Abstract
Background
Egg-laying in Caenorhabditis elegans has been well studied at the genetic and behavioral levels. However, the neural basis of egg-laying behavior is still not well understood; in particular, the roles of specific neurons and the functional nature of the synaptic connections in the egg-laying circuit remain uncharacterized.
Results
We have used in vivo neuroimaging and laser surgery to address these questions in intact, behaving animals. We have found that the HSN neurons play a central role in driving egg-laying behavior through direct excitation of the vulval muscles and VC motorneurons. The VC neurons play a dual role in the egg-laying circuit, exciting the vulval muscles while feedback-inhibiting the HSNs. Interestingly, the HSNs are active in the absence of synaptic input, suggesting that egg-laying may be controlled through modulation of autonomous HSN activity. Indeed, body touch appears to inhibit egg-laying in part by interfering with HSN calcium oscillations.
Conclusions
The egg-laying motor circuit comprises a simple three-component system combining feed-forward excitation and feedback inhibition. This microcircuit motif is common in the C. elegans nervous system as well as in the mammalian cortex; thus, understanding its functional properties in C. elegans may provide insight into its computational role in more complex brains.
INTRODUCTION
How cells in a neural circuit interact with each other and with sensory inputs to give rise to a behavioral output is a fundamental question in neuroscience. Recent studies have focused on possible circuit mechanisms in a range of neurological abnormalities, including drug addiction and Parkinson’s disease (Bergman and Deuschl, 2002; Lesch, 2005); however, the complexity of the neural circuits involved in these disorders has prevented full elucidation of their underlying neural mechanisms. Several simpler neural circuits have been characterized at the cellular level, including the crustacean stomatogastric ganglion [1–3] and the forward swimming circuits in the leech [4] and lamprey [5–7]. However, none of these model systems is genetically accessible, limiting any study of the molecular machinery that may be conserved among different species and important in human disease. Recent advances in non-invasive laser surgery and in vivo imaging of neural activity in a genetically tractable model organism, the nematode C. elegans [8–10], make integrated studies of the genetic, cellular, and circuit-level processes governing behavior possible.
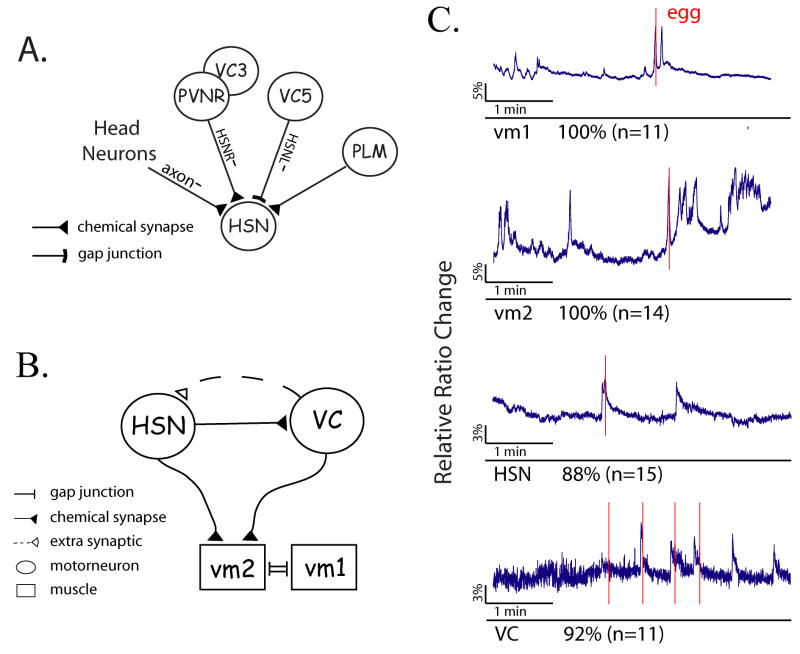
Among the anatomically simplest neural circuits in C. elegans is the one involved in the control of egg-laying behavior. Individual egg-laying events occur through contraction of the vulval muscles, which causes transient opening of the vulva and allows eggs to be expelled. The anatomical structure of the egg-laying circuit [11] is relatively simple, composed of three critical cell types: the vulval muscles (vm1 and vm2), and two sets of hermaphrodite-specific motorneurons, the two HSNs and six VCs (Figure 1A, 1B). Both the HSNs and VCs (in particular the vulval-proximal VC4 and VC5 neurons) make neuromuscular synapses with the vm2 vulval muscles, and the HSNs make many neuron-to-neuron synapses with the VCs. Laser ablation studies have revealed that the vm2 vulval muscles are essential for egg-laying (M. Stern, personal communication). In addition, ablation of the HSNs severely decreases egg-laying, though egg-laying still occurs at a low frequency [12, 13]. The role of the VCs is more ambiguous, as ablation of the VCs has a weak egg-laying constitutive phenotype on its own [14] but enhances the egg-laying defect of animals lacking the HSNs [15]. In addition, recent studies [14] found evidence that the cholinergic VC neuron may inhibit HSN activity extrasynaptically through a muscarinic acetylcholine receptor GAR-2.
Figure 1. Egg-laying is temporally correlated with motorneuron and muscle activity.
(A, B) Structure of the egg-laying circuit. Synaptic connectivity information is derived from White et al (1986). (C) Egg-laying events are coupled with the neural activities in vm2, vm1, HSN and VC cells. The blue lines are time-lapse cameleon signals, the relative change in its YFP/CFP ratio, which indicates the relative intracellular calcium concentrations. The percentage of active traces and total number of recordings for the indicated cell are shown. Red lines indicate the times egg-laying events occurred. Scale bars for cameleon signals are as indicated.
Both classes of motorneurons are notable for expressing of multiple neurotransmitters and neuromodulators. The HSNs synthesize both acetylcholine [16] and serotonin (Desai et al., 1988; Horvitz et al., 1982), as well as several neuropeptides [17, 18]. The VCs are cholinergic [16] and express at least one RF-amide neuropeptide [17]. Both acetylcholine and serotonin have been found to have both stimulatory and inhibitory effects on egg-laying [19], making their study by classical genetic methods quite complicated.
Like most C. elegans behaviors, egg-laying is regulated by a diverse set of environmental cues. Egg-laying events occur in a specific temporal pattern [15, 20] in which eggs are laid in bursts separated by inactive periods averaging 20 minutes in duration. When food is less abundant, these inactive periods become much longer, an effect that requires neuropeptides encoded by the flp-1 gene [21]. Other genes affecting the modulation of egg-laying by food have been identified [22, 23], but the neural basis for this regulation is not well understood. Vibrational stimulation also inhibits egg-laying; this response has been shown to require the ALM and PLM touch receptor neurons [24].
Although the putative components of the egg-laying circuitry have been identified, fundamental questions remain about how this circuit controls egg-laying behavior. For example, it is not known whether the synapses onto and between the egg-laying motorneurons are excitatory or inhibitory. Likewise, the functional significance of these connections in the generation of egg-laying behavior is not well understood. Here, we describe a functional characterization of the neural circuit regulating C. elegans egg-laying behavior using in vivo calcium imaging and femtosecond laser ablation. By combining visible light microscopy with in vivo calcium imaging, we were able to correlate cellular activity with egg-laying behavior and use genetic and pharmacological manipulations to study the roles of individual neurons and neurotransmitters in the control of egg-laying. In this way, we have been able to define the neural mechanisms by which the core egg-laying circuit generates behavior .
RESULTS
Temporal correlation between motorneuron activity and egg-laying behavior
To obtain more information about the roles of individual muscles and neurons in the egg-laying circuit, we imaged their activities in behaving animals. In a previous study [25], we imaged vulval muscle and egg-laying motorneuron activity in intact immobilized worms; however, the conditions under which these experiments were performed were not permissive for egg-laying. We determined that if animals were immobilized on low osmolarity medium, they laid eggs efficiently under our neuroimaging conditions. To study egg-laying behavior and neural activity simultaneously, we also developed a technique of combined fluorescence and visible-light microscopy, which allowed us to identify within a single video frame when egg-laying events occurred, and to correlate these events with calcium transients detected by cameleon expressed in egg-laying neurons or muscle cells (Figure 1C).
We first investigated how egg-laying events correlated with the activities of each component of the egg-laying circuit. We observed that all egg-laying events occurred rapidly within a single 100 ms frame (Supplemental Figure 1); thus, for our purposes, each egg-laying event could be regarded as an instantaneous event. In these experiments, we observed that egg-laying events were temporally correlated with Ca2+ spikes in both vm1 (n=11/11 egg-laying events) and vm2 (n=14/14 egg-laying events) vulval muscles (Figure 1, 2). Most egg-laying events occurred after the onset of a calcium surge but before began returning to baseline. Calcium spikes in the vulval muscles can occur singly or in clusters (Figure 2), and egg-laying can coincide with either single calcium spikes or at any time during cluster spiking activity (Figure 2). Although egg-laying was never observed without a calcium spike in vulval muscles, many vulval muscle calcium transients were observed that were not associated with egg-laying events or even detectable muscle contraction (data not shown). The there was no obvious difference in the dynamics of calcium spikes that successfully resulted in egg-laying compared to those that did not, and the functional significance of vulval muscle calcium transients not associated with egg-laying is not known (see discussion).
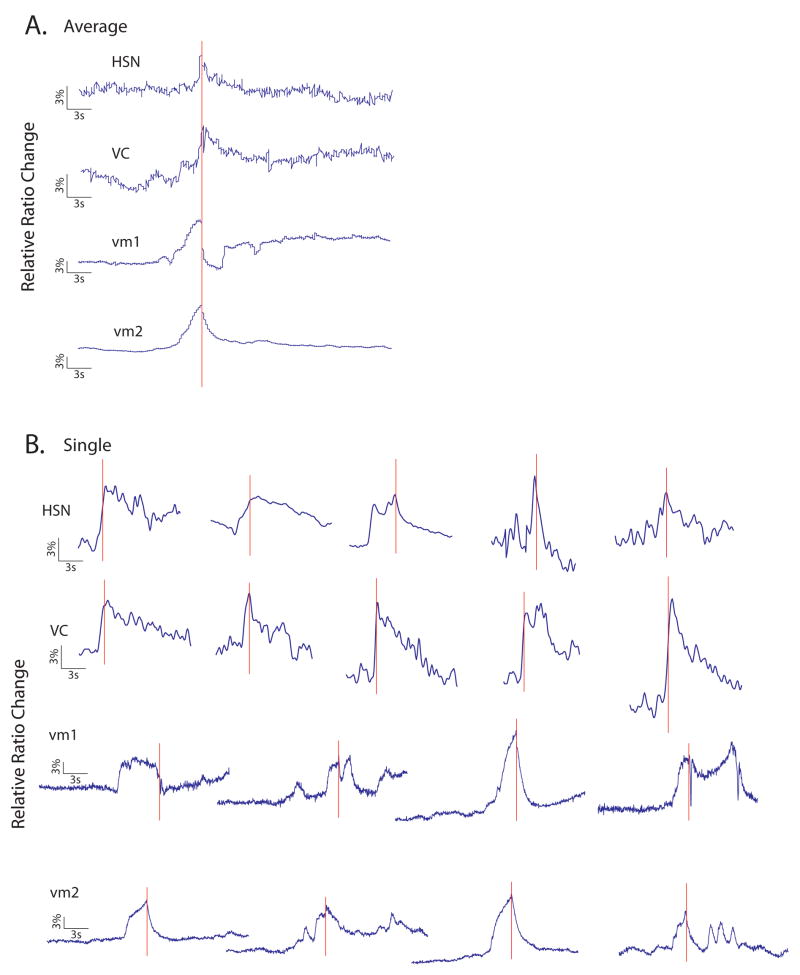
Figure 2. Timing of motorneuron and vulval muscle calcium transients relative to egg-laying.
(A). Shown are averaged calcium recordings of HSN, VC, vm1 and vm2 cells phase-locked to egg-laying events. The onset of calcium transients in all cells clearly precedes egg-laying. Interestingly, vulval muscle calcium influx often occurs before the neuronal calcium transient, suggesting that while neuronal activity may be important for evoking egg-laying, it is not essential for vulval muscle calcium transients. (B) Sample individual calcium traces around egg-laying events. In vulval muscle cells, an egg-laying event can happen during a single calcium spike in (3rd in the vm1 and vm2 rows) or any time during a prolonged excitation (1st, 2nd and 4th in the rows). In contrast, egg-laying events tend to be associated with single spikes in the HSN and VC motorneurons. Scale bars are indicated.
In addition, we also observed strong temporal correlation between egg-laying events and calcium transients in both the HSN (n=15/17 egg-laying events) and VC (n=11/12 egg-laying events) motorneurons (Figure 1, 2). In general, neuronal calcium transients were more predictive of egg-laying events than those in vulval muscles; for example, under moderately permissive conditions (150 mOsm), only 10% of vulval muscle calcium transients were associated with egg-laying events, while the majority of calcium spikes in HSN or VC were egg-laying-associated (52% and 68%, respectively; data not shown). The onset of the neuronal calcium transient preceded the egg-laying event by an average of approximately 1 second for both motorneuron classes (Figure 2), suggesting that neural activity in both the HSNs and VCs triggers egg-laying events rather than the converse.
To further investigate the causal links between motorneuron activity and egg-laying, we imaged HSN and VC calcium transients in an egl-15(n484) mutant, in which vulval muscles fail to properly develop due to a defect in sex myoblast migration. In these animals, both the HSN and VC neurons still showed significant calcium activity under low osmolarity conditions (Supplemental Figure 2), indicating that muscle contraction is not required to activate either neuron class. Together, these results suggest that one or both of these motorneuron classes and the vulval muscles is important for the generation of egg-laying events, most likely through fast excitatory neurotransmission.
Effects of osmolarity on egg-laying
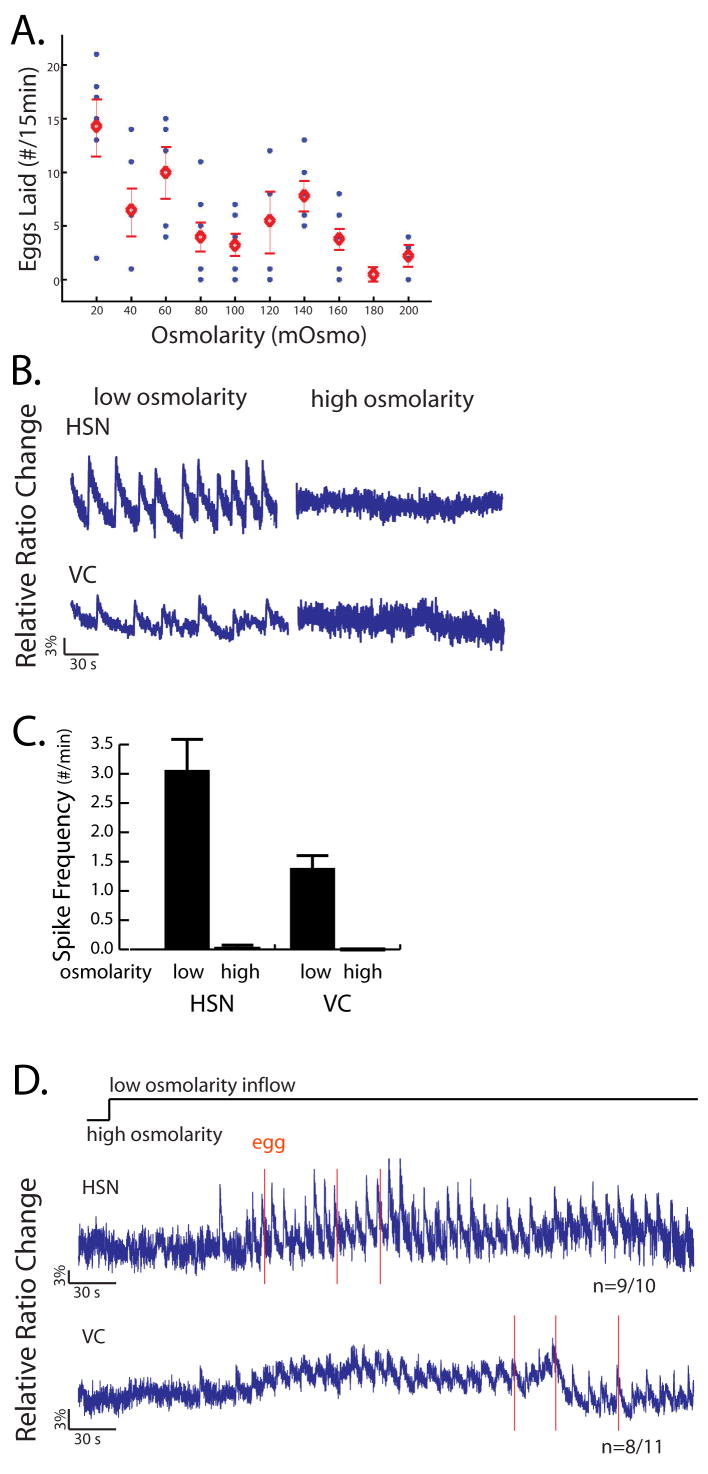
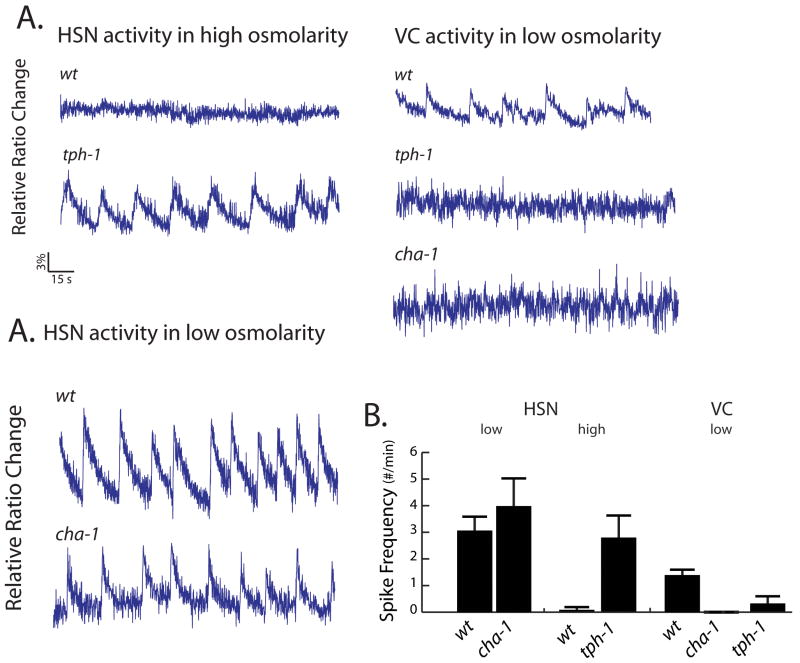
We investigated in more detail how osmolarity affected egg-laying behavior and the egg-laying circuitry. By varying the concentrations of ions in the buffer individually and also in combinations, we found that it was the overall osmolarity of the solution, rather than the concentration of a particular ion, that affects the rate of egg-laying (data not shown). We therefore tested dose responses of egg-laying behavior to different osmotic solutions made from sucrose (Figure 3A). These results showed gradual, dose-dependent decrease in egg-laying rate as the external osmolarity increased from 20 to 200 mOsm (M9 is 320 mOsm and NGM plates are around 150 mOsm-180 mOsm). When we performed calcium imaging experiments in high or low osmolarity, we observed that in the HSN and VC neurons, the calcium spike frequencies were likewise high in low osmolarity and silent in high osmolarity conditions (Figure 3B, C), indicating that osmolarity affects motorneuron activity. At low osmolarity, we found that both HSN and VC neurons exhibited calcium spikes in a strikingly rhythmic pattern, with periods ranging from 6 to 12 seconds. We also analyzed the response of the cells in the circuit to an acute osmolarity change during one continuous 10 minute Ca2+ imaging recording and observed an activation of HSN and VC activity following the shift to low osmolarity (Figure 3D). Together, these results indicate that the regulatory effect of osmolarity on egg-laying involves the control of motorneuron activity.
Figure 3. Osmolarity affects egg-laying behavior and neural activity.
(A) Egg-laying rates in different osmolarity buffers made from sucrose. Each blue dot represents one worm; the red circles indicate the mean number of eggs laid. Standard errors of means (SEM) are shown as extended red lines. (B-C) Responses of HSN and VC neurons to osmolarity. B shows representative traces of HSN and VC calcium of wild type worms in extreme high (200 mOsm) or low (0 mOsm) osmolarity conditions.as measured by cameleon. In C, the mean and SEM of spike frequencies of multiple imaged animals are plotted to illustrate the HSN and VC responses to different osmolarities. (D) The HSN and VC responses to acute low osmotic shock. A low osmolarity inflow was opened at 30 seconds after recording begins. N represents the number of worms that showed similar responses as illustrated here. All blue traces are cameleon signals representing relative intracellular calcium concentrations. Scale bars are as indicated.
We also analyzed the effect of osmolarity on vulval muscle activity. Like the HSNs and VCs, the vm1 and vm2 muscles showed higher activity in low osmolarity media (Supplemental Figure 3A, B), as expected from the higher rate of egg-laying under those conditions. However, even in high osmolarity conditions under which the motorneurons were nearly silent, we observed significant activity in the vulval muscles (Supplemental Figure 3). Interestingly, there was a significant difference in the degree to which the activity of individual vm2 muscle cells was correlated under different osmolarity conditions. In low osmolarity solution, the vm2 showed a 52 % within-type correlation (p<0.001), and the vm1s showed a 80 % within-type correlation (p<0.001); in contrast, in high osmolarity the vm2s showed a 15 % correlation (p > .05), and the vm1s showed a 59% correlation (p<0.001). The differences in vm2’s within-type correlations suggest that the neural activity specifically observed under low osmolarity conditions may serve to coordinate vm2 activity. In contrast, this neural activity may be less important for preserving within-type correlation of the vm1s, which are connected to each other through gap junctions but do not receive direct synaptic input [11].
The functional nature of HSN/VC connectivity
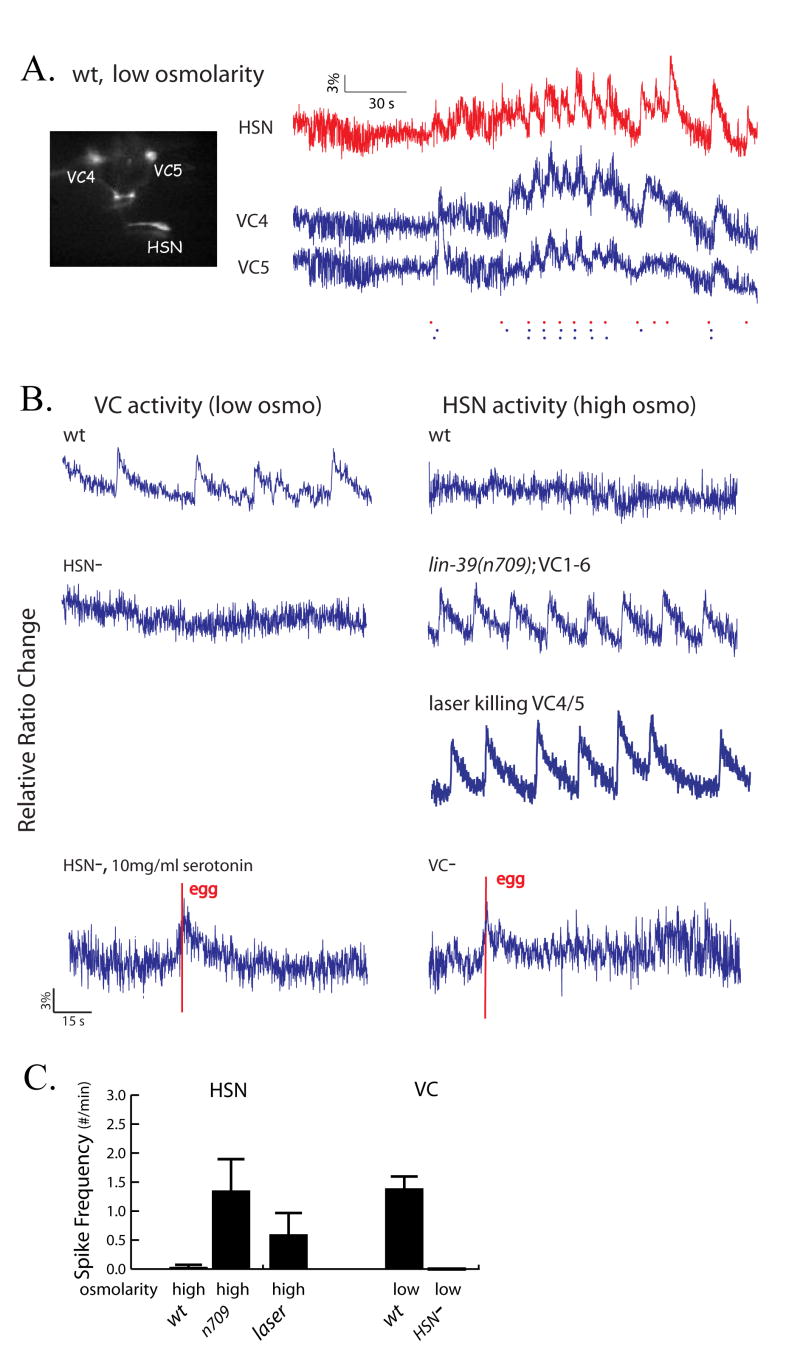
Since HSN and VC activities were both temporally correlated with egg-laying behavior, and since the HSNs direct synaptic output to the VCs, we reasoned that HSN activity might likewise be correlated with VC activity. To investigate this possibility directly, we used a slide rotating device (Suzuki et al., 2008) to bring both HSNs and VCs into the same focal plane and image their activity simultaneously. HSN and VC showed 70 % (p<0.001) correlation in the timing of their calcium transients (Figure 4A), suggesting that the HSNs might excite the VCs to drive their activity, or alternatively, that the VCs might excite the HSNs. With our temporal resolution of 0.1s, we were unable to resolve the temporal order of HSN and VC spikes; the onset of HSN and VC spikes typically occurred simultaneously within one or two frames.
Figure 4. Functional connectivity between the HSN and VC motorneurons.
(A) Both the HSN and VC activities are simultaneously captured with the help of a worm rotating device [45]. Shown are intracellular calcium traces for HSN (red trace), VC4 and VC5 (blue traces) as measured by cameleon. Dashed lines indicate the correlated spikes. (B) Effects of genetic ablations of HSN or VC neurons on the activity of the remaining motorneuron class. HSN- refers to animals carrying an egl-1 mutation, which causes inappropriate HSN apoptosis in hermaphrodites. The lin-39 mutation leads to the absence of all VC neurons; the vulval-proximal VCs (VC4 and VC5) were eliminated by laser ablation. (C) Histogram showing the mean and SEM of the spike frequencies in wild-type, HSN- and VC- animals as described in part (B). Loss of VC neurons leads to constitutive significantly higher activity in the HSNs, (p< 0.5 for lin-39 and for VC4/5 ablation; Mann Whitney rank test) indicating that the VCs normally inhibit the HSNs. Loss of HSN neurons leads to significantly reduced VC activity (p< .05; Mann Whitney rank test), indicating that the HSNs normally excite the VCs.
To investigate causal relationships between HSN and VC activities, we investigated how eliminating one class of motorneuron affected the activity of the other. We first analyzed egl-1(n986) mutants, in which the HSNs specifically and inappropriately undergo programmed cell death in hermaphrodites [26]. We observed that the VC neurons in egl-1 worms were largely inactive even under low-osmolarity conditions when VC is originally hyperactive (Figure 3B, C), suggesting that the HSNs are required to efficiently activate the VCs. We also analyzed lin-39(n709) mutant animals [27], in which the VC neurons undergo inappropriate cell death. Surprisingly, in these animals the HSN neurons showed increased activity compared to wild-type animals even in high osmolarity conditions when HSN is originally hypoactive (Figure 4B,C). We also found that laser ablation of VC4 and VC5 neurons cause hyperactivity in HSN calcium dynamics (Figure 4B,C). These data suggest that the VCs inhibit, rather than excite, the HSNs.
The egl-1 and lin-39 mutant strains also allowed us to determine whether neuromuscular excitation in the egg-laying circuit is mediated by the HSNs, the VCs, or both. We observed that in lin-39 mutant animals, which lack VC neurons, egg-laying events were still strongly correlated with HSN activity (Figure 3B), suggesting that the synapses from HSN to vulval muscles are excitatory and capable of inducing egg-laying events. Performing the analogous experiment in egl-1 worms was difficult since these animals have severely defective egg-laying behavior and nearly silent VCs under all conditions. To surmount this problem, we imaged VCs in egl-1 worms in the presence of exogenous serotonin, which is known to stimulate egg-laying in the absence of HSN neurons. In the presence of serotonin, VC activity was partially restored, and this activity was temporally coupled with egg-laying events (p<0.001), with egg-laying following the onset of the calcium transient by an average of 1.4 sec (Figure 4B). Together with the previous finding that exogenous serotonin does not stimulate egg-laying events if VC is ablated in the egl-1 background [15], our results suggest that the VCs directly excite the vulval muscles to induce egg-laying events.
Effects of serotonin and acetylcholine on the egg-laying circuit
We next investigated the roles of serotonin and acetylcholine, two neurotransmitters that are expressed in the HSN and VC motorneurons. Previous neuroimaging studies indicated that exogenous serotonin inhibits HSN activity [25]. To further investigate the role of serotonin, we analyzed a mutant defective in the essential serotonin biosynthetic gene tph-1. We observed that tph-1(mg280) null mutant worms (defective in tryptophan hydroxylase) [28] showed hyperactivity in the HSN neurons (Figure 5A), which correlated with egg-laying events (data not shown), and reduced activity in VCs (Figures 5A and 5B). Together with our previous finding (Figure 4B) that exogenous serotonin stimulates VC activity in HSN-deficient egl-1 mutants, these results support the hypothesis that serotonin directly enhances VC activity. Serotonin may therefore inhibit HSN activity either directly, or alternatively indirectly by promoting the VCs’ inhibitory effect on HSN.
Figure 5. Effect of serotonin and acetylcholine on HSN and VC activity.
(A) Representative calcium traces of HSN and VC neuronal cell bodies in tph-1 mutants (defective in serotonin synthesis) and cha-1 mutants (conditionally defective in acetylcholine synthesis). cha-1 animals were shifted to the restrictive temperature and recorded 7 minutes later.. (B) Histogram of calcium spike frequencies (mean/SEM) of spike frequencies in HSN and VC neurons. Serotonin deficient animals showed elevated HSN activity and silenced VC activity, and acetylcholine-deficient animals showed reduced VC activity (all p<.05 according to the Mann Whitney rank test).
To investigate the role of acetylcholine in the egg-laying circuit, we used a temperature-sensitive allele of the choline acetyltransferase gene, cha-1(y226), which upon transfer from 15 to 21°C rapidly undergoes a coiled paralysis characteristic of a strong defect in cholinergic neurotransmission. When we imaged neural activities in the egg-laying circuit approximately 7 minutes following a temperature shift from 15°C to 21°C, we observed significant activity in the HSNs but no activity in the VCs (Figure 5A, B). Moreover, under these conditions egg-laying behavior was strongly reduced (0.5 ± 0.4 eggs/15 minutes compared to 12.8 ± 1.3 for wild-type). These results suggest that acetylcholine is necessary for excitation of the VCs and the triggering of egg-laying events by the HSNs. Interestingly, calcium transients were still observed in both the vm1 and vm2 vulval muscles in temperature-shifted cha-1 mutants. In the vm1 cells, these transients were frequent and highly synchronous (Supplemental Figure 4A; 4C) while in the vm2s, we found lower frequency transients that were nevertheless highly synchronous (Supplemental Figure 4B, 4C). It is worth noting that these ACh-independent calcium transients do not lead to egg-laying. Together, these results suggest ACh and 5-HT may function as cotransmitters at egg-laying neuromuscular synapses, with 5-HT acting through a GPCR to modulate muscle activity and ACh acting through a nicotinic receptor to evoke individual egg-laying events.
Gentle body touch transiently inhibits HSN activity
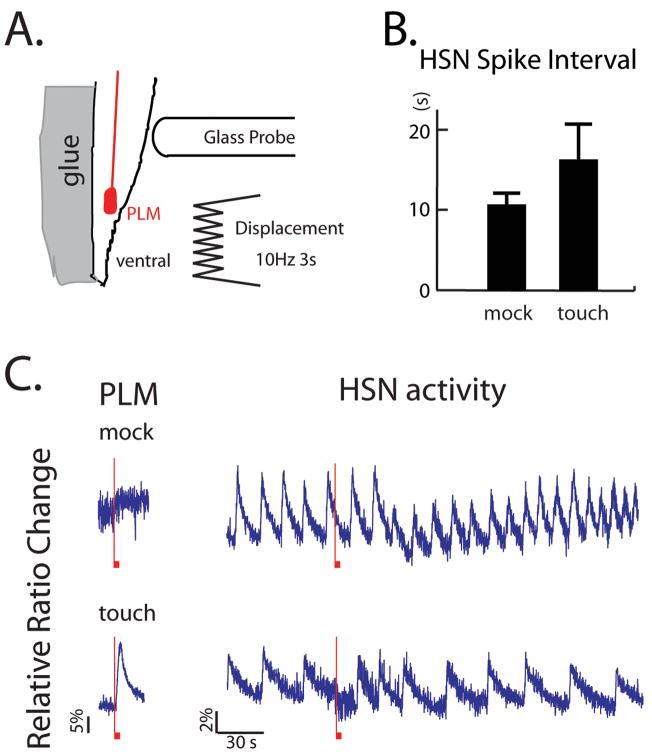
We were next interested in investigating the neural mechanisms by which egg-laying behavior might be controlled by sensory stimuli. It was shown previously that mechanical vibrations, sensed by the body touch mechanoreceptor neurons, inhibit egg-laying [24]. Since one of these neurons, the posterior touch receptor PLM, directly synapses onto the HSN cell body [11], we wondered whether activation of PLM might affect HSN activity. We stimulated PLM by applying gentle touch stimulation to the posterior body region, which is in the receptive field of PLM but not the other body touch receptors [9], and imaged HSN activity (Figure 6A). As expected, PLM was immediately activated upon mechanical stimulation (Figure 6C). In contrast, while HSN showed significant activity before and after touch stimulation, closer analysis of the HSN recording revealed that there was a small but significant increase in the duration of the interval between calcium transients containing the mechanical stimulation compared to mock-stimulated animals (p<0.05) (Figure 6B). These results indicate that PLM transiently inhibits HSN activity, an effect that may contribute to the inhibition of egg-laying behavior by gentle body touch.
Figure 6. The PLM posterior mechanoreceptor neuron transiently inhibits HSN.
(A) Diagram of the protocol for mechanical stimulation of PLM mechanoreceptor neurons. During the stimulus, the probe was moved against the animal’s skin in the indicated position for 3 seconds. (B) Average interval between stimulation (or mock stimulation) and the next calcium spike in HSN. The time lag from the stimulus to the first HSN calcium spike was significantly longer in stimulated animals than in mock-stimulated animals according to the Mann-Whitney rank sum test (p<.05). (C) Representative traces of cell body calcium for the PLM and HSN neurons during the mechanosensory stimulation protocol. The red vertical bars indicate the beginning of the stimulations and the boxes at the bottom indicate the time period of the stimulation.
HSN activity is not dependent on presynaptic excitation
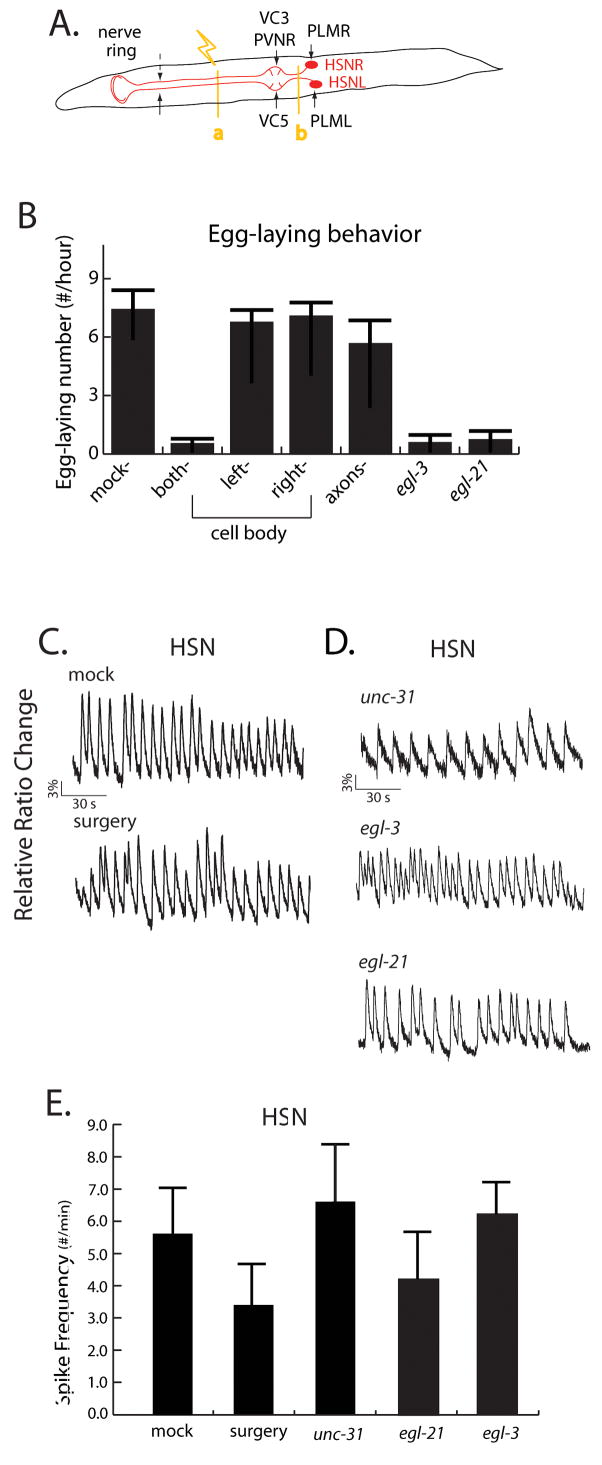
The finding that mechanosensory inputs negatively regulate egg-laying by inhibiting the HSNs raised the general question of how HSN activity is generated and controlled. In principle, HSN could be driven by excitatory inputs from presynaptic partners or from extrasynaptic neuromodulation. Alternatively, the HSNs could be autonomously active but modulated by neuromodulators and sensory cues. To investigate these possibilities, we first focused on whether the HSNs required extrinsic synaptic or extrasynaptic input for its activity. Most morphologically identified synapses to HSN occur within the nerve ring, a few (from PVNR, VC3 and VC5) occur on the neuronal processes near the vulva, and single synapses from mechanosensory neurons are made on the HSN cell body (Figure 1A, 7A) [11].
Figure 7. Independence of HSN activity from synaptic and neuropeptide inputs.
(A) An illustration of synaptic inputs on the HSN cell bodies and axons as described by White et al. (1986). The targets for femtosecond laser axotomy (a and b) are indicated by the yellow lines. (B) Egg-laying behavior of laser operated worms. Elimination of HSN inputs from the nerve ring (cutting at position a) does not cause a detectable egg-laying defect, consistent with the hypothesis that HSN is autonomously active. HSN doubly-ablated animals and egl-3 and egl-21 mutants laid significantly fewer eggs than mock-ablated animals (p<.05; Mann Whitney rank test). (C) Calcium transients in the HSN cell bodies of axotomized and mock-ablated animals. Axons were severed near the cell body (position b), and emission ratios from cameleon expressed in the HSN cell body were measured under low osmolarity conditions. (D) Calcium transients in the HSN cell bodies of unc-31(e928), egl-3(ok979) and egl-21(n476) mutant worms. Animals were imaged under permissive, low osmolarity conditions. While significant variability was observed in all types, mutant neurons showed oscillation frequencies comparable to or sometimes higher than wild-type. (E) Average spike frequencies in HSN for axotomized and mutant animals.
To determine the effect of the nerve ring input to HSN activity, we severed the HSN axons using femtosecond laser ablation [10, 29, 30]. We observed animals ablated between the nerve ring and the vulva (indicated as the point “a” in Figure 7A) showed mostly normal egg-laying behavior (Figure 7B), suggesting that the HSNs were still active in these animals. We also ablated either the HSNL cell body, which should eliminate the input from PVNR and VC3, or the HSNR cell body, which should eliminate the input from VC5. These ablated animals laid eggs normally, suggesting that neither of the PVNL, VC3 or VC5 neurons was necessary to drive HSN activity. Finally, we directly imaged HSN activity in animals with severed axons. For these experiments, the HSN axons of animals expressing cameleon in the HSNs were cut between the cell body and the vulva (indicated as the point “b” in Figure 7A), which eliminates all synapses to and from the HSNs except for the PLM synapses (already inferred to be inhibitory). When calcium transients in the HSN cell body were imaged under permissive low-osmolarity conditions, we observed normal oscillatory activity patterns in both operated and intact animals (Figure 7C, 7E). Taken together, these results suggest that HSN activity does not require activation by excitatory presynaptic input.
Neuropeptides are not essential for HSN activity
Next, we investigated whether HSN activity is dependent on neuroendocrine or extrasynaptic signaling. C. elegans uses at least four monoamine neuromodulators (serotonin, dopamine, octopamine and tyramine) as well as diverse neuropeptides (Husson et al., 2005; Kim and Li, 2004; Li et al., 2004; Li et al., 1999; Li et al., 2003; McVeigh et al., 2005; Nathoo et al., 2001; Pierce-Shimomura et al., 2001). To assess the possible roles of these molecules in HSN activation, we first imaged HSN calcium transients in an unc-31 null mutant, which is specifically defective in dense core vesicle release [31, 32]. The HSNs showed robust and rhythmic activity in these mutant animals (Figure 7D, E), indicating that neuropeptides and monoamines are not essential for HSN activity. We also analyzed a deletion allele of egl-3, which encodes a proprotein convertase required for the processing of all known C. elegans neuropeptides [33–35], and a null allele of egl-21, a carboxypeptidase also known to be important for neuropeptide processing (Jacob and Kaplan, 2003). Again, HSN cell bodies in these animals showed robust and rhythmic calcium transients (Figure 7D, E), suggesting that neuropeptides are not necessary for HSN activity. We had shown previously that a severe loss-of-function allele of unc-13, functioning in fast synaptic vesicle release, likewise does not compromise HSN activity [36]. Taken together, these results suggest that HSN activity does not require direct neuroendocrine or extrasynaptic input, and are consistent with the possibility that the HSNs are autonomously active.
DISCUSSION
Our imaging studies have allowed us to understand more precisely the roles of specific neurons in the egg-laying circuit. As expected, the neurons most critically important for egg-laying are the HSN motorneurons. We observed that HSN activity is highly correlated with egg-laying, even when the VCs have been eliminated, indicating that HSN activity directly evokes individual egg-laying events. The HSNs are also highly correlated in activity with the VCs, and are necessary for VC activation. Thus, the HSNs appear to activate the egg-laying muscles both directly and indirectly through the VCs.
Calcium spikes in the motor neurons and (perhaps surprisingly) the vulval muscles do not necessarily correlate with egg-laying events. It is not clear from vulval muscle calcium imaging what distinguishes transients that lead to egg-laying from those that do not. Interestingly, interfering with acetylcholine neurotransmission appears to block egg-laying but does not completely suppress vulval muscle calcium transients. This, along with the correlation between motorneuron spikes and egg-laying, is consistent with the possibility that egg-laying events require cholinergic neurotransmission from the motorneurons. Previous behavioral analyses likewise have suggested that ACh is involved in inducing individual egg-laying events during periods of active egg-laying (Waggoner et al., 1998). Potentially, cholinergic excitation may be necessary to coordinate simultaneous excitation of all eight vulval muscles, which may be required for a successful egg-laying event.
Interestingly, HSN appears to exhibit spontaneous activity that does not require extrinsic neuronal input. This suggests that the control of egg-laying is accomplished by modulation of the intrinsic activity of the HSNs. In fact, we observed that touch stimuli inhibit egg-laying by transiently suppressing the spontaneous activity of the HSN neurons. This may represent a general mechanism for regulating egg-laying in C. elegans. For example, EGL-47 encodes a G-protein coupled receptor whose wild-type activity negatively regulates egg-laying through control of HSN activity [37]. Although the EGL-47 ligand is not known, it is thought to be a neuropeptide that might act either synaptically or humorally to control egg-laying in response to sensory or homeostatic cues. In principle other peptide or monoamine neuromodulators [22, 38, 39] might also exert their effects on egg-laying by regulating the activity of the HSNs.
Neuropeptides and monoamine neuromodulators may also act directly on the vulval muscles. Consistent with this possibility, egl-3 and egl-21 mutants, while strongly egg-laying defective, exhibited normal patterns of HSN activity. This suggests that one or more neuropeptides may be required to potentiate the ability of the HSNs and VCs to evoke egg-laying events. The mode of regulation we have observed in the egg-laying circuit, in which hormones and neuromodulators modify the activity of an autonomously active motor circuit, is conceptually and mechanistically similar to the control of involuntary motor programs by the autonomic nervous system in mammals. Thus, egg-laying in C. elegans may serve as a useful model for understanding how neural circuits of this type respond to environmental context and behavioral state.
Our results also provide information about the role of the VC motorneurons in the egg-laying circuit. We found that calcium transients in the VCs were dependent on the HSNs and were temporally correlated with HSN calcium transients. These findings lead us to infer that the HSNs excite the VCs. Additionally, VC calcium transients are temporally correlated with egg-laying events, even in the absence of the HSNs; thus, the VCs appear to play a redundant role in exciting the vulval muscles. These data help explain the apparently paradoxical findings that VC ablation can confer either an egg-laying defective or an egg-laying constitutive phenotype, depending on the experimental context. Our results indicating that the VCs negatively regulate egg-laying by inhibiting HSN activity are consistent with previous genetic results indicating the HSNs as a target of negative regulation by the VCs [14]. Likewise, the apparent redundancy of the VCs stimulatory effect on the vulval muscles is consistent with the earlier observation that VC ablation leads to slower egg-laying most clearly in animals lacking functional HSNs [15].
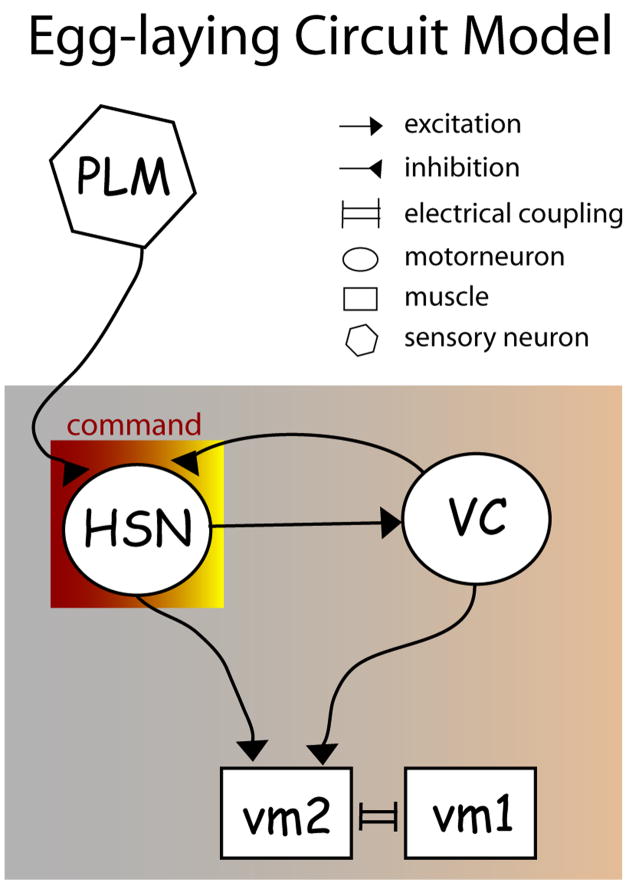
The functional architecture of the egg-laying circuit (Figure 8) has intriguing parallels in C. elegans as well as in other organisms. A recent analysis [40] found that the connectivity pattern seen in the egg-laying motor circuit, in which two reciprocally-connected cells direct synaptic output to the same target, was significantly overrepresented in the C. elegans nervous system. It is thought that such overrepresented motifs may represent functional units, analogous to circuit elements in a computer, that perform key computational functions [41]. In the case of the egg-laying circuit, this circuit combines the properties of a negative feedback loop (the HSNs excite the VCs, which in turn feedback inhibit the HSNs) and feed-forward stimulation (the HSNs excite the vulval muscles both directly and indirectly through the VCs). Interestingly, the aforementioned connectivity pattern was recently shown to also be overrepresented in the mammalian cerebral cortex [42]. Thus, studies of the C. elegans egg-laying circuit may provide fundamental insights into the properties of microcircuits that underlie the workings of much more complicated brains.
Figure 8. Functional connectivity in the egg-laying motor circuit.
Shown are the excitatory and inhibitory connections in the egg-laying circuit inferred from these studies. Both the HSNs and VCs excite the vulval muscles, and the HSNs excite the VCs, generating a feed-forward circuit exciting the vulval muscles. The VCs also inhibit the HSNs, creating a negative feedback loop between the HSNs and VCs. The PLMs inhibit the HSNs to mediate touch inhibition of egg-laying.
EXPERIMENTAL METHODS
General Methods and Strains
All nematodes were grown at 20.5+0.5°C on standard Nematode Growth Medium (NGM) seeded with E. coli strain OP50 as the food source, except the cha-1(y226) temperature sensitive mutant, which was maintained at 15°C. Nematodes were assayed 24 hours after late L4 stage at 20.5+0.5°C, except cha-1(y226), which was assayed 30 hours after later L4 stage. Integrated transgenic arrays IjIs25[myo-3::YC2.0] and ljIs19[cat-1::iYC2.1] were generated by gamma irradiation (5000 rad) of existing transgenic lines (kyEx302 and ljEx65 respectively), followed by 7 and 6 generations of back-crossing respectively. Neither line showed obvious differences compared to the wild type N2 strain in egg-laying behavior. Mutant nematode strains, egl-1(n986), lin-39 (n709ts), tph-1(mg280) and cha-1(y226), were crossed to ljIs25 to generate cameleon lines for Ca2+ imaging experiments. For surgery experiments, we used the transgenic strain SK4013, a wild-type N2 background strain with the integrated transgene zdIs13[tph-1::gfp] [28, 43], obtained from Scott Clark.
Ca2+ Imaging Experiments
Combined fluorescent and visible microscopy was used to view both the Ca2+ signal and egg-laying events simultaneously. The microscope equipment was largely as described previously [9], briefly, a Zeiss Axioskop 2 upright microscope equipped with a Hamamatsu Orca ER CCD camera, a Hamamatsu W-View emission image splitter and a Uniblitz Shutter (Vincent Associates). Filter/dichroic pairs were: excitation, 420/40; excitation dichroic 455; CFP emission, 480/30; emission dichroic 505; YFP emission, 535/30 (Chroma). A minimal level of fluorescent light was used in order to reduce photobleaching and phototoxicity; for our microscope, this involved using 20–60% of the maximal power to the lamp with a neutral density filter (ND) of 2.0. Weak visible light, barely revealing egg-laying events, was used to minimize interference with the cameleon fluorescent signal (as shown in Supplemental Figure 1). Fluorescent images were acquired and saved using MetaVue 4.6 (Universal Imaging) at a frequency of either 33hz or 10 Hz for muscle and neuron imaging respectively (binning 4×4), using a 63X Zeiss Achroplan water immersion objective. Photobleaching was corrected by fitting the baseline YFP/CFP ratio to a single exponential decay; bleedthrough was corrected using an RCFP value of 0.6 [44].
Worms were immobilized with Nexaband S/C cyanoacrylate veterinary glue on a small agarose pad with the buffer of choice freshly made on a microscopy slide. The worms were then quickly covered with the buffer of choice and immediately moved under the microscope for recording. For HSN and vulval muscle imaging, the agarose pad was made in the same buffer as the bath. For VC imaging, a 2% agarose pad in M9 was used, regardless of the bath buffer, due to the fast adaptation of VCs to low osmolarity conditions. Agarose pads were made immediately before slide preparation in order to minimize the loss of focus in long recordings (10 mins), which usually is caused by swelling of the agarose pad. Worms were allowed to equilibrate for 2–4 mins before the start of recording.
Acute osmotic shock experiments
A flow chamber was created by connecting a small reservoir (RC-26GLP, Warner Instruments) to inflow buffer and vacuum lines, which allowed for continuous buffer exchange. The chamber was originally filled with 1 ml of the starting buffer and gradually changed over 1–2 mins (flow rate of about 0.3–0.4 ml/min) to the new buffer after the syringe and vacuum connections were opened at the indicated time, 30 seconds after the beginning of the recording.
PDMS mold for rotating and imaging worms
We poured liquid unpolymerized polydimethylsiloxane (PDMS) from Sylgard 184 Silicone Elastomer Kit on top of a 12-inch long-playing vinyl record to make a negative replica mold of the record channels. We polymerized the PDMS mold by baking overnight at 50C. By fabricating agarose pads against ridges of the PDMS mold, we formed channels on the surface of the agarose pad. L4 to young adult worms fit within the channels, which hold them in a fixed orientation for imaging or surgery. By observing body markings, worms can be quickly rotated by pick to a desired orientation. At the L4 to young adult stage, the vulva protrudes significantly from the rest worm’s body. As the coverslip is placed on top of worms on a flat agarose pad, the protruding vulva is pushed to the side, forcing the worm into a lateral imaging orientation. The channels lower the worm into the agarose, decreasing the force of the coverslip against the vulva. When the coverslip is introduced, the worm will retain their orientation.
Femtosecond Laser Ablation of HSN cell bodies and Axons
Femtosecond laser ablation of the HSN neurons was performed on young adult worms using established procedures. Young adult worms were anesthetized using 1.5 mM sodium azide and imaged on an epi-fluorescent microscope for surgery. A regeneratively-amplified Titanium:sapphire laser (■ = 800 nm) delivered a10 -kHz train of 80–90-fs pulses, which were attenuated to 2–3-nJ pulse energy and tightly focused by a 1.4-NA, 63x oil-immersion objective onto the GFP-fluorescent HSN neurons. We severed HSN axons by irradiation for less than 0.5 s, typically at a location halfway between the cell body and the nerve ring. We ablated HSN cell bodies by irradiation of the soma two or three times for 1 s. Usually the disappearance of only the nuclear fluorescence was noted within the first second of irradiation. We confirmed the surgeries by fluorescent reimaging after assay.
Microscopic PLM activation
Experiments were performed as described previously [9]. Briefly, a smooth glass rod about 15 um in diameter was mounted on a three-axis manipulator through a M-111.1DG microtranslation stage (Polytec PI) and positioned perpendicular to and barely touching the ventral side of an immobilized worm, about 5–10 um anterior to the PLM cell body. A “buzz” stimulus, in which the probe was continuously vibrated aganist the body with 10 μm of displacement at 10 Hz for 3 seconds, was delivered as described [9]. A LED light flashed once to indicate the beginning of the stimulation.
To test the statistical significance of the delay in HSN activity induced by PLM activation, we took advantage of the fact that all spike intervals except the one just following the stimulation from single worm recording are found to follow a normal distribution, as confirmed by both the Lilliefors test and the Jarque-Bera test. Thus, a fitted normal distribution was calculated for each experiment. The time interval just following the stimulation was then compared to the fitted distribution of that single worm and the standard Z-score is calculated. All the Z-scores from the stimulating experiment and also the mock experiment were then compared using the Mann Whitney rank test.
Supplementary Material
Acknowledgments
We would like to thank Prof. Peter Swoboda (Karolinska Institute, Department of Biosciences and Nutrition, Södertörn University College) for discussions, Calvin Mok for technical assistance, and Robyn Branicky, Marina Ezcurra and Prof. Rainer Breitling (Groningen Bioinformatics Centre, University of Groningen) for helpful input and critical readings of this manuscript. Some strains were provided by the Caenorhabditis Genetics Center. This work was supported by grants to WRS from NIDA and HFSP. SHC is supported by NSF DMR-0213805 and PHY-0555583.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marder E, Bucher D, Schulz DJ, Taylor AL. Invertebrate central pattern generation moves along. Curr Biol. 2005;15:R685–699. doi: 10.1016/j.cub.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 2.Nusbaum MP, Beenhakker MP. A small-systems approach to motor pattern generation. Nature. 2002;417:343–350. doi: 10.1038/417343a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selverston AI. A neural infrastructure for rhythmic motor patterns. Cell Mol Neurobiol. 2005;25:223–244. doi: 10.1007/s10571-005-3154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill AA, Masino MA, Calabrese RL. Intersegmental coordination of rhythmic motor patterns. J Neurophysiol. 2003;90:531–538. doi: 10.1152/jn.00338.2003. [DOI] [PubMed] [Google Scholar]
- 5.Cangiano L, Grillner S. Mechanisms of rhythm generation in a spinal locomotor network deprived of crossed connections: the lamprey hemicord. J Neurosci. 2005;25:923–935. doi: 10.1523/JNEUROSCI.2301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grillner S. The motor infrastructure: from ion channels to neuronal networks. Nat Rev Neurosci. 2003;4:573–586. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- 7.Hellgren J, Grillner S, Lansner A. Computer simulation of the segmental neural network generating locomotion in lamprey by using populations of network interneurons. Biol Cybern. 1992;68:1–13. doi: 10.1007/BF00203132. [DOI] [PubMed] [Google Scholar]
- 8.Kerr R, Lev-Ram V, Baird G, Vincent P, Tsien RY, Schafer WR. Optical imaging of calcium transients in neurons and pharyngeal muscle of C. elegans. Neuron. 2000;26:583–594. doi: 10.1016/s0896-6273(00)81196-4. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki H, Kerr R, Bianchi L, Frokjaer-Jensen C, Slone D, Xue J, Gerstbrein B, Driscoll M, Schafer WR. In vivo imaging of C. elegans mechanosensory neurons demonstrates a specific role for the MEC-4 channel in the process of gentle touch sensation. Neuron. 2003;39:1005–1017. doi: 10.1016/j.neuron.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Chung SH, Clark DA, Gabel CV, Mazur E, Samuel AD. The role of the AFD neuron in C. elegans thermotaxis analyzed using femtosecond laser ablation. BMC Neurosci. 2006;7:30. doi: 10.1186/1471-2202-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White J, Southgate E, Thomson J, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Phil Trans R Soc Lond (Biol) 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 12.Trent C, Tsung N, Horvitz HR. Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics. 1983;104:619–647. doi: 10.1093/genetics/104.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai C, Horvitz HR. Caenorhabditis elegans mutants defective in the functioning of the motor neurons responsible for egg laying. Genetics. 1989;121:703–721. doi: 10.1093/genetics/121.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bany IA, Dong MQ, Koelle MR. Genetic and cellular basis for acetylcholine inhibition of Caenorhabditis elegans egg-laying behavior. J Neurosci. 2003;23:8060–8069. doi: 10.1523/JNEUROSCI.23-22-08060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waggoner LE, Zhou GT, Schafer RW, Schafer WR. Control of alternative behavioral states by serotonin in Caenorhabditis elegans. Neuron. 1998;21:203–214. doi: 10.1016/s0896-6273(00)80527-9. [DOI] [PubMed] [Google Scholar]
- 16.Duerr JS, Han HP, Fields SD, Rand JB. Identification of major classes of cholinergic neurons in the nematode Caenorhabditis elegans. J Comp Neurol. 2008;506:398–408. doi: 10.1002/cne.21551. [DOI] [PubMed] [Google Scholar]
- 17.Schinkmann K, Li C. Localization of FMRFamide-like peptides in Caenorhaditis elegans. J Comp Neurol. 1992;316:251–260. doi: 10.1002/cne.903160209. [DOI] [PubMed] [Google Scholar]
- 18.Nathoo AN, Moeller RA, Westlund BA, Hart AC. Identification of neuropeptide-like protein gene families in Caenorhabditiselegans and other species. Proc Natl Acad Sci U S A. 2001;98:14000–14005. doi: 10.1073/pnas.241231298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schafer WF. Genetics of egg-laying in worms. Annu Rev Genet. 2006;40:487–509. doi: 10.1146/annurev.genet.40.110405.090527. [DOI] [PubMed] [Google Scholar]
- 20.Zhou GT, Schafer WR, Schafer RW. A three-state biological point process model and its parameter estimation. IEEE Trans on Signal Processing. 1998;46:2698–2707. [Google Scholar]
- 21.Waggoner LE, Hardaker LA, Golik S, Schafer WR. Effect of a neuropeptide gene on behavioral states in Caenorhabditis elegans egg-laying. Genetics. 2000;154:1181–1192. doi: 10.1093/genetics/154.3.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alkema MJ, Hunter-Ensor M, Ringstad N, Horvitz HR. Tyramine functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron. 2005;46:247–260. doi: 10.1016/j.neuron.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 23.Daniels SA, Ailion M, Thomas JH, Sengupta P. egl-4 acts through a transforming growth factor-beta/SMAD pathway in Caenorhabditis elegans to regulate multiple neuronal circuits in response to sensory cues. Genetics. 2000;156:123–141. doi: 10.1093/genetics/156.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawin ER. PhD Thesis thesis. M. I. T; Cambridge, MA: 1996. Genetic and cellular analysis of modulated behaviors in Caenorhabditis elegans. [Google Scholar]
- 25.Shyn SI, Kerr R, Schafer WR. Serotonin and Go modulate functional states of neurons and muscles controlling C. elegans egg-laying behavior. Curr Biol. 2003;13:1910–1915. doi: 10.1016/j.cub.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 26.Conradt B, Horvitz HR. The C. elegans protein EGL-1 is required for programmed cell death and interacts with the Bcl-2-like protein CED-9. Cell. 1998;93:519–529. doi: 10.1016/s0092-8674(00)81182-4. [DOI] [PubMed] [Google Scholar]
- 27.Li C, Chalfie M. Organogenesis in C. elegans: positioning of neurons and muscles in the egg-laying system. Neuron. 1990;4:681–695. doi: 10.1016/0896-6273(90)90195-l. [DOI] [PubMed] [Google Scholar]
- 28.Sze JY, Victor M, Loer C, Shi Y, Ruvkun G. Food and metabolic signaling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature. 2000;403:560–564. doi: 10.1038/35000609. [DOI] [PubMed] [Google Scholar]
- 29.Yanik MF, Cinar H, Cinar HN, Chisholm AD, Jin Y, Ben-Yakar A. Neurosurgery: functional regeneration after laser axotomy. Nature. 2004;432:822. doi: 10.1038/432822a. [DOI] [PubMed] [Google Scholar]
- 30.Shen N, Datta D, Schaffer CB, LeDuc P, Ingber DE, Mazur E. Ablation of cytoskeletal filaments and mitochondria in live cells using a femtosecond laser nanoscissor. Mech Chem Biosyst. 2005;2:17–25. [PubMed] [Google Scholar]
- 31.Hammarlund M, Watanabe S, Schuske K, Jorgensen EM. CAPS and syntaxin dock dense core vesicles to the plasma membrane in neurons. J Cell Biol. 2008;180:483–491. doi: 10.1083/jcb.200708018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Speese S, Petrie M, Schuske K, Ailion M, Ann K, Iwasaki K, Jorgensen EM, Martin TF. UNC-31 (CAPS) is required for dense-core vesicle but not synaptic vesicle exocytosis in Caenorhabditis elegans. J Neurosci. 2007;27:6150–6162. doi: 10.1523/JNEUROSCI.1466-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomez-Saladin E, Luebke AE, Wilson DL, Dickerson IM. Isolation of a cDNA encoding a Kex2-like endoprotease with homology to furin from the nematode Caenorhabditis elegans. DNA Cell Biol. 1997;16:663–669. doi: 10.1089/dna.1997.16.663. [DOI] [PubMed] [Google Scholar]
- 34.Husson SJ, Clynen E, Baggerman G, Janssen T, Schoofs L. Defective processing of neuropeptide precursors in Caenorhabditis elegans lacking proprotein convertase 2 (KPC-2/EGL-3): mutant analysis by mass spectrometry. J Neurochem. 2006;98:1999–2012. doi: 10.1111/j.1471-4159.2006.04014.x. [DOI] [PubMed] [Google Scholar]
- 35.Husson SJ, Janssen T, Baggerman G, Bogert B, Kahn-Kirby AH, Ashrafi K, Schoofs L. Impaired processing of FLP and NLP peptides in carboxypeptidase E (EGL-21)-deficient Caenorhabditis elegans as analyzed by mass spectrometry. J Neurochem. 2007;102:246–260. doi: 10.1111/j.1471-4159.2007.04474.x. [DOI] [PubMed] [Google Scholar]
- 36.Yeh E, Ng S, Zhang M, Bouhours M, Wang Y, Wang M, Hung W, Aoyagi K, Melnik-Martinez K, Li M, Liu F, Schafer WR, Zhen M. A putative cation channel, NCA-1, and a novel protein, UNC-80, transmit neuronal activity in C. elegans. PLoS Biol. 2008;6:e55. doi: 10.1371/journal.pbio.0060055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moresco JJ, Koelle MR. Activation of EGL-47, a Galpha(o)-coupled receptor, inhibits function of hermaphrodite-specific motor neurons to regulate Caenorhabditis elegans egg-laying behavior. J Neurosci. 2004;24:8522–8530. doi: 10.1523/JNEUROSCI.1915-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacob TC, Kaplan JM. The EGL-21 carboxypeptidase E facilitates acetylcholine release at Caenorhabditis elegans neuromuscular junctions. J Neurosci. 2003;23:2122–2130. doi: 10.1523/JNEUROSCI.23-06-02122.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kass J, Jacob TC, Kim P, Kaplan JM. The EGL-3 proprotein convertase regulates mechanosensory responses of Caenorhabditis elegans. J Neurosci. 2001;21:9265–9272. doi: 10.1523/JNEUROSCI.21-23-09265.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reigl M, Alon U, Chklovskii DB. Search for computational modules in the C. elegans brain. BMC Biol. 2004;2:25. doi: 10.1186/1741-7007-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milo R, Shen-Orr S, Itzkovitz S, Kashtan N, Chklovskii D, Alon U. Network motifs: simple building blocks of complex networks. Science. 2002;298:824–827. doi: 10.1126/science.298.5594.824. [DOI] [PubMed] [Google Scholar]
- 42.Song S, Sjostrom PJ, Reigl M, Nelson S, Chklovskii DB. Highly nonrandom features of synaptic connectivity in local cortical circuits. PLoS Biol. 2005;3:e68. doi: 10.1371/journal.pbio.0030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark SG, Chiu C. C. elegans ZAG-1, a Zn-finger-homeodomain protein, regulates axonal development and neuronal differentiation. Development. 2003;130:3781–3794. doi: 10.1242/dev.00571. [DOI] [PubMed] [Google Scholar]
- 44.Kerr RA. Imaging the activity of neurons and muscles. WormBook. 2006:1–13. doi: 10.1895/wormbook.1.113.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki H, Thiele TR, Faumont S, Ezcurra M, Lockery SR, Schafer WR. Functional asymmetry in Caenorhabditis elegans taste neurons and its computational role in chemotaxis. Nature. 2008;454:114–117. doi: 10.1038/nature06927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kerr RA. Imaging the activity of neurons and muscles. Wormbook, ed. the C. elegans Research Community, Wormbook. 2006 doi: 10.1895/wormbook.1.113.1. http://wormbook.org. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.