Abstract
Herein we describe detailed characterization of four common mutations (L302P, H421Y, R496L and ΔR608) within the acid sphingomyelinase (ASM) gene causing Types A and B Niemann-Pick disease (NPD). In vitro and in situ enzyme assays revealed marked deficiencies of ASM activity in NPD cell lines homoallelic for each mutation, although western blotting and fluorescent microscopy showed that the mutant ASM polypeptides were expressed at normal levels and trafficked to lysosomes. Co-immunoprecipitation of the polypeptides with the ER chaperone, BiP, confirmed these findings, as did in vitro expression of the mutant cDNAs in reticulocyte lysates. We further developed a computer assisted, three-dimensional model of human ASM based on homologies to known proteins, and used this model to map each NPD mutation in relation to putative substrate binding, hydrolysis and zinc binding domains. Lastly, we generated transgenic mice expressing the R496L and ΔR608 mutations on the complete ASM knock-out background (ASMKO), and established breeding colonies for the future evaluation of enzyme enhancement therapies. Analysis of these mice demonstrated that the mutant ASM transgenes were expressed at high levels in the brain, and in the case of the ΔR608 mutation, produced residual ASM activity that was significantly above the ASMKO background.
Keywords: Lysosomal storage disease, mutations, transgenic mouse
Introduction
Types A and B Niemann-Pick disease (NPD) are two clinically distinct forms of a lysosomal storage disorder resulting from the inherited deficiency of acid sphingomyelinase activity (ASM, sphingomyelin phosphodiesterase, EC 3.1.4.12) [1]. Type A NPD is the severe, neurodegenerative form characterized by progressive psychomotor retardation early in infancy, hepatosplenomegaly and death by 3 years of age. In contrast, type B NPD patients display little or no CNS involvement, and often survive into adulthood. However, type B NPD is clinically heterogeneous, and patients may present in childhood or adulthood with a variety of symptoms including hepatosplenomegaly, growth retardation, respiratory problems and hematological abnormalities. Both forms of NPD are panethnic, although most reported cases of the type A form occur among Ashkenazi Jewish individuals. In contrast, type B NPD is most prevalent in individuals of Turkish, Arabic or North African descent [2].
The genomic sequence and organization of the human ASM gene (SMPD1) has been determined [3], and numerous mutations causing types A and B NPD have been published [e.g. 4 – 12]. Among these, several common SMPD1 mutations have been identified in individual populations. For example, two common missense mutations (L302P and R496L) account for ~60 % of the mutant SMPD1 alleles in Ashkenazi Jewish patients with type A NPD [5, 7]. ΔR608, on the other-hand, is a panethnic mutation that always results in type B NPD (i.e., neuroprotective) [6]. ΔR608 occurs on ~15 % of all mutant type B NPD alleles in North America [2], and on a much higher percentage of mutant alleles among type B NPD patients from North Africa [13]. In addition, the H421Y missense mutation is the most common NPD mutation in Saudi Arabia and leads to a particularly severe, non-neurological form of the disorder [2].
At present there are no known treatments available for NPD. Bone marrow transplantation may provide visceral organ correction [14, 15], although complications arising from the transplant procedure are often severe and HLA-compatible donors are difficult to find. Enzyme replacement therapy remains a viable alternative for non-neurological NPD patients [16], and a clinical trial evaluating the safety of this approach is currently underway.
Other treatment options include small molecule therapies that act to increase the residual activity of the mutant ASM polypeptides [17]. However, the development of such enzyme enhancement approaches are currently hampered by the lack of data on how individual mutations in the SMPD1 gene affect ASM stability and trafficking, as well as mouse models that express the specific mutant polypeptides. Computer-assisted structural models are also necessary to help predict the effect of individual SMPD1 mutations on ASM function. To date, one model of ASM’s structure has been developed based on similarities to kidney bean purple acid phosphatase [18].
To gain a better understanding of how individual SMPD1 mutations influence the ASM polypeptide, as well as to improve genotype/phenotype correlations for this disorder, we have studied the structural and functional properties of four common NPD mutations (L302P, H421Y, R496L and ΔR608) using primary skin fibroblasts harvested from homoallelic patients. We demonstrate that the mutant ASM proteins are expressed at normal levels and trafficked to lysosomes, and that the only differences observed were in enzymatic activity. We have also developed a three-dimensional model of human ASM based on the known crystal structures of human saposin and β-glucuronidase, as well as kidney bean purple acid phosphatase. The substrate binding and catalytic sites were further identified by homologies with human phospholipases A2 and C, and the earthworm sphingomyelin-binding protein, lysenin. Lastly, we have constructed two new transgenic mouse models expressing the R496L and ΔR608 mutations on the complete ASMKO background, and studied the transgene expression and residual ASM activity in two clinically relevant organs, liver and brain.
Materials and methods
Cell culture
Human skin fibroblasts and HEK293T17 cells were maintained in DMEM containing 2 mM L-glutamine and supplemented with 10 % (v/v) FBS and 1 % (v/v) penicillin-streptomycin (Invitrogen, Carlsbad, CA). For serum starvation experiments, skin fibroblasts were cultured in DMEM containing 2 mM L-glutamine and supplemented with 2 % (v/v) FBS and 1 % (v/v) penicillin-streptomycin. The normal and NPD skin fibroblasts were established from biopsies obtained at the Mount Sinai Medical Center after informed consent and with the approval of our Institutional Review Board.
Total protein determination
Protein levels were quantified using the Bio-Rad Protein Assay Kit (Bio-Rad Laboratories, Hercules, CA) using BSA as a standard.
In vitro acid sphingomyelinase assay
Assays were performed as previously described [19]. Fibroblast cell extracts were prepared by freeze-thawing (x 3) in 100 µl PBS/0.2 % (v/v) Triton X-100. Tissue extracts were prepared by homogenizing in 1 ml 20 mM HEPES/0.2 % (v/v) Igepal. The homogenates were centrifuged at 13,000 x g for 30 min at 4°C and the cleared supernatants removed. Aliquots were then used for protein determination. The remaining supernatants were mixed with an equal volume of BODIPY-C12-sphingomyelin (B12SM; 100 µM final concentration) (Molecular Probes, Eugene, OR) that had been previously diluted in assay buffer (100 mM sodium acetate pH 5, 0.1 mM ZnCl2 and 0.2 % (v/v) Igepal CA-630), and the reactions were incubated at 37°C for 60 min. The reactions were then terminated by the addition of 100 % (v/v) ethanol, and the hydrolytic BODIPY-C12-ceramide (B12CER) product was detected and quantified by HPLC analysis using a reversed-phase column (Aquasil C-18, Keystone Scientific, Inc., St. Marys, PA).
In situ acid sphingomyelinase assay
B12SM was diluted in cell culture medium (2.3 nmole/ml final concentration) and added to the human skin fibroblasts. The cells were incubated at 37°C for 5 h. The labeling solution was then replaced with fresh culture medium and the cells were incubated for a further 16 h at 37°C. Following this “pulse/chase” labeling, fibroblast cell extracts were prepared as above, and aliquots were removed for protein determination. Lipids were extracted from the remainder of the extracts by the addition of 500 µl of chloroform:methanol:water [4:2:1 (v/v)] and sonicating for 5 min at room temperature. The extracts were centrifuged at 5000 x g for 2 min at room temperature, and the chloroform layer was removed and evaporated. The dried lipid extracts were resuspended in 50 µl of 100 % ethanol, and the hydrolytic B12CER products were detected and quantified by HPLC as described above.
Immunoblotting
Fibroblast cell extracts were prepared as above and aliquots were removed for protein determination. Cell extracts (10 µg) or purified, human recombinant ASM (hrASM) (100 ng) as a control were mixed with 2 x Sample Loading Buffer (Bio-Rad Laboratories, Hercules, CA), resolved by SDS-PAGE and transferred onto nitrocellulose membranes. Membranes were blocked by overnight incubation at 4°C in 20 % (w/v) milk powder dissolved in PBS and 0.1 % (w/v) Tween 20 (PBST). They were then rinsed in PBST and incubated at room temperature for 1 h in the presence of a polyclonal anti-ASM antibody (1:5000) [20] in 5 % (w/v) milk powder in PBST. The membrane was washed again in PBST, and incubated at room temperature for 1 h in the presence of a HRP conjugated anti-rabbit IgG antibody (1:5000) suspended in 5 % (w/v) milk powder in PBST. The membrane was then washed and developed using the ECL Plus Western Blotting Detection System (GE Healthcare, Buckinghamshire, UK). It was subsequently stripped at 60°C for 1 h by incubating in 62 mM Tris-HCl (pH 7.4), 100 mM β-mercaptoethanol, 2 % (w/v) SDS, and re-probed using β-actin antibody as a loading control (see above).
BiP Immunoprecipitation
Dynabead Protein A beads (Dynal Biotech Inc., Lake Success, NY) were vortexed for 2 min and a 100 µl aliquot was removed. The beads were washed and resuspended in 500 µl PBS using the Dynal MPC-S apparatus (Dynal Biotech Inc., Lake Success, NY). A 100 µl aliquot of the washed beads was added to 1 µg BiP antibody (BD Biosciences, San Jose, CA), and the reaction was incubated for 1 h at room temperature on a rotary mixer to allow antibody capture. The BiP-Protein A complexed beads were then washed and resuspended in 500 µl PBS/0.1 % (v/v) Tween 20. Fibroblast cell extracts were prepared as above and aliquots were removed for protein determination. Cell extract (100 µg) was added to 100 µl of BiP-Protein A complexed beads and the reaction was incubated at 4°C overnight. The beads were then washed with 100 µl ice cold PBS and resuspended in 10 µl of 1 x Sample Loading Buffer (Bio-Rad Laboratories, Hercules, CA). The samples were resolved by SDS-PAGE and immunoblotted using anti-ASM antibody as described above.
Immunostaining
Human skin fibroblasts grown on glass chamber slides were fixed in 4 % (v/v) paraformaldehyde for 25 min at room temperature. The slides were washed in PBS, and then blocked for 2 h at room temperature in PBS containing 10 % (v/v) normal goat serum and 0.1 % (v/v) Tween 20. The slides were briefly rinsed with PBS and then incubated at 4°C overnight with primary antibodies for ASM (1:2000), Calnexin (1:100) (Abcam Inc., Cambridge, MA) and Eea1 (1:100) (BD Biosciences, San Jose, CA) in PBS containing 5 % (v/v) normal goat serum and 0.1 % (v/v) Tween 20. The slides were washed in PBS and incubated for 1 h at room temperature with anti-rabbit or mouse secondary antibodies conjugated to Cy2 (green) or Cy3 (red) fluorophores (1:200) (Jackson Immunoresearch, West Grove, PA). Slides were washed in PBS, mounted in Vectashield containing 4',6-diamidino-2-phenylindole (DAPI) (Vector Laboratories Inc., Burlingame, CA), and analyzed by fluorescence microscopy. For lysosomal staining, lysotracker (Invitrogen, Carlsbad, CA) was diluted in cell culture medium (50 µm final concentration) and added to the fibroblasts grown on glass chamber slides. The cells were incubated at 37°C for 2 h and then processed for ASM immunostaining as described above.
Mutagenesis
Total RNA was extracted from normal human skin fibroblasts using the TRI reagent (Sigma, St. Louis, MO), and cDNA was synthesized from 500 ng of total RNA using the AccuScript High Fidelity First Strand cDNA Synthesis Kit (Stratagene, La Jolla, CA). PCR reactions were performed using the Pfu Turbo Polymerase Kit (Stratagene, La Jolla, CA) and contained 500 ng cDNA template and 0.5 µm of hASM forward (5′-CGCGAATTCGAACCAGCCCCGTGTAGG-3′) and reverse (5′-CGCAAGCTTAGCATTTGGGCTTTTTCACC-3′) primers. Amplified human ASM cDNA products were cut with Eco RI and Hind III and ligated into pGEM-7ZF(−) (Promega Corporation, Madison, WI). Recombinant plasmids were isolated using the QIAprep Spin Miniprep Kit (Qiagen Inc., Valencia, CA) and sequenced. The four NPD mutations were introduced into the cloned human ASM cDNA using the GeneEditor In Vitro Site Directed Mutagenesis System (Promega Corporation, Madison, WI) and the following mutagenic oligonucleotides: L302P (5′-ACCACCGTCACAGCACCTGTGAGAAGTTCCTGGGG-3′), H42IY (5′-GACAAAGTGTATATAATTGGC-3′), R496L (5′-CCTGGTTACCTTGT-GTACCAAATAGATGG-3′) and ΔR608 (5′-GACAGCCCTGCTCTGTGC⇓CACC-TGATGCCAGATGGG-3′). Plasmids were isolated and sequenced as described above.
Reticulocyte translation
cDNAs encoding normal human ASM and the selected NPD mutations were translated using the TnT Coupled Reticulocyte Lysate System (Promega Corporation, Madison, WI) and 35S labeled methionine (Perkin Elmer, Waltham, MA). The reactions were mixed with 2 x Sample Loading Buffer (Bio-Rad Laboratories, Hercules, CA) and resolved by SDS-PAGE. Gels were incubated in PBS containing 7 % (v/v) acetic acid/7 % (v/v) methanol/1 % (v/v) glycerol for 5 min at room temperature, dried and exposed to x-ray film at −80°C overnight.
Protein Sequence Information
Information from six proteins was used for the modeling. Three were used to predict the three-dimensional structure of ASM (accession numbers correspond to the NCBI protein database): human saposin (2RB3 A), human β-glucuronidase (AAD14101), and kidney bean purple acid phosphatase (4KBP D). Another three were used to predict the substrate binding and catalytic sites: human phospholipase A2 (AAF09020), human phospholipase C (BAA07688) and earthworm lysenin (O18423).
Model building and energy minimization
Three-dimensional modeling was carried out using a computer program (Modeller) designed for comparative protein structure modeling by satisfaction and spatial restraints [24]. Published structures of saposin, β-glucuronidase, and kidney bean purple acid phosphatase were used to generate the model. Identical residue pairs were assumed to have the same conformation as in the templates, and were kept. The conformation information for the remaining, unmatched residues was obtained from the database throughout the initial model building stages. The model was subjected to structure qualification using the RCSB PDB Validation Server and Biotech PDB Validation Service. Structure refinement was performed using the Swiss-PDB Viewer, and energy minimization was determined using the SCULP2.6 software (Interactive Simulations Inc., San Diego, CA).
Protein sequence alignments and secondary structure
To identify regions of similarity between ASM, phospholipases A2 and C, and lysenin (Fig. 4), alignments were generated using the Blast 2.0 program at the National Center for Biotechnology Information Server (NCBI) and the ClustalW (version 1.7) program at the GenomeNet ClustalW Server. To obtain a consensus secondary structure, the following programs were used: SOPMA [21], Multivariate Linear Regression Combination [22] and Conserved Domains from NCBI [23]. All known zinc-binding sites were extracted from the PDB and classified into several groups according to structural motifs. The zinc-binding motifs in ASM were predicted by aligning the amino acid sequence against the structural patterns of each group using the Iditis software.
Fig. 4. Protein alignment of ASM with lysenin and phospholipases A2 and C.
Sequences were extracted from the SWISSPROT database and the Protein Data Bank (PDB), and aligned using ClustalW (Version 1.7). (A) Predicted substrate binding site of ASM aligned with lysenin (LYS). Numbers above the ASM sequence indicate the position of the amino acids in the ASM protein. Asterisks under the sequence represent amino acids bearing similar biochemical properties. (B) Predicted active site of ASM aligned with phospholipase C (PLC). Numbers above the ASM sequence indicate the position of the amino acids in the ASM protein. Asterisks under the sequence represent amino acids bearing similar biochemical properties. Colons under the sequence represent conserved histidine residues involved in metal binding. (C) Homology between ASM and the predicted active site region of phospholipase A2 (PA2). The underlined area indicates the critical active site region of phospholipase A2. Numbers above the ASM sequence indicate the position of the amino acids in the ASM protein. Asterisks under the sequence represent amino acids bearing similar biochemical properties.
Generation of transgenic mouse models
A 2.2 kb genomic fragment including the mouse SMPD1 promoter region was digested with the Acc I restriction enzyme. The released 1.6 kb fragment mapped to the −11 to −1679 bp position relative to the previously described translation initiation codon of the human ASM cDNA [25], and was designated mSMPD1p. This fragment was subcloned into the pGL3 Basic vector (Promega Corporation, Madison, WI) and the recombinant plasmid was isolated as described above. The pGL3 mSMPD1p plasmid (4 µg) was transfected into HEK293T17 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) and incubated overnight to allow for maximal luciferase expression. Cell extracts were prepared as described above and luciferase activities were measured using the Dual-Luciferase Reporter Assay Kit (Promega Corporation, Madison, WI) to confirm promoter function.
Transgenic constructs were subsequently assembled in a pNEB193 vector (New England Biolabs, Ipswich, MA) by placing the 1.6 kb mSMPD1p fragment upstream of the human cDNAs encoding the R496L and ΔR608 NPD mutations coupled to a simian virus 40 polyadenylation signal. Transgene fragments were released from the vector by restriction digest and microinjected into one-cell stage embryos derived from the superovulation of ASM knock-out (ASMKO) mice [26]. Founder mice were crossed onto C57BL/6 mice to generate stable lines for analysis. All founder mice and transgene carrying progeny were identified by two independent PCR assays. The genotype of the ASMKO SMPD1 allele was determined as described previously [26], and the presence of the transgene was confirmed by the use of GoTaq PCR Core system (Promega Corporation, Madison, WI) and 0.5 µm of human ASM Tg forward (5′-AGCCCAAATGCTTCTAGAGTCGGG-3′) and ASM Tg reverse (5′-AAACAGTCGACGGATCCTTATCGATTTTACC-3′) primers in conjunction with the MIT2681 (5′-CGTCGAAGCTGGGTCTTGGC-3′) and MIT3325 (5′-CAGCAACCCCTCTTACTGCTGTTTG-3′) primers, which acted as an internal control to assess DNA integrity.
Northern Blot Analysis
Total RNA was extracted from tissues using the RNeasy Mini Kit (Qiagen Inc., Valencia, CA). Northern blots containing 10 µg of total RNA were performed as described previously [27]. Membranes were probed using a randomly primed [α32P]-dCTP radiolabelled SV40 cDNA fragment (300 bp) to detect the transgenes. Hybridizations were performed at 68°C for 2 h in QuikHyb solution (Stratagene, La Jolla, CA). The membranes were washed for 2 × 30 min at 42°C and 65°C in 0.1 × SSC/0.1 % (w/v) SDS and exposed to BIOMAX film (Eastman Kodak Company, Rochester, NY) at −80°C.
Results
Skin fibroblasts from a normal individual and four NPD patients were cultured under standard growth conditions and the ASM activities in cell extracts were measured in vitro using a fluorescent substrate, BODIPY-C12 sphingomyelin (B12SM) (Fig. 1A). Each of the cell lines was homozygous for a particular SMPD1 mutation (L302P, R496L, H421Y and ΔR608) and, as expected, the residual ASM activities were extremely low (<2 % compared to normal cells). Patients homozygous for the ΔR608 mutation have a much less severe, non-neurological form of NPD than those with the other three mutations, and also had slightly higher (albeit insignificant) residual ASM activities. We then determined the residual ASM activities in the NPD cell lines using an in situ assay system that monitors the degradation of B12SM in live cells over time [28]. Similar to the results obtained with the in vitro system, the residual ASM activity in each NPD cell line was very low, although the ΔR608 cells had activities that were ~4-fold above those in the other three (data not shown).
Fig. 1. ASM activities and protein levels in normal and NPD human skin fibroblasts and reticulocyte lysates.
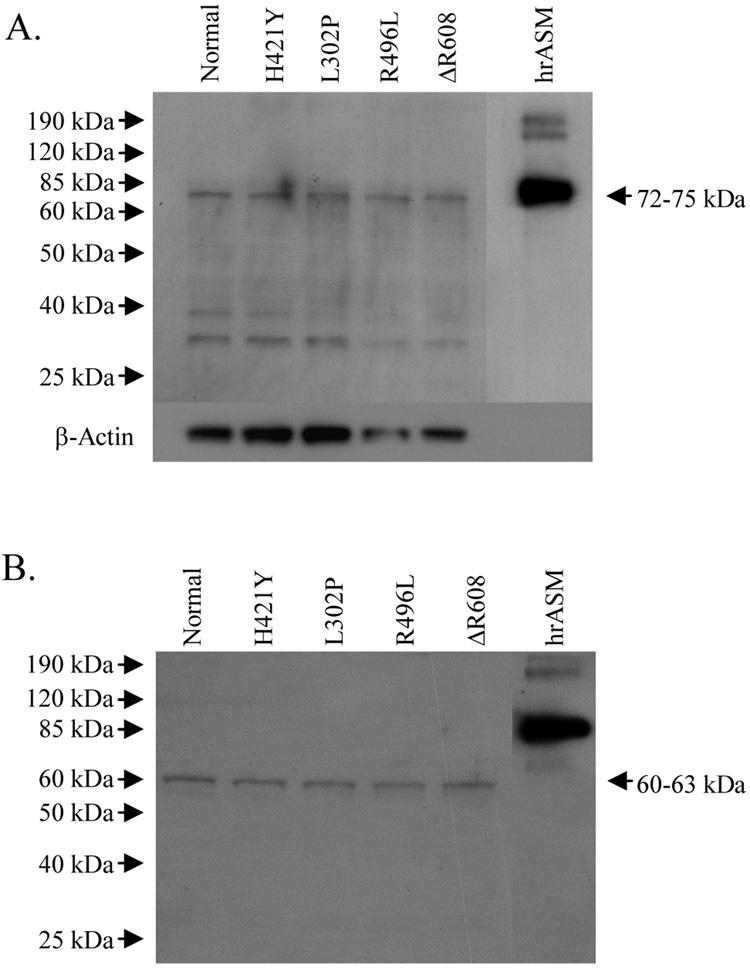
(A) Normal and NPD human skin fibroblasts were harvested and the in vitro ASM activities were determined using B12SM. All reactions contained 1–10 µg of cell extract protein and 100 µM B12SM substrate (final concentration). Data are presented as percentage means ± SD (n = 3). The value calculated for normal skin fibroblasts was set as 100 %. (B) Expression of ASM polypeptides in normal and NPD human skin fibroblasts grown under standard culturing conditions (DMEM containing 2 mM glutamine supplemented with 10 % (v/v) FBS and 1 % (v/v) penicillin-streptomycin). Lanes contained 10 µg of cell extract. Human recombinant ASM (hrASM) (100 ng) was used as a positive control. β-actin was used as a loading control. (C) Recombinant clones harboring normal human ASM or NPD mutant cDNAs were translated using a reticulocyte lysate system containing 35S methionine. Proteins were resolved by SDS-PAGE and exposed to x-ray film. Lanes contained 10 µg of reticulocyte extract.
Immunoblotting experiments were then performed to evaluate the levels of the mutant ASM polypeptides in each cell line. Cell extracts were prepared and analyzed by Western blots using an anti-ASM antibody that specifically recognizes the human enzyme [20]. These results revealed that normal levels of ASM protein were detected in each of the NPD cell lines (Fig. 1B). A predominant band of ~72 – 75 kDa was observed that migrated at the same rate as the normal, recombinant ASM control. Several lower molecular weight bands also were observed that probably corresponded to a combination of degraded ASM protein and non-specific binding of the antibody. Importantly, the same minor bands were found in the normal and NPD cells. To confirm these immunoblotting results, the experiment was performed with a commercial antihuman ASM antibody (Santa Cruz #11352), and the same results were obtained (i.e., equivalent signals from normal and NPD cells; data not shown).
We then used an independent technique to assess the levels of ASM expression from each of the mutations. For these studies the wild-type, human ASM cDNA was cloned from normal skin fibroblasts, and the required NPD mutations (L302P, H421Y, R496L and ΔR608) were introduced by site-directed mutagenesis. The clones were then in vitro translated in a reticulocyte system in the presence of 35S-labeled methionine. A single band of ~60 – 63 kDa was detected in all sample lanes that corresponded to the unglycosylated form of ASM (Fig. 1C) [30]. Note that the level of expressed protein from each of the mutant cDNAs was equivalent to wild-type.
The normal and NPD skin fibroblasts were then cultured for 5 days in reduced serum conditions (2 % (v/v) FBS) to stress the cells, and immunoblot analysis was again performed. Similar results were observed to those described above; i.e., a single, predominant band of ~72 – 75 kDa that migrated at the same rate as the hrASM control was detected (Fig. 2A). These data further indicated that the level of the mutant ASM polypeptides in the NPD cells was similar to that of wild-type. To extend these observations, an immunoprecipitation experiment was performed using the ER chaperone protein, BiP [29]. Cell extracts were prepared from the wild-type and NPD fibroblasts, and complexed with BiP antibody. The captured proteins were then separated by SDS-PAGE and immunoblotted with anti-ASM antibody. No differences in BiP-associated ASM protein levels were detected between the normal and NPD cells following co-immunoprecipitation (Fig. 2B), suggesting that the mutant ASM polypeptides were not being retained in the ER. A predominant band of approximately ~60 – 63 kDa was detected in all sample lanes, which was equivalent to that expressed in reticulocyte lyates (Fig. 1C) and corresponded to the unglycosylated form of ASM [30].
Fig. 2. Stability of ASM proteins in normal and NPD skin fibroblasts and association with BiP.
(A) Expression of ASM in normal and NPD human skin fibroblasts grown under reduced serum culturing conditions (DMEM containing 2 mM glutamine supplemented with 2 % (v/v) FBS and 1 % (v/v) penicillin-streptomycin). Lanes contained 10 µg of cell extract. Human recombinant ASM (hrASM) (100 ng) was used as a positive control. β-actin was used as a loading control. (B) Co-immunoprecipitation of ASM with BiP/GRP78. Normal and NPD human skin fibroblasts were cultured under standard conditions (DMEM containing 2 mM glutamine supplemented with 10 % (v/v) FBS and 1 % (v/v) penicillin-streptomycin). Cell extracts (100 µg) were incubated with BiP antibody and the immunoprecipitated fraction was immunoblotted using ASM antibody. Human recombinant ASM (hrASM) (100 ng) was used as a positive control.
Immunostaining experiments were then performed on the skin fibroblasts to confirm these observations and determine whether the mutant ASM polypeptides reached the lysosomes. Cells were stained with antibodies against ASM alone or in conjunction with cell markers for colocalization: lysotracker (lysosomal marker), Eea1 (early endosome marker) and calnexin (ER marker) (Fig. 3). These analyses confirmed the fact that each of the mutant ASM polypeptides was present in the NPD skin fibroblasts at normal or near normal levels, and further revealed that the majority of the mutant proteins were found in lysosomes, similar to normal (note the predominant yellow color in the merged anti-ASM [red] and lysotracker [green] image). Minor amounts of the ASM protein also colocalized with Eea1 in the early endosome. Little or no ASM was detected in the ER of normal or NPD cells.
Fig. 3. Subcellular localization of ASM in normal and NPD skin fibroblasts.
Normal and NPD human skin fibroblasts were immunostained with ASM antibody and the following cell markers for colocalization were used: Lysotracker (lysosomal marker), Eea1 (early endosome marker) and Calnexin (ER marker). Cells were mounted in Vectashield mounting medium containing 4',6-diamidino-2-phenylindole (DAPI) and analyzed by fluorescence microscopy. Colocalization is indicated by a yellow color following merging of the ASM (Cy3: red) and cell marker (Cy2: green) immunostaining.
Drawing from the above data it was concluded from several independent experiments that the mutant ASM polypeptides were expressed at normal or near normal levels and trafficked to the correct cellular compartment. The only observable differences in the NPD cells were in the levels of residual enzymatic activity. To further investigate these mutations and to develop a new tool to assist in the evaluation of other NPD mutations, a three-dimensional model of human ASM was developed based on the known crystal structures of human saposin, human β-glucuronidase, and kidney bean purple acid phosphatase. In addition, regions of similarities between the primary amino acid sequences of ASM and human phospholipases A2 and C, and the earthworm sphingomyelin binding protein, lysenin [31], were used to predict the catalytic and substrate binding sites, respectively. Alignments of these regions are shown in Fig. 4.
Human ASM consists of two conserved domains: a saposin B-like domain (depicted in gold, Fig. 5A) [32] that connects to a metallophosphoesterase-like domain (depicted in blue) via a proline-rich linker region. Each of the NPD mutations studied in this manuscript was within the metallophosphoesterase-like domain.
Fig. 5. Three-dimensional model of human ASM.
All images were built using the Swiss-PDB Viewer and POV-Ray programs. (A) Domain structure of human ASM. The saposin B-like domain is in gold, and the metallophosphoesterase-like domain is in blue. (B) Locations of functional regions within the metallophosphoesterase-like domain. The putative active site motif is in red, the putative sphingomyelin-binding motif is in green, and one of the three putative zinc-binding motifs (HXXGHXXXGH) is in pink. Note that these three motifs are adjacent to one another, and form an “active site pocket” in the center of the domain. (C) Depiction of the H421Y and R496L mutations. The putative active site pocket backbone is in red. Two of the three histidines involved in the critical zinc-binding site are shown in pink (H425 and H430), and the two NPD mutations are depicted in purple. Note that the H421Y mutation alters the first histidine in this important zinc-binding site, and the R496L mutation is close to the active site. (D) Depiction of the ΔR608 mutation, which lies within 3 Å of two cysteine residues involved in intramolecular disulfide bonds.
Importantly, regions of similarity between ASM and lysenin (i.e., predicted substrate binding site; ASM residues 354–388), as well as phospholipases A2 and C (i.e., predicted catalytic site; ASM residues 384–503 and 201–617, respectively) were nearby one another and formed an “active site pocket” within our three-dimensional model (Fig. 5B). Three putative zinc-binding sites also were identified within the ASM sequence [18, 33], and one of these (ASM residues 421–430) was within the active site region (Fig. 5B). As shown in Fig. 5C, the H421Y mutation disrupts the first histidine of the critical zinc-binding site within this region, and the R496L mutation alters a residue that is also nearby this site.
In contrast, the L302P and ΔR608 mutations were located on the surface of the protein according to this model (not shown). To examine the consequences of these later two mutations further, we investigated the cysteine residues of ASM using our model. ASM possesses six intramolecular disulphide bonds [34], and our model predicts that R608 was in close proximity (less than 3 Å) to two of the cysteines involved in these bonds (Cys588 and Cys607). Thus, deletion of this amino acid (leading to a histidine at position 608) could potentially disrupt either one of these bonds (Fig. 5D).
Finally, to investigate the behavior of these mutations in vivo and for the future evaluation of potential treatment approaches based on reactivation of the mutant polypeptides, we generated transgenic mice expressing two of the mutations, R496L and ΔR608. As described in the Methods, to accomplish this goal mutant human transgenes were constructed under the control of the mouse SMPD1 promoter (Fig. 6A) and microinjected into blastocysts from ASMKO mice. The functionality of the mouse SMPD1 promoter fragment was first validated by reporter assays in HEK293T17 cells (Fig. 6B). Following microinjection, individual founder animals were generated for each mutation and breeding colonies established. Figure 6C shows representative genotyping of F2 offspring from each founder line.
Fig. 6. Construction of transgenic NPD mice expressing the R496L and ΔR608 ASM mutations.
(A) Schematic diagram of the mouse SMPD1 promoter region (mSMPD1p). The 1.6 kb Acc I fragment was cloned into the pGL3 Basic vector upstream of a luciferase cassette (pGL3 mSMPD1p). This fragment also was cloned into the pNEB193 vector upstream of cDNAs encoding human ASM harboring the R496L and ΔR608 mutations. An SV40 polyadenylation signal was introduced downstream (Transgenic Constructs). (B) The pGL3 Basic and pGL3 mSMPD1p vectors were transfected into HEK293T17 cells. Cell extracts were prepared and the in vitro luciferase activity was measured. Data are presented as means ± SD (n = 3). (C) Transgene constructs were microinjected into one-cell stage embryos derived from superovulation of ASM knock-out mice and individual founder mice carrying the R496L and ΔR608 transgenes were identified. PCR genotyping of representative F2 offspring from each founder line is shown. (D). Northern blot analysis of total RNA prepared from liver (L) and brain (B) of wild-type (WT), ASM knockout (KO) and representative F2 transgenic mice from each founder line (R496L and ΔR608). Note that the probe used was derived from the SV40 polyadenylation sequence, and thus only the transgenes were detected. (E). Livers and brains were harvested from wild-type (WT), ASM knockout (KO) and several F2 transgenic mice (Tg) for each mutation, and the in vitro ASM activities were measured. All reactions contained 1–10 µg of protein and 100 µM B12SM substrate (final concentration). Data are presented as percentage means ± SD (n = 3). The value calculated for wild-type mice was set as 100 %. Note that the mean activity values in the brains of the ΔR608 transgenic animals were significantly greater (P < 0.01) than the ASMKO background.
Total RNA was then prepared from the livers and brains of representative F2 transgenic animals from each founder line, and expression was determined by Northern blotting. As shown in Fig. 6D, robust expression was found in the brain for both mutant transgenes. In contrast, very low-level expression was detected in the liver. RNA expression was not detected in tissues of wild-type animals by Northern blotting unless very long exposure times were used (data not shown). Tissue extracts were then prepared from wild-type, ASMKO and several F2 transgenic mice (four from each founder line), and ASM activities were determined. As shown in Fig. 6E, mice expressing the ΔR608 mutation had levels of residual ASM activity in the brain that were significantly (P < 0.01) above the ASMKO background levels, consistent with the RNA expression results and less severe phenotype of patients harboring this mutation. In contrast, despite high levels of RNA expression in the brains of transgenic mice expressing the R496L mutation, no significant residual ASM activity was found, again consistent with the very severe neurological phenotype of patients harboring this mutation. Long-term studies are underway to evaluate the effect of these mutations of the biochemical, pathological and clinical phenotypes of these new transgenic mouse models.
Discussion
Currently, our understanding of how individual SMPD1 mutations affect ASM stability, trafficking and catalytic function is very limited. In this manuscript we focused our efforts on characterizing four common SMPD1 mutations that occur at high frequencies in specific NPD populations. Three of these mutations cause very severe disease (L302P, R496L and H421Y), and one is associated with mild disease (ΔR608). We performed comprehensive structure-function studies on cell lines homoallelic for each mutation, created a three-dimensional model of ASM based on homologies to known proteins to assist in predicting the outcome of individual mutations, and constructed transgenic mice expressing two of the mutations on the complete ASM knock-out background.
Primary fibroblasts were grown from normal and homoallelic NPD patients, and the ASM activities were measured in vitro and in situ. As expected, the residual ASM activity in each of the NPD cell lines was very low compared to normal cells. Surprisingly, however, western blotting analysis (performed on cells grown under normal or reduced serum conditions) revealed that normal or near normal levels of (mutant) ASM polypeptides were present. Expression of the mutant cDNAs in reticulocyte lysates confirmed these findings, as did co-immunmoprecipitation studies with anti-BiP antibodies and immunolocalization studies. These latter experiments also suggested that the majority of the mutant ASM polypeptides were correctly targeted to lysosomes or late endosomes.
These results were somewhat unexpected since three of the mutations studied (e.g., L302P, R496L and H421Y) caused severe NPD. We therefore anticipated that one or more of these mutations might have resulted in low-level protein expression and/or reduced stability. The findings have important implications for enzyme replacement therapy (ERT) and the development of new enzyme enhancement approaches for types A and B NPD. Regarding ERT, the fact that NPD patients have normal or near normal levels of residual ASM protein predicts that the frequency and/or severity of immunologically related infusion reactions may be less severe than in other diseases where deletion mutations are common. In addition, the fact that the mutant proteins are expressed in NPD cells suggests that approaches to enhance the activity of these proteins with small molecules are worthy of investigation.
Human ASM consists of two domains: a “saposin-like” domain that connects to a “metallophosphoesterase-like” domain via a proline-rich linker region [32]. Each of the NPD mutations studied in this manuscript resides within the metallophosphoesterase-like domain. Although no structural information is available for ASM from any species, we developed a new, computer-generated model of the human enzyme’s three-dimensional structure based on the known structures of three proteins: human saposin (to predict the “saposin” domain), human β-glucuronidase (another lysosomal hydrolase), and kidney bean acid phosphatase, which has been used previously to generate a partial model of ASM (see below). To predict this three-dimensional structure the Modeller software package was used [24].
The three-dimensional model was further refined by predicting the substrate-binding site of ASM based on a region of homology to lysenin (ASM residues 354–388), a sphingomyelin-specific binding protein from the earthworm [31]. In addition, the active site was predicted based on homologies between ASMases from human, mouse and C. elegans (data not shown), as well as human phospholipases A2 and C. Three putative zinc-binding sites also were identified within the ASM sequence, and it was notable that one of these (ASM residues 421–430) was within the predicted active site region. That fact that three critical components (substrate binding site, zinc binding site, and active site) were within the same region and formed an “active site pocket” helped to validate our three-dimensional model.
In addition, six intramolecular disulfide bonds have been identified in ASM [34]. We mapped the twelve cysteine residues forming these bonds onto our three-dimensional model, and found that the residues forming three bonds (Cys120/131, 385/431, 594/607) were in close proximity. It is also notable that R608 (deleted in patients with the ΔR608 mutation) was in close proximity to residues forming two of the disulfide bonds (Cys 588 and 607) (Fig. 5D). In addition, C629, the only cysteine residue in ASM that is not involved in intramolecular bonds [34], was far from all of the other cysteines and located on the surface of the protein according to our model.
It should be recognized that another partial model predicting ASM’s structure has been described [18] based on sequence and structural homologies to kidney bean purple acid phosphatase only. Two of the mutations studied here were assessed using this model, L302P and H421Y. Although no predictions could be made about the H421Y mutation, it was suggested that the L302P mutation might result in an unstable protein. In our model the L302P mutation sits on the surface of the protein, away from the active site pocket. It was also suggested previously that the H421Y mutation lies between two central β sheets, and was not in the proximity of the dimetal center. In our model this mutation is within the active site pocket, and alters the first histidine of a critical zinc-binding site in this region. Lastly, it should be noted that in our model another important amino acid residue, W391 (commonly mutated to G391 in gypsy NPD populations) [35], also was located within the active site pocket (data not shown).
Thus, the model presented here constitutes a new tool to assess the influence of individual mutations on ASM activity, and might assist in future studies aimed at improving genotype/phenotype correlations. It is important to acknowledge, however, that in the absence of precise structural information, molecular modeling such as this provides approximations only, and should not be used to draw definitive conclusions. Any conclusions based on either of these models must be validated by experimental investigation. Such studies are currently underway, as are studies aimed at solving the crystal structure of ASM.
Finally, an underlying, long-term goal of the present investigation was to determine whether any of the four common NPD mutations we investigated was suitable for enzyme enhancement therapies. Although the fact that the stability and trafficking of the mutant ASM polypeptides appeared to be normal, we cannot rule out the possibility of subtle folding and/or stability abnormalities that were not discerned using our techniques. Thus, it remains possible that enzyme enhancement might be accomplished, either using chaperones or other approaches (e.g., co-factor supplementation). We therefore constructed transgenic mice expressing two of the mutations (R496L and ΔR608) on the complete ASM knock-out background. This was feasible since the ASMKO mice are viable and 1-cell embryos could be harvested for microinjection. Individual founder lines for each of the mutations were established, and the mRNA expression levels in the brain and liver were assessed. High-level expression was only observed in the brain of these animals, not the liver. Compared to wild-type mice, the relative level of expression in brain versus liver was similar (i.e., wild-type mice also have several fold higher SMPD1 expression levels in the brain than liver; data not shown), suggesting that the promoter fragment was functioning normally. However, the overall levels of expression in the transgenic animals were significantly greater than wild-type. Based on Southern blotting and segregation analysis in the breeding colonies, multiple copies of the transgenes were inserted in both transgenic lines on a chromosome distinct from SMPD1 (located on murine chromosome 7), perhaps explaining the higher than normal levels of expression (data not shown).
We next assessed residual ASM activity in the liver and brain of the mutant transgenic mice. As predicted from the Northern blotting experiments, neither transgenic line expressed significant residual ASM activity in the liver as compared to the ASMKO animals. In contrast, however, the ΔR608 mice expressed ASM activity in the brain that was ~8-fold above the ASMKO controls (i.e., ~1–2 % of normal). In contrast, despite high-level transgene expression in the brain, the R496L mice did not have detectable levels of residual ASM activity in the brain. These results are consistent with the phenotype of NPD patients (i.e., patients harboring the ΔR608 mutation have a much less severe neurological phenotype than patients harboring the R496L mutation). In the future it will be important to follow the biochemical, pathological, and clinical consequences of expressing these mutations in the transgenic mouse lines, and to use these animals to evaluate in vivo approaches to enhancing the residual ASM activities.
Acknowledgements
We acknowledge the MSSM-Microscopy Shared Resource Facility for assistance with the fluorescent microscopy. Transgenic mice were created at the MSSM-Mouse Genetics Shared Resource Facility.
Sources of funding
Funding for this work was provided to E.H.S. by the NIH (R01 HD28607) and by a grant from the National Niemann-Pick Disease Foundation. The MSSM-Microscopy Shared Resource facility was supported, in part, by an NIH-NCI shared resources grant (5R24 CA095823-04), a Major Research Instrumentation grant (DBI-9724504) from the NSF, and a NIH shared instrumentation grant (1 S10 RR0 9145-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schuchman EH, Desnick RJ. Niemann-Pick disease types A and B: acid sphingomyelinase deficiencies. In: Scriver CR, Beaudet AL, Valle D, Sly WS, editors. The Metabolic and Molecular Bases of Inherited Disease. 8th Edition. New York: McGraw Hill; 2001. pp. 3589–3610. [Google Scholar]
- 2.Simonaro CM, Desnick RJ, McGovern MM, Wasserstein MP, Schuchman EH. The demographics and distribution of type B Niemann-Pick disease: novel mutations lead to new genotype/phenotype correlations. Am. J. Hum. Genet. 2002:1413–1419. doi: 10.1086/345074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuchman EH, Levran O, Pereira LV, Desnick RJ. Structural organization and complete nucleotide sequence of the gene encoding human acid sphingomyelinase (SMPD1) Genomics. 1992;12:197–205. doi: 10.1016/0888-7543(92)90366-z. [DOI] [PubMed] [Google Scholar]
- 4.Ferlinz K, Hurwitz R, Sandhoff K. Molecular basis of acid sphingomyelinase deficiency in a patient with Niemann-Pick disease type A. Biochem. Biophys. Res. Comm. 1991;179:1187–1191. doi: 10.1016/0006-291x(91)91697-b. [DOI] [PubMed] [Google Scholar]
- 5.Levran O, Desnick RJ, Schuchman EH. Niemann-Pick Disease: a frequent missense mutation in the acid sphingomyelinase gene of Ashkenazi Jewish type A and B patients. Proc. Natl. Acad. Sci. USA. 1991;88:3738–3752. doi: 10.1073/pnas.88.9.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levran O, Desnick RJ, Schuchman EH. Niemann-Pick Type B Disease: Identification of a single codon deletion in the acid sphingomyelinase gene and genotype/phenotype correlations in type A and B patients. J. Clin. Invest. 1991;88:806–810. doi: 10.1172/JCI115380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levran O, Desnick RJ, Schuchman EH. Identification and expression of a common missense mutation (L302P) in the acid sphingomyelinase gene of Ashkenazi Jewish Type A Niemann-Pick disease patients. Blood. 1992;8:2081–2087. [PubMed] [Google Scholar]
- 8.Takahashi T, Desnick RJ, Takada G, Schuchman EH. Identification of a missense mutation (S436R) in the acid sphingomyelinase gene from a Japanese patient with type B Niemann-Pick disease. Hum. Mutat. 1992;1:70–71. doi: 10.1002/humu.1380010111. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi T, Suchi M, Desnick RJ, Takada G, Schuchman EH. Identification and expression of five mutations in the human acid sphingomyelinase gene causing types A and B Niemann-Pick disease. Molecular evidence for genetic heterogeneity in the neuronopathic and non-neuronopathic forms. J. Biol. Chem. 1992;267:12552–12558. [PubMed] [Google Scholar]
- 10.Levran O, Desnick RJ, Schuchman EH. Identification of a 3' acceptor splice site mutation (g2610c) in the acid sphingomyelinase gene of patients with Niemann-Pick disease. Hum. Mol. Genet. 1993;2:205–206. doi: 10.1093/hmg/2.2.205. [DOI] [PubMed] [Google Scholar]
- 11.Levran O, Desnick RJ, Schuchman EH. Type A Niemann-Pick disease: a frameshift mutation in the acid sphingomyelinase gene (fsP330) occurs in Ashkenazi Jewish patients. Hum. Mutat. 1993;2:317–319. doi: 10.1002/humu.1380020414. [DOI] [PubMed] [Google Scholar]
- 12.Schuchman EH. Two new mutations in the acid sphingomyelinase gene causing type a Niemann-pick disease: N389T and R441X. Hum. Mutat. 1995;6:352–354. doi: 10.1002/humu.1380060412. [DOI] [PubMed] [Google Scholar]
- 13.Vanier MT, Ferlinz K, Rousson R, Duthel S, Louisot P, Sandhoff K, Suzuki K. Deletion of arginine (608) in acid sphingomyelinase is the prevalent mutation among Niemann-Pick disease type B patients from Northern Africa. Hum. Genet. 1993;92:325–330. doi: 10.1007/BF01247328. [DOI] [PubMed] [Google Scholar]
- 14.Bayever E, Kamani N, Ferreira P, Machin GA, Yudkoff M, Conrad K, Palmieri M, Radcliffe J, Wenger DA, August CS. Bone marrow transplantation for Niemann-Pick type 1A disease. J. Inherit. Metab. Dis. 1992;15:919–928. doi: 10.1007/BF01800234. [DOI] [PubMed] [Google Scholar]
- 15.Victor S, Coulter JB, Besley GT, Ellis I, Desnick RJ, Schuchman EH, Vellodi A. Niemann-Pick disease: sixteen-year follow-up of allogeneic bone marrow transplantation in a type B variant. J. Inherit. Metab. Dis. 2003;26:773–785. doi: 10.1023/B:BOLI.0000009950.81514.c8. [DOI] [PubMed] [Google Scholar]
- 16.Miranda SR, He X, Simonaro CM, Gatt S, Dagan A, Desnick RJ, Schuchman EH. Infusion of recombinant human acid sphingomyelinase into Niemann-Pick disease mice leads to visceral, but not neurological, correction of the pathophysiology. FASEB J. 2000;14:1988–1995. doi: 10.1096/fj.00-0014com. [DOI] [PubMed] [Google Scholar]
- 17.Beck M. New therapeutic options for lysosomal storage disorders: enzyme replacement, small molecules and gene therapy. Hum. Genet. 2007;121:1–22. doi: 10.1007/s00439-006-0280-4. [DOI] [PubMed] [Google Scholar]
- 18.Seto M, Whitlow M, McCarrick MA, Srinivasan S, Zhu Y, Pagila R, Mintzer R, Light D, Johns A, Meurer-Ogden JA. A model of the acid sphingomyelinase phosphoesterase domain based on its remote structural homolog purple acid phosphatase. Protein Sci. 2004;13:3172–3186. doi: 10.1110/ps.04966204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He X, Chen F, Dagan A, Gatt S, Schuchman EH. A fluorescence-based, high-performance liquid chromatographic assay to determine acid sphingomyelinase activity and diagnose types A and B Niemann-Pick disease. Anal. Biochem. 2003;314:116–120. doi: 10.1016/s0003-2697(02)00629-2. [DOI] [PubMed] [Google Scholar]
- 20.He X, Miranda SRP, Xiong X, Dagan A, Gatt S, Schuchman EH. Characterization of human acid sphingomyelinase purified from the media of overexpressing Chinese hamster ovary cells. Biochim. Biophys. Acta. 1999;1432:251–264. doi: 10.1016/s0167-4838(99)00069-2. [DOI] [PubMed] [Google Scholar]
- 21.Geourjon C, Deleage G. SOPMA: significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Comput. Appl. Biosci. 1985;11:681–684. doi: 10.1093/bioinformatics/11.6.681. [DOI] [PubMed] [Google Scholar]
- 22.Guermeur Y, Geourjon C, Gallinari P, Deleage G. Improved performance in protein secondary structure prediction by inhomogeneous score combination. Bioinformatics. 1999;15:413–421. doi: 10.1093/bioinformatics/15.5.413. [DOI] [PubMed] [Google Scholar]
- 23.Marchler-Bauer A, Bryant SH. CD-Search: protein domain annotations on the fly. Nucleic Acids Res. 2004;32:327–331. doi: 10.1093/nar/gkh454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eswar N, Webb B, Mari-Renom MA, Madhusudhan MS, Eramian D, Shen M, Pieper U, Sali A. Comparative protein structure modelling using Modeller. Current Protocols in Bioinformatics. 2006;15:5.6.1–5.6.30. doi: 10.1002/0471250953.bi0506s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuchman EH, Suchi M, Takahashi T, Sandhoff K, Desnick RJ. Human acid sphingomyelinase. Isolation, nucleotide sequence and expression of the full-length and alternatively spliced cDNAs. J. Biol. Chem. 1991;13:8531–8539. [PubMed] [Google Scholar]
- 26.Horinouchi K, Erlich S, Perl DP, Ferlinz K, Bisgaier CL, Sandhoff K, Desnick RJ, Stewart CL, Schuchman EH. Acid sphingomyelinase deficient mice: a model of types A and B Niemann-Pick disease. Nat. Genet. 1995;10:288–293. doi: 10.1038/ng0795-288. [DOI] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch EF, Maniatis T. A Laboratory Manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1989. Molecular Cloning. [Google Scholar]
- 28.Darroch PI, Dagan A, Granot T, He X, Gatt S, Schuchman EH. A lipid analogue that inhibits sphingomyelin hydrolysis and synthesis, increases ceramide, and leads to cell death. J. Lipid. Res. 2005;46:2315–2324. doi: 10.1194/jlr.M500136-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Le Fourn V, Siffroi-Fernandez S, Ferrand M, Franc JL. Competition between calnexin and BiP in the endoplasmic reticulum can lead to the folding or degradation of human thyroperoxidase. Biochemistry. 2006;45:7380–7388. doi: 10.1021/bi060415i. [DOI] [PubMed] [Google Scholar]
- 30.Bartelsen O, Lansmann S, Nettersheim M, Lemm T, Ferlinz K, Sandhoff K. Expression of recombinant human acid sphingomyelinase in insect Sf21 cells: purification, processing and enzymatic characterization. J. Biotechnol. 1998;63:29–40. doi: 10.1016/s0168-1656(98)00070-4. [DOI] [PubMed] [Google Scholar]
- 31.Shakor AB, Czurvio EA, Sobota A. Lysenin, a unique sphingomyelin-binding protein. FEBS Lett. 2003;542:1–6. doi: 10.1016/s0014-5793(03)00330-2. [DOI] [PubMed] [Google Scholar]
- 32.Kolzer M, Ferlinz K, Bartelsen O, Hoops SL, Lang F, Sandhoff K. Functional characterization of the postulated intramolecular sphingolipid activator protein domain of human acid sphingomyelinase. J. Biol. Chem. 2004;385:1193–1195. doi: 10.1515/BC.2004.154. [DOI] [PubMed] [Google Scholar]
- 33.Klabunde T, Strater N, Frohlich R, Witzel H, Krebs B. Mechanism of Fe(III)-Zn(II) purple acid phosphatase based on crystal structures. J. Mol. Biol. 1996;259:737–748. doi: 10.1006/jmbi.1996.0354. [DOI] [PubMed] [Google Scholar]
- 34.Lansmann S, Schuette CG, Bartelsen O, Hoernschemeyer J, Linke T, Weisgerber J, Sandhoff K. Human acid sphingomyelinase. Eur. J. Biochem. 2003;270:1076–1088. doi: 10.1046/j.1432-1033.2003.03435.x. [DOI] [PubMed] [Google Scholar]
- 35.Mihaylova V, Hantke J, Sinigerska I, Cherninkova S, Raicheva M, Bouwer S, Tincheva R, Khuyomdziev D, Bertranpetit J, Chandler D, Angelicheva D, Kremensky I, Seeman P, Tourney I, Kalaydjieva L. Highly variable neural involvement in sphingomyelinase-deficient Niemann-Pick disease caused by an ancestral Gypsy mutation. Brain. 2007;130:1050–1061. doi: 10.1093/brain/awm026. [DOI] [PubMed] [Google Scholar]