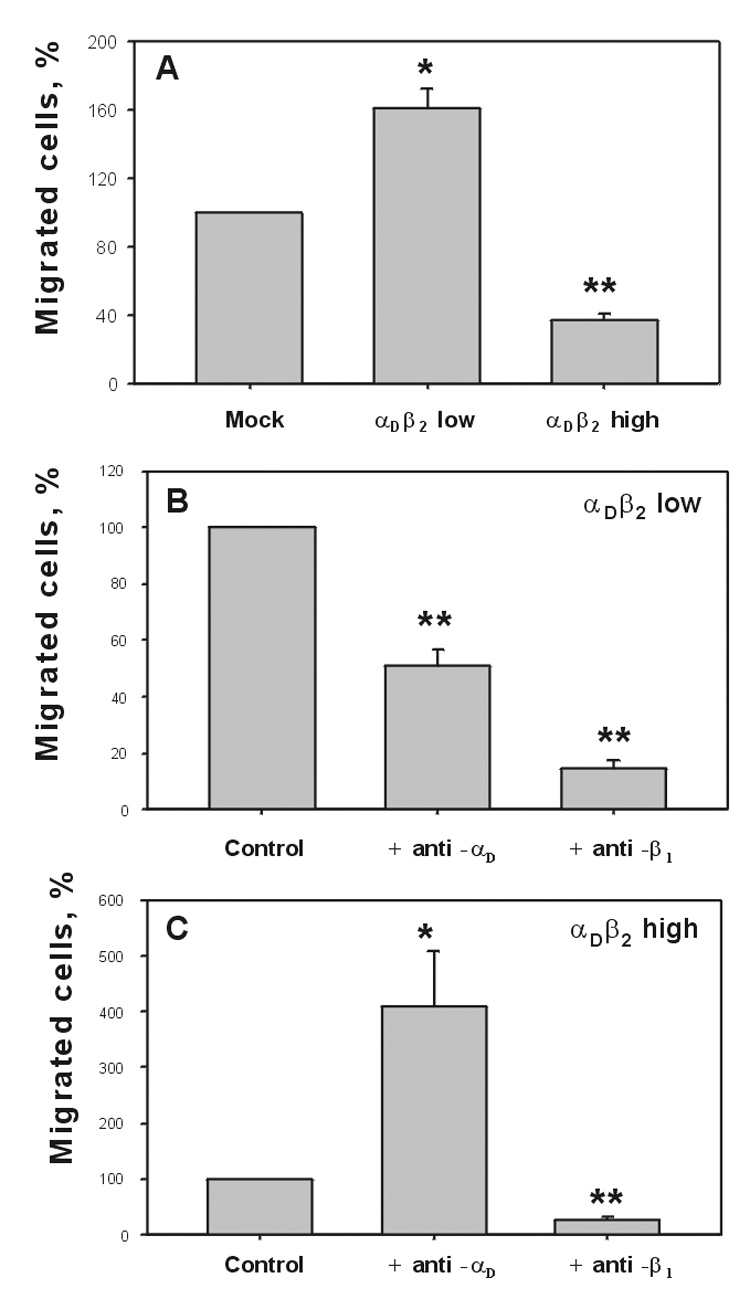
A, HEK 293 cells expressing different densities of αDβ2 were analyzed for their ability to migrate through the Transwell inserts having a pore size of 8 µM and 6.5 mm in diameter. Cells were allowed to migrate toward 50 µg/ml of vitronectin placed in the lower chamber of the Transwell system for 18 hours at 37°C in 5% CO2. Two hours prior to the completion of the migration assay, cells were labeled with calcein AM and fluorescence of cells that migrated to the undersurface of the filter was determined. The cells expressing low (B) and high (C) densities of αDβ2 were analyzed for their ability to migrate to 50 µg/ml of vitronectin in the presence of function blocking polyclonal anti-αD antibody or blocking anti-β1 mAb. Cells were preincubated with 20 µg/ml of each antibody for 20 min before their addition to the upper chamber of Transwell plates. Migration data are expressed as a percentage of the migration of mock-transfected cells (A) or control αDβ2 transfected cells in the absence of antibodies (B, C). Results are the mean ± SD from three to five separate experiments, each with duplicate measurements. (*, P<0.05; **, P<0.01 compared to mock transfected cells in (A) or control (B,C)).
To confirm that the rate of migration in this system depends on the density of αDβ2 but not on the nature of the adhesive ligand, we examined cell migration to different ECM proteins. Previous studies have demonstrated that fibronectin, fibrinogen and CCN1 are ligands for αDβ2 [13]. Furthermore, fibronectin and fibrinogen can interact with the β1 integrin α5β1 [22,23], and CCN1 can engage α6β1 [24]. As shown in Fig.4, pretreatment of cells expressing a high density of αDβ2 with anti-αD antibody significantly enhanced migration to all proteins while the anti-β1 specific antibody inhibited migration. These results indicate that as with vitronectin, the increased density of αDβ2 results in the decline of β1-driven migration to other ligands and that the retarded migration could be restored by the removal of the restraint imposed by the excess of the αDβ2 - ligand bonds. However, the extent to which ligation of αDβ2 by function-blocking anti-αD increased migration was different, which may reflect the relative contribution of αDβ2 and β1 integrins to adhesion. For instance, cell adhesion to CCN1 is mediated predominantly by αDβ2 with a small contribution of β1 integrins [13], and, accordingly, anti-αD exerted a strong stimulating effect on cell migration. In contrast, anti-αD caused a moderate effect on cell migration to fibronectin because both α5β1 and αDβ2 mediate cell adhesion to this ligand [13]. Nevertheless, regardless of these differences the stimulating effect of anti-αD was detected with all ligands suggesting that the increased density of αDβ2 inhibits cell migration to various ECM proteins.
Fig.4. Migration of HEK 293 cells expressing a high density of αDβ2 to various ECM proteins.

HEK 293 cells expressing a high density of αDβ2 were analyzed for their ability to migrate to 50 µg/ml fibrinogen, fibronectin and CCN1 in the absence or in the presence of blocking polyclonal anti-αD antibody or blocking anti-β1 mAb. Cells were preincubated with 20 µg/ml antibodies for 20 min before their addition to the upper chamber of Transwell plates. Cells were allowed to migrate toward ligands for 18 hours at 37°C in 5% CO2 and the extent of cell migration was assessed as described in Materials and Methods. Data are expressed as a percentage of migration; cell migration in the absence of blocking antibodies was assigned a value of 100%. Values represent the means ± SD with duplicate wells for each experiment from three or more individual experiments (*, P<0.05; **, P<0.01 compared to control).
Migratory properties of αDβ2 expressed on the macrophage cell line IC-21
Because macrophage-derived foam cells that are present in atherosclerotic lesions express high levels of αDβ2 [14], we examined the role of αDβ2 in monocyte/macrophage migration. In previous studies, we have demonstrated that the mouse macrophage cell line IC-21 expresses similar levels of αMβ2 and αDβ2 [13]. After stimulation of these cells with 100 ng/ml PMA for 48 h, the level of αDβ2 was upregulated by more than 5-fold while expression of αMβ2 did not change (Fig.5). After 72 h, expression of αDβ2 remained high and gradually returned to the basal levels after 7 days in culture.
Fig.5. Analyses of integrin αDβ2 and αMβ2 expression on mouse IC-21 macrophages after PMA stimulation.

IC-21 cells were stimulated with 100ng/ml PMA for different periods of time and the levels of αMβ2 (open bars) and αDβ2 (black bars) integrins were assessed by flow cytometry using anti-αM mAb M1/70 and polyclonal anti-αD antibody. Results are the mean ± SD values of mean fluorescence from three independent experiments (*, P<0.05; **, P<0.01 compared to αD expression before PMA treatment.)
Since resting and PMA-stimulated IC-21 cells can model the two cell lines with low and high densities of αDβ2, respectively, these cells were tested for their ability to support migration to vitronectin. At least three other integrins on the surface of IC-21 cells, including αVβ1, αVβ3, and αMβ2, can potentially mediate migration to vitronectin. Expression of αM, αV, β1 and β3 integrins did not change significantly after 72 hours of stimulation with PMA (not shown) suggesting that only up-regulation of αDβ2 can modulate cell adhesiveness. Both cell lines were able to migrate to vitronectin but migration of resting IC-21 was reproducibly higher than that of PMA-stimulated cells (Fig. 6A,B) and when compared in the same experiment (not shown). Preincubation of cells with the Fab preparation of the anti-αD polyclonal antibody inhibited migration of resting macrophages indicating that αDβ2 support migration (Fig.6A). No inhibition of migration by control Fab of rabbit IgG was detected. In these studies, we have utilized Fab preparations of anti-αD because preliminary experiments demonstrated that intact rabbit IgG at the concentration 20 µg/ml inhibited migration of IC-21 cells, an effect often encountered in such assays that is attributed to the Fc portion of the antibody molecule. Pretreatment of resting macrophages with either intact anti-αM mAb M1/70 or its F(ab’)2 inhibited migration by ~45%, indicative of the contribution of αMβ2 to cell migration. As a control, rat IgG did not inhibit cell migration. Function blocking antibodies against murine αV, the common subunit for integrins αVβ1 and αVβ3, also inhibited cell migration indicating that either αVβ1 or αVβ3 or both can support migration (Fig. 6A).
Fig.6. Migration of IC-21 cells expressing different levels of αDβ2 to vitronectin.
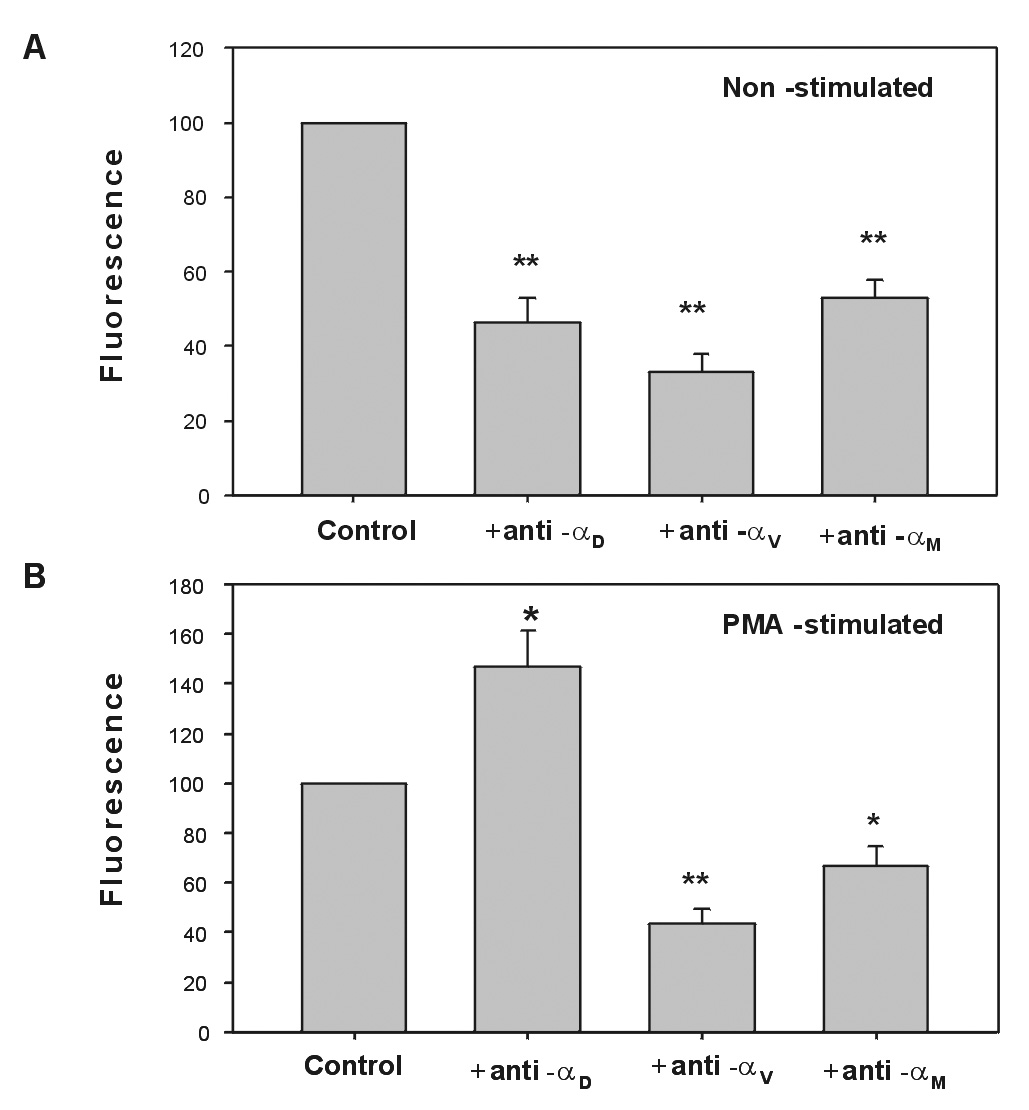
Unstimulated (A) and PMA-stimulated for 72 h (B) IC-21 cells were analyzed for their ability to migrate through the Transwell inserts to 50 µg/ml of vitronectin either in the absence or presence of either Fab preparations of anti-αD or mAb M1/70. Cells were preincubated with 20 µg/ml of each antibody for 20 min before their addition to the upper chambers of Transwell plates. Cells were allowed to migrate toward vitronectin coated on the undersurface of the filter for 18 hours at 37°C in 5% CO2. Data are expressed as a percentage of migrated cells in the absence of blocking antibodies. Results are the mean ± SD values from three to five individual experiments with duplicate wells in each experiment for each reagent tested (*, P<0.04; **, P<0.01 compared to control).
In contrast to resting cells, preincubation of PMA-stimulated macrophages with Fab preparations of anti-αD resulted in an increase of their migration which was significant (Fig.6B). These results are consistent with the effect of anti-αD on migration of HEK 293 cells expressing a high density of αDβ2 and suggest that this integrin can be responsible for the increased adhesiveness of stimulated macrophages as well. By contrast, anti-αM mAb M1/70 produced the opposite effect, i.e., it inhibited macrophage migration. Hence, like in resting macrophages, αMβ2 contributes to migration. Collectively, these data demonstrate that upregulation of αDβ2 in a cell line which naturally expresses this receptor leads to decreased cell migration. In addition, these results distinguish the opposite roles of two integrins in PMA-stimulated macrophages: while αMβ2 promotes migration, αDβ2 inhibits it.
Effect of anti-αD antibody on migration of macrophages to mice peritoneum
To assess the role of αDβ2 in monocyte migration in vivo, we examined the effect of anti-αD antibody on emigration of monocytes into the peritoneal cavity of mice with thioglycollate-induced inflammation. Previous studies demonstrated that significant numbers of monocytes arrive into the peritoneum by 72 h after thioglycollate injection [25], where they apparently differentiate into macrophages. To determine whether αDβ2 upregulation occurs in an environment such as that in inflamed peritoneum, we characterized expression of the integrin on monocytes at 72 h after thioglycollate injection and on monocytes in the circulation. Expression of several other macrophage integrins, including αM, αD, αX, β1 and β3 have also been determined. Fig.7 shows that the levels of αDβ2 and αMβ2 were increased by ~4- and 1.5-fold, respectively, compared to basal levels on peripheral blood monocytes. No increase in the expression of other integrin subunits was detected. Resident macrophages, which constitute approximately 5% the total number of recruited macrophages, contained a low level of αDβ2 and elevated levels of αMβ2 (data not shown). Thus, αDβ2 is the major integrin on the surface of stimulated macrophages elicited in response to inflammatory stimulation.
Fig.7. Analyses of integrin expression on mouse monocytes isolated from peripheral blood and on peritoneal macrophages.
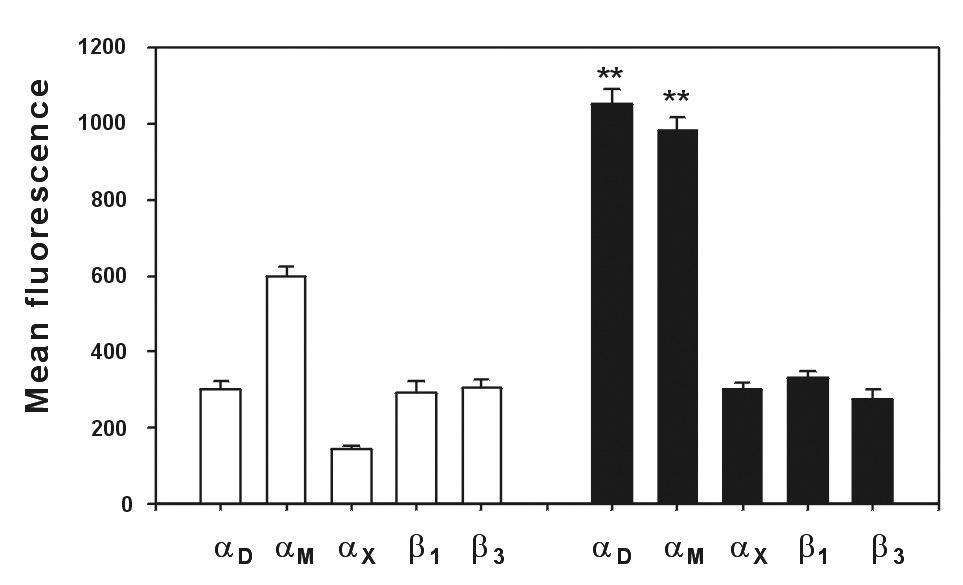
Peripheral blood monocytes were isolated from mouse blood collected by cardiac puncture. Monocytes were obtained as a monocyte-lymphocyte phase after separation using a Ficoll-Paque gradient. Peritoneal macrophages were collected at 72 hours after injection of 0.5 ml 3% thioglycollate by washing peritoneum cavity with 5 ml of PBS. The integrin densities on monocytes (open bars) and macrophages (black bars) were determined by flow cytometry using selected FITC-conjugated mAbs against specific integrin subunits. Monocytes isolated from the peripheral blood and macrophages in the peritoneal lavage were detected using back-gaiting with phycoerythrin-conjugated mAbs against CD14 and F4/80, respectively. Results are the mean ± SD values of mean fluorescence in three or more independent experiments (**, P<0.01 compared to αD and αM expression on monocytes).
To analyze the role of αDβ2 in accumulation of macrophages in the peritoneum, mice were injected with 100 µg of anti-αD antibody 4 hours before injection of thioglycollate. After 72 hours, macrophages in the peritoneal exudate were counted and compared with macrophage levels in a control group injected with rabbit IgG or PBS. The amount of macrophages in the mice treated with anti-αD antibody was increased by 44 ± 8% compared to the control group. Fig.8 shows a representative of four independent experiments. In each experiment, 7 mice were used for the control (non-treated) and 7 mice were used for the treated group. The difference between the groups in each experiment was statistically significant. The results indicate that the blockade of αDβ2 with anti-αDβ2 antibody resulted in the increased numbers of monocyte/macrophage recovered from the peritoneum (Fig.8). In contrast, both mAb M1/70 or its F(ab’) 2 preparation inhibited monocyte migration slightly and this effect was not significant (data not shown). These data suggest that up-regulation of αDβ2 on activated macrophages might either suppress their migration into the peritoneum or enhance their adhesion to the peritoneal wall. Furthermore, αDβ2 and αMβ2 may have different roles in monocyte migration and macrophage accumulation in the peritoneum.
Fig.8. Effect of anti-αD antibodies on macrophage accumulation in the peritoneum.
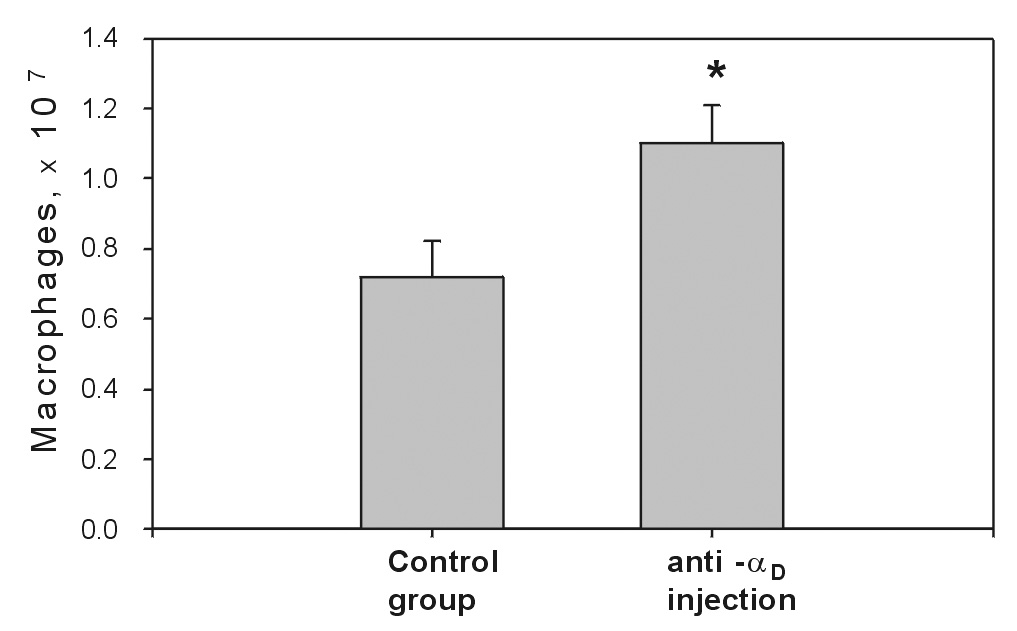
Mice were treated with affinity purified anti-αD polyclonal antibody (100 µg) or rabbit IgG 4 hours before the development of peritonitis. Peritonitis was induced by intraperitoneal injection of 3% Brewer thioglycollate medium (0.5 ml). 72 hours later, the mice were euthanized by isoflurane inhalation, and their peritoneal cavities were lavaged with 5 ml PBS. The numbers of migrated macrophages in the peritoneum were determined by nonspecific esterase assay. The data shown are representative of four separate experiments. The data represent the means ± SD for the control and the treated groups with seven mice in each group. Asterisk denotes the statistically significant difference between the control and treated groups (P < 0.02). This experiment was replicated 4 times with the same results.
Discussion
The β2 integrins play a central role in the inflammatory response by mediating adhesive reactions of leukocytes during their migration from the peripheral blood to extravascular sites of injury. In a previous study we have demonstrated that two members of the group, αMβ2 and the most recently discovered integrin αDβ2, have similar recognition specificity in that they are capable of supporting adhesion to numerous ECM proteins [13]. In addition to their salient multiligand binding properties, another distinctive feature of both integrins is that they are upregulated to high densities on the surface of leukocytes. When coming into play, these qualities may influence adhesive andmigratory properties of leukocytes in unusual ways. Indeed, previous studies proposed that a gradual increase of αMβ2 density on the surface of stimulated neutrophils can serve as a “brake” in the overall process of neutrophil migration [3,12]. The commonality in properties between αMβ2 and its sister integrin αDβ2 suggests that upregulation of αDβ2 may perform a similar function in monocyte/macrophage migration. To define the specific contribution of αDβ2 to the process of macrophage migration, we have utilized αDβ2-expressing HEK 293 cells as well as a murine IC-21 macrophage cell line expressing different levels of αDβ2 and we also compared αDβ2 function with that of αMβ2. In addition, using anti-αD blocking antibodies we have examined the role of αDβ2 in a mouse model of peritonitis. The main findings of our study are that: 1) when expressed at a low density, integrin αDβ2 cooperates with β1/β3 integrins in supporting cell migration; 2) when expressed at a high density, αDβ2 inhibits β1/β3-governed cell migration; and 3) activation of macrophages in vitro and in vivo is accompanied by strong upregulation of αDβ2.
Inhibition of cell migration by integrins expressed at high density is well established. As shown experimentally in studies with transfected cells and based on generated mathematical models, cell migration exhibits a bell-shaped dependence on the cell-substratum adhesiveness with the maximal speed occurring at its intermediate value [26,27,28]. This is an adhesiveness at which the cell can form new attachments at the cell front but break bonds at the rear. The total cell-substratum adhesiveness is a resultant of three variables, including ligand concentration, integrin activation and integrin density [28]. Under conditions when two other parameters are constant, a moderate increase in integrin density enhances the strength of cell adhesion and promotes migration. However, as the receptor density continues to raise, this leads to an increased number of adhesive bonds between integrins and the substratum, which inhibits migration. The behavior of αDβ2 observed in our experiments fits this general model well, i.e. αDβ2 expressed at a low level promotes migration while high density αDβ2 inhibits migration. The conclusion that the increase in the αDβ2-mediated adhesiveness is responsible for inhibition of migration is based on the effect of anti-αD function-blocking antibodies which enhance cell migration to multiple αDβ2 ligands. Thus, disruption the excessive integrin-substrate bonds diminish cell adhesion and restores cell migration.
Although the relationships between integrin density and the rate of migration are evident, they have been established for recombinant cells transfected with only one integrin [28]. A unique feature of myeloid leukocytes (which is also modeled in our experiments with the αDβ2-expressing HEK 293 cells) is that they express different types of integrins, β1 and β2, both of which can engage the same proteins in the ECM. Furthermore, these receptors are expressed differently on various leukocyte populations. While circulating neutrophils express low levels of β1 integrins and their levels are only modestly induced on stimulated cells [29,7], αMβ2 is strongly upregulated on neutrophils migrated into inflamed tissues [10]. Furthermore, as we demonstrate here, β1 and β3 integrins are not induced on migrating monocytes whereas the levels of αDβ2 are increased several fold. Therefore, although both β1/β3 and β2 integrins can support adhesion to the same ligands their function in migration appears to be different: β1/β3 integrins drive migration whereas β2 integrins, through alterations in their density, modulate the β1/β3-driven migration. The role of β1 integrins as primary migratory receptors is also supported by our data with anti-β1 function-blocking mAbs which invariably inhibited migration of the αDβ2-transfectd HEK 293 cells to various ECM proteins. In addition, blocking the common αV subunit of αVβ1 and αVβ3 blocked migration of IC-21 macrophages. However, αDβ2, when expressed at a low density, can contribute to migration as evidenced by the following data: 1) anti-αD antibodies partially inhibited cell migration; and 2) migration of the αDβ2-transfected cells expressing a low density of integrin was enhanced compared to mock-transfected cells (Fig.3A). In this regard, previous in vivo studies demonstrated that anti-αDβ2 antibody inhibited the accumulation of monocytes and neutrophils at sites of spinal cord injury [30]. Since infiltration of leukocytes was determined 48 h after injury, the period when αDβ2 seems not to be significantly upregulated, the anti-αDβ2 antibody might exert the inhibitory effect on leukocyte extravasation. Thus, depending on its density, αDβ2 may have a dual role in monocyte/macrophage migration, both pro-migratory and anti-migratory.
Previous studies demonstrated that expression of αDβ2 requires de novo synthesis and occurs during in vitro differentiation of human peripheral blood monocytes to macrophages in the presence of selected cytokines [15]. Moreover, oxLDL and AcLDL have been identified as stimuli that augment αDβ2 gene expression [15]. Also, activation with PMA can induce αD mRNA in HL60 monocytic cells [15] and upregulate αDβ2 surface expression in isolated eosinophils [31]. Consistent with these findings, culturing murine macrophages with PMA led to a 5-fold increase in αDβ2 levels after 48–72 h whereas the levels of αMβ2 remained unaltered (Fig.5). Our data that both anti-αD and anti-αM antibodies inhibited cell migration of non-stimulated cells in vitro suggest that both αMβ2 and αDβ2 support migration. However, on PMA-stimulated cells, over-expressed αDβ2 suppressed migration while αMβ2 still supported migration. Thus, in these cells αMβ2 appears to provide no added contribution to the total cell adhesiveness which arises mainly from the excess of αDβ2.
An important finding of the present study is that macrophages elicited by thioglycollate injection upregulated αDβ2 by ~4-fold after 72 h indicating that physiologic activators in the inflamed peritoneum also induce αDβ2 synthesis in vivo. The anti-αD antibodies increased the number of macrophages recruited in the peritoneal cavity after 72 h of inflammatory stimulation. Thus, the data with blocking anti-αD antibodies in vitro and in vivo are entirely consistent and, therefore, the blockade of excessive adhesive bonds formed between αDβ2 and its ligands may explain the increased recovery of macrophages from the peritoneal cavity. However, different mechanisms may account for this effect. First, the anti-αD antibodies potentially may inhibit increased αDβ2-mediated monocyte adhesion to the ECM proteins in the mesenteric wall as monocytes move from circulation into the peritoneal cavity, thus resulting in their augmented migration. Nevertheless, a pattern of αDβ2 upregulation seems to argue against the involvement of αDβ2 during post-endothelial monocyte migration. Since αDβ2 synthesis occurs slowly over a 72 h period it is reasonable to assume that monocytes upregulate αDβ2 only after they have traversed the peritoneal wall and develop into inflammatory macrophages. Therefore, the most probable interpretation is that αDβ2 is responsible for enhanced macrophage adhesion to the peritoneal wall and their retention in the peritoneal cavity. Hence, the increased number of macrophages recovered from the peritoneal fluid likely reflects the inhibitory effect of anti-αD antibodies on adhesion of inflammatory macrophages to the peritoneal wall. Further studies using different models of inflammation may help to define how αDβ2 contributes to monocyte/macrophage recruitment and their retention at sites of inflammation.
The increased expression of αDβ2 on macrophage foam cells [14] suggests that αDβ2 may play roles in the development of atherosclerosis. Based upon our present results, it is tempting to speculate that upregulation of αDβ2 on differentiated macrophages can increase cell adhesiveness, resulting in the retention of macrophages in the atherosclerotic lesions and thereby evoking the development of atherosclerosis. It appears that αDβ2 upregulation on inflammatory macrophages contrasts that of αMβ2. Analogous to our data that αMβ2 is not upregulated on IC-21 cells, surface expression of this integrin on elicited peritoneal macrophages was augmented to a lesser extent than that of αDβ2 (1.5- versus 4-fold). Furthermore, expression of αMβ2 has been downregulated in atherosclerotic lesion-derived macrophages [32] and αMβ2-deficiency has not influenced the development of atherosclerosis [33]. However, since β2-deficiency does protect against atherosclerosis [34], other β2 integrins appear to play roles in lesion progression. Given the remarkable pattern of αDβ2 upregulation by atherogenic lipids, as well as overexpression of αDβ2 ligands such as CCN1 in the atherosclerotic lesions [35] the potential role of this integrin in disease progression merits further investigation.
Acknowledgments
Supported by the National Institutes of Health (TPU and SC-TL), and the American Heart Association (TPU and VPY).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kishimoto TK, Baldwin ET, Anderson DC. Inflammation: Basic Principles and Clinical Correlates. 3rd. 1999. pp. 537–569. [Google Scholar]
- 2.Henderson RB, Lim LHK, Tessier PA, Gavins FNE, Mathies M, Perretti M, Hogg N. The use of lymphocyte function-associated antigen (LFA)-1-deficient mice to determine the role of LFA-1, Mac-1, and α4 integrin in the inflammatory response of neutrophils. J.Exp.Med. 2001;194:219–226. doi: 10.1084/jem.194.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding ZM, Babensee JE, Simon SI, Lu HF, Perrard JL, Bullard DC, Dai XY, Bromley SK, Dustin ML, Entman ML, Smith CW, Ballantyne CM. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J.Immunol. 1999 Nov 1;163:5029–5038. [PubMed] [Google Scholar]
- 4.Issekutz TB. In vivo blood monocyte migration to acute inflammatory reactions, IL-1a, TNF-a, INF-g, and C5a utilize LFA-1, Mac-1, and VLA-4. J.Immunol. 1995;154:6533–6540. [PubMed] [Google Scholar]
- 5.Henderson RB, Hobbs JAR, Mathies M, Hogg N. Rapid recruitment of inflammatory monocytes is independent of neutrophil migration. Blood. 2003;102:328–335. doi: 10.1182/blood-2002-10-3228. [DOI] [PubMed] [Google Scholar]
- 6.Shang XZ, Issekutz AC. β2 (CD18) and β1(CD29) integrin mechanisms in migration of human polymorphonuclear leucocytes and monocytes through lung fibroblast barriers: shared and distinct mechanisms. Immunology. 1997;92:527–535. doi: 10.1046/j.1365-2567.1997.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Werr J, Johansson J, Eriksson EE, Hedqvist P, Ruoslahti E, Lindbom L. Integrin α2β1 (VLA-2) is a principal receptor used by neutrophils for locomotion in extravascular tissue. Blood. 2000;95:1804–1809. [PubMed] [Google Scholar]
- 8.Ibbotson GC, Doig C, Kaur J, Gill V, Ostrovsky L, Fairhead T, Kubes P. Functional alpha4-integrin: a newly identified pathway of neutrophil recruitment in critically ill septic patients. Nature Med. 2002;7:465–470. doi: 10.1038/86539. [DOI] [PubMed] [Google Scholar]
- 9.Coxon A, Rieu P, Barkalow FJ, Askari S, Sharpe AH, Von Andrian UH, Arnaout MA, Mayadas TN. A novel role for the beta 2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity. 1996;5:653–666. doi: 10.1016/s1074-7613(00)80278-2. [DOI] [PubMed] [Google Scholar]
- 10.Lu H, Smith CW, Perrard J, Bullard D, Tang L, Entman ML, Beaudet AL, Ballantyne CM. LFA-1 is sufficient in mediating neutrophil emigration in Mac-1 deficient mice. J.Clin.Invest. 1997;99:1340–1350. doi: 10.1172/JCI119293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prince JE, Brayton CF, Fosset MC, Durand JA, Kaplan SL, Smith CW, Ballantyne CM. The differential roles of LFA-1 and Mac-1 in host defense against systemic infection with Streptococcus pneumoniae. J.Immunol. 2001;166:7362–7369. doi: 10.4049/jimmunol.166.12.7362. [DOI] [PubMed] [Google Scholar]
- 12.Lishko VK, Yakubenko VP, Ugarova TP. The interplay between Integrins αMβ2 and α5β1 during cell migration to fibronectin. Exp.Cell Res. 2003;283:116–126. doi: 10.1016/s0014-4827(02)00024-1. [DOI] [PubMed] [Google Scholar]
- 13.Yakubenko VP, Yadav SP, Ugarova TP. Integrin αDβ2, an adhesion receptor up-regulated on macrophage foam cells, exhibits multiligand binding properties. Blood. 2006;107:1643–1650. doi: 10.1182/blood-2005-06-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van der Vieren M, Le Trong H, St.John T, Staunton DE, Gallatin WM. A novel leukointegrin, αdβ2, binds preferentially to ICAM-3. Immunity. 1995;3:683–690. doi: 10.1016/1074-7613(95)90058-6. [DOI] [PubMed] [Google Scholar]
- 15.Noti JD. Expression of the myeloid-specific leukocyte integrin gene CD11d during macrophage foam cell differentiation and exposure to lipoproteins. International Journal of Molecular Medicine. 2002;10:721–727. [PubMed] [Google Scholar]
- 16.Vuento M, Vahery A. Purification of fibronectin from human plasma by affinity chromatography under non-denaturing conditions. Biochem.J. 1979;183:331–337. doi: 10.1042/bj1830331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yatohgo T, Izumi M, Kashiwagi H, Hayashi M. Novel purification of vitronectin from human plasma by heparin affinity chromatography. Cell Struct.Funct. 1988;13:281–292. doi: 10.1247/csf.13.281. [DOI] [PubMed] [Google Scholar]
- 18.Kireeva ML, Mo FE, Yang GP, Lau LF. Cyr61, a product of a growth factor-inducible immediate-early gene, promotes cell proliferation, migration, and adhesion. Mol.Cell.Biol. 1996;16:1326–1334. doi: 10.1128/mcb.16.4.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yakubenko VP, Lishko VK, Lam SCT, Ugarova TP. A molecular basis for integrin αMβ2 ligand binding promiscuity. J.Biol.Chem. 2002;277:48635–48642. doi: 10.1074/jbc.M208877200. [DOI] [PubMed] [Google Scholar]
- 20.Forsyth CB, Solovjov DA, Ugarova TP, Plow EF. Integrin αMβ2-mediated cell migration to fibrinogen and its recognition peptides. J.Exp.Med. 2001;193:1123–1133. doi: 10.1084/jem.193.10.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang L, Ugarova TP, Plow EF, Eaton JW. Molecular determinants of acute inflammatory responses to biomaterials. J.Clin.Invest. 1996;97:1329–1334. doi: 10.1172/JCI118549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pytela R, Pierschbacher MD, Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985;40:191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- 23.Suehiro K, Gailit J, Plow EF. Fibrinogen is a ligand for integrin α5β1 on endothelial cells. J.Biol.Chem. 1997;272:5360–5366. doi: 10.1074/jbc.272.8.5360. [DOI] [PubMed] [Google Scholar]
- 24.Leu SJ, Lam SC, Lau LF. Pro-angiogenic activities of Cyr61 (CCN1) mediated through integrins alphavbeta3 and alpha6beta1 in human umbelical vein endothelial cells. J.Biol.Chem. 2002;277:46248–46255. doi: 10.1074/jbc.M209288200. [DOI] [PubMed] [Google Scholar]
- 25.Ploplis VA, French EL, Carmeliet P, Collen D, Plow EF. Plasminogen deficiency differentially affects recruitment of inflammatory cell populations in mice. Blood. 1998;91:2005–2009. [PubMed] [Google Scholar]
- 26.DiMilla PA, Barbee K, Lauffenburger DA. Mathematical model for the effects of adhesion and mechanics on cell migration speed. Biophys.J. 1991;60:15–37. doi: 10.1016/S0006-3495(91)82027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DiMilla PA, Stone JA, Quinn JA, Albelda SM, Lauffenburger DA. Maximal migration of human smooth muscle cells on fibronectin and type IV collagen occurs at an intermediate attachment strength. J.Cell Biol. 1993;122:729–737. doi: 10.1083/jcb.122.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 29.Werr J, Xie X, Hedqvist P, Ruoslahti E, Lindbom L. β1 integrins are critically involved in neutrophil locomotion in extravascular tissue in vivo. J.Exp.Med. 1998;187:2091–2096. doi: 10.1084/jem.187.12.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mabon PJ, Weaver LC, Dekaban GA. Inhibition of monocyte/macrophage migration to a spinal cord injury site by an antibody to the integrin alphaD: a potential new anti-inflammatory treatment. Exp.Neurol. 2000;166:52–64. doi: 10.1006/exnr.2000.7488. [DOI] [PubMed] [Google Scholar]
- 31.Grayson MH, Van der Vieren M, Sterbinsky SA, Gallatin WM, Hoffman PA, Staunton DE, Bochner BS. αDβ2 Integrin is expressed on human eosinophils and functions as an alternative ligand for vascular cell adhesion molecule 1 (VCAM-1) J.Exp.Med. 1998;188:2187–2191. doi: 10.1084/jem.188.11.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gray JL, Shankar R. Down regulation of CD11b and CD18 expression in atherosclerotic lesion- derived macrophages. Am.Surg. 1995;61:674–679. [PubMed] [Google Scholar]
- 33.Kubo N, Boisvert WA, Ballantyne CM, Curtiss LK. Leukocyte CD11b expression is not essential for the development of atherosclerosis in mice. J.Lipid Res. 2000;41:1060–1066. [PubMed] [Google Scholar]
- 34.Nageh MF, Sandberg ET, Marotti KR, Lin AH, Melchior EP, Bullard DC, Beaudet AL. Deficiency of inflammatory cell adhesion molecules protects against atherosclerosis in mice. Arterioscler.Thromb.Vasc.Biol. 1997;17:1517–1520. doi: 10.1161/01.atv.17.8.1517. [DOI] [PubMed] [Google Scholar]
- 35.Schober JM, Chen N, Grzeszkiewicz T, Emeson EE, Ugarova TP, Ye RD, Lau LF, Lam SCT. Identification of integrin αMβ2 as an adhesion receptor on peripheral blood monocytes for Cyr61 and connective tissue growth factor, immediate-early gene products expressed in atherosclerotic lesions. Blood. 2002;99:4457–4465. doi: 10.1182/blood.v99.12.4457. [DOI] [PubMed] [Google Scholar]


